Abstract
So far, among the different non-invasive neurostimulation methods, only transcutaneous supraorbital nerve stimulation (t-SNS) with the Cefaly® (Cefaly Technology sprl, Herstal, Belgium) device has randomized controlled trial-based evidence for safety and efficacy and obtained American Food and Drug Administration approval for the prevention of episodic migraine. In a double-blinded, randomized, sham-controlled trial on 67 episodic migraine patients (mean pre-treatment migraine days/month: 6.9), the 50% responder rate after 3 months was significantly higher in the active group (38.2%) than in the sham group (12.1%); attack frequency and total headache days were also significantly reduced, but not headache severity. Acute anti-migraine drug intake was reduced by 36.7% in the active group. Statistical sub-analysis suggested that t-SNS was more effective in patients with a higher attack frequency. In a large survey on 2313 Cefaly users about safety and satisfaction only 4.3% of subjects reported side effects, all of which were minor and fully reversible, the most frequent being intolerance to the paresthesia feeling and the most severe an allergic skin reaction to the electrode gel. The efficacy/safety ratio of the Cefaly device was therefore most favorable, especially when compared to preventive anti-migraine drugs. The therapeutic efficacy of t-SNS with Cefaly with low-frequency migraine (≤5 attacks/month) was recently confirmed in an open randomized trial. No published data are available in chronic migraine. According to preliminary results of a fluorodeoxyglucose-positron emission tomography study, Cefaly might exert its effect in migraine by increasing activity in crucial areas of the limbic system and salience matrix such as orbitofrontal and anterior cingulate cortices.
Electronic supplementary material
The online version of this article (doi:10.1007/s40122-015-0039-5) contains supplementary material, which is available to authorized users.
Keywords: Cefaly®, External trigeminal nerve stimulation (e-TNS), Headache, Migraine, Migraine prophylaxis, Neuromodulation, Neurostimulation, Preventive therapy, Transcutaneous supraorbital nerve stimulation (t-SNS), Trigeminal nerve
Introduction
Migraine is the most prevalent neurological disorder and is characterized by recurrent headache attacks associated with gastrointestinal symptoms and sensoriphobia [1–3]. Due to the disabling headache and associated symptoms, migraine significantly impairs quality of life [4, 5] and is the sixth most disabling disease worldwide according to the Global Burden of Disease Study 2013 [6]. At present, there is no definitive cure for migraine, but various treatments are available to manage the disease. Migraine management includes acute and preventive treatments: acute treatments aim at interrupting an attack and restore normal function [7, 8], while preventive treatments have the disease-modifying objective of reducing attack frequency and severity [8–11]. Currently, migraine is mostly managed using pharmacologic treatments [10]. The most commonly used drugs to stop migraine attacks are analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), and triptans [8, 10–13]. Effective preventive drugs include beta-blockers (e.g., metoprolol, propranolol, bisoprolol, candesartan), calcium channel blockers (such as flunarizine or verapamil), and the anticonvulsants topiramate and valproate [8–10, 12–14], while nutraceuticals like riboflavin, co-enzyme Q10, magnesium or feverfew have lower efficacy [15].
Most of preventive anti-migraine drugs have contraindications and are associated with moderate to severe side effects [9, 12, 13, 16–19]. Importantly, these side effects, combined with insufficient efficacy, frequently lead to dissatisfaction and discontinuation [17, 18, 20]. In a large United States health insurance survey, 73.4%, 70.2%, and 67.6% of patients who initiated migraine prophylaxis with antidepressants, anticonvulsants, and beta-blockers, respectively, were designated non-adherent after 6 months [21].
In patients with frequent and/or prolonged migraine attacks, excessive consumption of acute anti-migraine drugs may lead to headache chronification, i.e., medication overuse headache [13, 18], which worsens the patients’ condition [8, 10, 22].
Moreover, some patients, in particular those suffering from chronic migraine, become resistant to conventional medications used to treat and prevent migraine and thus do not achieve sufficient pain relief [22–27].
To sum up, 80% of patients are willing to change their current medication for a treatment with similar efficacy but fewer side effects [20]. Medication-related adverse effects and lack of efficiency thus underscore the need for better anti-migraine treatments and have created a niche for non-pharmacologic therapies such as neurostimulation.
Peripheral nerve stimulation (PNS) represents an emerging, neurostimulation alternative to treat and prevent migraine [22, 24, 27]. Percutaneous sub-occipital nerve stimulation (ONS) was first considered as a treatment for chronic headaches in 1999 [22, 28, 29]. Many non-controlled studies reported reduction in headache frequency, severity and headache-related disability after ONS in chronic migraine, but the global effect in three sham-controlled trials was not overwhelming [25, 30–35]. The combination of percutaneous ONS and supraorbital nerve stimulation (SNS) was claimed to have a better effect, but a randomized controlled trial is lacking [22, 36, 37]. Stimulation of the vagus nerve (VNS) with implantable electrodes in the neck was found effective for migraine prevention in a few case reports and stimulation of the sphenopalatine ganglion with a microstimulator implanted in the pterygopalatine fossa is being studied for migraine treatment [19, 22, 24, 38]. The common denominator of these neurostimulation methods is that they are invasive and applicable only to the most disabled patients with frequent, severe and drug refractory migraine [16, 27]. For instance, ONS that requires implantation of leads in the neck and of a battery in a subcutaneous pocket is associated with major adverse events (AE), chiefly electrode migration, battery depletion and replacement or infections, leading to multiple surgical interventions [19, 22, 25, 26, 28].
The development of non-invasive transcutaneous stimulators opened the field of neurostimulation therapy to all migraine patients without consideration of disability or drug refractoriness [19, 23]. These methods can be subdivided into transcranial neurostimulations, chiefly magnetic or direct current, and transcutaneous stimulations of pericranial nerves. Transcranial direct current stimulation (tDCS) showed encouraging results in migraine prevention [19, 22]. Transcranial magnetic stimulation (TMS) was approved by the American Food and Drug Administration (FDA) for acute treatment of migraine with aura attacks [39]. Promising results were also associated with TMS and repetitive TMS (rTMS) for preventive treatment of migraine, although large sham-controlled trials are lacking [19, 22]. Regarding VNS, a transcutaneous device applied in the neck was found effective for acute migraine treatment in two open studies [40, 41]. Concerning SNS, the transcutaneous Cefaly® device (Cefaly Technology sprl, Herstal, Belgium) showed a significant reduction in both migraine attacks and headache days in a randomized sham-controlled trial [16]. Consequently, in March 2014, Cefaly was the first medical device approved by the FDA for the prevention of migraine.
In this paper, we will review in detail the available data for Cefaly device as a migraine treatment including technical aspects, effect size, and safety, as well as possible explanations for its mode of action. These data will provide better insight into the therapeutic potentials of the device as a non-pharmaceutical, non-invasive migraine treatment, and allow determination of how this product fits into the available armamentarium for the management of migraine patients. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Technical Aspects
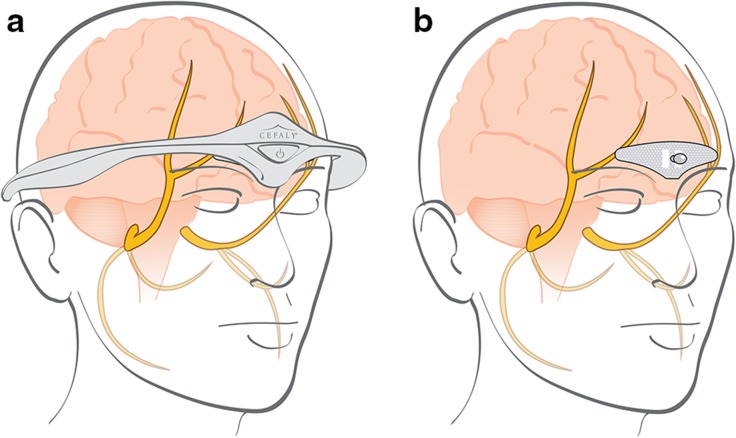
Cefaly is an external neurostimulator designed for transcutaneous SNS (t-SNS), also known as external trigeminal nerve stimulation (e-TNS) [42]. It is a constant current generator for a maximum skin impedance of 2.2 kOhms. In practice, the electrical impulses generated by the Cefaly headband are transmitted transcutaneously via a self-adhesive, supraorbital electrode to excite (trigger action potentials) on the supratrochlear and supraorbital branches of the ophthalmic nerve (V1) located under the skin of the forehead (Fig. 1). Parameters of the generated electrical impulses are as follows: rectangular biphasic compensated impulses with an electrical mean equal to zero, impulse width of 250 µS, frequency of 60 Hz, maximum intensity of 16 mA with a progressive slope from 1 to 16 mA over 14 min.
Fig. 1.
The electrical impulses generated by the Cefaly® device are transmitted via an electrode to excite the upper branches of the ophthalmic nerve (V1). These pictures are the property of Cefaly Technology
The patient has to use the device in daily sessions of 20 min preferably in the evening. The multiuse electrode has a central pin that interlocks with the median part of the device to get the metallic contact blades in connection with the conductive areas of the electrode. Cefaly operates on direct electrical energy provided by two 1.5 V AAA batteries. As the current intensity progressively increases, the patient has the possibility to stabilize the intensity when the tingling or prickling forehead sensation becomes uncomfortable.
The Cefaly device has an in-built software that allows monitoring of the number of completed sessions, and thus compliance.
Clinical Trials
Pilot Trials
Between 2007 and 2008, a pilot trial performed in 10 patients to assess safety and efficacy of Cefaly showed a decrease of the average migraine attack frequency from 3.9 to 2.8 per month and 5 out of 8 patients were satisfied with the device [43]. In the same pilot trial, when Cefaly was used as an acute treatment (total of 30 attacks), total relief without rescue medication was obtained in 13% of attacks, postpone drug intake in 20% of attacks and partial relief with additional drug treatment in 45% of attacks. In 2008, an unpublished safety and acceptance trial was conducted by Laboratoire Spincontrol (Tours, France) on 32 subjects using Cefaly. The device was considered “simple to use” by 94% of subjects. No adverse effects related to Cefaly were reported during these pilot trials.
The PREMICE Trial
An investigator-driven randomized controlled trial with Cefaly in migraine prevention was conducted between 2009 and 2011 under the auspices of the Belgian Headache Society. This PREvention of MIgraine using Cefaly (the PREMICE) study was a prospective, multicenter, double-blinded, randomized and sham-controlled trial conducted in five Belgian headache clinics. The study included 67 patients with at least 2 migraine attacks per month. After a 1-month baseline period, patients were randomized to verum or sham stimulation for a 3-month treatment period [16].
The intention-to-treat analysis showed a significant reduction in migraine days (P = 0.023) and in headache days (P = 0.011) between baseline and the third month of treatment in the verum group, but not in the sham group (Fig. 2). On average, patients in the verum group had a reduction of 29.7% in migraine days and 32.3% in headaches days, while respective reductions in the sham group were 4.9% and 3.4%; the differences between the two groups were significant for headache days but slightly missed the level of significance for migraine days. The percentage of patients having at least 50% reduction in monthly migraine days between baseline and third month of treatment (i.e., the 50% responder rate) was significantly greater in the verum group than in the sham group (38.2% versus 12.1%, respectively, P = 0.023). The number of migraine attacks was also significantly reduced in the verum group while attack severity was reduced but not significantly so. Using Cefaly induced a significant 36.7% reduction in acute anti-migraine drug intake (P = 0.006) compared to the sham group where there was on average a slight 0.4% increase of drug intake. Overall, the PREMICE study provided evidence that Cefaly is a safe and effective preventive treatment for migraine that reduces considerably disease burden and acute anti-migraine drug intake [16].
Fig. 2.
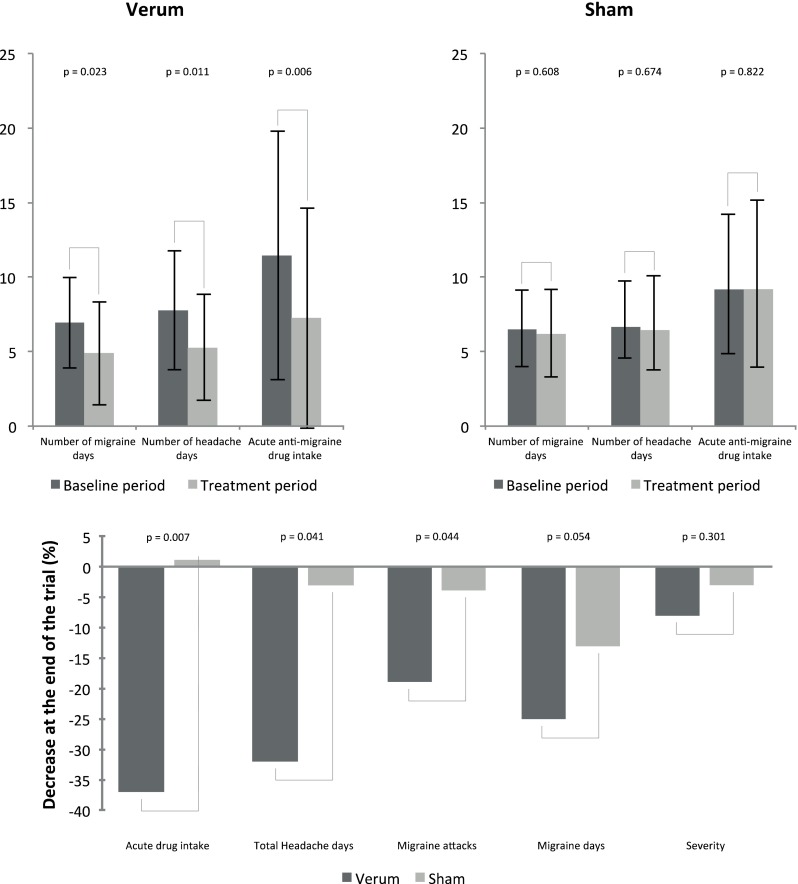
Results of the PREMICE study. Results are presented as means or means ± standard deviations where appropriate. Baseline and treatment period were compared using the Wilcoxon signed rank test for paired samples. Comparison of relative changes between verum and sham groups was assessed using the Wilcoxon rank sum test (also known as Mann–Whitney U test). P values < 0.05 are considered as significant
Additional statistical analyses showed that age and disease duration did not impact on outcome, but that the effect size was directly related to the number of migraine days during the baseline period [44]. When taking into account this correlation, the difference in migraine day reduction between verum and sham groups reached the level of statistical significance (P = 0.044, instead of 0.054). This result suggests that Cefaly might be more beneficial for patients with more frequent migraines. In the PREMICE trial, patients had on average four attacks or seven migraine days per month during baseline, which is in line with most trials in episodic migraine [16]. However, in clinical practice patients with very frequent or chronic migraine are the most disabled and might thus benefit from t-SNS Cefaly. Trials targeting this population of migraineurs are underway (ClinicalTrials.gov identifier, NCT02342743).
The percentage of satisfied patients was significantly higher in the verum group (70.59%; P = 0.009) than in the sham group (51.52%) [16]. Compliance was acceptable for the two groups of patients. The verum group had a slightly better compliance than the sham group (55.54 sessions vs. 49.00 sessions per patient, on average) but this difference was not significant, which suggests that blinding was preserved. Partial unblinding was considered unlikely, since the sham response within the first months was within the expected range, based on previous studies. As mentioned in the original article [16], “To decrease the risk of unblinding due to the difference in sensory perception between verum and sham stimulators, the investigators and their staff members committed themselves to the following: not interrogate patients about sensory perceptions, not enroll patients acquainted with each other and avoid physical contact between patients during visits.”
There were no adverse effects in either group, indicating that the Cefaly device is safe [16].
Post-Marketing Survey of 2313 Users
A survey was conducted to confirm the safety of Cefaly and assess the users’ satisfaction in a large cohort of headache sufferers who rented the Cefaly device via the Internet and used the device for a testing period of 58.2 days on average. The study was performed between September 2009 and June 2012 and included 2313 subjects who were assumed to suffer from migraine on the basis that they were using triptans for acute treatment [17].
During the test period, 99 patients (4.3%) reported a total of 104 AEs. The most frequent AE was intolerance to the local paresthesia induced by the electrical stimulation (n = 31, 1.3% of users), despite the possibility to interrupt the gradual current intensity increase as soon as the forehead sensation becomes uncomfortable. All patients reporting paresthesia intolerance stopped using Cefaly and only four patient users presented persistent paresthesia for several hours after the end of the stimulation. The second most frequent AE (n = 19, 0.8% of users) was arousal and sleep change: 15 users reported sleepiness or a feeling of fatigue during the stimulation while 4 subjects reported insomnia. These findings were consistent with the sedative effect of the Cefaly device described in the later mentioned study with high frequency stimulation. Some users (n = 12, 0.5%) reported tension-type headache after using Cefaly. Five users reported reversible skin irritation due to the electrode and two subjects presented an allergic skin reaction at the electrode site. This allergy was attributed to the acrylate component of the electrode gel. Consequently, new electrodes with hypoallergenic high quality gel without acrylate were developed and are currently available on the market to replace the previous model in allergic subjects. All the aforementioned AEs were minor and completely reversible supporting that Cefaly is safe and has excellent tolerability [17].
At the end of the trial period, users were offered to return the device or to purchase it. 1077 subjects (46.6%) returned the device and were thus considered to be dissatisfied. Table 1 illustrates compliance indexed by the time of use determined thanks to the in-built software for the returned devices. The analysis revealed that 48 subjects did not even switch on the device and 157 subjects did not use the Cefaly device for more than 60 min (3 session of 20 min), while they were assumed to use it once a day (20 min/day) during 40 days. Using the Cefaly device for more than 400 min would probably have been required to achieve a therapeutic effect over the rental period. Less than half of subjects who returned the device (n = 431, 40.0%) used it for more than 400 min; the true (“per protocol”) non-responder rate could thus be 18.6%. Among the 646 subjects who used the device for less than 400 min, 56 (8.7%) reported AEs, twice more than in the total group of 2313 subjects (4.28%). This rather high prevalence of AEs that probably reduced compliance and the lack of a therapeutic effect partly because of insufficient use may explain why 46.6% of subjects returned the device [17].
Table 1.
Compliance of returned Cefaly® devices after the rental period
| Total time of use (min) | Number of patients (% out of the 1077 patients) |
|---|---|
| 0 | 48 (4.46%) |
| 1 to 20 | 58 (5.39%) |
| 21 to 40 | 46 (4.27%) |
| 41 to 60 | 53 (4.92%) |
| 61 to 100 | 78 (7.24%) |
| 101 to 200 | 174 (16.16%) |
| 201 to 400 | 189 (17.55%) |
| >400 | 431 (40.02%) |
Reproduced from [17]: Magis D, Sava S, d’Elia TS, Baschi R, Schoenen. Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2313 headache sufferers in the general population. J Headache Pain. 2013;14:95. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0)
The remaining 1236 users (53.4%) purchased the Cefaly device after the rental period, which could be taken as an indicator of their satisfaction [17]. This satisfaction rate is lower than the 70.6% observed in the PREMICE study [16]. The difference may have two main reasons. First, the satisfaction rate was influenced by the willingness to pay in the current survey. The decision to return the device may also be influenced by the financial aspect in addition to lack of efficacy or satisfaction. Second, the subjects had to decide to keep the device after an average period of 58 days, while the PREMICE study shows that the maximal reduction in migraine frequency only occurs after 3 months of treatment with the Cefaly device. Therefore, the rental period may have been too short for some users to achieve a sufficient level of efficacy.
The survey confirmed that the Cefaly device is a safe, well-tolerated treatment for migraine headaches and that 53.4% of subjects purchased the device at the end of the testing period. Only 4.3% of users reported AEs that were all minor and completely reversible [17].
Very recently an open trial was conducted on 24 episodic migraine without aura, having ≤5 attacks per month with an average of 4.5 ± 0.24 migraine days per month, and having never been treated with migraine preventive medication. t-SNS with the Cefaly device was applied for 2 months with a daily session of 20 min. Per protocol analysis was performed on the 20 compliant patients, i.e., those who used the device for at least two-thirds of the recommend total time (i.e., ≥800 min of t-SNS neurostimulation). Between run-in and the second month, the 50% responder rate for migraine days was 75% (P < 0.001) and 81% for migraine attacks (P < 0.001); intake of acute migraine drugs was as well significantly reduced [45].
Studies on Possible Modes of Action
The precise mode of action of pericranial nerve neurostimulation methods in migraine is not determined. The initial rationale for their use postulated that convergence of somatic afferents from the trigeminal or the C2 territories with visceral trigeminovascular afferents on spinal trigeminal nucleus nociceptors may block ascending impulses in the pain pathway. This hypothesis was not confirmed in studies exploring the effect of per- or transcutaneous ONS on trigeminal nociceptor sensitivity [18]. In a fluorodeoxyglucose-positron emission tomography (FDG-PET) study of refractory cluster headache patients before and after long-term percutaneous ONS treatment [46], the only significant difference in responders was an increase in metabolism in the perigenual anterior cingulate gyrus, which suggests that ONS may act by changing activity in top-down pain control. Few physiological studies have been performed with the Cefaly device. The three studies described below do not solve the question about its mechanisms of action, but they may stimulate the discussion and further research.
Trial on Sedative Effects
A sedative effect was demonstrated by a double-blinded, crossover, sham-controlled study on 30 healthy volunteers who underwent a series of 4 stimulation protocols in random order: no stimulation (blank control, BC), sham stimulation (sham control, SC), low frequency (2.5 Hz) neurostimulation (LFN), and high frequency (120 Hz) neurostimulation (HFN). t-SNS sessions using the Cefaly device lasted 20 min. The sedative effect related to each of the four different conditions was assessed using the Psychomotor Vigilance Task (PVT), the Critical Flicker Fusion Frequency (CFFF), the d2 test, and the Fatigue Visual Numeric Scale (FVNS). These tests are described in detail in [47].
The results showed that the HFN with the Cefaly device induced a significant increase in PVT reaction time and FVNS score (P < 0.001), as well as a significant decrease in CFFF (P < 0.001), all indicating a mild decrease in vigilance during stimulation. The three other conditions (BC, SC, and LFN) were not associated with sedative effects. On the whole, this study showed that HFN with the Cefaly device induces a sedative effect in healthy volunteers.
Study of Pericranial Electromyography Activity
Recently, a study evaluated in 23 chronic migraine patients the effect of t-SNS with the Cefaly device on pericranial and neck muscle activity using quantitative electromyography (EMG) recordings [48]. Activity in frontalis, anterior temporalis, auricularis posterior, and middle trapezius muscles was recorded with surface EMG before and during stimulation with the Cefaly device. The results showed that the neurostimulation induced an increase of median frequency and amplitude of the myoelectrical signal in all muscles recorded except in frontalis muscles. The significance of this finding for the mode of action of the Cefaly device is doubtful, the more so that is unlikely that pericranial muscle activity plays a role in chronic migraine [49].
Cerebral FDG Uptake Changes
Preliminary results of an FDG-PET study in episodic migraine patients before and after 3 months of daily t-SNS with the Cefaly device were most recently presented at the International Headache Society Congress (IHC 2015, May 14–17, Valencia, Spain) [50]. Fourteen migraine patients recorded before and after neurostimulation were compared to 14 healthy untreated volunteers. Compared to the latter, at baseline patients were hypometabolic in the fronto-temporal regions (P < 0.001), especially in the orbitofrontal and perigenual anterior cingulate cortex. There was no significant effect on brain metabolism after one session with the Cefaly device. In compliant patients (n = 10), daily t-SNS with the Cefaly device for 3 months was followed by a normalization of the orbitofrontal hypometabolism [MNI (Montreal Neurological Institute) coordinates: −10, 32, −22, familywise error-corrected P value < 0.01]. In previous studies, the orbitofrontal cortex was found hypometabolic [51] or hypotrophic [52] in medication overuse headache, particularly so in patients not responding to treatment [53]. Like ONS in cluster headache, t-SNS with the Cefaly device might thus exert its beneficial effect in migraine via slow neuromodulatory mechanisms involving limbic and pain control areas in the cerebral cortex.
Discussion
Migraine is one of the most frequent disabling diseases. Developing a safe, effective migraine treatment is thus a high-priority issue. In this context, the Cefaly device may represent a first-line non-pharmacologic preventive migraine treatment. The potential benefits, advantages, and limitations of neurostimulation with the Cefaly device are identified and discussed.
t-SNS using the Cefaly device was shown to be efficient by decreasing migraine and headache days significantly more than sham stimulation. The device also reduced the number of migraine attacks. Patients using the Cefaly device reported no severe side effects and all the infrequent AEs collected were minor and completely reversible. Consequently, the Cefaly device allows to efficiently and safely prevent and treat episodic migraine.
For the discussion, the therapeutic results obtained with the Cefaly device can be compared to the pooled results obtained in placebo-controlled trials with one of the most potent anti-migraine preventive drugs, topiramate. The 50% responder rate is 38.2% for the Cefaly device, 45.3% for topiramate [54]. The responder rate for the sham Cefaly device is 12.1%, 21.8% for the topiramate placebo [54]. Therefore, the therapeutic gain is 26.1% for the Cefaly device but 23.5% for the topiramate. Topiramate is more effective than the Cefaly device in reducing migraine days and attacks. However, in the trials with topiramate half of patients had drug-related side effects and 25% of patients abandoned the drug because of intolerable adverse effects [54]. According to these results, the Cefaly device has a superior efficacy/safety ratio, which indicates that it merits to be proposed for episodic migraine prevention prior to prescribing topiramate. As for other non-invasive neurostimulation methods, compliance could be a problem with the Cefaly device, although it can be optimized by sustained patient education.
As the Cefaly device is a non-pharmacologic treatment, it represents an alternative for patients resistant to common anti-migraine drugs or intolerant to their side effects. It can also be combined without reluctance with pharmacological treatments. The device has very limited contraindications compared to preventive anti-migraine drugs. Only patients with recent brain or facial trauma cannot use the device. Some patients may be electro-hypersensitive and not tolerate the sensation induced by electrical stimulation, but they represent a minority of the headache sufferer population. For patients allergic to acrylate, hypoallergenic electrodes are now available. Overall, the Cefaly device could be proposed to patients who prefer non-pharmacologic treatments, or who have contraindications to the usual preventive anti-migraine drugs or do not tolerate them. Last but not least, the Cefaly device allows to significantly reduce acute anti-migraine medication use and therefore reduces the risk for chronification of migraine by acute medication overuse, which represents a pharmaco-economical advantage.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funding was received for publication of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
FR reports no disclosures with regard to this work. SP is an employee of Cefaly Technology. JS is a consultant for Cefaly Technology.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition (beta version) Ceph Int J Headache. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 2.Rossi HL, Recober A. Photophobia in primary headaches. Headache. 2015;55:600–604. doi: 10.1111/head.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35:6619–6629. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Bakar N, Tanprawate S, Lambru G, et al. Quality of life in primary headache disorders: a review. Cephalalgia. 2015 doi: 10.1177/0333102415580099. [DOI] [PubMed] [Google Scholar]
- 5.Lantéri-Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31:837–850. doi: 10.1177/0333102411398400. [DOI] [PubMed] [Google Scholar]
- 6.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougherty C, Silberstein SD. Providing care for patients with chronic migraine: diagnosis, treatment, and management. Pain Pract. 2014 doi: 10.1111/papr.12243. [DOI] [PubMed] [Google Scholar]
- 8.Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(Suppl 2):103–122. doi: 10.1111/head.12505_2. [DOI] [PubMed] [Google Scholar]
- 9.Silberstein SD. Preventive migraine treatment. Neurol Clin. 2009;27:429–443. doi: 10.1016/j.ncl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Steiner TJ, Paemeleire K, Jensen R, et al. European principles of management of common headache disorders in primary care. J Headache Pain. 2007;8(Suppl 1):S3–S47. doi: 10.1007/s10194-007-0366-y. [DOI] [PubMed] [Google Scholar]
- 11.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the american headache society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3–20. doi: 10.1111/head.12499. [DOI] [PubMed] [Google Scholar]
- 12.Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur J Neurol. 2009;16:968–981. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 13.Lanteri-Minet M, Valade D, Geraud G, et al. Revised French guidelines for the diagnosis and management of migraine in adults and children. J Headache Pain. 2014;15:2. doi: 10.1186/1129-2377-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16:86–92. doi: 10.1007/s11916-011-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoenen J, Magis D. Herbal medicines and vitamins. In: Migraines and other headache disorders, Chap 23. New York: Eds Lipton RB & Bigal ME. Informa Healthcare; 2006. p. 363–374.
- 16.Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697–704. doi: 10.1212/WNL.0b013e3182825055. [DOI] [PubMed] [Google Scholar]
- 17.Magis D, Sava S, d’ Elia TS, et al. Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2313 headache sufferers in the general population. J Headache Pain. 2013;14:95. doi: 10.1186/1129-2377-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1300–1311. doi: 10.1111/head.12154. [DOI] [PubMed] [Google Scholar]
- 19.Magis D, Jensen R, Schoenen J. Neurostimulation therapies for primary headache disorders: present and future. Curr Opin Neurol. 2012;25:269–276. doi: 10.1097/WCO.0b013e3283532023. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher RM, Kunkel R. Migraine medication attributes important for patient compliance: concerns about side effects may delay treatment. Headache. 2003;43:36–43. doi: 10.1046/j.1526-4610.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 21.Berger A, Bloudek LM, Varon SF, Oster G. Adherence with migraine prophylaxis in clinical practice. Pain Pract. 2012;12:541–549. doi: 10.1111/j.1533-2500.2012.00530.x. [DOI] [PubMed] [Google Scholar]
- 22.Magis D, Schoenen J. Advances and challenges in neurostimulation for headaches. Lancet Neurol. 2012;11:708–719. doi: 10.1016/S1474-4422(12)70139-4. [DOI] [PubMed] [Google Scholar]
- 23.Cruccu G, Aziz TZ, Garcia-Larrea L, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952–970. doi: 10.1111/j.1468-1331.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 24.Lambru G, Matharu MS. Peripheral neurostimulation in primary headaches. Neurol Sci. 2014;35(Suppl 1):77–81. doi: 10.1007/s10072-014-1748-y. [DOI] [PubMed] [Google Scholar]
- 25.Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31:271–285. doi: 10.1177/0333102410381142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwedt TJ. Occipital nerve stimulation for medically intractable headache. Curr Pain Headache Rep. 2008;12:62–66. doi: 10.1007/s11916-008-0012-7. [DOI] [PubMed] [Google Scholar]
- 27.Schwedt TJ. Neurostimulation for primary headache disorders. Curr Neurol Neurosci Rep. 2009;9:101–107. doi: 10.1007/s11910-009-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartsch T, Paemeleire K, Goadsby PJ. Neurostimulation approaches to primary headache disorders. Curr Opin Neurol. 2009;22:262–268. doi: 10.1097/WCO.0b013e32832ae61e. [DOI] [PubMed] [Google Scholar]
- 29.Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217–221. doi: 10.1046/j.1525-1403.1999.00217.x. [DOI] [PubMed] [Google Scholar]
- 30.Popeney CA, Aló KM. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43:369–375. doi: 10.1046/j.1526-4610.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- 31.Oh MY, Ortega J, Bellotte JB, et al. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a c1-2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004;7:103–112. doi: 10.1111/j.1094-7159.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 32.Matharu MS, Bartsch T, Ward N, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220–230. doi: 10.1093/brain/awh022. [DOI] [PubMed] [Google Scholar]
- 33.Schwedt TJ, Dodick DW, Hentz J, et al. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153–157. doi: 10.1111/j.1468-2982.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 34.Dodick DW, Silberstein SD, Reed KL, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2015;35:344–358. doi: 10.1177/0333102414543331. [DOI] [PubMed] [Google Scholar]
- 35.Silberstein SD, Dodick DW, Saper J, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2012;32:1165–1179. doi: 10.1177/0333102412462642. [DOI] [PubMed] [Google Scholar]
- 36.Reed KL, Black SB, Banta CJ, Will KR. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia. 2010;30:260–271. doi: 10.1111/j.1468-2982.2009.01996.x. [DOI] [PubMed] [Google Scholar]
- 37.Reed KL. Peripheral neuromodulation and headaches: history, clinical approach, and considerations on underlying mechanisms. Curr Pain Headache Rep. 2013;17:305. doi: 10.1007/s11916-012-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tepper SJ, Rezai A, Narouze S, et al. Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache. 2009;49:983–989. doi: 10.1111/j.1526-4610.2009.01451.x. [DOI] [PubMed] [Google Scholar]
- 39.Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373–380. doi: 10.1016/S1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- 40.Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986–993. doi: 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 41.Barbanti P, Grazzi L, Egeo G, et al. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 2015;16:542. doi: 10.1186/s10194-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Pilot study of trigeminal nerve stimulation (TNS) for epilepsy: a proof-of-concept trial. Epilepsia. 2006;47:1213–1215. doi: 10.1111/j.1528-1167.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 43.Gérardy P, Fabry D, Fumal A, Schoenen J. A pilot study on supra-orbital surface electrotherapy in migraine. Cephalalgia. 2009;29:134. [Google Scholar]
- 44.Schoenen J. Addendum to “Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial” [electronic response to Schoenen et al., Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial]. Neurology. 2015. http://www.neurology.org/content/80/8/697/reply#neurology_el;64113. Accessed 6 Oct 2015.
- 45.Russo A, Tessitore A, Conte F, et al. Transcutaneous supraorbital neurostimulation in “de novo” patients with migraine without aura: the first italian experience. J Head Pain. 2015;16:69. doi: 10.1186/s10194-015-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magis D, Bruno M-AA, Fumal A, et al. Central modulation in cluster headache patients treated with occipital nerve stimulation: an FDG-PET study. BMC Neurol. 2011;11:25. doi: 10.1186/1471-2377-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piquet M, Balestra C, Sava SL, Schoenen JE. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135. doi: 10.1186/1471-2377-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Didier HA, Di Fiore P, Marchetti C, et al. Electromyography data in chronic migraine patients by using neurostimulation with the Cefaly(®) device. Neurol Sci. 2015;36(Suppl 1):115–119. doi: 10.1007/s10072-015-2154-9. [DOI] [PubMed] [Google Scholar]
- 49.Coppola G, Schoenen J. Cortical excitability in chronic migraine. Curr Pain Headache Rep. 2012;16:93–100. doi: 10.1007/s11916-011-0231-1. [DOI] [PubMed] [Google Scholar]
- 50.D’Ostillo K, Thibaut A, Laureys S, et al. Cerebral FDG uptake changes after supraorbital transcutaneous electrical stimulation with the Cefaly(R) device in patients with migraine. Poster at international headache conference, Valencia, Spain, 14–17 May 2015.
- 51.Fumal A, Laureys S, Di Clemente L, et al. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain. 2006;129:543–550. doi: 10.1093/brain/awh691. [DOI] [PubMed] [Google Scholar]
- 52.Riederer F, Marti M, Luechinger R, et al. Grey matter changes associated with medication-overuse headache: correlations with disease related disability and anxiety. World J Biol Psychiatry. 2012;13:517–525. doi: 10.3109/15622975.2012.665175. [DOI] [PubMed] [Google Scholar]
- 53.Riederer F, Gantenbein AR, Marti M, et al. Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J Neurosci. 2013;33:15343–15349. doi: 10.1523/JNEUROSCI.3804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bussone G, Diener H-CC, Pfeil J, Schwalen S. Topiramate 100 mg/day in migraine prevention: a pooled analysis of double-blind randomised controlled trials. Int J Clin Pract. 2005;59:961–968. doi: 10.1111/j.1368-5031.2005.00612.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.