Summary
Invariant natural killer T (iNKT) cells recognize glycolipids as antigens and diversify into NKT1 (IFN-γ), NKT2 (IL-4), and NKT17 (IL-17) functional subsets while developing in the thymus. Mechanisms that govern the balance between these functional subsets are poorly understood due partly to the lack of distinguishing surface markers. Here we identified the heparan sulfate proteoglycan syndecan-1 (sdc1) as a specific marker of naïve thymic NKT17 cells and that sdc1 deficiency significantly increased thymic NKT17 cells at the expense of NKT1 cells, leading to impaired iNKT cell-derived IFN-γ, both in vitro and in vivo. Using surface expression of sdc1 to identify NKT17 cells, we confirmed differential tissue localization and interstrain variability of NKT17 cells and uncovered that NKT17 cells expressed high TCRβ, preferentially use Vβ8, and display high sensitivity to ɑ-GalCer than to CD3/CD28 stimulation. These findings provide a novel non-invasive simple method for identification and viable sorting of naïve NKT17 cells from unmanipulated mice and suggest that sdc1 expression negatively regulates homeostasis iNKT cells. In addition, they lay the groundwork for investigating the mechanisms by which sdc1 regulates NKT17 cells.
Keywords: iNKT cells, Syndecan-1, CD138, NKT17, IFN-γ, IL-17
Introduction
The invariant natural killer T (iNKT) cell is a unique innate-like cell type that expresses an invariant T cell receptor α-chain (TCRVα14-Jα18 in mice) and recognizes glycolipids, instead of peptides, as antigens [1, 2]. While developing in the thymus, the iNKT cell acquires an effector ability to rapidly secrete large amounts of IFN-γ, IL-4, and IL-17 [3] that are prototypically produced by activated CD4 T helper cells after differentiation into Th1, Th2, or Th17 cells, respectively, depending on cytokines in milieu and nature of instigating pathogen [4]. By rapidly secreting these cytokines, iNKT cells play critical roles in regulating innate and adaptive immune responses and driving autoimmune diseases [4]. However, the mechanisms controlling the generation and relative ratio of the functional iNKT cell subsets are yet to be fully understood due, at least partly, to the lack of bona fide physiologic surface markers that can be used to visualize and fractionate iNKT cells into viable subsets without resorting to terminal intracellular staining of transcription factors and cytokines.
Syndecan 1 (sdc1, CD138) is a heparan sulfate proteoglycans (HSPGs) that regulates multiple cellular functions, including cell proliferation, differentiation, and survival of adherent cells and tumors by using its heparan sulfate side chains to mediate interactions with multiple ligands, including cell matrix proteins, growth factors, cytokines, and chemokines [5-7]. In addition, sdc1 has been implicated in triglyceride clearance [8] and modulating chemotaxis and inflammatory responses [6]. Sdc1 is normally expressed by epithelial and other adherent cells [5]. Among normal immune cells, only plasma cells and developing B cells are known to express sdc1 [7].
Here we identified sdc1 as an unexpected and specific marker of IL-17-producing NKT17 cells that also appears to play an important role in regulating their frequency relative to that of IFN-γ-producing NKT1 during thymic development.
Results and Discussion
Surface expression of sdc1 identifies IL-17-producing iNKT (NKT17) cells
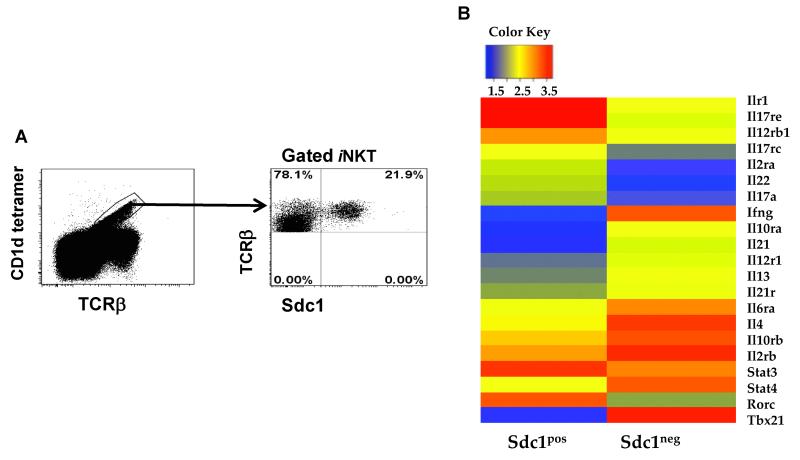
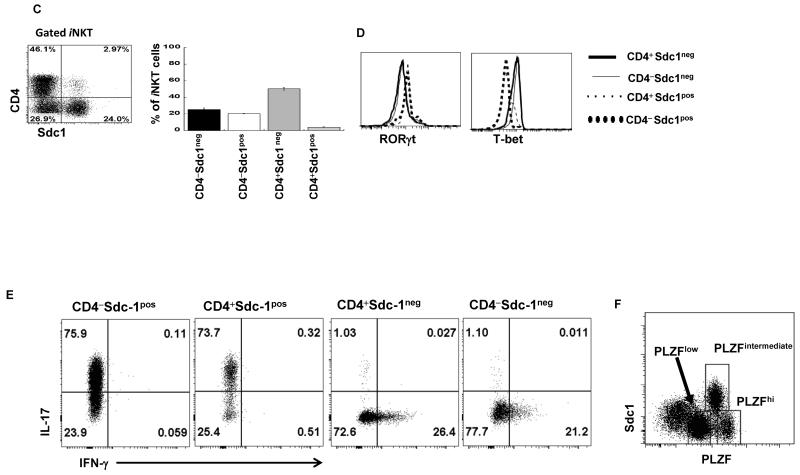
We have detected a discrete novel subset of thymic iNKT cells that specifically expressed sdc1 (sdc1pos iNKT cells) (Fig. 1A). To identify this subset and its relationship to the sdc1neg subset, we isolated sdc1pos and sdc1neg subsets from Balb/c mice and conducted genome-wide gene profiling using Affymetrix DNA microarrays. The genes associated with IL-17 and IFN-γ lineages were differentially expressed by the sdc1pos and sdc1neg subsets, respectively (Fig. 1B), suggesting that sdc1 expression distinguishes between IL-17 and IFN-γ-producing subsets. Both subsets had CD4+ and CD4− subpopulations, but the CD4+ cell subpopulation of the sdc1pos subset was very small (Fig. 1C). Flow cytometric analysis validated the microarray data as RORγt was exclusively expressed by the sdc1pos cells, whereas T-bet was expressed by the sdc1neg cells, irrespective of the CD4 expression (Fig. 1D). Likewise, sdc1pos and sdc1neg subsets differentially expressed IL-17 and IFN-γ after PMA/ionomycin stimulation (Fig. 1E). To exclude the possibility that sdc1 is also expressed by IL-4-producing NKT2 cells (identified by high PLZF expression), we related PLZF expression to that of sdc1. Only PLZFintermediate iNKT cells (NKT17), but not PLZFhigh (NKT2) or PLZFlow (NKT1) cells expressed sdc1 (Fig. 1F). Taken together, these results identify sdc1 as an exclusive marker of thymic NKT17 cells.
Figure 1. Comparative analysis of sdc1pos and sdc1neg subsets of iNKT cells.
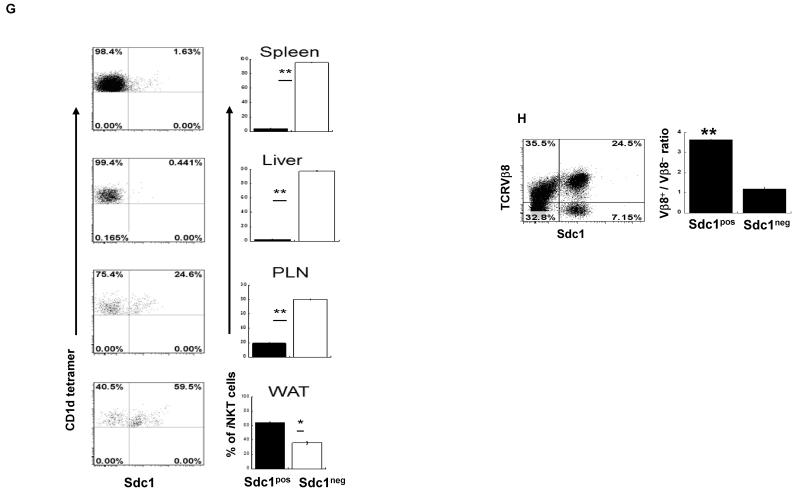
(A) Thymic iNKT cells were stained with CD1d tetramer and analyzed for sdc1 expression by FACS. (B) Transcript profiles of sorted sdc1pos and sdc1neg subsets were determined using affymetrix DNA microarray. Heat map shows differential expression of genes encoding IL-17, IFN-γ, IL-4, and associated transcription factors. (C) Surface staining and percentages of sdc1pos and sdc1neg subsets that express CD4. Results (mean ± s.e.m) are derived from three independent experiments; n= 15-20 mice pooled. (D-E) iNKT cells (pooled from 10 to 15 mice per experiment) were sorted into the indicated subpopulations and analyzed for the expression of T-bet and RORγt (D) or intracellular IL-17 and IFN-γ (E) after rapid stimulation with PMA/ionomycin. Cumulative data are from one of at least three independent experiments with similar results. (F) Relative expression of sdc1 by PLZFlow (NKT1), PLZFintermediate (NKT17), and PLZFhigh (NKT2) subsets of gated iNKT cells. Dot plot is from one of two independent experiments with similar results (n= 3-5 mice per experiment). (G) iNKT cells were isolated from the indicated organs and analyzed for sdc1 expression as shown in dot plots. Average frequency of sdc1pos and sdc1neg subsets were determined using data from three independent experiments; n = 15 mice. (H) Thymic iNKT cells were isolated and analyzed for TCRVβ8 usage by sdc1pos and sdc1neg subsets. Dot plot shows surface expression of TCRVβ8+ (TCRVβ8.1+8.2) and sdc1 by gated iNKT cells. Ratio of TCRVβ8+ versus TCRVβ8− (which are mainly Vβ2+β7 in iNKT cells) usage by sdc1pos and sdc1neg subsets are derived from three independent experiments; n= 9 mice. Error bars indicate s.e.m. *P < 0.05, **P < 0.01 by Student’s t test.
The ability to identify NKT17 cells using surface sdc1 staining allowed us to confirm properties of NKT17 that would otherwise require intracellular staining. These include rarity of NKT17 cells in C57BL/6 mice [9] and their relative abundance in the C3H/HeJ and NOD/LtJ mice (Supporting information Fig. 1). We were also able to monitor thymic NKT17 cells in mice of different ages and show that they plateau around the age of 10 weeks (Supporting information Fig. 2). Furthermore, sdc1pos cells resided mainly in peripheral lymph nodes and visceral adipose tissue (Fig. 1G), consistent with published results [10]. While selective sdc1 expression in certain tissues is indicative of specific staining, additional negative controls are included (Supporting information Fig. 3). In addition, we discovered that sdc1pos cells preferentially utilize TCRVβ8 chain at approximately 3:1 ratio relative to Vβ8− chains, whereas sdc1neg cells use Vβ8+ : Vβ8− at approximately 1:1 ratio (Fig. 1H). These data together with the report that NKT2 cells preferentially utilize Vβ2 and β7 chains [9] are strongly supportive of differential utilization of Vβ chains by the functional subsets of iNKT cells.
NKT17 cells are highly responsive to α-GalCer compared to anti-CD3/CD28 stimulation
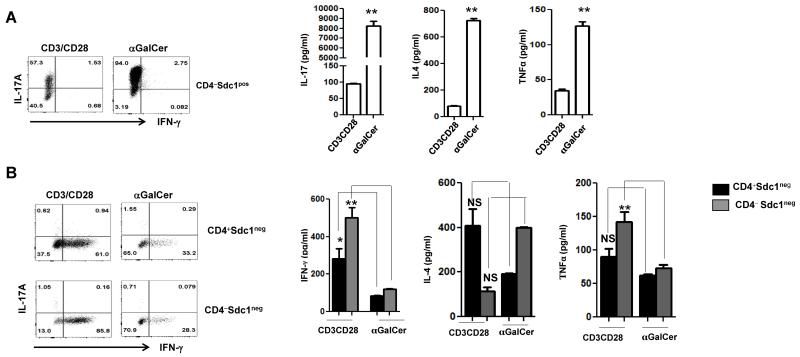
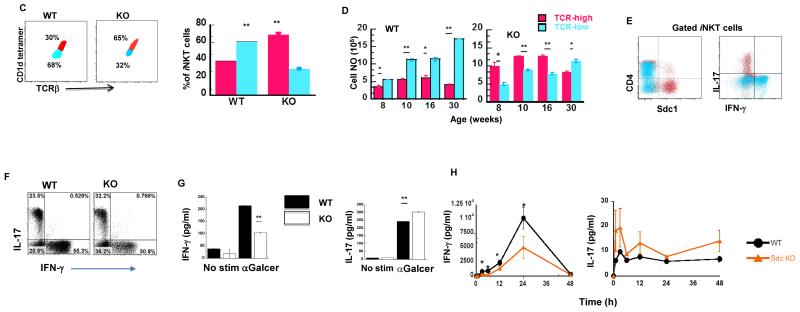
Vβ usage influences antigen affinity of iNKT cells [3, 11]. In light of skewed Vβ usage by thymic sdc1pos versus sdc1neg subset and our new capacity to FACS-sort them into viable status, we compared the ability of each subset to produce its signature cytokine (IL-17 and IFN-γ, respectively) as well as common cytokines (IL-4, TNFα) in response to the stimulation with αGalCer glycolipid and anti-CD3/CD28 beads. Due to rarity of CD4+ sdc1pos cells, we limited our analysis of the sdc1pos subset to the CD4− subpopulation. Surprisingly, αGalCer was significantly more potent than anti-CD3/CD28 in eliciting cytokine secretion by NKT17 cells (Fig. 2A). More than 90% of sdc1pos cells expressed IL-17 in response to αGalCer compared to about 57% in response to anti-CD3/CD28 stimulation (Fig. 2A, dot plots). Quantitatively, αGalCer reproducibly elicited about 1000-fold more IL-17 than anti-CD3/CD28 stimulation (Fig. 2A, left graph). Production of IL-4 and TNFα by NKT17 cells followed the same pattern as for IL-17, although the differences were less dramatic (Fig. 2A, middle and right graphs). Differential responses were also observed in the case of sdc1neg cells, albeit in the opposite direction and largely limited to IFN-γ as both CD4+ and CD4− subpopulations produced significantly more IFN-γ in response to anti-CD3/CD28 than to αGalCer stimulation (Fig. 2B, dot plots and left graph). CD4+ sdc1neg cells showed similar trends in the cases of IL-4 and TNFα, but the differences were not statistically significant (Fig. 2B, middle and right graphs). CD4− sdc1neg cells produced significantly more TNFα after anti-CD3/CD28 as compared to αGalCer stimulation, but not in the case of IL-4 (Fig. 2B, middle and right graphs). Why production of common cytokines particularly of IL-4 by sdc1neg cells did not follow the observed pattern for IFN-γ is currently unknown, but likely related to the presence of NKT2 cells within sdc1neg subset (Fig. 1F) and the possibility that NKT2 are more responsive to αGalCer than CD3/CD28 stimulation - will be examined in the future. Expression or lack of CD4 is a second potential confounding factor [12, 13]. Taken together, these results show that NKT17 cells, which primarily reside in white adipose tissue and lymph nodes and preferentially expressed Vβ8, were highly responsive to αGalCer at least in regard to the tested cytokines, whereas NKT1 cells, which primarily reside in the liver and spleen, were more responsive to CD3/CD28 stimulation as measured by IFN-γ production, their signature cytokine. αGalCer and CD3/CD28 also differed in their ability to induce shedding of surface sdc1. Only a few sorted sdc1pos cells retained sdc1 expression after stimulation with anti-CD3/CD28 beads (13% ± 9), but the majority (81% ± 3) retained expression when stimulated with αGalCer, raising the possibility that NKT17 cells might retain sdc1 expression after activation with some but not all ligands. It is noteworthy that PMA/ionomycin stimulation led to rapid loss of sdc1 by NKT17 cells (Supporting information Fig. 4), as described for epithelial cells [14]. The differential ability of αGalCer and anti-CD3/CD38 to elicit cytokine production by iNKT subsets could have important implications for choosing and testing therapeutic ligands and for clues that may lead to identification of natural ligands.
Figure 2. Expression and secretion of cytokines by thymic NKT17 and NKT1 cells in response to stimulation with αGalCer or anti-CD3/CD28 beads.
Thymic iNKT cells were sorted into CD4−sdc1pos (A) and sdc1neg subset into a CD4+ sdc1neg, and CD4− sdc1neg subpopulation (B). Sorted cells were stimulated in duplicates for 72 h with αGalCer or anti-CD3/CD28 beads and analyzed for intracellular IL-17 and IFN-γ and secreted IL-17 and IFN-γ, IL-4, and TNFα. Results are from one of at least three independent experiments with similar results; n= 10-15 mice. Comparison of cytokine concentrations by sdc1pos cells were compared using unpaired t test with Welch’s correction. Secretion of cytokines by sdc1neg cells were compared using one-way ANOVA followed by Bonferroni’s Multiple Comparison Test. Error bars indicate s.e.m. *P < 0.05, **P < 0.001.
Sdc1-deficiency increases the frequency of NKT17 cells at the expense of NKT1 cells
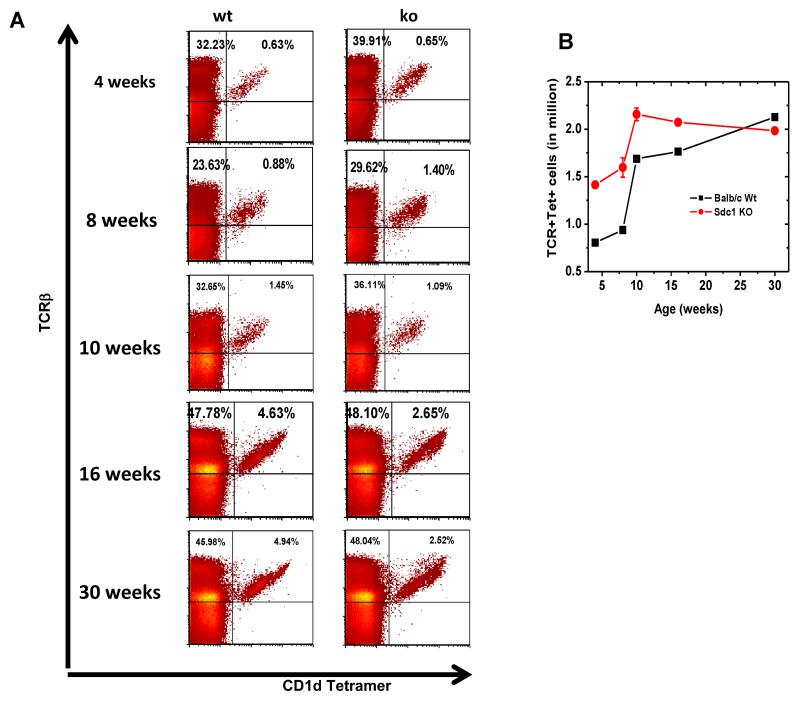
To analyze the impact of sdc1 deficiency on iNKT cell development, we compared their frequency and total numbers in sdc1KO and WT controls. Sdc1 was not essential for iNKT cell development as they were present in thymi of sdc1KO mice of various ages although their frequency varied slightly from that of age-matched WT mice (Fig. 3A). The absolute numbers of iNKT cells were also slightly higher in sdc1KO mice than in WT controls up to the age of 16 weeks (Fig. 3B). Furthermore, we observed that thymic iNKT cells could be divided into TCRβhi (mean fluorescent intensity, MFI of 2002 ± 41), and TCRβlo, (MFI of 854 ± 51) subpopulations. The ratio and absolute numbers of TCRβhi and TCRβlo were virtually reversed in WT and sdc1KO mice at all ages tested (Fig. 3C-D, Supporting information Fig. 5). Moreover, NKT17 cells resided almost exclusively within the TCRβhi subpopulation, where NKT1 cells resided within the TCRβlo subpopulation. This was based on differential expression of sdc1 and intracellular IL-17 and IFN-γ by the two subpopulations (Fig. 3E). The increased percentage of TCRβhi subpopulation in sdc1KO mice correlated with significant increase in the frequency of NKT17 cells at the expense of the NKT1 cells (Fig. 3F), but not in the frequency of IL-4-expressing cells (Supporting information Fig. 6) or PLZFhi cells (Supporting information Fig. 7). Functionally, this translated into significantly reduced IFN-γ and increased IL-17 production by sdc1KO after in vitro and in vivo activation with αGalCer (Fig. 3G-H) with no measurable effect on serum IL-4 (Supporting information Fig. 8). Thus, sdc1 deficiency significantly increases thymic NKT17 cells and raises their proportion at the expense of NKT1 cells.
Figure 3. Impact of sdc1 deficiency on development and cytokine secretion by thymic iNKT cells.
(A-B) Thymocytes were analyzed from WT and sdc1KO mice of indicated ages and frequency and absolute number of iNKT cells of age-matched mice compared. (A) Representative dot plots and (B) absolute numbers; n= 3-5 mice per age group. (C-D) iNKT cells from 8- to 10-week-old WT and sdc1KO mice were analyzed. (C) Percentages of TCRβhi (red-colored) and TCRβlo (blue-colored) in 8- to 10-week-old WT and sdc1KO mice. (D) Absolute numbers in WT and sdc1KO of increasing ages; n= 10-15 per age group. (E) Thymic iNKT cells from WT mice were gated and analyzed for the expression of CD4 and sdc1 (left) or IL-17 and IFN-γ (right) by gated TCRβhi (red dots) and TCRβlo (blue dots) subpopulations. (F-G) Thymic iNKT cells from age-matched WT and sdc1KO mice were sorted, stimulated for 72 h in triplicates with αGalCer and then analyzed intracellular (F) and secreted IFN-γ and IL-17(G). Results are from three independent experiments; n= 5-10 mice. (H) Sdc1KO and WT mice were treated with αGalCer and serum levels of IFN-γ and IL-17 measured at the indicated time points using CBA. Results are derived from 6 to 8 mice per group. Error bars indicate s.e.m. *P < 0.05, **P < 0.01 by Mann-Whitney test.
Concluding Remarks
These results identify sdc1 as a specific surface marker of naïve NKT17 cells. By providing a simple flow cytometric assay for distinguishing NKT17 from NKT1 and NKT2 cells, sdc1 expression provides a novel approach for detection and live sorting of NKT17 necessary for functional analysis. These results lay the groundwork for analysis of sdc1 expression by human iNKT cells and opportunities for modulating NKT17 cells for novel therapeutics.
Materials and Methods
Mice
Wild type Balb/c mice, C57BL/6, NOD-LtJ, and C3H/HeJ mice were purchased from the Jackson Laboratory. Sdc-1−/− Balb/c mice, generated as described [15] were a gift from Mary Ann Stepp (George Washington University). Jα18−/− mice [16] backcrossed onto Balb/c [17] were a gift from M. Taniguchi (Riken Research Center for allergy and Immunology) and R. Blumberg and D. Umetsu (Harvard University). Unless otherwise noted, female mice between the age of 8 and 10 weeks were used. All mice were bred and kept in specific pathogen-free conditions at the Johns Hopkins Animal Care Facility and all experimental procedures were approved by Institutional Animal Care and Use Committee.
Thymus-derived iNKT cell enrichment and sorting
Thymic iNKT cells were MACS enriched by depleting DP cells using the CD8α-MACS isolation kits (Miltenyi Biotec), surface-stained with mAbs specific for sdc1 (clone 281-2), CD4 (clone GK1.5), and TCRβ (clone H57-597) mAbs, and CD1d-PBS-57 tetramer (hereafter referred to as CD1d tetramer) and gated iNKT cells were sorted as whole or divided into sdc1pos and sdc1neg subsets using Mo-Flo FACS sorter. Unloaded tetramers were always used for specific gating.
Isolation of lymphocytes from different organs
Lymphocytes were isolated from lymphoid organs [18], liver [19] and visceral adipose tissues [20] using standard procedures.
Analysis of intracellular and secreted cytokines
Intracellular cytokines were analyzed after stimulation for 5 h with PMA and ionomycin using standard procedure. Cytokines were also measured after 72 h with anti-CD3/CD28 beads or with αGalCer. FACS-sorted iNKT cells were cultured (2 × 104 / well) and stimulated in duplicates with anti-CD3/28 beads (2 × 104) or 100 ng/ml αGalCer for 72 h in the presence of Jα18−/− feeders. BD Th1/Th2/Th17 cytometric bead array (CBA) was used to measure cytokines in tissue culture supernatants according to the manufacturer’s instruction. Data were acquired on LSRII (BD Biosciences) and analyzed with FlowJo software (TreeStar).
In vivo stimulation with αGalCer
Wild type and sdc-1KO Balb/c mice were injected i.p. with α-Galcer (2 μg mouse). Sera collected at baseline, 6, 12, 24, and 48 h were assayed for cytokines, using BD Th1/Th2/Th17 cytokine kit according to the manufacturer’s instruction.
Statistical analysis
Data are expressed as arithmetic mean ± s.e.m. Statistical significance was evaluated using the two-tailed unpaired Student’s t test, Mann-Whitney U test, and one-way ANOVA followed by Bonferroni post hoc test for comparing means of more than two groups with a confidence level of 95%. A p value ≤0.05 was considered significant. Data were analyzed using Prism 5 Statistical Software package (GraphPad).
Supplementary Material
Acknowledgments
We thank the NIH tetramer Core Facility for the provision of CD1d tetramers, Dr. Mary Ann Stepp of George Washington University for sdc1−/− KO mice and Dr. Hao Zhang (Bloomberg School of Public Health flow Cytometry Core) for sorting. Supported by public health service grant AI099027 and by P&F grant from Mid-Atlantic Nutrition and Obesity Research Center.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 2.Kinjo Y, Kitano N, Kronenberg M. The role of invariant natural killer T cells in microbial immunity. J Infect Chemother. 2013;19:560–570. doi: 10.1007/s10156-013-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald BD, Constantinides MG, Bendelac A. Polarized effector programs for innate-like thymocytes. Nat Immunol. 2013;14:1110–1111. doi: 10.1038/ni.2739. [DOI] [PubMed] [Google Scholar]
- 4.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 5.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett AH, Hayashida K, Park PW. Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol Cells. 2007;24:153–166. [PubMed] [Google Scholar]
- 7.Khotskaya YB, Dai Y, Ritchie JP, MacLeod V, Yang Y, Zinn K, Sanderson RD. Syndecan-1 is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo. J Biol Chem. 2009;284:26085–26095. doi: 10.1074/jbc.M109.018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doisne JM, Soulard V, Becourt C, Amniai L, Henrot P, Havenar-Daughton C, Blanchet C, Zitvogel L, Ryffel B, Cavaillon JM, Marie JC, Couillin I, Benlagha K. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J Immunol. 2011;186:662–666. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- 11.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203:1197–1207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamad A, Srikrishnan A, Mirmonsef P, Broeren C, June C, Pardoll D, Schneck J. Lack of coreceptor allows survival of chronically stimulated double negative αβ Tcells: Implications for autoimmunity. J Exp Med. 2001;193:1113–1121. doi: 10.1084/jem.193.10.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamad AR, Schneck JP. Antigen-induced T cell death is regulated by CD4 expression. Int Rev Immunol. 2001;20:535–546. doi: 10.3109/08830180109045577. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Gotte M, Bernfield M, Reizes O. Constitutive and accelerated shedding of murine syndecan-1 is mediated by cleavage of its core protein at a specific juxtamembrane site. Biochemistry. 2005;44:12355–12361. doi: 10.1021/bi050620i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 16.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 17.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamood AS, Bargatze D, Xiao Z, Jie C, Yagita H, Ruben D, Watson J, Chakravarti S, Schneck JP, Hamad AR. Fas-Mediated Apoptosis Regulates the Composition of Peripheral alphabeta T Cell Repertoire by Constitutively Purging Out Double Negative T Cells. PLoS ONE. 2008;3:e3465. doi: 10.1371/journal.pone.0003465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X, Hua J, Mohamood AR, Hamad AR, Ravi R, Li Z. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology. 2007;46:1519–1529. doi: 10.1002/hep.21823. [DOI] [PubMed] [Google Scholar]
- 20.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.