Abstract
The repertoire of antigens associated with the development of an autoimmune response in breast cancer has relevance to detection and treatment strategies. We have investigated the occurrence of autoantibodies associated with the development of triple-negative breast cancer (TNBC) in the before diagnosis setting and in samples collected at the time of diagnosis of TNBC. Lysate arrays containing protein fractions from the TNBC MDA-MB-231 cell line were hybridized with TNBC plasmas from the Women's Health Initiative cohort, collected before clinical diagnosis and with plasmas from matched controls. An immune response directed against spliceosome and glycolysis proteins was observed with case plasmas as previously reported in estrogen receptor+ breast cancer. Importantly, autoantibodies directed against networks involving BRCA1, TP53, and cytokeratin proteins associated with a mesenchymal/basal phenotype were distinct to TNBC before diagnosis samples. Concordant autoantibody findings were observed with mouse plasma samples collected before occurrence of palpable tumors from a C3(1)-T triple negative mouse model. Plasma samples collected at the time of diagnosis of stage II TNBC and from matched healthy controls were subjected to proteomic analysis by mass spectrometry to identify Ig-bound proteins yielding a predominance of cytokeratins, including several associated with a mesenchymal/basal phenotype among cases compared with controls. Our data provide evidence indicative of a dynamic repertoire of antigens associated with a humoral immune response reflecting disease pathogenesis in TNBC.
Introduction
The use of samples collected in before diagnosis setting, obtained before onset of symptoms, from prospective cohorts has the potential to identify biomarkers that are particularly relevant for early cancer detection (1). Moreover, refined genetically engineered mouse (GEM) models of human cancer that recapitulate their human counterparts offer a potential discovery and filtering engine for prioritization of candidate markers discovered in parallel using human specimens (2). A promising source of circulating cancer biomarkers stems from harnessing the humoral immune response directed against tumor antigens for the development of marker panels that have use for early cancer detection (3–6). The biologic significance of the humoral immune response in promoting or suppressing tumor development remains unclear (7, 8). Nevertheless, the discovery of potential antigens associated with an autoimmune response has led in some cases to successful validation studies aimed at assessing their potential diagnostic use (6, 9).
The full repertoire of antigens and epitopes associated with the development of autoantibodies and their specificity to particular cancer types remain largely undetermined (10, 11). We previously assessed the autoantibody repertoire exhibited in before diagnosis samples from subjects that subsequently developed estrogen receptor (ER)+ progesterone receptor (PR)+ breast cancer from a longitudinal cohort and the autoantibody repertoire of a mouse model engineered to develop ER+ breast cancer (12). We provided evidence for the occurrence of circulating autoantibodies directed against glycolytic pathway proteins preceding a diagnosis of ER+/PR+ breast cancer in samples collected up to 6 months before diagnosis (1). However, there is a paucity of data about the humoral immuneresponse in triple-negative breast cancer (TNBC) and the extent of similarities and differences in the antigenic repertoire between TNBC and ER+ breast cancer. TNBC represents an aggressive type with poor prognosis and has been associated with distinct gene expression signatures with a predominance of a basal expression subtype (13). The basal subtype correlates with high histologic grade at the time of diagnosis (14).
In the present study, we addressed the occurrence of autoantibodies associated with TNBC. High-density protein arrays prepared from lysate proteins from the MDA-MB-231 cell line were probed with plasma samples collected before clinical diagnosis of TNBC from participants in the Women's Health Initiative (WHI) cohort (15). The goal was to identify antigens that exhibited differential reactivity with circulating immunoglobulins among cases compared with controls. We also constructed in parallel arrays prepared from lysate proteins of an immortalized cell line derived from the C3(1)-T mouse model that were probed with mouse plasma samples collected before palpable tumor from the same mouse model and from tumor-free control mouse plasmas (16). We further examined using mass spectrometry (MS), the occurrence of antigen–antibody complexes using plasma samples collected at the time of diagnosis of early-stage TNBC. The potential occurrence of a distinctive autoantibody repertoire in TNBC was examined through a comparison with our prior findings in ER+PR+ breast cancer.
Materials and Methods
Plasma samples
Prediagnostic EDTA plasma samples were collected as part of the WHI observational study (Table 1). Plasma was available from 13 women who subsequently developed TNBC. Plasma from an equal number of controls from the same cohort matched on age, time of blood collection, and hormone therapy use was analyzed in parallel. EDTA plasma samples were collected from newly diagnosed women with stage II TNBC and used for MS analysis of proteins bound to Ig. Separate analyses were performed using plasmas from 9 premenopausal and 9 postmenopausal women diagnosed with TNBC and from an equal number of control women matched for menopausal status and blood collection and storage conditions.
Table 1. Human subject characteristics.
| WHI samples | ||
|---|---|---|
|
| ||
| Case | Control | |
| Sample number | 13 | 13 |
| Average age, y | 61 (50–69) | 61 (50–69) |
| Stage | ||
| I | 0 | – |
| II | 8 (61.5%) | – |
| III | 5 (38.5%) | – |
| IV | 0 | – |
| Avg. days to diagnosis | 138.5 (6–240) | – |
Plasma samples used for autoantibody studies were collected from a C3(1)-T mouse model that has been engineered to develop hormone-independent, invasive tumors in the mammary epithelium (16). Plasma was collected at baseline, at 8 weeks, and before palpable tumor, on average around 120 days from 19 mice.
Array construction
One hundred fifty milligrams of protein derived from MDA-MB-231 and from C3(1)-T cell lysates was each subjected to orthogonal two-dimensional (2D) high-performance liquid chromatography (HPLC) fractionation in an automated system (Shimadzu Corporation; ref. 17). Fractionation was based on anion exchange (SAX/10 column, 7.5 mm ID × 150 mm, Column Technology Inc) using a 40 step-elution, followed by a second-dimension reversed-phase separation (RP/5D column, 4.6 mm ID × 150 mm, Column Technology Inc). Fractions (n = 2,430) were collected from the 2D separation. The first-dimension anion-exchange chromatography mobile phase A was 20 mmol/L Tris, pH 8.5, and mobile phase B was 20 mmol/L Tris, 1 mol/L NaCl, pH 8.5. The second-dimension reversed-phase chromatography mobile phase A was 95% water, 5% acetonitrile, 0.1% TFA and mobile phase B was 90% acetonitrile, 10% water, 0.1% TFA.
A total of 300 μL from each fraction was lyophilized and resuspended in 30 μL of printing buffer (250 mmol/L Tris-HCl, pH 6.8, 0.5% SDS, 25% glycerol, 0.05% Triton X-100, 62.5 mmol/L dithiothreitol). Fractions (n =1,950), together with printing buffer as negative controls and positive controls, were printed onto nitrocellulose-coated slides using a contact printer, as previously described (6, 18). Plasma samples were hybridized with an individual microarray at a dilution of 1:150. Reactivity was quantified using an indirect immunofluorescence protocol. Local background subtracted median spot intensities were generated using GenePix Pro 6.1 and used for downstream statistical analysis using R 2.9.0. Spot intensities were log (base 2) transformed before statistical analysis. P values were calculated using the Student t test.
Western blots
Individual fractions (100 μL) were lyophilized and resuspended in 40 μL of loading buffer. Fractions were run in separate lanes of a 4% to 12% Bis-Tris Criterion XT Precast Gel. Gels were transferred to polyvinylidene difluoride (PVDF) membranes for 1.5 hours at 80 V. Membranes were blocked in 3% BSA at room temperature for 1 hour. Plasma samples were diluted 1:500 in 3% BSA and incubated with the membrane at 4°C overnight. Samples were removed and membranes were washed with 0.1% PBST 5 times for 5 minutes each. Horseradish peroxidase–labeled anti-mouse or anti-human IgG at a 1:2,000 dilution was incubated with the membrane at room temperature for 1 hour. Solutions were removed and membranes were washed with 0.1% PBST 5 times for 5 minutes each. Membranes were exposed to enhanced chemiluminescence (ECL) for 1 minute and exposed to ECL hyperfilm for varied lengths of time. Films were developed and scanned for qualitative analysis.
Identification of immunogenic proteins in microarray reactive fractions
Immunogenic proteins were identified as previously described (1, 19). Briefly, significantly elevated fractions and neighboring fractions were grouped into “fraction clusters” on the basis of microarray reactivity and subjected to further analysis. Western blot analysis was used to determine the molecular weight of proteins with autoantibody reactivity in plasma samples by matching observed bands to microarray reactivity data. MS analysis matched total peptide count from individual fractions to microarray reactivity data to determine protein identifications. When no reactive bands were observed in Western blot analyses, protein identification was based solely on results from the MS analysis of fractions.
On the basis of the protein microarray analysis, 50 μL of each reactive fraction from the 2D-HPLC was lyophilized using a freeze drying system (Labconco). The lyophilized protein samples were dissolved in 100 mmol/L NH4HCO3 (pH 8.5) followed by overnight in-solution digestion with trypsin at 37° C. The digestion was quenched by adding 5 μL of 1.0% formic acid solution before LC-MS/MS analysis as described previously (20). Briefly, peptides were separated by reversed-phase chromatography using a nano HPLC system (Eksigent) coupled online with a LTQ-FT mass spectrometer (Thermo Fisher Scientific, Inc.). MS parameters were spray voltage 2.5 kV, capillary temperature 200°C, FT resolution 100,000, FT target value 8 × 105, LTQ target value 104, 1 FT microscan with 850 ms injection time, and 1 LTQ microscan with 100 ms injection time. Mass spectra were acquired in a data-dependent mode with the m/z range of 400 to 2,000. The full mass spectrum (MS scan) was acquired by the FT and tandem mass spectrum (MS/MS scan) was acquired by the LTQ with a 35% normalized collision energy. Acquisition of each full mass spectrum was followed by the acquisition of MS/MS spectra for the 5 most intense +2 or +3 ions within a 1-second duty cycle. The minimum signal threshold (counts) for a precursor occurring during a MS scan was set at 1,000 for triggering a MS/MS scan.
The acquired LC-MS/MS data were processed by the Computational Proteomics Analysis System (9, 21, 22). Briefly, LC-MS/MS data were first converted to mzXML format using ReAdW software (version 1.2) to generate the peak list for protein database searching. The X!Tandem search engine (version 2005.12.01) parameters included cysteine (Cys) alkylated with iodoacetamide (57.02146@C) as a fixed modification and methionine (Met) oxidation (15.99491@M) as a variable modification. Data were searched against the International Protein Index (IPI) human protein knowledgebase (version v3.57), which contained entries for 76,542 proteins. The minimum criterion for peptide matching was a Peptide Prophet Score >0.2. Peptides meeting this criterion were grouped to protein sequences using the Protein Prophet algorithm at an error rate of ≤5%. Total peptide count in each fraction was used as a measure of protein concentration within that fraction.
Analysis of Ig-bound proteins in plasma by MS
IgG-bound proteins from a total of 100 μL of plasma for each experimental condition were extracted with Protein A/G Agarose (Thermo Scientific, #20421 20422 20423). The collected proteins were treated with 25 mmol/L TCEP for Cys reduction and subsequently alkylated with acrylamide. The samples were next fractionated at the protein level by reverse-phase chromatography followed by desalting for 5 minutes with 95% mobile phase A (0.1%TFAin 95% H2O) at a flow rate of 3 mL/min. Proteins were eluted from the column and collected into 12 fractions, at a flow rate of 2.1 mL/min, with a gradient elution that included an increase from 5% to 70% mobile phase B (0.1% TFA in 95% acetonitrile) over 25 minutes, 70% to 95% mobile phase B for 3 minutes, a wash step to hold at 95% mobile phase B for 2 minutes, and a re-equilibration step at 95% mobile phase A for 5 minutes. The collected fractions were lyophilized and digested with trypsin before analysis by MS using a SYNAPT G2–S instrument and Waters Masslynx (version 4.1, SCN 851). The mass spectrometer was operated in V-mode with a typical resolving power of at least 20,000. All analyses were performed using positive mode ESI using a NanoLockSpray source. The lock mass channel was sampled every 60 seconds. The mass spectrometer was calibrated with a fibrinopeptide solution (300 fmol/μL) delivered through the reference sprayer of the NanoLockSpray source. Accurate mass LC-HDMSE data was collected in an alternating, low-energy (MS) and high-energy (MSE) mode of acquisition with mass scan range from m/z 50 to 1800. The spectral acquisition time in each mode was 1.0 second with a 0.1-second interscan delay. In low-energy HDMS mode, data were collected at constant collision energy of 2 eV in both Trap cell and Transfer cell. In high-energy HDMSE mode, the collision energy was ramped from 25 to 55 eV in the Transfer cell only. The RF applied to the quadrupole mass analyzer was adjusted such that ions from m/z 300 to 2,000 were efficiently transmitted, ensuring that any ions observed in the LC-HDMSE data less than m/z 300 were known to arise from dissociations in the Transfer collision cell. The acquired LC-HDMSE data were processed and searched against protein knowledge database (Uniprot) through ProteinLynx Global Server (PLGS, Waters Company) with false discovery rate (FDR) 4%.
Results
Autoantibody profiles in human TNBC prediagnostic plasmas
Prediagnostic plasmas were available from 13 women who were participants in the postmenopausal WHI cohort study and who were diagnosed with TNBC up to 240 days subsequent to blood collection (Table 1). We also obtained from the same cohort, plasmas from an equal number of matched healthy controls that remained cancer-free during the period of followup. The prediagnostic period of 240 days was similar to the 259 days of the prior WHI ER+ plasma autoantibody study (1). Plasma samples were each individually hybridized with MDA-MB-231–derived protein arrays. Of the 2,300 printed fractions from the MDA-MB-231 human breast cancer cell line, 229 individual fractions had significantly elevated IgG reactivity (P < 0.01 and mean ratio >2) in preclinical plasmas compared with control plasmas. Concordant reactivity among some neighboring fractions suggested elution of reactive antigens over sequential chromatographic fractions. These peak reactivity profiles were used to select clusters around statistically significant fractions. MS yielded identification of 49 reactive proteins (Table 2).
Table 2. Immunogenic proteins identified by autoantibody responses from patients with TNBC to MDA-MB-231 cancer cell lysate.
| Gene | Description | Subcellular localization | Case-to-control ratio | q | AUC |
|---|---|---|---|---|---|
| ACTA1 | Actin, alpha 1, skeletal muscle | Cytoplasm | 2.17 | 1.59E–03 | 0.825 |
| ACTA2 | Actin, alpha 2, smooth muscle, aorta | Cytoplasm | 2.17 | 1.59E–03 | 0.825 |
| ACTB | Actin, beta | Cytoplasm | 2.34 | 4.30E–05 | 0.959 |
| ACTC1 | Actin, alpha, cardiac muscle 1 | Cytoplasm | 2.34 | 4.30E–05 | 0.959 |
| ACTG1 | Actin, gamma 1 | Cytoplasm | 2.17 | 1.59E–03 | 0.825 |
| ACTG2 | Actin, gamma 2, smooth muscle, enteric | Cytoplasm | 2.17 | 1.59E–03 | 0.825 |
| AHNAK | AHNAK nucleoprotein | Nucleus | 2.50 | 4.73E–03 | 0.846 |
| BAD | BCL2-associated agonist of cell death | Cytoplasm | 2.55 | 2.06E–05 | 0.923 |
| CALU | Calumenin | Cytoplasm | 2.13 | 4.58E–03 | 0.811 |
| CLTA | Clathrin, light chain A | Plasma Membrane | 2.41 | 4.46E–03 | 0.861 |
| CLTB | Clathrin, light chain B | Plasma Membrane | 2.29 | 8.71E–05 | 0.899 |
| DCD | Dermcidin | Extracellular Space | 2.00 | 2.32E–03 | 0.837 |
| DNAH11 | Dynein, axonemal, heavy chain 11 | Cytoplasm | 2.41 | 4.46E–03 | 0.861 |
| EIF4B | Eukaryotic translation initiation factor 4B | Cytoplasm | 2.71 | 1.62E–04 | 0.923 |
| HIST1H1C | Histone cluster 1, H1c | Nucleus | 2.34 | 4.30E–05 | 0.959 |
| HIST1H1D | Histone cluster 1, H1d | Nucleus | 2.14 | 2.18E–03 | 0.855 |
| HIST1H1E | Histone cluster 1, H1e | Nucleus | 2.07 | 2.80E–04 | 0.876 |
| HIST1H1T | Histone cluster 1, H1t | Nucleus | 2.79 | 1.17E–-03 | 0.852 |
| HNRNPK | Heterogeneous nuclear ribonucleoprotein K | Nucleus | 2.06 | 2.54E–-03 | 0.834 |
| HSPA5 | Heat shock 70-kDa protein 5 | Cytoplasm | 2.49 | 8.01E–03 | 0.802 |
| KRT1 | Keratin 1 | Cytoplasm | 2.08 | 1.17E–03 | 0.828 |
| KRT10 | Keratin 10 | Cytoplasm | 2.10 | 7.97E–03 | 0.858 |
| KRT14 | Keratin 14 | Cytoplasm | 2.04 | 1.42E–03 | 0.899 |
| KRT16 | Keratin 16 | Cytoplasm | 2.77 | 2.09E–03 | 0.899 |
| KRT2 | Keratin 2 | Cytoplasm | 2.28 | 4.96E–03 | 0.817 |
| KRT5 | Keratin 5 | Cytoplasm | 2.04 | 1.42E–03 | 0.899 |
| KRT6A | Keratin 6A | Plasma membrane | 2.77 | 2.09E–03 | 0.899 |
| KRT9 | Keratin 9 | Cytoplasm | 2.07 | 5.13E–04 | 0.882 |
| LASP1 | LIM and SH3 protein 1 | Cytoplasm | 2.38 | 1.92E–03 | 0.879 |
| MRPS36 | Mitochondrial ribosomal protein S36 | Cytoplasm | 2.37 | 6.47E–03 | 0.817 |
| NUCB1 | Nucleobindin 1 | Cytoplasm | 2.37 | 2.01E–03 | 0.828 |
| P4HB | Prolyl 4-hydroxylase, beta polypeptide | Cytoplasm | 2.55 | 2.06E–05 | 0.923 |
| PDIA3 | Protein disulfide isomerase family A, member 3 | Cytoplasm | 2.06 | 2.54E–03 | 0.834 |
| PRKCSH | Protein kinase C substrate 80K-H | Cytoplasm | 2.02 | 2.28E–03 | 0.831 |
| RCN1 | Reticulocalbin 1, EF-hand calcium-binding domain | Cytoplasm | 2.17 | 8.84E–05 | 0.923 |
| RPLP2 | Ribosomal protein, large, P2 | Cytoplasm | 2.36 | 1.02E–04 | 0.932 |
| RPS21 | Ribosomal protein S21 | Cytoplasm | 2.29 | 8.71E–05 | 0.899 |
| RPS27A | Ribosomal protein S27a | Cytoplasm | 2.13 | 3.81E–04 | 0.911 |
| SF3B1 | Splicing factor 3b, subunit 1, 155 kDa | Nucleus | 2.33 | 2.02E–04 | 0.893 |
| SLIRP | SRA stem-loop interacting RNA-binding protein | Cytoplasm | 2.37 | 7.66E–03 | 0.811 |
| SFRS1 | Serine/arginine-rich splicing factor 1 | Nucleus | 2.23 | 2.02E–04 | 0.914 |
| SFRS2 | Serine/arginine-rich splicing factor 2 | Nucleus | 2.00 | 6.69E–04 | 0.882 |
| SFRS3 | Serine/arginine-rich splicing factor 3 | Nucleus | 2.79 | 1.17E–03 | 0.852 |
| SFRS7 | Serine/arginine-rich splicing factor 7 | Nucleus | 2.36 | 1.02E–04 | 0.932 |
| SFRS9 | Serine/arginine-rich splicing factor 9 | Nucleus | 2.79 | 1.17E–03 | 0.852 |
| UBA52 | Ubiquitin A-52 residue ribosomal protein fusion product 1 | Cytoplasm | 2.13 | 3.81E–04 | 0.911 |
| UBB | Ubiquitin B | Cytoplasm | 2.53 | 6.71E–03 | 0.846 |
| UBC | Ubiquitin C | Cytoplasm | 2.53 | 6.71E–03 | 0.846 |
| ZYX | Zyxin | Plasma membrane | 2.06 | 2.54E–03 | 0.834 |
Ingenuity network analysis of reactive proteins identified in human samples revealed top network functions that included cancer and cellular morphology. Top scoring networks exhibited major nodes of PI3K and TP53, genes commonly mutated in TNBC (Fig. 1A and B). An enrichment was also observed for cytoskeletal proteins consisting of actins, cytokeratins, DNAH11, LASP1, and ZYX (FDR= 3.8E–9). Reactive cytokeratins consisted of KRT1, 2, 5, 6a, 9, 10, 14, and 16. The cytokeratin signature was not previously observed in ER+ breast cancer (1). An enrichment was also observed for spliceosome proteins represented by HNRNPK, SF3B1, SFRS1, 2, 3, 7, and 9 (FDR = 7.5E–4) as previously observed in ER+ breast cancer (1). We also investigated circulating protein complexes of proteins in the glycolytic pathway by Western blotting using WHI TNBC case samples and matched controls, which revealed an increase in circulating immune complexes involving glycolytic proteins, notably PKM2, ENO1, and TPI, in cases compared with controls as we previously observed in ER+ breast cancer, in samples collected 0 to 33 weeks before diagnosis compared with farther from diagnosis (Fig. 2A; ref. 1).
Figure 1.
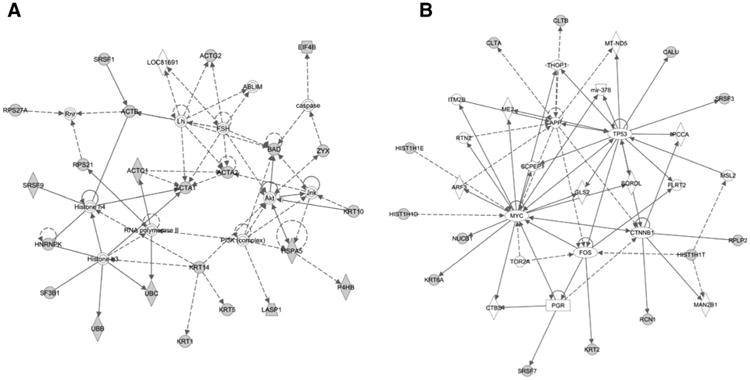
A and B, top two networks of proteins eliciting a significantly elevated (P < 0.01; mean ratio > 2) autoimmune response in WHI human prediagnostic TNBC samples, based on Ingenuity Pathway Analysis.
Figure 2.
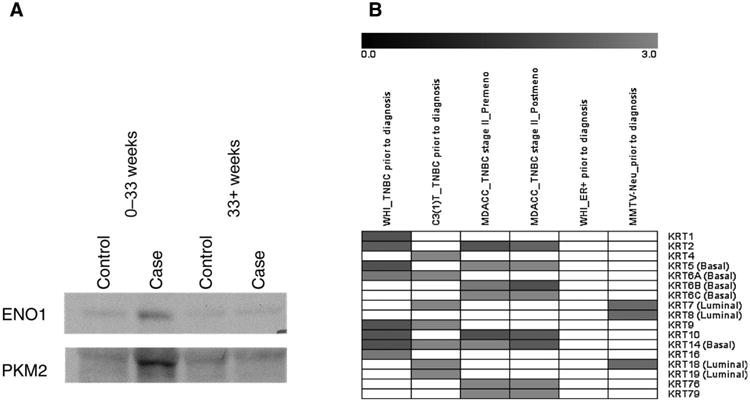
Signatures of autoimmune response in plasma. A, Western blot analysis of immunodepleted fractions from pooled prediagnostic TNBC samples collected within 33 weeks before diagnosis and greater than 33 weeks before diagnosis compared with matched controls for glycolysis pathway proteins ENO1 and PKM2. Increased reactivity is observed in samples collected within 33 weeks before diagnosis. B, cytokeratin autoantibody panel in TNBC/basal and ER+/luminal type. Gray indicates statistically significant and elevated in case. WHI ER+ and MMTV-Neu dataset was from our previous study (1).
Autoantibody profiles in plasmas from a TNBC mouse model before occurrence of palpable tumor
Plasma samples collected at baseline and before palpable tumor from 19 individual mice were each hybridized with mouse breast cancer cell lysate protein arrays. Of the 2,800 printed fractions, 148 individual fractions exhibited significantly increased Ig binding (P < 0.01 and mean ratio >2) in preclinical samples compared with baseline. Analysis of reactive clusters yielded identification of 33 reactive proteins (Table 3) from the C3(1)-T mouse model. A spliceosome autoantibody signature was observed in plasmas from the C3(1)-T mouse model, as we observed with human prediagnostic TNBC samples and as we previously reported in preclinical plasmas from an ER+ breast cancer mouse model and ER+ breast cancer subjects. The spliceo-some signature consisted of Hnrnpk, Hnrnpm, Sfrs2, and U2af2. The glycolysis signature previously reported in ER+ breast cancer was similarly observed in the C3(1)-T mouse preclinical samples, with 3 proteins (Eno1, Pkm2, and Tpi1) associated with glycolysis exhibiting autoantibody reactivity.
Table 3. Immunogenic proteins identified in C3(1)-T mouse cancer cell lysate.
| Gene | Description | Subcellular localization | Case-to-control ratios | qa | |
|---|---|---|---|---|---|
|
| |||||
| Time point #1 | Time point #2 | ||||
| Adamts2 | ADAM metallopeptidase with thrombospondin type 1 motif, 2 | Extracellular space | 4.98 | 2.52E–03 | 2.38E–03 |
| Dkc1 | Dyskeratosis congenita 1, dyskerin | Nucleus | 4.98 | 2.52E–03 | 2.38E–03 |
| Eif5a | Eukaryotic translation initiation factor 5A | Cytoplasm | 4.48 | 2.49E–03 | 7.04E–03 |
| Eno1 | Enolase 1, (alpha) | Cytoplasm | 6.23 | 7.39E–04 | 1.89E–03 |
| Hnrnpk | Heterogeneous nuclear ribonucleoprotein k | Nucleus | 4.08 | 1.49E–03 | 6.70E–04 |
| Hnrnpm | Heterogeneous nuclear ribonucleoprotein m | Nucleus | 4.74 | 1.50E–03 | 4.36E–03 |
| Hspa5 | Heat shock 70-kDa protein 5 (glucose-regulated protein, 78 kDa) | Cytoplasm | 4.95 | 2.49E–03 | 6.14E–03 |
| Hspa9 | Heat shock 70-kDa protein 9 (mortalin) | Cytoplasm | 4.15 | 6.64E–03 | 2.69E–03 |
| Idh3a | Isocitrate dehydrogenase 3 (NAD+) alpha | Cytoplasm | 3.86 | 6.31E–05 | 5.75E–05 |
| Krt14 | Keratin 14 | Cytoplasm | 8.80 | 1.09E–03 | 9.39E–03 |
| Krt18 | Keratin 18 | Cytoplasm | 4.46 | 1.23E–04 | 4.51E–03 |
| Krt19 | Keratin 19 | Cytoplasm | 3.69 | 1.22E–04 | 8.42E–05 |
| Krt4 | Keratin 4 | Cytoplasm | 6.91 | 2.97E–03 | 2.89E–03 |
| Krt6a | Keratin 6A | Plasma membrane | 4.74 | 1.50E–03 | 4.36E–03 |
| Krt7 | Keratin 7 | Cytoplasm | 4.10 | 1.52E–03 | 1.39E–03 |
| Krt9 | Keratin 9 | Cytoplasm | 3.69 | 1.22E–04 | 8.42E–05 |
| Nup153 | Nucleoporin 153 kDa | Nucleus | 6.91 | 2.97E–03 | 2.89E–03 |
| Ogfr | Opioid growth factor receptor | Plasma membrane | 4.98 | 2.52E–-03 | 2.38E–03 |
| Pdia3 | Protein disulfide isomerase family A, member 3 | Cytoplasm | 5.12 | 1.97E–03 | 2.43E–03 |
| Pdia6 | Protein disulfide isomerase family A, member 6 | Cytoplasm | 4.31 | 1.33E–03 | 5.46E–04 |
| Pkm2 | Pyruvate kinase, muscle | Cytoplasm | 5.12 | 1.97E–03 | 2.43E–03 |
| Psmc3 | Proteasome (prosome, macropain) 26S subunit, ATPase, 3 | Nucleus | 3.86 | 6.31E–-05 | 5.75E–05 |
| Ptbp1 | Polypyrimidine tract-binding protein 1 | Nucleus | 4.11 | 3.03E–04 | 2.40E–03 |
| S100a11 | S100 calcium-binding protein A11 | Cytoplasm | 5.54 | 3.15E–03 | 4.78E–03 |
| Sfrs12 | Serine/arginine-rich splicing factor 12 | Nucleus | 3.86 | 6.31E–-05 | 5.75E–05 |
| Sfrs2 | Serine/arginine-rich splicing factor 2 | Nucleus | 3.87 | 2.22E–03 | 3.12E–03 |
| Stxbp3a | Syntaxin-binding protein 3 | Plasma membrane | 4.87 | 2.17E–03 | 1.90E–03 |
| Tpi1 | Triosephosphate isomerase 1 | Cytoplasm | 4.70 | 5.50E–03 | 7.21E–03 |
| U2af2 | U2 small-nuclear ribonucleoprotein auxiliary factor (U2AF) 2 | Nucleus | 3.86 | 6.31E–05 | 5.75E–05 |
| Ubb | Ubiquitin B | Cytoplasm | 3.87 | 2.22E–03 | 3.12E–03 |
| Ubc | Ubiquitin C | Cytoplasm | 3.87 | 2.22E–-03 | 3.12E–03 |
| Vim | Vimentin | Cytoplasm | 5.89 | 4.27E–04 | 5.10E–05 |
| Whsc1 | Probable histone-lysine N-methyltransferase NSD2 | Nucleus | 4.48 | 2.49E–03 | 7.04E–03 |
NOTE: Case-to-control ratios are an average of two analyzed blood draws.
q values based on all 2,808 arrayed fractions.
Identified Ingenuity Network Analysis of antigenic proteins from the C3(1)-T mouse was done as with human TNBC antigenic proteins. The top two scoring networks (P ∼ 10−30 and P ∼ 10−10) included major nodes commonly associated with an invasive, triple-negative, or basal phenotype of breast cancer, encompassing TP53, MDM2, BRCA1, and CDKN1A and cytokeratins (Fig. 3A and B). Gene ontology analysis of proteins associated with autoantibodies (23) yielded a signature of cytoskeletal proteins (FDR = 0.0056), consisting mainly of cytokeratins and vimentin. This signature that included KRT4 6a, 7, 9, 14, 18, and 19 was not previously observed in ER+ breast cancer mouse or human samples but was concordant with the cytokeratin autoantibody signature observed in prediagnostic TNBC plasmas.
Figure 3.
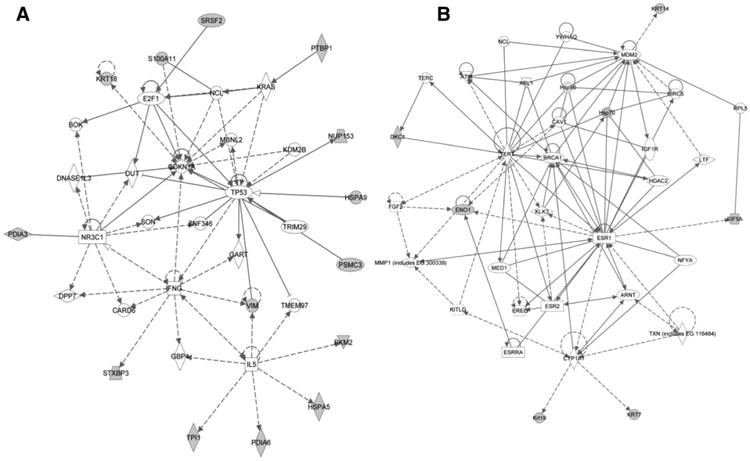
A and B, top two networks of proteins eliciting a significantly elevated (P < 0.01; mean ratio > 2) response in C3(1)-T TNBC mouse model samples collected before palpable tumor compared with baseline, based on Ingenuity Pathway Analysis.
Ig-bound proteins in plasmas from subjects with newly diagnosed TNBC
The occurrence of immune complexes associated with TNBC was further investigated using a MS approach applied to plasmas from subjects with newly diagnosed TNBC and healthy controls matched for menopausal status. The approach was based on capture of the Ig fraction from plasmas followed by MS to identify Ig-bound proteins. The quantitation was based on the MS counts calculated from the cumulative number of the MS2 spectra assigned to each protein accession. To this effect, 9 plasma samples from premenopausal women and 9 samples from post-menopausal newly diagnosed women with stage II TNBC and an equal number of plasmas from healthy pre- and postmenopausal women were separately pooled, and individual pools were subjected to MS analyses of Ig-bound fractions. We identified and quantified a total of 90 and 45 Ig-bound proteins from premenopausal stage II TNBC case plasmas versus healthy, respectively, and 133 and 123 distinct proteins from postmen-opausal stage II TNBC cases plasmas versus healthy, respectively. Of these proteins, 10 met the criteria of 2-fold increase in MS counts in case versus control, as a measure of the amount of protein bound to IG (24) in both the premenopausal and postmenopausal samples (Table 4). These 10 proteins were identified with two or more peptides each. Remarkably, 9 of the 10 common proteins consisted of cytokeratins, including four basal type of cytokeratins (KRT5, 6B, 6C, and 14). Four additional cytokeratins (KRT 15, 16, 17, and 19) met these criteria only in postmenopausal cases.
Table 4. Plasma IgG-bound proteins in healthy controls and newly diagnosed TNBC stage II patients.
| Protein | Healthy premenopausal MS counts | TNBC premenopausal MS counts | Healthy postmenopausal MS counts | TNBC postmenopausal MS counts |
|---|---|---|---|---|
| KRT2 | 60 | 130 | 100 | 243 |
| KRT5 | 5 | 40 | 18 | 110 |
| KRT6B | 16 | 46 | 34 | 70 |
| KRT6C | 2 | 28 | 15 | 50 |
| KRT10 | 93 | 188 | 139 | 312 |
| KRT14 | 10 | 21 | 47 | |
| KRT25 | 5 | 28 | 15 | 33 |
| KRT76 | 12 | 4 | 21 | |
| KRT79 | 1 | 12 | 5 | 30 |
| LATS1 | 3 | 10 | 4 | 12 |
Discussion
We have investigated the immune response in the form of autoantibodies in TNBC and have compared findings with a previously published study of ER+ breast cancer that used a similar approach as in this study (1). TNBC is a more aggressive, invasive cancer than receptor-positive breast cancers (25). Concordant findings were observed with human TNBC cancer plasmas and plasmas from the TNBC mouse model. The proteins that exhibited immunoreactivity both in prediagnostic and mouse model plasmas represented two major groupings. One grouping consisted of spliceosome and glycolysis proteins, which was the major grouping of reactive proteins previously observed in ER+ breast cancer. However, the top scoring networks in prediagnostic TNBC samples exhibited major nodes of TP53 and PI3K, genes commonly mutated in TNBC.
The finding of autoantibodies to antigens related to TP53 and PI3K networks reflects disease pathogenesis in TNBC. Tumors with germline mutations in BRCA1 are more often hormone receptor–negative and are more likely to overexpress TP53 protein (26–28). TNBC shares clinical and pathologic features with hereditary BRCA1-related breast cancers (29). MDM2 is an important negative regulator of TP53 and has been shown to be integral to metastasis and tumor growth (30). Moreover studies have shown that inhibition of MDM2 reduced systemic inflammation and reduced autoantibody production (31). A network involving PI3K also included CDKN1A and IL5 as additional nodes. The PI3K pathway has been investigated as a therapeutic target for TNBC and basal-type cancers (32, 33). Genetic variants of CDKN1A have been associated with breast cancer and TNBC cell proliferation (34). IL5 plays an important role in immune response by stimulating B cells and increasing immunoglobulin secretion (35) and has been shown to promote malignant pleural effusion formation, which in breast cancer is associated with poor prognosis and reduced survival (36). It is therefore likely that altered pathways in TNBC impact the immune response in the form of autoantibodies.
One feature that differentiates TNBC from ER+ breast cancer is the frequent occurrence of epithelial-to-mesenchymal transition (EMT), a mechanism whereby epithelial cells gain invasive traits. EMT has been correlated with high histologic grade, triple-negative phenotype, and basal-like phenotype breast cancers (37, 38). Alterations in cytoskeletal protein levels have been linked to increases in cell invasion and have been implicated in EMT (39–41).
It is interesting that many of the immunoreactive proteins we have identified are considered to be intracellular proteins. One possible mechanism for their association with an immune response in the form of autoantibodies may be through their occurrence in exosome (42). A recent proteomic study of breast cancer–derived vesicles revealed a large number of glycolysis pathway proteins present, including ALDOA, ENO1, GAPDH, PGK1, and TPI1 (43). Searching against the database Exocarta (44), we found that 35 of the 49 proteins (71%) identified from analysis of human TNBC samples occur in exosomes. In addition, 22 of the 33 (67%) identified proteins from the C3(1)-T mouse also occur in exosomes. Thus, exosomes may contribute as an important antigen-presenting source.
A major finding from our study is the occurrence of a cytokeratin autoantibody signature in prediagnostic human TNBC plasmas as well as in mouse preclinical samples. Moreover, in samples collected at the time of diagnosis, on the basis of the analysis of stage II TNBC samples, cytokeratins represented the most predominant proteins bound to Ig. The autoantibody response in the mouse model and in the prediagnostic samples was investigated using natural protein arrays whereas in samples from newly diagnosed subjects we relied on MS to identify Ig-bound proteins. The concordance of findings in independent sample sets and with the use of two different methodologies provides strong support for a basal cytokeratin autoantibody signature associated with TNBC. Multiple cytokeratin family members may be expressed in a given cell and are characteristic of the cell type and differentiation state. Cytokeratins are used for the fingerprinting of carcinomas in general. In breast tissue, the luminal epithelial cells express cytokeratins 8/18, 7, and 19, whereas basal/myoepithelial cells express cytokeratins 5/6, 14, and 17 predominantly (45). Cytokeratins 8 and 18 have been identified as tumor antigens with elevated serum levels of corresponding autoantibodies in patients with several cancer types (46, 47). Several of the cytokeratins found to be associated with autoantibodies in this study have been associated with TNBC and basal type of breast cancer. In one study, a series of 995 invasive breast cancers were investigated using immunohistochemical staining for four basal cytokeratins (48). Expression was demonstrated for KRT5, 6, 14, and 17. Another study found that in three-dimensional organoid assays, collective invasion was led by specialized cancer cells that were defined by their expression of basal epithelial genes, including cytokeratins 5 and 14 (49). Relocalization of KRT5 and KRT14 to the cell surface “leading edge” may contribute to the development of autoantibodies.
A comparison of cytokeratin autoantibodies we previously observed in ER+/luminal type of breast cancer with TNBC cytokeratin autoantibodies in this study as displayed in Fig. 2B reveals that luminal cytokeratins KRT7, 8, and 18 were commonly observed. Whereas basal cytokeratins were limited to TNBC mouse and human samples providing support for autoantibody signatures reflecting molecular characteristics of TNBC and disease pathogenesis.
Acknowledgments
Grant Support: This work was supported by an NCI grant U01CA141539 and a Komen Foundation grant. S. Hanash was supported by a Rubenstein Family endowed professorship. M.L. Disis was supported by the Athena Distinguished Professorship of Breast Cancer Research and a Komen Leadership Award.
Footnotes
Disclosure of Potential Conflicts of Interest: M.L. Disis reports receiving a commercial research grant from EMD Serono and Ventirx and has an ownership interest in Eptihany, Ventirx, and the University of Washington.
Authors' Contributions: Conception and design: H. Katayama, J.J. Ladd, T. Chao, M.L. Disis, S. Hanash
Development of methodology: H. Katayama, J.J. Ladd, T. Chao, J. Suo, M.L. Disis
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H. Katayama, J.J. Ladd, M.M. Johnson, T. Chao, M. Capello, J. Suo, J. Mao, J.A.E. Manson, R. Prentice, F. Esteva, H. Wang, M.L. Disis
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): H. Katayama, C. Boldt, J.J. Ladd, T. Chao, M. Capello, J.A.E. Manson, F. Esteva, M.L. Disis
Writing, review, and/or revision of the manuscript: H. Katayama, J.J. Ladd, J.A.E. Manson, R. Prentice, F. Esteva, M.L. Disis
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): H. Katayama, M.L. Disis
Study supervision: H. Katayama, M.L. Disis, S. Hanash
References
- 1.Ladd JJ, Chao T, Johnson MM, Qiu J, Chin A, Israel R, et al. Autoantibody signatures involving glycolysis and splicesome proteins precede a diagnosis of breast cancer among postmenopausal women. Cancer Res. 2013;73:1502–13. doi: 10.1158/0008-5472.CAN-12-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taguchi A, Politi K, Pitteri SJ, Lockwood WW, Faca VM, Kelly-Spratt K, et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer Cell. 2011;20:289–99. doi: 10.1016/j.ccr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brichory F, Beer D, Le Naour F, Giordano T, Hanash S. Proteomics-based identification of protein gene product 9.5 as a tumor antigen that induces a humoral immune response in lung cancer. Cancer Res. 2001;61:7908–12. [PubMed] [Google Scholar]
- 4.Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, et al. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci U S A. 2001;98:9824–9. doi: 10.1073/pnas.171320598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–94. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 6.Qiu J, Madoz-Gurpide J, Misek DE, Kuick R, Brenner DE, Michailidis G, et al. Development of natural protein microarrays for diagnosing cancer based on an antibody response to tumor antigens. J Proteome Res. 2004;3:261–7. doi: 10.1021/pr049971u. [DOI] [PubMed] [Google Scholar]
- 7.Chaput N, Conforti R, Viaud S, Spatz A, Zitvogel L. The Janus face of dendritic cells in cancer. Oncogene. 2008;27:5920–31. doi: 10.1038/onc.2008.270. [DOI] [PubMed] [Google Scholar]
- 8.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauch A, Bellew M, Eng J, Fitzgibbon M, Holzman T, Hussey P, et al. Computational Proteomics Analysis System (CPAS): an extensible, open-source analytic system for evaluating and publishing proteomic data and high throughput biological experiments. J Proteome Res. 2006;5:112–21. doi: 10.1021/pr0503533. [DOI] [PubMed] [Google Scholar]
- 10.Heo CK, Bahk YY, Cho EW. Tumor-associated autoantibodies as diagnostic and prognostic biomarkers. BMB Rep. 2012;45:677–85. doi: 10.5483/BMBRep.2012.45.12.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MA, O'Leary JJ, Cahill DJ. Assessment of the humoral immune response to cancer. J Proteomics. 2012;75:4573–9. doi: 10.1016/j.jprot.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Lu H, Ladd J, Feng Z, Wu M, Goodell V, Pitteri SJ, et al. Evaluation of known oncoantibodies, HER2, p53, and cyclin B1, in prediagnostic breast cancer sera. Cancer Prev Res. 2012;5:1036–43. doi: 10.1158/1940-6207.CAPR-11-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Eirew P, Mullaly SC, Aparicio S. The omics of triple-negative breast cancers. Clin Chem. 2014;60:122–33. doi: 10.1373/clinchem.2013.207167. [DOI] [PubMed] [Google Scholar]
- 14.Rody A, Karn T, Liedtke C, Pusztai L, Ruckhaeberle E, Hanker L, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97. doi: 10.1186/bcr3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama H, Paczesny S, Prentice R, Aragaki A, Faca VM, Pitteri SJ, et al. Application of serum proteomics to the Women's Health Initiative conjugated equine estrogens trial reveals a multitude of effects relevant to clinical findings. Genome Med. 2009;1:47. doi: 10.1186/gm47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green JE, Shibata MA, Yoshidome K, Liu ML, Jorcyk C, Anver MR, et al. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene. 2000;19:1020–7. doi: 10.1038/sj.onc.1203280. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Hanash S. Multi-dimensional liquid phase based separations in proteomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:11–8. doi: 10.1016/s1570-0232(02)00335-5. [DOI] [PubMed] [Google Scholar]
- 18.Madoz-Gurpide J, Kuick R, Wang H, Misek DE, Hanash SM. Integral protein microarrays for the identification of lung cancer antigens in sera that induce a humoral immune response. Mol Cell Proteomics. 2008;7:268–81. doi: 10.1074/mcp.M700366-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Qiu J, Choi G, Li L, Wang H, Pitteri SJ, Pereira-Faca SR, et al. Occurrence of autoantibodies to annexin I, 14-3-3 theta and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol. 2008;26:5060–6. doi: 10.1200/JCO.2008.16.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Hanash SM. Increased throughput and reduced carryover of mass spectrometry-based proteomics using a high-efficiency nonsplit nanoflow parallel dual-column capillary HPLC system. J Proteome Res. 2008;7:2743–55. doi: 10.1021/pr700876g. [DOI] [PubMed] [Google Scholar]
- 21.MacLean B, Eng JK, Beavis RC, McIntosh M. General framework for developing and evaluating database scoring algorithms using the TANDEM search engine. Bioinformatics. 2006;22:2830–2. doi: 10.1093/bioinformatics/btl379. [DOI] [PubMed] [Google Scholar]
- 22.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 23.Thomas PD, Kejariwal A, Campbell MJ, Mi H, Diemer K, Guo N, et al. PANTHER: a browsable database of gene products organized by biological function, using curated protein family and subfamily classification. Nucleic Acids Res. 2003;31:334–41. doi: 10.1093/nar/gkg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen CT, Ostergaard O, Stener L, Iversen LV, Truedsson L, Gullstrand B, et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012;64:1227–36. doi: 10.1002/art.34381. [DOI] [PubMed] [Google Scholar]
- 25.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 26.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 27.Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96:11–5. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20:2310–8. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–32. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 30.Shi W, Meng Z, Chen Z, Hua Y, Gao H, Wang P, et al. RNA interference against MDM2 suppresses tumor growth and metastasis in pancreatic carcinoma SW1990HM cells. Mol Cell Biochem. 2014;387:1–8. doi: 10.1007/s11010-011-1208-4. [DOI] [PubMed] [Google Scholar]
- 31.Allam R, Sayyed SG, Kulkarni OP, Lichtnekert J, Anders HJ. Mdm2 promotes systemic lupus erythematosus and lupus nephritis. J Am Soc Nephrol. 2011;22:2016–27. doi: 10.1681/ASN.2011010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimbung S, Biskup E, Johansson I, Aaltonen K, Ottosson-Wadlund A, Gruvberger-Saal S, et al. Co-targeting of the PI3K pathway improves the response of BRCA1 deficient breast cancer cells to PARP1 inhibition. Cancer Lett. 2012;319:232–41. doi: 10.1016/j.canlet.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Moulder SL. Does the PI3K pathway play a role in basal breast cancer? Clin Breast Cancer. 2010;10(Suppl 3):S66–71. doi: 10.3816/CBC.2010.s.014. [DOI] [PubMed] [Google Scholar]
- 34.De Santi M, Galluzzi L, Lucarini S, Paoletti MF, Fraternale A, Duranti A, et al. The indole-3-carbinol cyclic tetrameric derivative CTet inhibits cell proliferation via overexpression of p21/CDKN1A in both estrogen receptor-positive and triple-negative breast cancer cell lines. Breast Cancer Res. 2011;13:R33. doi: 10.1186/bcr2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–13. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 36.dos Santos GT, Prolla JC, Camillo ND, Zavalhia LS, Ranzi AD, Bica CG. Clinical and pathological factors influencing the survival of breast cancer patients with malignant pleural effusion. J Bras Pneumol. 2012;38:487–93. doi: 10.1590/s1806-37132012000400011. [DOI] [PubMed] [Google Scholar]
- 37.Jeong H, Ryu YJ, An J, Lee Y, Kim A. Epithelial-mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology. 2012;60:E87–95. doi: 10.1111/j.1365-2559.2012.04195.x. [DOI] [PubMed] [Google Scholar]
- 38.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–97. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 39.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition:a cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588–93. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 42.Klein-Scory S, Kubler S, Diehl H, Eilert-Micus C, Reinacher-Schick A, Stuhler K, et al. Immunoscreening of the extracellular proteome of colorectal cancer cells. BMC Cancer. 2010;10:70. doi: 10.1186/1471-2407-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palazzolo G, Albanese NN, DIC G, Gygax D, Vittorelli ML, Pucci-Minafra I. Proteomic analysis of exosome-like vesicles derived from breast cancer cells. Anticancer Res. 2012;32:847–60. [PubMed] [Google Scholar]
- 44.Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 45.Lane EB, Alexander CM. Use of keratin antibodies in tumor diagnosis. Semin Cancer Biol. 1990;1:165–79. [PubMed] [Google Scholar]
- 46.Ahlemann M, Schmitt B, Stieber P, Gires O, Lang S, Zeidler R. Evaluation of CK8-specific autoantibodies in carcinomas of distinct localisations. Anticancer Res. 2006;26:783–9. [PubMed] [Google Scholar]
- 47.Le Naour F, Brichory F, Misek DE, Brechot C, Hanash SM, Beretta L. A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Mol Cell Proteomics. 2002;1:197–203. doi: 10.1074/mcp.m100029-mcp200. [DOI] [PubMed] [Google Scholar]
- 48.Alshareeda AT, Soria D, Garibaldi JM, Rakha E, Nolan C, Ellis IO, et al. Characteristics of basal cytokeratin expression in breast cancer. Breast Cancer Res Treat. 2013;139:23–37. doi: 10.1007/s10549-013-2518-x. [DOI] [PubMed] [Google Scholar]
- 49.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–51. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


