Abstract
Two tick-borne diseases with expanding case and vector distributions are ehrlichiosis (transmitted by Amblyomma americanum) and rickettiosis (transmitted by A. maculatum and Dermacentor variabilis). There is a critical need to identify the specific habitats where each of these species is likely to be encountered to classify and pinpoint risk areas. Consequently, an in-depth tick prevalence study was conducted on the dominant ticks in the southeast. Vegetation, soil, and remote sensing data were used to test the hypothesis that habitat and vegetation variables can predict tick abundances. No variables were significant predictors of A. americanum adult and nymph tick abundance, and no clustering was evident because this species was found throughout the study area. For A. maculatum adult tick abundance was predicted by NDVI and by the interaction between habitat type and plant diversity; two significant population clusters were identified in a heterogeneous area suitable for quail habitat. For D. variabilis no environmental variables were significant predictors of adult abundance; however, D. variabilis collections clustered in three significant areas best described as agriculture areas with defined edges. This study identified few landscape and vegetation variables associated with tick presence. While some variables were significantly associated with tick populations, the amount of explained variation was not useful for predicting reliably where ticks occur; consequently, additional research that includes multiple sampling seasons and locations throughout the southeast are warranted. This low amount of explained variation may also be due to the use of hosts for dispersal, and potentially to other abiotic and biotic variables. Host species play a large role in the establishment, maintenance, and dispersal of a tick species, as well as the maintenance of disease cycles, dispersal to new areas, and identification of risk areas.
Introduction
The roles of Amblyomma americanum (lone star tick), Amblyomma maculatum (Gulf coast tick), and Dermacentor variabilis (American dog tick) in tick-borne disease (TBD) transmission has been directed at host association studies [1–6], but field studies investigating habitat use and niche partitioning where these species co-exist are severely lacking. Currently, these tick species’ distributions overlap with one another [5–8]. What makes these species of particular interest is the fact that they transmit a number of pathogens that affect humans and animals. Amblyomma americanum is a competent vector of Ehrlichia ewingii and E. chaffeensis causing ehrlichiosis [9–11], of Francisella tularensis causing tularemia [12, 13], and of the newly identified Heartland virus [14]. Amblyomma maculatum is the vector of R. parkeri causing American Boutonouse fever [11, 15] and E. ruminantium causing heartwater in ruminants [16]. Dermacentor variabilis is the vector of agents causing spotted fever group Rickettsiae and is a known vector of R. rickettsii [17, 18]. Within the southeastern United States both Ehrlichia and Rickettsia diagnoses are increasing, likely due, in part, to increasing tick numbers and expanding ranges [19].
Integrated tick management (ITM) programs include the application of acaricides to animals and vegetation, and various methods of habitat disturbance [20]. Acaricides alone are often not effective long-term when applied to existing vegetation because tick populations re-establish quickly as hosts move through the habitat; however, vegetation removal along with acaricides can produce conditions not suitable for tick survival and establishment [21]. Mechanical clearing of vegetation has been shown to result in an immediate reduction of local tick populations, though long-term reduction was not demonstrated [22]. Reduced A. americanum populations have been associated with landscape alterations including mechanical removal of vegetation such as clearing undergrowth and overstory, which reduce relative humidity and soil moisture [20]. Various results have been obtained in experiments that examined controlled burning as a means of tick control. Hoch et al. [23] found that controlled burning of woodlots was not effective in long-term control of A. americanum ticks, while other studies observed short-term reductions in the populations of some species [22, 24, 25]. Successful habitat treatments and a sustained lowering of tick populations are not single application procedures; as these sites become neglected, natural plant succession occurs and the site generally reverts to previous conditions.
Both abiotic and biotic components of the environment influence tick populations [26–28]. Because vegetation type influences the presence and movement of host species, it is likely that vegetative composition and structure are related to the presence, density, or persistence of tick species and to the probability of successful host acquisition. Microclimates and associated environmental habitats have been reported for a number of ticks; and these variables include humidity, temperature, and day light hours [26, 27]. For example, ticks will desiccate if isolated from microclimates with high temperatures [21]. The larval stage of ticks is the stage most susceptible to desiccation, and questing activity is “self”-restricted to periods when potential for desiccation is reduced [29]. After brief periods of questing on vegetation for passing hosts, the potential for desiccation forces the tick to travel back into the leaf litter [30]. Some ticks have adapted to longer questing periods and hotter temperatures. Dermacentor variabilis is considered desiccation-tolerant, I. scapularis can directly absorb fluids from saturated and mildly sub-saturated atmospheres, and Amblyomma ticks have a thickened waxy coating on their cuticles to prevent water loss [30]. It is often assumed that most ticks prefer secondary growth woodland habitats with a dense understory [26, 27], but these studies did not directly compare adjacent habitats with equal host opportunities. Ixodes scapularis populations have been associated with deciduous, dry to mesic forests and alfisol-type soils of sandy or loam-sand textures overlying sedimentary rock [28], and these data have been used to identify potential locations with Lyme disease (aka risk areas). Thus, the habitat’s microclimate, vegetation, and soil type likely have significant effects on tick abundance and questing activity and on the dynamics of TBDs. Habitat suitability also includes other stages in a tick’s life, including overwintering, molting, and oviposition sites.
Previous work at Ames Plantation in southwestern Tennessee and within the previously identified Rocky Mountain Spotted fever hot spot [31] during the summer of 2012 indicated that A. americanum abundance was positively associated with increasing basal area and ground cover [7]. Additionally, A. americanum specimens were identified with three Ehrlichia species (E. ewingii, Panola Mountain Ehrlichia, E. chaffeensis) and Anaplasma odocoilei. While these infected ticks were collected from a variety of habitats, positive collections were primarily in summer months (June), and no spatial clustering of positive ticks was evident [7]. Interestingly, Ehrlichia and Anaplasma were detected only in sites where both A. americanum and D. variabilis were present [7]. Additionally at that site, R. parkeri was identified in questing and host-collected A. maculatum (unpublished). Since the questing site is the location where pathogen transmission begins [32], it is essential to identify questing locations to minimize tick encounters and subsequent tick bites. There is a critical need to identify the landscape and vegetation features where each tick species is likely encountered to classify and pinpoint risk areas. Consequently, an in-depth tick prevalence study focused on the ticks of the southeastern United States was conducted during June 2014 when TBD cases peak in Tennessee [33, 34].
The objectives of this study were to specify the summer questing habitats of ticks commonly encountered in the southeastern United States through classification of vegetative and landscape characteristics. Additionally, we attempted to identify indicator plant species that inferred the presence of one or more tick species. The overarching hypothesis was that habitat and vegetation variables that influence questing behavior and host availability predict tick abundances. Identifying preferred habitats for questing ticks is the first step for an overall ITM program, which then includes identification of infected-questing habitats to study disease transmission, identify management options, and help with disease diagnosis.
Materials and Methods
Site Selection
Ames Plantation (35.115366 N, -89.216735 W) is a University of Tennessee-managed research center in western Tennessee. Permission was obtained from the director, R. Carlisle, and coauthor A. Houston to use the site. It is a 7,446 hectare contiguous tract (74.5 km2) containing an array of land-use types, including commodity row crops (e.g., cotton, soybean, corn, grain, wheat), pastures for horses and beef cattle, native warm season grasslands, and forests, including loblolly pine plantations, bottomland hardwoods, and upland hardwoods. These land uses are underlain with a broad spectrum of physiography, ranging from mesic bottomlands associated with the North Fork of the Wolf River to xeric upland sites, which provides significantly different ecological systems and suitable habitats for a number of different animals, including potential tick hosts. From the Ames’ Quality Deer Management Program, which includes an observation and harvest grid system with each grid ~40.5 ha, 76 tick-sampling sites were randomly stratified. Sites included 15 bottomland deciduous sites, 28 upland deciduous sites, 15 coniferous sites, and 18 open grasslands (Fig 1). Each of the 76 sites contained one plot, 100m long by 20m wide. Three 100m transects were placed side-by-side in these plots, 10m apart. Sampling from multiple sites provided an unbiased and representative proportion of the different environmental variables.
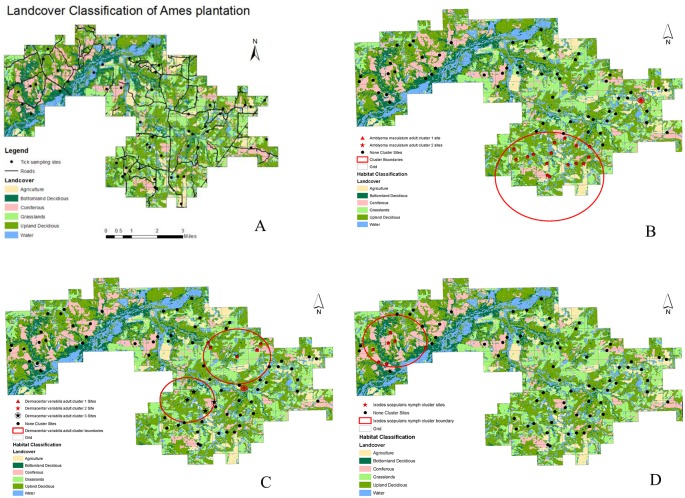
Fig 1. Ticks were collected from a variety of land cover classifications (A), and spatial clustering analysis indicated Amblyomma maculatum adults had two clusters (B), Dermacentor variabilis adults had three clusters (C), and Ixodes scapularis nymphs had one cluster (D).
Land cover classification is generated from the Landsat 8 OLI image downloaded at http://earthobservatory.nasa.gov
Tick Collection
Based upon a trapping methods comparison (unpublished), CO2-dry ice traps, conventional dragging, CO2-dragging, and CO2-flagging were used for tick sampling during June of 2014 when tick-borne diseases peak in Tennessee [7, 33, 34]. One dry ice trap was set at the middle of each center 100m transect and left overnight. The remaining three collection methods were randomly assigned to each 100m transect and checked for ticks every 20m along each transect. All encountered ticks were removed, counted, and placed in vials of 80% ethanol. Ticks were identified in the laboratory to life-stage, species, and sex [35–37].
Vegetation Characterization
Vegetation was sampled in a 1m2 quadrat at the center point of each tick drag along the center transect at each site. Therefore, plant community composition and structure were sampled at 10, 30, 50, 70, and 90m along each centerline. Within each sampling area, each plant species was identified and percent cover of each species was visually estimated with an adapted Daubenmire cover scale (<1%, 1–5%, 5–10%, 10–25%, 25–50%, 50–75%, 75–95%, > 95%) and transformed to median values for community similarity analyses. Transect data for each site were combined for both diversity and composition pattern analyses. To determine percentage canopy openness and leaf area index (LAI), hemispherical photographs taken in the transect center (50m) with a fisheye lens on a 1m tripod were analyzed with Gap Light Analyzer software [38]. All photographs were taken on cloudless days in late May between 0830 and 1330 h. To determine vertical structure, basal area was estimated using a handheld prism, identifying and including tree species with a diameter at breast height (dbh) > 5cm at each sampling point along the transect. All basal area data were combined for each site for analysis.
Landscape Characterization
At each site, three-soil core samples were taken every 5m along the middle transect. Soil samples were combined together to produce one composite sample for each 20m segment of the transect, and placed in a single labeled bag. Samples were stored on ice, brought back to the laboratory, and stored in a freezer at -20°C until analyzed. In the laboratory, soil pH was determined by mixing soil and deionized water in a 1:1 volume by weight ratio, shaking samples, and allowing them to settle for an hour before reading pH using a pH meter [39].
Remote Sensing and GIS methods were used to characterize four general site properties: 1) Normalized Difference Vegetation Index (NDVI as proxy for photosynthetic activity), 2) proximity to roads, 3) proximity to water body and 4) patchiness of land cover. Landsat 8 OLI scene (WRS-2 Path 22 Row 36) taken on April 9, 2014 was used for land cover classification and calculation of NDVI. The vegetation survey for 76 tick-sampling sites and high-resolution aerial photograph from ESRI World Imagery were used to create training sites for the six land cover types of water/marsh, agriculture, grass/pasture, bottomland hardwoods, upland hardwoods, and coniferous plantation. In addition, a field survey was conducted during mid-July 2014 across Ames Plantation and a total of 84 GPS validation points were identified on the ground when tick-borne diseases peak in Tennessee [7, 33, 34]. Land cover classification was then carried out using per-pixel classification methods and an error matrix was also created. NDVI was calculated using Landsat red and near-infrared regions of spectral reflectance as NIR-Red/NIR+Red to indicate the overall photosynthetic activity level of the surface [40]. Because proximity of study sites to roads may influence the presence of ticks as a major CO2 source (as an attractant) or as a site for road kill (where ticks would detach) [41], roads found within the boundary of Ames Plantation were identified as secondary roads, local roads, four-wheel-drive roads, service roads, and private roads. Many tick-sampling sites were located close to several roads, so we applied a weighted overlay method in grid analysis to give larger and more frequently used roads a larger weight when calculating the proximity value. The weight values are from AEH’s knowledge at Ames Plantation as secondary roads were weighted at 50%, local roads weighted at 20%, and the remaining three types were weighted at 10% each. The Ames Plantation landscape has several kinds of water features, such as creeks, lakes, and marshes. Because proximity of study sites to different kinds of water bodies may relate to tick abundance [42] the Euclidian distance was calculated from each study site to the closest water body as a proximity to water. Patchiness of land cover (mixed type of land covers vs. single types), or the spatial complexity of the environment, was calculated by summing the edges of the land cover classification polygons within a 300m buffer around each study site.
Statistical Analysis
Habitat types and the presence of each tick species were compared with contingency tests (X 2 tests), and habitat preference was determined with an ANOVA on log (x+1) transformed tick counts from each site. To determine the relationship between soil pH, vegetation characteristics, landscape measures, and each tick’s presence, a multivariate analyses with a significance level of α = 0.05 was used. A PCA was generated to visualize how sites and habitat groups differed with respect to the different predictor variables, but not to examine their relationship (S1 Fig). ANOVA models consisted of continuous (e.g., pH, vegetation height) and habitat type as a categorical variable. For tick species that had multiple life stages represented in five or more sites (A. americanum nymphs and adults), MANOVA was used to examine habitat use. For MANOVAs, the dependent variable was the number of individuals in each stage class (adults or nymphs) at a site. For tick species in which only adults were well represented (A. maculatum and D. variabilis), an ANOVA was used with number of adult ticks at a site as the dependent variable. Dependent variables were log-transformed to meet model assumptions. In both MANOVA and ANOVA, our predictor variables were habitat type, plant diversity (Shannon Index), plant evenness (Eh), basal area, canopy openness, soil pH, NDVI, distance to water, distance to roads, and patchiness. We tested all continuous predictor variables for an interaction with habitat type. While I. scapuarlis was collected, a model was not built for I. scapularis because it occurred in only four sites and did not meet the five or more site minimum. Datasets are provided in S1 Table.
Cluster Detection and Identification
Spatial scan statistics, implemented in SaTScan [43] were used to detect locations of statistically significant spatial clusters of collected tick species and life stages. The spatial scan statistic uses a circular window of variable radius that moves across the study area. The radius of the window was set to vary from 0 up to a maximum value that included 50% of the population under investigation [44]. As the window of the statistic moves across the study area (Ames Plantation), it defines a set of different neighboring sampling sites each of which is a candidate for a potential cluster [45]. Clusters are assessed by comparing the number of specimens (adults, nymphs, or total) within the window with the number that would be expected if the specimens were randomly distributed in the study area. The test of significance of identified clusters is based on a likelihood ratio test whose P-value is obtained through Monte Carlo testing. Clusters were assessed under the discrete Poisson model assumption and 999 Monte Carlo replications were used for significance testing. The null hypothesis of no clusters was rejected when the simulation P ≤ 0.05. Results of cluster detection were then imported into ArcGIS 10.1 [46], and the spatial distribution of identified clusters and sites was displayed in maps.
Results
Tick Collection
A total of 5050 ticks were collected in June 2014 from the 76 sites consisting of 4904 (97.11%) A. americanum, 128 (2.53%) D. variabilis, 11 (0.22%) A. maculatum, and 7 (0.14%) I. scapularis (Table 1). Of the A. americanum, 4166 (84.95%) were nymphs, 387 (7.89%) were adult females, 340 (6.93%) were adult males, and 11 (0.22%) were larvae. The remaining ticks were 71 D. variabilis females, 57 D. variabilis males, 9 A. maculatum males, and 2 A. maculatum females. Additional A. americanum larvae were encountered throughout the study by the investigators, but were not included in the analysis because the investigators could not guarantee where each was acquired due to size for detection and walking into sites.
Table 1. A total of 5050 ticks were collected from 76 sites consisting of bottomland deciduous, upland deciduous, coniferous, and grassland habitats.
A majority of the specimens were Amblyomma americanum, A. maculatum nymphs, D. variabilis nymphs, and I. scapularis adults were not collected.
| Habitat (no. of sites) | No. ticks collected (mean ± SEM) | |||||
|---|---|---|---|---|---|---|
| Amblyomma americanum | Amblyomma maculatum | Dermacentor variabilis | Ixodes scapularis | Total | ||
| Nymph | Adult | Adult | Adult | Nymph | All life stages | |
| Bottomland Deciduous (n = 15) | 233 (15.53 ± 5.005) | 77 (2.57 ± 0.717) | 0 (NA) | 29 (0.97 ± 0.398) | 1 (0.07 ± 0.067) | 340 (22.67 ± 6.035) |
| Upland Deciduous (n = 28) | 2837 (101.32 ± 48.122) | 358 (6.39 ± 1.406) | 0 (NA) | 52 (0.93 ± 0.264) | 5 (0.18 ± 0.146) | 3262 (116.5 ± 49.940) |
| Coniferous (n = 15) | 913 (60.87 ± 20.186) | 238 (7.93 ± 1.188) | 0 (NA) | 32 (1.07 ± 0.339) | 1 (0.07 ± 0.067) | 1185 (79.0 ± 21.400) |
| Grasslands (n = 18) | 183 (10.17 ± 2.795) | 54 (1.50 ± 0.384) | 11 (0.31 ± 0.158) | 15 (0.42 ± 0.163) | 0 (NA) | 263 (14.61 ± 3.343) |
| Total (n = 76) | 4166 (54.82 ± 18.563) | 727 (4.78 ± 0.657) | 11 (0.07 ± 0.040) | 128 (0.84 ± 0.148) | 7 (0.09 ± 0.057) | 5050 (66.45 ± 19.390) |
*An additional 11 larvae were recorded from upland deciduous (10 specimens) and a coniferous site (1 specimen).
From the 76 sites sampled, a mean of 66.5 (± 19.39) specimens were collected (range: 1 to 1216 specimens). Twenty-four (31.6%) sites had only 1 species (23 sites had only A. americanum and 1 site had only D. variabilis), 46 (60.5%) sites had 2 species (at all of these sites A. americanum was the dominant species), and 6 (7.9%) sites had 3 species. Four of the sites with three tick species were grassland sites with A. americanum, A. maculatum, and D. variabilis. The remaining two sites were upland deciduous and harbored A. americanum, D. variabilis, and I. scapularis. Collections from forested sites (bottomland deciduous, upland deciduous, and coniferous) were similar to one another and were primarily composed of A. americanum, D. variabilis, and I. scapularis. In contrast, grassland sites did not have any I. scapularis and were the only sites where A. maculatum were collected. Amblyomma americanum and D. variabilis were also collected at grassland sites.
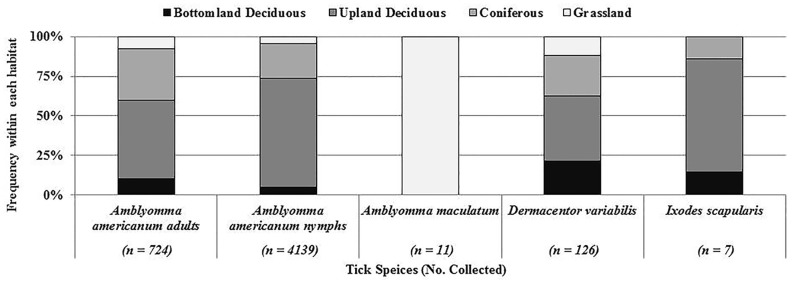
Different habitats were significantly associated with the presence of each tick species (represented by means) (Table 1, Fig 2). At one upland deciduous site, 1186 A. americanum nymphs were collected, which was 518 more specimens than the next population dense site. Population size indicated by mean abundance suggested significantly more ticks were collected from coniferous sites (79.0 ± 82.90) than grassland sites (14.6 ± 3.34), but not upland deciduous sites (116.5 ± 49.94). Bottomland deciduous sites (22.7 ± 6.04) were not significantly different from either upland deciduous or grassland sites (F = 6.71; df = 3, 72; P = 0.0005). The presence of A. americanum did not differ among habitats (X 2 = 4.12; df = 3; P = 0.249), but population size indicated by abundance did suggest significantly more A. americanum were collected from upland deciduous (114.5 ± 49.94) and coniferous (76.8 ± 21.06) sites, than from bottomland deciduous (20.7 ± 5.95), and those collections were significantly greater than grassland sites (13.2 ± 3.38) (F = 7.26; df = 3, 72; P = 0.0003). The presence of A. maculatum was significantly different across habitats (X 2 = 24.8; df = 3, 72; P < 0.001), as all 11 specimens were collected from grassland sites (0.6 ± 0.22). The presence of D. variabilis did not differ between habitats (X 2 = 4.54; df = 3; P = 0.209), with 1.9 ± 0.73 ticks in bottomland deciduous, 1.9 ± 0.47 in upland deciduous, 2.1 ± 0.58 in coniferous, and 0.8 ± 0.28 in grassland sites. The presence of I. scapularis did not differ across habitats (X 2 = 1.32; df = 3; P = 0.725), and neither did population sizes (F = 0.4724; df = 3, 72; P = 0.7025).
Fig 2. Frequency of each tick species and life stage (number collected) among the different habitat types.
Comparisons of A. americanum life stages mirrored total A. americanum collections. A total of 727 adults were collected of which significantly more were collected at coniferous sites (15.9 ± 2.12) and upland deciduous sites (12.8 ± 2.79) than bottomland deciduous sites (5.1 ± 1.30) and grassland sites (3.0 ± 0.72) (F = 12.34; df = 3, 72; P < 0.0001). This pattern was also true for the 4166 nymphs collected from the 76 sites as coniferous sites (60.9 ± 20.19) had significantly more ticks than grassland sites (10.2 ± 2.80), but not more than upland deciduous sites (101.3 ± 48.12) or bottomland deciduous sites (15.5 ± 5.01) (F = 3.2112; df = 3, 72; P = 0.028).
Vegetation Characterization
There were no differences among site types for any predictor variables including Shannon-Weiner Diversity index (F = 1.146; df = 3, 72; P = 0.366), evenness (F = 0.928; df = 3, 72; P = 0.432), leaf area index (F = 1.595; df = 3, 72; P = 0.198), openness (F = 2.092; df = 3, 72; P = 0.109), and basal area (F = 1.427; df = 3, 72; P = 0.242). PCA also showed that these predictor variables do not group based on habitat type (S1 Fig); this is likely an artifact of habitat site designations. A Detrended Correspondence Analysis (DCA) was conducted to determine whether habitat types were distinguishable based on vegetation measures (S2 Fig). DCA indicated two vegetation habitat types (grassland and forest) instead of four (grassland, bottomland deciduous, upland deciduous, and coniferous) (S2 Fig). To keep consistent with tick sampling protocol and other predictor variables, we conducted our analyses using four habitat types (upland deciduous, bottomland deciduous, coniferous, and grassland). For all predictor variables there were no significant pairwise Tukey comparisons.
Landscape Characterization
Landcover classification (Fig 1) conducted with maximum-likelihood methods indicated that Ames Plantation consists of agriculture (7%), bottomland deciduous (14%), grasslands/pastures (19%), coniferous (12%), upland deciduous (32%), and water/marsh (12%). The overall classification accuracy against 160 ground truth data (76 tick-sampling sites and 84 ground-truth points) was 70% and Kappa value—accuracy that taking a random chance into account—was 62% (Table 2). The largest classification confusion occurred between bottomland deciduous and upland deciduous cover type as these two have similar canopy reflectance values from satellite imagery (error of commission or percentage of misclassified pixel against ground truth data, was 0.48 for bottom land deciduous and 0.31 for upland deciduous). Mean NDVI values for all 76 tick-sampling sites was 0.26, while overall NDVI for Ames plantation was 0.18 which reflected negative NDVI values for water bodies. The closest site to water was within 10m while the furthest site from a water body was located 1.3 km away. Mean distance to water was 313m (SD = 247m). Distance to roads calculated by weighted average by different road types is a unit less measure and produced a mean of 2608 (SD = 1604). Mean patchiness was 9173m (SD = 2129m).
Table 2. Error matrix for landcover classification in AMES plantation showed overall accuracy of 0.7 (Kappa value of 0.62) and the largest confusion found between bottomland deciduous and upland deciduous.
| Ground-truth land covers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Agriculture | Bottomland Deciduous | Grass/ Pasture | Coniferous | Upland Deciduous | Water/ marsh | Total | *Error of Commission | |
| Agriculture | 21 | 3 | 4 | 0 | 1 | 1 | 30 | 0.30 |
| Bottomland Deciduous | 3 | 17 | 2 | 4 | 7 | 0 | 33 | 0.48 |
| Grass/pasture | 1 | 1 | 14 | 0 | 0 | 1 | 17 | 0.17 |
| Coniferous | 1 | 2 | 0 | 20 | 4 | 1 | 28 | 0.28 |
| Upland Deciduous | 1 | 5 | 1 | 3 | 22 | 0 | 32 | 0.31 |
| Water/marsh | 0 | 0 | 1 | 0 | 0 | 19 | 20 | 0.05 |
| Total | 27 | 28 | 22 | 27 | 34 | 22 | 160 | |
| **Error of Omission | 0.22 | 0.39 | 0.36 | 0.25 | 0.35 | 0.13 | 0.70 | |
*Error of commission: percentage of misclassified pixel against ground truth data
**Error of omission: percentage of omitted pixel against ground truth data
Models of Habitat Use
MANOVA for A. americanum indicates that no variables were significant predictors of adult and nymph tick abundance (Table 3). Adult A. maculatum tick abundance was predicted by NDVI (F = 5.07; df = 1, 35; P = 0.03) and by the interaction between habitat type and plant diversity (F = 3.75; df = 3, 35; P = 0.02); combined, these significant predictors account for 22% of the total Sum of Squares (Table 4). For D. variabilis, no variables were significant predictors of adult tick abundance (Table 5).
Table 3. MANOVA table for abundances of Amblyomma americanum adults and nymphs indicates that no variables were significant predictors of adult and nymph tick abundance.
| Potential Predictor | Df | Pillai | Approximate F value | Num. Df | Den. Df | P value |
|---|---|---|---|---|---|---|
| habitat type | 3 | 0.133553 | 0.8348 | 6 | 70 | 0.54717 |
| plant diversity | 1 | 0.122842 | 2.38078 | 2 | 34 | 0.10773 |
| plant evenness | 1 | 0.044821 | 0.79772 | 2 | 34 | 0.4586 |
| basal area | 1 | 0.074734 | 1.37309 | 2 | 34 | 0.26701 |
| distance to roads | 1 | 0.040149 | 0.71108 | 2 | 34 | 0.49827 |
| Patchiness | 1 | 0.031333 | 0.54989 | 2 | 34 | 0.58206 |
| Openness | 1 | 0.010384 | 0.17838 | 2 | 34 | 0.8374 |
| NDVI | 1 | 0.005285 | 0.09032 | 2 | 34 | 0.91386 |
| soil pH | 1 | 0.130813 | 2.5585 | 2 | 34 | 0.09224 |
| Water | 1 | 0.065209 | 1.18588 | 2 | 34 | 0.3178 |
| habitat type: plant diversity | 3 | 0.234116 | 1.54673 | 6 | 70 | 0.17591 |
| habitat type: plant evenness | 3 | 0.05912 | 0.35537 | 6 | 70 | 0.90444 |
| habitat type: basal area | 3 | 0.157268 | 0.99569 | 6 | 70 | 0.43517 |
| habitat type: distance to roads | 3 | 0.109011 | 0.67255 | 6 | 70 | 0.6721 |
| habitat type: patchiness | 3 | 0.025544 | 0.15094 | 6 | 70 | 0.98829 |
| habitat type: openness | 3 | 0.214825 | 1.40395 | 6 | 70 | 0.22541 |
| habitat type: NDVI | 3 | 0.123006 | 0.76456 | 6 | 70 | 0.60023 |
| habitat type: soil pH | 3 | 0.088152 | 0.53793 | 6 | 70 | 0.77761 |
| habitat type: water | 3 | 0.118362 | 0.73388 | 6 | 70 | 0.62398 |
| Residuals | 35 |
Table 4. ANOVA table for abundances of Amblyomma maculatum adults indicate A. maculatum presence can be predicated by NDVI and by interaction between habitat type and plant diversity.
| Potential Predictor | Df | Sum Sq | Mean Sq | F value | P value |
|---|---|---|---|---|---|
| habitat type | 3 | 0.164 | 0.0547 | 0.721 | 0.5463 |
| plant diversity | 1 | 0.0263 | 0.0263 | 0.346 | 0.5601 |
| plant evenness | 1 | 0.1009 | 0.1009 | 1.331 | 0.2565 |
| basal area | 1 | 0.1694 | 0.1694 | 2.234 | 0.1439 |
| distance to roads | 1 | 0.0101 | 0.0101 | 0.133 | 0.718 |
| patchiness | 1 | 0.006 | 0.006 | 0.079 | 0.7804 |
| openness | 1 | 0.0005 | 0.0005 | 0.007 | 0.9336 |
| NDVI | 1 | 0.3844 | 0.3844 | 5.068 | 0.0308* |
| soil pH | 1 | 0.0041 | 0.0041 | 0.054 | 0.8172 |
| water | 1 | 0.0056 | 0.0056 | 0.074 | 0.7868 |
| habitat type: plant diversity | 3 | 0.853 | 0.2843 | 3.749 | 0.0195* |
| habitat type: plant evenness | 3 | 0.1389 | 0.0463 | 0.611 | 0.6126 |
| habitat type: basal area | 3 | 0.0265 | 0.0088 | 0.117 | 0.9498 |
| habitat type: distance to roads | 3 | 0.3715 | 0.1238 | 1.633 | 0.1994 |
| habitat type: patchiness | 3 | 0.0427 | 0.0142 | 0.188 | 0.9041 |
| habitat type: openness | 3 | 0.4738 | 0.1579 | 2.082 | 0.1203 |
| habitat type: NDVI | 3 | 0.0119 | 0.004 | 0.053 | 0.9839 |
| habitat type: soil pH | 3 | 0.1233 | 0.0411 | 0.542 | 0.6568 |
| habitat type: water | 3 | 0.1516 | 0.0505 | 0.666 | 0.5784 |
| Residuals | 35 | 2.6543 | 0.0758 |
Bolded values are significant (* P < 0.05).
Table 5. ANOVA table for abundances of Dermacentor variabilis adults indicate D. variabilis populations can be predicted by the interaction of habitat type and basal area.
| Potential Predictor | Df | Sum Sq | Mean Sq | F value | P value |
|---|---|---|---|---|---|
| habitat type | 3 | 0.577 | 0.1922 | 0.356 | 0.785 |
| plant diversity | 1 | 0.001 | 0.0008 | 0.001 | 0.97 |
| plant evenness | 1 | 0.021 | 0.0211 | 0.039 | 0.844 |
| basal area | 1 | 0.147 | 0.1471 | 0.273 | 0.605 |
| distance to roads | 1 | 0.325 | 0.325 | 0.602 | 0.443 |
| patchiness | 1 | 0.126 | 0.1256 | 0.233 | 0.632 |
| openness | 1 | 1.385 | 1.3848 | 2.567 | 0.118 |
| NDVI | 1 | 0.163 | 0.1632 | 0.303 | 0.586 |
| soil pH | 1 | 0.248 | 0.2478 | 0.459 | 0.502 |
| water | 1 | 0.012 | 0.0125 | 0.023 | 0.88 |
| habitat type: plant diversity | 3 | 0.103 | 0.0343 | 0.064 | 0.979 |
| habitat type: plant evenness | 3 | 0.113 | 0.0376 | 0.07 | 0.976 |
| habitat type: basal area | 3 | 2.877 | 0.959 | 1.778 | 0.169 |
| habitat type: distance to roads | 3 | 2.313 | 0.7711 | 1.429 | 0.251 |
| habitat type: patchiness | 3 | 1.643 | 0.5478 | 1.015 | 0.398 |
| habitat type: openness | 3 | 1.817 | 0.6057 | 1.123 | 0.353 |
| habitat type: NDVI | 3 | 2.913 | 0.9709 | 1.8 | 0.165 |
| habitat type: soil pH | 3 | 0.785 | 0.2618 | 0.485 | 0.695 |
| habitat type: water | 3 | 0.577 | 0.1924 | 0.357 | 0.785 |
| Residuals | 35 | 18.882 | 0.5395 |
Cluster Detection and Identification
Spatial clusters were not identified for A. americanum total counts, adult or nymphal counts indicating the species and life stages were found throughout Ames Plantation (P > 0.05). Analysis of A. maculatum adults identified two statistically significant clusters, one cluster with 1 site (RR = 64.6, P = 0.05) and a second cluster with sixteen sites (RR = 7.3; P = 0.028) (Fig 1b). Analysis of D. variabilis adults identified three statistically significant clusters, one cluster with five sites (RR = 3.1, P = 0.0005), a second cluster with one site (RR = 6.8, P = 0.0016), and a third cluster with four sites (RR = 3.1; P = 0.029) (Fig 1). While only seven I. scapularis were collected from four sites, these seven were found in a single statistically significant cluster (RR = 80.0, P = 0.001) (Fig 1d).
Discussion
A thorough understanding of tick populations and their pathogens is essential to the accurate and timely diagnosis of TBDs, the development of risk assessments, and advancement of management plans to control ticks and reduce TBDs. This study identified few vegetation and landscape variables associated with tick presence or density, and this is likely due to ticks using hosts for dispersal and limiting our environmental variables to vegetation and landscape features to one tick season and study site. This study focused on vegetative and landscape features because these data are easier to obtain over broad geographic regions and because these traits likely influence ticks directly through abiotic factors and indirectly through their effects on host species. While some variables, such as NDVI and the interaction between habitat type and plant diversity, were significantly associated with one tick species, the amount of variation explained was low and not useful for predicting presence or density reliably. The NDVI coefficient was extremely small (9x10-14) and likely an artifact of the large number of 0s (tick absence); while statistically significant, the association is weak and not biologically useful for prediction. This low amount of variation explained by our models is likely due to the use of hosts for dispersal [47, 48] and potentially other environmental variables such relative humidity [49, 50] and soil conditions [28]. Host species play a large role in the establishment, maintenance, and dispersal of a tick species, as well as the maintenance of disease cycles and associated pathogens [29, 32, 51]. Identification of preferred host species for each tick species in western Tennessee will help determine the factors that aide in the establishment and maintenance of tick populations and identify potential mechanisms (i.e., host agents) of tick (and pathogen) dispersal.
Although we were unable to identify specific environmental variables associated with each tick species and/or life stage, we found significant spatial clusters for A. maculatum, D. variabilis, and I. scapularis. Again, we speculate that this clustering might be due to 1) uncharacterized environmental variables, 2) the need for additional seasonal sampling and replication, and 3) the use of hosts for dispersal. Other constraints that might favor or limit tick populations include the assemblage of host species and habitat parameters. This might include not just using hosts for dispersal and a food source, but also for “directed dispersal” to a suitable habitat that provides protection from the elements and permits successful molting to the next instar. The significant I. scapularis cluster was found in the western region of Ames, an area with established coniferous and deciduous stands, and habitat to a plentiful turkey and white-tailed deer population, both common I. scapularis hosts [6, 52, 53]. Ixodes scapularis, here found only in wooded areas of the plantation, likely spend a majority of its life in the shade of trees (both on the host and off the host in the environment). This tick is notably susceptible to desiccation and must use humidity to reabsorb fluids from the atmosphere [30]. The three D. variabilis clusters were located in the middle of the plantation, and this species is commonly collected from raccoons [2, 6, 54]. The location of the D. variabilis clusters is largely conventional agriculture with crops, field, and woods, but all with generally hard, clearly defined edges. For a cosmopolitan tick such as D. variabilis, which is considered desiccation-tolerant [30], conventional agricultural land represents an island habitat with habitat patches (heterogeneous in nature), but well defined with definite vegetative typing possible. For Amblyomma ticks with thickened waxy coatings on their epicuticle, host species were likely more responsible for distributions and clustering as A. americanum was found throughout the plantation (and use turkeys and white-tailed deer), and A. maculatum was collected only in small clusters that were defined as a heterogeneous blend of habitats. Amblyomma maculatum prefer to feed on a number of hosts including cattle and quail [29, 55], and the identified clusters in the southern region of Ames is home to the National Bird dog trials, where quail are released and habitat is managed for those quail. This area is a highly heterogeneous, patchy mix of woods, fields, and native grasslands. Together, these data and findings lead to additional questions about macro- and micro-scale parameters associated with tick presence and abundance. Ultimately, these spatial clusters provide useful information to guide future studies investigating factors responsible for the identified higher densities and could likely serve as sites for host trapping studies. Such studies could also include reasons for pathogen presence/absence at different sites, similar to work conducted by Ostfeld et al. [56] which identified small hosts with large populations and r-life histories as excellent reservoirs for Borrelia burgdorferi, Babesia microti, and A. phagocytophilum.
Due to the lack of significant environmental relationships, we have begun to examine host populations directly. Preliminary data on birds and small mammals were collected to identify tick-host-habitat relationships at the study site. Investigator Kennedy (unpublished) collected primarily the white-footed mouse (Peromyscus leucopus) from a variety of habitats with I. scapularis, D. variabilis, and A. maculatum as ectoparasites, and the hispid cotton rat (Sigmodon hispidus) only from grassland sites with D. variabilis and A. maculatum as ectoparasites. Investigator Collins (unpublished) mist-netted birds, and ticks were found on two individuals (0.65%). An adult female Indigo Bunting (Passerina cyanea) caught in a coniferous site had a rabbit tick (Haemaphysalis leporispalustris), and an adult female Carolina Wren (Thryothorus ludovicianus) netted in a bottomland hardwood site had two Ixodes brunneus. Both bird species nest near the ground (Indigo Buntings <1m and Carolina Wrens <2m) and both specimens were adult females with brood patches. Bird-habitat and small mammal-habitat associations were also identified; some host species were collected in all site types (white-footed mouse), while others were specific to open/grassland sites (hispid cotton rat, Indigo bunting).
It can be assumed from the literature [4–6, 35, 53–65] and from our findings that tick hosts have different tick communities, hosts have preferred habitats, and hosts often have a different ectoparasite community from questing collections (as A. americanum dominated questing collections and were absent from the host collections). Here, our observations might suggest that potential hosts nesting on or near the ground (e.g., mice, wrens) are more likely to harbor and transport ticks and TBDs than animals nesting or living in canopy settings (e.g., squirrels, nuthatches). Additionally, one could speculate that those animals (specifically birds) that migrate and nest near the ground could transport ticks further and introduce them to new areas. Nevertheless, future research into the use of niche-defined hosts and as indicators should be further investigated. Moreover, the patterns predicted by tick susceptibility to desiccation are likely correlated with host-habitat associations as well.
It is plausible that habitat use by potential hosts also varies across our field site. Generalist hosts, such as deer and turkeys, will use all four habitats, have a defined home range, and can be found throughout the plantation. It will be difficult to develop habitat models for ticks (such as A. americanum) that use these generalist hosts as primary hosts, so these tick species are either strongly influenced by a specific abiotic/biotic variable, or have the ability to use multiple habitats. Mesomammals such as raccoons, skunks, and opossums can also be found throughout the plantation, and these species have a generalized affinity for habitats, and there are likely some occasional seasonal tendencies, but few will be predictable. Ticks using these animals as primary hosts will probably be found in mixed environments, and true predictability with repeatability will be rare. Hosts with small home ranges, such as birds and small mammals, will likely have the greatest impact on tick populations. These animals commonly prefer specific vegetation types, and other abiotic/biotic parameters. As a part of the preliminary host studies, white-footed deer mice were collected in field, hardwood, and pine habitats; however, hispid cotton rats were only collected in field grass habitats. Hispid cotton rats are noted as primary hosts for immature A. maculatum [29, 55]. This leads us to hypothesize that host-habitat specificity will also likely influence tick presence and absence.
Although large numbers of ticks were collected, a majority of the questing ticks were A. americanum. The critical next step is to determine 1) how the environment influences a tick’s ability to locate a host, 2) how the environment influences the presence and abundance of potential tick hosts, and 3) how environmental variables and host community jointly influence population sizes and dispersal of each tick species (and subsequent pathogens). As with I. scapularis and Lyme disease, it is likely that the environment provides shelter and food sources for southeastern ticks with the ability to transmit Ehrlichia and/or Rickettsia pathogens. Moreover, the environment provides questing sites for tick attachment, and questing sites are essentially where pathogen transmission begins. Additional drivers into tick range expansion likely include climate warming and/or habitat change as both will affect the plant composition and subsequent host composition for ticks and their pathogens. Further studies into this system that include hosts, vectors, and pathogens [66] that describe the nidus of pathogen transmission [67] such as those presented by Simon et al. [68] are necessary for these southern TBDs.
These data serve as groundwork for commonly encountered ticks and for tick-habitat associations in the southeastern United States and demonstrate a need for 1) continued work on tick-habitat associations that include multiple seasons and sampling efforts, 2) inclusion of hosts in future studies, and 3) concurrent pathogen detection studies to identify areas with pathogen-infected ticks. These findings will assist future endeavors at field sites and serve as foundational data for tick distribution models for the region. Consequently, these findings serve as the basis for determining species distribution, identifying local tick habitats, and analyzing tick biological patterns. With additional tick and pathogen surveillance, these and additional data could contribute to preparing relative risk maps for both Ehrlichia and Rickettsia within the southeast. Identification of these sites assists administrators in developing practical and cost-effective strategies for tick control, so managers can monitor and treat areas with tick and infected-tick populations. The next step is to sample host communities (when ticks are actively questing and feeding), additional seasons (when other species and life stages are questing), and replicate the project over multiple years and sites to validate and expand on the model.
Supporting Information
PCA results depict how sites and habitat groups differed with respect to the different predictor variables, but not to examine their relationship.
(TIF)
DCA results indicated habitat types were distinguishable based on vegetation measures; two vegetation habitat types (grassland and forest) instead of four (grassland, bottomland deciduous, upland deciduous, and coniferous).
(TIF)
Data files for MANOVA and ANOVA analyses.
(ZIP)
Acknowledgments
We thank the board of trustees and the employees of Ames Plantation, particularly Larry Teague and James Morrow for their assistance with collections throughout the project. We would also like to thank University of Tennessee (UT) Medical and Veterinary Entomology Lab members Dave Paulsen, Chelsea Casteel, Sarah Mays, Megan Noseda, and Cassie Urquhart for assistance in collecting, identifying, and processing tick specimens. We would also like to thank a plethora of graduate and undergraduate students who helped with field collections and identifications from Christian Brothers University for plant identifications (Colton Terhune and Desire’ Smith), from the UTIA Urban Forestry Lab for assistance with collection of soil samples (Thomas Turnbull, Benjamin Reichert, and Thomas Jennings), and field assistants from Rhodes College.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This project was funded by the University of Tennessee and the Department of Entomology and Plant Pathology, Ames Plantation Research and Education Center, Mellon Foundation, Rhodes College, as well as AKC Canine Health Foundation (01864-A) and USDA Tennessee Hatch Project (TEN00433).
References
- 1. Jordan BE, Onks KR, Hamilton SW, Hayslette SE, Wright SM. Detection of Borrelia burgdorferi and Borrelia lonestari in birds in Tennessee. J Med Entomol. 2009; 46: 131–138. [DOI] [PubMed] [Google Scholar]
- 2. Kollars TM Jr, Kengluecha A. Spotted fever group Rickettsia in Dermacentor variabilis (Acari: Ixodidae) infesting raccoons (Carnivora: Procyonidae) and opossums (Marsupialia: Didelphimorphidae) in Tennessee. J Med Entomol. 2001; 38: 601–602. [DOI] [PubMed] [Google Scholar]
- 3. Moncayo AC, Cohen SB, Fritzen CM, Huang E, Yabsley MJ, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010; 83: 653–657. 10.4269/ajtmh.2010.09-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trout RT, Steelman CD. Ticks parasitizing canines and deer in Arkansas. J Entomol Sci. 2010; 45: 140–149. [Google Scholar]
- 5. Zimmerman RH, McWherter GR, Bloemer SR. Medium-sized mammal hosts of Amblyomma americanum and Dermacentor variabilis (Acari:Ixodidae) at Land between the Lakes, Tennessee, and effects of integrated tick management on host infestations. J Med Entomol. 1988; 25: 461–466. [DOI] [PubMed] [Google Scholar]
- 6. Cohen SB, Freye JD, Dunlap BG, Dunn JR, Jones TF, Moncayo AC. Host associations of Dermacentor, Amblyomma, and Ixodes (Acari: Ixodidae) ticks in Tennessee. J Med Entomol. 2010; 47: 415–420. [DOI] [PubMed] [Google Scholar]
- 7.Hendricks BM. Identification and characterization of peak activity, environmental variables, and bacterial pathogens in A. americanum L. at Ames Plantation, west Tennessee [thesis]. Knoxville (TN): University of Tennessee; 2013.
- 8. Pagac BB, Miller MK, Mazzei MC, Nielsen DH, Jiang J, Richards AL. Rickettsia parkeri and Rickettsia montanensis, Kentucky and Tennessee, USA. Emerg Infect Dis. 2014; 20: 1750–1752. 10.3201/eid2010.140175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, McDade JE. Human infection with Ehrlichia canis, a luekocytic Rickettsia. NE J Med. 1987; 316: 853–856. [DOI] [PubMed] [Google Scholar]
- 10. Dumler JS, Brouqui P, Aronson J, Taylor JP, Walker DH. Identification of Ehrlichia in human tissue. NE J Med. 1991; 325: 1109–1110. [DOI] [PubMed] [Google Scholar]
- 11. Goddard J, Varela-Stokes AS. Role of the lone star tick. Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009; 160: 1–12. 10.1016/j.vetpar.2008.10.089 [DOI] [PubMed] [Google Scholar]
- 12. Calhoun EL. Natural occurrence of tularemia in the Lone star tick, Amblyomma americanum (L), and in dogs in Arkansas. Am J Trop Med Hyg. 1954; 3: 360–366. [DOI] [PubMed] [Google Scholar]
- 13. Hopla CE. The transmission of tularemia organisms by ticks in the southern states. Southern Med J. 1960; 53: 92–97. [DOI] [PubMed] [Google Scholar]
- 14. Savage HM, Godsey MS Jr, Lambert A, Panella NA, Burkhalter KL, Harmon JR, et al. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013; 89: 445–452. 10.4269/ajtmh.13-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, et al. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007; 13: 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burridge MJ, Simmons LA, Peter TF, Mahan SM. Increasing risks of introduction of heartwater onto the American mainland associated with animal movements. Ann NY Acad Sci. 2002; 969: 269–274. [DOI] [PubMed] [Google Scholar]
- 17. Price WH. The epidemiology of Rocky Mountain Spotted fever. I. The characterization of strain virulence of Rickettsia rickettsii . Am J Hyg. 1956; 58: 248–268. [DOI] [PubMed] [Google Scholar]
- 18. Weiss E. History of rickettsiology Vol 1 In Biology of rickettsial diseases. Edited by Walker DH. CRC Press, Inc; Boca Raton FL: 1988. p. 15–32. [Google Scholar]
- 19. Stromdahl EY, Hickling GJ. Beyond Lyme: etiology of tick-borne human diseases with emphasis on the southeastern United States. Zoo Public Health. 2012; 59: 48–64. [DOI] [PubMed] [Google Scholar]
- 20. Ginsberg HS, Stafford KC III. Management of ticks and tick-borne diseases In: Goodman J. L., et al. editors. Tick-borne diseases of humans. Washington DC: ASM Press; 2005. p. 65–86. [Google Scholar]
- 21. Hoch AL, Barker RW, Hair JA. Further observations on the control of lone star ticks (Acarina: Ixodidae) through integrated control procedures. J Med Entomol. 1971; 8: 731–734. [DOI] [PubMed] [Google Scholar]
- 22. Wilson ML. Reduced abundance of adult Ixodes dammini (Acari: Ixodidae) following destruction of vegetation. J Econ Entomol. 1986; 79: 693–696. [DOI] [PubMed] [Google Scholar]
- 23. Hoch AL, Semtner PJ, Barker RW, Hair JA. Preliminary observations on controlled burning for lone star tick (Acarina: Ixodidae) control in woodlots. J Med Entomol. 1972; 9: 446–451. [DOI] [PubMed] [Google Scholar]
- 24. Cully JF Jr. Lone star tick abundance, fire, and bison grazing in tallgrass prairie. J Range Managmt. 1999; 52: 139–144. [Google Scholar]
- 25. Gleim ER, Conner LM, Yabsley MJ. The effect of Solenopsis invicta (Hymenoptera: Formicidae) and burned habitat on the survival of Amblyomma americanum (Acari: Ixodidae) and Amblyomma maculatum (Acari: Ixodidae). J Med Entomol. 2013; 50: 270–276. [DOI] [PubMed] [Google Scholar]
- 26. Clymer BC, Howell DE, Hair JA. Environmental alteration in recreational areas by mechanical and chemical treatment as a means of lone star tick control. J Econ Entomol. 1970; 63: 504–509. [DOI] [PubMed] [Google Scholar]
- 27. Patrick CD, Hair JA. White-tailed deer utilization of three different habitats and its influence on lone star tick populations. J Parasitol. 1978; 64: 1100–1106. [Google Scholar]
- 28. Guerra M, Walker E, Jones C, Paskewitz S, Cortinas MR, Stancil A, et al. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north central United States. Emerg Infect Dis. 2002; 8:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Teel PD, Ketchum HR, Mock DE, Wright RE, Stray OF. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J Med Entomol. 2010; 45:707–722. [DOI] [PubMed] [Google Scholar]
- 30. Sonenshine DE. The biology of tick vectors of human disease In Tick-borne Diseases of humans. Edited by Goodman JL, Dennis DT, Sonenshine DE. ASM Press, Washington, D.C. 2005. p. 12–36. [Google Scholar]
- 31. Adjemian JZ, Krebs J, Mandel E, McQuiston J. Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001–2005. Am J Trop Med Hyg. 2009; 80: 72–77. [PubMed] [Google Scholar]
- 32. Paddock CD, Yabsley MJ. Ecological havoc, the rise of the white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. CTMI. 2007; 315: 289–324. [DOI] [PubMed] [Google Scholar]
- 33.(CDCa) Centers for Disease Control and Prevention [Ehrlichiosis], National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-borne Diseases. [updated 2013 November 15]. Available: www.cdc.gov/ehrlichiosis/.
- 34.(CDCb) Centers for Disease Control and Prevention [Rocky Mountain spotted fever] Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-borne Diseases. [updated 2013 November 21]. Available: www.cdc.gov/rmsf/.
- 35. Cooley RA, Kohls GM. The genus Amblyomma (Ixodidae) in the United States. J Parasitol. 1944; 30: 77–111. [Google Scholar]
- 36. Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi river. J Med Entomol. 1989; 26: 435–448. [DOI] [PubMed] [Google Scholar]
- 37. Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J Med Entomol. 1998; 35: 489–495. [DOI] [PubMed] [Google Scholar]
- 38. Frazer GW, Canham CD, Lertzman KP. Gap light analyzer (GLA), Version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, user’s manual and program documentation. Simon Fraser University, Burnaby, BC: and the Institute of Ecosystem Studies, Millbrook, New York: 1999. [Google Scholar]
- 39. Jean-Philippe SR, Franklin JA, Buckley DS, Hughes K. The effect of mercury on trees and their mycorrhizal fungi. Environ Poll. 2011; 159: 2733–2739. [DOI] [PubMed] [Google Scholar]
- 40. Wylie BK, Johnson DA. Laca E, Saliendra NZ, Gilmanov TG, Reed BC, et al. Calibration of remotely-sensed coarse resolution NDVI to CO2 fluxes. Remote Sensing Environ. 2003; 85: 243–255. [Google Scholar]
- 41. McEnroe WD. The effect of automobile traffic on American dog tick distribution (Dermacentor variabilis, Say: Acarina, Ixodidae). Environ Pollution. 1971; 2: 135–143. [Google Scholar]
- 42. Bunnell JE, Price SD, Das A, Shields TM, Glass GE. Geographic information systems and spatial analysis of adult Ixodes scapularis (Acari: Ixodidae) in the middle Atlantic region of the U.S.A. J Med Entomol. 2003; 40: 570–576. [DOI] [PubMed] [Google Scholar]
- 43.Kulldorff M, Mostashari F. The SaTScan™ software version 9.3. 2014.
- 44. Kulldorf M. A spatial scan statistic. Communications in Statistics: Theory and Methods. 2007; 26: 1481–1496. [Google Scholar]
- 45. Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995; 14: 799–810. [DOI] [PubMed] [Google Scholar]
- 46. ESRI. ArcGIS Version 10.1 Envioronmental Systems Research Institute, Inc. Redlands, California, USA: 2012. [Google Scholar]
- 47. Wilson ML, Adler GH, Spielman A. Correlation between abundance of deer and that of the deer tick, Ixodes dammini (Acari: Ixodidae). Ann Entomol Soc Am. 1985; 78: 172–176. [Google Scholar]
- 48. Wilson ML, Telford SRIII, Piesman J, Spielman A. Reduced abundance of immature Ixodes dammini (Acari: Ixodidae) following elimination of deer. J Med Entomol. 1988; 25: 224–228. [DOI] [PubMed] [Google Scholar]
- 49. Vail SG, Smith G. Vertical movement and posture of blacklegged tick (Acari: Ixodidae) nymphs as a function of temperature and relative humidity in laboratory experiments. J Med Entomol. 2002; 39: 842–846. [DOI] [PubMed] [Google Scholar]
- 50. Ginsberg HS, Rulison EL, Azevedo A, Pang GC, Muczag IM, Tsao JI, et al. Comparison of survival patterns of northern and southern genotypes of the North American tick Ixodes scapularis (Acari: Ixodidae) under northern and southern conditions. Parasites Vectors. 2014; 7: 394–404. 10.1186/1756-3305-7-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barré N, Uilenberg G. Spread of parasites transported with their hosts: case study of two species of cattle tick. Revue Scient Et Technique. 2010; 29:149–160. [PubMed] [Google Scholar]
- 52. Rand PW, Lubelczyk C, Lavigne GR, Elias S, Holman MS, Lacombe EH, et al. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2003; 40: 179–184. [DOI] [PubMed] [Google Scholar]
- 53. Cortinas MR, Kitron U. County-level surveillance of white-tailed deer infestation by Ixodes scapularis and Dermacentor albopictus (Acari: Ixodidae) along the Illinois River. J Med Entomol. 2006; 43: 810–819. [DOI] [PubMed] [Google Scholar]
- 54. Kollars TM Jr. Ticks (Acari: Ixodidae) infesting medium-sized wild mammals in southwestern Tennessee. J Med Entomol. 1993; 20: 896–900. [DOI] [PubMed] [Google Scholar]
- 55. Semtner PJ, Hair JA. Distribution, seasonal abundance, and hosts of the Gulf Coast tick in Oklahoma. Ann Entomol Soc Am. 1973; 66: 1264–1268. [Google Scholar]
- 56. Ostfeld RS, Levi T, Jolles AE, Martin LB, Hosseini PR, Keesing F. Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PLoS ONE. 2014; 9: e107387 10.1371/journal.pone.0107387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Apperson CS, Levine JF, Nicholson WL. Geographic occurrence of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) infesting white-tailed deer in North Carolina. J Wildlife Dis. 1990; 26: 550–553. [DOI] [PubMed] [Google Scholar]
- 58. Richardson ML, Demarais S. Parasites and condition of coexisting populations of white-tailed and exotic deer in south-central Texas. J Wildlife Dis. 1992; 28: 485–489. [DOI] [PubMed] [Google Scholar]
- 59. Hasle G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front Cell Infect Microbiol. 2013. 3:48 10.3389/fcimb.2013.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Florin DA, Brinkerhoff RJ, Gaff H, Jiang J, Robbins RG, Eickmeyer W, et al. Additional U.S. collections of the Gulf Coast tick, Amblyomma maculatum, (Acari: Ixodidae), from the state of Delaware, the first reported field collections of adult specimens from the state of Maryland, and data regarding this tick from surveillance of migratory songbirds in Maryland. Sys App Acarol. 2014; 19: 257–262. [Google Scholar]
- 61. Bishopp FC, Hixon H. Biology and economic importance of the Gulf Coast tick. J Econ Entomol. 1936; 29: 1067–1076. [Google Scholar]
- 62. Bishopp FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945; 31: 1–54. [Google Scholar]
- 63. Mather TN, Wilson ML, Moore SI, Ribeiro JMC, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am J Clin Epidemiol. 1989; 130: 143–150. [DOI] [PubMed] [Google Scholar]
- 64. Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB, Morshed MG. Birds dispersed ixodid (Acari: Ixodidae) and Borrelia burgdorferi–infected ticks in Canada. J Med Entomol. 2001; 38: 493–500. [DOI] [PubMed] [Google Scholar]
- 65. Piesman J, Gern L. Lyme Borreliosis in Europe and North America. Parasitol. 2004; 129: S191–S220. [DOI] [PubMed] [Google Scholar]
- 66. Ogden NH, Tsao JI. Biodiversity and Lyme disease; dilution or amplification? Epidemics. 2009; 1: 196–206. 10.1016/j.epidem.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 67. Reisen WK. Landscape epidemiology of vector-borne diseases. Ann Rev Entomol. 2010; 55: 461–483. [DOI] [PubMed] [Google Scholar]
- 68. Simon JA, Marrotte RR, Desrosiers N, Fiset J, Gaitan J, Gonzalez A, et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at northeastern limit of its distribution. Evol App. 2014; 7: 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCA results depict how sites and habitat groups differed with respect to the different predictor variables, but not to examine their relationship.
(TIF)
DCA results indicated habitat types were distinguishable based on vegetation measures; two vegetation habitat types (grassland and forest) instead of four (grassland, bottomland deciduous, upland deciduous, and coniferous).
(TIF)
Data files for MANOVA and ANOVA analyses.
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.