Abstract
Background
Improvement in renal function and decreases in serum uric acid (SUA) have been reported following prolonged high-intensity statin (HMG-CoA reductase inhibitor) therapy. This post hoc analysis of the SAGE trial examined the effect of intensive versus less intensive statin therapy on renal function, safety, and laboratory parameters, including SUA, in elderly coronary artery disease (CAD) patients (65–85 years) with or without chronic kidney disease (CKD).
Methods
Patients were randomized to atorvastatin 80 mg/day or pravastatin 40 mg/day and treated for 12 months. Patients were stratified using Modification of Diet in Renal Disease (MDRD) estimated glomerular filtration rates (eGFRs) in CKD (eGFR <60 mL/min/1.73 m2) and non-CKD populations.
Results
Of the 893 patients randomized, 858 had complete renal data and 418 of 858 (49 %) had CKD (99 % Stage 3). Over 12 months, eGFR increased with atorvastatin and remained stable with pravastatin (+2.38 vs. +0.18 mL/min/1.73 m2, respectively; p < 0.0001). MDRD eGFR improved significantly in both CKD treatment arms; however, the increased eGFR in patients without CKD was significantly greater with atorvastatin (+2.08 mL/min/1.73 m2) than with pravastatin (−1.04 mL/min/1.73 m2). Modest reductions in SUA were observed in both treatment arms, but a greater fall occurred with atorvastatin than with pravastatin (−0.52 vs. −0.09 mg/dL, p < 0.0001). Change in SUA correlated negatively with changes in eGFR and positively with changes in low-density lipoprotein cholesterol. Reports of myalgia were rare (3.6 % CKD; 5.7 % non-CKD), and there were no episodes of rhabdomyolysis. Elevated serum alanine and aspartate transaminase to >3 times the upper limit of normal occurred in 4.4 % of atorvastatin- and 0.2 % of pravastatin-treated patients.
Conclusion
Intensive management of dyslipidemia in older patients with stable coronary heart disease may have beneficial effects on renal function and SUA.
Key Points
| This post hoc analysis of the SAGE trial suggests that intensive treatment of dyslipidemia over 1 year in older patients with stable coronary artery disease had beneficial effects on renal function, based on dual assessment of estimated glomerular filtration rate and on serum uric acid. |
| Consistent with longer-term studies, relatively short-term treatment of dyslipidemia with high-dose statin (HMG-CoA reductase inhibitor) therapy appears to preserve renal function and slow progression of chronic kidney disease in a high-risk population of older patients. |
Introduction
For nearly 100 years, dyslipidemia has been implicated as a cause or a contributing factor to renal injury [1]. Analyses of multiple clinical trials [2–9] and meta-analyses [1, 10–12] have suggested that, in addition to preventing and/or reducing cardiovascular events and mortality, intensive treatment of dyslipidemia with statins (HMG-CoA reductase inhibitors) may stabilize or improve renal function in patients with vascular disease, with or without pre-existing renal impairment. More specifically, stabilization or improvement in the estimated glomerular filtration rate (eGFR) has been observed in post hoc analyses across a broad range of patients with coronary heart disease (CHD) or vascular disease with or without chronic kidney disease (CKD) [2, 4–6], and in patients with CHD with or without prior stroke [13].
Several reports have demonstrated that high-intensity statin therapy may have additional renoprotective effects in patients with cardiovascular disease, as statins have been shown to stabilize or preserve renal function, reduce the quantity of albuminuria and proteinuria, and reduce the occurrence of contrast-induced acute kidney injury [9, 14–16], without increasing serious renal-related adverse events (AEs) [17].
It has long been recognized that renal function declines with aging [18–21], and the prevalence of CKD is higher in older individuals [21, 22]. Analyses of large epidemiologic databases and clinical studies have indicated that at least 44 % of subjects aged 65 years and older have CKD [21, 22]; the prevalence being highest for those patients aged ≥80 years and for those older subjects with co-morbidities [21]. Importantly, declines in eGFR and worsening of CKD status have also been reported in these older cohorts of subjects [21]. A recent meta-analysis from the CKD Prognosis Consortium demonstrated that mortality and the risk of end-stage renal disease increase with a 10 % or greater reduction in eGFR over a 2-year period [23].
Few clinical trials have examined the safety or impact of intensive statin therapy on renal function in an older high-risk cohort with established CHD and vascular disease. This post hoc analysis of the SAGE (Study Assessing Goals in the Elderly) trial was designed to examine the effect of statin therapy with atorvastatin 80 mg/day or pravastatin 40 mg/day on renal function and laboratory parameters, including changes in serum uric acid (SUA), in an older population with symptomatic CHD over 12 months of treatment.
Methods
The design of the SAGE trial, including eligibility criteria, has been described fully elsewhere [24, 25]. The SAGE trial was conducted in compliance with the ethical principles originating from the Declaration of Helsinki (Revised South Africa, 1996) and in compliance with the institutional review board/independent ethics committee, informed consent regulations, and International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) guidelines. Participants were aged 65–85 years with a documented history of clinically stable coronary artery disease (CAD) and one or more episodes of myocardial ischemia with a total ischemia duration ≥3 min during 48-h ambulatory ECG at screening, and with baseline low-density lipoprotein cholesterol (LDL-C) 100–250 mg/dL [24, 25]. Patients who satisfied all recruitment criteria were randomized (double-blind) to atorvastatin 80 mg/day or pravastatin 40 mg/day for 12 months, with 48-h ambulatory ECG at 3 and 12 months after randomization [24, 25].
Serum creatinine was measured at baseline and Month 12 from an eGFR and was analyzed at a central laboratory using a modified Jaffé alkaline picrate method using a Roche 747 analyzer [26]. The 4-component Modification of Diet in Renal Disease (MDRD) equation was used to calculate an eGFR based on serum creatinine [27]. MDRD was the recommended established standard used for assessment of eGFR at the time the study was conducted, in line with US National Kidney Foundation guidelines [28].
For the present analysis, the Kidney Disease Outcomes Quality Initiative (KDOQI) criteria for the classification and stratification of kidney disease [28, 29] were used and patients with an eGFR <60 mL/min/1.73 m2 at baseline were classified as having CKD. CKD was subcategorized according to KDOQI 2012 guidelines [29] as follows: Stage 3a = eGFR 45–59 mL/min/1.73 m2; Stage 3b = 30–44 mL/min/1.73 m2; Stage 4 = 15–29 mL/min/1.73 m2; and Stage 5 ≤15 mL/min/1.73 m2. No patients had Stage 5 CKD. Other biochemical parameters including SUA—measured by enzyme colorimetry using a Roche 747 analyzer—as well as sodium and potassium levels were measured at baseline and Month 12 at a central laboratory. Diabetes mellitus status was defined from either a history of diabetes or a baseline blood glucose value of >126 mg/dL.
Mean changes in eGFR between treatment groups, overall, and by CKD status, diabetes status, and sex, were compared in an analysis of covariance (ANCOVA) model, using treatment, baseline eGFR, sex, and study center as covariates. Additional analyses adjusted for the presence of hypertension and/or diabetes at baseline to assess whether these conditions had any impact on the primary observation (change in eGFR over 1 year). For the analysis by sex, the model included baseline eGFR and center as covariates. If 12-month data were unavailable, the last observation was carried forward.
An exploratory analysis investigated the effects of baseline LDL-C and changes from baseline LDL-C on treatment in models adjusted for these variables. The patient cohort was grouped according to quartiles of change in LDL-C values from baseline to Month 12. The effect of LDL-C change from baseline on change in eGFR was then evaluated by ANCOVA analysis with adjustments for baseline eGFR, sex, center, and LDL-C quartile. In another exploratory analysis, mean changes in SUA between treatment groups, overall, and by CKD status, diabetes status, and sex were compared using an ANCOVA model with treatment and baseline SUA as covariates. Pearson correlations were used to examine the association between changes from baseline in eGFR with LDL-C and with SUA, and between change in SUA and LDL-C.
Safety was monitored throughout SAGE by assessment of AEs for all patients who took one or more doses of study medication and had follow-up information [25]. The incidence of AEs relating to kidney injury was determined through review of the AE database. We selected from a standardized list of AE terms to evaluate for safety outcomes consistent with the Medical Dictionary for Regulatory Activities (MedDRA®)/Pfizer Narrow Renal Standardised MedDRA Query (SMQ), including: “Renal impairment”, “Renal disorder”, “Renal failure”, “Renal failure acute”, “Nephritis”, “Nephropathy”, “Renal tubular disorder”, or “Renal tubular necrosis”.
Results
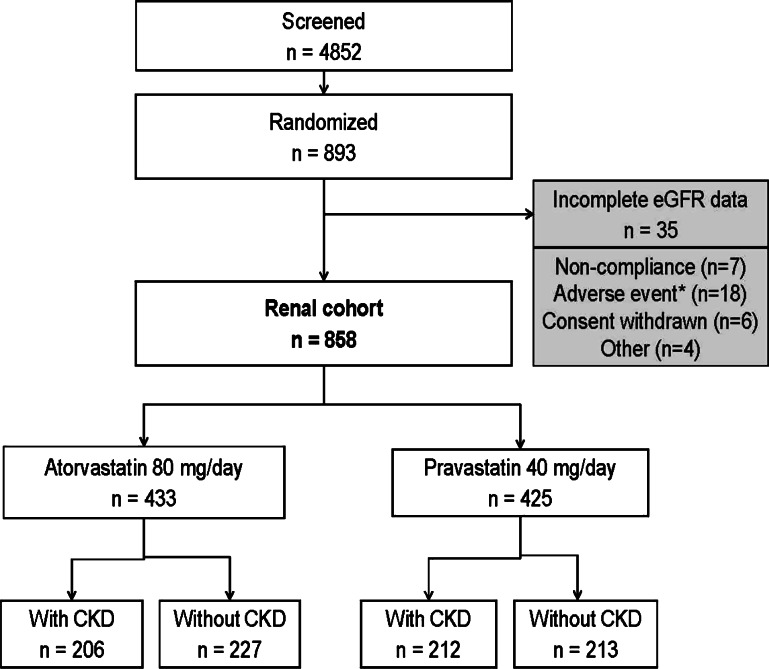
This post hoc analysis of SAGE consisted of 858 participants who had both baseline and Month 12 eGFR measurements (Fig. 1). Thirty-five SAGE patients were excluded from the renal cohort due to incomplete data; the majority of discontinuations were due to AEs (n = 18, including five deaths [atorvastatin n = 1, pravastatin n = 4]) or due to non-compliance (n = 7) (see Fig. 1). The mean (±standard deviation) age of the renal cohort was 72.4 ± 5.1 years, with 262 patients (30.5 %) aged >75 years. At baseline 49 % of the participants had CKD (eGFR ≤60 mL/min/1.73 m2), with 99 % (415/418) classified as Stage 3 (eGFR 30–60 mL/min/1.73 m2) (Table 1). The majority of patients with Stage 3 CKD were categorized as Stage 3a (84 %), according to KDOQI criteria [29].
Fig. 1.
Patient enrollment in the SAGE trial and inclusion in the renal analysis cohort. *Includes two patients with cardiac events. CKD chronic kidney disease, eGFR estimated glomerular filtration rate
Table 1.
Demographic and clinical characteristics of patients included in the renal analysis cohort [patients with both baseline and Month 12 estimated glomerular filtration rate (eGFR) data]
| Characteristics | Patients without CKD (eGFR ≥60 mL/min/1.73 m2) | Patients with CKD (eGFR <60 mL/min/1.73 m2) | p valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 440) | Atorvastatin (n = 227) | Pravastatin (n = 213) | p valuea | All (n = 418) | Atorvastatin (n = 206) | Pravastatin (n = 212) | p valuea | ||
| Age (years) | 71.8 ± 5.0 | 71.6 ± 4.9 | 72.2 ± 5.1 | 0.444 | 73.1 ± 5.2 | 73.2 ± 5.1 | 73.0 ± 5.3 | 0.825 | 0.0002 |
| Age >75 years | 118 (26.8) | 58 (25.6) | 60 (28.2) | 0.536 | 144 (34.5) | 71 (34.5) | 73 (34.4) | 0.995 | 0.015 |
| Males | 365 (83.0) | 185 (81.5) | 180 (84.5) | 0.402 | 230 (55.0) | 114 (55.3) | 116 (54.7) | 0.898 | <0.0001 |
| White | 430 (97.7) | 221 (97.4) | 209 (98.1) | 0.779 | 402 (96.2) | 199 (96.6) | 203 (95.8) | 0.582 | 0.092 |
| Weight (kg) | 77.3 ± 12.6 | 78.5 ± 13.4 | 76.0 ± 11.6 | 0.043 | 75.1 ± 12.1 | 76.0 ± 12.9 | 74.3 ± 11.2 | 0.167 | 0.011 |
| BMI (kg/m2) | 27.0 ± 4.0 | 27.3 ± 4.5 | 26.6 ± 3.3 | 0.054 | 27.3 ± 3.6 | 27.4 ± 3.6 | 27.2 ± 3.7 | 0.511 | 0.184 |
| Baseline eGFR (mL/min/1.73 m2) | 70.8 ± 7.9 | 70.4 ± 7.8 | 70.6 ± 7.9 | 0.790 | 51.8 ± 6.5 | 51.6 ± 6.5 | 52.0 ± 6.5 | 0.530 | <0.0001 |
| Smoking status: current smoker | 27 (6.1) | 15 (6.6) | 12 (5.6) | 0.909 | 25 (6.0) | 8 (3.9) | 17 (8.0) | 0.117 | 0.0007 |
| Baseline lipids (mg/dL) | |||||||||
| LDL-C | 144.3 ± 29.3 | 146.3 ± 29.1 | 142.2 ± 29.4 | 0.146 | 147.7 ± 32.5 | 148.9 ± 31.8 | 146.4 ± 33.1 | 0.431 | 0.115 |
| HDL-C | 46.3 ± 10.9 | 46.0 ± 11.5 | 46.6 ± 10.2 | 0.558 | 45.9 ± 12.3 | 44.9 ± 11.7 | 46.8 ± 12.9 | 0.111 | 0.627 |
| Triglycerides | 149.8 ± 67.8 | 151.3 ± 63.3 | 148.2 ± 72.4 | 0.629 | 171.6 ± 79.8 | 177.6 ± 77.4 | 165.7 ± 81.9 | 0.131 | <0.0001 |
| Total cholesterol | 220.6 ± 33.8 | 222.6 ± 34.4 | 218.6 ± 33.1 | 0.219 | 227.8 ± 38.4 | 229.3 ± 38.7 | 226.3 ± 38.2 | 0.425 | 0.004 |
| Diabetes mellitus | 99 (22.5) | 50 (22.0) | 49 (23.0) | 0.806 | 101 (24.2) | 46 (22.3) | 55 (25.9) | 0.388 | 0.565 |
| Hypertension | 256 (58.2) | 135 (59.5) | 121 (56.8) | 0.571 | 299 (71.5) | 152 (73.8) | 147 (69.3) | 0.314 | <0.0001 |
| Metabolic syndrome | 146 (33.2) | 87 (38.3) | 59 (27.7) | 0.018 | 200 (47.9) | 103 (50.0) | 97 (45.8) | 0.3850 | <0.0001 |
| Medical history | |||||||||
| Premature CHD | 76 (17.3) | 41 (18.1) | 35 (16.4) | 0.6513 | 68 (16.3) | 30 (14.6) | 38 (17.9) | 0.3519 | 0.6938 |
| CVA | 13 (3.0) | 5 (2.2) | 8 (3.8) | 0.336 | 20 (4.8) | 4 (1.9) | 16 (7.6) | 0.007 | 0.164 |
| Angina | 414 (94.1) | 213 (93.8) | 201 (94.4) | 0.813 | 392 (93.8) | 197 (95.6) | 195 (92.0) | 0.122 | 0.849 |
| CABG | 139 (31.6) | 65 (28.6) | 74 (34.7) | 0.169 | 113 (27.0) | 49 (23.8) | 64 (30.2) | 0.141 | 0.143 |
| MI | 191 (43.4) | 94 (41.4) | 97 (45.5) | 0.382 | 205 (49.0) | 103 (50.0) | 102 (48.1) | 0.700 | 0.098 |
| Stage 3 CKDc | 204 (99.0) | 211 (99.5) | 0.619 | ||||||
| Stage 3a | 172 (83.5) | 181 (85.4) | |||||||
| Stage 3b | 32 (15.5) | 30 (14.1) | |||||||
| Stage 4 CKDd | 2 (1.0) | 1 (0.5) | |||||||
Values are given as number of patients (%) or mean ± standard deviation
BMI body mass index, CABG coronary artery bypass graft, CHD coronary heart disease, CKD chronic kidney disease, CVA cerebrovascular accident, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, MI myocardial infarction
a p value for atorvastatin vs. pravastatin
b p value for all patients with CKD vs. all patients without CKD
cStage 3a = eGFR 45–59 mL/min/1.73 m2; Stage 3b = 30–44 mL/min/1.73 m2
dStage 4 = 15–29 mL/min/1.73 m2
Baseline triglycerides and total cholesterol were higher in patients with versus without CKD (Table 1), but lipids were similar across treatment arms within each CKD or non-CKD cohort. Across all patients in this renal cohort (both those with and without CKD), treatment with atorvastatin resulted in significantly greater decreases in total cholesterol (−40.9 vs. −21.7 %), LDL-C (−56.1 vs. −32.0 %), and triglycerides (−28.5 vs. −9.2 %) than did pravastatin after 3 months of follow-up (p < 0.001).
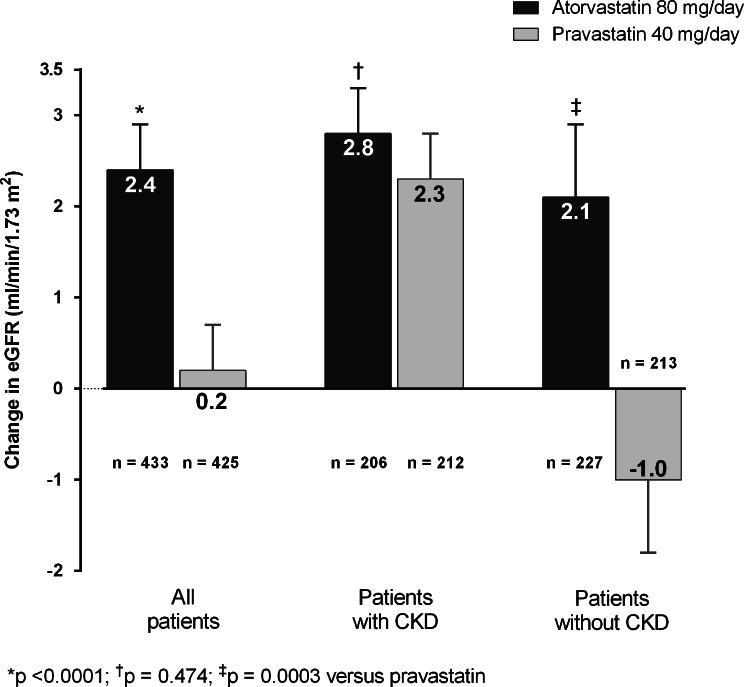
There were no significant differences in baseline eGFR between the treatment groups, or within treatment for patients with or without CKD (Table 1). Overall, eGFR increased over 12 months with atorvastatin (+2.38 mL/min/1.73 m2) and remained stable with pravastatin (+0.18 mL/min/1.73 m2) (Fig. 2; treatment difference p < 0.0001). In patients with CKD, eGFR improved in both treatment arms (atorvastatin: +2.8 mL/min/1.73 m2; pravastatin: +2.3 mL/min/1.73 m2) (treatment difference p = 0.474). In patients without CKD, a significant increase in eGFR from baseline was observed with atorvastatin (+2.08 mL/min/1.73 m2; p = 0.009) but not with pravastatin (−1.04 mL/min/1.73 m2; p = 0.196) (treatment difference p = 0.0003) (Fig. 2).
Fig. 2.
Changes in estimated glomerular filtration rate (eGFR) from baseline (least square mean ± standard error) following 12 months’ treatment with atorvastatin 80 mg/day or pravastatin 40 mg/day in all patients in the SAGE renal cohort and according to chronic kidney disease status. LDL-C low-density lipoprotein cholesterol
Increases in eGFR were seen with both treatments when analyzed by diabetes status; however, the difference between statin treatments was significant only in patients without diabetes (Table 2). The overall increase in eGFR was significantly greater among males than females (p = 0.003). A significant treatment effect with atorvastatin versus pravastatin was observed in males only (Table 2). No notable differences in the primary assessment (change in eGFR) were noted for patients with or without CKD when data were adjusted for baseline hypertension or diabetes status in addition to baseline eGFR and sex (data not shown).
Table 2.
Baseline estimated glomerular filtration rate (eGFR) and changes in eGFR following 12 months’ treatment with atorvastatin 80 mg/day or pravastatin 40 mg/day according to diabetes mellitus status and sex
| eGFR | Patients with diabetesa | Patients without diabetes | Males | Females | ||||
|---|---|---|---|---|---|---|---|---|
| Atorvastatin (n = 105) | Pravastatin (n = 119) | Atorvastatin (n = 328) | Pravastatin (n = 306) | Atorvastatin (n = 299) | Pravastatin (n = 296) | Atorvastatin (n = 134) | Pravastatin (n = 129) | |
| Baseline eGFRb (mL/min/1.73 m2) | 61.8 ± 13.1 | 60.4 ± 12.8 | 61.3 ± 11.5 | 61.7 ± 11.4 | 64.0 ± 11.6 | 63.3 ± 11.3 | 55.6 ± 10.4 | 56.7 ± 11.5 |
| Change in eGFRc (mL/min/1.73 m2) | 2.2 ± 1.0 | 0.7 ± 1.0 | 2.2 ± 0.5 | 0.2 ± 0.5 | 3.7 ± 0.6 | 1.1 ± 0.5 | 0.6 ± 0.8 | −0.1 ± 0.8 |
| p valued | 0.3065 | 0.0014 | 0.0001 | 0.4969 | ||||
aMedical history of diabetes or baseline glucose >126 mg/dL
bMean ± standard deviation
cLeast square mean ± standard error
dAll p values are for treatment effect of atorvastatin vs. pravastatin
There was a weak but significant correlation between change in eGFR and change in LDL-C (r = −0.11; p = 0.002). Increases in eGFR were significantly greater in the patients with greater reductions in LDL-C (p for trend = 0.049) (Fig. 3). After adjustment for baseline LDL-C or change in LDL-C, the treatment effect on eGFR remained significant.
Fig. 3.
Changes in estimated glomerular filtration rate (eGFR) from baseline (least square mean ± standard error) following 12 months’ treatment with atorvastatin 80 mg/day or pravastatin 40 mg/day, grouped by quartile ranges of changes in low-density lipoprotein cholesterol from baseline. CKD chronic kidney disease, eGFR estimated glomerular filtration rate
Baseline SUA values were within a normal range in both treatment groups, and with treatment a significantly greater reduction in mean SUA was seen with atorvastatin than with pravastatin treatment (p < 0.0001). These findings were similar when analyzed by CKD status, diabetes status, and sex (Table 3). There was a significant (negative) correlation between the change in SUA and the change in eGFR, regardless of CKD status (CKD patients, r = −0.373; p < 0.0001; non-CKD patients, r = −0.344; p < 0.0001). There was also a positive correlation between the change in LDL-C versus change in SUA, regardless of CKD status (CKD patients, r = 0.181; p = 0.0002; non-CKD patients, r = 0.262; p < 0.0001).
Table 3.
Baseline serum uric acid (SUA) and changes following 12 months treatment with atorvastatin 80 mg/day or pravastatin 40 mg/day according to chronic kidney disease (CKD) status, diabetes mellitus status, and sex
| Atorvastatin | Pravastatin | Atorvastatin | Pravastatin | |
|---|---|---|---|---|
| All patients | ||||
| n | 433 | 425 | ||
| Baseline SUAa (mg/dL) | 6.30 ± 1.34 | 6.15 ± 1.36 | ||
| Change in SUAb (mg/dL) | −0.52 ± 0.04 | −0.09 ± 0.04 | ||
| p valuec | <0.0001 | |||
| Patients without CKD | Patients with CKD | |||
| n | 227 | 213 | 206 | 212 |
| Baseline SUAa (mg/dL) | 5.92 ± 1.14 | 5.92 ± 1.32 | 6.71 ± 1.41 | 6.39 ± 1.35 |
| Change in SUAb (mg/dL) | −0.43 ± 0.05 | −0.02 ± 0.06 | −0.61 ± 0.07 | −0.18 ± 0.07 |
| p valuec | <0.0001 | <0.0001 | ||
| Patients without diabetes | Patients with diabetes | |||
| n | 328 | 306 | 105 | 119 |
| Baseline SUAa (mg/dL) | 6.33 ± 1.32 | 6.15 ± 1.33 | 6.19 ± 1.40 | 6.15 ± 1.43 |
| Change in SUAb (mg/dL) | −0.52 ± 0.05 | −0.085 ± 0.05 | −0.54 ± 0.09 | −0.10 ± 0.09 |
| p valuec | <0.0001 | <0.0001 | ||
| Males | Females | |||
| n | 299 | 296 | 134 | 129 |
| Baseline SUAa (mg/dL) | 6.40 ± 1.31 | 6.40 ± 1.27 | 6.07 ± 1.37 | 5.59 ± 1.38 |
| Change in SUAb (mg/dL) | −0.55 ± 0.05 | −0.14 ± 0.05 | −0.48 ± 0.09 | 0.03 ± 0.09 |
| p valuec | <0.0001 | <0.0001 | ||
aMean ± standard deviation
bLeast square mean ± standard error
cAll p values are for treatment effect of atorvastatin vs. pravastatin
Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels ≥3 times the upper limit of normal were reported in 19 atorvastatin patients (11/214 [5.1 %] with CKD; 8/232 [3.4 %] without CKD) and one pravastatin patient without CKD (1/227 [0.4 %]). There were no persistent elevations in liver enzymes (AST, ALT), as levels returned to normal for all patients who were followed up for a repeat test, including for the one pravastatin patient who permanently discontinued study medication (not due to liver function abnormality).
Myalgia was the most common muscle-related AE, which occurred in 25 patients without CKD (20 atorvastatin; five pravastatin) and 15 patients with CKD (three atorvastatin; 12 pravastatin). Myalgia led to discontinuation of six CKD (one atorvastatin; five pravastatin) and seven non-CKD (seven atorvastatin) patients. Myopathy was reported in one CKD patient receiving atorvastatin. Rhabdomyolysis was not reported.
Twenty-six deaths were reported in the overall renal cohort during the study, with more deaths recorded for patients with CKD (n = 15 [3.48 %]) than without CKD at baseline (n = 11 [2.40 %]). A greater number of cardiovascular deaths were recorded in the CKD population (n = 12) than in the non-CKD population (n = 3). A higher number of deaths were recorded in the renal cohort in patients receiving pravastatin (n = 18) than in those receiving atorvastatin (n = 8), for patients with and without CKD, many of which were adjudicated as cardiovascular deaths (pravastatin: n = 10; atorvastatin: n = 5). Investigator-reported renal AEs were less frequent in the atorvastatin (n = 1 [renal failure]) than in the pravastatin arm (n = 5 [renal failure, n = 2; acute renal failure, n = 1; renal impairment, n = 2]) for CKD patients. Investigator-reported renal AEs were very infrequent in both treatment arms for non-CKD patients (atorvastatin: n = 0; pravastatin: n = 1 [acute renal failure]).
Discussion
This post hoc analysis of the SAGE trial demonstrated that 1 year of intensive atorvastatin therapy (80 mg/day) improved renal function in a high-risk cardiovascular population of older patients with stable CAD and silent ischemia, regardless of the presence of CKD or diabetes, while moderate therapy with pravastatin (40 mg/day) stabilized renal function over the same period of follow-up. This observation is consistent with other analyses which demonstrate that renoprotection varies between atorvastatin and other lipid-lowering agents in subjects with vascular disease [30–32].
The mechanism by which statin therapy may have a beneficial effect on renal function remains unknown, and may differ between statin type. We noted a weak correlation between increases in eGFR with atorvastatin-associated reduction in LDL-C, so that the greatest improvement in renal function was correlated with more intense lipid lowering. Pravastatin had a more modest effect on renal function, possibly secondary to a smaller effect on lipid lowering when compared with atorvastatin [25]. The stabilizing effect on eGFR with pravastatin also aligned with findings in patients with or at high-risk of CHD over follow-up of nearly 5 years [4]. However, statin-associated renoprotection may be related to other pleiotropic effects, and not directly related to lipid lowering. In agreement, in a recent analysis of the prospective evaluation of proteinuria and renal function in diabetic patients with progressive renal disease (PLANET) I and II trials, high-dose atorvastatin (80 mg) was deemed to be more renoprotective over a 1-year treatment period than high-dose rosuvastatin (40 mg), despite rosuvastatin lowering plasma lipid concentrations to a greater extent than atorvastatin 80 mg in both CKD patients with end-stage renal disease and diabetes [33]. Furthermore, in a post hoc analysis of statin type, atorvastatin 80 mg lowered the urine protein:creatinine ratio significantly more than both low- and high-dose rosuvastatin in these patients [33].
The hypothesis that different statins have different renal protective effects is further supported by both non-clinical and clinical observations [34–43]. Statins have been observed to modify endothelial cell and vascular physiology in animal models [36] and show anti-inflammatory properties [37], both of which could have renoprotective effects. We report that in patients with CKD, eGFR improved with atorvastatin or pravastatin, while in those without CKD, eGFR improved in atorvastatin-treated patients but was only stabilized with pravastatin treatment. This suggests that the renoprotective effects of statins are more prominent in CKD patients than in non-CKD patients. The reasons underlying these differences are unclear; however, a number of differences in cellular physiology are known between CKD and non-CKD patients that could affect the outcomes observed herein [38–43]. For example, patients with CKD have been reported to have higher levels of inflammatory markers, such as C-reactive protein, interleukin-1β, and tumor necrosis factor-α, than an age-matched control population with normal renal function [38]. Treatment with atorvastatin can significantly lower these inflammatory markers in patients with CKD; furthermore, atorvastatin treatment may improve endothelial function and stabilize plaque-limiting atheroembolic renal disease [38, 39]. However, other statins may not have the same effect. In certain populations, evidence suggests that statins may influence blood flow [34, 35, 40–43], and this potential effect may contribute to changes in eGFR. However, the literature is somewhat conflicting regarding any effect, with some studies indicating that statins increase blood flow, but only in patients with normal endothelial function [43], whereas other studies suggest no change in healthy subjects [41], patients with hypercholesterolemia [40] or patients with peripheral arterial disease [42]. Given the diversity of populations studied, the small number of patients in the trials, and the known influence of patient characteristics (including CKD) and other risk factors on hemodynamics and endothelial function (as discussed earlier), further study is needed to clarify any statin-mediated influence on blood flow in CKD and non-CKD patient populations.
CKD progresses rapidly in patients with coexisting CHD, and this rapid progression leads to an increased risk of cardiovascular events [11, 15, 44, 45]. In our 12-month study of patients aged 65–85 years, the increase in eGFR observed with intensive atorvastatin therapy was in accordance with the renal benefits reported for the same regimen in longer trials of younger CHD populations [5, 6]. In addition, the improvement in renal function among patients with diabetes and/or CKD following intensive atorvastatin therapy is consistent with that reported in other post hoc analyses of atorvastatin treatment regimens over longer follow-up [7, 46], and including in patients with prior stroke or transient ischemic attack, both with and without CKD [13]. Statin-associated renoprotection was not seen in SHARP (Study of Heart and Renal Protection) [31, 32]. This prospective clinical study was designed to determine the effect of a statin–ezetimide combination on cardiovascular and renal endpoints, which included progression to end-stage renal disease, doubling of serum creatinine, and death, in patients with advanced CKD [31]. An exploratory SHARP renal analysis failed to show any benefit from a 1 mmol/L reduction in LDL-C on slowing progression of kidney disease in a wide variety of patients with CKD [32]. There are important differences between the patients studied in SHARP and our analysis of the SAGE trial. SHARP recruited a population of patients with well-characterized renal disease including patients with glomerulonephritis and cystic renal disease, and excluded subjects with prior myocardial infarction or coronary revascularization [31]. Patients with specific renal disease diagnoses were excluded from SAGE [25]. Another recent analysis of the SHARP cohort demonstrated that the type of kidney disease was a significant factor affecting progression of renal disease, as 23 % of patients with cystic renal disease versus 10 % with glomerulonephritis and 12 % with diabetic nephropathy progressed to end-stage renal disease [47]. The degrees of renal impairment differed at baseline and could be another factor that may account for the lack of renoprotection in SHARP, as nearly half of the patients in SHARP had an eGFR <15 mL/min/1.73 m2 [31], while there were only three such subjects in the SAGE renal cohort.
Guidelines now recommend statins or statin/ezetimide for adults aged ≥50 years with eGFR ≤60 mL/min/1.73 m2 who are not receiving long-term dialysis or for those who have had a kidney transplant [48, 49]. Recommendations for lipid-lowering therapy were justified on the basis of the high cardiovascular risk status of patients with coexisting CHD/CKD. Further incremental increases in cardiovascular risk occur with aging [19] or coexisting co-morbid conditions such as diabetes and hypertension [18, 44]. Despite recognized evidence for the benefits of statin therapy in younger patients with coexisting CHD and CKD [1], data surrounding the benefits of statin therapy for older patients with these co-morbidities are less clear. Our data demonstrate that an intensive atorvastatin-based treatment regimen can stabilize or improve renal function in a high-risk population of older patients with stable CAD and silent ischemia, with or without CKD, and that renoprotection was evident after 1 year. Overall, atorvastatin was more effective than pravastatin in stabilizing or improving renal function; however, significant improvement in renal function was also observed with pravastatin in this cohort of patients with CKD.
The renal benefits seen with atorvastatin in SAGE are consistent with those reported in other trials that have examined the effect of atorvastatin on renal function [2, 5–7, 13, 33, 46]. Although older subjects with CKD are less likely than younger populations to progress to end-stage renal disease, attempts should be made to modify risk factors and stabilize renal function. Hemmelgarn and colleagues [18] examined the changes in renal function that occurred in a cohort of 10,184 subjects aged 66 years or older over a 2-year period. Absolute reductions in eGFR in the cohort of 3191 subjects with baseline eGFR between 30 and 59 mL/min/1.73 m2 were 5.1 and 7.2 mL/min/1.73 m2 in female and male patients, respectively, with CKD and co-morbid diabetes, compared with 2.0 and 3.5 mL/min/1.73 m2 for female and male CKD patients, respectively, without diabetes [18]. The current analysis suggests that lipid-lowering therapy may be an effective strategy for limiting the expected decline in renal function in older CHD subjects with CKD and with or without coexisting diabetes.
Improvements in renal function following intensive atorvastatin treatment are further supported by dual assessment of change in eGFR alongside SUA. The observed small (−0.52 mg/dL) but statistically significant decrease in SUA with atorvastatin was also in agreement with those reported from CHD patients aged up to 75 years treated with atorvastatin (10–80 mg/day) [2]. The greater fall in SUA observed with atorvastatin treatment could be secondary to improved filtration, whereas only a stabilizing effect was observed with pravastatin treatment. A significant negative correlation was noted between the change in SUA and change in eGFR; thus, as eGFR improved, SUA fell. SUA has been reported as an independent risk factor for cardiovascular mortality, with risk increasing as SUA increases, even within the normal range [22, 50]. Although improvements in the glomerular filtration rate may account for the modest reduction in SUA, the exact mechanisms whereby atorvastatin and pravastatin lower SUA remain to be determined. In addition, given that the reduction in SUA was small, although statistically significant, over the 1-year study period, the clinical relevance of this observation needs to be confirmed.
In the entire SAGE population [25] and in this renal cohort, both atorvastatin 80 mg/day and pravastatin 40 mg/day were generally well-tolerated, and the AEs recorded were as expected in a cohort of older patients [51]. The current analysis also demonstrated that in this cohort of older CHD patients with CKD, there did not appear to be a greater risk of muscle-related AEs than in those without CKD. This is in line with a recently published analysis including 149,882 patient-years of follow-up that failed to show any increase in renal-related serious AEs with statins compared with controls [17]. Thus, although safety perceptions have been cited as a reason for not prescribing statin therapy to older patients with or without CKD [51], the findings of this study and others do not support the view that safety risks outweigh the cardiovascular benefits of intensive atorvastatin therapy for this high-risk elderly population [17, 52, 53]. Current guidelines for treating dyslipidemia in CKD acknowledge the potential benefits of high-dose statins in patients with diabetes and mild-to-moderate CKD (Stages 1–3); however, dose modification is also recommended for some statins and certain other lipid-lowering medicines in moderate-to-advanced CKD (Stages 3–5) [29].
Limitations
This post hoc analysis of a randomized controlled prospective study comparing two treatment regimens has some limitations. CKD staging was based on eGFR because data on proteinuria were not available, and albuminuria data were not analyzed. There was no placebo arm for comparison, and patient numbers were relatively small. There were more male than female patients in the non-CKD cohort (83 % male); eGFR is known to differ by sex, and the Cockroft-Gault equation is adjusted accordingly. In addition, observations have been published suggesting that SUA is higher in male than in female subjects both in the general population [22] and in subjects (mean age 61 years) with CKD [54]. Although our analyses were adjusted for sex and baseline eGFR, the uneven male:female ratio may have influenced the observations from the non-CKD analyses. The findings of this analysis should, therefore, be interpreted accordingly. Nevertheless, the results were in accordance with longer analyses and similar trials of younger patient groups [2, 5–7, 13, 33, 46]. Finally, although a number of our observations were statistically significant, the clinical relevance in a larger patient sample cannot be inferred.
Conclusions
This post hoc analysis of the SAGE trial extended the observation of renal benefits with statins to a high-risk population of older patients with a history of stable CAD with silent ischemia, and in particular to those with co-existent CKD. These findings suggest that relatively short-term (1 year), intensive management of dyslipidemia in older patients with stable CAD might be associated with preservation of renal function and slowed CKD progression without increasing the risk of muscle symptoms or other renal AEs.
Acknowledgments
This study was sponsored by Pfizer. Medical writing support was provided by Karen Burrows, MPhil, CMPP and Shuang Li of Engage Scientific Solutions and was funded by Pfizer.
Compliance with Ethical Standards
Conflict of interest
PCD has received research grants and consulting fees/other remuneration from Pfizer, Amgen, and Sanofi/Regeneron. PHS has received research grants from Boston Scientific and AstraZeneca. RSF and REL are employees of Pfizer. DJW was an employee of Pfizer at the time the study was conducted.
Funding source
This study was funded by Pfizer.
Footnotes
For the SAGE Investigators.
References
- 1.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59(1):260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Athyros VG, Elisaf M, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, et al. Effect of statins versus untreated dyslipidemia on serum uric acid levels in patients with coronary heart disease: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Am J Kidney Dis. 2004;43(4):589–599. doi: 10.1053/j.ajkd.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Rahman M, Baimbridge C, Davis BR, Barzilay J, Basile JN, Henriquez MA, et al. Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Am J Kidney Dis. 2008;52(3):412–424. doi: 10.1053/j.ajkd.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonelli M, Isles C, Craven T, Tonkin A, Pfeffer MA, Shepherd J, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112(2):171–178. doi: 10.1161/CIRCULATIONAHA.104.517565. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, et al. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol. 2007;2(6):1131–1139. doi: 10.2215/CJN.04371206. [DOI] [PubMed] [Google Scholar]
- 6.Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis. 2009;53(5):741–750. doi: 10.1053/j.ajkd.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am J Kidney Dis. 2009;54(5):810–819. doi: 10.1053/j.ajkd.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol. 2010;55(12):1266–1273. doi: 10.1016/j.jacc.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome) J Am Coll Cardiol. 2014;63(1):71–79. doi: 10.1016/j.jacc.2013.04.105. [DOI] [PubMed] [Google Scholar]
- 10.Strippoli GF, Navaneethan SD, Johnson DW, Perkovic V, Pellegrini F, Nicolucci A, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336(7645):645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157(4):263–275. doi: 10.7326/0003-4819-157-4-201208210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou W, Lv J, Perkovic V, Yang L, Zhao N, Jardine MJ, et al. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J. 2013;34(24):1807–1817. doi: 10.1093/eurheartj/eht065. [DOI] [PubMed] [Google Scholar]
- 13.Amarenco P, Callahan A, 3rd, Campese VM, Goldstein LB, Hennerici MG, Messig M, et al. Effect of high-dose atorvastatin on renal function in subjects with stroke or transient ischemic attack in the SPARCL trial. Stroke. 2014;45(10):2974–2982. doi: 10.1161/STROKEAHA.114.005832. [DOI] [PubMed] [Google Scholar]
- 14.Han Y, Zhu G, Han L, Hou F, Huang W, Liu H, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63(1):62–70. doi: 10.1016/j.jacc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Nitta K. Clinical assessment and management of dyslipidemia in patients with chronic kidney disease. Clin Exp Nephrol. 2012;16(4):522–529. doi: 10.1007/s10157-012-0655-x. [DOI] [PubMed] [Google Scholar]
- 16.Hoshi T, Sato A, Kakefuda Y, Harunari T, Watabe H, Ojima E, et al. Preventive effect of statin pretreatment on contrast-induced acute kidney injury in patients undergoing coronary angioplasty: propensity score analysis from a multicenter registry. Int J Cardiol. 2014;171(2):243–249. doi: 10.1016/j.ijcard.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Bangalore S, Fayyad R, Hovingh GK, Laskey R, Vogt L, DeMicco DA, et al. Statin and the risk of renal-related serious adverse events: analysis from the IDEAL, TNT, CARDS, ASPEN, SPARCL, and other placebo-controlled trials. Am J Cardiol. 2014;113(12):2018–2020. doi: 10.1016/j.amjcard.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69(12):2155–2161. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 19.Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, et al. A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol. Epub. 2014 doi: 10.1007/s40620-014-0077-9. [DOI] [PubMed] [Google Scholar]
- 20.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2010;55(3 Suppl 2):S23–S33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283(18):2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 23.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deedwania PC. Effect of aggressive versus moderate lipid-lowering therapy on myocardial ischemia: the rationale, design, and baseline characteristics of the Study Assessing Goals in the Elderly (SAGE) Am Heart J. 2004;148(6):1053–1059. doi: 10.1016/j.ahj.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Deedwania P, Stone PH, Bairey Merz CN, Cosin-Aguilar J, Koylan N, Luo D, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the Study Assessing Goals in the Elderly (SAGE) Circulation. 2007;115(6):700–707. doi: 10.1161/CIRCULATIONAHA.106.654756. [DOI] [PubMed] [Google Scholar]
- 26.Junge W, Wilke B, Halabi A, Klein G. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffe method. Clin Chim Acta. 2004;344(1–2):137–148. doi: 10.1016/j.cccn.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 28.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 29.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17(7):2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 31.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haynes R, Lewis D, Emberson J, Reith C, Agodoa L, Cass A, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014;25(8):1825–1833. doi: 10.1681/ASN.2013090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol. 2015;3(3):181–190. doi: 10.1016/S2213-8587(14)70246-3. [DOI] [PubMed] [Google Scholar]
- 34.Hafez K, Inman S, Stowe N, Novick A. Renal hemodynamic effects of lovastatin in a renal ablation model. Urology. 1996;48(6):862–867. doi: 10.1016/S0090-4295(96)00314-7. [DOI] [PubMed] [Google Scholar]
- 35.van Dijk M, Kamper A, van Veen S, Souverijn J, Blauw G. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2001;16(11):2152–2157. doi: 10.1093/ndt/16.11.2152. [DOI] [PubMed] [Google Scholar]
- 36.Zhou MS, Jaimes EA, Raij L. Atorvastatin prevents end-organ injury in salt-sensitive hypertension: role of eNOS and oxidant stress. Hypertension. 2004;44(2):186–190. doi: 10.1161/01.HYP.0000136395.06810.cf. [DOI] [PubMed] [Google Scholar]
- 37.Yokota N, O’Donnell M, Daniels F, Burne-Taney M, Keane W, Kasiske B, et al. Protective effect of HMG-CoA reductase inhibitor on experimental renal ischemia-reperfusion injury. Am J Nephrol. 2003;23(1):13–17. doi: 10.1159/000066301. [DOI] [PubMed] [Google Scholar]
- 38.Goicoechea M, de Vinuesa SG, Lahera V, Cachofeiro V, Gomez-Campdera F, Vega A, et al. Effects of atorvastatin on inflammatory and fibrinolytic parameters in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(12 Suppl 3):S231–S235. doi: 10.1681/ASN.2006080938. [DOI] [PubMed] [Google Scholar]
- 39.Scolari F, Ravani P, Pola A, Guerini S, Zubani R, Movilli E, et al. Predictors of renal and patient outcomes in atheroembolic renal disease: a prospective study. J Am Soc Nephrol. 2003;14(6):1584–1590. doi: 10.1097/01.ASN.0000069220.60954.F1. [DOI] [PubMed] [Google Scholar]
- 40.Ott C, Ritt M, Titze SI, Schaufele T, Schmieder RE. Rosuvastatin does not affect intrarenal hemodynamics in patients with hypercholesterolemia. J Nephrol. 2009;22(5):675–681. [PubMed] [Google Scholar]
- 41.Paulsen L, Holm C, Bech JN, Starklint J, Pedersen EB. Effects of statins on renal sodium and water handling: acute and short-term effects of atorvastatin on renal haemodynamics, tubular function, vasoactive hormones, blood pressure and pulse rate in healthy, normocholesterolemic humans. Nephrol Dial Transplant. 2008;23(5):1556–1561. doi: 10.1093/ndt/gfm807. [DOI] [PubMed] [Google Scholar]
- 42.Alnaeb ME, Youssef F, Mikhailidis DP, Hamilton G. Short-term lipid-lowering treatment with atorvastatin improves renal function but not renal blood flow indices in patients with peripheral arterial disease. Angiology. 2006;57(1):65–71. doi: 10.1177/000331970605700109. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Gong D, Li S, Zhou X. Meta-analysis of the effects of statin therapy on endothelial function in patients with diabetes mellitus. Atherosclerosis. 2012;223(1):78–85. doi: 10.1016/j.atherosclerosis.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009;20(12):2625–2630. doi: 10.1681/ASN.2009050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rein P, Saely CH, Vonbank A, Boehnel C, Drexel H. Usefulness of serial decline of kidney function to predict mortality and cardiovascular events in patients undergoing coronary angiography. Am J Cardiol. 2014;113(2):215–221. doi: 10.1016/j.amjcard.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 46.Shepherd J, Kastelein JP, Bittner VA, Carmena R, Deedwania PC, Breazna A, et al. Intensive lipid lowering with atorvastatin in patients with coronary artery disease, diabetes, and chronic kidney disease. Mayo Clin Proc. 2008;83(8):870–879. doi: 10.1016/S0025-6196(11)60763-5. [DOI] [PubMed] [Google Scholar]
- 47.Haynes R, Staplin N, Emberson J, Herrington WG, Tomson C, Agodoa L, SHARP Collaborative Group et al. Evaluating the contribution of the cause of kidney disease to prognosis in CKD: results from the Study of Heart and Renal Protection (SHARP) Am J Kidney Dis. 2014;64(1):40–48. doi: 10.1053/j.ajkd.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonelli M, Wanner C. Lipid management in chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2013 clinical practice guideline. Ann Intern Med. 2014;160(3):182. doi: 10.7326/M13-2453. [DOI] [PubMed] [Google Scholar]
- 49.Food and Drug Administration. Summary minutes of the Endocrinologic and Metabolic Drugs Advisory Committee, November 2, 2011. Silver Spring, MD. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM290774.pdf. Accessed 26 Jun 2014.
- 50.Kalaitzidis RG, Bakris GL. Serum creatinine vs. albuminuria as biomarkers for the estimation of cardiovascular risk. Curr Vasc Pharmacol. 2010;8(5):604–611. doi: 10.2174/157016110792006914. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson TA. Overcoming ‘ageism’ bias in the treatment of hypercholesterolaemia: a review of safety issues with statins in the elderly. Drug Saf. 2006;29(5):421–448. doi: 10.2165/00002018-200629050-00005. [DOI] [PubMed] [Google Scholar]
- 52.Wenger NK, Lewis SJ, Herrington DM, Bittner V, Welty FK. Outcomes of using high- or low-dose atorvastatin in patients 65 years of age or older with stable coronary heart disease. Ann Intern Med. 2007;147(1):1–9. doi: 10.7326/0003-4819-147-1-200707030-00002. [DOI] [PubMed] [Google Scholar]
- 53.Koren MJ, Feldman T, Mendes RA. Impact of high-dose atorvastatin in coronary heart disease patients age 65 to 78 years. Clin Cardiol. 2009;32(5):256–263. doi: 10.1002/clc.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshitomi R, Fukui A, Nakayama M, Ura Y, Ikeda H, Oniki H, et al. Sex differences in the association between serum uric acid levels and cardiac hypertrophy in patients with chronic kidney disease. Hypertens Res. 2014;37(3):246–252. doi: 10.1038/hr.2013.134. [DOI] [PubMed] [Google Scholar]