Abstract
Objective
Large randomized trials have failed to show a beneficial effect of statin treatment in chronic HF. The investigators tried to evaluate the long-term effects of statin therapy in patients with new onset heart failure (HF) following acute myocardial infarction (AMI).
Methods
Between January 2008 and December 2011, a total of 13,616 AMI patients were enrolled in the Korea Acute Myocardial Infarction Registry (KAMIR) which was a prospective, multi-center, nationwide, web-based database of AMI in Korea. From this database, we studied 1,055 patients with AMI who had newly developed severe acute HF [left ventricular ejection fraction ≤ 40%] and were discharged alive. The patients were divided into two groups, a statin group (n = 756) and a no-statin group (n = 299). We investigated the one-year major adverse cardiovascular events (MACEs), including all-cause mortality, MI, and any revascularization of each group. We then performed a propensity-score matched analysis.
Results
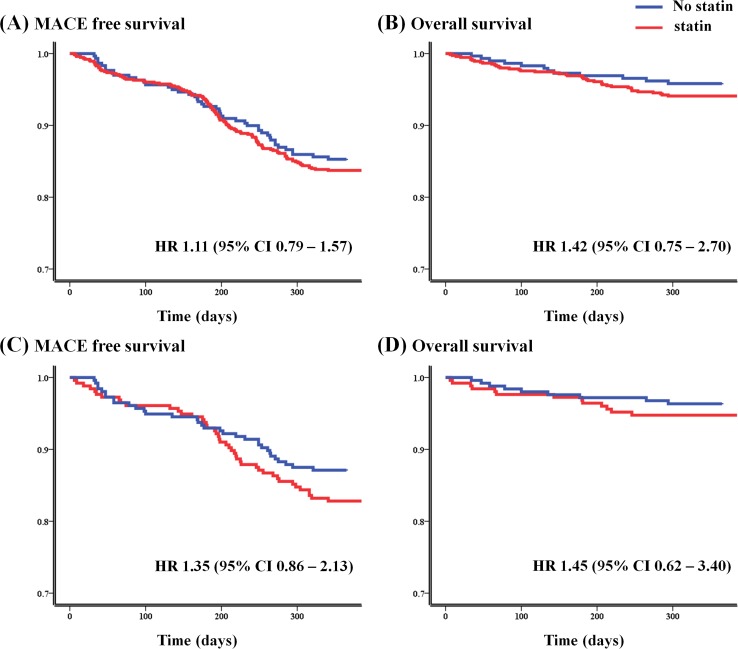
In the original cohort, one-year MACEs were similar between the two groups (16.5% vs. 14.7% in the statin or no-statin groups; p = 0.47). Propensity-score matching yielded 256 pairs, and in that population we observed comparable results in terms of MACEs (18.0% vs. 12.5% in the statin or no-statin groups, p = 0.11) and mortality (5.1% vs. 3.5% in the statin or no-statin groups, p = 0.51). Cox-regression analysis revealed that statin therapy was not an independent predictor for occurrence of a MACE [Hazard ratio (HR) 1.11, 95% CI 0.79–1.57, p = 0.54] or all-cause mortality (HR 1.42, 95% CI 0.75–2.70, p = 0.28).
Conclusion
Statin therapy was not associated with a reduction in the long-term occurrence of MACEs or mortality in survivors of AMI with severe acute HF in this retrospective cohort study.
Introduction
The 3-hydroxy-3-methylglutaryl coenzyme-A inhibitors or statins are widely used, and statin therapy has been shown to reduce adverse cardiovascular (CV) events and all-cause mortality in atherosclerotic coronary artery disease [1,2]. However, most clinical trials of statins have excluded patients with acute or chronic heart failure (HF), so the effects of statin therapy on outcomes in HF remain unclear. Although some clinical studies have shown that statin therapy is associated with improved outcomes in patients with chronic HF and that statin therapy reduces the development of HF after acute coronary syndrome [3,4,5,6], two large randomized clinical trials failed to demonstrate a beneficial effect of statin add-on therapy (rosuvastatin) in chronic HF patients who were medically well-controlled [7,8]. Beyond the lipid-lowering effect of statins, there are abundant pleiotropic effects such as improving endothelial function, enhancing stabilization of atheromatous plaque, decreasing oxidative stress, and decreasing vascular inflammation which would be translated to immediate benefits for patients with acute myocardial infarction (AMI) [9,10]. Even statin therapy would not be beneficial in patients with chronic and established HF, we hypothesized that there would be different results in new onset HF patients by its pleiotropic effects. Additionally, there is a lack of data demonstrating the effect of on clinical outcomes of statin therapy on clinical outcomes in patients with severe acute HF who survived an AMI. To address this issues, we performed an observational study using a nationwide registry to ascertain whether statin therapy improves long-term clinical outcomes, including major adverse cardiac events (MACEs) and mortality, in survivors from acute ischemic HF. We also analyzed clinical outcomes of acute ischemic HF patients by intensity of statin dosage.
Methods
Ethics statement
Our study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki, as revised in 2000. The study protocol was approved by the institutional review board of all centers, and the approval number was 05–49 of Chonnam National University Hospital. Participating sites are listed in an appendix. Given the retrospective design of the project, this institutional review board waived the need for consent. The study was conducted using the format recommended by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [11].
Data Source
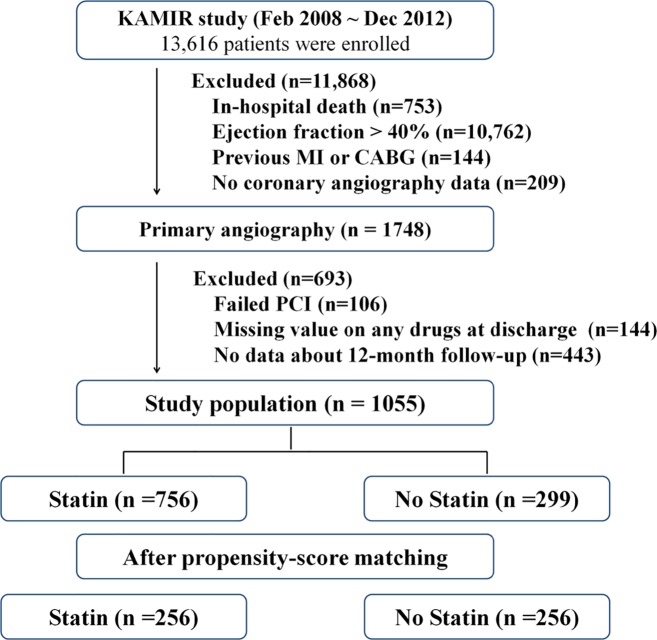
The Korea Acute Myocardial Infarction Registry (KAMIR) was developed to establish a standardized practice guideline for AMI patients in Korea. The KAMIR was a prospective, multi-center nationwide web-based database supported by the Korean Society of Cardiology that collects precise data with regard to demographic characteristics, treatment strategies in the emergency room, detailed angiographic findings, in-hospital medications, and the clinical outcomes of AMI patients. The detailed was described in previously [12]. It began in January 2008, and 53 centers that were capable of performing a large number of primary percutaneous coronary interventions (PCIs) participated. As shown in Fig 1, a total of 443 (25%) patients were excluded this study because of omitting 12-month clinical follow-up data. In this study, the data completeness of demographics including previous medical history were 97% and 85~95% for laboratory data. But discharge medications, clinical follow-up data were completed.
Fig 1. Study flow chart.
KAMIR, The Korea Acute Myocardial Infarction Registry; MI, myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention
Study Design and Population
This is a retrospective observational pooled analysis of nationwide registry data. Between January 2008 and December 2011, a total of 13,616 AMI patients were enrolled in the KAMIR registry. As shown in Fig 1, a total of 1,748 patients with a left ventricular ejection fraction (LVEF) less than 40% were eligible. Patients who had a past medical history of previous myocardial infarction (MI) or coronary artery bypass grafting (CABG) surgery were not included in order to exclude patients with chronic HF. Additionally, we did not include patients who died in hospital in order to only include AMI survivors in the study. We also excluded patients with missing data about medication and clinical follow-up. Ultimately, 1,055 patients with newly developed severe ischemic HF who presented with AMI were enrolled in this study. The patients were then divided into the following two groups according to the whether a statin was included among their discharge medications: treated with a statin group (statin group, n = 756) and not treated with a statin group (no-statin group, n = 299). The mean study follow-up duration was 336 ± 78 days. We also performed a propensity score matched analysis for treatment with statin as described in below.
Study outcomes
All of the definitions of the clinical outcomes in this study were based on the recommendations of Academic Research Consortium [13]. The primary endpoint of this study was a 12-month composite of MACEs, which was composed of all-cause mortality, recurrent MI, and any repeat PCI or coronary artery bypass grafting (CABG). All-cause mortality included cardiac and noncardiac deaths. Recurrent MI was defined as recurrent symptoms with new electrocardiographic changes compatible with MI or cardiac markers at least twice the upper limit of normal. Stent thrombosis was defined as definite and probable stent thrombosis according to the Academic Research Consortium definition [13]. Target lesion revascularization was defined as any repeat PCI of the target lesion or coronary bypass surgery of the target vessel or other complications related to the target lesion. The target lesion was defined as the treated segment within a stent or within 5 mm proximal or distal to the stent. Target vessel revascularization was defined as any repeat PCI or coronary bypass surgery of any segment of the target vessel. The target vessel was defined as the entire major coronary vessel proximal and distal to the target lesion, which included upstream and downstream branches and the target lesion itself. The secondary endpoint included clinical outcomes according to the intensities of statin therapy.
Statistical Analysis
All descriptive data are expressed as the mean ± SEM. To compare continuous and categorical variables between groups, Student’s t-test (2-sided) and Chi-square test or Fisher's exact test were used, respectively. An unadjusted Cox proportional-hazards model was used to evaluate the association between statin treatment and clinical outcomes and to calculate hazard ratios (HR) with 95% confidence intervals (CI). The cumulative MACE and mortality rates were estimated using the Kaplan-Meier method with the log rank test, and the time-dependent effect of statin therapy as a covariate on clinical outcomes was analyzed using a Cox proportional hazards regression model.
To overcome differences in baseline characteristics and discharge medications between the two groups and to minimize any selection bias, we performed a propensity-score matched analysis, which yielded 256 well-matched pairs. Briefly, the propensity-score of each patient, which indicated the estimated probability of receiving statin treatment, was calculated from the equation of a logistic regression model. Variables included in the logistic regression model used to deduce the propensity-score were age; gender; body mass index; systolic and diastolic blood pressure; heart rate; hypertension; diabetes mellitus; dyslipidemia; family history of heart disease; smoking history; specific medical history, including coronary artery disease, cerebrovascular accident, peripheral artery disease, and statin use before index admission; and lipid profiles, including total cholesterol, triglyceride, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein cholesterol. The c-statistic for the propensity score derivation was 0.85. Analyses were carried out using SPSS for Windows, version 19.0 (SPSS Inc., Chicago, Illinois).
Results
Baseline characteristics, angiographic results and medications
Baseline demographic characteristics and laboratory findings of the patients are demonstrated in Table 1. Systolic blood pressure (BP), diastolic BP, medical history of hyperlipidemia, and previous statin use were significantly different between the two groups. Among laboratory findings, the serum creatinine level was significantly lower in the statin group. However, serum total cholesterol, triglyceride and LDL cholesterol levels were significantly higher in the statin group. LVEF measured by echocardiography was not different between the two groups (33.8 ± 5.9% in the statin group vs. 33.9 ± 6.2% in the no-statin group, p = 0.72). In Angiographic results, lesion characteristics showed more severe in the statin group, but pre-procedural Thrombolysis in Myocardial Infarction score; pre-procedural lesion stenosis; and implanted stent number, diameter, and length were not significantly different between the two groups (S1 Table). In the propensity score-matched populations, all baseline characteristics, laboratory findings, and angiographic results were balanced between the two groups. In discharge medications, statin group patients more frequently had aspirin and clopidogrel among their discharge medications. However, in the propensity score-matched populations, discharge medications were not significantly different between the two groups.
Table 1. Baseline characteristics.
| Total population | Propensity-matched population | |||||
|---|---|---|---|---|---|---|
| No Statin(n = 299) | Statin(n = 756) | p value | No Statin(n = 256) | Statin(n = 256) | p value | |
| Age | 66.6 ± 11.7 | 66.8 ± 12.4 | 0.83 | 66.7 ± 11.5 | 66.2 ± 12.0 | 0.64 |
| Gender (male) | 218 (73%) | 517 (68%) | 0.15 | 72 (28%) | 72 (28%) | 0.99 |
| Body mass index (kg/m2) | 23.3 ± 3.5 | 23.5 ± 3.2 | 0.35 | 23.1 ± 3.5 | 22.9 ± 2.8 | 0.54 |
| Systolic blood pressure (mmHg) | 122.6 ± 31.6 | 127.8 ± 26.9 | < 0.01 | 124.0 ± 28.7 | 127.8 ± 27.1 | 0.13 |
| Diastolic blood pressure (mmHg) | 76.2 ± 19.9 | 78.6 ± 16.6 | 0.04 | 77.1 ± 18.2 | 78.1 ± 15.7 | 0.53 |
| Heart rate (bpm) | 84.1 ± 23.3 | 86.5 ± 21.4 | 0.11 | 84.4 ± 22.5 | 85.9 ± 21.4 | 0.45 |
| Final diagnosis (STEMI) | 172 (57%) | 452 (60%) | 0.50 | 103 (40%) | 110 (43%) | 0.59 |
| Killip class (more than II) | 140 (49%) | 316 (44%) | 0.15 | 124 (50%) | 109 (44%) | 0.18 |
| Previous statin use | 14 (5%) | 62 (25%) | 0.03 | 11 (4%) | 9 (3%) | 0.82 |
| Cardiovascular risk factors | ||||||
| Hypertension | 158 (53%) | 410 (55%) | 0.61 | 136 (53%) | 133 (52%) | 0.86 |
| Diabetes mellitus | 110 (37%) | 255 (34%) | 0.36 | 94 (37%) | 97 (38%) | 0.85 |
| Hyperlipidemia | 14 (5%) | 112 (15%) | < 0.01 | 10 (4%) | 12 (5%) | 0.83 |
| Current smoking | 122 (42%) | 284 (38%) | 0.29 | 108 (42%) | 108 (42%) | 0.99 |
| Previous coronary artery disease | 46 (15%) | 144 (19%) | 0.16 | 38 (15%) | 34 (13%) | 0.70 |
| Laboratory findings | ||||||
| Ejection fraction | 33.9 ± 6.2 | 33.8 ± 5.9 | 0.72 | 34.3 ± 6.0 | 34.3 ± 5.8 | 0.89 |
| Glucose (mg/dL) | 194.9 ± 103.4 | 187.7 ± 93.9 | 0.27 | 201.1 ± 110.4 | 197.1 ± 105.5 | 0.68 |
| Creatinine (mg/dL) | 1.4 ± 1.7 | 1.2 ± 1.1 | 0.02 | 1.2 ± 1.2 | 1.3 ± 1.6 | 0.38 |
| Max-CK-MB (mg/dL) | 154.6 ± 213.6 | 144.3 ± 184.7 | 0.44 | 146.8 ± 189.4 | 157.4 ± 213.1 | 0.55 |
| Max-cTnI (mg/dl) | 79.5 ± 250.4 | 72.2 ± 190.1 | 0.64 | 83.0 ± 181.2 | 77.8 ± 234.3 | 0.79 |
| Total cholesterol (mg/dL) | 169.1 ± 36.9 | 182.9 ± 48.4 | < 0.01 | 170.9 ± 45.6 | 168.8 ± 36.4 | 0.58 |
| Triglyceride (mg/dL) | 105.6 ± 63.3 | 120.3 ± 86.0 | < 0.01 | 109.1 ± 68.9 | 103.5 ± 62.5 | 0.34 |
| HDL-cholesterol (mg/dL) | 45.5 ± 26.7 | 44.1 ± 12.9 | 0.28 | 44.0 ± 14.0 | 45.6 ± 27.8 | 0.43 |
| LDL-cholesterol (mg/dL) | 108.4 ± 58.1 | 117.9 ± 41.9 | < 0.01 | 105.4 ± 38.3 | 104.6 ± 33.8 | 0.80 |
| hsCRP (mg/dL) | 7.7 ± 25.1 | 8.1 ± 26.6 | 0.81 | 11.3 ± 36.8 | 8.3 ± 26.6 | 0.31 |
| NT-proBNP (ng/dL) | 5510.2 ± 8927.4 | 4915.7 ± 7700.0 | 0.35 | 5853.1 ± 9139.0 | 5693.0 ± 9213.9 | 0.86 |
| HbA1C (%) | 6.8 ± 1.7 | 6.6 ± 1.5 | 0.13 | 6.7 ± 1.4 | 6.8 ± 1.7 | 0.37 |
| Discharge medications | ||||||
| Aspirin | 289 (97%) | 753 (99%) | < 0.01 | 250 (98%) | 254 (99%) | 0.28 |
| Clopidogrel | 287 (96%) | 745 (98%) | 0.01 | 248 (97%) | 251 (98%) | 0.58 |
| Calcium-channel blocker | 14 (5%) | 42 (6%) | 0.57 | 13 (5%) | 18 (7%) | 0.46 |
| Beta-blocker | 237 (79%) | 627 (83%) | 0.16 | 208 (81%) | 208 (81%) | 0.99 |
| ACE inhibitor | 158 (53%) | 435 (57%) | 0.17 | 136 (53%) | 144 (56%) | 0.53 |
| Angiotensin-receptor blocker | 80 (27%) | 199 (26%) | 0.88 | 71 (28%) | 62 (24%) | 0.42 |
| Angiographic findings | ||||||
| Stenotic vessels | ||||||
| 1 vessel disease | 93 (32%) | 258 (35%) | 0.38 | 81 (32%) | 85 (33%) | 0.78 |
| 2 vessel disease | 88 (30%) | 234 (31%) | 0.67 | 75 (29%) | 80 (31%) | 0.70 |
| 3 vessel disease | 97 (33%) | 233 (30%) | 0.32 | 87 (34%) | 80 (21%) | 0.57 |
| Left main coronary artery involved | 15 (5%) | 30 (4%) | 0.44 | 13 (5%) | 11 (4%) | 0.83 |
| ACC/AHA lesion class | ||||||
| Type A or B1 | 98 (32%) | 113 (17%) | < 0.01 | 83 (32%) | 80 (31%) | 0.55 |
| Type B2 or C | 201 (67%) | 554 (83%) | < 0.01 | 173 (68%) | 176 (69%) | 0.55 |
| Pre TIMI flow 0/1 | 183 (66%) | 425 (60%) | 0.38 | 148 (58%) | 136 (56%) | 0.65 |
| Post TIMI flow 3 | 246 (93%) | 647 (94%) | 0.59 | 218 (93%) | 226 (95%) | 0.44 |
| Target stent length | 24.2 ± 6.1 | 24.4 ± 6.9 | 0.59 | 24.1 ± 6.6 | 24.1 ± 6.1 | 0.85 |
| Target stent diameter | 3.1 ± 0.4 | 3.1 ± 0.4 | 0.89 | 3.1 ± 0.4 | 3.1 ± 0.4 | 0.77 |
| Total stent number | 1.5 ± 0.9 | 1.6 ± 0.8 | 0.40 | 1.6 ± 0.8 | 1.5 ± 0.9 | 0.49 |
CK-MB, creatine kinase MB; cTnI, cardiac troponin I; cTnT, cardiac troponin T; GFR, glomerular filtration rate; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reaction protein; LDL, low-density lipoprotein; NT-pro BNP, N-terminal prohormone of brain natriuretic peptide; ACC/AHA, American College of Cardiology/American Heart Association; TIMI, Thrombolysis in Myocardial Infarction
The effects of statin therapy on clinical outcomes
As shown in Table 2, the prevalence of one-year MACEs and one-year all-cause mortality were not significantly different between the two groups (Fig 2A and 2B). Similarly, one-year MACE-free and one-year all-cause survival rates in the propensity score-matched populations were not statistically different (Fig 2C and 2D). Cox proportional hazard regression analysis revealed that long-term statin use, as indicated by its inclusion in the discharge medications, did not reduce the incidence of MACEs or all-cause mortality during follow-up. Likewise, in the propensity-score matched populations, statin use did not reduce the incidence of MACEs or all-cause mortality during one-year of follow-up.
Table 2. Overall clinical outcomes.
| Total population | Propensity-matched population | |||||
|---|---|---|---|---|---|---|
| No Statin (n = 299) | Statin (n = 756) | p value | No Statin (n = 256) | Statin (n = 256) | p value | |
| Overall MACE * | 44 (14.7%) | 125 (16.5%) | 0.47 | 32 (12.5%) | 46 (18.0%) | 0.11 |
| All-cause mortality | 12 (4.0%) | 43 (5.7%) | 0.27 | 9 (3.5%) | 13 (5.1%) | 0.51 |
| Cardiac death | 9 (3.0%) | 32 (4.2%) | 0.35 | 6 (2.3%) | 9 (3.5%) | 0.60 |
| Non-cardiac death | 3 (1.0%) | 11 (1.5%) | 0.56 | 3 (1.2%) | 4 (1.6%) | 0.99 |
| Myocardial infarction | 6 (2.0%) | 16 (2.1%) | 0.91 | 6 (2.3%) | 5 (2.0%) | 0.99 |
| Repeated Revascularization | 24 (8.0%) | 55 (7.3%) | 0.68 | 17 (6.6%) | 23 (9.0%) | 0.41 |
| TLR | 12 (4.0%) | 28 (3.7%) | 0.81 | 10 (3.9%) | 13 (5.1%) | 0.67 |
| TVR | 4 (1.3%) | 8 (1.1%) | 0.70 | 4 (1.6%) | 2 (0.8%) | 0.69 |
| CABG | 2 (0.7%) | 6 (0.8%) | 0.83 | 1 (0.4%) | 0 (0%) | 0.99 |
| Stent thrombosis | 1 (0.3%) | 7 (0.9%) | 0.32 | 1 (0.4%) | 5 (2%) | 0.22 |
*Composed of all-cause mortality, myocardial infarction and repeated revascularization.
CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular event; TLR, target-lesion revascularization; TVR, target-vessel revascularization
Fig 2. Event-free survival curves.
The Kaplan-Meier curves demonstrate no significant differences between statin and no-statin groups for either 12-month major adverse cardiovascular events (A, overall population; C, propensity-score matched population) or 12-month all cause mortalities (B, overall population; D, propensity-score matched population). MACE, major adverse cardiovascular event; HR, hazard ratio
The statins prescribed at discharge and the intensities of statin therapy are demonstrated in Table 3. Atorvastatin was the most common statin included in the discharge medication lists, followed by rosuvastatin, pitavastatin, and simvastatin. However, the kind of statin did not have a significant effect on clinical outcomes in terms of MACEs or all-cause mortality (p = 0.70, 0.88, respectively). The intensities of statin therapy were also not an independent predictor of MACEs or all-cause mortality (p = 0.79, 0.43, respectively), and the same tendency was observed in the propensity score-matched populations.
Table 3. The effects of statin therapy on clinical outcomes.
| Total population | Propensity-matched population | |||
|---|---|---|---|---|
| *Composites of MACE | All-cause mortality | *Composites of MACE | All-cause mortality | |
| Kinds of statin | ||||
| Atorvastatin | 74 / 432 (17.1%) | 25 / 432 (5.8%) | 29 / 145 (20.0%) | 6 / 145 (4.1%) |
| Rosuvastatin | 26 / 193 (13.5%) | 12 / 193 (6.2%) | 9 / 63 (14.3%) | 5 / 63 (7.9%) |
| Pitavastatin | 12 / 68 (17.6%) | 2 / 68 (2.9%) | 5 / 28 (17.9%) | 0 / 28 (0%) |
| Simvastatin | 9 / 41 (22.0%) | 2 / 41 (4.9%) | 1 / 13 (7.7%) | 0 / 13 (0%) |
| Pravastatin | 3 / 17 (17.6%) | 2 / 17 (11.8%) | 2 / 7 (28.6%) | 2 / 7 (28.6%) |
| Fluvastatin | 1 / 5 (20.0%) | 0 / 5 (0%) | - | - |
| Intensities of statin | ||||
| Low | 2 / 11 (18.2%) | 1 / 11 (9.1%) | 1/6 (16.7%) | 1/3 (33.3%) |
| Moderate | 102 / 615 (16.6%) | 33 / 615 (5.4%) | 21/222 (9.5%) | 8/213 (3.8%) |
| High | 21 / 130 (16.2%) | 9 / 130 (6.9%) | 5/62 (8.1%) | 4/40 (10.0%) |
*Composed of all-cause mortality, myocardial infarction (MI), any repeated revascularization. MACE, major adverse cardiovascular event
Discussion
In this registry-based observational study, we found that long-term statin therapy did not reduce one-year MACEs or all-cause mortality in patients with AMI complicated by severe acute HF. Additionally, neither the type nor the intensity of statin therapy affected the one-year clinical outcomes of enrolled patients.
Although all of the patients in our study suffered from new onset acute HF, the results of our study are comparable to other prospective randomized controlled trials, such as Controlled Rosuvastatin Multinational Trial in HF trial (CORONA) or GISSI-Heart Failure trial (GISSI-HF), that enrolled patients with chronic systolic HF [7,8]. In contrast, several studies suggested that statin therapy could reduce MACEs in patients with acute ischemic HF complicating AMI. The post-hoc analysis of Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) study revealed that all-cause mortality occurred more in patients belonging to the group that did not receive a statin than the group that received a statin (18% vs. 12%, p < 0.01), and after adjustment for various confounding factors, the relative risk of all-cause mortality was lower in patients belonging to the group that received statin therapy (HR 0.80, 95% CI 0.69–0.92, p < 0.01) [14]. In addition, a small retrospective study demonstrated a lower all-cause mortality rate in the group that was prescribed a statin at discharge compared with a no-statin group (28.5% vs. 53.3%, p < 0.01). Statin therapy has also been shown to independently reduce HF re-admissions (7.0% vs. 32.0%, p < 0.01) and death from HF (4.0% vs 13.0%, p < 0.01) [15]. However, these reports are retrospective and have small sample sizes, and there is a lack of randomized data demonstrating the efficacy of statin therapy in patients with AMI complicated by acute HF.
Recent clinical studies have suggested that the potential benefits of statins in HF patients can be partially explained by their lipid-independent pleiotropic effects. These pleiotropic effects include stabilizing atherosclerotic plaques, improving endothelial function, and alleviating vascular oxidation and inflammation [16]. On the other hand, the mechanisms underlying the harmful effects of statin therapy in HF patients remain poorly understood. Some studies have demonstrated that low serum cholesterol levels in HF patients are related to adverse clinical outcomes [17,18], but whether a low serum cholesterol level itself induces deterioration of HF is unclear. Additionally, it has been proposed that statin therapy could increase susceptibility to infections in patients with HF by diminishing lipoproteins that eliminate bacterial lipopolysaccharides [19]. Other researchers have suggested that statins could damage cardiac myocytes by lowering levels of serum coenzyme Q10. Coenzyme Q10 is a key element in mitochondrial oxidative phosphorylation, and its depletion has been reported to be associated with increased mortality [20,21], but McMurray et al. demonstrated that neither statin (rosuvastatin) nor coenzyme Q10 is an independent prognostic factor in patients with HF [22].
According to the latest guidelines for dyslipidemia management, there is no obvious evidence that supports a beneficial effect of statin therapy in patients with New York Heart Association (NYHA) class II-IV HF [23,24]. Additionally, in the International Atherosclerosis Society guideline and National Institute for Health and Care Excellence guideline, there is no special mention of statin use in patients with HF [25,26]. According to this observational study and previous two large randomized trials [7,8], there was no benefit from initiating statin therapy in patients with severe HF (mean LVEF were less than 33 percent). But this results could not be generalized to mild HF patients or HF with preserved EF. Further prospective randomized controlled trials are needed to validate the role of statin therapy in acute ischemic HF patients.
Our study had a number of limitations. First, it was a retrospective, observational study based on a registry data. There was a lack of data that cannot be ignored although we encouraged to fill-up the missing values. Undetected selection bias may remain despite our cautious use of propensity-score matching with excluding patients with missing data. We could not controlled non-cardiac related medications and other patients' condition such as nutritional status or life style because of inherent limitations of registry data. Second, the number of patients participating in this study was relatively small. Third, there was the possibility of including patients with pre-existing non-ischemic HF despite of our careful review. Fourth, we have no detailed follow-up data with regard to lipid profiles or echocardiographic parameters of registered patients. Finally, we were not able to acquire safety information for the statins prescribed to the patients, such as the incidence rates of serious adverse effects or compliance data. Despite these drawbacks, we are able to present some negative evidence with regard to statin therapy in patients with MI complicated by severe acute ischemic HF. We also found that the type of statin and intensity of statin therapy did not exert an influence on clinical outcomes.
In conclusion, statin therapy had no significant effect on the one-year incidence of MACEs or mortality in survivors with acute severe ischemic HF following AMI.
Appendix
Institutional Review Board of Participating Sites
The Korea Acute Myocardial Infarction Registry (KAMIR) was approved by the institutional review board (IRB) at each participating center (Kyunghee University IRB, Asan Medical Center IRB, Ajou University Hospital IRB, Busan Hanseo Hospital IRB, Busan National University Hospital IRB, Choongbook National University Hospital IRB, Chungnam National University Hospital IRB, Catholic University Hospital IRB, Chonbuk National University Hospital IRB, Chosun University Hospital IRB, Chung-Ang University Hospital IRB, Dankook University Hospital IRB, Daegu Catholic University Medical Center IRB, Dong-A University Medical Center IRB, Daejeon St. Mary's Hospital IRB, Dongguk University Gyeongju Hospital IRB, Gyeongsang National University Hospital IRB, Hallym University Kandong Sacred Heart Hospital IRB, Hallym University Sacred Heart Hospital IRB, Inje University Haeundae Paik hospital IRB, Inje University Sanggye Paik Hospital IRB, Inha University Hospital IRB, Jeju National University Hospital IRB, Kyungpook National University Hospital IRB, Keimyung University Hospital IRB, Korea University Guro Hospital IRB, Konyang University Hospital IRB, Kwangju Christian Hospital IRB, Maryknoll Medical Center IRB, National Health Insurance Corporation Ilsan Hospital IRB, Yeungnam University Hospital IRB, Yonsei University Severance Hospital IRB, Yonsei University Wonju Hospital IRB, Presbyterian Medical Center IRB, Seoul National University Hospital IRB, Seoul National University Bundang Hospital IRB, Soon Chun Hyang University Bucheon Hospital IRB, Soon Chun Hyang University Cheonan Hospital IRB, Samsung Medical Center IRB, Sun General Hospital IRB, Saint Carollo Hospital IRB, Wonkwang University Hospital IRB and Chonnam National University Hospital IRB).
Supporting Information
(DOC)
Acknowledgments
Korea Acute Myocardial infarction Registry (KAMIR) Investigators
Myung Ho Jeong MD, Young Keun Ahn MD, Sung Chull Chae MD, Jong Hyun Kim MD, Seung Ho Hur MD, Young Jo Kim MD, In Whan Seong MD, Dong Hoon Choi MD, Jei Keon Chae MD, Taek Jong Hong MD, Jae Young Rhew MD, Doo Il Kim MD, In Ho Chae MD, Jung Han Yoon MD, Bon Kwon Koo MD, Byung Ok Kim MD, Myoung Yong Lee MD, Kee Sik Kim MD, Jin Yong Hwang MD, Myeong Chan Cho MD, Seok Kyu Oh MD, Nae Hee Lee MD, Kyoung Tae Jeong MD, Seung Jea Tahk MD, Jang Ho Bae MD, Seung Woon Rha MD, Keum Soo Park MD, Chong Jin Kim MD, Kyoo Rok Han MD, Tae Hoon Ahn MD, Moo Hyun Kim MD, Ki Bae Seung MD, Wook Sung Chung MD, Ju Young Yang MD, Chong Yun Rhim MD, Hyeon Cheol Gwon MD, Seong Wook Park MD, Young Youp Koh MD, Seung Jae Joo MD, Soo Joong Kim MD, Dong Kyu Jin MD, Jin Man Cho MD, Byung Ok Kim MD, Sang-Wook Kim MD, Jeong Kyung Kim MD, Tae Ik Kim MD, Deug Young Nah MD, Si Hoon Park MD, Sang Hyun Lee MD, Seung Uk Lee MD, Hang-Jae Chung MD, Jang Hyun Cho MD, Seung Won Jin, MD, Yang Soo Jang MD, Jeong Gwan Cho, MD and Seung Jung Park MD.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–504. Epub 2004/03/10. 10.1056/NEJMoa040583 [pii]. . [DOI] [PubMed] [Google Scholar]
- 2. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–35. Epub 2005/03/10. NEJMoa050461 [pii] 10.1056/NEJMoa050461 . [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Nye R, Levy WC. Statin therapy is associated with lower mortality among patients with severe heart failure. Am J Cardiol. 2004;93(9):1124–9. Epub 2004/04/28. 10.1016/j.amjcard.2004.01.039 S0002914904001298 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Clark AL, Winkler R, Zugck C, Cicoira M, Ponikowski P, et al. Statin use and survival in patients with chronic heart failure—results from two observational studies with 5200 patients. Int J Cardiol. 2006;112(2):234–42. Epub 2006/07/19. S0167-5273(06)00468-2 [pii] 10.1016/j.ijcard.2006.03.057 . [DOI] [PubMed] [Google Scholar]
- 5. Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296(17):2105–11. Epub 2006/11/02. 296/17/2105 [pii] 10.1001/jama.296.17.2105 . [DOI] [PubMed] [Google Scholar]
- 6. Krum H, Latini R, Maggioni AP, Anand I, Masson S, Carretta E, et al. Statins and symptomatic chronic systolic heart failure: a post-hoc analysis of 5010 patients enrolled in Val-HeFT. Int J Cardiol. 2007;119(1):48–53. Epub 2006/10/20. S0167-5273(06)00893-X [pii] 10.1016/j.ijcard.2006.07.106 . [DOI] [PubMed] [Google Scholar]
- 7. Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248–61. Epub 2007/11/07. NEJMoa0706201 [pii] 10.1056/NEJMoa0706201 . [DOI] [PubMed] [Google Scholar]
- 8. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1231–9. Epub 2008/09/02. S0140-6736(08)61240-4 [pii] 10.1016/S0140-6736(08)61240-4 . [DOI] [PubMed] [Google Scholar]
- 9. Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 Suppl 1):III39-43. Epub 2004/06/17. 10.1161/01.CIR.0000131517.20177.5a 109/23_suppl_1/III-39 [pii]. . [DOI] [PubMed] [Google Scholar]
- 10. Kushner FG, Hand M, Smith SC Jr., King SB 3rd, Anderson JL, Antman EM, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54(23):2205–41. Epub 2009/11/28. S0735-1097(09)03518-9 [pii] 10.1016/j.jacc.2009.10.015 . [DOI] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. Epub 2007/12/08. S0140-6736(07)61602-X [pii] 10.1016/S0140-6736(07)61602-X . [DOI] [PubMed] [Google Scholar]
- 12. Kim HK, Jeong MH, Lee SH, Sim DS, Hong YJ, Ahn Y, et al. The scientific achievements of the decades in Korean Acute Myocardial Infarction Registry. Korean J Intern Med. 2014;29(6):703–12. Epub 2014/11/08. 10.3904/kjim.2014.29.6.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51. Epub 2007/05/02. 115/17/2344 [pii] 10.1161/CIRCULATIONAHA.106.685313 . [DOI] [PubMed] [Google Scholar]
- 14. Dobre D, Rossignol P, Murin J, Parkhomenko A, Lamiral Z, Krum H, et al. Statin therapy and clinical outcomes in myocardial infarction patients complicated by acute heart failure: insights from the EPHESUS trial. Eur J Heart Fail. 2013;15(2):221–7. Epub 2012/08/09. hfs128 [pii] 10.1093/eurjhf/hfs128 . [DOI] [PubMed] [Google Scholar]
- 15. Sankaranarayanan R, Maini S, James MA, Burtchaell S, Chatterjee AK. Do statins improve heart failure outcome in post-myocardial infarction patients with moderate to severe left ventricular dysfunction? Congest Heart Fail. 2010;16(4):181–6. Epub 2010/07/29. CHF165 [pii] 10.1111/j.1751-7133.2010.00165.x . [DOI] [PubMed] [Google Scholar]
- 16. Ramasubbu K, Estep J, White DL, Deswal A, Mann DL. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51(4):415–26. Epub 2008/01/29. S0735-1097(07)03348-7 [pii] 10.1016/j.jacc.2007.10.009 . [DOI] [PubMed] [Google Scholar]
- 17. Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8(4):216–24. Epub 2002/10/25. S1071916402000180 [pii]. . [DOI] [PubMed] [Google Scholar]
- 18. Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42(11):1933–40. Epub 2003/12/10. S0735109703012002 [pii]. . [DOI] [PubMed] [Google Scholar]
- 19. Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356(9233):930–3. Epub 2000/10/19. S0140-6736(00)02690-8 [pii] 10.1016/S0140-6736(00)02690-8 . [DOI] [PubMed] [Google Scholar]
- 20. Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52(18):1435–41. Epub 2008/11/20. S0735-1097(08)02722-8 [pii] 10.1016/j.jacc.2008.07.044 . [DOI] [PubMed] [Google Scholar]
- 21. Deichmann R, Lavie C, Andrews S. Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010;10(1):16–21. Epub 2011/05/24. [PMC free article] [PubMed] [Google Scholar]
- 22. McMurray JJ, Dunselman P, Wedel H, Cleland JG, Lindberg M, Hjalmarson A, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J Am Coll Cardiol. 2010;56(15):1196–204. Epub 2010/10/05. S0735-1097(10)02710-5 [pii] 10.1016/j.jacc.2010.02.075 . [DOI] [PubMed] [Google Scholar]
- 23. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-45. Epub 2013/11/14. 01.cir.0000437738.63853.7a [pii] 10.1161/01.cir.0000437738.63853.7a . [DOI] [PubMed] [Google Scholar]
- 24. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818. Epub 2011/06/30. ehr158 [pii] 10.1093/eurheartj/ehr158 . [DOI] [PubMed] [Google Scholar]
- 25. Grundy SM. An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia. J Clin Lipidol. 2013;7(6):561–5. Epub 2013/12/10. S1933-2874(13)00301-2 [pii] 10.1016/j.jacl.2013.10.001 . [DOI] [PubMed] [Google Scholar]
- 26. Rabar S, Harker M, O'Flynn N, Wierzbicki AS. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: summary of updated NICE guidance. BMJ. 2014;349:g4356 Epub 2014/07/19. 10.1136/bmj.g4356 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.