Abstract
Background
Obesity is associated with both increased breast cancer risk and poorer prognosis after disease onset. However, little is known about the effect of obesity on treatment efficacy. We evaluated the association of obesity with outcomes and with tamoxifen efficacy in women with early-stage, hormone-responsive breast cancer participating in a multicenter cancer cooperative group clinical trial.
Methods
The cohort consisted of 3385 women enrolled in National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-14, a randomized, placebo-controlled trial evaluating tamoxifen for lymph node–negative, estrogen receptor (ER)–positive breast cancer. Hazards of breast cancer recurrence, contralateral breast tumors, other new primary cancers, and several mortality endpoints were evaluated in relation to body mass index (BMI), using statistical modeling to adjust for other prognostic factors. Median follow-up time was 166 months. All statistical tests were two-sided.
Results
The hazard of breast cancer recurrence was the same among obese (BMI ≥30.0 kg/m2) women as compared with underweight and normal-weight women (BMI <25.0; hazard ratio [HR] = 0.98, 95% confidence interval [CI] = 0.80 to 1.18). Contralateral breast cancer hazard was higher in obese women than in underweight/normal-weight women (HR = 1.58, 95% CI = 1.10 to 2.25), as was the risk of other primary cancers (HR = 1.62, 95% CI = 1.16 to 2.24). Compared with normal-weight women, obese women had greater all-cause mortality (HR = 1.31, 95% CI = 1.12 to 1.54) and greater risk of deaths due to causes unrelated to breast cancer (HR = 1.49, 95% CI = 1.15 to 1.92). Breast cancer mortality was not statistically significantly increased for obese women (HR = 1.20, 95% CI = 0.97 to 1.49). Tamoxifen reduced breast cancer recurrence and mortality, regardless of BMI.
Conclusions
For women with lymph node–negative, ER-positive breast cancer, obesity was not associated with a material increase in recurrence risk or a change in tamoxifen efficacy. However, because obesity was associated with increased risks of contralateral breast cancer, of other primary cancers, and of overall mortality, it may influence long-term outcomes for breast cancer survivors.
The relationship between obesity and prognosis after breast cancer has been investigated extensively, and it is generally accepted that women with a greater body mass index (BMI) or with other anthropometric measures indicative of high body fat have less favorable outcomes than lean women. A review of studies through 1988 found a moderately poorer prognosis for obese patients, particularly those who were postmenopausal at diagnosis (1). However, several of the larger studies reviewed reported the effect to be small (2–4). Several subsequent studies (5–8) have also found obesity to be associated with a less favorable prognosis, whereas in others (9–12) the association was modest or absent. A recent comprehensive review (13) supports the view that obesity is associated with poorer prognosis in both premenopausal and postmenopausal women.
Obesity is prognostic in part because of its association with less favorable disease features at diagnosis, such as larger tumors and a greater number of involved lymph nodes (14–20). However, the predominant hypothesis relating obesity to prognosis concerns the influence of adipose tissue volume on hormone levels. Specifically, high fat volume is associated with elevated levels of serum estrogen; this elevation is mediated both by the conversion of androgens by the aromatase enzyme in adipose fat (21–23) and by a decrease in sex hormone–binding globulin, which lowers estrogen activity (21,24,25). The resulting increased estrogen availability can potentially influence both the characteristics and the growth rate of any breast tumor that develops. If this hypothesis is correct, then the prognostic effect of obesity may depend on menopausal status, because premenopausal and postmenopausal women differ in their hormonal milieus, and also on whether the breast tumor expresses estrogen receptors.
An additional question concerning obesity and breast cancer is whether obesity affects treatment efficacy. In a recent review, Chlebowski et al. (13) pointed out that few studies have addressed interactions among adjuvant therapy, obesity, and clinical outcomes. Numerous mechanisms have been suggested by which obesity may influence the efficacy of cancer therapeutic agents, but we are aware of only one study that has rigorously investigated (i.e., in a large sample under randomized treatment assignment) whether obesity is associated with a material alteration in the benefits and risks associated with adjuvant therapies in common use (12). In that study, among lymph node–positive breast cancer patients, obesity was not associated with any decrease in the efficacy of chemotherapy (12). Chlebowski et al. (13) also noted that, although survivorship among those with early-stage breast cancer is high, the risk of subsequent recurrence remains elevated for 10–15 years following diagnosis, suggesting a potentially beneficial role for additional interventions such as weight reduction and maintenance of a lower body weight. Moreover, high breast cancer survivorship necessitates a shift of attention to outcomes such as other cancers and deaths from noncancer causes. Although it has long been recognized that having had breast cancer increases a woman’s risk for subsequent cancers of the breast and other sites (26–28), there has been growing appreciation of the association between obesity and risk of several major cancers (29). Because obesity is prevalent in women with breast cancer (12,30), it may influence the long-term welfare of survivors of the disease.
To address some of the outstanding questions about obesity and outcomes after breast cancer, we analyzed a cohort of women with lymph node–negative, estrogen receptor (ER)–positive breast cancer, a disease type representing approximately 40% of new breast cancers diagnosed in the United States in recent years (31). The study cohort consisted of women who participated in a multicenter clinical trial evaluating the use of tamoxifen as adjuvant therapy after surgery. We examined the association of obesity with breast cancer recurrence, with second primary cancers of the contralateral breast, with the incidence of other second primary cancers, and with several mortality endpoints. We also examined whether the effect of tamoxifen on breast cancer recurrence and mortality differed according to obesity status at diagnosis.
Patients and Methods
The NSABP B-14 Trial
The NSABP is a National Cancer Institute–sponsored multicenter cooperative clinical trials group that has evaluated strategies for the treatment of breast and colorectal cancer for more than 40 years. In 1982, NSABP Protocol B-14 was begun to evaluate the efficacy of tamoxifen after surgery among women with ER-positive breast tumors and negative lymph nodes. Women who did not have serious concomitant disease that would preclude treatment were eligible to enter the trial if they had operable breast tumors that were ER-positive (≥10 fmol/mg cytosol protein) and axillary lymph nodes that were determined to be negative for tumor cells on histologic examination. After written informed consent was obtained, patients were randomly assigned (in a double-blind fashion) after surgery to receive either tamoxifen (20 mg daily by mouth) or placebo for 5 years. Entry to the original protocol began in January 1982 and was terminated in January 1988, after 2892 patients had been randomly assigned. An additional 1235 patients meeting the same entry criteria entered a tamoxifen registration arm of the trial from January through October 1988. Meanwhile, in April 1987, a second trial was initiated to evaluate the effectiveness of tamoxifen for 10 years compared with 5 years, with selected patients from the tamoxifen randomization and registration arms of the original B-14 trial participating after they had completed 5 years on study. Institutional Review Board approval of the B-14 trial was obtained at each participating center. Additional study design details and major findings of the trial have been presented previously (32–34).
For this analysis, we included all randomized and registered patients in NSABP B-14 who had follow-up information after entry, who met the eligibility requirement for tumor estrogen receptors and lymph node status, and for whom height and weight information was recorded in original study data forms (3385 patients; 2355 from the randomized trial and 1030 from the registration arm). Comparisons of this cohort with all B-14 participants (4127 patients) revealed no systematic differences in characteristics or outcomes (data not shown). Median follow-up was 166 months, and active follow-up continues for all living patients. Results presented here reflect information received at the NSABP data coordinating center through September 30, 2001.
Endpoints and Statistical Methods
BMI, defined as weight in kilograms divided by height in meters squared was used as the determinant of obesity. For our primary analyses, we used BMI categories defined by the Centers for Disease Control and Prevention (CDC) (35): underweight, <18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; and obese, ≥30 kg/m2. Patient and tumor characteristics were compared by BMI category using Fisher’s exact test.
In the B-14 trial, the primary endpoints were disease-free survival (DFS), defined as length of time after surgery that the woman was free of breast cancer recurrence or a new primary cancer at any site or death prior to these events (i.e., time to first event of any kind), and overall survival. For this analysis, we used competing risks methods to evaluate the events constituting DFS, specifically the hazard rate and cumulative incidence of 1) breast cancer recurrence, defined as tumor recurrence at any local, regional, or distant anatomic site; 2) contralateral breast tumors; 3) second primary cancers of the endometrium; 4) second primary cancers at other sites; and 5) death prior to any of these events. Although second primary cancers may occur after breast cancer recurrence and multiple second primary tumors may occur in an individual, the analyses presented in this article considered first events only, classified into the mutually exclusive and exhaustive categories defined here. Additional analyses of all second primary cancers did not yield materially different results (data not shown).
Overall survival time, defined as time from surgery until death from any cause, was the primary mortality endpoint for the B-14 trial. In addition to total survival, we examined cause-specific mortality. It is often difficult to unequivocally establish cause of death in multicenter clinical trials (36,37). Therefore, to address whether mortality following diagnosis and treatment of breast cancer was due to the disease or to some other cause, a simple classification scheme was used that did not require extensive review of available materials to establish cause of death. Deaths were classified as either 1) following breast cancer recurrence or second primary contralateral breast cancer (because all such events were reliably recorded) or 2) not following such an event. The majority of deaths of the first type are due to breast cancer, whereas almost all deaths of the latter type are due to noncancer causes or to second primary cancers of other organ sites. There is precedent for this type of classification system in other instances in which cause of death may be unavailable or inconsistently reported (38).
The cumulative probabilities of breast cancer recurrence, second primary cancers, and cause-specific mortality were estimated using cumulative incidence estimators to correctly account for competing risks (39). The Kaplan–Meier estimator was used to estimate total survival (40). In this report, we cite 10-year percentages from these time-to-event distributions.
The prognostic influence of BMI and other patient and tumor characteristics on cause-specific event hazards was assessed using the Cox proportional hazards model (41). The proportionality assumption was evaluated by examining interactions between hazard ratios (HRs) for BMI and follow-up time and was found to be satisfactorily met. From the Cox model, event hazard ratios with 95% confidence intervals (CIs) were obtained for patients classified as underweight, overweight, or obese relative to normal-weight patients, taking into account potential confounding by age and menopausal status at diagnosis, race, tumor size, and tumor estrogen and progesterone receptor content. Because BMI may have a nonlinear relationship with failure risk for some endpoints, we also used spline models and similar methods to explore appropriate functional forms for modeling BMI on a continuous scale in relation to event hazards (42) (data not shown). The main effect for BMI and interaction effects between BMI, menopausal status, and tamoxifen use were evaluated using likelihood ratio tests, for which two-sided P values are reported.
Results
BMI and Other Patient and Disease Characteristics
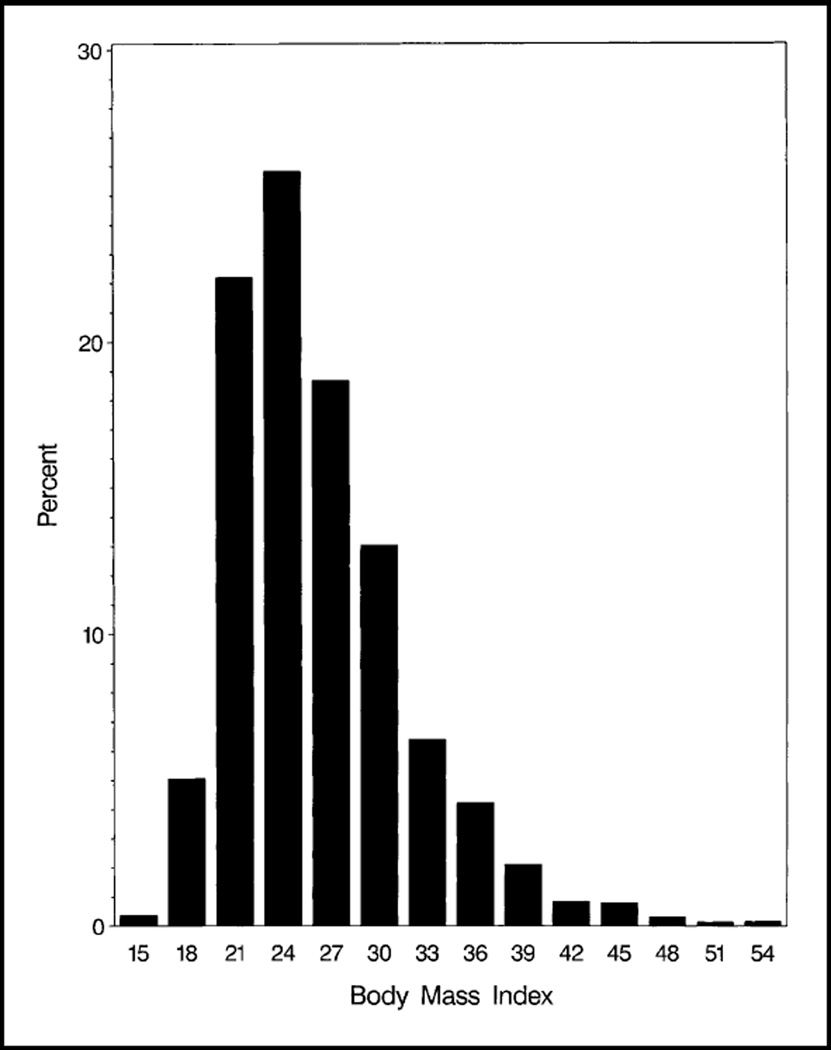
BMI among women in the cohort ranged from 13.8 to 55.2 kg/m2 (mean, 26.2 kg/m2; median, 25.1 kg/m2). Thus, approximately 50% of the women were overweight or obese according to the CDC definition (35), with 20% classified as obese (Fig. 1). Only 2.5% of the women were underweight at diagnosis. Women who were older at diagnosis, had larger tumors, or were African American were more likely to be overweight or obese (Table 1). Women who had lower tumor ER and progesterone receptor (PR) levels were more likely to be underweight.
Fig. 1.
Distribution of body mass index (BMI) defined as [weight in kilograms]/[height in meters]2 among the 3385 women in the NSABP B-14 trial included in this analysis.
Table 1.
Patient and tumor characteristics according to body mass index (BMI) classification*
| Characteristic | BMI category† | All patients, No. (%) (N = 3385) |
P‡ | |||
|---|---|---|---|---|---|---|
| Underweight, % (n = 83) |
Normal weight, % (n = 1593) |
Overweight, % (n = 1022) |
Obese, % (n = 687) |
|||
| Treatment | .16 | |||||
| Placebo | 31.3 | 36.4 | 35.5 | 31.7 | 1187 (35.1) | |
| Tamoxifen | 68.7 | 63.6 | 64.5 | 68.3 | 2198 (64.9) | |
| Age at diagnosis, y | <.001 | |||||
| <40 | 18.1 | 11.1 | 5.3 | 5.3 | 282 (8.3) | |
| 40–49 | 33.7 | 26.4 | 20.1 | 16.7 | 769 (22.7) | |
| 50–59 | 26.5 | 28.7 | 30.5 | 32.0 | 1011 (29.9) | |
| ≥60 | 21.7 | 33.8 | 44.1 | 45.9 | 1323 (39.1) | |
| Menopausal status at diagnosis | <.001 | |||||
| Pre-/perimenopausal | 42.2 | 37.5 | 25.7 | 20.4 | 1035 (30.6) | |
| Postmenopausal | 57.8 | 62.5 | 74.3 | 79.6 | 2350 (69.4) | |
| Race | <.001 | |||||
| White | 85.5 | 92.7 | 90.7 | 88.5 | 3082 (91.1) | |
| Black | 4.8 | 2.9 | 4.9 | 6.8 | 146 (4.3) | |
| Other/unknown | 9.6 | 4.4 | 4.5 | 4.7 | 157 (4.6) | |
| Clinical tumor size, cm | .002 | |||||
| ≤2.0 | 65.1 | 61.8 | 62.8 | 54.7 | 2056 (60.7) | |
| 2.1–4.0 | 28.9 | 34.3 | 33.1 | 38.1 | 1170 (34.6) | |
| ≥4.1 | 6.0 | 3.9 | 4.1 | 7.2 | 159 (4.7) | |
| Tumor ER level, fmol/mg | <.001 | |||||
| 10–49 | 59.0 | 48.3 | 42.1 | 36.5 | 1500 (44.3) | |
| 50–99 | 20.5 | 21.0 | 20.7 | 23.6 | 725 (21.4) | |
| ≥100 | 20.5 | 30.7 | 37.2 | 39.9 | 1160 (34.3) | |
| Tumor PR level, fmol/mg | <.001 | |||||
| 0–9 | 27.7 | 24.4 | 20.0 | 17.9 | 738 (21.8) | |
| 10–49 | 30.1 | 23.4 | 23.3 | 21.0 | 780 (23.0) | |
| 50–99 | 18.1 | 14.0 | 14.7 | 16.6 | 502 (14.8) | |
| ≥100 | 24.1 | 38.2 | 42.1 | 44.5 | 1365 (40.3) | |
BMI is defined as weight in kilograms divided by height in meters squared; ER = estrogen receptor; PR = progesterone receptor.
Centers for Disease Control and Prevention BMI categories: underweight = <18.5 kg/m2, normal weight = 18.5–24.9 kg/m2, overweight = 25.0–29.9 kg/m2, obese = ≥30.0 kg/m2.
Two-sided Fisher’s exact test of association between BMI category and the characteristic.
Outcomes in Relation to Obesity
Breast cancer recurrence
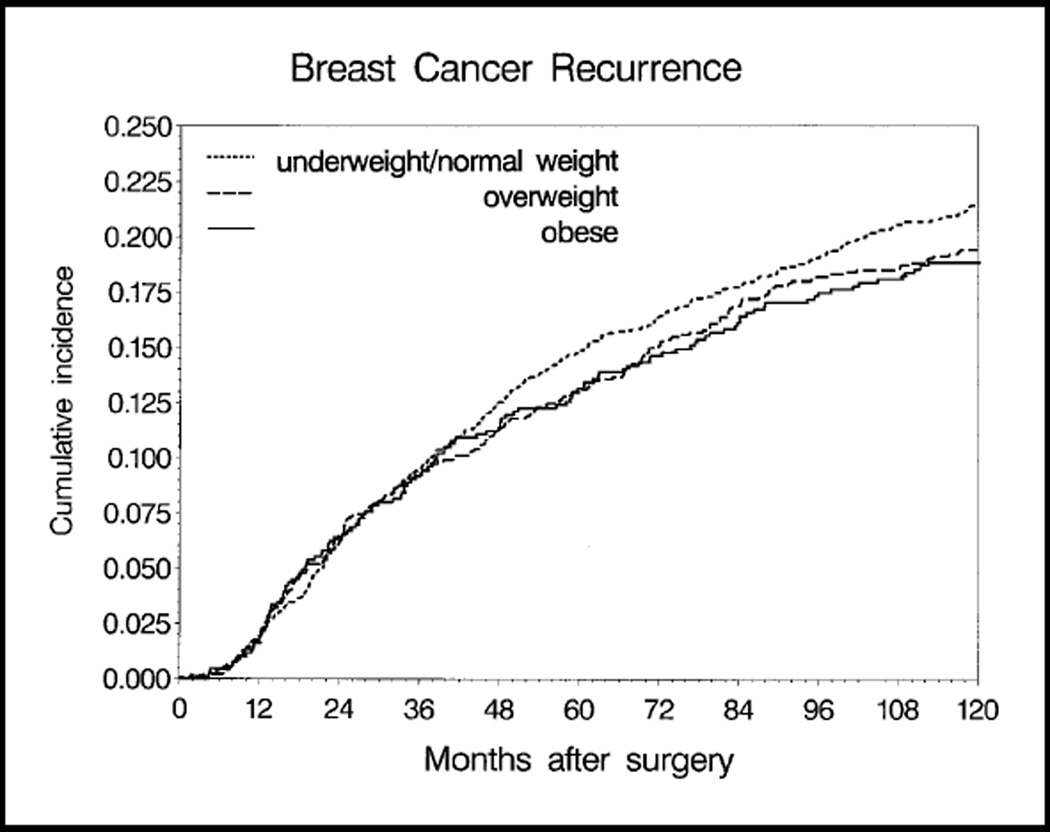
Cumulative incidence of breast cancer recurrence was determined for underweight/normal-weight, overweight, and obese women (Fig. 2). (Underweight and normal-weight women did not differ in incidence of recurrence, of contralateral breast cancers, or of second primary cancers and were therefore combined for analyses of these endpoints.) At 10 years after surgery, the cumulative probability of recurrence ranged from 18.8% in obese women to 21.4% in underweight/normal-weight women. In a multivariable model for breast cancer recurrence hazard, obesity was not associated with a higher risk of recurrence (Table 2). The association of obesity with recurrence risk did not differ by menopausal status at diagnosis (menopausal status by BMI interaction test P = .35). Tamoxifen use, older age at diagnosis, and smaller tumors were associated with lower recurrence hazard (data not shown).
Fig. 2.
Cumulative incidence of breast cancer recurrence by body mass index, unadjusted for patient and disease characteristics. At 10 years after surgery, the numbers of patients remaining in each weight group were underweight/normal-weight women (≤24.9 kg/m2), 1073 patients; overweight women (25.0–29.9 kg/m2), 643 patients; obese women (≥30.0 kg/m2), 395 patients. The cumulative incidences of recurrence (with 95% confidence intervals [CIs]) were 0.214 (95% CI = 0.194 to 0.234) for underweight/normal-weight women, 0.194 (95% CI = 0.170 to 0.219) for overweight women, and 0.188 (95% CI = 0.159 to 0.218) for obese women.
Table 2.
Relative hazard of breast cancer events according to body mass index (BMI) classification*
| Hazard ratio† (95% confidence interval) |
||
|---|---|---|
| Breast cancer recurrence (787 events) |
Contralateral breast tumors (193 events) |
|
| BMI‡ | ||
| Underweight/normal weight§ | 1.00 (referent) | 1.00 (referent) |
| Overweight | 1.01 (0.86 to 1.19) | 1.22 (0.87 to 1.71) |
| Obese | 0.98 (0.80 to 1.18) | 1.58 (1.10 to 2.25) |
| P‖ | .94 | .04 |
BMI is defined as weight in kilograms divided by height in meters squared.
Hazard ratio from the Cox model, adjusted for treatment, age, menopausal status, race, tumor size, estrogen receptor level, and progesterone receptor level.
Centers for Disease Control and Prevention categories of BMI: underweight = <18.5 kg/m2, normal weight = 18.5–24.9 kg/m2, overweight = 25.0–29.9 kg/m2, obese = ≥30.0 kg/m2.
Underweight and normal-weight women were combined for these analyses because results for these endpoints did not differ.
Global likelihood ratio test of statistical significance for BMI.
Second primary cancers of the contralateral breast
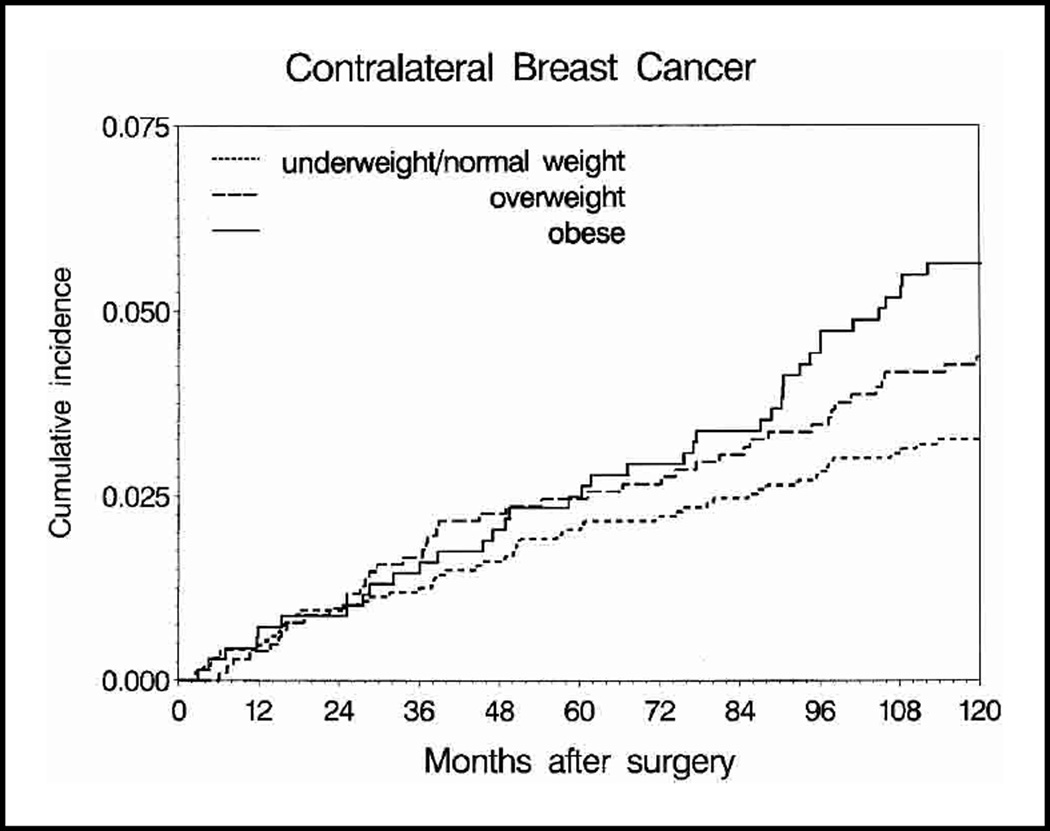
Tenyear cumulative incidence of contralateral breast cancer ranged from 3.3% in underweight/normal-weight women to 5.6% in obese women (Fig. 3). Adjusting for other patient and tumor characteristics, obese women had a statistically significant 1.58-fold greater hazard of contralateral breast tumors relative to underweight/normal-weight women (Table 2). The excess contralateral breast tumor hazard for obese women compared with underweight/normal-weight women was similar in women who were premenopausal at study entry (HR = 1.52, 95% CI = 0.77 to 3.03) and in those who were postmenopausal at study entry (HR = 1.63, 95% CI = 1.07 to 2.51) (interaction test P = .96).
Fig. 3.
Cumulative incidence of contralateral breast cancer by body mass index, unadjusted for patient and disease characteristics. At 10 years after surgery, the numbers of patients remaining in each weight group were underweight/normal weight women (≤24.9 kg/m2), 1073 patients; overweight women (25.0–29.9 kg/m2), 643 patients; obese women (≥30.0 kg/m2), 395 patients. The cumulative incidences of contralateral breast cancer (with 95% confidence intervals [CIs]) were 0.033 (95% CI = 0.024 to 0.041) for underweight/normal weight women, 0.044 (95% CI = 0.031 to 0.057) for overweight women, and 0.056 (95% CI = 0.039 to 0.074) for obese women.
Other second primary cancers
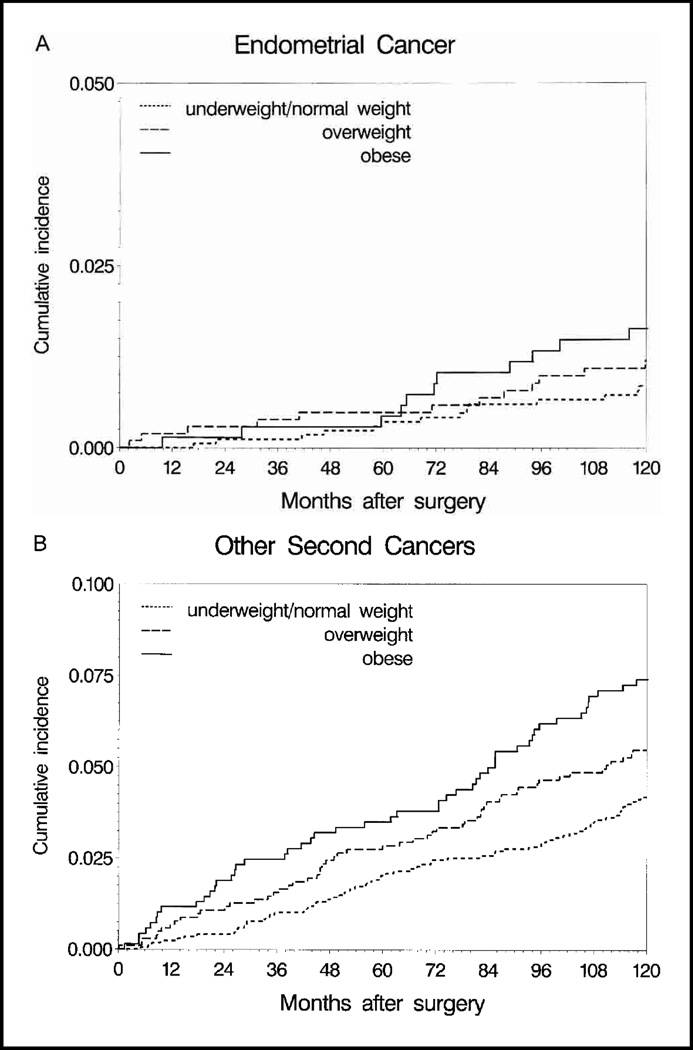
We examined obesity in relation to occurrence of endometrial cancer (51 events) and other second primary cancers (232 events) (Fig. 4). There was some indication of increased endometrial cancer risk among obese women compared with underweight/normal-weight women, but the results did not achieve statistical significance (HR = 1.45, 95% CI = 0.72 to 2.94). Hazard of other second primary cancers (which were seen at 37 different anatomic sites) was increased among overweight (HR = 1.29, 95% CI = 0.95 to 1.76) and obese (HR = 1.62, 95% CI = 1.16 to 2.24) women as compared with underweight/normal-weight women (global test for BMI P = .02).
Fig. 4.
Cumulative incidence of endometrial cancer (A) and other second primary cancers (B) by body mass index, unadjusted for patient and disease characteristics. Note that the y-axis scale differs between the two plots. At 10 years after surgery, the numbers of patients remaining in each weight group were underweight/normal weight women (≤24.9 kg/m2), 1073 patients; overweight women (25.0–29.9 kg/m2), 643 patients; obese women (≥30.0 kg/m2), 395 patients. The cumulative incidences (with 95% confidence intervals [CIs]) of endometrial cancer (A) and other cancers (B) were 0.009 (95% CI = 0.004 to 0.013) and 0.042 (95% CI = 0.032 to 0.052), respectively, for underweight/normal-weight women; 0.012 (95% CI = 0.005 to 0.019) and 0.055 (95% CI = 0.041 to 0.070), respectively, for overweight women; and 0.016 (95% CI = 0.007 to 0.026) and 0.074 (95% CI = 0.054 to 0.094), respectively, for obese women.
Mortality endpoints
Unadjusted 10-year overall survival percentages were 72.8% for underweight women, 81.4% for normal-weight women, 79.9% for overweight women, and 75.2% for obese women. Adjusting for other prognostic covariates, there was a statistically significant, 31% greater mortality hazard for obese women relative to normal-weight women, whereas underweight women had a statistically significant 79% greater mortality hazard (Table 3). The effect of BMI on mortality did not differ by menopausal status (interaction test P = .88).
Table 3.
Relative hazard of death according to body mass index (BMI) classification*
| Hazard ratio† (95% confidence interval) | |||
|---|---|---|---|
| Total mortality (983 events) |
Deaths following breast cancer events (595 events) |
Other deaths (388 events) |
|
| BMI‡ | |||
| Normal weight | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Underweight | 1.79 (1.25 to 2.55) | 1.08 (0.64 to 1.81) | 3.50 (2.13 to 5.73) |
| Overweight | 1.08 (0.93 to 1.25) | 1.02 (0.84 to 1.23) | 1.19 (0.94 to 1.52) |
| Obese | 1.31 (1.12 to 1.54) | 1.20 (0.97 to 1.49) | 1.49 (1.15 to 1.92) |
| P§ | <.001 | .36 | <.001 |
BMI is defined as weight in kilograms divided by height in meters squared.
Hazard ratio from the Cox model, adjusted for treatment, age, menopausal status, race, tumor size, estrogen receptor level, and progesterone receptor level.
Centers for Disease Control and Prevention categories of BMI: underweight = <18.5 kg/m2, normal weight = 18.5–24.9 kg/m2, overweight = 25.0–29.9 kg/m2, obese = ≥30.0 kg/m2.
Global likelihood ratio test of statistical significance for BMI.
Analysis of mortality hazards was conducted for deaths classified into two mutually exclusive groups: those preceded by tumor recurrence or contralateral breast cancer, most of which were likely due to breast cancer, and those not preceded by breast cancer events, most of which were likely due to other cancers or to noncancer causes (Table 3). Assignment of cause of death by this rule and the reported cause of death (death certificates with International Classification of Disease codes were available for 75% of deaths) were highly concordant with respect to presumed cause of death. Of 983 deaths observed, 595 deaths (60.5%) followed breast cancer recurrence or second breast cancers. Relative hazards for these deaths did not differ among underweight, overweight, or obese women relative to women of normal weight (P = .36). By contrast, there was a 3.5-fold increase in hazard of death attributed to other cancers or to noncancer causes among underweight women relative to normal- weight women and an approximately 1.5-fold excess hazard for obese women relative to normal-weight women (Table 3). In a similar analysis in which we considered death prior to recurrence or any second primary cancer occurrence (i.e., death as a first event, n = 243) as a mortality endpoint, hazard ratios were similar for obese (HR = 1.37, 95% CI = 0.99 to 1.89) and underweight (HR = 3.47, 95% CI = 1.90 to 6.34) women relative to women of normal weight. These deaths, which were mostly attributable to noncancer causes (e.g., other chronic diseases), occurred primarily among older patients and accounted for only 6% of all deaths in the premenopausal women as compared with 32% of all deaths in the postmenopausal women.
Tamoxifen Efficacy and Obesity
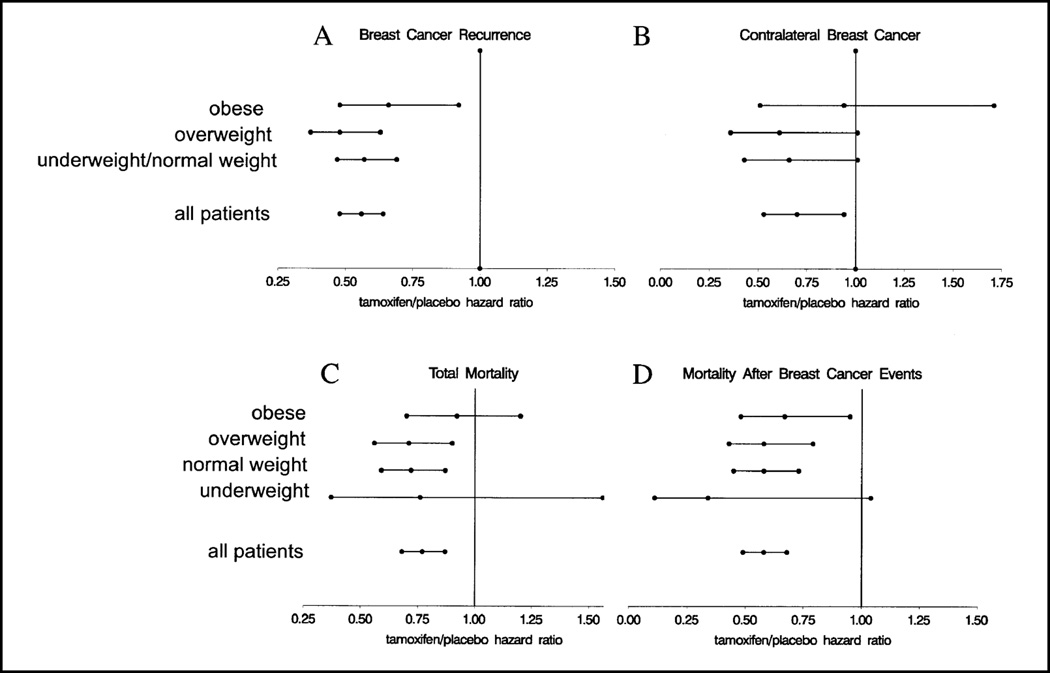
We examined whether the effect of tamoxifen differed according to obesity by testing for interaction effects between BMI and treatment group in the statistical models evaluating the prognostic significance of BMI described earlier. The statistically significant reduction in breast cancer recurrence hazard associated with tamoxifen use did not vary across BMI groups (interaction test P = .34). By computing tamoxifen/placebo breast cancer recurrence hazard ratios we found that, within each BMI category, women receiving tamoxifen had an approximately 40% reduction in the hazard of breast cancer recurrence compared with women receiving placebo (Fig. 5, A).
Fig. 5.
Tamoxifen/placebo hazard ratios according to BMI. Hazard ratios with 95% confidence intervals were computed within BMI categories for breast cancer recurrence (A) and contralateral breast cancer (B). Because underweight and normal-weight women had similar outcomes for these endpoints, these categories were combined. Hazard ratios with 95% confidence intervals were computed within BMI categories (obese, ≥30.0 kg/m2; overweight, 25.0–29.9 kg/m2; normal weight, 18.5–24.9 kg/m2; underweight, ≤18.5 kg/m2) for all deaths (C) and deaths preceded by breast cancer recurrence or occurrence of contralateral breast tumor (D). For all panels, hazard ratios of less than 1.0 indicate a more favorable outcome for women receiving tamoxifen.
There were relatively few contralateral breast cancer events for treatment comparisons stratified by BMI and, thus, these estimates are more variable than those for other endpoints (Fig. 5, B). Nevertheless, a formal test for interaction between tamoxifen use and BMI did not suggest a statistically significant difference in hazard ratios among the groups (P = .35).
Overall, women who used tamoxifen had a 23% reduction in overall mortality relative to women who took placebo, and this effect did not differ by BMI group (interaction test P = .43), although the plotted tamoxifen/placebo mortality hazard ratios suggest that the mortality reduction is minimal in obese women (Fig. 5, C). For deaths following breast cancer events, women receiving tamoxifen had lower mortality than women receiving placebo in each BMI group (Fig. 5, D).
Because determinations of treatment efficacy should ideally be based strictly on comparisons of randomized groups, we repeated these analyses among randomized patients only (1187 in the placebo arm, 1168 in the tamoxifen arm)—that is, excluding the 1030 patients who entered the tamoxifen registration arm of the trial after randomization ended in January 1988. Results were similar to those for the entire patient group (data not shown).
Discussion
Data from clinical trials offer a number of advantages over data from other sources for assessing the effects of obesity on breast cancer prognosis. Patients in clinical trials are homogeneous with respect to disease stage at diagnosis, as defined in the protocol entry criteria, and have minimal concurrent serious morbidity at study entry. Treatment delivery is uniform and quality controlled, and detailed information on clinical and pathologic disease features is available. A particular advantage of the B-14 trial is the large number of patients with long follow-up duration, which yielded adequate statistical power to test the association of obesity with multiple endpoints as well as to evaluate whether obesity has a differential prognostic effect depending on menopausal status or treatment. The restriction of the study population to patients with ER-positive tumors confined to the breast allowed us to focus on the potential contribution of obesity to breast cancer prognosis among women with putatively hormone-sensitive tumors while reducing the confounding effects of disease stage, different treatment regimens, and other extraneous factors.
Numerous investigations [reviewed in (13)] have reported a 1.25- to 2.5-fold increase in breast cancer recurrence and mortality hazard for obese versus lean women. In our study of women with early-stage breast cancer, by contrast, obesity was not associated with increased recurrence risk. Several reasons for why our results differ from those of the earlier studies can be identified. First, our population consisted only of patients with operable tumors and negative axillary lymph nodes, whereas most other studies included patients from a broader spectrum of disease extent. Obesity has been found to be associated with a more advanced stage of disease and unfavorable features such as a greater number of tumor-positive lymph nodes, and these effects are eliminated in this patient cohort [although it should be noted that some studies (8,43,44) have found a larger prognostic influence specifically among obese women with negative lymph nodes]. In addition, all patients in this study had ER-positive tumors, which are generally associated with a more favorable prognosis. Whereas some studies (45–49) have suggested that obese women have a higher frequency of ER-positive tumors than non-obese women, other studies (50–53) have found a greater frequency of ER-negative tumors among obese women; because ER-negative tumors are generally associated with poorer prognosis, such a phenomenon could account for the less favorable prognosis among obese women. Finally, our cohort consisted entirely of clinical trial participants, who may represent a generally healthier group of breast cancer patients, because the serious comorbid conditions associated with extreme obesity would render an individual ineligible for the trial. Nevertheless, 50% of the women in this cohort were classified as overweight or obese according to current CDC guidelines. Uniform treatment and follow-up care might also contribute to better outcomes for the obese women in our study. Indeed, other studies among single institutions or clinical trial databases have also found that BMI has a relatively modest effect or no effect on prognosis (9,12).
A traditional hypothesis for how obesity affects breast cancer recurrence risk is based on the mitogenic effect of excess estrogen produced in fat cells via the enzymatic conversion of adrenal steroids to estrogen. It might be anticipated that obesity would be prognostic via this mechanism only in postmenopausal women, because in premenopausal women ovarian estrogen production and the large changes in circulating hormone levels coinciding with menstrual cycles might overwhelm any estrogen contribution due to obesity. Furthermore, some studies (54,55) have suggested that obesity is associated with decreased, rather than increased, serum estradiol levels in premenopausal women. In our study, neither premenopausal nor postmenopausal obese women had increased recurrence risk. The lack of an association between obesity and breast cancer recurrence hazard, even in postmenopausal women, might imply that the increased circulating estrogens attributable to obesity are not sufficient to increase the risk of recurrence. Alternatively, it is possible that intracellular estrogen production in neoplastic breast cells overshadows any effect of variation in circulating estrogens. In support of this hypothesis are the findings that estrogen levels in breast cancers from postmenopausal women are 10-fold higher than plasma estrogen concentrations (56–58) and that the intratumoral activity of aromatase is high in patients responding to endocrine therapy (59).
Body composition with respect to fat has long been implicated as a breast cancer risk factor, partly as a result of a strong correlation observed between breast cancer incidence and fat content in diets worldwide (60). Although obesity has been convincingly associated with breast cancer risk in numerous studies (61–66), the specific findings are often complex and involve additional factors (67–71). For example, results from the Nurses’ Health Study suggested that weight gain after age 18 years, interacting with hormone replacement therapy use, may account for as many as one-third of postmenopausal breast cancer cases (69). A recent pooled analysis of data from seven cohort studies estimated a 26% excess breast cancer risk for postmenopausal obese women (72). As with the association between obesity and breast cancer prognosis, a variety of underlying mechanisms linking obesity with increased breast cancer risk can be invoked, including exposure to carcinogens contained in dietary fat, effects of energy balance and calorie restriction on cancer risk throughout life, elevated exposure to endogenous estrogen secondary to androgen conversion by the aromatase enzyme in adipose tissue, and effects on sex hormone– binding globulin, progesterone, insulin, and insulin-like growth factor. Whatever the mechanism(s) of action are, obesity is of particular interest as a breast cancer risk factor because the risks it poses can potentially be modified.
In contrast to our findings for breast cancer recurrence, we found, among both premenopausal and postmenopausal women, greater risk of contralateral breast tumors in obese women as compared with women of normal or low weight. For postmenopausal women, this finding is consistent with those of the numerous population-based case–control and cohort studies cited earlier (69,72), as well as with the finding of an earlier study (73) that examined contralateral breast cancer in African American and Caucasian women participating in NSABP trials. Interestingly, obesity has been associated with decreased breast cancer risk among premenopausal women in many studies (63,67,69, 72,74,75), but our study did not show a decreased contralateral breast tumor risk for obese women who were premenopausal at diagnosis. However, risk factors for subsequent breast cancers among breast cancer patients and the population at large may be different. Results of the recent pooled analysis by van den Brandt et al. (72) suggested that only those premenopausal women with BMIs greater than about 32 had decreased breast cancer risk, and only a small proportion of women in our study (13%) had a BMI in this range. The observation that the obese women in our study had a greater risk of contralateral tumors but not of recurrence suggests that obesity may act as an indicator of risk incurred cumulatively or at some earlier time in life, rather than imparting risk through enhanced estrogen exposure over some time period after the diagnosis of the first breast tumor.
In this study, obese women did have a modestly increased mortality risk relative to normal-weight women, and the small proportion of women classified as underweight also had increased mortality relative to normal-weight women. When considering mortality in relation to body composition, it is of interest to determine which causes of death are affected. Although detailed documentation is obtained from participating centers for disease recurrence and adverse events, including the occurrence of second primary cancers, information on cause of death is less reliable and does not adhere to nosology coding standards. Studies (36,37) have shown that problems can arise when reported cause-of-death information from clinical trials is used without careful review. As a practical remedy, therefore, we classified deaths according to whether the death was preceded by breast cancer recurrence or occurrence of a contralateral breast tumor, treating deaths with preceding breast cancer events as breast cancer–related deaths and the other deaths as due to other causes. Our analysis by cause of death suggests that the increased mortality associated with obesity was due specifically to death from causes other than breast cancer (i.e., other chronic diseases or other cancers).
Our study has several limitations. First, we did not have information pertaining to weight or obesity throughout different periods of life, including adolescence and early adult years, which could be informative, given that some researchers have suggested that these measures may have substantial influence on current risk (72). Second, we also lacked information on socioeconomic measures, dietary history, and other factors that could have contributed to obesity at the time of study entry. However, with regard to dietary factors, findings of previous studies (3,7,9,17,76,77) of the association between pre-disease diet and either tumor characteristics or eventual prognosis have been inconsistent. Finally, our study lacked longitudinal measurements of obesity during and after treatment, and weight change has been found to be a potentially important prognostic factor in some chemotherapy trials (13,78). However, results of earlier analyses of tamoxifen-associated adverse events (32,79,80) suggested that tamoxifen use, unlike chemotherapy, is not associated with substantial weight gain or loss; if so, BMI at trial entry can be considered a reasonable estimate of post-disease body composition. Serial obesity measures could lend insight into whether weight changes after breast cancer can alter breast cancer prognosis or risk, although a study the size of NSABP B-14 would likely be underpowered to detect such effects. Large prospective studies, such as the dietary modification portion of the Women’s Health Initiative trial (81), may eventually provide information on this question.
Few studies reporting a relationship between breast cancer prognosis and obesity have examined the question of whether there is a differential benefit of systemic adjuvant therapy among lean and obese women, and no study has specifically evaluated the effect of obesity on treatment efficacy among women with ER-positive, lymph node–negative breast cancer. In a study of chemotherapy regimens in lymph node–positive patients, obesity in and of itself did not influence treatment efficacy, but dose alterations due to concerns about drug toxicity may have led to worse prognoses among obese women (12). Our findings similarly suggest that obesity is not associated with a material alteration in the effectiveness of tamoxifen for breast cancer recurrence and mortality. Nonetheless, because tamoxifen may increase the risk of certain adverse events, such as pulmonary embolism and other thromboembolic events (75), and because obesity may represent an additional risk factor for these events, such risks must be weighed against the benefits of tamoxifen for obese women. The suggestion of increased endometrial cancer risk among overweight and obese women in our study and in the study by Bernstein et al. (82) might also provide guidance for monitoring and risk management of this cancer among women using tamoxifen.
In light of the increased risks of contralateral breast cancer and other cancers associated with obesity, behavioral interventions combined with agents such as tamoxifen might offer the best approach to breast cancer risk reduction among obese women. Because obesity is associated with increased estrogen availability, one might conjecture that antiestrogen therapy might be of greater benefit to obese women than non-obese women. Indeed, an analysis of raloxifene use and breast cancer incidence stratified by estrogen-level measures suggested a somewhat larger risk reduction for raloxifene in women with higher BMI (83). However, a randomized study (84) exploring tamoxifen dose reduction found no difference in drug or metabolite levels according to BMI, although the extent to which obese women were represented in that study was not stated. Thus, it is unclear whether benefit from antiestrogens might vary according to surrogate or direct measures of estrogen levels. At present, our findings support the use and benefits of tamoxifen in breast cancer patients of all body types, subject to appropriate cautions for the health conditions that often accompany obesity.
Acknowledgments
E. P. Mamounas is on the advisory board and speaker’s bureau for AstraZeneca, makers of tamoxifen.
Supported by Public Health Service grants NCI-U10-CA-69651, NCI-U10-CA-12027, NCI-P30-CA-14599, and NCI-R03-CA-99508 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and by a grant to J. J. Dignam from the University of Chicago Cancer Research Foundation Women’s Board.
We thank Barbara Good, PhD, for editorial review and improvements and John Bryant, PhD, for insightful comments.
References
- 1.Goodwin PJ, Boyd NF. Body size and breast cancer prognosis: a critical review of the evidence. Breast Cancer Res Treat. 1990;16:205–214. doi: 10.1007/BF01806329. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg ER, Vessey MP, McPherson K, Doll R, Yeates D. Body size and survival in premenopausal breast cancer. Br J Cancer. 1985;51:691–697. doi: 10.1038/bjc.1985.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregorio DI, Emrich LJ, Graham S, Marshall JR, Nemoto T. Dietary fat consumption and survival among women with breast cancer. J Natl Cancer Inst. 1985;75:37–41. [PubMed] [Google Scholar]
- 4.Goodwin PJ, Panzarella T, Boyd NF. Weight gain in women with localized breast cancer--a descriptive study. Breast Cancer Res Treat. 1988;11:59–66. doi: 10.1007/BF01807559. [DOI] [PubMed] [Google Scholar]
- 5.Mohle-Boetani JC, Grosser S, Whittemore AS, Malec M, Kampert JB, Paffenbarger RS., Jr Body size, reproductive factors, and breast cancer survival. Prev Med. 1988;17:634–642. doi: 10.1016/0091-7435(88)90056-4. [DOI] [PubMed] [Google Scholar]
- 6.Coates RJ, Clark WS, Eley JW, Greenberg RS, Huguley CM, Jr, Brown RL. Race, nutritional status, and survival from breast cancer. J Natl Cancer Inst. 1990;82:1684–1692. doi: 10.1093/jnci/82.21.1684. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. Cancer. 1995;76:275–283. doi: 10.1002/1097-0142(19950715)76:2<275::aid-cncr2820760218>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Newman SC, Lees AW, Jenkins HJ. The effect of body mass index and oestrogen receptor level on survival of breast cancer patients. Int J Epidemiol. 1997;26:484–490. doi: 10.1093/ije/26.3.484. [DOI] [PubMed] [Google Scholar]
- 9.Jain M, Miller AB, To T. Premorbid diet and the prognosis of women with breast cancer. J Natl Cancer Inst. 1994;86:1390–1397. doi: 10.1093/jnci/86.18.1390. [DOI] [PubMed] [Google Scholar]
- 10.Bastarrachea J, Hortobagyi GN, Smith TL, Kau SW, Buzdar AU. Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med. 1994;120:18–25. doi: 10.7326/0003-4819-120-1-199401010-00004. [DOI] [PubMed] [Google Scholar]
- 11.den Tonkelaar I, de Waard F, Seidell JC, Fracheboud J. Obesity and subcutaneous fat patterning in relation to survival of postmenopausal breast cancer patients participating in the DOM-project. Breast Cancer Res Treat. 1995;34:129–137. doi: 10.1007/BF00665785. [DOI] [PubMed] [Google Scholar]
- 12.Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from Cancer and Leukemia Group B study 8541. J Clin Oncol. 1996;14:3000–3008. doi: 10.1200/JCO.1996.14.11.3000. [DOI] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 14.Zumoff B, Dasgupta I. Relationship between body weight and the incidence of positive nodes at mastectomy in breast cancer. J Surg Oncol. 1983;22:217–220. doi: 10.1002/jso.2930220402. [DOI] [PubMed] [Google Scholar]
- 15.Verreault R, Brisson J, Deschenes L, Naud F. Body weight and prognostic indicators in breast cancer. Am J Epidemiol. 1989;129:260–268. doi: 10.1093/oxfordjournals.aje.a115131. [DOI] [PubMed] [Google Scholar]
- 16.Jones BA, Kasl SV, McCrea-Curnen MG, Owens PH, Dubrow R. Severe obesity as an explanatory factor for the black/white difference in stage at diagnosis of breast cancer. Am J Epidemiol. 1997;146:394–404. doi: 10.1093/oxfordjournals.aje.a009292. [DOI] [PubMed] [Google Scholar]
- 17.Jain M, Miller AB. Tumor characteristics and survival of breast cancer patients in relation to premorbid diet and body size. Breast Cancer Res Treat. 1997;42:43–55. doi: 10.1023/a:1005798124538. [DOI] [PubMed] [Google Scholar]
- 18.Madarnas Y, McCready D, Pritchard KI, Koo J, Trudeau M, Hood N, et al. Can body size or diet predict tumor characteristics in an incident cohort of women with locoregional breast cancer? [abstract 415] Breast Cancer Res Treat. 1999;57:101. [Google Scholar]
- 19.Moorman PG, Jones BA, Millikan RC, Hall IJ, Newman B. Race, anthropometric factors, and stage at diagnosis of breast cancer. Am J Epidemiol. 2001;153:284–291. doi: 10.1093/aje/153.3.284. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y, Whiteman MK, Flaws JA, Langenberg P, Tkaczuk KH, Bush TL. Body mass and stage of breast cancer at diagnosis. Int J Cancer. 2002;98:279–283. doi: 10.1002/ijc.10209. [DOI] [PubMed] [Google Scholar]
- 21.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45:277–282. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 22.Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129:1120–1131. doi: 10.1093/oxfordjournals.aje.a115234. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 24.Selby C. Sex hormone binding globulin: origin, function, and significance. Ann Clin Biochem. 1990;27:532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15:48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 26.Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. J Natl Cancer Inst. 1995;87:645–651. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- 27.Fowble B, Hanlon A, Freedman G, Nicolaou N, Anderson P. Second cancers after conservative surgery and radiation for stages I–II breast cancer: identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679–690. doi: 10.1016/s0360-3016(01)01665-0. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AG, Ragheb NE, Swanson GM, Satariano WA. Racial and age differences in multiple primary cancers after breast cancer: a population-based analysis. Breast Cancer Res Treat. 1989;14:245–254. doi: 10.1007/BF01810741. [DOI] [PubMed] [Google Scholar]
- 29.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 30.Madarnas Y, Sawka CA, Franssen E, Bjarnason GA. Are medical oncologists biased in their treatment of the large woman with breast cancer? Breast Cancer Res Treat. 2001;66:123–133. doi: 10.1023/a:1010635328299. [DOI] [PubMed] [Google Scholar]
- 31.Ries LA, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al., editors. Bethesda (MD): National Cancer Institute; 2002. [Last accessed August 19, 2003]. SEER Cancer Statistics Review, 1973–1999. Available at: http://seer.cancer.gov/csr/1973_1999/. [Google Scholar]
- 32.Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 33.Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 35.United States Department of Health and Human Services. Rockville (MD): Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [Last accessed: August 19, 2003]. The Surgeon General’s call to action to prevent and decrease overweight and obesity. Available at: http://www.surgeongeneral.gov/topics/obesity/calltoaction/CalltoAction.pdf. [PubMed] [Google Scholar]
- 36.Remington RD. Who should code cause of death in a clinical trial? Control Clin Trials. 1984;5:241–244. doi: 10.1016/0197-2456(84)90027-8. [DOI] [PubMed] [Google Scholar]
- 37.Messite J, Stellman SD. Accuracy of death certificate completion: the need for formalized physician training. JAMA. 1996;275:794–796. [PubMed] [Google Scholar]
- 38.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 39.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11:813–829. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 41.Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 42.Gray R. Flexible methods for analyzing survival data using splines, with applications to breast cancer prognosis. J Am Stat Assoc. 1992;87:942–951. [Google Scholar]
- 43.Donegan WL, Hartz AJ, Rimm AA. The association of body weight with recurrent cancer of the breast. Cancer. 1978;41:1590–1594. doi: 10.1002/1097-0142(197804)41:4<1590::aid-cncr2820410449>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 44.Tretli S, Haldorsen T, Ottestad L. The effect of pre-morbid height and weight on the survival of breast cancer patients. Br J Cancer. 1990;62:299–303. doi: 10.1038/bjc.1990.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donegan WL, Johnstone MF, Biedrzycki L. Obesity, estrogen production, and tumor estrogen receptors in women with carcinoma of the breast. Am J Clin Oncol. 1983;6:19–24. [PubMed] [Google Scholar]
- 46.Hislop TG, Coldman AJ, Elwood JM, Skippen DH, Kan L. Relationship between risk factors for breast cancer and hormonal status. Int J Epidemiol. 1986;15:469–476. doi: 10.1093/ije/15.4.469. [DOI] [PubMed] [Google Scholar]
- 47.Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;151:703–714. doi: 10.1093/oxfordjournals.aje.a010265. [DOI] [PubMed] [Google Scholar]
- 48.Enger SM, Ross RK, Paganini-Hill A, Carpenter CL, Bernstein L. Body size, physical activity, and breast cancer hormone receptor status: results from two case-control studies. Cancer Epidemiol Biomarkers Prev. 2000;9:681–687. [PubMed] [Google Scholar]
- 49.Sellers TA, Davis J, Cerhan JR, Vierkant RA, Olson JE, Pankratz VS, et al. Interaction of waist/hip ratio and family history on the risk of hormone receptor-defined breast cancer in a prospective study of postmenopausal women. Am J Epidemiol. 2002;155:225–233. doi: 10.1093/aje/155.3.225. [DOI] [PubMed] [Google Scholar]
- 50.Papatestas AE, Panveliwalla D, Pertsemlidis D, Mulvihill M, Aufses AH., Jr Association between estrogen receptors and weight in women with breast cancer. J Surg Oncol. 1980;13:177–180. doi: 10.1002/jso.2930130213. [DOI] [PubMed] [Google Scholar]
- 51.McTiernan A, Thomas DB, Johnson LK, Roseman D. Risk factors for estrogen receptor-rich and estrogen receptor-poor breast cancers. J Natl Cancer Inst. 1986;77:849–854. [PubMed] [Google Scholar]
- 52.Stanford JL, Szklo M, Boring CC, Brinton LA, Diamond EA, Greenberg RS, et al. A case-control study of breast cancer stratified by estrogen receptor status. Am J Epidemiol. 1987;125:184–194. doi: 10.1093/oxfordjournals.aje.a114519. [DOI] [PubMed] [Google Scholar]
- 53.Yoo KY, Tajima K, Miura S, Takeuchi T, Hirose K, Risch H, et al. Breast cancer risk factors according to combined estrogen and progesterone receptor status: a case-control analysis. Am J Epidemiol. 1997;146:307–314. doi: 10.1093/oxfordjournals.aje.a009271. [DOI] [PubMed] [Google Scholar]
- 54.Thomas HV, Key TJ, Allen DS, Moore JW, Dowsett M, Fentiman IS, et al. Re: Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. 1997;89:396–398. doi: 10.1093/jnci/89.5.396. [DOI] [PubMed] [Google Scholar]
- 55.Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. 1996;88:756–758. doi: 10.1093/jnci/88.11.756. [DOI] [PubMed] [Google Scholar]
- 56.van Landegham AA, Portman J, Nabauurs M. Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res. 1985;45:2900–2906. [PubMed] [Google Scholar]
- 57.Thorsen T, Tangen M, Stoa KF. Concentrations of endogenous estradiol as related to estradiol receptor sites in breast tumor cytosol. Eur J Cancer Clin Oncol. 1982;18:333–337. doi: 10.1016/0277-5379(82)90002-5. [DOI] [PubMed] [Google Scholar]
- 58.Brodie A, Long B, Lu Q. Aromatase expression in the human breast. Breast Cancer Res Treat. 1998;49(Suppl 1):S85–S91. doi: 10.1023/a:1006029612990. [DOI] [PubMed] [Google Scholar]
- 59.Miller WR, Hawkins RA, Forrest AP. Significance of aromatase activity in human breast cancer. Cancer Res. 1982;42(8 Suppl):3365s–3368s. [PubMed] [Google Scholar]
- 60.Carroll K. Dietary factors in hormone-dependent cancers. Curr Concepts Nutr. 1977;6:25–40. [PubMed] [Google Scholar]
- 61.Lubin F, Ruder AM, Wax Y, Modan B. Overweight and changes in weight throughout adult life in breast cancer etiology. A case-control study. Am J Epidemiol. 1985;122:579–588. doi: 10.1093/oxfordjournals.aje.a114137. [DOI] [PubMed] [Google Scholar]
- 62.Pathak DR, Whittemore AS. Combined effects of body size, parity, and menstrual events on breast cancer incidence in seven countries. Am J Epidemiol. 1992;135:153–168. doi: 10.1093/oxfordjournals.aje.a116268. [DOI] [PubMed] [Google Scholar]
- 63.Kampert JB, Whittemore AS, Paffenbarger RS., Jr Combined effect of childbearing, menstrual events, and body size on age-specific breast cancer risk. Am J Epidemiol. 1988;128:962–979. doi: 10.1093/oxfordjournals.aje.a115070. [DOI] [PubMed] [Google Scholar]
- 64.Chu SY, Lee NC, Wingo PA, Senie RT, Greenberg RS, Peterson HB. The relationship between body mass and breast cancer among women enrolled in the Cancer and Steroid Hormone Study. J Clin Epidemiol. 1991;44:1197–1206. doi: 10.1016/0895-4356(91)90152-y. [DOI] [PubMed] [Google Scholar]
- 65.den Tonkelaar I, Seidell JC, Collette HJ, de Waard F. Obesity and subcutaneous fat patterning in relation to breast cancer in postmenopausal women participating in the Diagnostic Investigation of Mammary Cancer Project. Cancer. 1992;69:2663–2667. doi: 10.1002/1097-0142(19920601)69:11<2663::aid-cncr2820691107>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 66.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87:190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 67.Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15:110–132. doi: 10.1093/oxfordjournals.epirev.a036096. [DOI] [PubMed] [Google Scholar]
- 68.Ursin G, Longnecker MP, Haile RW, Greenland S. A meta-analysis of body mass index and risk of pre-menopausal breast cancer. Epidemiology. 1995;6:137–141. doi: 10.1097/00001648-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 70.Hirose K, Tajima K, Hamajima N, Takezaki T, Inoue M, Kuroishi T, et al. Association of family history and other risk factors with breast cancer risk among Japanese premenopausal and postmenopausal women. Cancer Causes Control. 2001;12:349–358. doi: 10.1023/a:1011232602348. [DOI] [PubMed] [Google Scholar]
- 71.Lam PB, Vacek PM, Geller BM, Muss HB. The association of increased weight, body mass index, and tissue density with the risk of breast carcinoma in Vermont. Cancer. 2000;89:369–375. doi: 10.1002/1097-0142(20000715)89:2<369::aid-cncr23>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 72.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 73.McCaskill-Stevens W, Bryant J, Costantino J, Wickerham DL, Vogel V, Wolmark N. Incidence of contralateral breast cancer, endometrial cancer, and thromboembolic events in African-American women receiving tamoxifen for treatment of primary breast cancer [abstract 269] Proc ASCO. 2000;19:70a. [Google Scholar]
- 74.Willett WC, Browne ML, Bain C, Lipnick RJ, Stampfer MJ, Rosner B, et al. Relative weight and risk of breast cancer among premenopausal women. Am J Epidemiol. 1985;122:731–740. doi: 10.1093/oxfordjournals.aje.a114156. [DOI] [PubMed] [Google Scholar]
- 75.Hall IJ, Newman B, Millikan RC, Moorman PG. Body size and breast cancer risk in black women and white women: the Carolina Breast Cancer Study. Am J Epidemiol. 2000;151:754–764. doi: 10.1093/oxfordjournals.aje.a010275. [DOI] [PubMed] [Google Scholar]
- 76.Verreault R, Brisson J, Deschenes L, Naud F, Meyer F, Belanger L. Dietary fat in relation to prognostic indicators in breast cancer. J Natl Cancer Inst. 1988;80:819–825. doi: 10.1093/jnci/80.11.819. [DOI] [PubMed] [Google Scholar]
- 77.Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86:826–835. doi: 10.1002/(sici)1097-0142(19990901)86:5<826::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 78.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 79.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 80.Kumar NB, Allen K, Cantor A, Cox CE, Greenberg H, Shah S, et al. Weight gain associated with adjuvant tamoxifen therapy in stage I and II breast cancer: fact or artifact? Breast Cancer Res Treat. 1997;44:135–143. doi: 10.1023/a:1005721720840. [DOI] [PubMed] [Google Scholar]
- 81.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 82.Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–1662. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 83.Lippman ME, Krueger KA, Eckert S, Sashegyi A, Walls EL, Jamal S, et al. Indicators of lifetime estrogen exposure: effect on breast cancer incidence and interaction with raloxifene therapy in the multiple outcomes of raloxifene evaluation study participants. J Clin Oncol. 2001;19:3111–3116. doi: 10.1200/JCO.2001.19.12.3111. [DOI] [PubMed] [Google Scholar]
- 84.Decensi A, Gandini S, Guerrieri-Gonzaga A, Johansson H, Manetti L, Bonanni B, et al. Effect of blood tamoxifen concentrations on surrogate biomarkers in a trial of dose reduction in healthy women. J Clin Oncol. 1999;17:2633–2638. doi: 10.1200/JCO.1999.17.9.2633. [DOI] [PubMed] [Google Scholar]