Abstract
The alternative sigma factor RpoN is a unique regulator found among bacteria. It controls numerous processes that range from basic metabolism to more complex functions such as motility and nitrogen fixation. Our current understanding of RpoN function is largely derived from studies on prototypical bacteria such as Escherichia coli. Bacillus subtilis and Pseudomonas putida. Although the extent and necessity of RpoN-dependent functions differ radically between these model organisms, each bacterium depends on a single chromosomal rpoN gene to meet the cellular demands of RpoN regulation. The bacterium Ralstonia solanacearum is often recognized for being the causative agent of wilt disease in crops, including banana, peanut and potato. However, this plant pathogen is also one of the few bacterial species whose genome possesses dual rpoN genes. To determine if the rpoN genes in this bacterium are genetically redundant and interchangeable, we constructed and characterized ΔrpoN1, ΔrpoN2 and ΔrpoN1 ΔrpoN2 mutants of R. solanacearum GMI1000. It was found that growth on a small range of metabolites, including dicarboxylates, ethanol, nitrate, ornithine, proline and xanthine, were dependent on only the rpoN1 gene. Furthermore, the rpoN1 gene was required for wilt disease on tomato whereas rpoN2 had no observable role in virulence or metabolism in R. solanacearum GMI1000. Interestingly, plasmid-based expression of rpoN2 did not fully rescue the metabolic deficiencies of the ΔrpoN1 mutants; full recovery was specific to rpoN1. In comparison, only rpoN2 was able to genetically complement a ΔrpoN E. coli mutant. These results demonstrate that the RpoN1 and RpoN2 proteins are not functionally equivalent or interchangeable in R. solanacearum GMI1000.
Introduction
The alternative sigma factor σ54 or RpoN regulates an assortment of diverse biological processes and is an essential protein for some bacteria [1]. RpoN proteins are structurally and mechanistically distinct from sigma factors belonging to the σ70 family [2]. For example, RpoN proteins have a modular structure consisting of three domains (regions I, II & III). Region I is at the N-terminus and is involved in interactions with transcriptional regulator proteins known as enhancer-binding proteins or EBPs [3–6]. Region II serves as a linker between regions II and III; region II is absent in some RpoN proteins [7]. Region III (C-terminus) is involved in interacting with RNA polymerase [8, 9] and contains the DNA-binding motifs responsible for promoter recognition [9–11]. RpoN recognizes a unique −24/−12 promoter that has a consensus sequence of TGGCACG-N4-TTGC [12]. This sequence is remarkably conserved with some nucleotides in the −24 and −12 sites displaying >90% conservation across a number of bacterial species [12].
Our knowledge of RpoN as a regulator of gene expression is largely derived from studies on bacteria harboring a single rpoN gene, and thus, a single RpoN protein. Under these circumstances, the RpoN protein has evolved to interact efficiently with its arsenal of partner EBPs to direct transcription from −24/−12 promoters. At the center of these interactions is region I (residues 1–50) of RpoN and the conserved amino acid motif ‘GAFTGA’ localized in a loop structure in the RpoN-interaction domain of EBPs [13]. These two domains make direct contacts with one another, and therefore, play a significant role in allowing RpoN to communicate with EBPs that differ in overall structure and function.
The situation can be more complex when a bacterium possesses multiple RpoN proteins. For example, the photosynthetic bacterium Rhodobacter sphaeroides has four genes encoding for RpoN proteins (RpoN1–4), which share 50–60% homology to one another [14]. The RpoN3 and RpoN4 proteins had no observable function in this bacterium [14]. In contrast, RpoN1 was specifically required for transcription of genes associated with nitrogen fixation while RpoN2 was necessary for motility [14]. The specializations of the RpoN1 and RpoN2 proteins were a result of differences in their promoter recognition (at position −11) and EBP interactions [15].
A number of soil-dwelling and plant-associated bacteria do have genes encoding for dual RpoN proteins. For example, the two RpoN proteins in Bradyrhizobium japonicum are nearly identical (>80% homology) and were previously found to be interchangeable [16]. The plant pathogen Ralstonia solanacearum is widely known for its role as the causative agent of bacterial wilt disease in economically important plant species such as banana, tomato, potato and peanut [17]. The genome of this plant pathogen consists of a 3.7 Mb chromosome and a 2.1 Mb megaplasmid [18]. Interestingly, each of these replicons harbors an rpoN gene. The rpoN1 gene is chromosomal while rpoN2 is carried on the megaplasmid. Unlike B. japonicum, the RpoN1 and RpoN2 proteins in R. solanacearum GMI1000 are not identical. They share ∼60% homology towards one another with the majority of differences being found in regions I and II. Indeed, a recent study showed that the rpoN genes in R. solanacearum GMI1000 are not genetically redundant [19]. Specifically, phenotypic traits such as nitrate utilization, natural competence, twitching motility and virulence were dependent only on the rpoN1 gene. Inactivation of the rpoN2 gene had no affect on these phenotypes.
In an effort to expand on these previous findings, and therefore, more fully understand metabolic functions dependent on RpoN in R. solanacearum GMI1000, we constructed a series of rpoN-deletion mutants (ΔrpoN1, ΔrpoN2 and ΔrpoN1 ΔrpoN2) and subsequently tested their growth on an array of compounds. In addition to being essential for nitrate utilization, the rpoN1 gene was required for the assimilation of dicarboxylates, ethanol, ornithine, proline, propionate and xanthine. We did not observe any metabolic role for the rpoN2 gene. However, despite the lack of an observable role for metabolic function in R. solanacearum GMI1000, heterologous expression of the rpoN2 gene did genetically complement a ΔrpoN mutant of Escherichia coli. In contrast, heterologous expression of rpoN1 had no effect on restoring RpoN function in ΔrpoN E. coli. Plasmid-derived expression of the rpoN1 gene but not rpoN2 completely rescued the growth defects of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 R. solanacearum GMI1000 mutants. These findings not only confirm that the rpoN genes are not genetically redundant, but importantly, demonstrate that RpoN1 and RpoN2 are not interchangeable (functionally equivalent proteins) in R. solanacearum GMI1000.
Materials and Methods
Bacteria, plasmids and general growth conditions
Bacteria and plasmids used in the study are given in Table 1. R. solanacearum was maintained in CPG media (1.0 g L-1 casamino acids, 10 g L-1 peptone, 5 g L-1 glucose) [20]. E. coli was grown in Lennox broth (10 g L-1 tryptone, 5 g L-1 yeast extract, 5 g L-1 NaCl). Antibiotic selection for R. solanacearum consisted of carbenicillin (Cb) 200 μg mL-1, gentamicin (Gm) 15 μg mL-1, kanamycin (Km) 50 μg mL-1 and tetracycline (Tc) 15 μg mL-1. Similar concentrations of antibiotics were used for selection of recombinant E. coli except for Cb, which was used at 100 μg mL-1.
Table 1. Bacteria, plasmids and oligonucleotides used in the current study.
| Strain, plasmid or oligo-nucleotides | Relevant Characteristics | Source | |
|---|---|---|---|
| Ralstonia solanacearum | |||
| GMI1000 | wild-type | ATCC | |
| ΔrpoN1 | rpoN1::Gmr | This study | |
| ΔrpoN2 | rpoN2::Gmr | This study | |
| ΔrpoN1 ΔrpoN2 | rpoN1::Gmr rpoN2::Tcr | This study | |
| Escherichia coli | |||
| Top10 | F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galE15 galK16 rpsL(StrR) endA1 λ- | Invitrogen | |
| BW25113 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ -, rph-1, Δ(rhaD-rhaB)568, hsdR514 | [21] | |
| JM3169-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ -, ΔrpoN730::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | [21] | |
| Plasmids | |||
| pCR-Blunt | Cloning plasmid; Kmr | Invitrogen | |
| pBBR1MCS-2 | Broad-host plasmid; Kmr | [22] | |
| pDONR221 | Cloning plasmid; Kmr | Invitrogen | |
| pEX18ApGW | Plasmid for gene deletions in P. aeruginosa; Cbr Gmr | [23] | |
| pPS856 | Plasmid harboring Gmr marker | [24] | |
| pBR322 | Cloning plasmid; Cbr Tcr | New England BioLabs | |
| pBRL533 | rpoN1::Gmr in pDONR221; Gmr Kmr | This study | |
| pBRL534 | rpoN2::Gmr in pDONR221; Gmr Kmr | This study | |
| pBRL535 | rpoN1::Gmr in pEX18ApGW; Cbr Gmr | This study | |
| pBRL536 | rpoN2::Gmr in pEX18ApGW; Cbr Gmr | This study | |
| pBRL557 | rpoN2::Tcr in pDONR221; Gmr Tcr | This study | |
| pBRL560 | rpoN2::Tcr in pEX18ApGW; Cbr Gmr Tcr | This study | |
| pBRL577 | rpoN1 gene in pCR-Blunt; Kmr | This study | |
| pBRL578 | rpoN2 in pCR-Blunt; Kmr | This study | |
| pBRL584 | rpoN2 gene in pBBR1MCS-2; Kmr | This study | |
| pBRL587 | rpoN1 gene in pBBR1MCS-2; Kmr | This study | |
| Oligonucleotides | |||
| BL462.f | 5’-tacaaaaaagcaggctatgaaacagtcgctccagctc-3’ | ||
| BL462.r | 5’-tcagagcgcttttgaagctaattcggtagtcgctgtcgaaatcgctg-3’ | ||
| BL463.f | 5’-aggaacttcaagatccccaattcggagctttttcacgcacggtg-3’ | ||
| BL463.r | 5’-tacaagaaagctgggtctataaagacttgcgcagattc-3’ | ||
| BL464.f | 5’-tacaaaaaagcaggctgtcaaagccgctctcgaaatg-3’ | ||
| BL464.r | 5’-tcagagcgcttttgaagctaattcgcattcgccgatgtcgctgtc-3’ | ||
| BL464.r2 | 5’-atcgatgataagctgtcaaacatgacattcgccgatgtcgctgtc-3’ | ||
| BL465.f | 5’-aggaacttcaagatccccaattcggccaaaatcaagggcaagtgg-3’ | ||
| BL465.f2 | 5’-cggattcaccactccaagaattggagccaaaatcaagggcaagtgt-3’ | ||
| BL465.r | 5’-tacaagaaagctgggtcctcgatcatctccttgagc-3’ | ||
| B568.f | 5’-gcaggtaccgatcgactgctgcagttgtg-3’ | ||
| BL568.r | 5’-gcatctagactataaagacttgcgcagattcac-3’ | ||
| BL569.f | 5’-gcaggtacccgacatgatcatgtagaaacgg-3’ | ||
| BL569.r | 5’-gcatctagatcagatctgccgccggag-3’ | ||
| Gm-F | 5’-cgaattagcttcaaaagcgctctga-3’ | ||
| Gm-R | 5’-cgaattggggatcttgaagttcct-3’ | ||
| Tc-F | 5’-tcatgtttgacagcttatcatcgat-3’ | ||
| Tc-R | 5’-tccaattcttggagtggtgaatccg-3’ | ||
| GW-attB1 | 5’-ggggacaagtttgtacaaaaaagcaggct-3’ | ||
| GW-attB2 | 5’-ggggaccactttgtacaagaaagctgggt-3’ | ||
Electroporation of R. solanacearum
A single colony of R. solanacearum was inoculated into 10 mL of CPG medium, and the culture was grown for 24 h at 30°C, 200 rpm. The entire 10 mL culture was centrifuged, and the cells were washed two times (1st wash 5.0 mL, 2nd wash 1.0 mL) with 10% (v/v) glycerol. After the second wash, cells were suspended in a final volume of 0.1 mL of 10% (v/v) glycerol. The cell suspension was given 0.05–1.0 μg of plasmid DNA and the plasmid-cell mixture was transferred to a 2-mm gap electroporation cuvette. Electroporation was performed at 2500 V in an ECM 399 (Harvard Apparatus). Cells were recovered in 1.0 mL of CPG at 30°C, 200 rpm for 1.0 h. Transformants were selected on CPG supplemented with the appropriate antibiotics.
Standard DNA procedures
DNA was purified using Promega nucleic acid purification kits. Restriction enzymes, ligases and polymerases were products of New England BioLabs. Phusion polymerase (New England BioLabs) was used for all PCR applications. PCR was done using cycling times and parameters as recommended for the Phusion polymerase. Oligonucleotides used for PCR applications were purchased from Integrated DNA Technologies and are listed in Table 1. Cloned DNA was verified by sequencing (Genewiz).
Cloning of the rpoN genes
The rpoN1 and rpoN2 genes were PCR amplified with the primers BL568.f/BL568.r and BL569.f/BL569.r, respectively. The desired rpoN1 and rpoN2 PCR products (~1.5 kb) were gel-purified and separately cloned into pCR-Blunt (Invitrogen) according to the manufacturer’s instructions. The rpoN1 and rpoN2 genes were then individually subcloned into the KpnI/XbaI sites of pBBR1MCS-2 [22] to give pBRL587 and pBRL584, respectively.
Deletion of rpoN genes in R. solanacearum GMI1000
A gene deletion methodology that was originally developed for Pseudomonas aeruginosa [23] was adopted and used to inactivate the rpoN1 and rpoN2 genes from R. solanacearum GMI1000. Construction of the rpoN deletion plasmids was done according to previously published procedures [23]. The 5’ and 3’ ends (~250 bp) of the rpoN1 ORF were PCR amplified with the primers BL462.f/BL462.r and BL463.f/BL463.r, respectively. Similarly, primers BL464.f/BL464.r and BL465.f/BL465.r were used to PCR amplify the 5’ and 3’ ends, respectively, of the rpoN2 ORF. The Gmr marker was PCR amplified from the pPS856 plasmid [24] with the primers Gm-F/Gm-R. The desired PCR products were gel-purified. The purified 5’ and 3’ end fragments of each rpoN ORF were fused to Gmr marker via PCR with the primers GW-attB1/GW-attB2. The resulting rpoN1::Gmr and rpoN2::Gmr cassettes were gel-purified and individually cloned into pDONR221 (Invitrogen) using BP clonase II (Invitrogen). Lastly, LR clonase II (Invitrogen) was used to shuttle the rpoN1::Gmr or rpoN2::Gmr cassette from pDONR221 into the gene replacement vector pEX18ApGW [23]. The final pBRL535 and pBRL536 plasmids carried the rpoN1::Gmr and rpoN2::Gmr cassette, respectively, in pEX18ApGW. For the deletion plasmid pBRL560 (rpoN2::Tcr pEX18ApGW), primers BL464.f/BL464.r2 and BL465.f2/BL465.r were used to PCR amplify the 5’ and 3’ ends of the rpoN2 ORF. The Tcr marker was PCR-amplified from pBR322 (NEB) with the primers Tc-F/Tc-R. Fusion PCR and subcloning of the rpoN2::Tcr cassette was done using identical procedures as described above.
To generate the single ΔrpoN1 and ΔrpoN2 mutants, 0.5–1.0 μg of pBRL535 or pBRL536 was electroporated into (0.1 mL) R. solanacearum GMI1000. Following a 1.0 h recovery period (30°C, 200 rpm), the entire culture was plated onto CPG supplemented with Gm. The plates were incubated at 30°C for 48 h. Gmr colonies were patched onto CPG supplemented with either Gm or Cb in order to differentiate from double and single crossovers, respectively. After 48 h at 30°C, patched clones displaying Gmr and Cbs were identified as putative ΔrpoN mutants. The rpoN1 and rpoN2 loci were PCR amplified from the ΔrpoN1 and ΔrpoN2 mutants. PCR products were gel-purified, cloned into pCR-Blunt (Invitrogen) and the inserts present in the recombinant plasmids were sequenced to verify the desired ΔrpoN mutation.
A similar procedure was used to create the double ΔrpoN1 ΔrpoN2 mutant. Briefly, the pBRL560 plasmid was electroporated into ΔrpoN1 R. solanacearum GMI1000, and colonies were selected for on CPG supplemented with Gm and Tc. Gmr Tcr colonies were patched onto CPG supplemented with Cb, and colonies displaying Cbs were identified as putative ΔrpoN1 ΔrpoN2 mutants. The rpoN loci were PCR-amplified, cloned and sequenced to verify the mutations in the double ΔrpoN1 ΔrpoN2 mutant.
Growth of ΔrpoN mutants of R. solanacearum GMI1000 on various carbon, nitrogen and sulfur sources
Growth analyses were done in triplicate. Strains were first grown on solid CPG at 30°C for 48 h. Single colonies were inoculated into 1.0 mL of liquid CPG, and the inoculums were grown at 30°C, 200 rpm for 24 h. Next, 10 μL of a CPG-grown seed culture was used to inoculate 1.0 mL of a low-salt minimal media (15 mM K phosphate, 0.7 mM Na citrate, 0.5 mM MgSO4, pH 7.0) [20]. For carbon-source testing, minimal media was supplemented with 20 mM NH4Cl and carbon sources were used at a final concentration of 20 mM except for benzaldehyde (5 mM), benzoate (10 mM), phenol (5 mM) and pectin [0.2% (w/v)], phenylalanine (5 mM), tryptophan (5 mM) and tyrosine (5 mM). For nitrogen-source testing, minimal media was supplemented with 20 mM glucose and nitrogen sources were used at a final concentration of 20 mM except for phenylalanine (5 mM), tryptophan (5 mM), and xanthine (0.5 mM). Lastly, minimal media consisting of 20 mM glucose, 20 mM NH4Cl and 0.5 mM MgCl2 (in exchange of MgSO4) was used to measure growth on sulfur compounds, which were provided at a final concentration of 0.5 mM. Compounds used in the experiments are listed in Table 2.
Table 2. Compounds tested as carbon, nitrogen and sulfur sources for wild-type, ΔrpoN1, ΔrpoN2 and ΔrpoN1 ΔrpoN2 R. solanacearum GMI1000.
| Growth Experiment | Compounds |
|---|---|
| Carbon Sources | Acetoin, Alanine a , b , Arginine a , b , Asparagine a , b , Aspartate a , b , Benzoate, Benzaldehyde, Cysteine b , c , Ethanol, Ethanolamine a , Fumarate, Galactose, Glutamate a , b , Glucose, Gluconate, Glucouronate, Glutamine a , b , Glycine a , Histidine a , b , Isoleucine a , b , α-Ketoglutarate (α-KG), Leucine a , b , Lysine a , b , Malate, Malonate, Methionine b , c , Octanoate, Ornithine a , b , Pectin, Phenol, Phenylalanine a , b , Proline a , b , Propionate, Serine a , b , Sorbitol, Succinate, Sucrose, Taurine c , Threonine a , b , Tryptophan a , b , Valine a , b , Xylose |
| Nitrogen Sources | Acetamide, Ammonium, Carnitine, Nitrate, Nitrite, Urea, Xanthine |
| Sulfur Sources | HEPES, Methanesulfonate, MOPS, PIPES, Sulfate |
aCompounds were tested as both sole carbon and sole nitrogen sources.
bOnly L-amino acids were tested.
cCompounds were tested as sole carbon, sole nitrogen and sole sulfur sources.
Genetic complementation experiments
Growth analyses were done in triplicate. Plasmids carrying rpoN1 (pBRL587), rpoN2 (pBRL584) or no insert (pBBR1MCS-2) were electroporated into ΔrpoN1 and ΔrpoN1 ΔrpoN2 R. solanacearum GMI1000. Following initial selection, recombinant strains were grown in 1.0 mL of CPG supplemented with Km at 30°C, 200 rpm for 24 h. Minimal media (1.0 mL) was inoculated with 10 μL of CPG-grown seed culture. For complementation experiments relating to carbon utilization, minimal media was supplemented with 20 mM NH4Cl and 20 mM α-KG, ethanol, fumarate, malate, propionate or succinate. For complementation experiments relating to nitrogen utilization, minimal media was supplemented with 20 mM glucose and 20 mM alanine, nitrate, ornithine, proline or serine. Minimal media cultures were grown at 30°C, 200 rpm for 24–96 h.
ΔrpoN E. coli BW25113 was transformed with pBBR1MCS-2, pBRL584 or pBRL587. Recombinant strains were selected on LB supplemented with Km. Individual colonies were inoculated into 1.0 mL of LB supplemented with Km, and the inoculums were grown at 37°C, 200 rpm for 24 h. Minimal media (1.0 mL) was inoculated with 10 μL of LB-grown seed culture. For carbon utilization experiments, minimal media was supplemented with 20 mM NH4Cl and acetoacetate (20 mM) or propionate (20 mM). For nitrogen utilization experiments, minimal media was supplemented with 20 mM glucose and arginine (20 mM), asparagine (20 mM), NH4Cl (0.5 mM) or xanthine (0.5 mM). Minimal media cultures were grown at 37°C, 200 rpm for 24–48 h.
Plant assays
Tomato (Solanum lycopersicum) cultivar (cv.) Bonny Best and cv. Hawaii 7996 were grown in growth chambers (Conviron) with 12 h light-and-dark cycles at 25°C with 70% relative humidity. R. solanacearum strains were grown on solid CPG supplemented with 0.005% (w/v) of 2,3,5-tripheny tetrazolium chloride (TTC) at 30°C for 48 h. Single colonies that displayed a virulent morphology (mucoid and pinkish-white in color) [20] were grown in liquid CPG at 30°C for 24 h. Cells from each of these cultures were harvested, washed and then diluted in sterile water to a cell density of 1 x 106 CFU mL-1. These inoculums were used for the virulence assays as described below.
Virulence of R. solanacearum strains for tomato cv. Bonny Best and cv. Hawaii 7996 was determined using standard procedures with some modifications [20, 25]. Briefly, stems of healthy plants were wounded by puncturing into pith at the leaf axial using a disposable pipette tip. A drop of bacterial inoculum (1 x 106 CFU mL-1) was applied using a pipet tip, and the tip containing 10 μL of inoculum was left at the puncture site. Infected plants were returned to the growth chamber, and the day temperature was increased to 30°C. After the inoculum was taken up by the plant (10–24 h), the pipet tip was discarded.
Disease symptoms were evaluated daily and scored on a 0–5 disease index scale as follows: 0, healthy or no leaf wilting; 1, 10% of the leaf area wilted; 2, 11 to 40% of the leaf area wilted; 3, 41 to 60% of the leaf area wilted; 4, 61 to 85% of the leaf area wilted, 5, 85% of the leaf area wilted or dead leaf. Disease wilting index (dwi) was calculated using the following formula [20]: dwi (%) = [(sum of rating 0–5)] x 0.2 x (number of leaves evaluated) x 100. Plant assays were performed at least in duplicates using 8–9 plants per treatment.
The plants were sampled at 4 d post inoculation (dpi) for cv. Bonny Best and 6–7 dpi for cv. Hawaii7996. One-centimeter stem pieces just above the infection site were cut using a sterile razor blade. The stem pieces were weighed, sterilized with 70% (v/v) ethanol and then rinsed twice with sterile water. The stem pieces were ground using pestle in 800 μL of sterile water and serial dilutions were plated on CPG supplemented with 0.005% (w/v) TTC. Plates were incubated at 30°C for 18–24 h, and the number of bacterial colonies present were recorded. All experiments were performed at least in duplicates for each bacterial stain for both tomato cultivars. Data was analyzed using student t-tests, and values reported represent a mean of the colonies (± SD) per gram of fresh tissue.
Results and Discussion
Assimilation of a limited but diverse group of compounds is dependent only on the rpoN1 gene in R. solanacearum GMI1000
RpoN is well known for being involved in the assimilation of a wide variety of compounds among bacteria. Therefore, the ΔrpoN1, ΔrpoN2 and ΔrpoN1 ΔrpoN2 mutants of R. solanacearum GMI1000 were challenged with an array of carbon, nitrogen and sulfur sources (Table 2). Preliminary experiments showed that all three rpoN mutants grew to cell densities (OD600nm values) equivalent to that of wild-type in minimal media supplemented with 20 mM glucose and 20 mM NH4Cl (S1 Table). Based on this result, glucose was chosen as the carbon source for nitrogen utilization experiments while NH4Cl served as the nitrogen source for carbon utilization experiments.
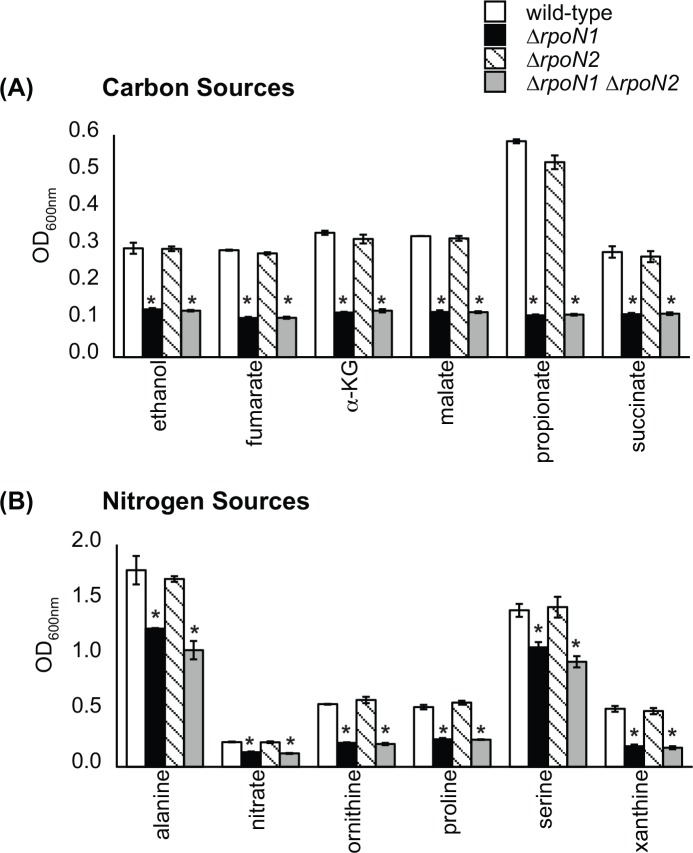
Surprisingly, only the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants were observed to have either zero or reduced growth on a limited number of compounds (Fig 1). The ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants could not grow on ethanol, propionate, fumarate, malate, succinate and α-KG as carbon sources. For nitrogen sources, nitrate, ornithine, proline and xanthine did not support the growth of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants. The ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants had decreased growth on alanine and serine when provided as nitrogen sources. The ΔrpoN2 mutant exhibited wild-type growth on all compounds. Furthermore, all rpoN mutants showed wild-type growth on aliphatic sulfur sources even though RpoN is known to regulate such assimilation in other bacteria (S1 Table) [26].
Fig 1. The rpoN1 gene was required for growth on a small number of compounds.
(A) The ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants failed to grow on C4-dicarboxylates, ethanol, α-KG and propionate when provided as sole carbon sources. (B) The ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants grew poorly when alanine, nitrate, ornithine, proline, serine and xanthine were provided as sole nitrogen sources. Out of the total >50 compounds tested, the utilization of only a dozen of them were found to require the rpoN1 gene in R. solanacearum GMI1000. [Data points represent mean values (n = 3) ± SD. Analysis of variance was done using Dunnett’s post hoc test (α-value of 0.05) to identify significant changes (P < 0.0001), which are marked with an asterisk].
The metabolic deficiencies of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants are in agreement with the putative EBPs and RpoN-controlled genes of R. solanacearum GMI1000 (Table 3). For example, the rpoN1 gene was necessary for the growth of R. solanacearum GMI1000 on C4-dicarboxylates and α-KG. In a number of bacteria, dicarboxylate utilization is dependent on RpoN and an EBP known as DctD, which together activate transcription of genes encoding for dicarboxylate transport proteins [27–29]. In R. solanacearum GMI1000, the Rsp0009 (dctD1) and Rsp0332 (dctD2) genes encode for homologs of DctD, and RpoN promoters are found upstream of genes encoding for C4-dicarboxylates and α-KG transport proteins. It is expected that R. solanacearum GMI1000 also uses an RpoN-DctD mechanism to regulate uptake of dicarboxylates.
Table 3. EBPs and their potential target genes of R. solanacearum GMI1000.
| EBP | Putative function | Potential regulated genes having RpoN promoters | ||
|---|---|---|---|---|
| Symbol | Name | Symbol | Function | |
| Rsc0222 a , b | rtcR | regulator of rtcB | Rsc0224 | RNA ligase (RtcB) |
| Rsc0332 b | dctD2 | dicarboxylate transport | Rsc0330 | C4-dicarboxylate transporter (DctA) |
| Rsc1186 b , c | – | – | – | – |
| Rsc1261 b , d | ntrC | nitrogen assimilation | RSc0381 | nitrate transporter (NasF) |
| Rsc1258 | glutamine synthetase (GlnA1) | |||
| RSc2118 | xanthine permease | |||
| Rsc3410 | amino acid-binding periplasmic protein | |||
| Rsp0886 | glutamine synthetase (GlnA2) | |||
| Rsp0942 | nitrogen assimilation transcriptional regulator (Nac) | |||
| Rsp1671 | RpoN2 | |||
| Rsp1223 | nitrate transporter | |||
| Rsc2807 b , e | pehR | type IV pilibiosynthesis | Rsc0558 | fimbrial pilin (PilA) |
| Rsc2827 | prepilin peptidase/methyltransferase protein (PilD) | |||
| Rsc3129 f | acoR | ethanol catabolism | Rsc3128 | acetaldehyde dehydrogenase (ExaC) |
| Rsp0009 b | dctD1 | dicarboxylate transport | Rsp0007 | α-ketoglutarate permease (KgtP2) |
| Rsp0123 b | prpR | propionate catabolism | Rsp0122 | 2-methylisocitrate lyase (PrpB) |
| Rsp0228 g | – | – | Rsp0229 | benzaldehyde dehydrogenase oxidoreductase |
| Rsp0959 b | – | – | Rsp0958 | iron-sulfur cluster repair protein |
| Rsp1079 b | – | – | Rsp1076 | glucose-fructose oxidoreductase |
| Rsp1667 f | – | – | Rps1668 | EBP |
| Rsp1671 | RpoN2 | |||
| Rsp1668 f | – | – | Rsp1668 | EBP |
| Rsp1671 | RpoN2 | |||
Because the RpoN-interaction domain is conserved in EBPs, the RpoN-interaction domain of E. coli NtrC was used in a BlastP search against the protein database of R. solanacearum GMI1000. This searched returned a total of thirteen putative EBPs, which is in agreement with a previously published assessment [30]. Nine EBPs possess the signature GAFTGA motif while the remaining four have GSFTGA or GAYTGA. A partial listing of potential gene targets regulated by RpoN and EBPs are given. Full listing of genes harboring putative RpoN promoters can be accessed and searched in the Sigma 54 Database (www.sigma54.ca) [31].
a. “Rsc” are chromosomal genes while “Rsp” indicates genes carried on megaplasmid.
b. EBP has GAFTGA motif in RpoN-interaction domain.
c. There are no genes near this EBP that possess RpoN promoters.
d. NtrC might regulate numerous genes. Only a select few are shown.
e. PehR shares homology with the pili-biosynthesis regulator EBP PilR. pehR mutants have been observed to be defective in type IV pili and twitching motility [19, 32].
f. EBP has GSFTGA motif in RpoN-interaction domain.
g. EBP has GAYTGA motif in RpoN-interaction domain.
RpoN and the EBP PrpR regulate the degradation of propionate in some bacteria [33, 34]. Indeed, the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants of R. solanacearum GMI1000 did not grow on propionate as a carbon source. Rsp0123 is a predicted homolog of PrpR, and there is an RpoN promoter upstream of an operon encoding for enzymes of a propionate catabolic pathway in R. solanacearum GMI1000. Propionate catabolism in R. solanacearum GMI1000 is likely regulated by RpoN1 and PrpR. Rsc3129 has homology to the EBP AcoR [35, 36] and is predicted to regulate transcription of an acetaldehyde dehydrogenase (ExaC), a key enzyme in the breakdown of acetoin, ethanol and ethanolamine [37, 38]. The rpoN1 gene was required for growth on ethanol, but we observed no growth for the wild-type strain or rpoN mutants on acetoin or ethanolamine.
Pectin, sucrose, galactose and hexoses in general are common substrates for R. solanacearum in the environment. Deletion of the rpoN genes had no impact on the consumption of these compounds. The utilization of nitrate, however, was completely dependent on the rpoN1 gene. This finding is in agreement with a previous study [19]. Nitrate assimilation was observed to be a crucial component for R. solanacearum in the host plant environment [39]. The NtrC-response was involved in nitrate utilization [39]. Nitrate might serve as a valuable nitrogen source for R. solanacearum during host plant interactions.
The rpoN genes were not required for the use of amino acids as carbon sources. However, ornithine and proline were the only amino acids that could not be used as nitrogen sources by the ΔrpoN1 mutants. The ΔrpoN1 mutants grew poorly on serine and alanine as nitrogen sources but nonetheless still produced wild-type cell densities after 72 h of incubation. The strict rpoN1-dependency for ornithine could be attributed to the Rsc3410 locus, which encodes for a protein sharing some homology to the glutamine transporter GlnH [40]. Ornithine transport might be mediated through Rsc3410. There are no putative RpoN-controlled genes encoding for catabolic enzymes of proline degradation in R. solanacearum GMI1000. Proline degradation is regulated by Nac (nitrogen assimilation regulator) in some bacteria [41, 42], and the nac gene in R. solanacearum GMI1000 is preceded by an RpoN promoter. This might account for the RpoN1-dependency of proline utilization.
RpoN1 and RpoN2 are not interchangeable
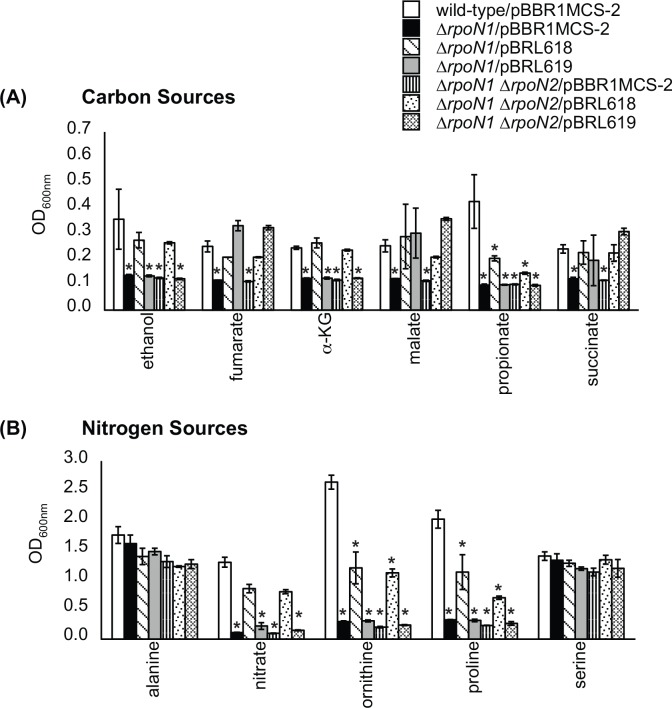
The rpoN1 and rpoN2 genes were individually cloned under the lac-promoter on the broad-host range plasmid pBBR1MCS-2 [22]. The plasmid-encoded rpoN1 and rpoN2 genes were electroporated into the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants, and the recombinant strains were challenged with compounds whose assimilation was previously found to be rpoN1-dependent. As shown in Fig 2, plasmid-derived expression of either rpoN1 or rpoN2 recovered the growth of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants on C4-dicarboxylates. Additionally, expression of rpoN1 rescued the growth of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants on ethanol, α-KG, nitrate, ornithine, proline and propionate. In sharp contrast, expression of rpoN2 did not allow for the growth of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants on these compounds. Complementation via RpoN2 was specific for C4-dicarboxylates, indicating that RpoN1 and RpoN2 are not equivalent proteins. Although this observation was unexpected at first, subsequent studies with the rpoN1/rpoN2 genes in ΔrpoN E. coli confirmed that the RpoN1 and RpoN2 proteins are not functionally identical.
Fig 2. Plasmid-derived expression of rpoN2 restored the growth of the ΔrpoN1 mutants on C4-dicarboxylates.
(A) Plasmid-derived expression of rpoN1 (pBRL618) or rpoN2 (pBRL619) enabled the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants to grow on C4-dicarboxylates as carbon sources. In contrast, expression of rpoN1 but not rpoN2 rescued the growth of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants on ethanol, α-KG and propionate. (B) Plasmid-derived expression of rpoN1 allowed for the utilization of nitrate, ornithine and proline as nitrogen sources in the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants. The inability of rpoN2 expression to completely genetically complement the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants is suggestive that the RpoN1 and RpoN2 proteins are not functionally equivalent. Note that we did not observe full recovery for some substrates. The rpoN genes were expressed from the lac promoter of pBBR1MCS-2. The weakness of the lac promoter might keep RpoN protein levels below what is needed for full complementation. [Data points represent mean values (n = 3) ± SD. Analysis of variance was done using Dunnett’s post hoc test (α-value of 0.05) to identify significant changes (P < 0.0001), which are marked with an asterisk].
RpoN1 and RpoN2 have different functionality in ΔrpoN E. coli
The RpoN1 and RpoN2 proteins share 63% and 55% homology to E. coli RpoN, respectively. Therefore, we decided to determine if the rpoN1 and rpoN2 genes were capable of genetically complementing a ΔrpoN E. coli strain [21]. The plasmid-encoded rpoN1 and rpoN2 genes were introduced into ΔrpoN E. coli strain, and the recombinant ΔrpoN E. coli strains were assayed under growth conditions known to be RpoN-dependent, including nitrogen assimilation (NtrC-regulated response) [43], propionate catabolism (PrpR-regulated response) [33] and acetoacetate metabolism (AtoC-regulated response) [44].
Heterologous expression of either the rpoN1 or rpoN2 gene did recover the growth of the ΔrpoN E. coli on nitrogen sources such as asparagine and xanthine (Fig 3). However, only the expression of rpoN2 completely rescued the growth of ΔrpoN E. coli under nitrogen limitation (NH4 < 0.5mM) and when arginine was the sole nitrogen source. Additionally, rpoN2 (but not rpoN1) restored the growth of ΔrpoN E. coli on propionate and acetoacetate to ∼25% and 100% of wild-type levels, respectively. These results indicate that the RpoN1 and RpoN2 proteins are not functionally equivalent.
Fig 3. RpoN1 and RpoN2 displayed different properties in ΔrpoN E. coli.
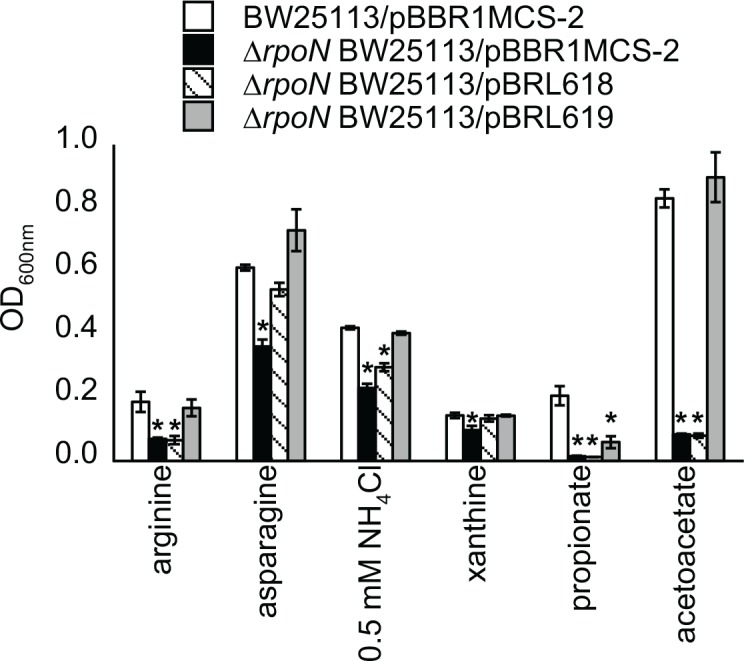
Plasmids carrying rpoN1 (pBRL618), rpoN2 (pBRL619) and no insert (pBBR1MCS-2) were introduced into ΔrpoN E. coli BW25113. As shown, expression of rpoN2 (and not rpoN1) restored the growth of ΔrpoN E. coli under conditions known to be RpoN dependent, including nitrogen limitation (0.5 mM NH4Cl), assimilation of nitrogenous compounds (arginine, asparagine and xanthine), acetoacetate utilization and propionate catabolism. RpoN2 and E. coli RpoN share weak homology in region II, which might account for RpoN2 being able to complement the ΔrpoN mutant. [Data points represent mean values (n = 3) ± SD. Analysis was done using Dunnett’s post hoc test (α-value of 0.05) to identify significant changes (P < 0.0001), which are marked with an asterisk].
Alignment of RpoN1, RpoN2 and E. coli RpoN revealed that all three proteins have ∼80% homology in region I (residues 1–50) and 60% homology in region III (C-terminal 350 residues). For RpoN2 and E. coli RpoN, they also share weak homology in region II (residues 51–100) (Fig 4). This homology does not exist between RpoN1 and E. coli RpoN, and thus, would explain why RpoN2 was more sufficient in complementing ΔrpoN E. coli than that of RpoN1 (Fig 3).
Fig 4. Alignment of regions I (residues 1–50) and II (residues 51–100) of E. coli RpoN with RpoN1 and RpoN2 of R. solanacearum GMI1000.
(A) E. coli RpoN (EcRpoN) and RpoN1 of R. solanacearum GMI1000 (RsRpoN1) (B) EcRpoN and R. solanacearum GMI1000 RpoN2 (RsRpoN2). (C) RsRpoN1 and RsRpoN2. E. coli RpoN and RpoN2 have homology in region II, which might enable RpoN2 to interact with EBPs of E. coli. Alignments were generated using EMBOSS.
Region II of RpoN has been implicated in EBP interactions [7], and NtrC of R. solanacearum GMI1000 has 70% homology to E. coli NtrC. Based on our results, it would appear that RpoN2 could function with NtrC of E. coli to facilitate nitrogen assimilation. RpoN1 can also interact with E. coli NtrC but to a much lesser extent than that of RpoN2 [as judged by RpoN1 being able to only partially compensate for nitrogen assimilation in ΔrpoN E. coli]. R. solanacearum NtrC exhibits 60% homology to E. coli AtoC. Expression of rpoN2 restored the growth of ΔrpoN E. coli on acetoacetate, suggesting that RpoN2 can partner with AtoC to regulate transcription from AtoC-controlled genes. R. solanacearum PrpR and E. coli PrpR have ∼50% homology. The differences in sequence between the PrpR EBPs are reflected in the inability of RpoN2 to fully restore the growth of ΔrpoN E. coli on propionate.
The rpoN1 gene is required for virulence of R. solanacearum GMI1000 on tomato
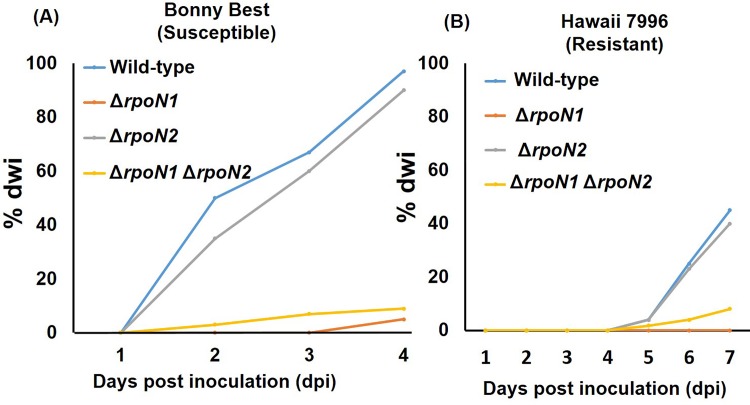
Dicarboxylates and nitrate were previously implicated as key nutrients for R. solanacearum in the host plant environment [25, 39]. Because the rpoN1 gene was needed for the growth of R. solanacearum GMI1000 on these compounds, it was suspected that rpoN1 might also be a necessary factor in the pathogenesis of this pathogen. Indeed, this suspicion was recently confirmed in a study, which found that the rpoN1 gene was necessary for virulence of R. solanacearum GMI1000 on tomato plants (Lycopersicum esculentum cv. Marmande)[19]. Therefore, we decided to determine if the ΔrpoN1 mutants generated in our study were also avirulent. To this end, tomato plants consisting of cv. Bonny Best (susceptible) and cv. Hawaii 7996 (resistant) were inoculated with a bacterial suspension comprising of wild-type, ΔrpoN1, ΔrpoN2 or ΔrpoN1 ΔrpoN2 R. solanacearum GMI1000. The inoculated plants were then examined on a daily basis for signs of wilt disease, which was quantified and used to calculate a disease-wilting index (dwi). Wilting was first observed on the older leaves of cv. Bonny Best plants inoculated with wild-type and ΔrpoN2 R. solanacearum GMI1000. The overall pattern of disease was similar for both tomato cultivars but the extent of wilting was severe in cv. Bonny Best compared to cv. Hawaii 7996 (Fig 5). Entire cv. Bonny Best plants wilted earlier (4 dpi) than cv. Hawaii 7996 (7 dpi). However, ΔrpoN1- and ΔrpoN1 ΔrpoN2-inoculated cv. Bonny Best and cv. Hawaii 7996 plants did not develop any significant wilting symptoms after 4 and 7 days, respectively (Fig 5). Mock plants treated with sterile water had no wilting symptoms (Fig 5). At 2 dpi, wild-type and ΔrpoN2-inoculated cv. Bonny Best plants had 35–50% dwi, which increased to 60–67% at 3 dpi. In contrast, progression of disease was reduced in ΔrpoN1- and ΔrpoN1 ΔrpoN2-inoculated cv. Bonny Best plants with dwi reaching only up to 0–7% at 3 dpi (Fig 6A). The wild-type and ΔrpoN2 inoculated cv. Hawaii 7996 population had 23–45% dwi at 6 dpi, which increased up to 40–45% at 7 dpi. ΔrpoN1- and ΔrpoN1 ΔrpoN2-inoculated cv. Hawaii-7996 population did not show any visible disease symptoms. At 6 dpi, the dwi for these plants was 0–4%, which did not significantly change at 7 dpi (0–8%) (Fig 6B).
Fig 5. The rpoN1 gene was required for wilt disease on tomato.
(A) cv. Bonny Best at 4 dpi. (B) cv. Hawaii 7996 at 7 dpi. Tomato plants infected with the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants did not show signs of wilting disease compared to the ΔrpoN2 mutant and wild-type R. solanacearum GMI1000.
Fig 6. Quantification of wilt disease caused by wild-type, ΔrpoN1, ΔrpoN2 and ΔrpoN1 ΔrpoN2 R. solanacearum GMI1000 on tomato.
(A) cv. Bonny Best. (B) cv. Hawaii 7996. Wilt symptoms were rated daily on a disease index scale, and the values were used to calculate a disease-wilting index (dwi). The dwi for both the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutant was significantly reduced compared to the ΔrpoN2 mutant and wild-type R. solanacearum GMI1000. Note that nine plants were used for each treatment.
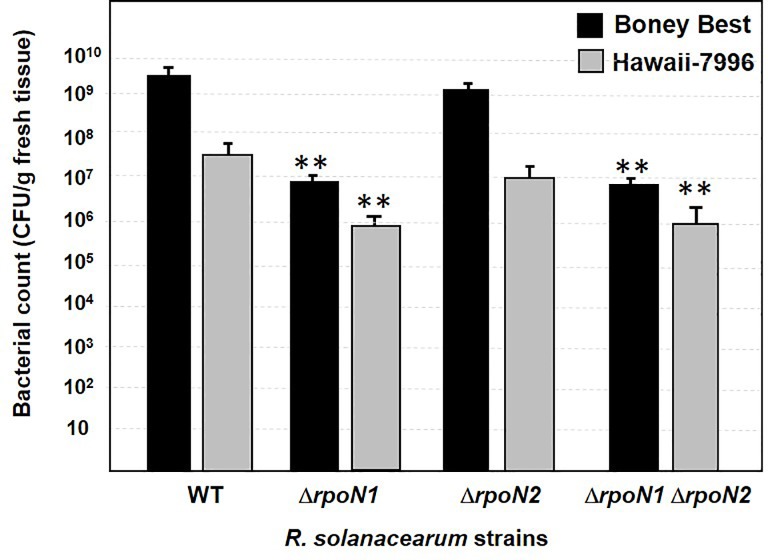
We next evaluated the in planta growth of the rpoN mutants. Plants were inoculated with each strain at a titer of 1 x 106 CFU mL-1 and bacterial growth (CFUs per gram of fresh tissue) was determined at 4 dpi for cv. Bonny Best and 7 dpi for cv. Hawaii 7996. The bacterial growth could not be determined at 7 dpi for the cv. Bonny Best plants, because all plants were dead by this particular dpi. At 4 dpi in cv. Bonny Best, wild-type cells and the ΔrpoN2 mutant produced ~1000-fold more CFUs than that of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants. Similarly, at 7 dpi in cv. Hawaii 7996, wild-type cells and the ΔrpoN2 mutant produced ~100-fold more CFUs than that of the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants (Fig 7). These results are consistent with the patterns of wilting disease caused by wild-type and ΔrpoN2 strains on both tomato cultivars. Collectively, these findings demonstrate that rpoN1 but not rpoN2 is critical for virulence of R. solanacearum on tomato and they are not functionally redundant for virulence.
Fig 7. In planta growth of wild type, ΔrpoN1, ΔrpoN2 and ΔrpoN1 ΔrpoN2 R. solanacearum GMI1000 in tomato.
Bacterial growth (CFUs per gram of fresh tissue) was determined at 4 dpi in cv. Bonny Best and 7 dpi in cv. Hawaii 7996. For both tomato cultivars, the ΔrpoN1 and ΔrpoN1 ΔrpoN2 mutants yielded lower CFUs (reduced growth) compared to the ΔrpoN2 mutant and wild-type R. solanacearum GMI1000. [Data points represent mean values (n = 8) ± SD. Analysis was done using Student t-test to identify significant changes (P < 0.005), which are marked with double asterisk].
Conclusions
Although two rpoN genes are present in R. solanacearum GMI1000, our results, in combination with previous findings clearly indicate that the rpoN1 and rpoN2 genes are not genetically redundant. Functions commonly associated with RpoN regulation were dependent only on the rpoN1 gene. Additionally, this study demonstrates that the RpoN1 and RpoN2 proteins are not equivalent or interchangeable. Why R. solanacearum GMI1000 possesses the genetic machinery to encode dual RpoN proteins that are not interchangeable is an intriguing question. It is possible that the EBPs of R. solanacearum GMI1000 have evolved to preferentially interact with RpoN1 to activate transcription from RpoN promoters while RpoN2 may have evolved to play more of an antagonistic role in R. solanacearum GMI1000, i.e., repressing transcription from genes possessing RpoN promoters. Future studies aimed at identifying the RpoN-EBP partnerships in R. solanacearum GMI1000 are expected to clarify the regulatory roles of the RpoN1 and RpoN2 proteins.
Supporting Information
Each strain (n = 3) was grown in minimal media in which the indicated compound served as either the sole carbon, nitrogen or sulfur source. The mean absorbance value at 600 nm (± standard deviation) is reported for each strain.
(DOCX)
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by National Institutes of Health (www.nih.gov) Grant R15 GM104880-01A1 awarded to CTN and National Science Foundation (www.nsf.gov) Grant CBET 1263905 awarded to CTN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Studholme DJ, Buck M. The biology of enhancer-dependent transcriptional regulation in bacteria: insights from genome sequences. FEMS Microbiol Lett. 2000;186: 1–9. [DOI] [PubMed] [Google Scholar]
- 2. Merrick MJ. In a class of its own—the RNA polymerase sigma factor sigma 54 (sigma N). Mol Microbiol. 1993;10: 903–9. [DOI] [PubMed] [Google Scholar]
- 3. Chaney M, Grande R, Wigneshweraraj SR, Cannon W, Casaz P, Gallegos MT, et al. Binding of transcriptional activators to sigma 54 in the presence of the transition state analog ADP-aluminum fluoride: insights into activator mechanochemical action. Genes Dev. 2001;15: 2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannon W, Gallegos MT, Casaz P, Buck M. Amino-terminal sequences of sigmaN (sigma54) inhibit RNA polymerase isomerization. Genes Dev. 1999;13: 357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sasse-Dwight S, Gralla JD. Role of eukaryotic-type functional domains found in the prokaryotic enhancer receptor factor sigma 54. Cell. 1990;62: 945–54. [DOI] [PubMed] [Google Scholar]
- 6. Syed A, Gralla JD. Identification of an N-terminal region of sigma 54 required for enhancer responsiveness. J Bacteriol. 1998;180: 5619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Southern E, Merrick M. The role of region II in the RNA polymerase sigma factor sigma(N) (sigma(54)). Nucleic Acids Res. 2000;28: 2563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gallegos MT, Buck M. Sequences in sigmaN determining holoenzyme formation and properties. J Mol Biol. 1999;288: 539–53. [DOI] [PubMed] [Google Scholar]
- 9. Wong C, Tintut Y, Gralla JD. The domain structure of sigma 54 as determined by analysis of a set of deletion mutants. J Mol Biol. 1994;236: 81–90. [DOI] [PubMed] [Google Scholar]
- 10. Taylor M, Butler R, Chambers S, Casimiro M, Badii F, Merrick M. The RpoN-box motif of the RNA polymerase sigma factor sigma N plays a role in promoter recognition. Mol Microbiol. 1996;22: 1045–54. [DOI] [PubMed] [Google Scholar]
- 11. Doucleff M, Pelton JG, Lee PS, Nixon BT, Wemmer DE. Structural basis of DNA recognition by the alternative sigma-factor, sigma54. J Mol Biol. 2007;369: 1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrios H, Valderrama B, Morett E. Compilation and analysis of sigma(54)-dependent promoter sequences. Nucleic Acids Res. 1999;27: 4305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bush M, Dixon R. The role of bacterial enhancer binding proteins as specialized activators of sigma54-dependent transcription. Microbiol Mol Biol Rev. 2012;76: 497–529. 10.1128/MMBR.00006-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poggio S, Osorio A, Dreyfus G, Camarena L. The four different sigma(54) factors of Rhodobacter sphaeroides are not functionally interchangeable. Mol Microbiol. 2002;46: 75–85. [DOI] [PubMed] [Google Scholar]
- 15. Poggio S, Osorio A, Dreyfus G, Camarena L. Transcriptional specificity of RpoN1 and RpoN2 involves differential recognition of the promoter sequences and specific interaction with the cognate activator proteins. J Biol Chem. 2006;281: 27205–15. [DOI] [PubMed] [Google Scholar]
- 16. Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, Fischer HM. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the sigma 54 gene (rpoN). J Bacteriol. 1991;173: 1125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayward AC. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum . Annu Rev Phytopathol. 1991;29: 65–87. [DOI] [PubMed] [Google Scholar]
- 18. Salanoubat M, Genin S, Artiguenave F, Gouzy J, Mangenot S, Arlat M, et al. Genome sequence of the plant pathogen Ralstonia solanacearum . Nature. 2002;415: 497–502. [DOI] [PubMed] [Google Scholar]
- 19. Ray SK, Kumar R, Peeters N, Boucher C, Genin S. rpoN1, but not rpoN2, is required for twitching motility, natural competence, growth on nitrate, and virulence of Ralstonia solanacearum . Front Microbiol. 2015;6: 229 10.3389/fmicb.2015.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denny TP, Hayward AC. Ralstonia In: Schaad NW, Jones JB, Chun W, editors. Laboratory Guide for Identification of Plant Pathogenic Bacteria: The American Phytopathological Society; 2001. p. 151–74. [Google Scholar]
- 21. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2: 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166: 175–6. [DOI] [PubMed] [Google Scholar]
- 23. Choi KH, Schweizer HP. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 2005;5: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212: 77–86. [DOI] [PubMed] [Google Scholar]
- 25. Jacobs JM, Babujee L, Meng F, Milling A, Allen C. The in planta transcriptome of Ralstonia solanacearum: conserved physiological and virulence strategies during bacterial wilt of tomato. MBio. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tralau T, Vuilleumier S, Thibault C, Campbell BJ, Hart CA, Kertesz MA. Transcriptomic analysis of the sulfate starvation response of Pseudomonas aeruginosa . J Bacteriol. 2007;189: 6743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valentini M, Storelli N, Lapouge K. Identification of C(4)-dicarboxylate transport systems in Pseudomonas aeruginosa PAO1. J Bacteriol. 2011;193: 4307–16. 10.1128/JB.05074-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundgren BR, Villegas-Peñaranda LR, Harris JR, Mottern AM, Dunn DM, Boddy CN, et al. Genetic analysis of the assimilation of C5-dicarboxylic acids in Pseudomonas aeruginosa PAO1. J Bacteriol. 2014;196: 2543–51. 10.1128/JB.01615-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yurgel SN, Kahn ML. Dicarboxylate transport by rhizobia. FEMS Microbiol Rev. 2004;28: 489–501. [DOI] [PubMed] [Google Scholar]
- 30. Studholme DJ, Dixon R. Domain architectures of sigma54-dependent transcriptional activators. J Bacteriol. 2003;185: 1757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conway K, Boddy CN. Sigma 54 Promoter Database. 2012; Available: http://www.sigma54.ca/.
- 32. Kang Y, Liu H, Genin S, Schell MA, Denny TP. Ralstonia solanacearum requires type 4 pili to adhere to multiple surfaces and for natural transformation and virulence. Mol Microbiol. 2002;46: 427–37. [DOI] [PubMed] [Google Scholar]
- 33. Palacios S, Escalante-Semerena JC. 2-Methylcitrate-dependent activation of the propionate catabolic operon (prpBCDE) of Salmonella enterica by the PrpR protein. Microbiology. 2004;150: 3877–87. [DOI] [PubMed] [Google Scholar]
- 34. Suvorova IA, Ravcheev DA, Gelfand MS. Regulation and evolution of malonate and propionate catabolism in proteobacteria. J Bacteriol. 2012;194: 3234–40. 10.1128/JB.00163-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fründ C, Priefert H, Steinbüchel A, Schlegel HG. Biochemical and genetic analyses of acetoin catabolism in Alcaligenes eutrophus . J Bacteriol. 1989;171: 6539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ali NO, Bignon J, Rapoport G, Debarbouille M. Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis . J Bacteriol. 2001;183: 2497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schobert M, Görisch H. Cytochrome c550 is an essential component of the quinoprotein ethanol oxidation system in Pseudomonas aeruginosa: cloning and sequencing of the genes encoding cytochrome c550 and an adjacent acetaldehyde dehydrogenase. Microbiology. 1999;145 (Pt 2): 471–81. [DOI] [PubMed] [Google Scholar]
- 38. Taniyama K, Itoh H, Takuwa A, Sasaki Y, Yajima S, Toyofuku M, et al. Group X aldehyde dehydrogenases of Pseudomonas aeruginosa PAO1 degrade hydrazones. J Bacteriol. 2012;194: 1447–56. 10.1128/JB.06590-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dalsing BL, Allen C. Nitrate assimilation contributes to Ralstonia solanacearum root attachment, stem colonization, and virulence. J Bacteriol. 2014;196: 949–60. 10.1128/JB.01378-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nohno T, Saito T, Hong JS. Cloning and complete nucleotide sequence of the Escherichia coli glutamine permease operon (glnHPQ). Mol Gen Genet. 1986;205: 260–9. [DOI] [PubMed] [Google Scholar]
- 41. Macaluso A, Best EA, Bender RA. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990;172: 7249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muse WB, Bender RA. The nac (nitrogen assimilation control) gene from Escherichia coli . J Bacteriol. 1998;180: 1166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, Peter BJ, et al. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci U S A. 2000;97: 14674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jenkins LS, Nunn WD. Regulation of the ato operon by the atoC gene in Escherichia coli . J Bacteriol. 1987;169: 2096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each strain (n = 3) was grown in minimal media in which the indicated compound served as either the sole carbon, nitrogen or sulfur source. The mean absorbance value at 600 nm (± standard deviation) is reported for each strain.
(DOCX)
Data Availability Statement
All relevant data are within the paper.