Abstract
Isolation and enrichment of low-abundant particles are essential steps in many bio-analytical and clinical applications. In this work, the capability of an insulator-based dielectrophoresis (iDEP) device for the detection and stable capture of low abundant polystyrene particles and yeast cells was evaluated. Binary and tertiary mixtures of particles and cells were tested, where the low-abundant particles had concentration ratios on the order of 1:10 000 000 compared to the other particles present in the mixture. The results demonstrated successful and stable capture and enrichment of rare particles and cells (trapping efficiencies over 99%), where particles remained trapped in a stable manner for up to 4 min. A device with four reservoirs was employed for the separation and enrichment of rare particles, where the particles of interest were first selectively concentrated and then effectively directed to a side port for future collection and analysis. The present study demonstrates that simple iDEP devices have appropriate screening capacity and can be used for handling samples containing rare particles; achieving both enrichment and isolation of low-abundant particles and cells.
I. INTRODUCTION
Low-abundant particles are of special interest in many bio-analytical and clinical applications, ranging from the detection of contaminants, to the screening of circulating tumor cells (CTCs) and pathogens from blood. There is a growing interest in the development of robust microfluidic techniques with strong screening capabilities to detect low-concentrated particles and isolate them from complex background solutions.1–3 Excellent reviews on the potential of microfluidics for rare cell separation and enrichment are available in the literature.4,5
Microfluidics has revolutionized the way many analytical assessments are performed; working on the microscale level has inherent advantages such as portability, quick processing times, and low cost. Electric field driven techniques such as electrophoresis (EP), electrorotation, and dielectrophoresis (DEP) have proven to be robust and reliable options for the assessment of a wide range of macromolecules, cells, and inert particles.6–9 Dielectrophoresis is particularly attractive due to its great flexibility; DEP allows for separation and enrichment of particles by exploiting polarization effects (not particle charge) with either alternating current (AC) or direct current (DC) electric fields. This flexibility has fostered the development of many successful DEP-based separations. Particle polarizability can be greater or lower than that of the suspending medium, producing two types of dielectrophoretic behavior: positive DEP occurs when particles have a greater polarizability than the medium and are therefore attracted to the regions with higher electric field gradients. Negative DEP occurs when the particle polarizability is lower than that of the suspending medium, producing particle movement away from the regions with higher electric field gradient.
Dielectrophoresis can be classified in two main groups: electrode-based DEP (eDEP) and insulator-based DEP (iDEP). Historically, DEP started with eDEP systems in 195110 and there is a significant wealth of knowledge in electrode designs for successful analysis and characterization of biological and inert particles. Electrode based systems have attractive advantages such as the requirement of low electric potentials and the possibility of exploiting the electric field frequency as a parameter to manipulate the type of dielectrophoretic response (positive or negative DEP).11 The use of insulators for producing DEP effects was discovered later;12 arrays of insulating structures in microchannels were employed for the first time in 2000.13,14 Insulator-based systems are simpler devices; usually, the entire device is made from a single substrate, so fabrication processes are inexpensive and quick. As an additional advantage, iDEP systems can use electroosmotic (EO) flow as means for pumping liquid and particles through the microchannels, making these systems even simpler. However, as a disadvantage, this type of systems require much greater electric potentials in order to produce strong enough dielectrophoretic forces.
The use of microfluidic devices, in particular, DEP-based systems, has been significantly explored for diagnostics and clinical applications. There are important reports on the use of eDEP systems for the detection and purification of rare cells. Gascoyne et al.2 reported the isolation of CTCs from blood employing a field-flow-fractionation DEP system. Samples containing concentration ratios of 1:1000 CTCs to normal blood cells were analyzed in a DEP device with embedded microelectrodes on the bottom of the channel. Tumor cells were separated from normal cells by action of negative DEP. Recovery rates from 48% to 92% were obtained, demonstrating the potential of this eDEP system for rare cell isolation. In a more recent study, Kuczenski et al.3 isolated Escherichia coli cells from blood employing a system with patterned microelectrodes. Samples containing a concentration ratio of 1:1 of E. coli cells to red blood cells (RBC) were employed. Bacterial cells were enriched (five-fold increase) and separated from RBC; demonstrating the potential of DEP for pathogen detection. Contactless-DEP is a hybrid system that uses both electrodes and insulators.15 In these systems, electrodes are located on the channels wall, separated by a thin polydimethylsiloxane (PDMS) layer from the sample, while insulating posts are patterned inside the channel. Elvington et al.16 separated ovarian cancer cells from a mixture with polystyrene particles at a concentration ratio of 1:1, the cancer cells exhibited positive DEP while the inert particles exhibited negative DEP. Similar systems had also been employed by this group17,18 to isolate prostate and leukemia cancer cells by exploiting their dielectrophoretic signature, i.e., their dielectric properties as a function of the electric potential frequency. Tumor initiating prostate cells were successfully collected without the need of extensive sample preparation or cell labeling,17 and leukemia cells were continuously sorted from diluted blood samples.18 Nakidde et al.19 employed a device that combined the characteristics of eDEP and iDEP by featuring 3D insulating cylindrical microposts and passivated electrodes. They coined this technique 3D πDEP. With this novel device, Nakidde et al.19 studied the trapping efficiency of biological cells (Staphylococcus aureus) using low applied electrical signals and varying frequencies. They found that by combining electrodes with insulating posts, the trapping efficiency for bacterial cells could be increased significantly at lower applied voltages than those required with conventional iDEP systems. They observed trapping efficiencies of 100% at flow rates as high as 350 μl/h and 70% at flow rates as high as 750 μl/h. They also demonstrated 100% trapping efficiency for live bacteria samples over a wide frequency range (50–400 kHz) with an applied signal of 200 Vpp. Luo et al.20 employed an iDEP system with devices made from PDMS and triangular insulating posts to explore the dielectrophoretic behavior of mouse hepatic mitochondria. They characterized the electric potential required for trapping mitochondria as 200 V in their system, and classified the observed mitochondrial behavior in three regimes: wiggling, trapping, and trap hopping. Negative dielectrophoretic behavior was obtained by employing DC and low frequency AC fields. Furthermore, using a different device with one constriction and five outlets, they were able to sort hepatic mitochondria particles into three groups with sizes ranging from ∼300 nm to 2 μm in diameter. This valuable fundamental study demonstrates the applicability of iDEP for the analysis of cell organelles.20 In a recent study, Marchalot et al.21 designed and studied the performance of a dielectrophoretic device with triangular-shaped electrodes used to analyze samples containing CTCs. The authors focused their study on the characterization of the dielectrophoretic trapping efficiency of these cells as a function of flow rate and channel height. By using numerical simulations, the authors were able to select the best locations for the electrodes inside the channel. They demonstrated trapping efficiencies for isolating MDA-MB-231 cells of 97% for a flow rate of 20 μl/h and 79% for a flow rate of 80 μl/h.
The excellent studies reviewed above are examples of the potential that DEP holds for rare cell detection and enrichment. The majority of these studies focused on the use of microelectrodes to energize the system and external pumps to generate liquid flow. The simplicity and ease of application of iDEP systems make them an attractive option for carrying out isolation and enrichment of low-abundant or rare particles. It has been demonstrated that iDEP systems can significantly enrich particle concentration up to three orders of magnitude.22 Other studies have analyzed the selectivity, particle trapping capacity, and the potential to scale down constrictions for the manipulation of nanoparticles.23–25 However, there is a need to characterize the limits of iDEP systems for the isolation and enrichment of low abundant particles in complex samples. It is particularly important to assess the discriminatory capabilities of iDEP for the capture of target particles and cells that may be present in extremely low concentrations. Applications such as sample preparation and point-of-care could benefit from a reliable technique that successfully discriminates and enriches rare target cells and particles. The limits and stability of separation schemes play a major role on state-of-the-art applications with complex mixtures. For instance, the detection and isolation of CTCs, although intensely studied during the last few years, represent a research area facing numerous challenges.26 One of the most prevalent challenges is the scarcity of CTCs in a blood sample for analysis.27 It has been reported that most patients with metastatic cancer have fewer than 10 CTCs per ml of blood.28 In addition, there are over 1 × 106 white blood cells (WBC) and 1 × 109 RBCs within the same blood volume.28 The relatively large size difference that the three types of cells exhibit (median diameter ∼10 μm for the WBCs,29 ∼7 μm for RBCs,30 and ∼15 μm for epithelial CTCs28) makes DEP-based techniques a strong candidate for performing this analysis, since DEP scales with the cube of particle radius. However, successful isolation should not only trap target cells but also retain the scarce number of CTCs in the sample, while a massive number of other cells are constantly streaming through the trapping region. Since non-target cells have the potential to disrupt the trapping of the low abundant CTCs, the large concentration difference poses a practical challenge for the development of effective separation technologies.26 In addition, the isolation process of CTCs should be gentle enough to avoid cells damage, so the CTCs can be used for further analysis.
The present study analyzes the potential of iDEP systems for the detection, capture, and enrichment of low abundant cells and particles in mixtures. A set of devices with cylindrical insulating posts fabricated from PDMS were employed. The experimental limits of iDEP for applications involving rare particles and cells were tested. Binary and tertiary mixtures were studied, where the low concentrated polystyrene particles and yeast cells had concentration ratios on the order of 1:10 000 000 compared to the non-target particles present in the mixture. Target particles and cells were trapped between the insulating posts due to DEP, while a significantly large number of non-target particles streamed through the post array; demonstrating that particle capture was stable and strong enough to withstand the disruption of a constant flow of non-target particles. The effectiveness and stability of iDEP were evaluated by varying both the particle concentration ratio and particle concentration. Automatic particle counting processes and fluorescence analyses were performed to evaluate both the trapping efficiency of the particles and the stability of the dielectrophoretic trapping. Furthermore, the potential for significant particle enrichment and isolation was tested employing a four-reservoir device where low abundant particles were first captured and enriched, and then effectively directed to a side port for future collection. The results of this study demonstrate that iDEP systems can be used for detecting, capturing, enriching, and isolating significantly low-concentrated particles in a mixture. Sample preparation and point-of-care assessments are some of the potential applications that could benefit from a simple technique that is able to discriminate one target particle from a mixture containing millions of non-target particles.
II. THEORY
Dielectrophoresis can only occur under the presence of a non-uniform electric field; the DEP force does not change direction with the local electric field, since it depends on the electric field gradient (∇E2), not the electric field itself. The dielectrophoretic force exerted on a spherical particle under a DC electric field is defined as31
| (1) |
where εm is the permittivity of the suspending medium, rp is the particle radius, ∇ is the gradient of the field strength squared, and Re(fCM) is the real part of the Clausius-Mossotti factor (fCM). The fCM is the parameter that accounts for particle polarizability relative to that of the suspending medium. Particles can exhibit positive or negative DEP, since the fCM ranges from −0.5 to 1 (for spherical particles). Under DC and low frequency AC-electric fields, the fCM can be expressed in terms of the real conductivities of the particle (σp) and the suspending medium (σm)11,32
| (2) |
Besides DEP, the other significant forces present in DC-iDEP systems are EP and EO flow. The superposition of EP and EO flow is referred to as the electrokinetic (EK) force. The expressions for the EK velocity () in terms of mobilities and zeta potentials are33
| (3) |
where μEK, μEO, and μEP are the EK, EO, and EP mobilities, respectively; η is the viscosity of the suspending medium; and ζwall and ζp are the zeta potentials of the microchannel wall and the particle, respectively. The devices for this study were made from PDMS. Since PDMS has a negative zeta potential,34 the application of a DC electric field produces an EO flow in the direction from the positive to the negative electrode (left to right, see Fig. 1(a)).
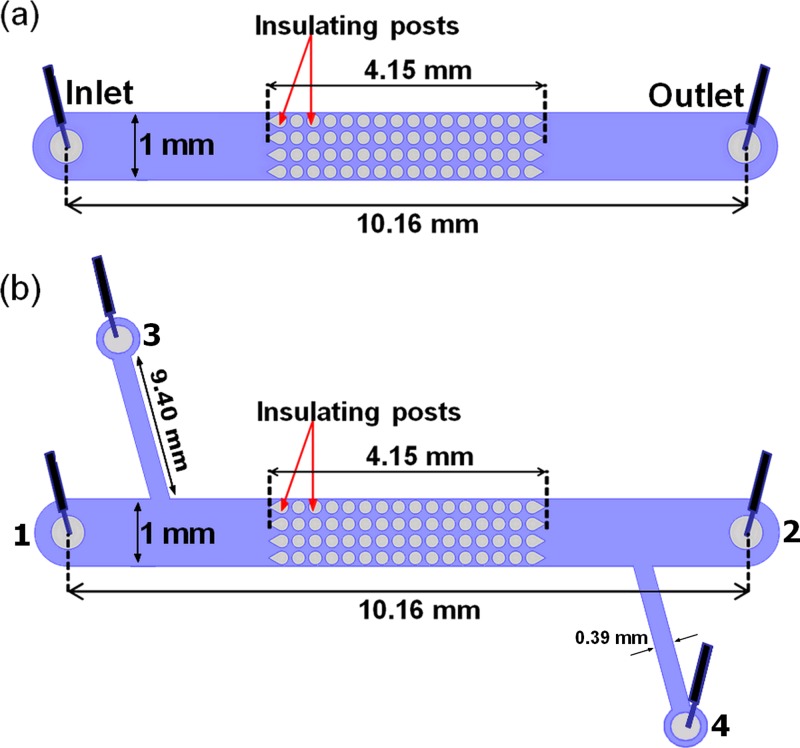
FIG. 1.
(a) Schematic representation of the iDEP channel employed in this study for the experiments in Sections IV A–IV C. (b) Schematic representation of the four-reservoir microchannel employed for the results reported in Section IV D.
Our group and others have published extensively on the modeling and improvement of iDEP systems; this is a well-studied topic.24,35–37 For further information on the mathematical modeling of iDEP systems, we refer the reader to studies available in the literature.24,35,38
III. MATERIALS AND METHODS
A. Devices
The microchannels employed in this study were made from PDMS (Dow Corning, Midland, MI) using standard soft-lithographic techniques.39 Briefly, a mold containing a negative replica of the microchannel and insulating structures was created on a 10-cm-diameter silicon wafer (Silicon Inc., Boise, ID) by utilizing SU-8 3050 photoresist (MicroChem, Newton, MA). PDMS was then casted onto the SU-8 negative replica, in order to produce the microchannels and insulating posts. The PDMS layer was activated using a plasma corona wand (Electro Technic Products, Chicago, IL) and sealed to a 10-cm glass wafer that had been previously coated with a thin layer of PDMS, in order to ensure that all the interior surfaces of the channel were covered by PDMS and had the same wall zeta potential. For results obtained in Sections IV A–IV C, each channel was 10.16-mm long, 1-mm wide, 40-μm deep, and contained one inlet and one outlet liquid reservoirs. The circular post shapes employed in this study were 200-μm in diameter and were arranged 250-μm center-to-center, i.e., the minimum spacing between posts was 50 μm. The spacing between the posts and the channel wall was 25-μm. The array of insulating structures was located at the center of the channel and consisted of 64 posts arranged in 16 columns of four posts each (Fig. 1(a)). For Section IV D, two extra liquid reservoirs were added, for a total of four liquid reservoirs (Fig. 1(b)). These extra reservoirs allowed for enriched particles to be effectively directed to a side port. Particles and cells were visualized by means of fluorescence microscopy. The effect of the light source on the PDMS posts results in a reflection that can be easily misinterpreted as particle fluorescence, since PDMS has inherent autofluorescence, which particularly shows at the border of the insulating posts. To depict the inherent autofluorescence of the PDMS insulating posts, an image of the post array in a channel that does not contain buffer nor particles has been included in the supplementary material,40 as Figure S2.
B. Biological cells and particles
Saccharomyces cerevisiae (ATCC 9763, Manassas, VA, USA) cells were cultured in Yeast Mold (YM) medium (Amresco, Albany, NY, Cat. 271120) at 30 °C in a shaking incubator for 16 h. The optical density of the yeast cells was measured using a spectrophotometer. From the optical density, using a calibration growth curve, the concentration of cells was determined to be ∼8.37 × 106 cells/ml. A volume of 1.75 ml of culture was then centrifuged at 10 000 rpm for 15 min, and the supernatant was discarded. The pellet was washed by re-suspending it in 1.75 ml of deionized (DI) water and then centrifuged at 10 000 rpm for 15 min and discarding the supernatant. The pellet was re-suspended in 1.75 ml of DI water, and a volume of 6 μl of Syto 11 green fluorescent (ex/em 508/527 nm) nucleic stain (Invitrogen, Eugene, OR) was added. The tube containing the cells was covered with aluminum foil and incubated at room temperature for 40 min. This solution was then centrifuged for 15 min, the supernatant was discarded, and the pellet of labeled cells was washed as previously described. A final volume of 1.47 ml of DI water was added to the pellet, resulting in a final concentration of 1 × 107 cells/ml. Average cell diameter for the yeast cells was measured as 6.3 ± 0.4 μm using bright-field microscopy.
Fluorescent polystyrene microspheres of three different sizes (Invitrogen, Eugene, OR) were employed: 500-nm diameter yellow-green (ex/em 505/515 nm) and red (ex/em 580/605 nm), 1-μm diameter yellow-green (ex/em 505/515 nm) and red (ex/em 580/605 nm), and 2-μm red (ex/em 580/605 nm). Microparticle stock suspensions were vortexed to disrupt aggregates and diluted in suspending medium to the required particle concentrations.
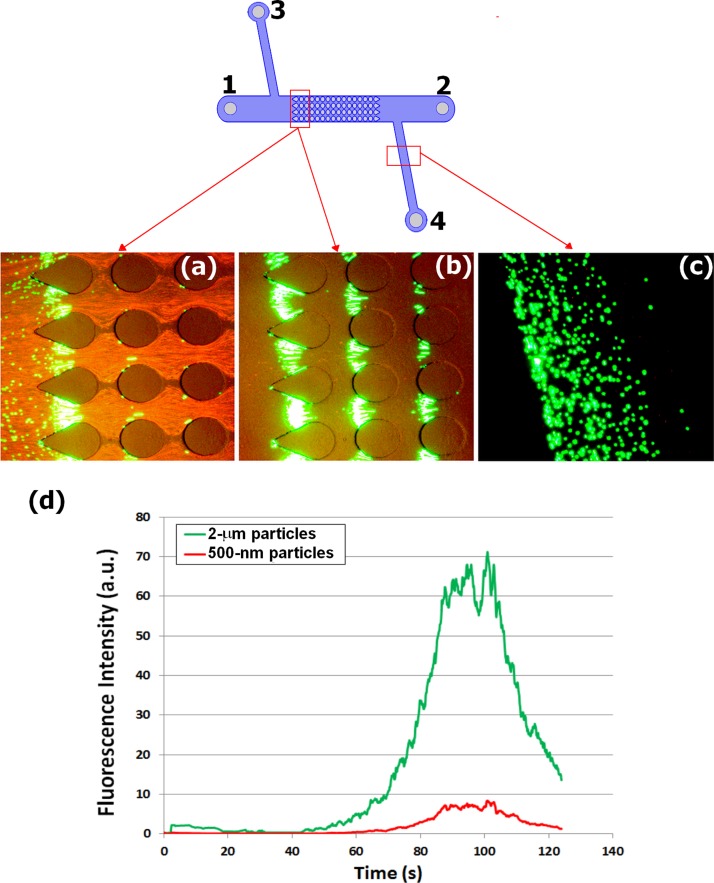
The suspending media used in experimentation consisted of DI water adjusted to a pH of 8 and a conductivity of 20 μS/cm. The pH and conductivity were adjusted by the addition of a 0.1 N KOH solution. In order to obtain a uniform and known concentration of particles and cells inside the microchannels, the particles or cells were added and mixed thoroughly with the suspending media before experimentation, i.e., the suspending media already contained the particles. Having a known and uniform particle and cell concentrations made the quantitative analysis easier to interpret. Only the experiments depicted in Figure 5 used a suspending medium without particles. For these sets of experiments, the particles were added to the microchannel after the suspending medium had been introduced. This was required since the experiments with the four reservoir channel needed a finite sample volume, in order to achieve isolation of the target particles.
FIG. 5.
Capture, enrichment, and isolation of low-abundant 2-μm (green) particles in a mixture with 500-nm (red) particles employing a four reservoir channel. A video of this experiment is available as supplementary material.40 A sample of 10 μl was introduced to reservoir 1 with a concentration ratio of 1:100 of 2-μm to 500-nm particles. Particle concentrations in the sample were 1 × 107 particles/ml and 1 × 109 particles/ml for the 2-μm and 500-nm particles, respectively. (a) Image showing selective capture and enrichment of 2-μm (green) particles while 500-nm (red) particles keep passing through; the electric potential was applied between reservoirs 1 and 2. (b) Image showing enrichment of 2-μm (green) particles after the 500-nm (red) particles were flushed out of the system; the electric potential was applied between reservoirs 3 and 2, which flushed out 500-nm particles to reservoir 2. (c) Image showing the isolation of enriched 2-μm (green) particles by directing them to reservoir 4; the image depicts the side channel to reservoir 4 where mainly only 2-μm particles can be seen; the electric potential was applied between reservoirs 1 and 4. (d) Dielectropherogram of the fluorescence signal vs. time obtained at the side channel, where enrichment of 2-μm can be observed as a peak of green fluorescence. A video depicting this experiment is included as supplementary material.40
C. Equipment and software
For Sections IV A–IV C, an Agilent waveform generator (Agilent 33500 Series, Santa Clara, CA) and a Trek high voltage amplifier (Model: PZD700A2, Trek Inc., Lockport, NY) were used to apply DC electric potentials. For Section IV D, a high voltage sequencer was used (Model HVS6000D; LabSmith, Livermore, CA). Electric potentials were applied employing platinum wire electrodes in all experiments. The iDEP experiments were carried out using a ZEISS Axiovert 40 CFL inverted microscope (Carl Zeiss Microscopy, Thornwood, NY). An Infinity 2 camera (Luminera, Ottawa, Canada) was used to visualize all experiments. The quantification of the experimental trapping efficiency was performed using the Fiji image processing package (a distribution of the open-source software ImageJ focused on biological-image analysis).41 A detailed description of the analysis performed using Fiji can be found in the supplementary material.40
D. Experimental procedure
Experiments started with a clean microchannel that was filled with the suspending medium. The channels were reversibly sealed to a vacuum chuck manifold (LabSmith, Livermore, CA) with a vacuum pump (Model 400-3910; Barnant Company, Barrington, IL). The manifold interfaces with slip tip syringes, which allowed a simple filling of the channels with the suspending medium using pressure. For the experiments reported in Sections IV A–IV C, the channel was filled with a solution containing the desired particle mixture in order to assure correct concentration. Platinum wire electrodes were placed at the channel reservoirs, and a DC electric potential was applied across the length of the microchannel by employing the waveform generator and high voltage amplifier. For the experiments in Section IV D, the channel was filled with the suspending medium without particles or cells. Next, a sample of 10 μl having a concentration of 1 × 107 and 1 × 109 particles/ml for 1-μm and 500-nm particles, respectively, was introduced to the channel into reservoir 1 (Fig. 1(b)). Particle response was observed to ensure normal dielectrophoretic behavior and recorded in the form of videos and pictures using the inverted microscope. The platinum wire electrodes were manually repositioned from reservoir to reservoir in order to achieve the desired flow direction. Between uses, it was necessary to re-condition the PDMS microchannels to ensure negative surface charge and stable EO flow.34,39 To do this, each channel was soaked in a solution of 0.1 N KOH for 2 h, rinsed, and then soaked in DI water for 1 h.
IV. RESULTS AND DISCUSSION
A. Trapping of target particles as function of particle size and concentration ratio in binary mixtures
One of the main objectives of the present study was to test the capability of an iDEP device (Fig. 1(a)) to selectively capture and enrich low-abundant particles in a mixture. That is, to test the limits of iDEP for the discrimination and separation of rare particles. Particle and cell concentration ratios up to 1:10 000 000 of polystyrene particles and yeast cells mixtures were investigated, where the larger particle in the mixture had the lower concentration. Figures 2(a)–2(d) show the results obtained using a mixture with yeast cells and 500-nm particles, with concentrations ratios up to 1:10 000 000. Figure 2(d), in particular, depicts how selective and reliable iDEP can be, where it was possible to trap the only cell introduced to the microchannel. Similar results were obtained using 2-μm particles as the low-abundant particle in mixtures with 1-μm (Figs. 2(e)–2(h)) and 500-nm (Figs. 2(i)–2(l)) particles; demonstrating that iDEP systems maintain their selectivity properties even when the target particles are closer in size to non-target particles in the mixture. These concentration ratios employed were high, up to 1:100 000 for the mixture with 1-μm particles and up to 1:1 000 000 for the mixture with 500-nm particles. Differences in particle size play an important role: the closer the particles are in size, the more challenging the separation becomes. However, Figure 2(h) demonstrates that with a difference in particle diameter of 1 μm, successful capture and retention of the particles of interest were possible despite employing a particle concentration difference of five orders of magnitude.
FIG. 2.
Images depicting selective dielectrophoretic capture of low-abundant particles in three different binary mixtures. Concentration ratios are shown in each image. First column, trapping of yeast cells (green) in a mixture with 500-nm (red) particles obtained at an average voltage of 600 V. The concentration of 500-nm particles was kept constant at 1 × 109 particles/ml. The following yeast cell concentrations were used: (a) 1 × 105, (b) 1 × 104, (c) 1 × 103, and (d) 1 × 102 cells/ml. Second column, trapping of 2-μm (red) particles in a mixture with 1-μm (green) particles obtained at an average voltage of 400 V. The concentration of 1-μm was kept constant at 1 × 108 particles/ml. The following 2-μm particle concentrations were used: (e) 1 × 106, (f) 1 × 105, (g) 1 × 104, and (h) 1 × 103 particles/ml. Third column, trapping of 2-μm (red) particles in a mixture with 500-nm (green) particles obtained at an average voltage of 500 V. The concentration of 500-nm was kept constant at 1 × 109 particles/ml. The following 2-μm particle concentrations were used: (i) 1 × 106, (j) 1 × 105, (k) 1 × 104, and (l) 1 × 103 particles/ml. All of these experiments were repeated at least three times each to ensure experimental reproducibility.
The results presented in Figure 2 show that iDEP devices have the capability to capture low-concentrated particles in binary mixtures with concentration differences in the order of 106. As mentioned, other EK forces (EP and EO flow) are present in these systems, so the dielectrophoretically captured particles are exposed to strong fluid flow due to EO; and still remain trapped by means of negative DEP at the constrictions between the posts. Furthermore, the trapped particles also endure a strong flow of the other particles present in the mixtures, as well as mutual particle interactions, which are present in very high concentrations. The images in Figure 2 illustrate that dielectrophoretic trapping of low-abundant particles is feasible, despite the presence of many other particles in the system. The trapping of the low-abundant particles is also stable. The stability of particle trapping is further studied in Section III C.
As the number of particles in the sample decreases, retaining these low abundant cells becomes more critical, in particular, if the target cells/particles are required for further analysis. Trapping efficiency was used to investigate how efficient and stable the particle trapping mechanism shown in Figure 2 is. Trapping efficiency (TE, Eq. (4)) was defined in this work as the relative difference in the number of incoming particles (IP) to the post array and the number of outgoing particles (OP) that escape after the third column of the post array. The region comprised by the three first columns of posts was selected for this analysis, since a great majority of the target low abundant particles and cells are trapped there.
| (4) |
Table I shows the quantitative TE calculated from three independent experiments for all cases shown in Figure 2. As observed, the minimum TE values obtained within the presented experimental setup are 91.6%, corresponding to the mixture with yeast and 500-nm particles at a concentration ratio of 1:100 000. In fact, considering all the sets of independent experiments, the technique has a median TE of 99%. The variability of the method presented here, as the standard deviation, has a maximum of only 6.1%. Thus, using iDEP devices for the capture of rare or low-concentrated particles in binary mixtures seems to be a feasible and robust option.
TABLE I.
Average trapping efficiency (TE) for the selective dielectrophoretic capture of low-abundant particles in the binary mixture experiments described in Figure 2 (n = 3).
| Mixture | Concentration ratio | TE average (%) |
|---|---|---|
| Yeast and 500-nm | 1:10 000 | 100.0 ± 0.0 |
| 1:100 000 | 91.6 ± 2.2 | |
| 1:1 000 000 | 100.0 ± 0.0 | |
| 1:10 000 000 | 100.0 ± 0.0 | |
| 2-μm and 1-μm | 1:100 | 96.5 ± 3.5 |
| 1:1000 | 98.9 ± 2.0 | |
| 1:10 000 | 100.0 ± 0.0 | |
| 1:100 000 | 100.0 ± 0.0 | |
| 2-μm and 500-nm | 1:1000 | 95.8 ± 2.5 |
| 1:10 000 | 98.5 ± 2.0 | |
| 1:100 000 | 96.5 ± 6.1 | |
| 1:1 000 000 | 100.0 ± 0.0 |
One important note about dielectrophoretic trapping is that it can be easily misinterpreted as particle adsorption. To demonstrate that particle capture occurs solely due to dielectrophoretic effects, a video has been included as supplementary material.40 This video shows a yeast cell that is trapped by applying an electric potential, and then released when the voltage is turned off. Additionally, a second video to support the results in Figure 5 has also been included. This second video40 shows the dielectrophoretic trapping followed by the release of a large number of 2-μm particles that are then collected in a side reservoir.
B. Trapping of target particles in tertiary mixtures
Tertiary particle mixtures were used to further test the limits of iDEP devices for the effective capture of low-abundant particles. Yeast cells labeled with a green fluorescent dye were used as the low abundant particles in a tertiary mixture with 1-μm (red) and 500-nm (green) polystyrene particles. Two different concentration ratios were tested, as illustrated in Figure 3. Successful trapping of yeast cells was achieved with these tertiary mixtures, demonstrating that iDEP is a selective technique for particle trapping. In particular, yeast cells were quite diluted in these samples. The channels have a small volumetric capacity (∼4 μl), therefore, only a few rare cells/particles are introduced into the channel for each experiment (∼400 cells through the entire system compared to 4 × 104 to 4 × 106 of the other particles); yet the technique does not fail in capturing these few cells. These results demonstrate that iDEP offers an effective and reliable alternative as a technique for the isolation of low concentrated particles of interest. The results in Figures 2 and 3 are encouraging, since iDEP devices are simple to fabricate and operate; opening the potential for a wide range of applications where effective capture of rare particles is required. Devices and operating conditions can be further optimized to trap particles of interest in more challenging conditions.
FIG. 3.
Experimental trapping of yeast cells (green) in a mixture with 1-μm (red) and 500-nm (green) polystyrene particles. Concentration ratios are depicted in each image in the following order yeast:1-μm:500-nm. Cell and particle concentrations (in particles/ml) were: (a) 1 × 105:1 × 107:1 × 107 for yeast cells, 1-μm, and 500-nm particles, respectively, at an applied voltage of 400 V. (b) 1 × 105: 1 × 108: 1 × 109 for yeast cells, 1-μm, and 500-nm particles, respectively, at an applied voltage of 350 V.
C. Stability of dielectrophoretic trapping
Adequate stability of particle trapping is essential for iDEP to become a prominent analytical and preparative technique for the analysis and processing of samples containing low-abundant cells or particles. A set of experiments was performed to test the stability of particle trapping; the results are shown in Figure 4. Yeast cells labeled green were used as the rare cells in a mixture with 1-μm red particles at an applied potential of 290 V. As it can be seen in Figures 4(a)–4(d), stable trapping of yeast was obtained. It is important to note that yeast cells are constantly being driven to the post array region, where they trap due to DEP. Hence, as more cells arrive to the trapping region over time, particle concentration locally increases in this region and its fluorescence increases. This is noted when comparing the time sequence pictures shown in Figures 4(a)–4(d). Moreover, these images demonstrate that once cells are captured they remained trapped, despite the strong fluid flow due to EO and despite the significant flow of 1-μm particles that keep pushing the trapped cells as they pass through the constrictions between the posts. Negative DEP is thus a stable way of retaining trapped cells or particles, since the negative dielectrophoretic traps form “barriers” that the particles or cells of interest simply cannot penetrate.24 These barriers are effective despite the presence of substantial fluid flow and particle flow through the constrictions between the insulating posts. As the yeast cells remained trapped during the 4 min of processing time, some agglomerates of 1-μm particles were also trapped. The results in Figure 4 clearly illustrate that particle trapping is stable, increasing the potential to achieve a higher enrichment factor of particles of interest by employing longer processing times. To be able to assess only the capture of yeast cells, additional images of these results were taken using a fluorescence filter which allowed the visualization of only the green particles. The images are included as Figure S1 in the supplementary material40 and are the same experimental images shown in Figures 4(a)–4(d). One important note about the stability of trapping is that pH changes, which commonly occur in DC-iDEP systems, do not seem to affect the capability of the device to trap the particles and keep them trapped during times of several minutes. It has been found earlier that the applications of electric potentials in the order of 3000 V can generate significant pH gradients in these types of systems.42 However, for these changes to affect cells, exposure times on the order of ∼2 h are required.43 The inherent pH changes start occurring at the positive electrode, where H+ ions are generated. These ions travel then into the channel. The speed at which these ions are produced and affect the system depends on the magnitude of the applied potential. For potentials on the order of 3000 V, significant changes occur in a time span of 1 min. For potentials on the order of 100 V, changes occur in a time span of 6–10 min. The processing times of the majority of these iDEP analyses are on the order of 1–2 min, while the potentials applied in the majority of our experiments are below 600 V. Therefore, it is expected that the inherent pH changes will not affect cell viability and the stability of the dielectrophoretic trapping of cells and particles.
FIG. 4.
Stability of the trapping of yeast cells (green) in a mixture with 1-μm (red) polystyrene particles as a function of time. A concentration ratio of 1:100 for yeast cells to 1-μm was employed. The following particle concentrations were employed: 1 × 105 yeast cells/ml and 1 × 107 1-μm particles/ml, respectively. Images were taken at four different times: (a) 1 min, (b) 2 min, (c) 3 min, and (d) 4 min. The applied potential was 290 V.
D. Capture, enrichment, and isolation of low-abundant particles in a four-reservoir device
In order to evaluate the potential of iDEP as a technique for enrichment and isolation of low abundant particles, a four-reservoir device was designed with the aim to direct the enriched and isolated rare particles to a specific liquid reservoir for future collection and analysis. This device is depicted in Figure 1(b) and at the top of Figure 5. A sample of 2-μm (green) and 500-nm (red) particles was employed at a concentration ratio of 1:100. The results are shown in Figure 5 and in a video available as supplementary material.40 The video has subtitles describing the experiment in detail. First, particle capture and enrichment were performed by applying 500 V between reservoirs 1 and 2; this potential produced selective capture of the 2-μm particles while the 500-nm particles moved along the channel due to EO (Fig. 5(a)). After a significant enrichment of 2-μm particles was achieved, the initial potential was removed and a second electric potential was then applied between reservoirs 3 and 2. This prevented new sample from entering the channel, and drove all 500-nm particles present in the channel to reservoir 2. Figure 5(b) illustrates the previously trapped and enriched 2-μm particles isolated, after the 500-nm particles were flushed out to reservoir 2. As a final step, the enriched and isolated 2-μm particles were successfully directed to reservoir 4, demonstrating a successful separation from the mixture. Figure 5(c) shows an image of the side channel to reservoir 4 depicting the flow of enriched 2-μm particles, with very few 500-nm particles present. The fluorescence signals obtained at the smaller side channel are plotted in the dielectropherogram shown in Figure 5(d). It can be observed that the 2-μm (green) particles have been enriched, when compared to the 500-nm (red) particles. By looking at the fluorescence peaks as a function of time, it can be noticed that the fluorescence from the 2-μm particles (green peak) is at least 10 times greater than that of the 500-nm particles (red peak). Although there is a non-linear relation between particle concentration and fluorescence intensity, the behavior of the fluorescence signal depicted in Figure 4(d) does correlate with an increase in particle concentration in the fluid passing through the fluorescence interrogation window as observed in the video submitted as supplementary material.40
Finally, an assessment of the trapping efficiency was performed by running an experiment employing a simple iDEP device (Fig. 1(a)) and adding 10 μl of the mixture with 2-μm and 500-nm particles at a concentration ratio of 1:100. One of the experiments is available as the third video in the supplementary material.40 This experiment started by applying 100 V to transport the sample through the system without trapping any particle. At time t = 30 s, a voltage of 500 V was continuously applied for 30 s (for a total experimental time of 1 min) in order to trap the 2-μm particles. While the 500 V were applied, only one 2-μm particle escaped at time t = 40 s. These time references are from the real-time video, included as a supplementary material video.40 It was found that, on average, over 99% of the 2-μm particles were captured by the device, supporting the statement that iDEP holds potential for applications that involve low-abundant cells.
V. CONCLUSIONS
The analysis of samples containing low-abundant particles is an important application in many fields, from bioanalytical applications to clinical analysis. In the present work, the potential of simple iDEP devices with circular insulating posts was tested as a technique for capturing, enriching, and isolating rare particles in binary and tertiary mixtures. Suspending media containing cells and particles were prepared prior to experimentation, in order to achieve uniform and known concentrations, which simplifies the quantification of the results. Yeast cells and polystyrene particles of different sizes (2-μm, 1-μm and 500-nm in diameter) were employed. The results demonstrated that iDEP systems can effectively and selectively capture rare cells in a sample, despite the presence of strong electroosmotic and non-target particles flow. Particle concentration ratios up to 1:10 000 000 were tested, where the iDEP systems were always able to detect and capture the rare particles with trapping efficiencies up to 99%. Careful assessment of particle and cell trapping efficiency was performed employing automatic cell and particle counting with the software Fiji (an update of ImageJ); revealing a minimum trapping efficiency of 92%, with a median of 99%; demonstrating that the system successfully traps and retains target particles and cells. Furthermore, the standard deviation obtained for the trapping efficiency was low, with a maximum of 6.1%, indicating that the technique is reproducible. The stability of particle trapping was assessed, demonstrating that the dielectrophoretically captured rare particles and cells successfully endure liquid and particle flow while remaining trapped at the constrictions between the insulating posts. An experiment, employing a four-reservoir device, further demonstrated successful isolation of low-abundant particles, where enriched rare target particles were driven to a side channel and collected in a separate reservoir. These results are encouraging, since the effective detection and manipulation of rare particles in a mixture can be performed with simple iDEP systems. Customized systems can be created for handling more complex samples, with several reservoirs for the collection of specific target particles for further analysis. This increases the possibility for iDEP as a technique of choice for the analysis of complex mixtures containing low-abundant particles in applications such as sample preparation and point-of-care analyses.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support provided by the National Science Foundation (Award No. CBET-1336160).
References
- 1. Huang C.-T., Amstislavskaya T. G., Chen G.-H., Chang H.-H., Chen Y.-H., and Jen C.-P., J. Med. Biol. Eng. 33, 51 (2013). 10.5405/jmbe.1177 [DOI] [Google Scholar]
- 2. Gascoyne P. R. C., Noshari J., Anderson T. J., and Becker F. F., Electrophoresis 30, 1388 (2009). 10.1002/elps.200800373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuczenski R. S., Chang H.-C., and Revzin A., Biomicrofluidics 5, 032005 (2011). 10.1063/1.3608135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y., Li P., Huang P.-H., Xie Y., Mai J. D., Wang L., Nguyen N.-T., and Huang T. J., Lab Chip 14, 626 (2014). 10.1039/c3lc90136j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pratt E. D., Huang C., Hawkins B. G., Gleghorn J. P., and Kirby B. J., Chem. Eng. Sci. 66, 1508 (2011). 10.1016/j.ces.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pethig R., Biomicrofluidics 4, 022811 (2010). 10.1063/1.3456626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castillo J., Dimaki M., and Svendsen W. E., Integr. Biol. 1, 30 (2009). 10.1039/B814549K [DOI] [PubMed] [Google Scholar]
- 8. Meighan M. M., Staton S. J. R., and Hayes M. A., Electrophoresis 30, 852 (2009). 10.1002/elps.200800614 [DOI] [PubMed] [Google Scholar]
- 9. Lapizco-Encinas B. H., Microfluidics in Detection Science: Lab-on-a-chip Technologies ( The Royal Society of Chemistry, 2015), p. 192. [Google Scholar]
- 10. Pohl H. A., J. Appl. Phys. 22, 869 (1951). 10.1063/1.1700065 [DOI] [Google Scholar]
- 11. Jones T. B., Electromechanics of Particles ( Cambridge University Press, USA, 1995). [Google Scholar]
- 12. Masuda S., Washizu M., and Nanba T., IEEE Trans. Ind. Appl. 25, 732 (1989). 10.1109/28.31255 [DOI] [Google Scholar]
- 13. Cummings E. B. and Singh A. K., in Proceedings of SPIE: Conference on Microfluidic Devices and Systems III, Santa Clara, CA, September 18–19 (2000), p. 164. [Google Scholar]
- 14. Cummings E. B. and Singh A. K., Anal. Chem. 75, 4724 (2003). 10.1021/ac0340612 [DOI] [PubMed] [Google Scholar]
- 15. Shafiee H., Caldwell J., Sano M., and Davalos R., Biomed. Microdevices 11, 997 (2009). 10.1007/s10544-009-9317-5 [DOI] [PubMed] [Google Scholar]
- 16. Elvington E. S., Salmanzadeh A., Stremler M. A., and Davalos R. V., J. Visualized Exp.: JoVE (79), e50634 (2013). 10.3791/50634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salmanzadeh A., Romero L., Shafiee H., Gallo-Villanueva R. C., Stremler M. A., Cramer S. D., and Davalos R. V., Lab Chip 12, 182 (2012). 10.1039/C1LC20701F [DOI] [PubMed] [Google Scholar]
- 18. Sano M. B., Caldwell J. L., and Davalos R. V., Biosens. Bioelectron. 30, 13 (2011). 10.1016/j.bios.2011.07.048 [DOI] [PubMed] [Google Scholar]
- 19. Nakidde D., Zellner P., Alemi M. M., Shake T., Hosseini Y., Riquelme M. V., Pruden A., and Agah M., Biomicrofluidics 9, 014125 (2015). 10.1063/1.4913497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo J., Abdallah B. G., Wolken G. G., Arriaga E. A., and Ros A., Biomicrofluidics 8, 021801 (2014). 10.1063/1.4866852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marchalot J., Chateaux J.-F., Faivre M., Mertani H. C., Ferrigno R., and Deman A.-L., Biomicrofluidics 9, 054104 (2015). 10.1063/1.4928703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lapizco-Encinas B. H., Davalos R., Simmons B. A., Cummings E. B., and Fintschenko Y., J. Microbiol. Methods 62, 317 (2005). 10.1016/j.mimet.2005.04.027 [DOI] [PubMed] [Google Scholar]
- 23. Chávez-Santoscoy A. V., Baylon-Cardiel J. L., Moncada-Hernández H., and Lapizco-Encinas B. H., Sep. Sci. Technol. 46, 384 (2011). 10.1080/01496395.2010.520295 [DOI] [Google Scholar]
- 24. Saucedo-Espinosa M. A. and Lapizco-Encinas B. H., Electrophoresis 36, 1086 (2015). 10.1002/elps.201400408 [DOI] [PubMed] [Google Scholar]
- 25. Chaurey V., Rohani A., Su Y.-H., Liao K.-T., Chou C.-F., and Swami N. S., Electrophoresis 34, 1097 (2013). 10.1002/elps.201200456 [DOI] [PubMed] [Google Scholar]
- 26. Alix-Panabières C., Schwarzenbach H., and Pantel K., Annu. Rev. Med. 63, 199 (2012). 10.1146/annurev-med-062310-094219 [DOI] [PubMed] [Google Scholar]
- 27. Yu M., Stott S., Toner M., Maheswaran S., and Haber D. A., J. Cell Biol. 192, 373 (2011). 10.1083/jcb.201010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haber D. A. and Velculescu V. E., Cancer Disc. 4, 650 (2014). 10.1158/2159-8290.CD-13-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin H. K., Zheng S., Williams A. J., Balic M., Groshen S., Scher H. I., Fleisher M., Stadler W., Datar R. H., Tai Y.-C., and Cote R. J., Clin. Cancer Res. 16, 5011 (2010). 10.1158/1078-0432.CCR-10-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park S., Zhang Y., Wang T.-H., and Yang S., Lab Chip 11, 2893 (2011). 10.1039/c1lc20307j [DOI] [PubMed] [Google Scholar]
- 31. Kirby B. J., Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices ( Cambridge University Press, New York, 2010). [Google Scholar]
- 32. Markx G. H., Dyda P. A., and Pethig R., J. Biotechnol. 51, 175 (1996). 10.1016/0168-1656(96)01617-3 [DOI] [PubMed] [Google Scholar]
- 33. Moncada-Hernandez H., Baylon-Cardiel J. L., Pérez-González V. H., and Lapizco-Encinas B. H., Electrophoresis 32, 2502 (2011). 10.1002/elps.201100168 [DOI] [PubMed] [Google Scholar]
- 34. Spehar A.-M., Koster S., Linder V., Kulmala S., de Rooij N. F., Verpoorte E., Sigrist H., and Thormann W., Electrophoresis 24, 3674 (2003). 10.1002/elps.200305624 [DOI] [PubMed] [Google Scholar]
- 35. Kwon J.-S., Maeng J.-S., Chun M.-S., and Song S., Microfluid. Nanofluid. 5, 23 (2008). 10.1007/s10404-007-0210-3 [DOI] [Google Scholar]
- 36. Jones P. V. and Hayes M. A., Electrophoresis 36, 1098 (2015). 10.1002/elps.201400504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhattacharya S., Chao T.-C., and Ros A., Electrophoresis 32, 2550 (2011). 10.1002/elps.201100066 [DOI] [PubMed] [Google Scholar]
- 38. Baylon-Cardiel J. L., Lapizco-Encinas B. H., Reyes-Betanzo C., Chávez-Santoscoy A. V., and Martínez Chapa S. O., Lab Chip 9, 2896 (2009). 10.1039/b906976c [DOI] [PubMed] [Google Scholar]
- 39. Duffy D. C., McDonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70, 4974 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- 40.See supplementary material at http://dx.doi.org/10.1063/1.4936371E-BIOMGB-9-011506 for (i) PDF containing a description of the analysis performed with Fiji software, and supplementary Figures S1 and S2. (ii) Video showing a single yeast cell (green) in a mixture with 500-nm (red) particles, the yeast cell is trapped when the voltage is turned on, and then released, when the voltage is turned off. (iii) Video showing a 4-reservoir device used for particle capture, enrichment, and isolation, where the enriched particles were diverted to a specific side reservoir. (iv) Video of the assessment of particle trapping efficiency, in this video the microscope is focused at the end of the post array, where it can be observed that only one 2-μm particle escapes after a voltage of 500 V is applied.
- 41. Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A., Nat. Methods 9, 676 (2012). 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gencoglu A., Camacho-Alanis F., Nguyen V. T., Nakano A., Ros A., and Minerick A. R., Electrophoresis 32, 2436 (2011). 10.1002/elps.201100090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moncada-Hernández H. and Lapizco-Encinas B. H., Anal. Bioanal. Chem. 396, 1805 (2010). 10.1007/s00216-009-3422-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4936371E-BIOMGB-9-011506 for (i) PDF containing a description of the analysis performed with Fiji software, and supplementary Figures S1 and S2. (ii) Video showing a single yeast cell (green) in a mixture with 500-nm (red) particles, the yeast cell is trapped when the voltage is turned on, and then released, when the voltage is turned off. (iii) Video showing a 4-reservoir device used for particle capture, enrichment, and isolation, where the enriched particles were diverted to a specific side reservoir. (iv) Video of the assessment of particle trapping efficiency, in this video the microscope is focused at the end of the post array, where it can be observed that only one 2-μm particle escapes after a voltage of 500 V is applied.