Summary
The Distance Constraint Model (DCM) is an ensemble-based biophysical model that integrates thermodynamic and mechanical viewpoints of protein structure. The DCM outputs a large number of structural characterizations that collectively allow for Quantified Stability/Flexibility Relationships (QSFR) to be identified and compared across protein families. Using five metallo-β-lactamases (MBLs) as a representative set, we demonstrate how QSFR properties are both conserved and varied across protein families. Similar to our characterizations on other protein families, the backbone flexibility of the five MBLs are overall visually conserved, yet there are interesting specific quantitative differences. For example, the plasmid-encoded NDM-1 enzyme, which leads to a fast spreading drug-resistant version of Klebsiella Pneumoniae, has several regions of significantly increased rigidity relative to the other four. In addition, the set of intramolecular couplings within NDM-1 are also atypical. While long-range couplings frequently vary significantly across protein families, NDM-1 is distinct because it has limited correlated flexibility, which is isolated within the active site S3/S4 and S11/H6 loops. These loops are flexibly correlated in the other members, suggesting it is important to function, but the others also have significant amounts of correlated flexibility throughout the rest of their structures.
Keywords: β-Lactamase, Distance constraint model, Protein flexibility, Allostery, Quantitative stability/flexibility relationships
1. Introduction
Feynman famously stated nearly 50 years ago that “Everything that living things do can be understood in terms of the jigglings and wigglings of atoms” (1). Since then, our appreciation of how these dynamical properties play out in proteins over a wide spectrum of timescales has been greatly expanded (2). However, the experimental and computational methods to interrogate and compare these jigglings and wigglings across many different proteins remain elusive (3). To that end, we have developed a powerful and computationally efficient Distance Constraint Model (DCM) (4-7) that allows us to efficiently compare protein stability and flexibility properties across many different proteins, which we refer to as Quantitative Stability/Flexibility Relationships (QSFR) (8, 9).
Starting with our initial QSFR comparisons in 2006 of a mesophilic and thermophilic RNase H pair (10), to our most recent comparisons of larger protein datasets (11-13), we have observed fairly consistent trends. That is, backbone flexibility is largely conserved across protein families, whereas intramolecular couplings (i.e., allostery) can be quite variable. Put otherwise, backbone flexibility is, in large part, engrailed by structure, whereas allostery is highly sensitive to small perturbations. While these qualitative observations are consistent and robust, many quantitative variations therein do occur. For example, single point mutations in lyosozyme have a frequent, large, and long-range effect on stability, backbone flexibility and intramolecular couplings (14, 15). In contrast, while they are indeed sensitive, allosteric couplings do quantitatively reflect similarities within protein structures. For example, we demonstrated that allosteric response in E. coli and S. typhimurium CheY orthologs was more similar than either was to the response in T. maritima, which is the naïve expectation based on evolutionary relationships (12).
Our most recent QSFR comparisons have focused on a dozen class-A β-lactamse (BL) structures, which is the enzyme that is primarily responsible for conferring resistance to penicillin and related antibiotics. There are four different BL classes, and the class-A enzymes are the most clinically relevant. Interestingly, the TEM-1 class-A enzyme has been shown to have an extremely rigid backbone (16), which is consistent with our results (17). Moreover, our backbone flexibility predictions are well conserved across the family, whereas allosteric couplings are overall quite variable. Similar to our earlier work on CheY, we further demonstrate that the systematic variations within the flexibility properties parallel the evolutionary history of the family. Large and systematic differences in both quantities occur between evolutionary out-groups, whereas properties are more conserved between close homologs.
While the class-A family is currently the most clinically relevant group of BL enzymes, the metallo-β-lactamases (MBLs) pose a looming pandemic threat. The MBLs (also referred to as class-B) are mechanistically distinct from the other BLs, and frequently have activity against carbapenems, which represent our last lines of antibiotic defense (18). For example, the plasmid-encoded New Delhi metallo-β-lactamase (NDM-1) has led to a number of patient deaths due to its incredible broad-spectrum activity (19). In this report, we describe how QSFR properties vary across five MBL enzymes, which is discussed as a representative of all of our comparative studies, while also revealing several specific and interesting details about NDM-1 that could lead to future antimicrobial strategies.
2. A Brief Summary of The Distance Constraint Model
From conception, the DCM has been designed to optimally balance computational efficiency with biophysical accuracy. To keep the method fast, it is fundamentally based on a free energy decomposition (FED) scheme where component enthalpies and entropies can be placed into look-up tables and accounted for quickly when present. However, FED descriptions of large, highly cross-linked macromolecules like proteins typically fail because of entropy nonadditivity (20, 21). As such, the critical component of the DCM is robustly accounting for nonadditivity within entropic components (22). The DCM models structure as a graph, where each chemical interaction is described by an edge. When present, an edge lowers the enthalpy, and also lowers the entropy when independent. When a constraint is placed into a flexible region, it is said to be independent because it removes a degree of freedom (DOF). Conversely, when a constraint is placed into a region that is already rigid, it is said to be redundant and does not further reduce the entropy because it is placed into a region that is already rigid, meaning all DOF have already been removed. For large atomic systems, a constraint is determined to be or not to be independent by a fast graph rigidity algorithm, called the Pebble Game (23, 24), which provides a complete and rigorous mechanical description of the molecular network (NewRef).
To account for thermal fluctuations, the DCM generates an ensemble of rigidity graphs where weak chemical interactions are allowed to fluctuate on and off. A Gibbs ensemble of rigidity graphs is modeled, each weighted by its free energy using the FED scheme described above. In the standard way, appropriate derivatives of the partition function provide a complete thermodynamic description of the protein. Subsequently, the partition function is used to weight the rigidity/flexibility descriptions of the protein, thus providing a feedback cycle that integrates mechanical and thermodynamic viewpoints. Put otherwise, thermodynamic characterizations are improved by distinguishing between independent and redundant constraints, whereas the calculated Boltzmann weights are used to appropriately average the mechanical properties. An important consequence of this approach is that the DCM appropriately models cooperativity because network rigidity is used as an underlying interaction that accounts for enthalpy-entropy compensations. That is, competition emerges between an enthalpically stabilized rigid structure with many redundant constraints and a flexible entropically stabilized unfolded state (25, 26).
In typical usage, the model is parameterized by reproducing experimental heat capacity curves (6, 7). Our current minimal DCM (mDCM) has three parameters (usol, vnat, δnat) that, respectively, describe the energy of forming a hydrogen bond to solvent, the energy associated with being in a native conformation, and the entropy associated with that native conformation. In the absence of the experimental data to fit to, which is the case for the MBL dataset considered here, the model is parameterized by systematically exploring the parameter space to find {usol, vnat, δnat} that is physically reasonable. For our dataset, three different parameter sets produced quantitatively reasonable two-state behavior. While full heat capacity characterizations are not available, the melting temperature of two of the five enzymes is known [need refs], which are well described using the central set with values usol = −2.6 kcal/mol, vnat = −0.5 kcal/mol, and δnat = 1.8 (cf. Fig. 1a). These model parameter values are well within the expected range established by our prior works across many different globular protein systems.
Fig. 1.
(a) Predicted heat capacity curves of each of the five enzymes. The referenced experimental melting temperatures are marked with dashed vertical lines. The Tm of VIM-4 and IMP-1 are, respectively, 332K and 345K. (b) Superposition of the five metallo-β-lactamase enzymes, which are color-coded the same as in panel (a). The average pairwise α-carbon RMSD across the dataset is 2.2 Å (standard deviation = 0.4 Å).
In addition to the thermodynamic quantities, the mDCM calculates a number of mechanical properties that are appropriately averaged by the thermodynamic ensemble. From the set of QSFR metrics, two are particularly useful. The first, called the Flexibility Index (FI), describes backbone flexibility. Positive FI values quantify the number of DOF within a local region, whereas negative values quantify the number of redundant constraints. When FI = 0, the backbone is said to be isostatically rigid, meaning it is marginally rigid (# of DOF = # redundant constraints = 0). The second, called Cooperativity Correlation (CC), depicts the complete set of residue-to-residue couplings. As described below, CC is described by an N × N matrix, where N is the number of residues in the protein. Each pixel (designating residue pair i,j) is colored based on the correlations therein. When a residue pair is likely to be included in the same rigid cluster, it is colored blue, whereas they are colored red when they are flexibly correlated. White indicates no correlation, but does not necessarily imply rigidity or flexibility. For example, residues i and j might always be rigid, but if their particular rigid clusters never overlap, the CC pixel i,j would be colored white.
3. Metallo-β-Lactamase QSFR
3.1. The Dataset
In this report, we compare five different MBL structures (27-31), all of which correspond to the B1-subfamily (18) (cf. Table 1). A superposition of the five structures is provided in Fig. 1b. Hydrogen atoms were added using H++ (32) with default parameters and the resulting structures were minimized using the AMBER 99 force field (33). MBLs have two zinc ions per monomer, which are bridged by a well-resolved water molecule. Interestingly, Kim et al. (34) have recently demonstrated that NDM-1 is functionally tolerant of non-zinc metals and a pH-dependent transition from a water molecule to a hydroxide anion. The mDCM is currently not parameterized to deal with metal ions. As such, we employ the same strategy that we used before with CheY (12), where constraints are added across the set of chelating residues to mimic the effect of metal binding within that region. The energy per chelating constraint is set such that the total binding energy is −4.5 kcal/mol per zinc ion, and the bridging water molecule is −3.0 kcal/mol.
Table 1.
Summary of the metallo-β-lactamase dataset
| Name | PDBID | Resolution (Å) |
R-Free | Structural similarity (Å)1 |
|---|---|---|---|---|
|
| ||||
| NDM-1 | 3ZR9 | 1.91 | 0.21 | -- |
| CcrA | 1HLK | 2.50 | 0.23 | 2.09 |
| BCII | 1BVT | 1.85 | 0.27 | 2.17 |
| VIM-4 | 2WHG | 1.90 | 0.25 | 2.33 |
| IMP-1 | 1DDK | 3.10 | 0.29 | 3.03 |
The pairwise α-carbon root mean square distance from each structure is calculated relative to NDM-1.
3.2. Backbone Flexibility is Mostly Conserved
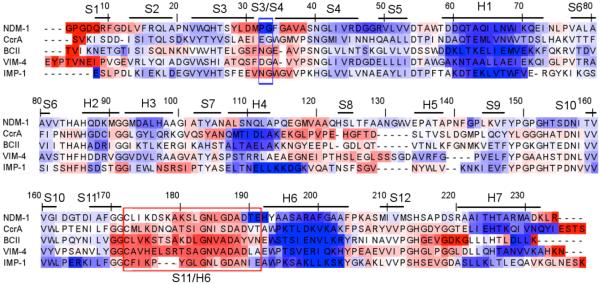
A multiple sequence alignment of the five MBLs color-coded by FI is provided in Fig. 2, and structural descriptions are provided in Fig. 3. The backbone flexibility properties are largely defined by secondary structure. For example, the most rigid portions of the protein uniformly correspond to α-helices, whereas the most flexible regions typically correspond to loops. Interestingly, compared to the Class-A BLs, the MBLs tend to be significantly less rigid, especially within the core regions of the protein. In fact, large portions of each structure are very close to isostatic. With the exception of the termini ends, the most consistently flexible portions of the protein correspond to the regions around the S3/S4 and S11/H6 loops (35), which is consistent with our results (cf. Fig. 2). This is particularly interesting because these two regions define the upper and lower borders of the active site. While there are several other structurally conserved loops within MBL, none are so flexibly conserved. Based on their active site proximity, this suggests that flexibility within these regions is critical to MBL function. Similar to our studies on earlier systems, no significant relationship could be found between the (dis)similarities within structure and backbone flexibility. This is an important result because it stresses, while backbone flexibility is in large part defined by secondary structure, it is largely impossible to relate quantitative differences to structural conformation based on the way rigidity propagates through the H-bond network (11).
Fig. 2.
Multiple sequence alignment of the five enzymes color-coded by backbone flexibility index (FI). Red indicates flexibility, whereas blue indicates rigidity. White corresponds to isostatic regions that are marginally rigid. Secondary structure information is provided above the alignment. In addition, the S3/S4 and S11/H6 loops are respectively indicated with blue and red boxes.
Fig. 3.
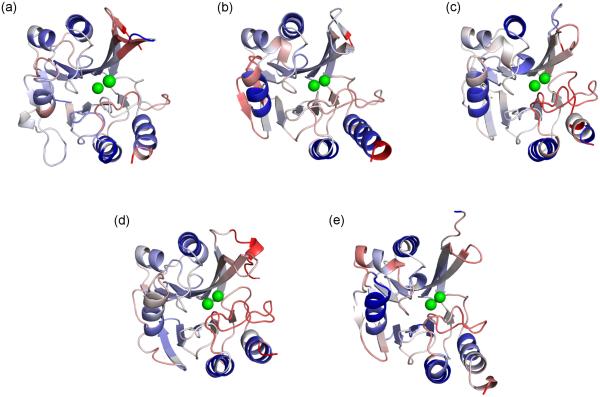
The five metallo-β-lactamase structures are colored by backbone flexibility index (FI), which are the identical values reported in Fig. 2. Red indicates flexibility, whereas blue indicates rigidity. White corresponds to isostatic regions that are marginally rigid. The structures are centered on the active site region, with the S3/S4 and S11/H6 loops just above and below, respectively, the metal ions. (a) NDM-1 (b) CcrA (c) BCII (d) VIM-4 (e) IMP-1.
3.3. Intramolecular Couplings are Highly Variable
Juxtaposed to the conservation within backbone flexibility, intramolecular couplings within the five MBL structures are visibly different (cf. Fig. 4) owing to the sensitivity within protein dynamics (15, 36). For example, the extensive blue coloring indicates that the NDM-1 structure is primarily composed of one large rigid cluster, with limited amounts of correlated flexibility. Conversely, much of N-terminal portion the VIM-4 structure is mostly co-rigid, whereas the C-terminal portion is primarily flexibly correlated. The three remaining structures have nearly equal amounts of rigidity and flexibility correlation. The observed variability is quite striking. Note that the large white swaths without any color shading correspond to gaps in the alignment.
Fig. 4.
Cooperativity Correlation (CC) plots reveal pairwise mechanical couplings within structure: (a) NDM-1 (b) CcrA (c) BCII (d) VIM-4 (e) IMP-1. That is, red indicates residue pairs that are flexibly correlated, whereas blue indicates residue pairs that are rigidly correlated. In each case, the matrices have been aligned so that all pixels are structurally equivalent. White indicates either residue pairs that are mechanically decoupled or gaps within the alignment. Notice that in NDM-1 the only appreciable correlated flexibility between loops occurs in the S3/S4 and S11/H6 regions. For reference, the structure of NDM-1 is shown in panel (f) with the S3/S4 (blue) and S11/H6 (red) loops highlighted.
Despite the large overall differences, many locally conserved features are observed within the CC plots. For example, the red swaths corresponding to loop L10 centered at alignment position 180 are observed in all five structures. Similarly, there is observable flexibility correlation between S3/S4 and S11/H6 loops in in all five structures. This is the only off-diagonal correlated flexibility that is observed in all five structures, which coupled with its active site location suggests that it is critical for enzyme function. Finally, the blue shading is always strongest in the N-terminal portion of the structure, with the region between residues 10 and ~100 being mostly co-rigid (with the exception of S3/S4 loop).
3.4. NDM-1
Taken together, the above results were described in the context of our collective results to represent what we have observed over several different comparative studies. However, due to the biomedical importance of NDM-1, we focus on what makes it special relative to the other MBLs. NDM-1 is a unique enzyme because it is functionally so promiscuous. It is active against nearly all known β-lactam antibiotics with exceptional efficiency (34). Moreover, the enzyme is functionally tolerant of substitutions in metal ion cofactors and changes in pH that cause the bridging water molecule to become deprotonated. Nevertheless, as discussed above, there is significantly more co-rigidity within NDM-1 compared to the other structures, and only the S3/S4 and S11/H6 loops are flexibly correlated. The backbone flexibility of the N-terminal half of L10 is significantly reduced in NDM-1 compared to the other structures, whereas the flexibility within the C-terminal portion of L10, which is closest to the active site rim, is similar to the others. Similarly, loop L11, strand β12, and a few other regions are significantly less flexible in NDM-1 as well. Taken together, these results indicate that flexibility and flexibility correlation within NDM-1 tends to be highly focused at the active site region, whereas flexibility and flexibility correlation is more widespread throughout the structure in the other four MBLs.
Another interesting difference is that there are a couple spots of intense rigidity within the NDM-1 structure. The first actually occurs within the S3/S4 loop. There is a sharp increase in flexibility due to the optimized β-turn that has both a proline in the second position and a glycine in the third. The NDM-1 H-bond network in the S3/S4 loop is among the strongest of the five enzymes, but the pronounced rigidity of this region is mostly attributed to proline residue therein, which is unique to NDM-1. While the NDM-1 β-turn is rigid, this does not necessarily imply that it is immobile. Flexibility identifies hinges, which are DOF that control motion elsewhere. In this case, the flexibility surrounding the β-turn allows the rigid body to move through space. This is analogous to a weight swinging from the motion provided by a pendulum. The pivot is flexible and immobile (assuming it is not moving through space), whereas the weight is simultaneously rigid and mobile. The second region of intense rigidity in the NDM-1 structure corresponds to the 310-capping helix, which is adjacent to the flexible portion of L10, which further underscores the isolation of flexibility within S3/S4 and S11/H6 loops of NDM-1.
It is tempting to speculate on the functional consequences of these observations. The fact that the S3/S4 and S11/H6 loops are flexibly correlated in all structures obviously suggests that it is clearly important to MBL function. No other correlation is strongly conserved across the family, which is not necessarily unexpected considering how different CC can be. Nevertheless, the unique isolation of correlated flexibility within NDM-1 does suggest that it is atypical compared to the other four. Based on this, we suggest that the distilling of the correlated flexibilities to the two active site loops contributes to the enzyme’s broad antibiotic resistance activity. However, it remains an open and potentially critical biomedical question of how the pronounced co-rigidity of NDM-1 is reconciled with its functional promiscuity (34).
4. Conclusions
Biology is an inherently comparative science. From Darwin’s finches to molecular sequence analysis, the paradigm of comparison and classification has driven biological insight. It follows that comparing the fundamental biophysical properties that govern structure and function within related proteins holds much promise for improved understanding. Unfortunately, however, the bulk of the experimental and computational methods needed to accurately describe these properties are prohibitive to large-scale analyses. To that end, we have developed the DCM in order to allow for robust and comprehensive comparisons of thermodynamic and dynamical properties within proteins. Over the past seven years, we have established the importance of our comparative QSFR characterizations on several different systems. Without exception, our results establish an intriguing mix of conservation and variation within QSFR properties. Typically, there is obvious similarity within protein structure backbone flexibility across members of the same family. Nevertheless, based on the complexity of the underlying H-bond network, there are simultaneously quantitative differences therein that are not obvious from structure. In contrast, the set of intramolecular couplings are much more sensitive to small perturbations, which can lead to dramatic differences across protein families. This result is entirely consistent with the growing appreciation for the sensitivity of allosteric response to mutation (12, 36-38) and other relatively minor perturbations (i.e., replacing one divalent metal ion for another (39)).
The MBLs characterized here are consistent with these general trends. The five structures are relatively conserved in shape and backbone flexibility. Yet, significant differences do occur across the set. Moreover, the similarities (and differences) within backbone flexibility do not simply parallel structural similarity. Nevertheless, manual analysis of the H-bond network can identify the origins of some of the difference, but not always due to the long-range nature of rigidity. While there are some conserved features within the CC plots, visually they are overall distinct. This is especially true in NDM-1, which is composed one large rigid cluster with correlated flexibility isolated in the active site S3/S4 and S11/H6 loops. Going forward, it will be important to determine if disruption of this isolated correlated flexibility has an impact on NDM-1’s broad-spectrum antibiotic resistance activity.
Acknowledgement
This work has been partially supported by NIH grants R01 GM073082 (to DJJ and DRL) and R15 GM101570 (to DRL). Key to the distance constraint model is the use of graph-rigidity algorithms, claimed in U.S. Patent 6,014,449, which has been assigned to the Board of Trustees Michigan State University. Used with permission.
References
- 1.Feynman R. The Feynman lectures on physics. Addison-Wesley Publishing Company; Reading, MA: 1963. [Google Scholar]
- 2.Henzler-Wildman KA, Lei M, Thai V, Kerns SJ, Karplus M, Kern D. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature. 2007;450:913–916. doi: 10.1038/nature06407. [DOI] [PubMed] [Google Scholar]
- 3.Livesay DR. Protein dynamics: dancing on an ever-changing free energy stage. Curr Opin Pharmacol. 2010;10:706–708. doi: 10.1016/j.coph.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs DJ. Recent Research Developments in Biophysics. Transworld Research Network; Trivandrum, India: 2006. Predicting protein flexibility and stability using network rigidity: a new modeling paradigm; pp. 71–131. [Google Scholar]
- 5.Jacobs DJ. Ensemble-based methods for describing protein dynamics. Curr Opin Pharmacol. 2010;10:760–769. doi: 10.1016/j.coph.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs DJ, Dallakyan S. Elucidating protein thermodynamics from the three-dimensional structure of the native state using network rigidity. Biophys J. 2005;88:903–915. doi: 10.1529/biophysj.104.048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livesay DR, Dallakyan S, Wood GG, Jacobs DJ. A flexible approach for understanding protein stability. FEBS Lett. 2004;576:468–476. doi: 10.1016/j.febslet.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs DJ, Livesay DR, Hules J, Tasayco ML. Elucidating quantitative stability/flexibility relationships within thioredoxin and its fragments using a distance constraint model. J Mol Biol. 2006;358:882–904. doi: 10.1016/j.jmb.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs DJ, Livesay DR, Mottonen JM, Vorov OK, Istomin AY, Verma D. Ensemble properties of network rigidity reveal allosteric mechanisms. In: Fenton AW, editor. Methods Mol Biol. Springer; 2012. pp. 279–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livesay DR, Jacobs DJ. Conserved quantitative stability/flexibility relationships (QSFR) in an orthologous RNase H pair. Proteins. 2006;62:130–143. doi: 10.1002/prot.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livesay DR, Huynh DH, Dallakyan S, Jacobs DJ. Hydrogen bond networks determine emergent mechanical and thermodynamic properties across a protein family. Chem Cent J. 2008;2:17. doi: 10.1186/1752-153X-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottonen JM, Jacobs DJ, Livesay DR. Allosteric response is both conserved and variable across three CheY orthologs. Biophys J. 2010;99:2245–2254. doi: 10.1016/j.bpj.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mottonen JM, Xu M, Jacobs DJ, Livesay DR. Unifying mechanical and thermodynamic descriptions across the thioredoxin protein family. Proteins. 2009;75:610–627. doi: 10.1002/prot.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma D, Jacobs DJ, Livesay DR. Predicting the melting point of human C-type lysozyme mutants. Curr Protein Pept Sci. 2010;11:562–572. doi: 10.2174/138920310794109210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma D, Jacobs DJ, Livesay DR. Changes in lysozyme flexibility upon mutation are frequent, large and long-ranged. PLoS Comput Biol. 2012;8:e1002409. doi: 10.1371/journal.pcbi.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savard PY, Gagne SM. Backbone dynamics of TEM-1 determined by NMR: evidence for a highly ordered protein. Biochemistry. 2006;45:11414–11424. doi: 10.1021/bi060414q. [DOI] [PubMed] [Google Scholar]
- 17.Verma D, Jacobs DJ, Livesay DR. Variations within class-A β-lactamase physiochemical properties reflect evolutionary, but not antibiotic specificity, patterns. 2013. In revision. [DOI] [PMC free article] [PubMed]
- 18.Cadag E, Vitalis E, Lennox KP, Zhou CL, Zemla AT. Computational analysis of pathogen-borne metallo beta-lactamases reveals discriminating structural features between B1 types. BMC Res Notes. 2012;5:96. doi: 10.1186/1756-0500-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma VK, Guleria R, Mehta V, Sood N, Singh SN. NDM-1 resistance: Fleming's predictions become true. Int J Appl Biol Pharm Tech. 2010;1:1244–1251. [Google Scholar]
- 20.Dill KA. Additivity principles in biochemistry. J Biol Chem. 1997;272:701–704. doi: 10.1074/jbc.272.2.701. [DOI] [PubMed] [Google Scholar]
- 21.Mark AE, van Gunsteren WF. Decomposition of the free energy of a system in terms of specific interactions. Implications for theoretical and experimental studies. J Mol Biol. 1994;240:167–176. doi: 10.1006/jmbi.1994.1430. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs DJ, Dallakyan S, Wood GG, Heckathorne A. Network rigidity at finite temperature: relationships between thermodynamic stability, the nonadditivity of entropy, and cooperativity in molecular systems. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:061109. doi: 10.1103/PhysRevE.68.061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs DJ, Rader AJ, Kuhn LA, Thorpe MF. Protein flexibility predictions using graph theory. Proteins. 2001;44:150–165. doi: 10.1002/prot.1081. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs DJ, Thorpe MF. Generic rigidity percolation: The pebble game. Phys Rev Lett. 1995;75:4051–4054. doi: 10.1103/PhysRevLett.75.4051. [DOI] [PubMed] [Google Scholar]
- 25.Vorov OK, Livesay DR, Jacobs DJ. Helix/coil nucleation: a local response to global demands. Biophys J. 2009;97:3000–3009. doi: 10.1016/j.bpj.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vorov OK, Livesay DR, Jacobs DJ. Nonadditivity in conformational entropy upon molecular rigidification reveals a universal mechanism affecting folding cooperativity. Biophys J. 2011;100:1129–1138. doi: 10.1016/j.bpj.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carfi A, Duee E, Galleni M, Frere JM, Dideberg O. 1.85 A resolution structure of the zinc (II) beta-lactamase from Bacillus cereus. Acta Crystallogr D Biol Crystallogr. 1998;54:313–323. doi: 10.1107/s0907444997010627. [DOI] [PubMed] [Google Scholar]
- 28.Concha NO, Janson CA, Rowling P, Pearson S, Cheever CA, Clarke BP, Lewis C, Galleni M, Frere JM, Payne DJ, Bateson JH, Abdel-Meguid SS. Crystal structure of the IMP-1 metallo beta-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry. 2000;39:4288–4298. doi: 10.1021/bi992569m. [DOI] [PubMed] [Google Scholar]
- 29.Payne DJ, Hueso-Rodriguez JA, Boyd H, Concha NO, Janson CA, Gilpin M, Bateson JH, Cheever C, Niconovich NL, Pearson S, Rittenhouse S, Tew D, Diez E, Perez P, De La Fuente J, Rees M, Rivera-Sagredo A. Identification of a series of tricyclic natural products as potent broad-spectrum inhibitors of metallo-beta-lactamases. Antimicrob Agents Chemother. 2002;46:1880–1886. doi: 10.1128/AAC.46.6.1880-1886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lassaux P, Traore DA, Loisel E, Favier A, Docquier JD, Sohier JS, Laurent C, Bebrone C, Frere JM, Ferrer JL, Galleni M. Biochemical and structural characterization of the subclass B1 metallo-beta-lactamase VIM-4. Antimicrob Agents Chemother. 2011;55:1248–1255. doi: 10.1128/AAC.01486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green VL, Verma A, Owens RJ, Phillips SE, Carr SB. Structure of New Delhi metallo-beta-lactamase 1 (NDM-1) Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67:1160–1164. doi: 10.1107/S1744309111029654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon JC, Myers JB, Folta T, Shoja V, Heath LS, Onufriev A. H++: a server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005;33:W368–371. doi: 10.1093/nar/gki464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponder JW, Case DA. Force fields for protein simulations. Adv Protein Chem. 2003;66:27–85. doi: 10.1016/s0065-3233(03)66002-x. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y, Cunningham MA, Mire J, Tesar C, Sacchettini J, Joachimiak A. NDM-1, the ultimate promiscuous enzyme: substrate recognition and catalytic mechanism. FASEB J. 2013 doi: 10.1096/fj.12-224014. fj.12-224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King DT, Worrall LJ, Gruninger R, Strynadka NCJ. New Delhi metallo-beta-lactamase: Structural insights into beta-lactam recognition and inhibition. J. Am. Chem. Soc. 2012;134:11363–11365. doi: 10.1021/ja303579d. [DOI] [PubMed] [Google Scholar]
- 36.Livesay DR, Kreth KE, Fodor AA. A critical evaluation of correlated mutation algorithms and coevolution within allosteric mechanisms. In: Fenton AW, editor. Methods Mol Biol. Springer; 2012. pp. 385–398. [DOI] [PubMed] [Google Scholar]
- 37.Conigrave AD, Franks AH. Allosteric activation of plasma membrane receptors--physiological implications and structural origins. Prog Biophys Mol Biol. 2003;81:219–240. doi: 10.1016/s0079-6107(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 38.Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 39.Fenton AW, Alontaga AY. The impact of ions on allosteric functions in human liver pyruvate kinase. Methods Enzymol. 2009;466:83–107. doi: 10.1016/S0076-6879(09)66005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]