Abstract
We have previously reported a small series of mixed efficacy μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist opioid peptidomimetics featuring a tetrahydroquinoline (THQ) scaffold, and showed the promise of this series as effective analgesics after intraperitoneal administration in mice. We report here expanded SAR of the pendant region of these compounds and focus in particular on the incorporation of heteroatoms into this side chain. These analogues provide new insight into the binding requirements for this scaffold at MOR, DOR, and the κ opioid receptor (KOR), and several (10j,k,m,n) significantly improve upon the overall MOR agonist/DOR antagonist profile of our previous compounds. In vivo data for 10j,k,m,n are also reported, and show the antinociceptive potency and duration of action of compounds 10j and 10m to be comparable to morphine.
Keywords: Opioid, mixed efficacy, intraperitoneal, dependence, tolerance, tetrahydroquinoline
Introduction
Opioid analgesics have long been the standard for the treatment of severe pain. Unfortunately, the use of opioids can lead to the development of a number of undesirable side effects, such as respiratory depression, constipation, and perhaps most problematically, dependence and tolerance [1]. There is therefore a great unmet need to develop agents that act as potent analgesics, but without the development of these side effects [2-3].
There is a growing body of evidence to suggest that the δ opioid receptor (DOR) plays a significant role in the modulation of the side effects related to the chronic use of opioid analgesics. Although the analgesic effects of traditional opioid agents such as morphine are associated with stimulation of the μ opioid receptor (MOR), the co-administration of DOR antagonists has been shown to maintain the desired antinocicepetive activity but with a reduced side effect profile as compared to a MOR agonist alone [4-7].
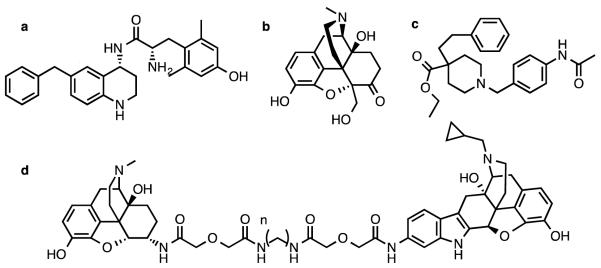
Because of these findings, our group and others have sought to develop bifunctional ligands that bind to both MOR and DOR, while only stimulating MOR. Several classes of compounds have been employed in this pursuit, including peptides [8], pseudopeptides [9,10] and small molecules [11,12]. MOR/DOR bivalent ligands developed by Portoghese and colleagues have been demonstrated to be effective analgesics with a diminished tolerance profile (Figure 1d) [13], and recently the MOR/DOR heteromer biased agonist CYM51010 was also shown to display reduced antinociceptive tolerance as compared to morphine (Figure 1c) [14]. MOR agonist/DOR antagonist compounds are also being developed clinically for the treatment of irritable bowel syndrome [15]. We have recently reported two MOR agonist/DOR antagonist compounds that are effective and bioavailable analgesics: a glycosylated cyclic pentapeptide [16] and a small molecule with a tetrahydroquinoline core (Figure 1a) [17-18]. Additionally, UMB425, a small molecule MOR agonist/DOR antagonist derived from thebaine, was also reported to display analgesia after subcutaneous administration, with reduced tolerance compared to morphine (Figure 1b) [19].
Figure 1. Bifunctional MOR/DOR Ligandsa.
a. Compound a from Ref. 17
The compounds reported in this paper build on the limited initial SAR done on our bioavailable THQ lead compound (S)-2-amino-N-((R)-6-benzyl-1,2,3,4-tetrahydroquinolin-4-yl)-3-(4-hydroxy-2,6-dimethylphenyl)propanamide (Figure 1a).
Initially, bulky, hydrophobic modifications to the side chain at position R1 were examined (Table 1) [17], as these substitutions were directly comparable to a previously reported series of MOR/DOR cyclic peptides [20-21], and were hypothesized to interact favorably with residues in the MOR active pocket to act as full MOR agonists while simultaneously behaving as DOR antagonists. These initial compounds are highly lipophilic, which is desirable for blood brain barrier penetration, but less than optimal for both aqueous solubility and metabolic stability [22]. We therefore explored a variety of polar side chains on this scaffold, for the purpose of improving these parameters and further probing the chemical space in this region of each of the receptors. Not only did simple heteroatom replacements have profound effects on receptor selectivity, these modifications also led to compounds that improved upon the in vivo profile of our initial THQ analogue. Compounds 10j and 10m both produced a maximum antinociceptive response in the mouse warm water tail withdrawal (WWTW) assay, and both improved significantly upon the duration of action of our lead peptidomimetic. These findings highlight the promise of this scaffold in vivo, and show that our SAR strategy focusing on polar side chains was effective for improving bioavailability. In vitro data for both binding affinity and efficacy are presented for all three opioid receptor types (MOR, DOR and the κ opioid receptor KOR).
Table 1.
Opioid receptor binding affinities and clogP values for analogues 10a-o.a

|
Binding, Ki (nM) | clogPc | ||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | MOR | DOR | KOR | |
| (Fig 1a) |

|
H | 0.22±0.02b | 9.4±0.8b | 68±2b | 3.7 |
| 10a |

|
H | 0.66±0.08 | 17±4 | 66±8 | 2.2 |
| 10b |

|
H | 0.3±0.1 | 120±29 | 29±9 | 2.7 |
| 10c |

|
H | 0.15±0.02 | 61±9 | 3.6±0.7 | 3.3 |
| 10d |

|
H | 0.6±0.1 | 140±67 | 170±32 | 1.4 |
| 10e |

|
H | 3.1±0.6 | 50±14 | 450±14 | 0.75 |
| 10f |

|
H | 0.8±0.2 | 18±6 | 20±3 | 2.9 |
| 10g |

|
H | 2.1±0.6 | 23±5 | 120±21 | 3.6 |
| 10h |

|
H | 0.10±0.02 | 1.5±0.2 | 16±4 | 3.6 |
| 10i |

|
H | 0.12±0.01 | 4.3±0.8 | 21±2 | 3.7 |
| 10j |

|
H | 0.15±0.08 | 15±5 | 2±1 | 3.1 |
| 10k |

|
H | 0.03±0.01 | 3.1±0.2 | 2.2±0.4 | 3.6 |
| 10l |

|
H | 0.15±0.01 | 4.8±0.9 | 37±8 | 4.1 |
| 10m |

|
Acetyl | 0.19±0.1 | 0.89±0.2 | 0.78±0.1 | 3.2 |
| 10n |

|
Acetyl | 0.32±0.09 | 2.6±0.2 | 7±3 | 2.7 |
| 10o |

|
Acetyl | 0.8±0.2 | 2±1 | 15±6 | 3.7 |
Binding affinities (Ki) were obtained by competitive displacement of [3H]diprenorphine in membrane preparations expressing either MOR, DOR or KOR. All values are mean ± SEM of three separate assays performed in duplicate.
Data from Ref. 17
Calculated using ChemBioDraw Ultra version 14.0
Chemistry
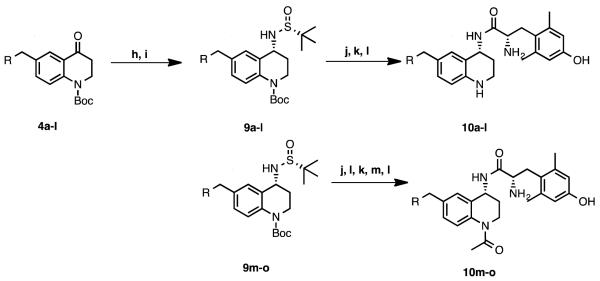
The synthesis of compounds 10a-c, 10f-o began with the acylation of p-toluidine with 3-bromopropionyl chloride (Scheme 1). The resulting alkyl bromide then underwent an intramolecular cyclization, followed by triflic acid mediated β-lactam rearrangement to give ketone 1 [23-24] which was subsequently Boc-protected on the THQ nitrogen to give 2. Ketone 2 was then brominated on the aryl methyl group as described previously [25] to give 3, onto which could be added the pendant of choice, either through a Suzuki coupling (4a, 4f-i) or through substitution with the appropriate secondary amine (4b,c 4j-o). It is important to note that 3 could be synthesized on a multi-gram scale, and all substitutions on this intermediate were high yielding. For the synthesis of compounds 10d and 10e, commercially available para-substituted anilines were then carried forward in a similar manner as in the synthesis of compound 2 to give intermediates 4d and 4e (Scheme 2).
Scheme 1. Synthesis of intermediates 4a-c, f-la.
a Reagents and conditions: (a) 3-bromopropionyl chloride, K2CO3, DCM, r.t.; (b) NaOtBu, DMF, r.t.; (c) TfOH, DCE, r.t.; (d) (Boc)2O, DMAP, DIPEA, DCM, reflux; (e) NBS, benzoyl peroxide, CCl4, reflux; (f) boronic acid or pinacol ester, Pd(dppf)Cl2, K2CO3, acetone, water, 100°C w/microwave irradiation; (g) secondary amine, K2CO3, DMF, r.t.
Scheme 2. Synthesis of intermediates 4d-e.a.
a Reagents and conditions: (a) 3-bromopropionyl chloride, K2CO3, DCM, r.t.; (b) NaOtBu, DMF, r.t.; (c) TfOH, DCE, r.t.; (d) (Boc)2O, DMAP, DIPEA, DCM, reflux.
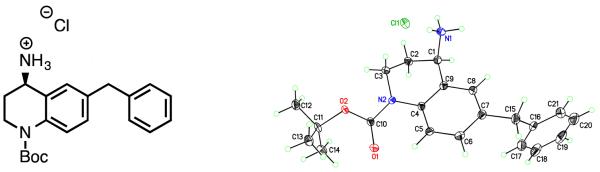
Ketones 4a-o were converted to the corresponding imines with (R)-(+)-2-methyl-2-propanesulfinamide and Ti(OEt)4, and could then be reduced asymmetrically with NaBH4 in situ to give tert-butanesulfinyl-protected amines 9a-o as single diastereomers, as previously described for analogous scaffolds [26,27]. Deprotection with concentrated HCl gave the corresponding primary, enantiomerically pure (R) amines as HCl salts. Stereochemistry of the HCl salt was verified by X-ray crystallography of 6-benzyl-1-(tert-butoxycarbonyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride, which was prepared by an identical synthetic route (Figure 2).
Figure 2.
Crystal structure of (R)-6-benzyl-1-(tert-butoxycarbonyl)-1,2,3,4-tetrahydroquinolin-4-aminium chloride
Boc-protected L-2,6-dimethyltyrosine could then be coupled to the chiral HCl salt, and deprotected with TFA (10a-l, Scheme 3). Analogues were then purified by RP-HPLC to provide enough material for in vitro and in vivo pharmacological evaluation (~5-10 mg). In the case of N-acetylated analogues 10m-o, Boc deprotection of the THQ nitrogen was performed prior to coupling to Boc-L-Dmt. After the amide coupling, the acetyl group was introduced by stirring the crude material in excess pyridine/acetic anhydride (1:1) overnight, followed by a second Boc deprotection and RP-HPLC purification. TFA content of final analogues was estimated by 19F-NMR as described previously, using a fluorinated analogue of 10m (see supporting information) and was found to be approximately 2.5 TFA molecules per final compound [28].
Scheme 3. Final steps in synthesis of 10a-oa.
a Reagents and conditions: (h) (R)-(+)-2-methyl-2-propanesulfinamide, Ti(OEt)4, THF, reflux; (i) NaBH4, THF; (j) conc. HCl, 1,4-dioxane, r.t.; (k) Boc-L-Dmt, PyBOP, DIPEA, HOBt-Cl, DMF, r.t.; (l) TFA, DCM, r.t.; (m) (Ac)2O, pyridine.
In Vitro Assays
Binding affinity (Ki) was measured by the competitive displacement of [3H]-diprenorphine (a non-selective opioid antagonist) in C6 cells stably expressing MOR or DOR, or Chinese Hamster Ovary (CHO) cells stably expressing KOR. In vitro potencies (EC50) and efficacies (as maximal % stimulation) were obtained by agonist-stimulated [35S]-GTPγS binding in the same cell types using previously described protocols [21, 29].
Results and Discussion
In previous reports, we described a mixed efficacy MOR agonist/DOR antagonist opioid peptidomimetic featuring a tetrahydroquinoline (THQ) scaffold and a benzyl pendant at ring position 6 [17,18] (Figure 1a). This compound was shown to be an effective analgesic in the warm water tail withdrawal (WWTW) assay after intraperitoneal administration, with a duration of action slightly shorter than morphine [17]. The initial SAR done on this lead compound was focused on several additional hydrophobic, aromatic substitutions at the 6 position, including 1-methylnaphthyl, 2-methylnaphthyl, 2-methylindanyl, and ethylphenyl. The side chains of these 4 compounds were chosen to mirror modifications made in a peptide series upon which the peptidomimetic scaffold was based [20] and as expected, modifications featuring a more extended pendant (2-methylnaphthyl, 2-methylindanyl, ethylphenyl) were compatible with the larger DOR inactive binding pocket, but not the smaller DOR active pocket, explaining the observed low efficacy at DOR. While these compounds displayed the desired MOR agonist/DOR antagonist efficacy profile, their binding profile was not optimal. Affinity for MOR for all 4 compounds was at least an order of magnitude higher than affinity for DOR, and the 2-methylnaphthyl compound showed over 2 orders of magnitude preference for MOR. Ligands with more balanced binding affinities at MOR and DOR would provide a better starting point for further development of this type of mixed efficacy opioid ligand [9,30]. Additionally, although we showed that an extended hydrophobic pendant translates to low DOR efficacy, changes in the electronic characteristics and polarity of the pendant were left unexplored.
To begin our expanded SAR, we first replaced the phenyl pendant of our lead compound (Fig. 1a) with a 3-pyridine (10a, Table 1). We observed not only a slight loss in binding affinity at both MOR and DOR (Table 1), but a significant loss in MOR efficacy and potency (Table 2). Although 10a adopts a similar conformation in the MOR active site to our lead compound, this loss in MOR binding and efficacy can be attributed to loss of hydrophobic contacts in this region of the receptor binding pocket (see Figure 3). Although this analogue did not improve upon the MOR agonist/DOR antagonist profile of our previous compounds, we were intrigued by the drastic consequences that a simple change in pendant electronics had on both binding and efficacy, and wished to explore this further. Compared to 10a and our lead compound, replacement with piperidine in analogue 10b widened the binding affinity preference for MOR over DOR even further, although this compound behaved as a moderately potent, full agonist at MOR, improving upon the MOR efficacy profile of 10a. Expansion of the piperidine ring in 10b to azepane (10c) resulted in improved binding at DOR and KOR. In contrast, morpholine analogue 10d displayed diminished binding affinities at DOR and KOR, and also decreased potency at MOR as compared to 10b. We next turned our attention to smaller aromatic systems, including a 1,2,4-triazole substitution (10e) and a 3-furan (10f). While the overall binding profile of 10f was comparable to the previous substitutions, 10e displayed a marked loss in binding affinity for MOR and KOR, and displayed no efficacy at MOR.
Table 2.
Opioid receptor efficacy for analogues 10a-o.a
| EC50 (nM) | % stimulation | |||||
|---|---|---|---|---|---|---|
| Compound | MOR | DOR | KOR | MOR | DOR | KOR |
| (Fig 1a) | 1.6±0.3b | 110±6b | 540±72b | 81±2b | 16±2b | 22±2b |
| 10a | 93±20 | dns | dns | 37±7 | dns | dns |
| 10b | 9±1 | dns | dns | 73±8 | dns | dns |
| 10c | 25±11 | dns | dns | 52±2 | dns | dns |
| 10d | 60±2 | dns | dns | 82±2 | dns | dns |
| 10e | dns | dns | dns | dns | dns | dns |
| 10f | 72±24 | dns | > 1000 | 18±2 | dns | > 40 |
| 10g | 23±13 | dns | dns | 34±6 | dns | dns |
| 10h | 2.2±0.9 | dns | dns | 84±6 | dns | dns |
| 10i | 14±3 | dns | dns | 36±3 | dns | dns |
| 10j | 3±1 | dns | 15±9 | 96±4 | dns | 14±2 |
| 10k | 0.4±0.1 | dns | 90±65 | 105±6 | dns | 25±4 |
| 10l | 2.0±0.5 | dns | 600±400 | 56±2 | dns | 14±1 |
| 10m | 6±2 | dns | 160±36 | 91±8 | dns | 46±5 |
| 10n | 0.9±0.4 | dns | 400±130 | 118±5 | dns | 32±1 |
| 10o | 40±20 | dns | > 2000 | 72±3 | dns | > 20 |
Efficacy data were obtained using agonist induced stimulation of [35S]GTPγS binding in membrane preparations expressing either MOR, DOR or KOR. Potency is represented as EC50 (nM) and efficacy as percent maximal stimulation relative to standard agonist DAMGO (MOR), DPDPE (DOR), or U69,593 (KOR) at 10 μM. All values are expressed as the mean ± SEM of three separate assays performed in duplicate. dns: does not stimulate.
Data from Ref. 17
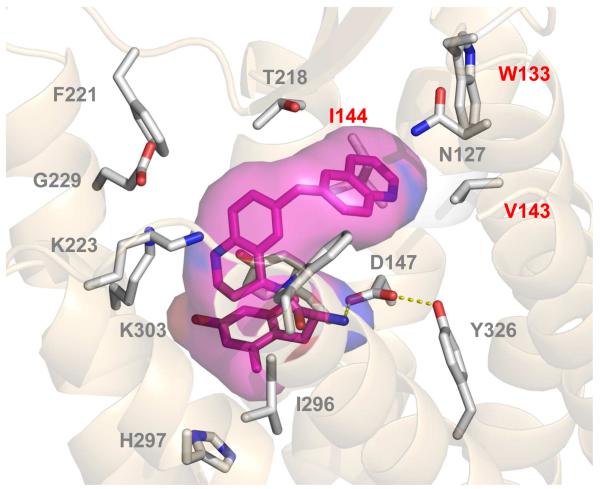
Figure 3. Docking of 10g in the MOR active site.a.
a. Key hydrophobic contacts are highlighted in red (I144, V143, W133).
In the initial series [17], the 2-methylnaphthyl modification resulted in the highest MOR efficacy, but the MOR/DOR binding balance favored MOR by over 2 orders of magnitude. To see if changes in electronics to the naphthyl system could improve DOR binding while maintaining low DOR efficacy, we first synthesized quinoline analogue 10g. Interestingly, the binding affinity of 6-quinoline analogue 10g at all three receptors was considerably lower than the previous bicyclic analogues. This finding suggests that both an extended pendant and pendant electronic characteristics are important for maintaining binding for this series at DOR. Using our previously published models of interactions of opioid ligands with the active states of the three receptors [20, 21], 10g was docked into the MOR active binding pocket. The quinoline nitrogen of 10g was found to extend much deeper into the hydrophobic pocket of the MOR active pocket, disrupting important contacts with hydrophobic residues W133, V143, and I144 as shown in Figure 3.
These initial data suggested that superior MOR efficacy (and low DOR efficacy) may result from a fused ring pendant in which the six-membered, non-heteroatom containing aromatic moiety is located in the most distal position from the THQ core. To test this hypothesis, analogues 10h-l were synthesized. 10h showed high efficacy at MOR, and improved DOR binding approximately tenfold as compared to the lead compound and 10g. 10j and 10k both behaved as potent, full MOR agonists that improved upon the efficacy of our original lead, with no efficacy at DOR. On the other hand, MOR efficacy is reduced in the case of 3,4-(methylenedioxy)phenyl analogue 10i. This is again consistent with the observation that distal electronegative substitutions adversely affect MOR efficacy. Reduction of the aromatic ring of 10k to give decahydroisoquinoline analogue 10l maintained a comparable, if slightly inferior in vitro profile as compared to 10k. While 10j and 10k showed potent stimulation at MOR (while exhibiting no efficacy at DOR), we still wished to improve the binding affinities of each at DOR. We reasoned that the THQ aniline was synthetically accessible and amenable to substitutions, and would be the next logical site for diversification. Preliminary studies in related analogues suggested that N-acetylation at the THQ core improved DOR affinity without increasing DOR efficacy (manuscript in preparation), so we likewise explored the effect of an acetyl substituent here, giving final analogues 10m-o. We were pleased to see that this modification not only improved DOR binding relative to the un-acetylated counterpart compounds (10j-l) but also that 10m showed similarly high affinity for MOR and DOR, and interestingly for KOR as well. As expected from its high binding affinity and lack of stimulation of [35S]GTPγS binding, 10m acted as an antagonist of DPDPE. 10m afforded a 7.8-fold rightward shift in the agonist concentration-response curve for DPDPE, giving an antagonist affinity constant (Ke) for 10m of 4.6 nM.
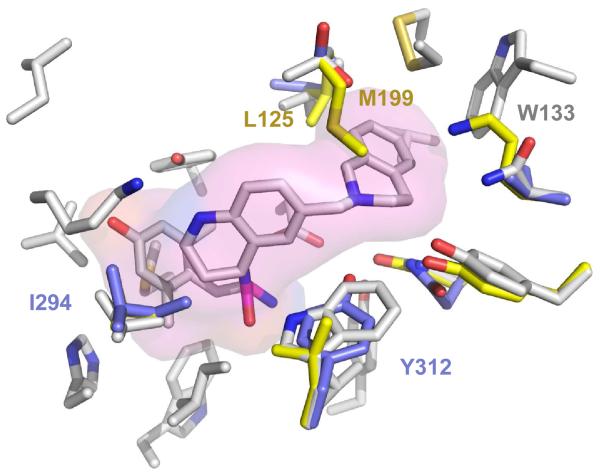
An overlay of 10k docked into the active site of all three receptors is shown in Figure 4. The compound fits nicely into the MOR active site, but clashes with M199 and L125 in the DOR active site. It is interesting to note that 10k and 10m, both featuring the 1,2,3,4-tetrahydroisoquinoline (THIQ) pendant, behave as partial KOR agonists. As shown in Figure 4, 10k fits in the KOR active site, but clashes slightly with I294 (and thus displays lower efficacy as compared to MOR). Additionally, the THIQ nitrogen of 10k is positioned to make a polar contact with Y312, a residue unique to the KOR binding pocket at this position, which may account for the high affinity of 10k and 10m for KOR. The MOR agonist/KOR agonist mixed efficacy profile has shown promise as a treatment for drug dependence, specifically cocaine addiction [31-33], and additional SAR on MOR/KOR agonist peptides has recently been reported [34]. Further substitutions on the THIQ pendant will have to be explored to fully optimize this profile, particularly for the purpose of improved potency at KOR.
Figure 4. Overlay of 10k in the MOR, DOR and KOR active site.a.
a. Grey residues = MOR, Yellow = DOR, Purple = KOR.
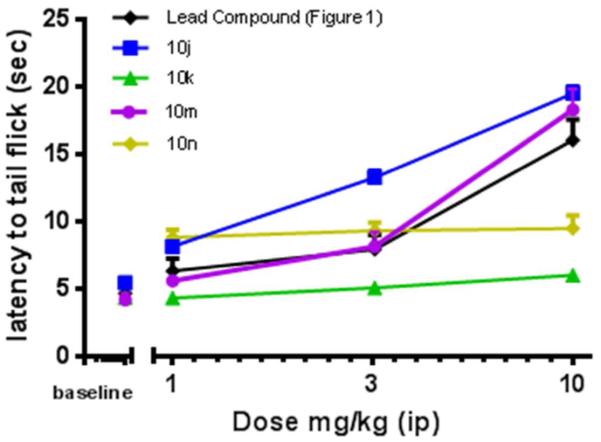
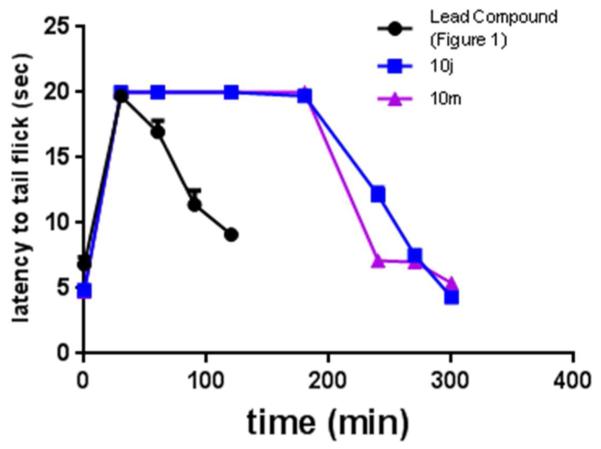
On the basis of their favorable in vitro profiles, compounds 10j, 10k, 10m, and 10n were chosen for in vivo studies. Effects of 10j, 10k, 10m, and 10n were compared with the lead compound by two-way ANOVA with Tukey’s multiple comparisons post hoc test. There was a significant interaction (F(12,76)=8.7, p< 0.0001) as well as significant main effects of dose (F(3,76)=82.7, p<0.0001) and compound (F(4,76)=24.6, p<0.0001). In the mouse warm water tail withdrawal (WWTW) assay (Figure 5), our benzyl pendant lead compound and compounds 10j and 10m were fully efficacious and produced dose-dependent increases in latency to tail flick, with 3.2 (at least p<0.05) and 10 mg/kg (p<0.001) significantly increasing latency times as compared with baseline. 10m was not statistically different from the lead compound, but 10j produced slight higher tail flick latencies at 3.2 (p<0.001) and 10 (p<0.05) mg/kg as compared with the lead compound. It is interesting to note that 10k, which lacks only the N-acetyl group of 10m, and 10n, which is the N-acetylated counterpart to 10j, did not significantly increase tail flick latency above baseline levels up to a dose of 10 mg/kg. To determine the duration of action of compounds 10j and 10m, tail withdrawal latencies were measured at intervals following the administration of the cumulative 10 mg/kg dose (Figure 6). Compounds 10j and 10m showed a full antinociceptive response for 200 minutes before returning to baseline. Compared with the lead compound (Figure 1a), these compounds both displayed a much longer duration of action after intraperitoneal injection.
Figure 5. Dose response curve for compounds 10j,k,m,n.a.
a Cumulative antinociceptive dose response curves in the mouse WWTW assay for 10j,k,m,n after intraperitoneal administration (n = 3-6) plotted as average ± SEM.
Figure 6. Time course for compounds 10j and 10m.a.
a. Time course of antinociceptive response in the mouse WWTW assay after a cumulative dose of 10 mg/kg ip administration over time for 10j and 10m. Plotted as average ± SEM.
In an effort to explain the unpredictable in vivo results for these structurally similar analogues, compounds 10j, 10k, and 10m were screened to determine their plasma stability, and were all found to be completely stable after 30 minutes at 37 °C (99.6, 100.3, and 95.3 mean % remaining respectively after 30 minutes as compared to positive control Eucatropine, 43.2%). There also appears to be no correlation between the predicted clogP values (Table 1) of these analogues with the observed in vivo data. While the explanation of the disparate in vivo results is unclear, differences in first pass metabolism after intraperitoneal administration [35] may be a contributing factor. To this end, additional synthetic and pharmacokinetic studies will need to be performed to shed light on how these subtle structural differences can have such a profound effect on the bioavailability of this scaffold.
Conclusions
We have described a series of opioid peptidomimetics with chemically diverse substitutions at the 6 position of the THQ scaffold, and have shown that changes in both pendant sterics and electronic characteristics can have profound impacts on receptor selectivity for both binding and efficacy. Intermediate 3, which is relatively simple to synthesize and can be done so on a multi-gram scale, represents a valuable building block for the expedient synthesis of a wide range of opioid small molecules, and makes possible the incorporation of diverse and readily available side chains (either through Suzuki coupling or SN2 substitution) that have not yet been explored in traditional opioid ligands. Compounds 10j and 10m also display promise in vivo, with efficacy and duration of action comparable to morphine, and improve upon the duration of action of our original lead.
Methods
General Synthetic Methods
All reagents and solvents were obtained from commercial sources and used without additional purification. Reactions were carried out in anhydrous solvents under an inert atmosphere unless otherwise specified. Suzuki couplings were performed on a Discover S-class (CEM) microwave in a closed vessel with maximum power input of 300 W. Flash column chromatography was carried out using P60 silica gel (230–400 mesh). Purification of final compounds was performed using a Waters semipreparative HPLC with a Vydac protein and peptide C18 reverse phase column, using a linear gradient of 10% solvent B (0.1% TFA in acetonitrile) in solvent A (0.1% TFA in water) to 60% solvent B in solvent A at a rate of 1% per minute. UV absorbance was monitored at 230 nm. Purity of synthesized compounds was determined on a Waters Alliance 2690 analytical HPLC instrument and a Vydac protein and peptide C18 reverse phase column, using a linear gradient of 0% solvent B in solvent A to 45% solvent B in solvent A in 45 min, measuring UV absorbance at 230 nm. Purities of the final compounds used for testing were ≥95% as determined by HPLC. 1H-NMR,13C-NMR, and 19F-NMR data were obtained on either a 400 or 500 MHz Varian instrument. In chloroform-d, shifts are referenced to TMS. If TMS peak was not visible in 13C-NMR spectra, shifts were referenced to the solvent peak (δ 77.16). Samples in CD3OD are unreferenced. Mass spec analysis was performed using an Agilent 6130 LC–MS mass spectrometer in positive mode.
In Vitro Assays
Binding affinity (Ki) was measured by the competitive displacement of [3H]-diprenorphine (a non-selective opioid antagonist) in C6 cells stably expressing MOR or DOR, or Chinese Hamster Ovary (CHO) cells stably expressing KOR. In vitro potencies (EC50) and efficacies (as maximal % stimulation) were obtained by agonist-stimulated [35S]-GTPγS binding in the same cell types using previously described protocols [21, 29, 36]. To determine the DOR antagonist activity of 10m a concentration response curve for DPDPE was obtained using the [35S]GTPγS binding assay in C6 cells expressing DOR in the presence or absence of 30 nM compound 10m, as previously described [21]. The ratio of potency (EC50) values of DPDPE in the presence and absence of 10m was determined to provide the dose-ratio. The antagonist affinity constant (Ke) for 10m was calculated using the equation Ke=[10m]/ DR-1. All concentration response curves were analyzed using GraphPad Prism (La Jolla, CA).
Animals
Adult male C57BL/6 mice, purchased from Harlan Laboratories (IN, USA) and weighing between 20-30g at 8-16 weeks old, were used for the described experiments. Mice were group-housed and had free access to food and water at all times. Experiments were conducted in the housing room, which was maintained on a 12h light/dark cycle (with lights on at 0700). Each mouse was used only once and experiments were conducted between 9 am and 5 pm. Studies were performed in accordance with the University of Michigan Committee on the Use and Care of Animals and the Guide for the Care and Use of Laboratory Animals [37].
Antinociception
All compounds were dissolved in sterile saline and administered by intraperitoneal (i.p.) injection in a volume of 10 mL/kg of body weight. Antinociceptive effects were evaluated in the warm water tail withdrawal (WWTW) assay. Tail withdrawal latencies were determined by briefly placing a mouse into a plastic, cylindrical restrainer and putting 2-3 cm of the tail tip into a water bath maintained at 50°C. The latency to tail withdrawal or rapidly flicking the tail back and forth was recorded with a maximum cutoff time of 20 sec. If the mouse did not remove its tail by the cutoff time, the experimenter removed its tail from the water to prevent tissue damage.
Acute antinociceptive effects were determined using a cumulative dosing procedure. Each animal received an injection of saline ip and then 30 min later, baseline withdrawal latencies (3-6 sec) were recorded. Following baseline determinations, increasing cumulative doses of the test compound were given ip at 30 min intervals. Thirty min after each injection, the tail withdrawal latency was measured as described above.
Plasma Stability
Plasma stability was assessed by Quintara Discovery (San Francisco, CA) Mouse plasma (K2 EDTA) was obtained from BioreclamationIVT. The assay was carried out in 96-well microtiter plates. Compounds were diluted into 200 uM in DMSO, then spiked into the plasma. After mixing, samples were immediately aliquoted into three 96-well plates. The time 0 plate was quenched immediately. The other two plates were incubated at 37°C. Reaction mixtures (20 μL) contained a final concentration of 1 μM test compound. The extent of metabolism was calculated as the disappearance of the test compound, compared to the 0-min control reaction incubations. Eucatropine was included as a positive control to verify assay performance.
At each of the time points, 150 μL of quench solution (100% acetonitrile with 0.1% formic acid) with internal standard was transferred to each well. Plates were sealed, vortexed, and centrifuged at 4°C for 15 minutes at 4000 rpm. The supernatant was transferred to fresh plates for LC/MS/MS analysis.
All samples were analyzed on LC/MS/MS using an AB Sciex API 4000 instrument, coupled to a Shimadzu LC-20AD LC Pump system. Analytical samples were separated using a Waters Atlantis T3 dC18 reverse phase HPLC column (10 mm × 2.1 mm) at a flow rate of 0.5 mL/min. The mobile phase consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in 100% acetonitrile (solvent B).
Supplementary Material
Acknowledgements
We are grateful to Dr. Irina Pogozheva for assistance with the ligand-receptor docking. Tyler Trask, Evan Schramm, Aaron Chadderdon and Chao Gao are thanked for additional in vitro studies. Dylan Kahl is also thanked for additional synthetic contributions, as is Brittany Van Koevering for in vivo contributions.
Funding
This study was supported by NIH grant DA003910 (H.I.M, E.M.J and J.R.T). JPA was supported by the Substance Abuse Interdisciplinary Training Program administered by NIDA: T32 DA007267 and N.W.G. by the Pharmacological Sciences Training Program administered by NIGMS: T32 GM007767
Footnotes
Author Contributions
Synthesis was carried out by A.M.B. Research project was designed by A.M.B, H.I.M and J.R.T. In vitro assays were carried out by N.W.G. In vivo studies were carried out by J.P.A and designed by J.P.A and E.M.J.
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information Available
Experimental procedures, 1H and 13C-NMR data, MS(EI) and HPLC data, X-ray crystallography data, and copies of NMR spectra are available free of charge at http://pubs.acs.org.
References
- 1.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid Complications and Side Effects. Pain Physician. 2008;11:S105–120. [PubMed] [Google Scholar]
- 2.Ananthan S. Opioid Ligands With Mixed μ/δ Opioid Receptor Interactions: An Emerging Approach to Novel Analgesics. The AAPS Journal. 2006;8:119–125. doi: 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller PW. Bi- or Multifunctional Opioid Peptide Drugs. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hepburn MJ, Little PJ, Gingras J, Kuhn CM. Differential Effects of Naltrindole on Morphine-Induced Tolerance and Physical Dependence in Rats. J. Pharm. Exp. Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- 5.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective Blockage of Delta Opioid Receptors Prevents the Development of Morphine Tolerance and Dependence in Mice. J. Pharm. Exp. Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 6.Kest B, Lee CE, McLemore GL, Inturrisi CE. An Antisense Oligodoxynucleotide to the Delta Opioid Receptor (DOR-1) Inhibits Morphine Tolerance and Acute Dependence in Mice. Brain Res. Bull. 1996;39:185–188. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, King MA, Schuller AGP, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of Supraspinal Delta-like Analgesia and Loss of Morphine Tolerance in δ Opioid Receptor Knockout Mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 8.Li T, Shiotani K, Miyazaki A, Tsuda Y, Ambo A, Sasaki Y, Jinsmaa Y, Marczak E, Bryant SD, Lazarus LH, Okada Y. Bifunctional [2′,6′-Dimethyl-L-tyrosine1]endomorphin-2 Analogues Substituted at Position 3 with Alkylated Phenylalanine Derivatives Yield Potent Mixed μ-Agonist/δ-Antagonist and Dual μ-Agonist/δ -Agonist Opioid Ligands. J. Med. Chem. 2007;50:2753–2766. doi: 10.1021/jm061238m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TMD, Lemieux C, Chung NN, Coderre TJ. The Opioid μ Agonist/δ Antagonist DIPP-NH2[ψ] Produces a Potent Analgesic Effect, No Physical Dependence, and Less Tolerance than Morphine in Rats. J. Med. Chem. 1999;42:3520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- 10.Balboni G, Salvadoria S, Trapella C, Knapp BI, Bidlack JM, Lazarus L,H, Peng X, Neumeyer JL. Evolution of the Bifunctional Lead μ Agonist/δ Antagonist Containing the 2′,6′-Dimethyl-L-tyrosine-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic Acid (Dmt-Tic) Opioid Pharmacophore. ACS Chem. Neurosci. 2010;1:155–164. doi: 10.1021/cn900025j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananthan S, Saini SK, Dersch CM, Xu H, McGlinchey N, Giuvelis D, Bilsky EJ, Rothman RB. 14-Alkoxy- and 14-Acyloxypyridomorphinans: μ Agonist/δAntagonist Opioid Analgesics with Diminished Tolerance and Dependence Side Effects. J. Med. Chem. 2012;55:8350–8363. doi: 10.1021/jm300686p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender AM, Clark MJ, Agius MP, Traynor JR, Mosberg HI. Synthesis and Evaluation of 4-Substituted Piperidines and Piperazines as Balanced Affinity μ Opioid Receptor (MOR) Agonist/δ Opioid Receptor (DOR) Antagonist Ligands. Bioorg. Med. Chem. Lett. 2014;24:548–551. doi: 10.1016/j.bmcl.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels DJ, Lenard NR, Etienne CL, Law P, Roerig SC, Portoghese PS. Opioid-Induced Tolerance and Dependence in Mice is Modulated by the Distance Between Pharmacophores in a Bivalent Ligand Series. Proc. Nat. Acad. Sci. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes I, Fujita W, Gupta A, Saldanha SA, Negri A, Pinello CE, Eberhart C, Roberts E, Filizola M, Hodder P, Devi LA. Identification of a μ-δ Opioid Receptor Heteromer-Biased Agonist With Antinociceptive Activity. Proc. Nat. Acad. Sci. 2013;110:12072–12077. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breslin HJ, Diamond CJ, Kavash RW, Cai C, Dyatkin AB, Miskowski TA, Zhang S, Wade PR, Hornby PJ, He W. Identification of a Dual δ OR Antagonist/μ OR Agonist as a Potential Therapeutic for Diarrhea-Predominant Irritable Bowel Syntrome (IBS-d) Bioorg. Med. Chem. Lett. 2012;22:4869–4872. doi: 10.1016/j.bmcl.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, Jutkiewicz EM. Development of a Bioavailable μ Opioid Receptor (MOPr) Agonist, δ Opioid Receptor (DOPr) Antagonist Peptide That Evokes Antinociception without Development of Acute Tolerance. J. Med. Chem. 2014;57:3148–3153. doi: 10.1021/jm5002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosberg HI, Yeomans L, Harland AA, Bender AM, Sobczyk-Kojiro K, Anand JP, Clark MJ, Jutkiewicz EM, Traynor JR. Opioid Peptidomimetics: Leads for the Design of Bioavailable Mixed Efficacy μ Opioid Receptor (MOR) Agonist/δ Opioid Receptor (DOR) Antagonist Ligands. J. Med. Chem. 2013;56:2139–2149. doi: 10.1021/jm400050y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, McFadyen IJ, Traynor J,R, Mosberg HI. Design of a High Affinity Peptidomimetic Opioid Agonist from Peptide Pharmacophore Modles. Bioorg. Med. Chem. Lett. 1998;8:2685–2688. doi: 10.1016/s0960-894x(98)00472-7. [DOI] [PubMed] [Google Scholar]
- 19.Healy JR, Bezawada P, Shim J, Jones JW, Kane MA, MacKerell AD, Jr., Coop A, Matsumoto RR. Synthesis, Modeling and Pharmacological Evaluation of UMB425, a Mixed μ Agonist/δ Antagonist Opioid Analgesic with Reduced Tolerance Liabilities. ACS Chem. Neurosci. 2013;4:1256–1266. doi: 10.1021/cn4000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anand JP, Purington LC, Pogozheva ID, Traynor JR, Mosberg HI. Modulation of Opioid Receptor Ligand Affinity and Efficacy Using Active and Inactive State Receptor Models. Chem. Biol. Drug Des. 2012;80:763–770. doi: 10.1111/cbdd.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purington LC, Sobczyk-Kojiro K, Pogozheva ID, Traynor JR, Mosberg HI. Development and In Vitro Characterization of a Novel Bifunctional μ-Agonist/δ-Antagonist Opioid Tetrapeptide. ACS Chem. Bio. 2011;6:1375–1381. doi: 10.1021/cb200263q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankovic Z. CNS Drug Design: Balancing Physiochemical Properties for Optimal Brain Exposure. J. Med. Chem. 2015;58:2584–2608. doi: 10.1021/jm501535r. [DOI] [PubMed] [Google Scholar]
- 23.Anderson KW, Tepe JT. Trifluoromethanesulfonic Acid Catalyzed Friedel-Crafts Acylation of Aromatics with β-Lactams. Tetrahedron. 2002;58:8475–8481. [Google Scholar]
- 24.Schmidt RG, Bayburt EK, Latshaw SP, Koenig JR, Daanen JF, McDonald HA, Bianchi BR, Zhong C, Joshi S, Honore P, Marsh KC, Lee C, Faltynek CR, Gomtsyan A. Chroman and Tetrahydroquinoline Ureas as Potent TRPV1 Antagonists. Bioorg. Med. Chem. Lett. 2011;21:1338–1341. doi: 10.1016/j.bmcl.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 25.Li SW, Nair MG, Edwards DM, Kisliuk RL, Gaumont Y, Dev IK, Duch DS, Humphreys J, Smith GK, Ferone R. Folate Analogues. 35. Synthesis and Biological Evaluation of 1-Deaza, 3-Deaza, and Bridge-Elongated Analogues of N10-Propargyl-5,8-dideazafolic Acid. J. Med. Chem. 1991;34:2746–2754. doi: 10.1021/jm00113a011. [DOI] [PubMed] [Google Scholar]
- 26.Tanuwidjaja J, Peltier HM, Ellman JA. One-Pot Asymmetric Synthesis of Either Diastereomer of tert-Butanesulfinyl-protected Amines from Ketones. J. Org. Chem. 2007;72:626–629. doi: 10.1021/jo0616512. [DOI] [PubMed] [Google Scholar]
- 27.Colyer JT, Anderson NG, Tedrow JS, Soukup TS, Faul MM. Reversal of Diastereofacial Selectivity in Hydride Reductions of N-tert-Butanesulfinyl Imines. J. Org. Chem. 2006;71:6859–6862. doi: 10.1021/jo0609834. [DOI] [PubMed] [Google Scholar]
- 28.Little MJ, Aubry N, Beaudoin M, Goudreau N, LaPlante SR. Quantifying Trifluoroacetic Acid as a Counterion in Drug Discovery by 19F NMR and Capillary Electrophoresis. J. Pharm. Biomed. Anal. 2007;43:1324–1330. doi: 10.1016/j.jpba.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 29.Harrison C, Traynor JR. The [35S]GTPγS Binding Assay: Approaches and Applications in Pharmacology. Life Sci. 2003;74:489–508. doi: 10.1016/j.lfs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham DJ, Lambert DG. Simultaneous Targeting of Multiple Opioid Receptors: A Strategy To Improve Side-Effect Profile. Br. J. Anaesth. 2009;103:38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- 31.Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. Effects of Mixed-Action kappa/mu Opioids on Cocaine Self-Administration and Cocaine Discrimination by Rhesus Monkeys. Neuropsychopharmacology. 2003;28:1125–1139. doi: 10.1038/sj.npp.1300105. [DOI] [PubMed] [Google Scholar]
- 32.Mello NK, Negus SS. Interactions Between Kappa Opioid Agonists and Cocaine. Preclinical Studies. Ann. N.Y. Acad. Sci. 2000;909:104. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 33.Bidlack JM. Mixed Kappa/Mu Partial Opioid Agonists as Potential Treatments for Cocaine Dependence. Advances in Pharmacology. 2014;69:387–418. doi: 10.1016/B978-0-12-420118-7.00010-X. [DOI] [PubMed] [Google Scholar]
- 34.Bai L, Li Z, Chen J, Chung NN, Wilkes BC, Li T, Schiller PW. Dmt1]DALDA Analogues with Enhanced μ Opioid Agonist Potency and With a Mixed μ/κ Opioid Activity Profile. Bioorg. Med. Chem. 2014;22:2333–2338. doi: 10.1016/j.bmc.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukas G, Brindle SD, Greengard P. The Route of Absorption of Intraperitoneally Administered Compounds. J. Pharm. Exp. Ther. 1971;178:562–566. [PubMed] [Google Scholar]
- 36.Traynor JR, Nahorski SR. Modulation by mu opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1995;47:848–854. doi: 10.1016/S0026-895X(25)08634-1. [DOI] [PubMed] [Google Scholar]
- 37.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th edition. National Academies Press (US); Washington (DC): 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK54050/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.