Abstract
Otto Warburg discovered that cancer cells exhibit a high rate of glycolysis in the presence of ample oxygen, a process termed aerobic glycolysis, in 1924 (Warburg et al., 1924). Since then we have significantly advanced our understanding of cancers’ fuel choice to meet their demands for energy and for the production of biosynthetic precursors. In this review, we will discuss the preferred nutrients of cancer cells and how they are utilized to satisfy their bioenergetic and biosynthetic needs. In addition, we will describe how cell intrinsic and extrinsic factors such as oncogene mutations, nutrient and oxygen availability and other microenvironmental factors influence fuel choice.
Introduction
The process of cellular proliferation requires the synthesis of new DNA, RNA, cellular membranes and protein (Vander Heiden et al., 2009). For this reason, rapidly proliferating cells, such as cancer cells, have increased demands for biosynthetic precursors for the generation of these macromolecules. In this section, we will discuss the fuels that are used to meet these demands and how they are used (Figure 1).
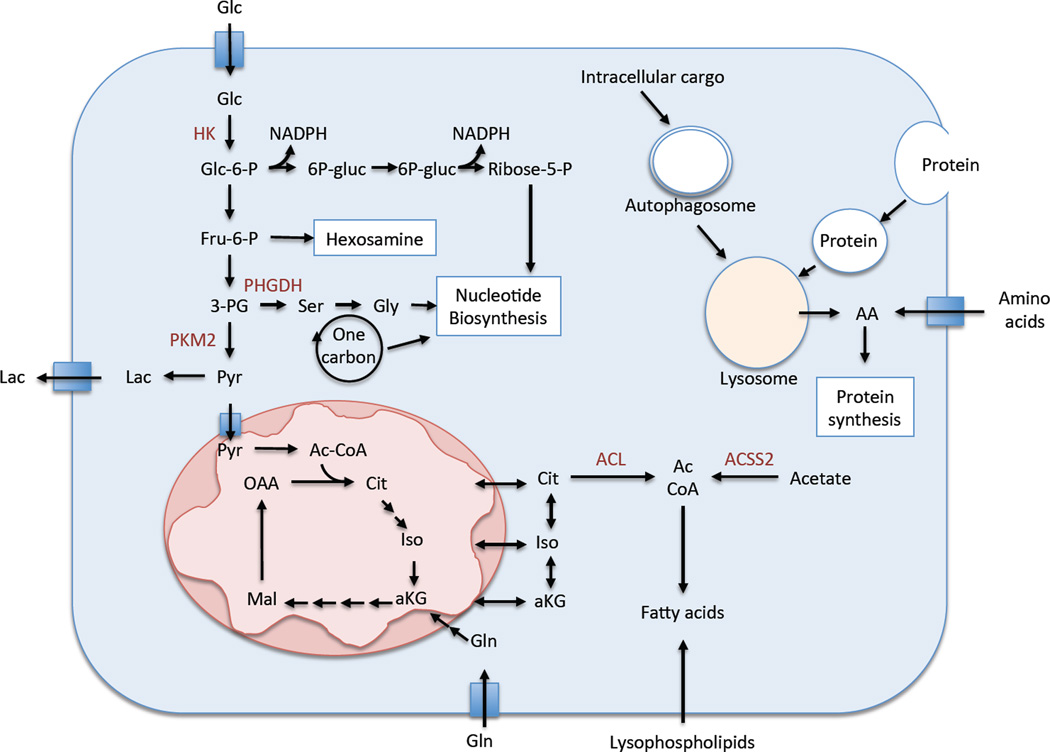
Figure 1.
Cancer’s fuel choice. Cancer cells can take up glucose, glutamine, amino acids, lysophospholipids, acetate, and extracellular protein and use these fuels to supply their pools of macromolecular precursors for cellular proliferation.
Glucose
Highly proliferating cells have a high demand for glucose and increased glycolytic activity compared to cells with a low rate of proliferation (Vander Heiden et al., 2009). Glucose is imported into cells via glucose transporters and phosphorylated by hexokinase to glucose-6-phosphate. This phosphorylation achieves two objectives: it traps glucose inside the cell and facilitates the entry of glucose into various pathways to provide energy for the cell as well as carbon atoms needed for biosynthetic processes. Most glucose enters glycolysis where it is metabolized to pyruvate, while a significant fraction is funneled into pathways for ribose synthesis, serine and glycine synthesis, phospho-glycerol synthesis and protein glycosylation. The pentose phosphate pathway supplies both NADPH, which is critical for defense against reactive oxygen species and for biosynthesis reactions, and ribose-5-phosphate, which forms the sugar base for nucleotide production for DNA and RNA synthesis. Ribose-5-phosphate can also be generated from glucose utilizing the transaldolase/transketolase pathway in an NADPH-independent manner. The hexosamine-phosphate pathway is particularly important for glycosylation of proteins that are secreted or placed on the surface of cancer cells. However, in most cancers, the majority of glucose is converted to pyruvate, the majority of which is converted to lactate by lactate dehydrogenase. This final step allows the NADH produced by glycolysis at the step of GAPDH to be converted back to NAD+, allowing glycolysis to proceed at a high rate. Although pyruvate can be converted to alanine by transaminases in the cytosol, most of the pyruvate that is not converted to lactate enters the TCA cycle for the generation of ATP and additional biosynthetic intermediates, including acetyl-CoA for fatty acid biosynthesis (discussed below). Thus, increased glycolytic flux is critical for more than just ATP production, as it supports many biosynthesis pathways for cellular proliferation.
Amino acids
Amino acids are divided into two groups: essential amino acids that cannot be synthesized de novo, and non-essential ones. Essential amino acids are supplied by dietary sources and include phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine and histidine. Non-essential amino acids are synthesized in the body and include arginine, cysteine, glycine, glutamine, proline, tyrosine, alanine, aspartic acid, asparagine, glutamate and serine. However, not all tissues are capable of synthesizing these amino acids and those that don’t rely on the blood supply for their delivery.
All 20 amino acids are used for protein synthesis, but some have additional biosynthetic roles to support cancer cell proliferation. Many types of cancer cells use glutamine as a major source of TCA cycle anapleurosis (Hensley et al., 2013). Glutamine is metabolized to glutamate via glutaminase and subsequently to the TCA cycle intermediate α-ketoglutarate by dehydrogenase or transaminase enzymes. Glutamine and glutamate are important nitrogen donors for the production of serine, alanine, aspartate, asparagine, proline and arginine. A recent study demonstrated the importance of glutamine-derived asparagine, as asparagine was sufficient to prevent glutamine withdrawal-induced apoptosis without restoring other TCA cycle intermediates or non-essential amino acids. Asparagine mediated survival by suppressing the induction of the stress-responsive protein CHOP without affecting the induction of the amino acid responsive transcription factor ATF4 (Zhang et al., 2014) These results demonstrate that asparagine is a key determinant of cellular adaptation to amino acid starvation. Glutamine also supports lipid generation via the TCA cycle intermediate citrate, and nucleotide biosynthesis. Glutamate plays an important role in amino acid exchange, such as through the glutamate/cystine exchanger xCT, which is essential for the uptake of cystine for glutathione synthesis. However, it is important to note that, unlike tumor cells in culture, tumors in vivo do not always demonstrate increased glutamine metabolism compared to normal tissue (Sellers et al., 2015).
The amino acids serine and glycine can be imported from the extracellular environment or synthesized de novo (Locasale, 2013). De novo synthesis occurs via metabolism of the glycolytic intermediate 3PG to serine. De novo serine synthesis is enhanced in some cancers due to the overexpression of the first enzyme in the serine biosynthesis pathway, PHGDH (Locasale et al., 2011; Possemato et al., 2011). Serine is an important precursor for many cellular metabolites including nucleotides, glutathione, cysteine, lipids, polyamines, methyl donors, and others. Serine metabolism to glycine occurs in the folate cycle, where serine donates the carbon atom frxom its side chain to folate, converting both serine to glycine and tetrahydrofolate (THF) to methyl-THF. The folate cycle supports the production of many macromolecular precursors, including methionine, thymidine and purine nucleotides, the methyl donor s-adenosylmethionine, and choline for lipid synthesis. The folate cycle also interacts with the transsulfuration cycle, which supports the production of cysteine from serine. Cysteine, together with glycine, is a critical amino acid for the synthesis of the antioxidant glutathione.
Protein
Membrane transporters that facilitate the active import of amino acids from the extracellular space supply much of the cellular amino acid pool. When extracellular concentrations are low, cells can turn to alternative fuel sources to meet their amino acid requirements. Pancreatic ductal adenocarcinoma (PDAC) demonstrates a hypovascular state relative to normal pancreas and other tumor types (Olive et al., 2009). As the blood supply delivers amino acids in addition to oxygen and glucose, the nutrient environment of PDAC is very limiting, Indeed, metabolomic comparisons of human PDAC and benign adjacent tissue revealed that bulk tumor tissue was low in nutrients such as glucose and the amino acids glutamine and serine (Kamphorst et al., 2015). Extracellular protein has been identified as an alternative supply source for cellular amino acids. Bar Sagi and colleagues demonstrated that oncogenic KRAS, which almost universally drives PDAC formation (Downward, 2003), induces the uptake of extracellular protein from the microenvironment in a process known as macropinocytosis (Commisso et al., 2013). Imported protein is subsequently degraded by the lysosome to supply intracellular amino acid pools. Macropinocytosis of albumin was found to support the growth of pancreatic cancer cells cultured under glutamine-depleted conditions. This study demonstrates that cells can turn to extracellular protein as a source of amino acids under nutrient limiting conditions. Although macropinocytosis of protein occurs in vivo in mouse pancreatic tumors (Commisso et al., 2013), the contribution of this process to tumor amino acid pools has not been established.
Interestingly, Wolpin and colleagues identified that elevated branch-chain amino acids (BCAAs) in human serum was a predictor for the future development of PDAC (Mayers et al., 2014). BCAAs are used primarily for protein synthesis; however, conversion of BCAAs to acetyl-coA can lead to their oxidation for energy production following their entry into the TCA cycle. PDAC mouse models also demonstrated an increase of BCAAs in the serum prior to tumor onset, which were derived from fast-twitch muscle and released as a consequence of protein breakdown (Mayers et al., 2014). PDAC is characterized by a musclewasting syndrome known as cachexia. These findings suggest that protein breakdown begins much earlier than previously thought and occurs prior to the onset of clinical cachexia, although the precise mechanisms regulating muscle breakdown remain to be determined. It is tempting to speculate that the demand for anabolic amino acids like glutamine and serine in cancer exceeds the demand for BCAAs, leading to BCAA accumulation in the serum following protein breakdown. Thus, pancreatic tumors rely on protein both from within the tumor and from skeletal muscle to supply amino acid requirements.
Fatty acids
In addition to glucose and glutamine, the oxidation of fatty acids can be a source of ATP. Caro et al. identified a subtype of diffuse large B cell lymphoma (DLBCL) that demonstrated increased expression of oxidative phosphorylation genes, a greater reliance on the mitochondria for ATP generation, and greater oxidation of palmitate than other subtypes (Caro et al., 2012). Palmitate specifically stimulated the growth of the “ox phos” subtype of DLBCL, and inhibition of fatty acid oxidation induced apoptosis of these cells. Thus, certain subsets of cancers demonstrate unique metabolic dependencies that could be exploited for cancer therapy.
Fatty acids are essential for proliferation because they are required for the generation of new cellular membranes. Fatty acid synthesis requires the generation of cytosolic acetyl-CoA, which is predominantly derived from mitochondrial citrate that is cleaved by ATP-citrate lyase in the cytosol. Under normoxia, this citrate is derived from glucose via pyruvate, which is converted to mitochondrial acetyl-CoA by pyruvate dehydrogenase. Under hypoxia, however, pyruvate dehydrogenase is inactivated by pyruvate dehydrogenase kinase, and reductive carboxylation of glutamine to citrate occurs. While many cancer cells synthesize fatty acids de novo from acetyl-CoA, some rely on exogenous sources. Kamphorst et al. found that hypoxia or expression of oncogenic RAS also increased fatty acid import in the form of lysolipids (Kamphorst et al., 2013). Thus, under hypoxia cells can scavenge lysophospholipids to meet their fatty acid requirements, and oncogenic RAS increases fatty acid scavenging.
While the metabolism of tumor cells has been well studied, the metabolism of cells that support tumor cell growth is less well understood. Schoors et al. examined the metabolism of the endothelial compartment and found that CTP1A is critical for vessel sprouting during angiogenesis (Schoors et al., 2015). CPT1A catalyzes the transfer of the acyl group of long-chain fatty acyl-CoA onto carnitine, an essential step for the import of long-chain fatty acids into mitochondria and their subsequent beta-oxidation. Surprisingly, fatty acid oxidation did not support ATP synthesis or redox homeostasis but rather supported dNTP synthesis for DNA replication. Interestingly, fatty acid carbons were found to contribute significantly to the TCA cycle and aspartate, which is used for the synthesis of both purines and pyrimidines. However, fatty acids cannot substitute for glucose or glutamine for TCA cycle anapleurosis as no net oxaloacetate can be generated from fatty acid-derived acetyl-CoA. Curiously, while palmitate could contribute to the citrate pool in some cancer cell lines, it could only contribute to dNTP synthesis in endothelial cells. These results demonstrate that tumor-supporting cells have unique metabolic requirements, and suggest inhibition of fatty acid oxidation may impair angiogenesis in tumors.
Acetate
Acetyl-CoA is an important metabolite for the function of the TCA cycle and is the sole carbon source for fatty acid and cholesterol biosynthesis. In addition to citrate, the cytosolic pool of acetyl-CoA can be supplied by the ligation of acetate and CoA by acetyl-CoA synthetase. In cultured cells under normal proliferating conditions, up to 95% of cytosolic acetyl-CoA is derived from citrate via glucose and glutamine (Kamphorst et al., 2014). Under hypoxia, however, this amount drops to as little as 50%. Even though BCAA and fatty acid degradation can generate acetyl-CoA, neither amino acids nor fatty acids were found to be major contributors to the acetyl-CoA pool under hypoxia. Interestingly, acetate was identified as the source of the remaining acetyl-CoA (Kamphorst et al., 2014). Acetate likely supplied the cytosolic acetyl-CoA pool via the cytosolic acetyl-CoA synthetase ACSS2. The notion that significant acetate conversion to acetyl-CoA may occur in human tumors is supported by the fact that some tumors can be 10 imaged by 11C-acetate PET (Grassi et al., 2012). This study demonstrated that cancer cells can utilize acetate to supply the cytosolic acetyl-CoA pool to support lipid biosynthesis. The ability of cells to scavenge lysophospholipids (e.g. RAS mutant) vs. synthesize their own from acetate (ACSS2 overexpressing) under hypoxia likely influences fuel choice when lipids or lipid precursors are limiting.
Factors influencing fuel choice
The metabolism of cancer cells is influenced by both the metabolic program of the cancer cells (cell-intrinsic regulation) and the metabolic microenvironment of the tumor in which those cells exist (cell-extrinsic regulation). In this section, we discuss how both factors cooperate to influence the fuel choice of the cancer cells and other cells in the tumor microenvironment.
Cell-intrinsic regulation of metabolism
Cancer cells engage metabolic programs that are different from their normal counterparts. In this section we discuss how cancer cell metabolism is influenced by cell-intrinsic factors including mutations in oncogenes and tumor suppressors, expression of non-coding RNAs, differentiation state, and alterations in mitochondrial activity. These factors impact both the choice of fuels and how cancer cells use those fuels.
Oncogenic alterations
Among the best-characterized regulators of cell metabolism are intracellular signaling pathways that are deregulated by oncogene or tumor suppressor alterations. Indeed, one of the first characterized effects of the proto-oncogenic tyrosine kinase SRC was inactivation of purified chicken-liver pyruvate kinase M2 in tumor lysates (Glossmann et al., 1981), leading to the hypothesis that SRC regulates glycolysis. We now know that PKM2 is a phosphotyrosine-binding protein, and binding of phosphotyrosine peptides to PKM2 results in inhibition of PKM2 enzymatic activity (Christofk et al., 2008). This regulation diverts glucose metabolites from energy production to anabolic processes for proliferation. Since this initial observation about SRC, many oncogenes have been found to regulate glycolysis including KRAS, BRAF, MYC, PI3K, AKT, and others (Iurlaro et al., 2014). Indeed, KRAS has profound effects on cellular metabolism by stimulating glucose uptake, redirecting glucose into the hexosamine and pentose phosphate pathways, and regulating glutamine metabolism for redox homeostasis (Son et al., 2013; Ying et al., 2012; Yun et al., 2009).
Tumor suppressor loss also results in metabolic deregulation. Mutations in the TCA cycle enzymes fumarate hydratase and succinate dehydrogenase are common in renal cell carcinoma (RCC) and can directly affect mitochondrial metabolism (Vander Heiden et al., 2009). Additionally, these mutations lead to activation of the HIF transcription factors (discussed below), which also occurs following loss of function of the tumor suppressor VHL, a common occurrence in RCC. Moreover, p53, the most commonly lost or mutated tumor suppressor in human cancer (Kandoth et al., 2013) and a major metabolic regulator, functions to limit glycolytic flux, enhance glutaminolysis, and support oxidative phosphorylation (reviewed in (Kruiswijk et al., 2015). Therefore, loss of p53 results in enhanced glycolysis and suppression of oxidative phosphorylation. p53 also regulates the cellular redox state through the modulation of the pentose phosphate pathway and malic enzyme (Kruiswijk et al., 2015). Furthermore, p53 can modulate cellular responses to nutrient starvation, and regulate alternative fuel choice by promoting fatty acid oxidation (Kruiswijk et al., 2015). Additionally, KEAP1 deficiency occurs frequently in non-small cell lung cancer (NSCLC) and results in the constitutive stabilization of the antioxidant transcription factor NRF2. In addition to inducing genes involved in ROS defense, NRF2 supports NADPH generation through activation of the pentose phosphate pathway and regulation of malic enzyme (Hayes and McMahon, 2009; Mitsuishi et al., 2012). Thus, many of the common genetic alterations in oncogenes and tumor suppressors result in metabolic alterations that can impose specific nutrient requirements on cancer cells.
While the effects of oncogenes and tumor suppressors on metabolism have been extensively studied, the effects of metabolism on oncogenic signaling are less well understood. Kang et al. identified a synthetic lethal interaction between oncogenic BRAFV600E and the ketogenic enzyme 3-hydroxy-3-methylglutaryl-CoA ligase (HMGCL) (Kang et al., 2015). BRAFV600E upregulates HMGCL leading to increased abundance of HMGCL in cancers harboring this mutation, and rendering them dependent on HMGCL expression. Surprisingly, the ketone product of HMGCL, acetoacetate, was found to enhance binding of BRAFV600E to MEK1 to promote MEK-ERK signaling. Thus, BRAFV600E rewires cellular metabolism in a feed-forward loop to enhance downstream signaling.
Fine-tuning of metabolism by non-coding RNAs
Alterations in oncogenic and tumor suppressive proteins are not the only regulatory events that modulate cancer cell metabolism. Recently, a role for non-coding RNAs has emerged. One of the first examples of the roles of miRNAs in cancer metabolism is the suppression of miR-23a by c-Myc to upregulate GLS and glutamine metabolism (Gao et al., 2009). Additionally, LIN28, which is overexpressed in tumors, has been described as a major regulator of cellular metabolism through the repression of the let-7 family of miRNAs, and through let- 7-independent mechanisms (Shyh-Chang et al., 2013; Zhu et al., 2011). Importantly, miRNAs may be a major part of the metabolic regulatory program. Ye et al. found that targeted disruption of Tsc1 led to a global reduction in total miRNA levels due to mammalian target of rapamycin (mTOR)-MDM2-mediated degradation of the critical miRNA biogenesis enzyme Drosha (Ye et al., 2015). Nutrient limitation, such as glucose starvation, inhibited the activity of mTOR and led to an increase in miRNA biogenesis. The authors identified 4 miRNAs, miR- 297, miR-376-3p, miR-567, and miR-627-5p, which were necessary and sufficient to protect cells from glucose starvation-induced apoptosis under conditions of mTOR hyperactivity. Surprisingly, miR-297 and miR-567 increased Drosha protein by 70%–90%, suggesting that these two miRNAs may protect cells by increasing Drosha itself. However, miR-376b-3p and miR-627-5p did not affect Drosha levels and protected against glucose starvation-induced apoptosis by an unknown mechanism. These results demonstrate that miRNA biogenesis is a part of the cellular response to nutrient limitation, and warrant further investigation into the regulation of, and requirement for miRNAs during limitation of other nutrients.
Influence of tumor stem cell state on metabolism
The differentiation state of cancer cells can have profound effects on their metabolism and fuel choice. Viale et al. found that a subpopulation of dormant tumor cells survived withdrawal of KrasG12D expression and led to the relapse of pancreatic tumors (Viale et al., 2014). These cells exhibited features of cancer stem cells and relied on oxidative phosphorylation (OXPHOS) for survival. They also demonstrated sensitivity to OXPHOS inhibitors, which prevented tumor recurrence. While it is unclear whether the unique metabolism of these cells is a cause or consequence of their stem-like state, there is evidence that embryonic stem cells maintain high levels of α-ketoglutarate to promote histone/DNA demethylation to maintain pluripotency (Carey et al., 2015). In many tumors, cells can undergo an epithelial-mesenchymal transition (EMT), which is associated with aggressiveness and stem-like properties. However, the metabolism associated with this transition is poorly understood. Sabatini and colleagues analyzed metabolic gene expression patterns in a large panel of cancer cell lines to identify a metabolic gene signature that is present in mesenchymal tumors. The authors identified a novel role for dihydropyrimidine dehydrogenase (DPYD), which catalyzes the rate-limiting step in pyrimidine degradation, in EMT during tumorigenesis (Shaul et al., 2014). EMT-promoting transcription factors induced the expression of DPYD, and the accumulation of dihydropyrimidines was required for cells to undergo EMT. Thus, the differentiation state can confer unique metabolic requirements on cancer cells to alter fuel choice.
Mitochondrial defects dictate cellular responses to nutrient limitation
Cellular metabolism can also be modulated by the ability of cells to respond to nutrient limitation. Sabatini and colleagues examined the metabolic dependencies of cancer cells in a chronically low glucose environment (Birsoy et al., 2014). The authors developed a continuous flow culture system (Nutrostat) for maintaining proliferating cells in constant nutrient conditions. The authors found that Jurkat cells cultured at a constant 0.75 mM glucose concentration proliferated exponentially at a rate that was only slightly less than in 10 mM glucose. However, profound metabolic changes were observed, including decreases in the rates of glucose consumption and lactate production, and in the levels of ATP and intermediates in the upper glycolysis and pentose-phosphate pathways. Cells that failed to increase oxidative phosphorylation due to mitochondrial DNA mutations or impaired glucose uptake demonstrated defects in proliferation under glucose-limiting conditions.
Mitochondrial impairment results in profound rewiring of cellular metabolism. In cells with intact mitochondria, pyruvate decarboxylation supplies the TCA cycle to generate citrate. During conditions of mitochondrial impairment, such as hypoxia or defects in the TCA cycle or the electron transport chain, glycolysis is enhanced and a significant portion of pyruvate is converted to lactate (Metallo et al., 2012; Mullen et al., 2012). The contribution of glucose to the TCA cycle is diminished, and glutamine can supply citrate through oxidative decarboxylation of α-ketoglutarate (Metallo et al., 2012; Mullen et al., 2012; Wise et al., 2011). Mullen et al. found that α-ketoglutarate oxidation was also required to supply reducing equivalents for reductive carboxylation (Mullen et al., 2014). These data demonstrate that bidirectional metabolism of glutamine-derived α-ketoglutarate supplies the TCA cycle in cells with mitochondrial defects, with oxidative metabolism producing reducing equivalents for reductive carboxylation reactions.
Mitochondrial pyruvate import determines fuel choice
Many cells in culture use both glucose and glutamine to supply the TCA cycle, with glucose supplying most of the acetyl-CoA via pyruvate, and glutamine supplying most of the α-ketoglutarate via glutamate. Entry of pyruvate into mitochondria serves as a gateway between glycolysis, gluconeogenesis and the TCA cycle. Recently, the proteins involved in pyruvate transport, MPC1 and MPC2, were identified and found to encode a multimeric complex embedded in the mitochondrial inner membrane (Bricker et al., 2012; Herzig et al., 2012). Expression of these proteins regulates pyruvate uptake into mitochondria. Recently, several groups characterized the effects of blocking entry of pyruvate into mitochondria by targeting the MPC proteins. Yang et al. found that blocking the entry of pyruvate into mitochondria enhanced the contribution of glutamine to acetyl-coA (Yang et al., 2014). This observation was shared by Metallo and colleagues, who sought to examine the role of the pyruvate carrier on metabolic flux and substrate selection in skeletal muscle (Vacanti et al., 2014). The authors found that suppression of pyruvate uptake into mitochondria resulted in a dramatic reduction in the oxidation of both glucose and pyruvate. Surprisingly, however, both cell growth and mitochondrial TCA cycle metabolism were maintained. The authors found that TCA cycle anapleurosis was supplied by glutamine, which also supplied the malate pool via malic enzyme. At high concentrations, pyruvate is capable of passively entering the mitochondrial matrix thereby bypassing the MPC. Vacanti et al. found that PDH activity was maintained in Mpc knockdown cells, and glutamine-derived pyruvate was metabolized to acetyl-CoA for lipid biosynthesis. However, the authors found a substantial increase in the oxidation of fatty acids to supply acetyl-CoA upon Mpc knockdown. The expression of MPC1 likely affects the fuel choice of tumors in vivo. Rutter and colleagues observed that MPC1 is deleted or underexpressed in multiple cancers, which correlates with poor prognosis (Schell et al., 2014). Reintroduction of MPC1 and MPC2 into tumor cells promoted pyruvate oxidation with no obvious effects on growth in adherent culture. Anchorage-independent growth and stem-like properties, however, were dramatically reduced. Collectively, these studies demonstrate that the mitochondrial pyruvate carrier is a major determinant of cancer cell metabolism, and its expression dictates the fuels used to feed the TCA cycle and produce associated metabolites.
Cell-extrinsic regulation of metabolism
While cell-intrinsic metabolic regulation can affect the dependence of cancer cells on, and the use of, particular nutrients for growth and proliferation, the tumor microenvironment influences the availability of those nutrients. While the metabolism of cancer cells has been extensively characterized, less is known about the metabolic alterations that occur in the tumor microenvironment. In this section, we discuss how cancer metabolism is affected by cell-extrinsic factors in the tumor microenvironment (Figure 2), and the current knowledge about the metabolism of the microenvironment itself.
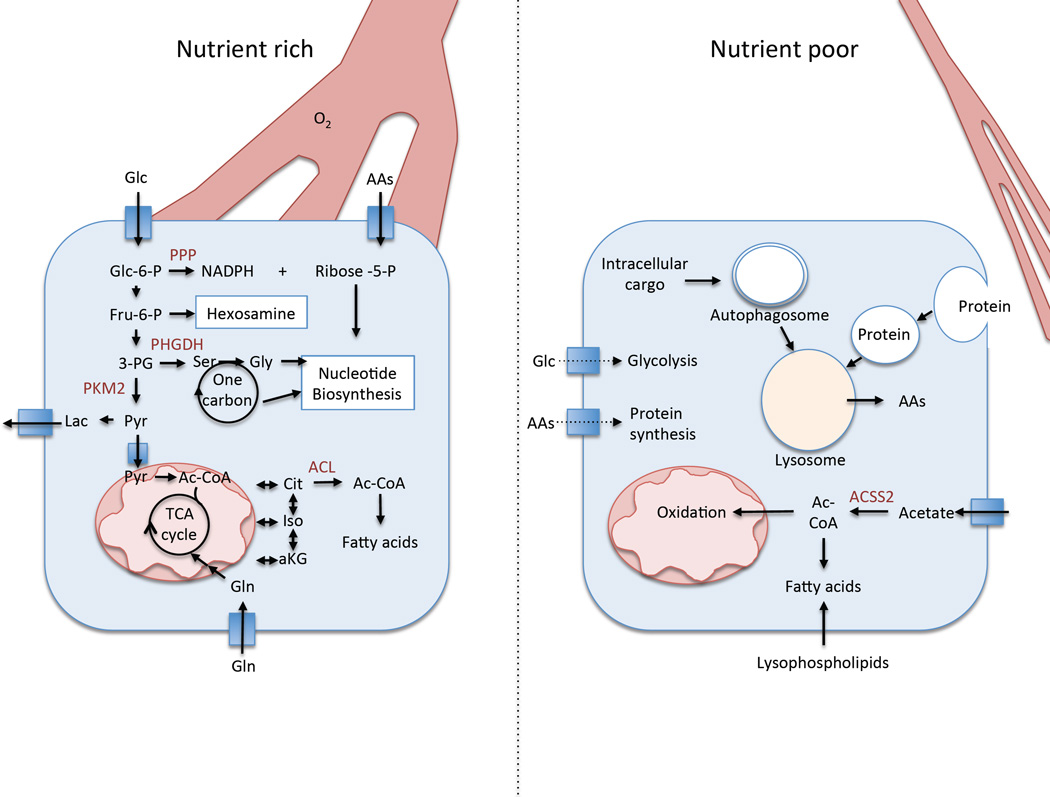
Figure 2.
Nutrient delivery can affect the metabolism of tumor cells. (Left) Under conditions of adequate nutrient delivery, glucose and glutamine are used as primary fuel sources to feed glycolysis and the TCA cycle to support proliferation. Amino acid uptake supports protein synthesis and other anabolic processes. (Right) Under conditions of poor nutrient delivery the cell relies on alternative fuel sources including lysosomal degradation of both intracellular cargo and extracellular protein obtained via macropinocytosis to supply intracellular amino acid pools. Fatty acid pools are obtained via scavenging lysophospholipids and de novo synthesis via acetate-derived acetyl-coA.
Nutrient availability
The availability of nutrients may affect tumor metabolism in vivo. Diet-induced insulin resistance can significantly increase the levels of blood glucose and the activity of the PI3K/AKT pathway in tumor cells, which increases glucose uptake. Indeed, Gunter et al. found that patients with high fasting insulin and glucose levels had a significantly increased risk of breast cancer (Gunter et al., 2015). Additionally, dietary limitation may slow tumor growth in vivo. Maddocks et al. found that feeding mice a diet lacking serine and glycine significantly impaired tumor growth (Maddocks et al., 2013). Furthermore, tumors may exhibit regional nutrient limitation. While some tumors are well vascularized, others demonstrate areas of poor perfusion, leading to decreased availability of certain nutrients and oxygen. In response to nutrient limitation or cellular stress, cells digest their organelles and macromolecules in a process known as autophagy. In this process organelles, proteins or protein aggregates are sequestered in a double membrane structure known as an autophagosome and targeted for degradation by the lysosome, resulting in the recycling of amino acids, lipids and nucleosides for cellular biosynthesis. The activity of autophagy is typically low in most tissues; however, it increases in response to various stresses. One of the most potent inducers of autophagy is nutrient deprivation, which induces autophagy through suppression of the mTOR pathway (Neufeld, 2010). Unsurprisingly, the activity of autophagy is increased in tumors, especially in nutrient-deprived regions (Degenhardt et al., 2006). Indeed, basal autophagy is particularly high in pancreatic cancer and is required for tumorigenesis (Yang et al., 2011). Perera et al. found that the MiT/TFE transcription factors are constitutively nuclear in pancreatic cancer and drive a lysosomal biogenesis program to promote autophagy (Perera et al., 2015). Nuclear translocation of MiT/TFE factors occurred despite intact mTOR signaling, which normally promotes cytoplasmic retention of these proteins. Furthermore, the authors found that MiT/TFE-dependent autophagy-lysosome activation was required for the maintenance of intracellular amino acid pools, which is likely especially critical in vivo under the nutrient poor conditions found in pancreatic cancer. The recycling of amino acids, fatty acids, nucleotides, and ATP supports cellular survival when these metabolites are limiting. However, in many tumors, the mTOR pathway is hyperactivated due to oncogenic signaling, which suppresses the activity of the autophagy pathway and the ability of cells to degrade both intracellular (macroautophagy) and extracellular (macropinocytosis) macromolecules. Thompson and colleagues found that under conditions of nutrient limitation, mTOR suppression actually increases tumor cell proliferation by enhancing lysosomal degradation of internalized protein through a mechanism that was distinct from its regulation of autophagy (Palm et al., 2015). The authors found that the well-vascularized portion of mouse pancreatic tumors was proliferative while the poorly vascularized portion was not proliferating. Treatment of mice with rapamycin suppressed the proliferation of the well-vascularized region of the tumor, but dramatically enhanced the proliferation of the poorly vascularized region. These studies demonstrate that lysosome-mediated substrate degradation is an important catabolic process to supply amino acids, and potentially other macromolecular precursors, in pancreatic cancer.
Oxygen availability
In addition to nutrient deprivation, tumors experience periods of low extracellular pH and hypoxia. Hypoxic areas, or areas of low oxygen, arise when oxygen consumption exceeds the supply (Vaupel and Harrison, 2004). In normal tissues, oxygen is supplied to match the metabolic requirements of the cells. However, the oxygen consumption in many tumors exceeds the supply, resulting in inadequate oxygen availability in some areas of the tumor. Tumor cells that are beyond the oxygen diffusion distance (> 70 µm from blood vessels) can rapidly exhaust the oxygen supply (Vaupel, 2004). In addition, tumor blood vessels are usually dilated, irregularly organized, and less functional. One mechanism by which cells adapt to hypoxia is activation of hypoxia inducible factors (HIF1α and HIF2α), which are kept at low levels in the presence of oxygen by von Hippel- Lindau protein (VHL)-mediated degradation (Semenza, 2012). In hypoxic conditions, HIF proteins are stabilized and regulate a number of genes including those involved in angiogenesis and glycolysis (Semenza, 2012). Because oxygen is the terminal electron acceptor during electron transport, limited oxygen availability impairs ATP synthesis. To compensate for this, HIF proteins upregulate glycolytic enzymes to maintain ATP levels. However, defective vasculature likely fails to deliver adequate nutrient supplies as well, and a failure to maintain ATP levels is the likely explanation for the necrotic regions observed in many tumor types (Zong and Thompson, 2006).
Recent studies have advanced our understanding of metabolic responses to hypoxia beyond the regulation of glycolysis by HIF proteins. The regulation of serine metabolism under hypoxia has recently been identified as an important mediator of cell survival. Ye et al. found that the mitochondrial enzyme SHMT2, which metabolizes serine to glycine to generate one carbon units in the folate cycle, is induced under hypoxia and is critical for maintaining NADPH levels and redox balance for survival (Ye et al., 2014). The hypoxic regulation of SHMT2 was also examined by Kim et al., who found that SHMT2 and the glycine cleavage enzyme GLDC were highly expressed in the pseudopalisading cells adjacent to necrotic foci in glioblastoma multiforme (Kim et al., 2015). SHMT2 was critical for hypoxic cell survival, but imposed a dependence on GLDC activity for glycine clearance, as excess glycine can be metabolized to the toxic byproducts aminoacetone and methylglyoxal. Thus, mitochondrial serine metabolism is critical for adaptation and survival under hypoxia.
Hypoxia also supports the production of fatty acids from acetate. ACSS2 is regulated by both low-oxygen and lipid-depleted conditions. Schug et al. found that nearly 40% of invasive ductal breast carcinomas have high expression of ACSS2, which allows them to utilize acetate for acetyl-CoA production (Schug et al., 2015). ACSS2 was upregulated in hypoxic regions of tumor xenografts and was transcriptionally controlled by synergy between HIF and SREBP transcription factors. Hypoxic induction of ACSS2 increased the incorporation of acetate into lipids. Normal plasma acetate levels are low (50–180µM) (Skutches et al., 1979; Tollinger et al., 1979) and are generated by gut microbes, liver ketogenesis, or ethanol metabolism in heavy drinkers. The contribution of gut microbiome-produced acetate to colon cancer growth is an intriguing possibility that remains to be explored. Furthermore, a shift in the composition of the gut microbiome toward higher acetate-producing species may increase serum acetate levels and promote tumorigenesis at distal sites in patients with ACSS2 overexpression.
Mashimo et al. found that patient glioblastomas derived less than 50% of their acetyl-CoA pool from glucose (Mashimo et al., 2014). Intriguingly, brain metastases derived from organs that do not demonstrate acetate uptake by 11C-acetate PET also oxidized acetate, suggesting it was an acquired property of tumor cells or adaptation to the brain microenvironment. Acetate was found to label the mitochondrial acetyl-CoA pool and can be used for ATP synthesis. Tumors were found to oxidize both acetate and glucose but not glutamine, and express ACSS2. Together, these studies suggest that acetate may supply both cytosolic and mitochondrial acetyl-CoA pools for both lipid/cholesterol synthesis and ATP production under hypoxia or when other substrates are limiting.
Composition of the stroma
The composition of the tumor stroma may affect the metabolism of the cancer cells in a variety of ways (Figure 3). The tumor microenvironment is composed of fibroblasts, immune cells, adipocytes, and/or endothelial cells that must co-exist with the tumor cells under conditions that may be nutrient limiting. Depending on the metabolism of these different cell types, they may compete with the tumor cells for metabolites, or they may work together with the tumor cells in a metabolic symbiosis to support their growth. Interestingly, the metabolic microenvironment may provide a disadvantage for immune effector cells, whose metabolism closely mirrors that of tumor cells (Wang et al., 2014). For example, naïve T-cells rely mainly on fatty acid oxidation and limited glycolysis to fulfill their energy requirements. Upon stimulation, activated T lymphocytes dramatically increase aerobic glycolysis and glutaminolysis, while decreasing lipid oxidation to support cell growth, proliferation, and cytokine production (Wang et al., 2014). Furthermore, the metabolism of Tregs, the immunosuppressive T-cell population that is increased in many tumors, is predominantly mitochondria-dependent oxidation of lipids and other metabolites (Wang et al., 2014). Additionally, forcing proliferating T cells to oxidize fatty acids enhances Treg differentiation. Therefore, the nutrient-limiting environment in tumors may promote immunosuppression due to competition for nutrients.
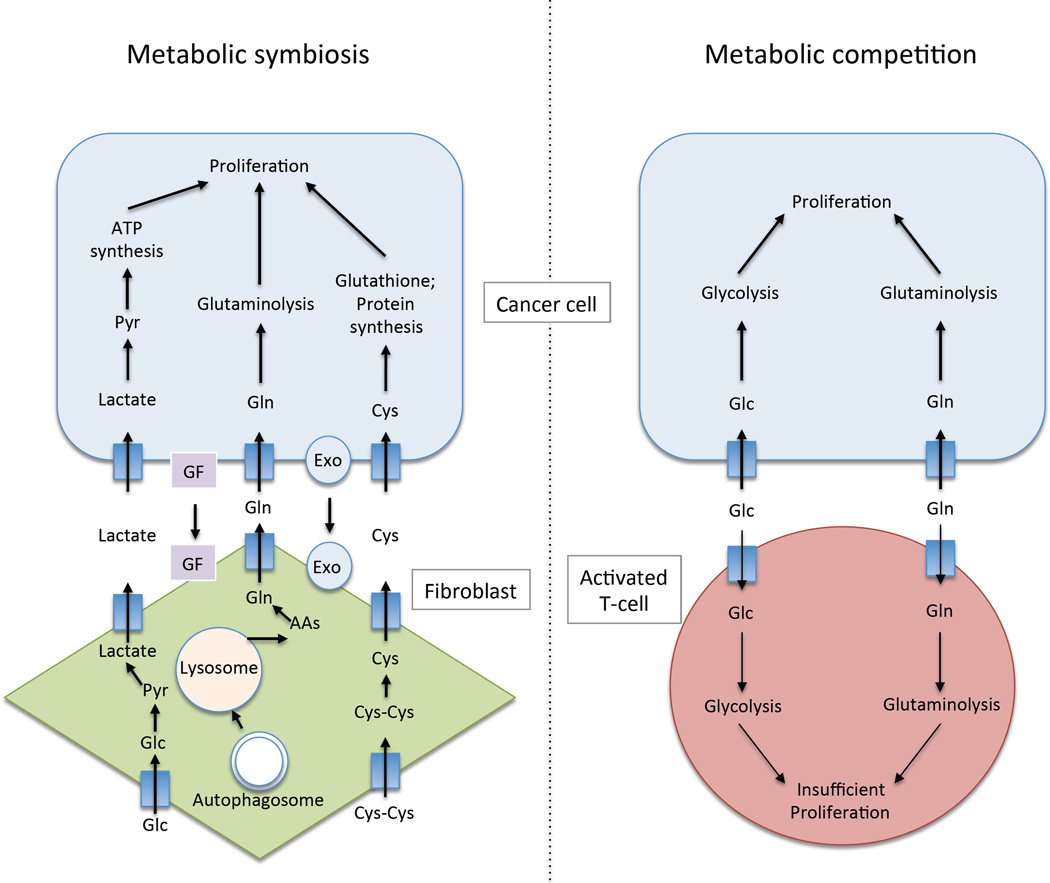
Figure 3.
The interaction of tumor cells with the microenvironment can be symbiotic or competitive. (Left) Metabolic symbiosis may occur between tumor cells and fibroblasts, whose metabolism may be reprogrammed by tumor cells due to secretion of growth factors (GF) or other signaling molecules such as exosomes (Exo). Fibroblasts may feed lactate, amino acids, and other metabolites to tumor cells. Lactate produced from fibroblasts via glycolysis may be taken up by tumor cells, converted to pyruvate, and metabolized in the TCA cycle. Amino acids such as glutamine may also be supplied to tumor cells following autophagic breakdown of fibroblast protein. Furthermore, fibroblasts may take up cystine from the microenviroment and reduce it to cysteine, which tumor cells use to make glutathione and protein. (Right). Tumor cells and activated T-cells compete for glucose and glutamine, which they both need for proliferation. This can result in an immunosuppressive environment if T-cell proliferation is inadequate.
By contrast, tumor cells may reprogram the metabolism of cancer-associated fibroblasts (CAFs), resulting in metabolite exchange between the two compartments to support tumor cell growth (Romero et al., 2015). Zhang et al. found that during the transition from naïve fibroblasts to CAFs cells switch from oxidative phosphorylation to aerobic glycolysis, which was mediated by downregulation of IDH3a and HIF-1α stabilization (Zhang et al., 2015). Treatment of fibroblasts with TGF-β1 or PDGF could induce this metabolic switch, suggesting that these growth factors may play a role in the metabolic reprogramming of CAFs by tumors. Metabolites that may be exchanged between tumors cells and CAFs include lactate, glutamine and cysteine, which may be taken up and used by tumor cells (Romero et al., 2015). However, the contribution of CAF-derived metabolites to tumor cell nutrient pools in vivo remains to be determined. Fibroblasts are not the only stromal cell that could supply nutrients to tumor cells. Adipocytes are prevalent in kidney, breast and ovarian cancer and they may supply fatty acids to tumor cells. Thus, tumor-induced metabolic reprogramming of stromal cells may supply nutrients to tumor cells to support their growth.
In addition, the stroma may affect the availability of nutrients and oxygen through its effects on the tumor vasculature. Studies in pancreatic cancer, one of the most stromal rich tumor types, have demonstrated that depletion of stromal cells using either pharmacological or genetic mechanisms results in tumors that are much better perfused and have higher blood vessel content (Olive et al., 2009; Rhim et al., 2014). Therefore, the cells within the microenvironment can modulate the nutrient and oxygen availability through their effects on the tumor vasculature.
In vivo measurement of metabolism provides novel insights
Recent technological advances have allowed for the measurement of metabolic pathway activity in vivo in human patients. While 2-[18F]fluoro-2-deoxy-D-glucose PET imaging has long been used to image glucose uptake in human tumors, additional PET tracers are now available to assay uptake of other carbohydrates, amino acids, fatty acids, and acetate (Lewis et al., 2015). Furthermore, the fate of metabolites in vivo can be examined using hyperpolarized substrates such as glucose, pyruvate, fumarate, and others (Brindle, 2015). Recently, Sellers et al. infused patients with early-stage NSCLC with uniformly 13C-labeled glucose prior to tissue resection to identify that tumors had enhanced pyruvate carboxylase activity compared to normal lung, which played an important role in TCA cycle anapleurosis (Sellers et al., 2015). Most research on TCA cycle anapleurosis in cancer cells has focused on the role of glutamine in cell culture. Given the importance of nutrient delivery and the tumor microenvironment, such experiments may not faithfully recapitulate the in vivo setting. Indeed, although Sellers et al. found that glutaminase was active in tumors, the activity was not elevated when compared to normal lung tissue. Thus, in vivo measurement of lung cancer metabolism has provided novel insight into the role of glucose in TCA cycle anapleurosis.
Future directions
Moving forward, the transition from a reductionist, cell culture-based analysis of metabolism to the direct measurement of tumor metabolism in vivo will provide greater insight into the fuel choice of both tumor cells and stromal cells in their complex natural environment. The interaction of cell intrinsic genetic and differentiation programs with cell extrinsic factors including nutrient and oxygen availability, coupled with contact with fibroblast, immune and other stromal cells will likely result in a very heterogeneous mixture of metabolic states within the same tumor. Additionally, tumor cells and stromal cells may compete for the same metabolite pools or instead form a metabolic symbiosis to support each other’s growth. The ability to measure the levels of metabolites and activities of metabolic pathways in these individual compartments will help distinguish these possibilities. To date, even the most elegant analyses of tumor metabolism in vivo have examined bulk tumor tissue comprised of a complex mixture of tumor and stromal cells. Technological advances that allow for the examination of cellular metabolism in these cellular compartments, as well as an in depth analysis of tumor heterogeneity as it relates to nutrient and oxygen availability, tumor grade, oncogene/tumor suppressor mutations and differentiation status will reveal the relative contribution of these parameters to tumor metabolism in vivo.
Acknowledgements
We would like to thank Florian Karreth for critical reading of the manuscript. We apologize to authors whose work we could not include due to space limitations. L.C.C. is supported by the NIH grant P01 CA117969. G.M.D. is supported by the Pancreatic Cancer Action Network-AACR Pathway to Leadership Grant. L.C.C. owns equity in, receives compensation from, and serves on the Board of Directors and Scientific Advisory Board of Agios Pharmaceuticals. Agios Pharmaceuticals is identifying metabolic pathways of cancer cells and developing drugs to inhibit such enzymes in order to disrupt tumor cell growth and survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen Y-C, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle KM. Imaging metabolism with hyperpolarized (13)C-labeled cell substrates. J. Am. Chem. Soc. 2015;137:6418–6427. doi: 10.1021/jacs.5b03300. [DOI] [PubMed] [Google Scholar]
- Carey BW, Finley LWS, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang T-C, Lee Y-S, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H, Presek P, Eigenbrodt E. Association of the src-gene product of Rous sarcoma virus with a pyruvate-kinase inactivation factor. Mol. Cell. Endocrinol. 1981;23:49–63. doi: 10.1016/0303-7207(81)90116-7. [DOI] [PubMed] [Google Scholar]
- Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. The clinical use of PET with (11)C-acetate. Am J Nucl Med Mol Imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- Gunter MJ, Xie X, Xue X, Kabat GC, Rohan TE, Wassertheil-Smoller S, Ho GYF, Wylie-Rosett J, Greco T, Yu H, et al. Breast cancer risk in metabolically healthy but overweight postmenopausal women. Cancer Res. 2015;75:270–274. doi: 10.1158/0008-5472.CAN-14-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, Veuthey J-L, Zamboni N, Westermann B, Kunji ERS, Martinou J-C. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- Iurlaro R, León-Annicchiarico CL, Muñoz-Pinedo C. Regulation of cancer metabolism by oncogenes and tumor suppressors. Meth. Enzymol. 2014;542:59–80. doi: 10.1016/B978-0-12-416618-9.00003-0. [DOI] [PubMed] [Google Scholar]
- Kamphorst JJ, Chung MK, Fan J, Rabinowitz JD. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab. 2014;2:23. doi: 10.1186/2049-3002-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H-B, Fan J, Lin R, Elf S, Ji Q, Zhao L, Jin L, Seo JH, Shan C, Arbiser JL, et al. Metabolic Rewiring by Oncogenic BRAF V600E Links Ketogenesis Pathway to BRAF-MEK1 Signaling. Mol Cell. 2015 doi: 10.1016/j.molcel.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520:363–367. doi: 10.1038/nature14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- Lewis DY, Soloviev D, Brindle KM. Imaging tumor metabolism using positron emission tomography. Cancer J. 2015;21:129–136. doi: 10.1097/PPO.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks ODK, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, Vousden KH. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, Boriack R, Rakheja D, Sullivan LB, Linehan WM, et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7:1679–1690. doi: 10.1016/j.celrep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld TP. TOR-dependent control of autophagy: biting the hand that feeds. Curr. Opin. Cell Biol. 2010;22:157–168. doi: 10.1016/j.ceb.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, Park Y, Wright K, Pavlova NN, Tuveson DA, Thompson CB. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015 doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, Lengrand J, Deshpande V, Selig MK, Ferrone CR, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015 doi: 10.1038/nature14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo H-K, Jang HG, Jha AK, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero IL, Mukherjee A, Kenny HA, Litchfield LM, Lengyel E. Molecular pathways: trafficking of metabolic resources in the tumor microenvironment. Clin Cancer Res. 2015;21:680–686. doi: 10.1158/1078-0432.CCR-14-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400–413. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, Bruning U, Missiaen R, Queiroz KCS, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Fox MP, Bousamra M, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125:687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul YD, Freinkman E, Comb WC, Cantor JR, Tam WL, Thiru P, Kim D, Kanarek N, Pacold ME, Chen WW, et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell. 2014;158:1094–1109. doi: 10.1016/j.cell.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM, Nguyen L, Asara JM, Cantley LC, Daley GQ. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155:778–792. doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest. 1979;64:708–713. doi: 10.1172/JCI109513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollinger CD, Vreman HJ, Weiner MW. Measurement of acetate in human blood by gas chromatography: effects of sample preparation, feeding, and various diseases. Clin. Chem. 1979;25:1787–1790. [PubMed] [Google Scholar]
- Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, Murphy AN, Metallo CM. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Suppl 5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sánchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liu G, Wang R. The Intercellular Metabolic Interplay between Tumor and Immune Cells. Front Immunol. 2014;5:358. doi: 10.3389/fimmu.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O, Posener K, Negelein E. Ueber den stoffwechsel der tumoren (Biochemische Zeitschrift) 1924 [Google Scholar]
- Wise DR, Ward PS, Shay JES, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell'antonio G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Fan J, Venneti S, Wan Y-W, Pawel BR, Zhang J, Finley LWS, Lu C, Lindsten T, Cross JR, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4:1406–1417. doi: 10.1158/2159-8290.CD-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Liu Y, Chen C, Tang F, Wu Q, Wang X, Liu C-G, Liu X, Liu R, Liu Y, et al. An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose-and amino acid-deprivation. Mol Cell. 2015;57:708–720. doi: 10.1016/j.molcel.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JKV, Markowitz S, Zhou S, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang Y, Shi Z, Liu J, Sun P, Hou X, Zhang J, Zhao S, Zhou BP, Mi J. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep. 2015;10:1335–1348. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205–218. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W-X, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]