Abstract
Sudden Cardiac Death (SCD) is a common cause of death in patients with structural heart disease, genetic mutations or acquired disorders affecting cardiac ion channels. A wide range of platforms exist to model and study disorders associated with SCD. Human clinical studies are cumbersome and are thwarted by the extent of investigation that can be performed on human subjects. Animal models are limited by their degree of homology to human cardiac electrophysiology including ion channel expression. Most commonly used cellular models are cellular transfection models, which are able to mimic the expression of a single ion channel offering incomplete insight into changes of the action potential profile. Induced pluripotent stem cell derived Cardiomyocytes (iPSC-CMs) resemble, but are not identical, to adult human cardiomyocytes, and provide a new platform for studying arrhythmic disorders leading to SCD. A variety of platforms exist to phenotype cellular models including conventional and automated patch clamp, multi-electrode array, and computational modeling. iPSC-CMs have been used to study Long QT syndrome, catecholaminergic polymorphic ventricular tachycardia, hypertrophic cardiomyopathy and other hereditary cardiac disorders. Although iPSC-CMs are distinct from adult cardiomyocytes, they provide a robust platform to advance the science and clinical care of SCD.
Keywords: induced pluripotent stem cells, cardiovascular diseases, disease modeling, drug discovery, drug-induced proarrhythmia, sudden cardiac death
Introduction
Sudden cardiac death (SCD) refers to death from an unexpected circulatory arrest, usually due to a cardiac arrhythmia occurring within a brief time period of the onset of symptoms.1 This condition is a common cause of death in patients with various forms of structural heart disease, genetic mutations, or acquired disorders affecting cardiac ion currents. Due to the lack of a uniform definition and systematic autopsy evaluations, the epidemiology and incidence of SCD are not accurately known, but the latter is believed to range between 184,000 and 462,000 cases per year in the United States.2 The subset of SCD patients without coronary or structural heart disease represents a substantial minority of cases that are difficult to identify and treat. Despite advances in risk stratification and elucidating mechanisms of SCD in patients without structural heart disease, significant knowledge gaps exist in the pathophysiology and risks associated with the individual disorders for this profile. There is a wide range of platforms to evaluate arrhythmic disorders leading to SCD, encompassing studying individual ion channel behavior to organism-level electrophysiology data. Induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) offer a novel modeling platform for studying these disorders. Furthermore, iPSC-CMs have a tremendous potential in advancing arrhythmia science and the clinical care of patients at risk for SCD. In this article, we explore potential applications of iPSC-CMs in the study of SCD in the context of the range of experimental platforms available.
Sudden Cardiac Death
Patients at risk for SCD are divided into three broad categories: (1) those with known structural heart disease such as coronary artery disease, left ventricular dysfunction, or hypertrophic cardiomyopathy that predisposes them to the development of arrhythmias; (2) those without structural heart disease, but who harbor an underlying genetic predisposition to the development of arrhythmias; and (3) those with no known predisposing factors who develop SCD in response to exogenous or acquired factors, most commonly drugs or metabolic disturbances.3, 4
Those with structural heart disease form the largest group of patients with SCD and are more likely to present with symptoms and to have traditional risk factors for SCD.3, 5 Non-invasive cardiac imaging has the potential to identify those patients if they are successfully screened, but there is no evidence to support population level screening for SCD. Nevertheless, tools are available to screen a subset of the population who are at high risk for SCD, such as those with coronary artery disease, post-myocardial infarction, or cardiomyopathy.
Patients who have SCD without structural heart disease tend to be younger, asymptomatic individuals and sometimes even elite athletes.4, 6 These patients may or may not have a family history of SCD, and SCD is often the initial presentation of their disease. This group represents 5–10% of the total cases of SCD and is a challenging group to identify and risk stratify. Efforts to identify screening tools for this population have proven arduous.7 The majority of these patients have underlying genetic cardiac ion channel disorders that predispose them to arrhythmic death.8 Even in the absence of family history, these individuals commonly have a genetic component contributing to their risk of SCD. The variable expression in the absence of affected relatives could be due to environmental triggers, germ line mutations, or modifier effects of polygenic disorders.
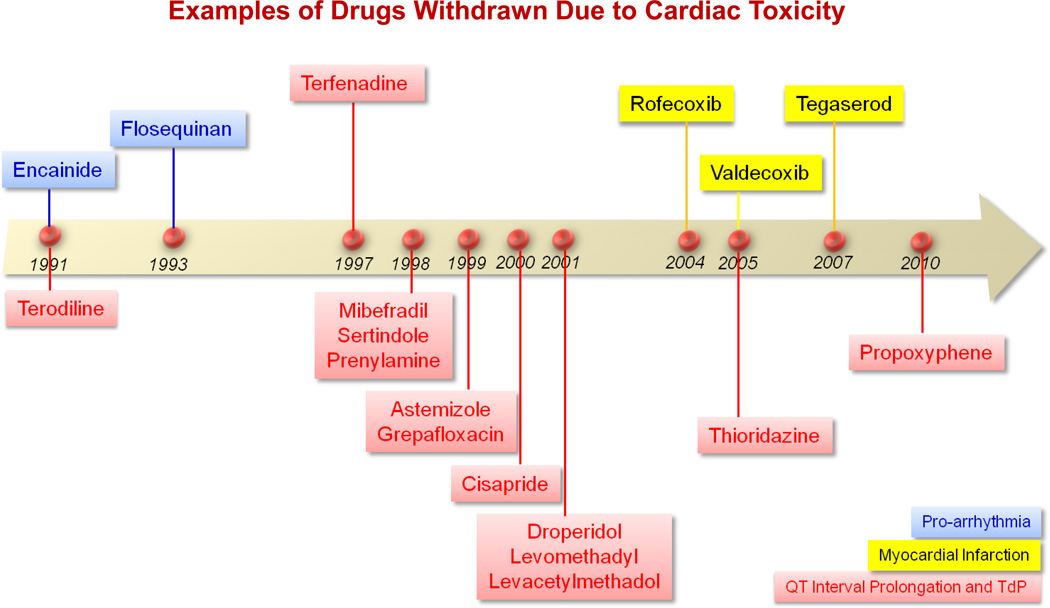
Drugs given to patients for a variety of purposes can cause cardiac arrhythmias and SCD. Drug-induced arrhythmia and SCD continue to be a significant concern in drug safety testing and have been a major reason for post-marketing drug warnings or drug withdrawal (Figure 1).9 In fact, the assessment of the potential risk of developing drug-induced Torsades de pointes (TdP) arrhythmia, by measuring drug effects on a surrogate marker (such as the QTc interval), is a mandatory component of drug testing prior to approval.10 Individuals who develop drug-induced arrhythmias may have an underlying genetic or structural heart disease that predisposes them to developing arrhythmias, but these arrhythmias may also be observed in patients with no known abnormalities.11–14 A low-event rate often delays awareness of the toxicity until data from large clinical trials or even post-marketing data are available. QT testing during drug development has been used to assess arrhythmia risk. This approach carries a high sensitivity but a low specificity for predicting arrhythmogenesis.15–17 This has led to a marked reduction in approval of drugs with unrecognized potential liability to cause arrhythmias. For example, drugs with possible favorable benefit-to-risk profiles that could potentially address major unmet medical needs have been discontinued from development solely on the basis of a QT prolonging effect.18 This concern has led to a major ongoing effort to directly measure a drug’s propensity to cause proarrhythmia instead of relying on the QT interval, which remains a low specificity surrogate.19
Figure 1. Examples of drugs that have been withdrawn due to cardiac safety issues.
Drugs withdrawn due to pro-arrhythmic effect include Encainide (1991) and Flosequinan (1993). Drugs withdrawn because of their potential to induce a myocardial infarction include Rofecoxib (2004), Valdecoxib (2005), and Tegaserod (2007). Drugs withdrawn due to QT interval prolongation effect and possible TdP include Terodiline (1991), Terfenadine (1997), Mibefradil (1998), Sertindole (1998), Prenylamine (1998), Astemizole (1999), Grepafloxacin (1999), Cisapride (2000), Droperidol (2001), Levomethadyl (2001), Levacetylmethadol (2001), Thioridazine (2005), and Propoxyphene (2010).
SCD without structural heart disease constitutes an important cohort of patients with SCD, and the spectrum of pathology underlying those cases remains an elusive and active area of scientific inquiry. The broad spectrum of pathologies leading to SCD has led to an equally extensive set of research tools to study these disorders. Here we will present traditional platforms available to study diseases predisposing to SCD, by highlighting the role of patient- and disease-specific iPSC-CMs as a novel platform with significant scientific and clinical potential in studying disorders related to SCD. The discussion of platforms available to study arrhythmic disorders will be conducted in the context of studying inherited or acquired disorders leading to SCD.
Platforms for Studying Arrhythmic Disorders Predisposing Patients to SCD and Cardiac-Safety Assessment of Drugs
The available platforms to study arrhythmic disorder and tailoring drug therapy include:
Organism Level (clinical studies and animal models);
Organ & Tissue Level (Langendorff technology, cardiac slices, and Purkinje fibers);
Cellular & Molecular Level (cardiomyocyte study, cardiac ion channels study, and iPSC-CMs).
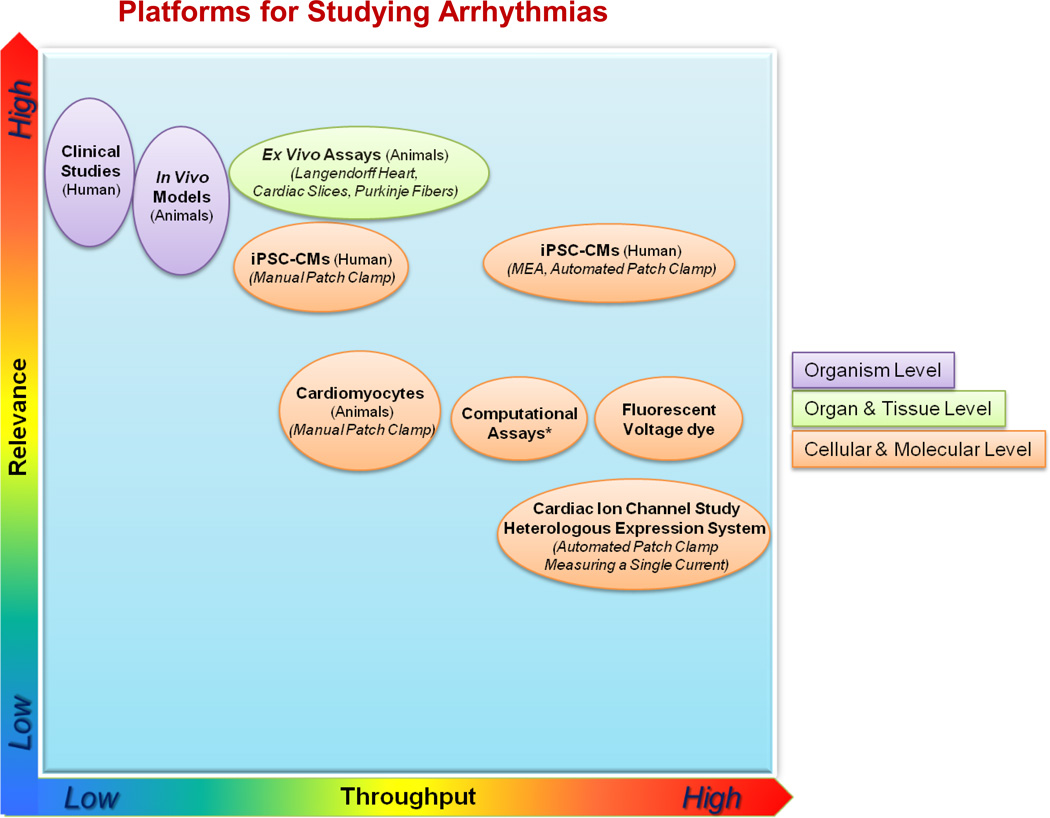
In the following section, the existing platforms employed to assess arrhythmic disorders predisposing patients to proarrhythmia liability of drugs20 (as listed in Figure 2) and to SCD are reviewed briefly. The relevance and throughput for tailoring drug therapy are discussed.
Figure 2. Platforms to assess arrhythmic disorders predisposing to sudden cardiac death and proarrhythmia liability of drugs.
Organism-level platforms include human clinical studies and in vivo animal models that could evaluate QT interval and TdP. The relevance of human and animal testing paradigms might not be very high with respect to specificity. Organ- and tissue-level platforms are mainly animal ex vivo assays, which include Langendorff technologies, cardiac slices, and Purkinje fibers. Cellular- and molecular-level platforms include human iPSC-CMs using manual and automated patch clamp and multielectrode arrays, animal cardiomyocytes using manual patch clamp measurement, fluorescent voltage dye, and computational assays. The relevance and throughput are approximate. *In silico proarrhythmia assessment would have more clinical relevance if the CiPA initiative is successful.19 Adapted from Heijman et al.20
Organism Level
Clinical Studies
The study of human subjects is central to understanding inherited and acquired human arrhythmic disorders. Resting, ambulatory, and exercise electrocardiographic findings are important in the diagnosis and management of inherited arrhythmic disorders.21 Such data from clinical studies have helped define the diagnostic features, natural history, and treatment strategies of arrhythmic disorders and drug-induced arrhythmias long before these entities were defined on a genetic and cellular level.22–24 Nevertheless, such ECG findings often have variable sensitivity and specificity in diagnosing these disorders.25, 26 Also, the relatively small number of patients with inherited arrhythmic disorders is often a barrier to creating large prospective clinical studies to guide risk stratification and therapy.1
Similarly, electrocardiographic evaluation via surface ECG and ambulatory rhythm monitoring is an integral component of the clinical assessment of cardiac safety of all compounds in development.27 These platforms provide information on drug-induced effects from cardiac electrophysiology, including cardiac repolarization,28 conduction defects, and incidence of arrhythmic events. The duration of ventricular depolarization and repolarization is shown as the QT interval on an ECG. QT interval prolongation has been associated with a potentially fatal arrhythmia, TdP.29 However, a prolonged QT is now recognized to be sensitive but not very specific for drug-induced proarrhythmia. In addition, ethical and regulatory standards limit the experimentation and testing that can be done in humans, and recommend that animal data of safety be validated before initial human drug testing.10 Thus, human clinical studies remain an important component of studying arrhythmic disorders, but technical and logistical challenges associated with such studies necessitate reliance on other models to elucidate the mechanism and risks of arrhythmia.
Animal Models
Animal models play a critical role in studying hereditary arrhythmic disorders and characterizing a drug's potential for proarrhythmic and cardiotoxic effects, prior to human administration. The need to define possible toxicities before experimental drugs are given to humans led to the adoption of animal model-based arrhythmia research utilizing small animals such as mice, rats, and rabbits. Similarly, hereditary arrhythmia studies extensively rely on mouse models by taking advantage of the relative ease of creating genetically modified small-animal strains that allow disease-specific modeling.30 Despite some degree of homology between many aspects of mouse and human cardiomyocytes, there are major fundamental electrophysiological and contractile differences between rodents and large mammals that limit the extrapolation of data generated from these models to humans. For example, the resting heart rate in mice is much higher than that of humans, and calcium-handling properties of mouse myocytes differ significantly from those of human myocytes.31–35 Most importantly, the ion channels responsible for determining action potential (AP) duration (and QT interval) in the mouse are not identical to those that determine action potential duration (APD) in human myocytes. By comparison, large animal models such as dogs,36 pigs,37 sheep,38 and primates39 have long APDs that more closely resemble human organ structure and function.40 Compared to the mouse model, humans and large animal models are more similar in heart rate,36, 41 APD and repolarization mechanisms, contractile filament isoforms, ion channels, and ion pumps,11, 18, 19, 42 all of which increase the validity of inferences made based on studies in large animal models for early stage clinical trials.43 However, despite the superior recapitulation of human cardiac physiology, translational failures still occur with large animal models, and no animal model system can fully replace human clinical trials.44 This is likely due to a host of factors, including inter-species variations in ion channel function,44 the relatively youthful state of animals used in research, and differences in drug metabolism between animals and humans.45
Organ & Tissue Level
The Langendorff technology, which uses an explanted perfused heart, allows access to all functional and electrical properties of an isolated heart. Limitations of this system include the absence of systemic neurohumoral regulation and the difficulty in maintaining normal function for prolonged periods.46 Cardiac slices preserve the functional syncytium properties of native myocardium to provide a platform for measuring signal propagation and conduction velocity. This model system bridges the gap between cellular and organ level assays. A limitation is the lack of natural electrical and mechanical cycles of the native heart due to the absence of natural pacemakers. However, this can be an advantage in the studies that require the exclusion of the influence of natural pacemakers.47 Furthermore, isolated cardiac tissue is only characteristic of the defined regions from which they are taken, such as Purkinje fiber and papillary muscle. This is especially useful in identifying regional specific electrophysiology effects of a disorder or a drug48, 49, 50 However, these models do not capture organ-level contributions or systemic contributions to arrhythmic disorders, including structural heart disease, autonomic tone, or adrenergic stimulation.51 This strengthens the relevance of organism-level models over organ-level and tissue-level models. Of course all of this depends on the questions being asked and the strategy should be to ensure that the model system used is best suited to answer the questions being posed. A combination of appropriate approaches is usually better than any single model alone in defining the nature of complex arrhythmias or the basis of drugs with complex electrophysiological effects.
Cellular & Molecular Level
Cardiomyocytes and Cardiac Ion Channels
Investigating primary ventricular cardiomyocytes allows a detailed study of ion channel behavior associated with physiological and pathophysiological functions of single cells. The difficulty in performing high-throughput drug screening in human-like ventricular cardiomyocytes led to the adoption of heterologous expression models such as introduction of hERG (the human Ether-à-go-go-Related Gene), which is a gene (KCNH2) that codes for a protein known as Kv11.1, the alpha subunit of a potassium ion channel. The hERG channel mediates the rapid delayed rectifier potassium current (IKr) and can be overexpressed in HEK293 (Human Embryonic Kidney 293) or CHO (Chinese Hamster Ovary) cell lines.52, 53 Isolated hERG inhibition has been shown to play an important role in TdP risk assessment in animals and in humans; mutations of this gene prolong the APD, and affected patients are prone to SCD.54 The hERG inhibition cellular screening assay is utilized at the early stages of drug development owing to the high-throughput and low cost of the platform, especially when screening a large number of compounds. However, it suffers from similar drawbacks as human QT testing because the drug-induced hERG block, while relatively sensitive, is not specific enough for predicting TdP. Some drugs that inhibit hERG at exposures reached in humans do not affect ventricular action potentials or result in TdP risk (despite APD/QT prolongation), due to the concomitant effect on one or more other ion currents in addition to IKr. The specific effects of drug-induced perturbations on multiple ionic channels (e.g., IKr, late INa, and ICa,L) likely explain why some QT prolonging drugs are not always proarrhythmic. One notable example is verapamil, which inhibits hERG in vitro but does not cause QT prolongation in vivo because of its additional calcium channel blocking properties.17 Although it inhibits hERG in vitro and causes QT prolongation, ranolazine is similarly free from proarrhythmia likely due to its additional late INa channel blocking properties.55 The traditional gold standard is to measure IKr in these heterologous expression systems by manual patch clamp, which is low throughput. Automated patch clamp systems with medium to high throughput therefore provide a better balance between productivity and data quality. Our conclusion is that the assessment of arrhythmia risk of a new drug by screening against a single cardiac ion channel is likely to lead to discarding potentially useful drugs while allowing others that can induce arrhythmias to move forward.
Induced Pluripotent Stem Cell-Derived Cardiomyocytes (iPSC-CMs)
More recently, iPSC-CMs have emerged as a new model capable of recapitulating many properties of human cardiomyocytes in vivo.56 Human iPSC-CMs express major cardiac ion channels naturally found in the human heart. The cells are made by reprogramming human somatic cells into pluripotent stem cells (PSCs) with transcription factors identified from embryonic stem cells (ESCs). The transcription factors are introduced into the somatic cells by viral transduction or non-viral transfection, or as soluble proteins.57 The resultant induced pluripotent stem cells (iPSCs) can be specifically differentiated into iPSC-CMs.58 The differentiated cardiomyocytes are beating cells that express many human cardiac ion channels and sarcomeric proteins.
A major advantage of the platform is that iPSC-CMs express the set of encoded cardiac genes that are not necessarily expressed by the original donor somatic cell (e.g., skin fibroblasts or peripheral blood mononuclear cells). Thus, in a case of an ion channel disorder, iPSC-CMs may express the abnormal ion channel gene and recapitulate the electrophysiologic abnormalities associated with the disorder. This has proven especially useful in studying inherited cardiac disorders because a virtually unlimited supply of cardiomyocytes expressing the phenotype encoded by a particular variant can be created. The platform has been used to study multiple inherited cardiac disorders, demonstrating good correlation with predicted adult human cardiomyocyte behavior.
The utility of single cell animal cardiomyocyte models is limited by different ion channel expression profile that may cause different electrophysiological response to drugs compared to human cardiomyocytes.59, 60 Similarly, hERG cellular transfection models have limitations in predicting drug toxicity related prolongation of ventricular repolarization.61, 62 Nevertheless, iPSC-CMs, while by no means a surrogate of adult ventricular myocytes, express a more complete panel of human cardiac ion channels associated with drug-induced cardiotoxicity.63, 64 With its higher degree of homology with human cardiomyocytes, iPSC models may provide a more comprehensive evaluation of ion channel function, action potential features, and arrhythmic potential compared to animal cardiomyocyte or heterologous transfection models.63, 65–68
A major strength of iPSC-CMs models is the ability to create a disease-specific system to develop therapeutics specifically targeted for that disorder and to define disease-specific drug toxicity. Although single-cell recording by conventional physiology techniques is generally low-throughput,69 newer single-cell approaches using automated patch clamp and multi-cellular recordings with multielectrode arrays (MEAs) have largely compensated for the differences in throughput and relevance.69–72 In addition, genetically encoded fluorescent voltage indicators are reported to faithfully demonstrate transmembrane potentials in iPSC-CMs. This new platform can be used in serial phenotyping of differentiating cardiomyocyte populations and in screening for drug-induced cardiotoxicity.73
Limitations of iPSC-CMs
It is important to note that iPSC-CMs, like any platform, have several limitations in modeling cardiac disorders. First, iPSC-CMs are single-cell models of cardiac disease and lack the complexity of cardiac tissue. Multicellular iPSC-CMs models might resolve some of these problems and some of these have shown promise in studying inherited and acquired disorders.74, 75 Another issue is that iPSC-CMs differentiate into heterogeneous patches of atrial-like, ventricular-like, and nodal-like precursors within the same preparation.76 The development of newer systems with chamber-specific or region-specific cells would improve the available models.77, 78 Lastly, as in the case with tissue level models, iPSC-CMs may not capture the complex organ-level or systemic interactions that could influence cardiac disorders.
Furthermore, iPSC-CMs are surrogates, not replicates of human adult cardiomyocytes; in particular, there are differences in ion channel and sarcomeric protein expression profile that suggest the cells are less mature than native adult myocytes.79 Important work has shown the possibility of advancing the maturity of the cell types, but at present iPSC-CMs remain closer in morphology to fetal cardiomyocytes.74, 80, 81 This is especially important given that ion channel function, AP properties, and arrhythmic potential will all be influenced by the cellular maturity and ion expression profile. In other words, instead of assuming they have analogous expression to adult human cardiomyocytes, iPSC-CMs must be carefully screened for expression of a gene of interest and the phenotype output signal. These limitations must be considered when drawing parallels to adult human myocyte physiology. Thus, iPSC-CMs are by no means a perfect model or a substitute for other science models of arrhythmia or human clinical testing. Instead, they represent a novel tool that can bridge some of the gaps between animal based models and adult human cardiomyocytes.
Electrophysiology Platforms
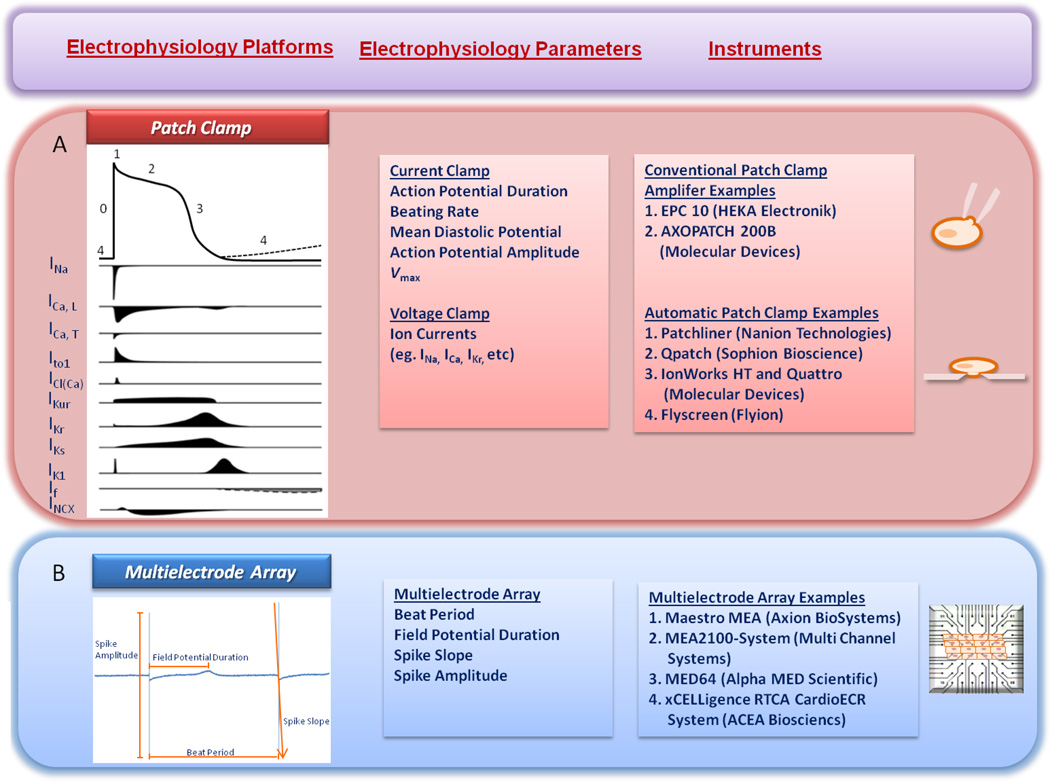
The electrophysiology platforms to study arrhythmic disorders and cardiac-safety assessment using cellular preparations will be discussed in detail as follows. Many of these platforms can be used to investigate iPSC-CMs and other cellular models as well (Figure 3).
Figure 3. The electrophysiology platforms to study arrhythmic disorders and cardiac-safety assessment using iPSC-CMs.
A. Patch Clamp. Raw trace, parameters (action potential duration, beating rate, mean diastolic potential, action potential amplitude, Vmax, and multiple ion currents), and instrument examples. B. Multielectrode array. Raw trace, parameters (beat period, field potential duration, spike slope, and spike amplitude), and instrument examples. Panel A is adapted from Hoekstra et al.82
Conventional Patch Clamping
Conventional patch clamp electrophysiology can be used to measure action potential properties (current clamp) or in-depth biophysical properties (voltage clamp) of cardiac myocytes. Parameters including APD, beating rate, mean diastolic potential and Vmax are easily measured with current clamp techniques. Individual families of ion channels can be characterized with cell voltage clamp techniques. The cardiac ventricular AP is divided into 5 phases (0–4) and is mediated by the coordinated opening and closing of sodium, calcium, and potassium channels.82–85 Phase 4 of the AP is the resting membrane potential that results from high K+ conductance due to inward rectifying K+ (IK1) channels. Voltage sensitive Na+ and Ca2+ channels are closed at the resting potential but remain available to activate with depolarization. The arrival of the depolarizing conducted AP causes the rapid opening of the inward Na+ current (INa), and the upstroke (phase 0) of the action potential. L-type Ca2+ (ICa,L) channels begin to activate during phase 0 of the AP. Phase 1 (initial repolarization) of the AP occurs immediately after the peak of depolarization, resulting from closure (inactivation) of the Na+ channels and opening (activation) of the transient outward potassium current (Ito). Phase 2 of the AP is the plateau phase during which the membrane potential changes slowly due to a balance between inward Ca2+ current through the L-type calcium channels and the outward K+ current through rapidly (IKr) and slowly (IKs) activating delayed rectifier K+ channels. Phase 3 of the AP is the final repolarization phase that occurs when repolarizing K+ currents (including the IKr, IKs, and IK1) sum to repolarize the membrane potential and reestablish the resting potential (Phase 4).86
The cardiac AP is a complex process that involves many different ionic currents, all of which are highly regulated. Prolongation of the APD can result from reduction of reporlarizing currents and/or increases in depolarizing currents. It is known that patients with genetic defects that cause prolongation of the APD are prone to SCD. Therefore, studying the effects of new drugs on APD could lead to useful approaches for predicting arrhythmia risk. In our view, these types of studies should be performed in adult cardiomyocytes with APDs similar to those of human cardiomyocytes.
Cells from patients with mutations that are known to be at risk of SCD can now be studied. Patient-specific iPSC-CMs exhibit distinct in vitro disease phenotypes associated with the patients from which they were derived. They can be used as models to study various disease phenotypes in affected patients. In addition, iPSC-CMs from normal humans could be used to identify drugs that have proarrhythmic properties, mainly by examining effects on APD morphology. However, increases in APD alone may not accurately predict arrhythmia risks of certain drugs. Additional approaches such as Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative19 might provide more substantial predictive value. The CiPA initiative adopts an integrated in vitro/in silico paradigm that emphasizes the repolarization changes that promote early afterdepolarizations (EADs), which are linked to TdP proarrhythmia.
In summary, patch clamp electrophysiology is considered as the gold standard for detailed biophysical studies of ion channels. It can be useful to define the detailed molecular bases of proarrhythmic drugs or ion channel mutations. However, these single cell approaches are labor intensive and selective ion channels must be studied while others are blocked. These requirements make voltage clamp approaches in intact cardiac myocytes a very low throughput approach for drug screening.
Automated Patch Clamp
Due to the low throughput and technically challenging nature of manual electrophysiology of intact cardiac myocytes, a series of devices to automate conventional patch clamp assays were developed in the 1990s, including the Robocyte,87 AutoPatch and RoboPatch,88,89 and OpusXpress 6000A90. Automated electrophysiology platforms provide a relatively high efficiency assessment of compounds against a single ion channel expressed in traditional cell lines (e.g., HEK293 and CHO) and in iPSC-CMs. The automated system requires large numbers of dissociated cells, with strict consistency and robustness of ion channel expression in order to achieve good reproducibility. While substantial data can be obtained with these automated approaches, the HEK293 and CHO cell systems cannot recapitulate the complexity of intact cardiac myocytes. Additionally, the automated techniques do not work well with adult cardiomyocytes because of their shape and fragility. Recently, the planar-array patch clamp has further improved the efficiency by coordinating parallel multi-well plate or chip recordings.91, 92 These systems automatically integrate the steps of cell giga-ohm sealing, perfusion handling, and stable recording. Some of these systems have been validated for drug safety evaluations, including Patchliner,93 IonWorks Quattro, PatchXpress, QPatch and IonWorks HTS . In such platforms, the number of data points that can be assessed depends largely on the type of plates used by the study.
Multielectrode Array (MEAs)
MEA technology provides non-invasive, long-term recordings of extracellular field potentials generated by electrically active cells.94, 95 The synchronous beating of a cultured cardiac myocyte monolayer preparation in vitro has some electrical patterns that relate to the ECG in vivo. The MEA may be most useful when used to evaluate not only the electrophysiological properties of cardiomyocytes, but also to correlate them to tissue networks. A series of MEAs including Maestro MEA, MEA2100-System, MED64, and xCELLgence RTCA CardioECR System have been developed. MEAs can be used to study AP propagation rates and the AP duration. Field potential duration (FPD) in MEA systems can be used to evaluate pro-arrhythmic effects of drugs. It represents the time between the upstroke of the action potential and final AP repolarization. FPDs reflect the same properties as the QT interval on the surface ECG. FPD prolongation is associated with QT prolongation, which has important predictive value for cardiac safety. MEAs have the advantage of measuring electrical behavior over long time frames. The spontaneous electrical activity can be measured to investigate chronotropic drug effects. MEAs can also be used to detect effects of drugs on the conduction of the action potential within the monolayer. As mentioned earlier, a limitation of this approach is that the myocytes employed often have an immature electrophysiological phenotype that limits translation of results to the adult heart.
Computational Modeling
In silico structure-affinity models of ion channels have emerged as a possible quick estimate of hERG affinity and other predicted electrophysiological models.96 These models provide a relatively efficient and inexpensive option to obtain a general prediction of a drug based on high-throughput data before launching the high-cost preclinical assessments. Encouragingly, the development of computer simulation methodologies has been reported to predict hERG-blocking and INa-blocking effects with whole-heart simulations,97 making it possibly more relevant to clinical studies. However, it should be noted that the prediction certainty of this new technology relies largely on the accuracy of the underlying experimental data used to build the model.
In silico arrhythmia modeling can also be used to predict proarrhythmia by utilizing existing data on a drug’s effect on specific ion channels. When in silico platforms integrate multiple ion currents, the models are better in predicting proarrhythmia than using hERG alone.98, 99 A number of evolving concepts are being evaluated in the modeling process, including using data from more than 2 or 3 channels, dose-response characteristics, and possibly kinetics of channel block. In silico arrhythmia modeling is a major focus of the evolving CiPA initiative, which utilizes an integrated nonclinical in vitro/in silico paradigm.19 Promising modeling approaches are evolving, and their appropriate validation with the human disease will be increasingly important.
Fluorescent Voltage Dyes
Considering the laborious nature of conventional electrophysiological recordings and strict cell quality requirements of automated patch clamp, platforms based on fluorescent voltage dyes have emerged as a high-throughput alternative. In addition, these dyes can be applied to organ-level approaches such as Langendorff, making them more relevant to clinical studies. However, some optical voltage indicators suffer from phototoxicity, which limits the recording time and degrades signal quality.100
Applying Electrophysiology Platforms to iPSC-CMs in Disorders Leading to SCD
Multiple studies have used iPSC-CMs to study arrhythmic disorders. These efforts have ranged from studying disease-specific models to investigation using iPSC-CMs from normal patients in screens for drug toxicity. We highlight some of the major efforts in this field in Table 1.
Table 1.
Summary of major efforts utilizing iPSC-CM to model hereditary arrhythmic disorders, platforms used to study disorders and most significant findings of modeled disorders.
| Disorder | Study | Gene (Variant/s) | Platforms |
Drug Testing | ||
|---|---|---|---|---|---|---|
| Patch Clamp | MEA | Fluorescent Based Calcium Imaging |
||||
| LQT1 | Moretti et al. (2010)70 | KCNQ1 (p.R190Q) |
|
- | - | Isoproterenol, Propranolol, |
| Egashira et al. (2012)96 | KCNQ1 (p.C1893del) | ↓IKs |
|
- | E4031, Chromanol, Isoproterenol, Propranolol, |
|
| Wang et al. (2014)76 | KCNQ1 (p.R190Q, p G269S, and p.G345E.) |
|
- | - | Nifedipine | |
| LQT2 | Itzhaki et al. (2011)72 | KCNH2 (p.A614V) |
|
Prolonged FPD EADs |
- | E4031, Cisapride, Nifedipine, Pinacidil, Ranolizine |
| Matsa et al. (2011)71 | KCNH2 (p.G1681A) |
|
Prolonged FPD, EADs |
- | E4031, Isoprenaline. Nicorandil, Nadolol, PD-118057, Propranolol |
|
| Lahti et al. (2012) | KCNH2 (p.R176W) |
|
Prolonged FPD | - | E4031, Cisapide, Erythromycin, Isoprenaline , Sotalol, |
|
| Wang et al. (2014)76 | KCNH2 (p.A614V) |
|
- | - | Nifedipine | |
| - | - | |||||
| LQT3 | Terrenoire et al. (2013)74 | SCN5A (p.F1473C) |
|
- | - | Mexiletine |
| Ma et al. (2013)73 | SCN5A (p.V1763M) |
|
- | - | Mexiletine | |
| LQT8 | Yazawa et al. (2011)75 | CACNA1C (p.G406R) |
|
- |
|
Roscovitine, |
| - | ||||||
| CPVT | Itzhaki et al. (2012)77 | RYR2 (p.M4109R) | DADs | - |
|
Flecanide Forskolin, Isoproterenol Thapsigargin |
| Fatima et al. (2011)95 | RYR2 (p.T7447A) | - | - |
|
Bay-K 8644, di-butryl cAMP, Isopreoterenol |
|
| Novak et al. (2012)94 | CASQ2 (p.D307H) | Longer APD, DADs, Oscillatory prepoentials |
- | Delayed after-contractions | Isopreoterenol, | |
| Jung et al. (2012)87 | RYR2 (p.S406L) | - | - |
|
Dantrolene | |
| HCM | Lan et al. (2013)51 | MYH7 (p.R663H) | DADs | - |
|
Isoproterenol, Propranolol, Verapamil, Lidocarine, Mexiletine, Ranolazine |
| Other | Liang et al. (2013)84 | KCNQ1 (p.G269S), MYH7 (p.R663H), TNNT2 (p.R173W) |
|
- | - | Alfuzosin Cisapride, Nicorandil. Verapamil,, |
| Braam et al. (2013)86 | KCNH2 (p.G1681A) | IKs, IKr | Drug induced prolonged FPD |
- | Bepridil Diltiazem Dofetilide, Levcromakalim, Moxifloxacin, Sotalol, Sparfloxacin, , Terfenadine, Verapamil, JNJ303, HMR1556 |
|
Long QT Syndrome
The first arrhythmic disorder to be modeled using iPSC-CMs was Long QT syndrome. This disease is characterized clinically by a prolonged QT interval on ECG, which predisposes patients to an unstable ventricular arrhythmia (e.g., TdP). There are over 10 loci associated with Long QT syndrome, with each locus being associated with a specific subtype that has unique ion channel derangements and clinical features.101
Moretti et al. reprogrammed fibroblasts from a patient with a KCNQ1 variant that is associated with Type I Long QT syndrome (LQT1) to create iPSCs, and subsequently differentiated them into iPSC-CMs.102 The authors found that the resulting iPSC-CMs recapitulated multiple features of LQT1 including reduced IKs currents, prolongation of APD, and increased incidence of spontaneous arrhythmias. A similar approach was undertaken by Matsa et al. and Izhaki et al. in studying Type II Long QT syndrome (LQT2).103, 104 These studies were also notable for confirming that FPD, as well as APD, was prolonged and validated the use of MEA to study tissue level phenotype in iPSC-CMs models.
Type III Long QT syndrome (LQT3) was modeled using iPSC-CMs from patients harboring SCN5A mutations by two groups, with both models showing good correlation with the clinical phenotype.105, 106 The study by Terrenoire et al. recruited a subject with a novel SCN5A variant predisposing the subject to LQT3, while also carrying a rare variant in KCNH2 that was of unclear clinical significance. The authors investigated the electrophysiological significance of this variant by studying the IKr current using voltage clamp and saw no abnormality, thus concluding that the patient’s presentation was secondary to the identified SCN5A variant without any contribution from the KCNH2 variant. A similar method was applied by Davis et al. in studying mice harboring a complex SCN5A variant (SCN5A1798insD/+), which encodes a sodium channel that exhibits combined characteristics of gain- and loss-of function. Such studies may provide a framework to investigate complex electrophysiology disorders or overlap syndromes. Specifically, these are disorders that do not fit into the classical channelopathies, often harboring features of more than one disorder. 107 More human studies are needed to validate the practice of functional testing of iPSC-CMs in overlap syndromes, including those caused by sodium channel variants.
Timothy Syndrome, also known as Type 8 Long QT syndrome (LQT8), is another inherited arrhythmic disorder associated with syndactyly, immune deficiency, and autism. Yazawa et al. created a model of the L-type calcium channel (Cav1.2) disorder using iPSC-CMs that mirrored the abnormal calcium homeostasis and increased arrhythmia associated with the disorder. 108 Another successful model of LQT was recently published by Wang et al.; the group created iPSC-CMs models of LQT1 and LQT2 using zinc finger nuclease (ZFN) genome editing of pathogenic variants into wild type iPSC.109 The advent of newer genome editing technology such as transcription activator-like effector nuclease (TALEN) and clustered, regularly interspaced, short palindromic repeat/Cas system (CRISPR/Cas) technology allows for highly targeted genome editing with minimal impact on non-targeted sites of the genome.110
The above genome editing techniques would be particularly useful in evaluating the functional significance of variants of uncertain significance (VUS) uncovered during genetic testing (Figure 4B). The classification of such variants usually relies on published human studies, in silico models, or animal models of the variant in question or similar variants. Instead of using a known disease causing variant, as was done by Wang et al.109, investigators can utilize a VUS suspected to cause LQT and evaluate the electrophysiology of the derived iPSC-CMs. Before attempting such a strategy, two criteria must be fulfilled: 1) iPSC-CMs must show stable and reliable expression of the encoded gene and protein similar to that of adult cardiomyocytes, and 2) iPSC-CMs must provide a reliable phenotype signal of the encoded protein that can be measured and has been shown to correlate with clinical disease.
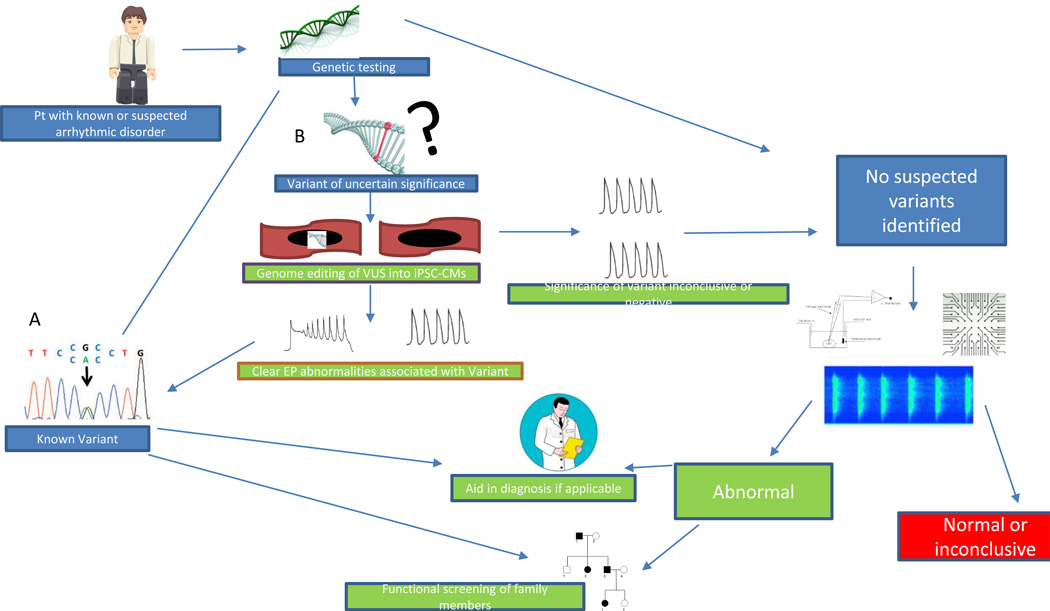
Figure 4. Flow diagram of how iPSC-CMs can augment the approach to genetic testing of patients with known or at risk for arrhythmic disorders.
Clinical genetic testing attempts to identify a rare variant in genes commonly associated with arrhythmic disorders. A. When a known variant is identified, this can sometimes aid the clinical diagnosis if one has not already been made, and genetic screening can be offered to family members to identify those at risk for developing the disorder. B. If a variant of uncertain significance (VUS) is identified, genome- edited lines with VUS can be made, and cellular EP testing can be done on the resultant iPSC-CMs to detect electrophysiological abnormalities and compare them to proband iPSC-CMs. This can potentially support the VUS being disease causing and place it in a similar category as a known variant. C. In cases where EP testing of the VUS is negative or inconclusive or no variants are identified on genetic testing, one can ignore the genetic component and use iPSC-CMs as the cellular functional testing platform (EP study in a dish). iPSC-CMs from the proband would be created and undergo comprehensive EP testing; abnormalities thus identified can then be used to aid in the diagnosis and management of the patient. Furthermore, family members can be offered the opportunity to have iPSC-CMs generated and screened for their arrhythmic predisposition. D. Much like genetic testing, iPSC-CM testing may not identify all arrhythmic abnormalities, and those patients are left to rely on clinical testing and screening. (Illustration credit: Ben Smith).
Catecholaminergic Polymorphic Ventricular Tachycardia
Several studies have evaluated the ability to model catecholaminergic polymorphic ventricular tachycardia (CPVT) using an iPSC-CM model. CPVT is an arrhythmic disorder characterized by ventricular tachycardia provoked by exercise or catecholamine surges. It is a disorder of cardiac calcium homeostasis often caused by variants in calcium regulation genes such as ryanodine receptor 2 (RYR2) and cardiac calsequestrin (CASQ2).101 The first model of CPVT by Itzhaki et al. created iPSC-CMs from a patient heterozygous for a RYR2 variant.111 Utilizing patch clamp techniques, the authors found an increased incidence of delayed afterdepolarizations (DADs) in response to adrenergic stimulation. Furthermore, the authors used dye-based calcium imaging to demonstrate abnormal calcium transients in CPVT iPSC-CMs, validating the long-standing hypothesis of the mechanism of arrhythmia of the disorder.112
Other Models
There have been multiple efforts to model other inherited cardiac disorders that are associated with a risk of SCD. These include familial dilated cardiomyopathy (DCM)113, arrhythmogenic right ventricular dysplasia (ARVD)78, 114, 115, LEOPARD Syndrome116, and Friedreich’s Ataxia.78, 113–117 However, none of the studies primarily focused on the arrhythmic potential of the disorder.
Future Directions
Mechanisms of Disease
The ability to study a human beating cardiomyocyte cellular model was limited prior to optimization of cardiac differentiation of pluripotent stem cells, because the primary tissue fails to survive adequately in vitro.118 As demonstrated by the models above, iPSC-CMs now offer the opportunity to study the cellular pathophysiology of arrhythmic disorders. The above models were concordant with known hypotheses of the underlying mechanism of the respective disorders (Table 1). Furthermore, Lan et al. were able to validate less common mechanistic hypotheses in hypertrophic cardiomyopathy (HCM).71 HCM is an inherited cardiac disorder clinically associated with one or more of the following: a mild to severe increase in muscle wall thickness, left ventricular cavity obstruction, and SCD.119 How these mutations in contractile filaments cause electrophysiological disturbances that promote sudden death has been studied.120, 121 Lan et al. created a model of the disorder and demonstrated relative hypertrophy of myocytes in response to adrenergic stimulation.71 Furthermore, the authors showed that HCM iPSC-CMs exhibited abnormal calcium handling and increased diastolic levels of calcium that were associated with an increase in DADs. This work was important in confirming the hypothesis that HCM is an arrhythmic disorder at a cellular level, independent of the degree of muscular hypertrophy or intracavitary obstruction in vivo.
Drug Testing
An emerging role for iPSC-CMs is in the field of drug testing, namely testing for arrhythmic toxicity of compounds. Currently hERG channel cellular transfection models serve as the standard platform for testing proarrhythmia toxicity. However, recent studies have highlighted a superior potential for iPSC-CMs in this field.
Liang et al.63 compared iPSC-CMs models and a hERG channel-transfected cell model, which is the current standard of pharmacological drug testing, to predict drug toxicity. The authors were able to demonstrate the superiority of iPSC-CMs in stratifying appropriate toxicity potentials of verapamil and alfuzosin. Furthermore, the study showed that there is a spectrum of toxicity dependent on the host genetic background; for example, iPSC-CMs from patients with Long QT syndrome were more predisposed to hERG-mediated toxicity compared to iPSC-CMs from control healthy patients. Similarly, Navarette et al. demonstrated that iPSC-CMs drug-induced arrhythmia can be documented by MEA.122
The concept of variable individual predisposition to arrhythmic toxicity, especially as it relates to TdP and the hypothesis of repolarization reserve, posits that a degree of repolarization current redundancy exists between IKs and IKr currents.123 The individual degree of repolarization reserve is likely dictated by a combination of genetic and environmental factors; the population heterogeneity of such factors likely explains the differential responses to QT prolonging drugs in individual patients. This was nicely demonstrated by Braam et al., who utilized iPSC-CMs to confirm the repolarization reserve hypothesis.124 The authors used MEA to assess iPSC-CM effects of 9 different anti-arrhythmic drugs on field potential duration. The authors note that the IKs blockers, HMR1556 and JNJ303, had minor effects on FPD. However, when the compounds were co-administered with sotalol (IKr blocker) or in LQT2 cells (genetically reduced IKr), the cells experienced a marked prolongation of FPD. The above studies support the versatility of iPSC-CMs as a platform for more comprehensive drug studies due to a broader recapitulation of adult ventricular myocyte ion channel expression compared to animal or cellular transfection models.
Efficacy testing is likely to be more challenging due to morphologic differences between iPSC-CMs and adult myocytes and the fact that anti-arrhythmic responses are often accompanied by competing pro-arrhythmic effects that sometimes are only unmasked in vivo.125 Furthermore, many arrhythmic disorders such as ARVD and Brugada Syndrome are thought to depend on multi-cellular and tissue level phenomenon that potentiate lethal arrhythmic events.78, 126 However, multiple investigators have shown consistent drug responses that are comparable with human cardiomyocyte responses in LQT1, LQT2, LQT3, and CPVT (Table 1). Thus, some evidence exists to utilize this platform for disease specific toxicity and efficacy testing. Larger studies are needed to validate the adoption of this platform for use in toxicity screening at the population level.
Drug Discovery
Because the electrophysiological and contractile phenotype of iPSC-CMs is similar to normal human cardiomyocytes, they are valuable research tools for drug discovery. Many of the studies modeling cardiac disorders have relied on known therapeutic or toxic drug responses as part of their repertoire of recapitulation of disease phenotype. However, one study took a prospective approach by testing a novel drug on iPSC-CMs. Jung et al. used iPSC-CMs made from a CPVT patient with a RYR2 variant to test the therapeutic efficacy of dantrolene.127 This old drug is often used to treat malignant hyperthermia and, in this case, was able to successfully abate the calcium-handling abnormalities and DADs seen in CPVT iPSC-CMs. This study suggests that a high throughput electrophysiology platform utilizing iPSC-CMs can be used to test the therapeutic and toxic potential of large chemical libraries in specific diseases and the general population.128
Clinical Testing of SCD Patients
Our understanding of inherited cardiac disorders has evolved with advances in genomic sequencing. For instance, it is now recognized that some of the variability in risks associated with inherited cardiac disorders is specific to the causative variant and modifier loci.101 Clinical genetic testing has proven to be a useful adjuvant tool to aid in diagnosis and management of some arrhythmic disorders. Furthermore, identifying a causative variant offers the ability to screen family members at risk for the disorder (Figure 4A). Unfortunately, with the exception of LQT, genetic testing yields causative variants in less than 50% of cases. Furthermore, clinical genetic testing often yields VUS, which are rare variants with unclear causal relationship to the observed clinical phenotype. Thus, in a large proportion of families with genetic arrhythmic disorders, we lack the ability to screen at risk members and possibly aid in the management of known affected individuals (Figure 4D).
iPSC-CMs have the potential to serve the role of a personalized medicine tool that can alleviate some of the limitations of our current approach. This owes to iPSC-CMs’ advantage of being donor specific, i.e., having the ability to recapitulate the genetic information of the original donor. As mentioned above, iPSC-CMs are not adult myocytes, and to utilize the platform in any investigational capacity, the following criteria must be met: 1) the gene in question must be functionally expressed; 2) the phenotype observed must be reliably measured; and 3) the phenotype measured must be comparable to adult cardiomyocytes to provide adequate clinical correlation or predictive ability. To date, multiple independent investigators have identified electrophysiologic abnormalities in iPSC-CMs that support criteria 1 and 2, but not definitively criterion 3 (Table 1). Population level studies are needed to prospectively test the sensitivity and specificity of iPSC-CMs as a diagnostic or risk stratification tool.
Validation of disease-specific electrophysiological characteristics of iPSC-CMs would enable the use of iPSC-CMs as an “EP study in a dish” platform. Using genome-editing methods such as ZFN109, TALEN129, and CRISPR/Cas110, this approach can be used to test the likelihood of candidate variants being disease causing (Figure 4B).109 Furthermore, this approach may prove useful in aiding the diagnosis of complex cases in which current clinical criteria fail to definitively make a diagnosis. Additionally, in cases that are clinically or genetically idiopathic, iPSC-CMs may provide vital clues as to the electrophysiological abnormalities underlying the disorder at hand. If abnormalities are indeed identified, iPSC-CMs derived from individual family members could serve as a valuable screening tool analogous to genetic testing (Figure 4C). Even if population studies prove iPSC-CMs to be reliable in diagnosing multiple disorders, this platform, similar to clinical genetic testing, will serve as an important adjuvant tool to aid the management and not define it.
Conclusions
Most of the heterogeneous disorders that lead to SCD in the absence of major structural heart disease share the common pathways of predisposition towards lethal arrhythmias. Multiple platforms have been used to study these disorders, ranging from cell-based to organism-based platforms, each providing important contributions to the field. Recently, the human iPSC-CM model has emerged as a novel beating cardiomyocyte model that provides several detailed and high throughput methods of electrophysiology assessment. This platform has already been used to elucidate several inherited and acquired rhythm disorders, and has shown excellent recapitulation of electrophysiology phenotype and drug efficacy and toxicity profile. With these advantages, iPSC-CMs are expected to provide valuable contributions to the science and clinical practice of arrhythmic disorders leading to SCD.
Supplementary Material
Acknowledgements
We gratefully acknowledge Joseph Gold and Blake Wu for critical reading and Ms. Amy Thomas for her assistance with illustration. Due to space limit, we are unable to include all of the important relevant papers; we apologize in advance to the investigators whose significant contributions to this field we have omitted here.
Sources of Funding
Funding support from National Institutes of Health (NIH) T32 training grant (KS), Leducq Foundation, NIH R01 HL113006, NIH R01 HL126527, NIH R01 HL123968, NIH R24 HL117756 (JCW), NIH R01 HL033921, and NIH P01 108806 (SRH).
Nonstandard Abbreviations and Acronyms
- SCD
sudden cardiac death
- iPSC-CMs
induced pluripotent stem cell-derived cardiomyocytes
- TdP
torsades de pointes
- AP
action potential
- APD
action potential duration
- hERG
human Ether-à-go-go-Related Gene
- HEK293
Human Embryonic Kidney 293
- CHO
Chinese Hamster Ovary
- PSCs
pluripotent stem cells
- ESCs
embryonic stem cells
- iPSCs
induced pluripotent stem cells
- MEAs
multielectrode arrays
- IK1
inward rectifying K+ current
- INa
inward Na+ current
- ICa,L
L-type Ca2+ current
- Ito
transient outward potassium current
- IKr
rapid delayed rectifier potassium current
- IKs
slow delayed rectifier potassium current
- CiPA
Comprehensive In Vitro Proarrhythmia Assay
- EADs
early afterdepolarizations
- FPD
field potential duration
- LQT1
Type I Long QT syndrome
- LQT2
Type II Long QT syndrome
- LQT3
Type III Long QT syndrome
- LQT8
Type 8 Long QT syndrome
- ZFN
zinc finger nuclease
- TALEN
transcription activator-like effector nuclease
- CRISPR/Cas
clustered, regularly interspaced, short palindromic repeat/Cas genes
- VUS
variants of uncertain significance
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- RYR2
ryanodine receptor 2
- EADs
early afterdepolarizations
- DCM
dilated cardiomyopathy
- ARVD
arrhythmogenic right ventricular dysplasia
- HCM
hypertrophic cardiomyopathy
- DADs
delayed afterdepolarizations
Footnotes
Disclosures
JCW is a co-founder of Stem Cell Theranostics.
References
- 1.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American College of Cardiology/American Heart Association Task F, European Society of Cardiology Committee for Practice G, European Heart Rhythm A, Heart Rhythm S. Acc/Aha/Esc 2006 Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): Developed in Collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 2.Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Siscovick D, Stevenson WG, Zipes DP American Heart A, American College of Cardiology F, Heart Rhythm S. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death: A Scientific Statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118:1497–1518. [PubMed] [Google Scholar]
- 3.Zipes DP, Wellens HJ. Sudden Cardiac Death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 4.Modi S, Krahn AD. Sudden Cardiac Arrest without Overt Heart Disease. Circulation. 2011;123:2994–3008. doi: 10.1161/CIRCULATIONAHA.110.981381. [DOI] [PubMed] [Google Scholar]
- 5.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting Sudden Death in the Population: The Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 6.Chugh SS, Kelly KL, Titus JL. Sudden Cardiac Death with Apparently Normal Heart. Circulation. 2000;102:649–654. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 7.Corrado D, Basso C, Thiene G. Pros and Cons of Screening for Sudden Cardiac Death in Sports. Heart. 2013;99:1365–1373. doi: 10.1136/heartjnl-2012-302160. [DOI] [PubMed] [Google Scholar]
- 8.Krahn AD, Healey JS, Chauhan V, Birnie DH, Simpson CS, Champagne J, Gardner M, Sanatani S, Exner DV, Klein GJ, Yee R, Skanes AC, Gula LJ, Gollob MH. Systematic Assessment of Patients with Unexplained Cardiac Arrest: Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (Casper) Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi ZP, Seoane-Vazquez E, Rodriguez-Monguio R, Stevenson KB, Szeinbach SL. Market Withdrawal of New Molecular Entities Approved in the United States from 1980 to 2009. Pharmacoepidemiol Drug Saf. 2011;20:772–777. doi: 10.1002/pds.2155. [DOI] [PubMed] [Google Scholar]
- 10.Food, Drug Administration HHS. International Conference on Harmonisation; Guidance on S7b Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (Qt Interval Prolongation) by Human Pharmaceuticals; Availability. Notice. Fed Regist. 2005;70:61133–61134. [PubMed] [Google Scholar]
- 11.Jamshidi Y, Nolte IM, Dalageorgou C, Zheng D, Johnson T, Bastiaenen R, Ruddy S, Talbott D, Norris KJ, Snieder H, George AL, Marshall V, Shakir S, Kannankeril PJ, Munroe PB, Camm AJ, Jeffery S, Roden DM, Behr ER. Common Variation in the Nos1ap Gene Is Associated with Drug-Induced Qt Prolongation and Ventricular Arrhythmia. J Am Coll Cardiol. 2012;60:841–850. doi: 10.1016/j.jacc.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaab S, Crawford DC, Sinner MF, Behr ER, Kannankeril PJ, Wilde AA, Bezzina CR, Schulze-Bahr E, Guicheney P, Bishopric NH, Myerburg RJ, Schott JJ, Pfeufer A, Beckmann BM, Martens E, Zhang T, Stallmeyer B, Zumhagen S, Denjoy I, Bardai A, Van Gelder IC, Jamshidi Y, Dalageorgou C, Marshall V, Jeffery S, Shakir S, Camm AJ, Steinbeck G, Perz S, Lichtner P, Meitinger T, Peters A, Wichmann HE, Ingram C, Bradford Y, Carter S, Norris K, Ritchie MD, George AL, Jr, Roden DM. A Large Candidate Gene Survey Identifies the Kcne1 D85n Polymorphism as a Possible Modulator of Drug-Induced Torsades De Pointes. Circ Cardiovasc Genet. 2012;5:91–99. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torp-Pedersen C, Moller M, Bloch-Thomsen PE, Kober L, Sandoe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm AJ. Dofetilide in Patients with Congestive Heart Failure and Left Ventricular Dysfunction Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–865. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 14.Sauer AJ, Newton-Cheh C. Clinical and Genetic Determinants of Torsade De Pointes Risk. Circulation. 2012;125:1684–1694. doi: 10.1161/CIRCULATIONAHA.111.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan A, Isbister GK, Kirkpatrick CM, Dufful SB. Drug-Induced Qt Prolongation and Torsades De Pointes: Evaluation of a Qt Nomogram. QJM. 2007;100:609–615. doi: 10.1093/qjmed/hcm072. [DOI] [PubMed] [Google Scholar]
- 16.Finlayson K, Witchel HJ, McCulloch J, Sharkey J. Acquired Qt Interval Prolongation and Herg: Implications for Drug Discovery and Development. Eur J Pharmacol. 2004;500:129–142. doi: 10.1016/j.ejphar.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between Preclinical Cardiac Electrophysiology, Clinical Qt Interval Prolongation and Torsade De Pointes for a Broad Range of Drugs: Evidence for a Provisional Safety Margin in Drug Development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 18.Bass AS, Darpo B, Valentin JP, Sager P, Thomas K. Moving Towards Better Predictors of Drug-Induced Torsades De Pointes. Br J Pharmacol. 2008;154:1550–1553. doi: 10.1038/bjp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N. Rechanneling the Cardiac Proarrhythmia Safety Paradigm: A Meeting Report from the Cardiac Safety Research Consortium. Am Heart J. 2014;167:292–300. doi: 10.1016/j.ahj.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Heijman J, Voigt N, Carlsson LG, Dobrev D. Cardiac Safety Assays. Curr Opin Pharmacol. 2014;15:16–21. doi: 10.1016/j.coph.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. Hrs/Ehra/Aphrs Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes: Document Endorsed by Hrs, Ehra, and Aphrs in May 2013 and by Accf, Aha, Paces, and Aepc in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Jervell A, Lange-Nielsen F. Congenital Deaf-Mutism, Functional Heart Disease with Prolongation of the Q-T Interval and Sudden Death. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 23.Brugada P, Brugada J. Right Bundle Branch Block, Persistent St Segment Elevation and Sudden Cardiac Death: A Distinct Clinical and Electrocardiographic Syndrome. A Multicenter Report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 24.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic Polymorphic Ventricular Tachycardia in Children. A 7-Year Follow-up of 21 Patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 25.Swan H, Saarinen K, Kontula K, Toivonen L, Viitasalo M. Evaluation of Qt Interval Duration and Dispersion and Proposed Clinical Criteria in Diagnosis of Long Qt Syndrome in Patients with a Genetically Uniform Type of Lqt1. J Am Coll Cardiol. 1998;32:486–491. doi: 10.1016/s0735-1097(98)00248-4. [DOI] [PubMed] [Google Scholar]
- 26.Veltmann C, Schimpf R, Echternach C, Eckardt L, Kuschyk J, Streitner F, Spehl S, Borggrefe M, Wolpert C. A Prospective Study on Spontaneous Fluctuations between Diagnostic and Non-Diagnostic Ecgs in Brugada Syndrome: Implications for Correct Phenotyping and Risk Stratification. Eur Heart J. 2006;27:2544–2552. doi: 10.1093/eurheartj/ehl205. [DOI] [PubMed] [Google Scholar]
- 27.Food, Drug Administration HHS. International Conference on Harmonisation; Guidance on E14 Clinical Evaluation of Qt/Qtc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; Availability. Notice. Fed Regist. 2005;70:61134–61135. [PubMed] [Google Scholar]
- 28.Rodriguez I, Erdman A, Padhi D, Garnett CE, Zhao H, Targum SL, Balakrishnan S, Strnadova C, Viner N, Geiger MJ, Newton-Cheh C, Litwin J, Pugsley MK, Sager PT, Krucoff MW, Finkle JK. Electrocardiographic Assessment for Therapeutic Proteins--Scientific Discussion. Am Heart J. 2010;160:627–634. doi: 10.1016/j.ahj.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Roden DM. Long Qt Syndrome: Reduced Repolarization Reserve and the Genetic Link. J Intern Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 30.Jucker M. The Benefits and Limitations of Animal Models for Translational Research in Neurodegenerative Diseases. Nat Med. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- 31.Yazawa M, Dolmetsch RE. Modeling Timothy Syndrome with Ips Cells. J Cardiovasc Transl Res. 2013;6:1–9. doi: 10.1007/s12265-012-9444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW, 2nd, MacLennan DH, Kremastinos DT, Kranias EG. Human Phospholamban Null Results in Lethal Dilated Cardiomyopathy Revealing a Critical Difference between Mouse and Human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raake PW, Zhang X, Vinge LE, Brinks H, Gao E, Jaleel N, Li Y, Tang M, Most P, Dorn GW, 2nd, Houser SR, Katus HA, Chen X, Koch WJ. Cardiac G-Protein-Coupled Receptor Kinase 2 Ablation Induces a Novel Ca2+ Handling Phenotype Resistant to Adverse Alterations and Remodeling after Myocardial Infarction. Circulation. 2012;125:2108–2118. doi: 10.1161/CIRCULATIONAHA.111.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Chen X, Gao E, MacDonnell SM, Wang W, Kolpakov M, Nakayama H, Zhang X, Jaleel N, Harris DM, Li Y, Tang M, Berretta R, Leri A, Kajstura J, Sabri A, Koch WJ, Molkentin JD, Houser SR. Increasing Cardiac Contractility after Myocardial Infarction Exacerbates Cardiac Injury and Pump Dysfunction. Circ Res. 2010;107:800–809. doi: 10.1161/CIRCRESAHA.110.219220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Lal H, Chen X, Shang X, Song J, Li Y, Kerkela R, Doble BW, MacAulay K, DeCaul M, Koch WJ, Farber J, Woodgett J, Gao E, Force T. Gsk-3alpha Directly Regulates Beta-Adrenergic Signaling and the Response of the Heart to Hemodynamic Stress in Mice. J Clin Invest. 2010;120:2280–2291. doi: 10.1172/JCI41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of Intracoronary and Transendocardial Delivery of Allogeneic Mesenchymal Cells in a Canine Model of Acute Myocardial Infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary Delivery of Autologous Cardiac Stem Cells Improves Cardiac Function in a Porcine Model of Chronic Ischemic Cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou X, Appleby N, Fuentes T, Longo LD, Bailey LL, Hasaniya N, Kearns-Jonker M. Isolation, Characterization, and Spatial Distribution of Cardiac Progenitor Cells in the Sheep Heart. J Clin Exp Cardiolog. 2012:S6. [PMC free article] [PubMed] [Google Scholar]
- 39.Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L, Bellabas L, Brinon B, Vanneaux V, Pradeau P, Peyrard S, Larghero J, Pouly J, Binder P, Garcia S, Shimizu T, Sawa Y, Okano T, Bruneval P, Desnos M, Hagege AA, Casteilla L, Puceat M, Menasche P. Composite Cell Sheets: A Further Step toward Safe and Effective Myocardial Regeneration by Cardiac Progenitors Derived from Embryonic Stem Cells. Circulation. 2010;122:S118–S123. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- 40.Dixon JA, Spinale FG. Large Animal Models of Heart Failure: A Critical Link in the Translation of Basic Science to Clinical Practice. Circ Heart Fail. 2009;2:262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gandolfi F, Vanelli A, Pennarossa G, Rahaman M, Acocella F, Brevini TA. Large Animal Models for Cardiac Stem Cell Therapies. Theriogenology. 2011;75:1416–1425. doi: 10.1016/j.theriogenology.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 42.Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between Human and Mouse Embryonic Stem Cells. Dev Biol. 2004;269:360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 43.Chong JJ, Murry CE. Cardiac Regeneration Using Pluripotent Stem Cells-Progression to Large Animal Models. Stem Cell Res. 2014 doi: 10.1016/j.scr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jost N, Virag L, Comtois P, Ordog B, Szuts V, Seprenyi G, Bitay M, Kohajda Z, Koncz I, Nagy N, Szel T, Magyar J, Kovacs M, Puskas LG, Lengyel C, Wettwer E, Ravens U, Nanasi PP, Papp JG, Varro A, Nattel S. Ionic Mechanisms Limiting Cardiac Repolarization Reserve in Humans Compared to Dogs. J Physiol. 2013;591:4189–4206. doi: 10.1113/jphysiol.2013.261198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Hara T, Rudy Y. Quantitative Comparison of Cardiac Ventricular Myocyte Electrophysiology and Response to Drugs in Human and Nonhuman Species. Am J Physiol Heart Circ Physiol. 2012;302:H1023–H1030. doi: 10.1152/ajpheart.00785.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Habbout A, Guenancia C, Lorin J, Rigal E, Fassot C, Rochette L, Vergely C. Postnatal Overfeeding Causes Early Shifts in Gene Expression in the Heart and Long-Term Alterations in Cardiometabolic and Oxidative Parameters. PLoS One. 2013;8:e56981. doi: 10.1371/journal.pone.0056981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer T, Stuerz K, Guenther E, Edamura M, Kraushaar U. Cardiac Slices as a Predictive Tool for Arrhythmogenic Potential of Drugs and Chemicals. Expert Opin Drug Metab Toxicol. 2010;6:1461–1475. doi: 10.1517/17425255.2010.526601. [DOI] [PubMed] [Google Scholar]
- 48.Ellinghaus P, Scheubel RJ, Dobrev D, Ravens U, Holtz J, Huetter J, Nielsch U, Morawietz H. Comparing the Global Mrna Expression Profile of Human Atrial and Ventricular Myocardium with High-Density Oligonucleotide Arrays. J Thorac Cardiovasc Surg. 2005;129:1383–1390. doi: 10.1016/j.jtcvs.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 49.Heijman J, Voigt N, Dobrev D. New Directions in Antiarrhythmic Drug Therapy for Atrial Fibrillation. Future Cardiol. 2013;9:71–88. doi: 10.2217/fca.12.78. [DOI] [PubMed] [Google Scholar]
- 50.Dobrev D, Nattel S. New Antiarrhythmic Drugs for Treatment of Atrial Fibrillation. Lancet. 2010;375:1212–1223. doi: 10.1016/S0140-6736(10)60096-7. [DOI] [PubMed] [Google Scholar]
- 51.Behr ER, Roden D. Drug-Induced Arrhythmia: Pharmacogenomic Prescribing? Eur Heart J. 2013;34:89–95. doi: 10.1093/eurheartj/ehs351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas P, Smart TG. Hek293 Cell Line: A Vehicle for the Expression of Recombinant Proteins. J Pharmacol Toxicol Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Gandini MA, Sandoval A, Felix R. Whole-Cell Patch-Clamp Recording of Recombinant Voltage-Sensitive Ca2+ Channels Heterologously Expressed in Hek-293 Cells. Cold Spring Harb Protoc. 2014;2014:396–401. doi: 10.1101/pdb.prot073213. [DOI] [PubMed] [Google Scholar]
- 54.Clancy CE, Rudy Y. Cellular Consequences of Herg Mutations in the Long Qt Syndrome: Precursors to Sudden Cardiac Death. Cardiovasc Res. 2001;50:301–313. doi: 10.1016/s0008-6363(00)00293-5. [DOI] [PubMed] [Google Scholar]
- 55.Verrier RL, Kumar K, Nieminen T, Belardinelli L. Mechanisms of Ranolazine's Dual Protection against Atrial and Ventricular Fibrillation. Europace. 2013;15:317–324. doi: 10.1093/europace/eus380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burridge PW, Keller G, Gold JD, Wu JC. Production of De Novo Cardiomyocytes: Human Pluripotent Stem Cell Differentiation and Direct Reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruetz T, Kaji K. Routes to Induced Pluripotent Stem Cells. Curr Opin Genet Dev. 2014;28C:38–42. doi: 10.1016/j.gde.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong SG, Hong WX, Huang M, Wu JC. Effect of Human Donor Cell Source on Differentiation and Function of Cardiac Induced Pluripotent Stem Cells. J Am Coll Cardiol. 2014;64:436–448. doi: 10.1016/j.jacc.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis RP, van den Berg CW, Casini S, Braam SR, Mummery CL. Pluripotent Stem Cell Models of Cardiac Disease and Their Implication for Drug Discovery and Development. Trends Mol Med. 2011;17:475–484. doi: 10.1016/j.molmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Zicha S, Moss I, Allen B, Varro A, Papp J, Dumaine R, Antzelevich C, Nattel S. Molecular Basis of Species-Specific Expression of Repolarizing K+ Currents in the Heart. Am J Physiol Heart Circ Physiol. 2003;285:H1641–H1649. doi: 10.1152/ajpheart.00346.2003. [DOI] [PubMed] [Google Scholar]
- 61.Shah RR. Drug-Induced Qt Interval Prolongation--Regulatory Guidance and Perspectives on Herg Channel Studies. Novartis Found Symp. 2005;266:251–280. discussion 280–255. [PubMed] [Google Scholar]
- 62.Obiol-Pardo C, Gomis-Tena J, Sanz F, Saiz J, Pastor M. A Multiscale Simulation System for the Prediction of Drug-Induced Cardiotoxicity. J Chem Inf Model. 2011;51:483–492. doi: 10.1021/ci100423z. [DOI] [PubMed] [Google Scholar]
- 63.Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC. Drug Screening Using a Library of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Reveals Disease-Specific Patterns of Cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kattman SJ, Koonce CH, Swanson BJ, Anson BD. Stem Cells and Their Derivatives: A Renaissance in Cardiovascular Translational Research. J Cardiovasc Transl Res. 2011;4:66–72. doi: 10.1007/s12265-010-9235-1. [DOI] [PubMed] [Google Scholar]
- 65.Honda M, Kiyokawa J, Tabo M, Inoue T. Electrophysiological Characterization of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. J Pharmacol Sci. 2011;117:149–159. doi: 10.1254/jphs.11038fp. [DOI] [PubMed] [Google Scholar]
- 66.Yokoo N, Baba S, Kaichi S, Niwa A, Mima T, Doi H, Yamanaka S, Nakahata T, Heike T. The Effects of Cardioactive Drugs on Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Biochem Biophys Res Commun. 2009;387:482–488. doi: 10.1016/j.bbrc.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 67.Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of Drug-Induced Cardiotoxicity Using Human Embryonic Stem Cell-Derived Cardiomyocytes. Stem Cell Res. 2010;4:107–116. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Caspi O, Itzhaki I, Kehat I, Gepstein A, Arbel G, Huber I, Satin J, Gepstein L. In Vitro Electrophysiological Drug Testing Using Human Embryonic Stem Cell Derived Cardiomyocytes. Stem Cells Dev. 2009;18:161–172. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- 69.Mercola M, Colas A, Willems E. Induced Pluripotent Stem Cells in Cardiovascular Drug Discovery. Circ Res. 2013;112:534–548. doi: 10.1161/CIRCRESAHA.111.250266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harris K, Aylott M, Cui Y, Louttit JB, McMahon NC, Sridhar A. Comparison of Electrophysiological Data from Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes to Functional Preclinical Safety Assays. Toxicol Sci. 2013;134:412–426. doi: 10.1093/toxsci/kft113. [DOI] [PubMed] [Google Scholar]
- 71.Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC. Abnormal Calcium Handling Properties Underlie Familial Hypertrophic Cardiomyopathy Pathology in Patient-Specific Induced Pluripotent Stem Cells. Cell Stem Cell. 2013;12:101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mordwinkin NM, Burridge PW, Wu JC. A Review of Human Pluripotent Stem Cell-Derived Cardiomyocytes for High-Throughput Drug Discovery, Cardiotoxicity Screening, and Publication Standards. J Cardiovasc Transl Res. 2013;6:22–30. doi: 10.1007/s12265-012-9423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leyton-Mange JS, Mills RW, Macri VS, Jang MY, Butte FN, Ellinor PT, Milan DJ. Rapid Cellular Phenotyping of Human Pluripotent Stem Cell-Derived Cardiomyocytes Using a Genetically Encoded Fluorescent Voltage Sensor. Stem Cell Reports. 2014;2:163–170. doi: 10.1016/j.stemcr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, Weisel RD, Keller G, Li RK. The Effect of Cyclic Stretch on Maturation and 3d Tissue Formation of Human Embryonic Stem Cell-Derived Cardiomyocytes. Biomaterials. 2014;35:2798–2808. doi: 10.1016/j.biomaterials.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 75.Kadota S, Minami I, Morone N, Heuser JE, Agladze K, Nakatsuji N. Development of a Reentrant Arrhythmia Model in Human Pluripotent Stem Cell-Derived Cardiac Cell Sheets. Eur Heart J. 2013;34:1147–1156. doi: 10.1093/eurheartj/ehs418. [DOI] [PubMed] [Google Scholar]
- 76.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High Purity Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Electrophysiological Properties of Action Potentials and Ionic Currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Witty AD, Mihic A, Tam RY, Fisher SA, Mikryukov A, Shoichet MS, Li RK, Kattman SJ, Keller G. Generation of the Epicardial Lineage from Human Pluripotent Stem Cells. Nat Biotechnol. 2014;32:1026–1035. doi: 10.1038/nbt.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen HS. Studying Arrhythmogenic Right Ventricular Dysplasia with Patient-Specific Ipscs. Nature. 2013;494:105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ivashchenko CY, Pipes GC, Lozinskaya IM, Lin Z, Xiaoping X, Needle S, Grygielko ET, Hu E, Toomey JR, Lepore JJ, Willette RN. Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Exhibit Temporal Changes in Phenotype. Am J Physiol Heart Circ Physiol. 2013;305:H913–H922. doi: 10.1152/ajpheart.00819.2012. [DOI] [PubMed] [Google Scholar]
- 80.Chan YC, Ting S, Lee YK, Ng KM, Zhang J, Chen Z, Siu CW, Oh SK, Tse HF. Electrical Stimulation Promotes Maturation of Cardiomyocytes Derived from Human Embryonic Stem Cells. J Cardiovasc Transl Res. 2013;6:989–999. doi: 10.1007/s12265-013-9510-z. [DOI] [PubMed] [Google Scholar]
- 81.Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T. Ultrastructural Maturation of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Long-Term Culture. Circ J. 2013;77:1307–1314. doi: 10.1253/circj.cj-12-0987. [DOI] [PubMed] [Google Scholar]
- 82.Hoekstra M, Mummery CL, Wilde AA, Bezzina CR, Verkerk AO. Induced Pluripotent Stem Cell Derived Cardiomyocytes as Models for Cardiac Arrhythmias. Front Physiol. 2012;3:346. doi: 10.3389/fphys.2012.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Wang F, Zhang X, Qi Z, Tang M, Szeto C, Zhang H, Chen X. Beta-Adrenergic Stimulation Increases Cav3.1 Activity in Cardiac Myocytes through Protein Kinase A. PLoS One. 2012;7:e39965. doi: 10.1371/journal.pone.0039965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang M, Zhang X, Li Y, Guan Y, Ai X, Szeto C, Nakayama H, Zhang H, Ge S, Molkentin JD, Houser SR, Chen X. Enhanced Basal Contractility but Reduced Excitation-Contraction Coupling Efficiency and Beta-Adrenergic Reserve of Hearts with Increased Cav1.2 Activity. Am J Physiol Heart Circ Physiol. 2010;299:H519–H528. doi: 10.1152/ajpheart.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim GH. Microrna Regulation of Cardiac Conduction and Arrhythmias. Transl Res. 2013;161:381–392. doi: 10.1016/j.trsl.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amin AS, Tan HL, Wilde AA. Cardiac Ion Channels in Health and Disease. Heart Rhythm. 2010;7:117–126. doi: 10.1016/j.hrthm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 87.Schnizler K, Kuster M, Methfessel C, Fejtl M. The Roboocyte: Automated Cdna/Mrna Injection and Subsequent Tevc Recording on Xenopus Oocytes in 96-Well Microtiter Plates. Receptors Channels. 2003;9:41–48. [PubMed] [Google Scholar]
- 88.Vasilyev DV, Merrill TL, Bowlby MR. Development of a Novel Automated Ion Channel Recording Method Using "inside-out" Whole-Cell Membranes. J Biomol Screen. 2005;10:806–813. doi: 10.1177/1087057105279481. [DOI] [PubMed] [Google Scholar]
- 89.Vasilyev D, Merrill T, Iwanow A, Dunlop J, Bowlby M. A Novel Method for Patch-Clamp Automation. Pflugers Arch. 2006;452:240–247. doi: 10.1007/s00424-005-0029-2. [DOI] [PubMed] [Google Scholar]
- 90.Papke RL. Estimation of Both the Potency and Efficacy of Alpha7 Nachr Agonists from Single-Concentration Responses. Life Sci. 2006;78:2812–2819. doi: 10.1016/j.lfs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 91.Milligan CJ, Moller C. Automated Planar Patch-Clamp. Methods Mol Biol. 2013;998:171–187. doi: 10.1007/978-1-62703-351-0_13. [DOI] [PubMed] [Google Scholar]
- 92.Kiss L, Bennett PB, Uebele VN, Koblan KS, Kane SA, Neagle B, Schroeder K. High Throughput Ion-Channel Pharmacology: Planar-Array-Based Voltage Clamp. Assay Drug Dev Technol. 2003;1:127–135. doi: 10.1089/154065803321537845. [DOI] [PubMed] [Google Scholar]
- 93.Farre C, Haythornthwaite A, Haarmann C, Stoelzle S, Kreir M, George M, Bruggemann A, Fertig N. Port-a-Patch and Patchliner: High Fidelity Electrophysiology for Secondary Screening and Safety Pharmacology. Comb Chem High Throughput Screen. 2009;12:24–37. doi: 10.2174/138620709787047966. [DOI] [PubMed] [Google Scholar]
- 94.Frey U, Egert U, Heer F, Hafizovic S, Hierlemann A. Microelectronic System for High-Resolution Mapping of Extracellular Electric Fields Applied to Brain Slices. Biosens Bioelectron. 2009;24:2191–2198. doi: 10.1016/j.bios.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 95.Spira ME, Hai A. Multi-Electrode Array Technologies for Neuroscience and Cardiology. Nat Nanotechnol. 2013;8:83–94. doi: 10.1038/nnano.2012.265. [DOI] [PubMed] [Google Scholar]
- 96.Rampe D, Brown AM. A History of the Role of the Herg Channel in Cardiac Risk Assessment. J Pharmacol Toxicol Methods. 2013;68:13–22. doi: 10.1016/j.vascn.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 97.Zemzemi N, Bernabeu MO, Saiz J, Cooper J, Pathmanathan P, Mirams GR, Pitt-Francis J, Rodriguez B. Computational Assessment of Drug-Induced Effects on the Electrocardiogram: From Ion Channel to Body Surface Potentials. Br J Pharmacol. 2013;168:718–733. doi: 10.1111/j.1476-5381.2012.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mirams GR, Cui Y, Sher A, Fink M, Cooper J, Heath BM, McMahon NC, Gavaghan DJ, Noble D. Simulation of Multiple Ion Channel Block Provides Improved Early Prediction of Compounds' Clinical Torsadogenic Risk. Cardiovasc Res. 2011;91:53–61. doi: 10.1093/cvr/cvr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davies MR, Mistry HB, Hussein L, Pollard CE, Valentin JP, Swinton J, Abi-Gerges N. An in Silico Canine Cardiac Midmyocardial Action Potential Duration Model as a Tool for Early Drug Safety Assessment. Am J Physiol Heart Circ Physiol. 2012;302:H1466–H1480. doi: 10.1152/ajpheart.00808.2011. [DOI] [PubMed] [Google Scholar]
- 100.Herron TJ, Lee P, Jalife J. Optical Imaging of Voltage and Calcium in Cardiac Cells & Tissues. Circ Res. 2012;110:609–623. doi: 10.1161/CIRCRESAHA.111.247494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. Hrs/Ehra Expert Consensus Statement on the State of Genetic Testing for the Channelopathies and Cardiomyopathies This Document Was Developed as a Partnership between the Heart Rhythm Society (Hrs) and the European Heart Rhythm Association (Ehra) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 102.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A, Laugwitz KL. Patient-Specific Induced Pluripotent Stem-Cell Models for Long-Qt Syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 103.Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A, Denning C. Drug Evaluation in Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells Carrying a Long Qt Syndrome Type 2 Mutation. Eur Heart J. 2011;32:952–962. doi: 10.1093/eurheartj/ehr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the Long Qt Syndrome with Induced Pluripotent Stem Cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 105.Ma D, Wei H, Zhao Y, Lu J, Li G, Sahib NB, Tan TH, Wong KY, Shim W, Wong P, Cook SA, Liew R. Modeling Type 3 Long Qt Syndrome with Cardiomyocytes Derived from Patient-Specific Induced Pluripotent Stem Cells. Int J Cardiol. 2013;168:5277–5286. doi: 10.1016/j.ijcard.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Terrenoire C, Wang K, Tung KW, Chung WK, Pass RH, Lu JT, Jean JC, Omari A, Sampson KJ, Kotton DN, Keller G, Kass RS. Induced Pluripotent Stem Cells Used to Reveal Drug Actions in a Long Qt Syndrome Family with Complex Genetics. J Gen Physiol. 2013;141:61–72. doi: 10.1085/jgp.201210899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Remme CA, Wilde AA. Scn5a Overlap Syndromes: No End to Disease Complexity? Europace. 2008;10:1253–1255. doi: 10.1093/europace/eun267. [DOI] [PubMed] [Google Scholar]
- 108.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using Induced Pluripotent Stem Cells to Investigate Cardiac Phenotypes in Timothy Syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Y, Liang P, Lan F, Wu H, Lisowski L, Gu M, Hu S, Kay MA, Urnov FD, Shinnawi R, Gold JD, Gepstein L, Wu JC. Genome Editing of Isogenic Human Induced Pluripotent Stem Cells Recapitulates Long Qt Phenotype for Drug Testing. J Am Coll Cardiol. 2014;64:451–459. doi: 10.1016/j.jacc.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu PD, Lander ES, Zhang F. Development and Applications of Crispr-Cas9 for Genome Engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Itzhaki I, Maizels L, Huber I, Gepstein A, Arbel G, Caspi O, Miller L, Belhassen B, Nof E, Glikson M, Gepstein L. Modeling of Catecholaminergic Polymorphic Ventricular Tachycardia with Patient-Specific Human-Induced Pluripotent Stem Cells. J Am Coll Cardiol. 2012;60:990–1000. doi: 10.1016/j.jacc.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 112.Nakajima T, Kaneko Y, Taniguchi Y, Hayashi K, Takizawa T, Suzuki T, Nagai R. The Mechanism of Catecholaminergic Polymorphic Ventricular Tachycardia May Be Triggered Activity Due to Delayed Afterdepolarization. Eur Heart J. 1997;18:530–531. doi: 10.1093/oxfordjournals.eurheartj.a015281. [DOI] [PubMed] [Google Scholar]
- 113.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-Specific Induced Pluripotent Stem Cells as a Model for Familial Dilated Cardiomyopathy. Sci Transl Med. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kleber AG, Saffitz JE. Identification of a New Modulator of the Intercalated Disc in a Zebrafish Model of Arrhythmogenic Cardiomyopathy. Sci Transl Med. 2014;6:240ra274. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma D, Wei H, Lu J, Ho S, Zhang G, Sun X, Oh Y, Tan SH, Ng ML, Shim W, Wong P, Liew R. Generation of Patient-Specific Induced Pluripotent Stem Cell-Derived Cardiomyocytes as a Cellular Model of Arrhythmogenic Right Ventricular Cardiomyopathy. Eur Heart J. 2013;34:1122–1133. doi: 10.1093/eurheartj/ehs226. [DOI] [PubMed] [Google Scholar]
- 116.Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-Specific Induced Pluripotent Stem-Cell-Derived Models of Leopard Syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hick A, Wattenhofer-Donze M, Chintawar S, Tropel P, Simard JP, Vaucamps N, Gall D, Lambot L, Andre C, Reutenauer L, Rai M, Teletin M, Messaddeq N, Schiffmann SN, Viville S, Pearson CE, Pandolfo M, Puccio H. Neurons and Cardiomyocytes Derived from Induced Pluripotent Stem Cells as a Model for Mitochondrial Defects in Friedreich's Ataxia. Dis Model Mech. 2013;6:608–621. doi: 10.1242/dmm.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brandenburger M, Wenzel J, Bogdan R, Richardt D, Nguemo F, Reppel M, Hescheler J, Terlau H, Dendorfer A. Organotypic Slice Culture from Human Adult Ventricular Myocardium. Cardiovasc Res. 2012;93:50–59. doi: 10.1093/cvr/cvr259. [DOI] [PubMed] [Google Scholar]
- 119.Jacoby DL, DePasquale EC, McKenna WJ. Hypertrophic Cardiomyopathy: Diagnosis, Risk Stratification and Treatment. CMAJ. 2013;185:127–134. doi: 10.1503/cmaj.120138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD, Morad M. Familial Hypertrophic Cardiomyopathy-Linked Mutant Troponin T Causes Stress-Induced Ventricular Tachycardia and Ca2+-Dependent Action Potential Remodeling. Circ Res. 2003;92:428–436. doi: 10.1161/01.RES.0000059562.91384.1A. [DOI] [PubMed] [Google Scholar]
- 121.Knollmann BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodelling of Ionic Currents in Hypertrophied and Failing Hearts of Transgenic Mice Overexpressing Calsequestrin. J Physiol. 2000;525(Pt 2):483–498. doi: 10.1111/j.1469-7793.2000.t01-1-00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Navarrete EG, Liang P, Lan F, Sanchez-Freire V, Simmons C, Gong T, Sharma A, Burridge PW, Patlolla B, Lee AS, Wu H, Beygui RE, Wu SM, Robbins RC, Bers DM, Wu JC. Screening Drug-Induced Arrhythmia [Corrected] Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Low-Impedance Microelectrode Arrays. Circulation. 2013;128:S3–S13. doi: 10.1161/CIRCULATIONAHA.112.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roden DM. Repolarization Reserve: A Moving Target. Circulation. 2008;118:981–982. doi: 10.1161/CIRCULATIONAHA.108.798918. [DOI] [PubMed] [Google Scholar]
- 124.Braam SR, Tertoolen L, Casini S, Matsa E, Lu HR, Teisman A, Passier R, Denning C, Gallacher DJ, Towart R, Mummery CL. Repolarization Reserve Determines Drug Responses in Human Pluripotent Stem Cell Derived Cardiomyocytes. Stem Cell Res. 2013;10:48–56. doi: 10.1016/j.scr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 125.Podrid PJ. Proarrhythmia, a Serious Complication of Antiarrhythmic Drugs. Curr Cardiol Rep. 1999;1:289–296. doi: 10.1007/s11886-999-0052-6. [DOI] [PubMed] [Google Scholar]
- 126.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada Syndrome: Report of the Second Consensus Conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 127.Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL. Dantrolene Rescues Arrhythmogenic Ryr2 Defect in a Patient-Specific Stem Cell Model of Catecholaminergic Polymorphic Ventricular Tachycardia. EMBO Mol Med. 2012;4:180–191. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mordwinkin NM, Lee AS, Wu JC. Patient-Specific Stem Cells and Cardiovascular Drug Discovery. JAMA. 2013;310:2039–2040. doi: 10.1001/jama.2013.282409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suzuki K, Yu C, Qu J, Li M, Yao X, Yuan T, Goebl A, Tang S, Ren R, Aizawa E, Zhang F, Xu X, Soligalla RD, Chen F, Kim J, Kim NY, Liao HK, Benner C, Esteban CR, Jin Y, Liu GH, Li Y, Izpisua Belmonte JC. Targeted Gene Correction Minimally Impacts Whole-Genome Mutational Load in Human-Disease-Specific Induced Pluripotent Stem Cell Clones. Cell Stem Cell. 2014;15:31–36. doi: 10.1016/j.stem.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.