Abstract
Background
Regulation of MMP expression by activation of mTOR signalling has been demonstrated for several tumor types, but has thus far not been confirmed in gastric cancer.
Findings
The study compromised 128 patients who underwent gastric resection for cancer (66.4 % male; 86 intestinal, 42 diffuse type). Immunohistochemical staining of MMPs was performed to analyse the topographical pattern of MMP expression at the tumor center and the invasive front, respectively. MMP2 showed higher expression at the invasive front compared to the tumor center, whereas MMP7 staining scores were higher in the tumor center, and there was no difference for MMP9. The expression of p-mTOR was higher in the tumor center than at the invasive front, with a similar trend for mTOR. For intestinal type gastric cancer there was a weak correlation of MMP9 with expression of mTOR in the tumor center. Otherwise, there was no correlation of the MMPs with mTOR. By treatment of MKN45 gastric cancer cells with rapamycin, a reduction of p-mTOR in the Western blot was achieved; however, expression of MMPs remained unaffected.
Conclusions
Expression of MMP2 and MMP7 in gastric cancer is not associated with mTOR, MMP9 expression might be related to mTOR signalling in a subset of tumors.
Electronic supplementary material
The online version of this article (doi:10.1186/s13000-015-0449-z) contains supplementary material, which is available to authorized users.
Keywords: Gastric cancer, mTOR, MMP2, MMP7, MMP9
Findings
The degradation of the extracellular matrix by matrix metalloproteinases (MMPs) is essential for invasive behaviour of gastric cancer [1–3]. Expression of MMPs can be induced by specific growth factors [4, 5], and depending on the cellular energy level growth factor dependent processes can be regulated by mammalian target of rapamycin (mTOR) related signalling [6]. Thus, also mTOR activation has a role in tumor invasion and metastatic spread [7, 8] and, unsurprisingly, its expression is identified in up to 64 % of gastric cancers [7–9]. The link between growth factor dependent activation of PI3K/Akt/mTOR signalling and downstream up-regulation of MMP gene expression has been shown in vitro for several cancer types [4, 10–12]. However, a direct association between mTOR activation and MMP expression has not been shown for gastric cancer so far. MMP2, MMP7 and MMP9 have been most extensively investigated in gastric cancer, but never previously in a direct comparison and in association with mTOR expression. The aim of this study was to investigate whether the expression of MMP2, MMP7, and MMP9 in humans is associated with the expression of mTOR in its “naïve” and its phosphorylated (active) form in different topographical regions of gastric adenocarcinomas. Separate assessment of the tumor center and the invasive front of the cancer has been performed to evaluate the involvement of this potential regulatory mechanism for invasive growth of gastric adenocarcinomas.
The clinicopathological characteristics of patients who underwent gastrectomy for gastric cancer between 1997 and 2009 were retrospectively identified from the archives of the Magdeburg University Hospital (Table 1). Patients with neoadjuvant treatment and with adenocarcinoma associated with Barrett’s metaplasia and/or location proximal at the esophagogastric junction (Siewert type 1) were excluded from the analysis. For statistical reasons, tumors showing a mixed type according to the Laurén classification (n = 14) or cancers with mucinous phenotype (n = 2) were combined with the group of diffuse type carcinomas (n = 26). Patients with diffuse type gastric cancer below the age of 50 years at diagnosis or who presented with a positive family history were assessed for mutations of the CDH1 gene, which was negative in all respective cases. Finally, paraffin embedded tissue for immunohistochemistry (IHC) was retrieved for 128 patients (Table 1). The study was approved by the ethics committee of our institution (Ref. 2004–98) and conducted according to the ethical guidelines of the declaration of Helsinki as revised in 1989. In an additional proof-of-principle approach, we measured the expression of MMP2, MMP7 and MMP9 in MKN45 gastric cancer cells before and after treatment with the mTOR inhibitor rapamycin to investigate the putative link between mTOR signalling and MMP expression in gastric cancer. MMP expression has been assessed by RT-PCR. The activity of mTOR signalling has been assessed by Western blot for the main downstream target of mTOR the P70S6K which is only active in its phosphorylated form (p-P70S6K). Please see Additional file 1 (supplementary methods) for further details. For statistical comparisons, non-parametrical tests have been applied using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). For group comparisons the Mann Whitney U-test was used, Wilcoxon's sign rank test for matched pair comparison between tumor center and invasion front. For correlation analysis Spearman’s rank correlation test was applied. For comparison of categorical data Fisher's exact test was applied. For all tests a two-sided significance level of p < 0.05 was considered significant.
Table 1.
Demographic and clinicopathological characteristics of the main study population
| Parameters | Intestinal (n = 86) | Diffuse (n = 42) | Total (N = 128) | p-value | |
|---|---|---|---|---|---|
| Age in years (median, IQR) | 79 (72–85) | 78 (68–84) | 78 (70–78) | 0.239 | |
| Sex (male) | 61 (70.9 %) | 24 (57.1 %) | 85 (66.4 %) | 0.163 | |
| T-stage | T1 | 13 (15.1 %) | 3 (7.1 %) | 16 (12.5 %) | 0.328 |
| T2 | 36 (41.9 %) | 15 (35.7 %) | 51 (39.8 %) | ||
| T3 | 31 (36.0 %) | 22 (52.4 %) | 53 (41.4 %) | ||
| T4 | 6 (7.0 %) | 2 (4.8 %) | 8 (6.2 %) | ||
| N-stage* | Positive (N1-3) | 57 (66.3 %) | 39 (92.9 %) | 96 (75.0 %) | 0.001 |
| M1 | Positive (M1) | 29 (33.7 %) | 20 (47.6 %) | 49 (38.3 %) | 0.175 |
| Grading* | G1 | 6 (7.0 %) | 0 (0) | 6 (4.7 %) | <0.001 |
| G2 | 45 (52.3 %) | 1 (2.4 %) | 46 (35.9 %) | ||
| G3 | 35 (40.7 %) | 41 (97.6 %) | 76 (59.4 %) | ||
| Localisation* | Cardia | 30 (34.9 %) | 6 (14.3 %) | 36 (28.1 %) | 0.042 |
| Corpus | 33 (38.4 %) | 23 (54.8 %) | 56 (43.8 %) | ||
| Antrum | 23 (26.7 %) | 13 (31.0 %) | 36 (28.1 %) |
Comparison between intestinal and diffuse type tumors was done by Fisher's exact test with significant differences (p < 0.05) marked by an asterisk. IQR: interquartile range; Grading: G1: well differentiated, G2: moderately differentiated, G3: poorly differentiated
IHC staining reaction of the tumor was present in 44-60 % for MMP2, MMP7, and MMP9, as well as in 96 % and 80 % for mTOR and p-mTOR, respectively (Additional file 2: Figure S1). As assessed by the immune-reactivity scores [13], only MMP2 was more markedly expressed at the invasive front, whereas MMP9 was homogenously expressed throughout the invasive front and the tumor center (Fig. 1, Table 2). In contrast, MMP7 staining was more pronounced in the tumor center relative to the invasive front. Both mTOR and p-mTOR staining was more pronounced in the tumor center than at the invasive front (Table 2).
Fig. 1.
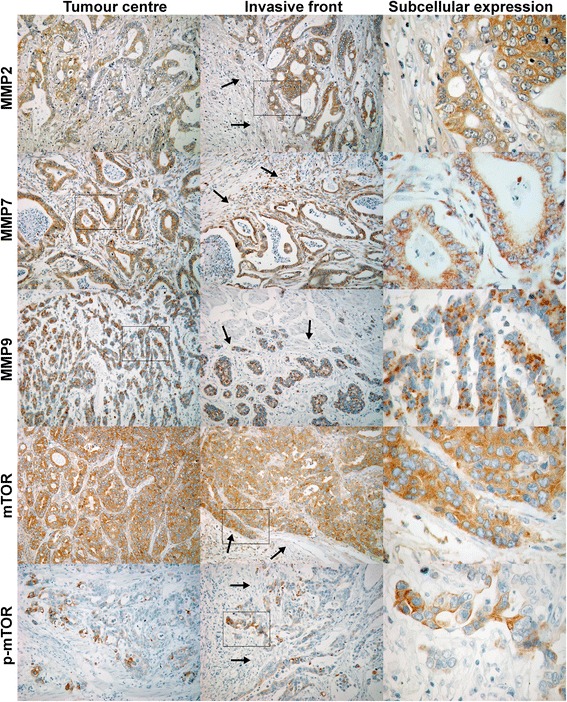
Immunohistochemical staining at the tumor center and the invasive front. Exemplary staining of MMP2, MMP7, MMP9, as well as mTOR and p-mTOR in the tumor center, at the invasive front (100×), and subcellular expression with larger magnification (400×). Arrows are marking the marginal zone of the tumor at the invasive front. Squares are marking the magnified area for each panel
Table 2.
Immune-reactivity score for expression of mTOR, p-mTOR, MMP2, MMP7, MMP9 in the tumor center and at the invasive front of type gastric cancer
| Targets | Intestinal type (n = 86) | Diffuse type (n = 42) | Overall (N = 128) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor center | Invasive front | p-value | Tumor center | Invasive front | p-value | Tumor center | Invasive front | p-value | |
| MMP2 | 4.10 ± 5.042 | 7.65 ± 7.782 | <0.001* | 2.93 ± 3.925 | 5.40 ± 6.688 | 0.005* | 3.72 ± 4.721 | 6.91 ± 7.490 | <0.001* |
| MMP7 | 5.43 ± 8.345 | 4.48 ± 7.680 | 0.008* | 5.10 ± 8.798 | 4.50 ± 7.613 | (0.128) | 5.32 ± 8.463 | 4.48 ± 7.628 | 0.002* |
| MMP9 | 4.57 ± 7.362 | 3.97 ± 7.058 | (0.227) | 5.62 ± 7.821 | 4.71 ± 6.656 | (0.144) | 4.91 ± 7.501 | 4.21 ± 6.912 | (0.097) |
| mTOR | 10.95 ± 7.543 | 9.79 ± 8.778 | 0.025* | 8.67 ± 6.091 | 9.12 ± 6.463 | (0.589) | 10.20 ± 7.156 | 9.57 ± 8.072 | (0.127) |
| p-mTOR | 3.42 ± 4.185 | 2.69 ± 4.610 | 0.008* | 2.13 ± 2.662 | 2.76 ± 4.667 | (0.545) | 3.00 ± 3.792 | 2.71 ± 4.610 | 0.013* |
Comparison between tumor center and invasive front has been done by the Mann–Whitney U-test with significant differences (p < 0.05) marked by an asterisk. Values are given as mean and standard deviation
The immune-reactivity scores for the expression of mTOR and p-mTOR as well as for MMP2, MMP7, and MMP9 in the tumor center correlated each with its expression at the invasive front (p < 0.001; Additional file 3: Figure S2a-e). Only for intestinal type tumors, there was a correlation of mTOR with MMP9 expression both in the tumor center (r = 0.251, p = 0.020) and at the invasive front (r = 0.254, p = 0.018; Additional file 4: Figure S3a). Otherwise, there was no association between mTOR and MMP2 or MMP7 as assessed by the IHC analysis. Staining of mTOR in the tumor center correlated with p-mTOR (r = 0.195, p = 0.028; Additional file 4: Figure S3b), and there was a positive association MMP2 with MMP9 at the invasive front (r = 0.214, p = 0.015; Additional file 4: Figure S3c).
mTOR (p = 0.003) and p-mTOR (p = 0.02) staining was associated with stage of disease, with lower staining scores in the tumor center of advanced stage cancers (T3 and T4) compared to early disease (T1 and T2). However, the immune-reactivity score for mTOR was higher in the tumor center of patients with evidence of distant metastases (p = 0.01), and MMP7 was more highly expressed in the tumors of patients with nodal involvement (tumor center: p = 0.01, invasive front: p = 0.019; data not shown). Otherwise there was no association of MMP staining with any tumor-associated parameter.
In a parallel proof-of-principle approach, we treated MKN45 gastric cancer cells with rapamycin to investigate the effect of mTOR inhibition on MMP expression. Rapamycin treatment led to an effective inhibition of mTOR signalling, mirrored by a reduction of p-P70S6K, the main downstream target of mTOR signalling (Additional file 5: Figure S4). Transcript levels of MMP2, MMP7 and MMP9 were clearly expressed in the cells at baseline and were not systematically affected by mTOR inhibition, as assessed by RT-PCR (data not shown).
To our knowledge, this is the first study that analyses the association of the expression of three specific MMPs and mTOR in its native and in its activated, phosphorylated form in human gastric cancer tissue. The expression pattern for MMP2 was consistent with previous reports [14, 15]. Surprisingly, MMP7 showed higher staining scores in the tumor center, but it must be taken into account that we scored only positive staining within the gastric cancer cells and not of stromal components which can also express MMP7 [16]. Expression of MMP2, MMP7 and MMP9 could be confirmed in the majority of gastric cancers, but there was no significant correlation with the presence of either mTOR or p-mTOR. The association of MMP9 with mTOR was only weak in intestinal type cancers, suggesting a probable interaction of other regulatory mechanisms, such as pathways that respond to inflammatory stimuli [11, 12, 17, 18]. There are further alternative mechanisms that may interfere at this level such as MMP2 being capable of activating MMP9 [19, 20]. In a previous study, MMP2 and MMP7 expression were reduced by the mTOR inhibitor rapamycin in human gastric NUGC4 cells that have been stimulated with CXCL12 [21]. MKN45 cells were chosen for this work because that are derived from a poorly differentiated gastric cancer and express both the respective MMPs and mTOR at baseline without the need for stimulation or transfection. Since both mTOR and the MMPs were expressed in MKN45 and the negative findings supported the IHC data showing no association between mTOR and MMP expression in gastric cancer no additional cell line validation was undertaken. The lack of an association of mTOR signalling and MMP expression might be due to the interplay with pathways that are involved in regulation of mucosal inflammation which are dependent on the very complex tissue microenvironment within gastric cancer. As mentioned above for MMP9, NFκB-dependent induction in response to inflammatory stimuli has been reported [11, 17, 18, 20, 22, 23], and MMP9 expression is raised in cag-dependent manner in the course of H. pylori infection. H. pylori induced inflammation can induce both MMP9 and MMP7 expression in the gastric mucosa mediated by tissue macrophages [24–27]. Furthermore, mTOR seems to have a stage-dependent impact, with the expression being higher in earlier stages of gastric carcinogenesis; however, our cohort mostly consisted of advanced stage cancers.
It has been reported that expression and functional activity of both mTOR and specific MMPs are associated with less favourable prognosis in gastric carcinoma, with overall heterogeneous results and stronger evidence for mTOR [3, 7, 28–31]. None of the parameters analysed were associated with overall survival in our cohort (data not shown). However, due to the retrospective nature of the study, survival data could not be gathered for all patients.
In summary, an association between the presence of MMP2 and MMP7 proteins and (p-)mTOR expression in gastric cancer could not be confirmed. A correlation of MMP9 with mTOR expression in intestinal type cancers was only of weak character and doesn't support mTOR as being the main regulating factor. Future studies should address alternative pathways and consider the influence of the inflammatory microenvironment of the surrounding mucosa.
Acknowledgements
The study was in part supported by a grant from the BMBF (BMBF-0315905D) in the frame of ERA-Net PathoGenoMics project to PM and institutional funds. We thank Ursula Stolz, Simone Philipsen and Ingrid Hegenbarth for their substantial contributions concerning the sample processing, RNA extraction and immunohistochemical staining.
Abbreviations
- H. pylori
Helicobacter pylori
- IHC
immunohistochemistry
- IRS
immune-reactivity score
- MMP
matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- RT-PCR
reverse transcription (real time) polymerase chain reaction
Additional files
(DOCX 25 kb)
Relative expression of mTOR and MMPs. Partition of samples with positive staining of mTOR, p-mTOR, MMP2, MMP7 and MMP9 each at the tumor center and at the invasive front. Comparison of both localisations have been done by Fisher's exact test. Significant differences (p<0.05) are marked with an asterisk. (TIF 447 kb)
Correlation of staining scores between tumor center and invasive front. Displayed are the patient-matched paired IRS for the staining reaction at the tumor center and the invasive front for (a) MMP2, (b) MMP7, (c) MMP9, (d) mTOR and (e) p-mTOR. Correlation of the IRS for tumor center and invasive front was done by Spearman's rank correlation test with p<0.05 considered as significant. (TIF 4658 kb)
Correlation of staining scores between different factors. Displayed are the patient-matched paired IRS for MMP9 and mTOR at the tumor center of intestinal type cancers (a), for mTOR and p-mTOR at the tumor center (b), and for MMP2 and MMP9 at the invasive front (c). Correlation analysis was done by Spearman's rank correlation test with p<0.05 considered as significant. (TIF 1937 kb)
mTOR inhibition by rapamycin in MKN45 gastric cancer cells. Western Blot of expression of p-P70S6K1, P70S6K1 and β-actin in MKN45 cells that have been treated with rapamycin (RAPA) for each 24h, 48h and 72h (a). Corresponding results of the PCR analysis of the transcript content MMP2, MMP7 and MMP9. There was no significant effect on MMP expression by treatment with rapamycin. For MMP9 only assessment after 48 hours gave consistent results. UC: untreated controls; DMSO: treatment with DMSO only. (TIF 2341 kb)
Footnotes
Competing interests
None of the authors has any conflict of interest to declare related to the data presented in this manuscript.
Authors’ contribution
JB designed the study with support from MS and PM, coordinated the IHC assessment and wrote major parts of this manuscript. TS performed the major part of the experimental and laboratory work for this study, and designed the graphs as part of her doctorial thesis. CL was involved in the laboratory work, especially sample preparation. AL and TW were involved in sample processing and the in vitro experiments. DJ and FM undertook the sample collection and clinicopathological description of the study cohort. MV undertook the immunohistochemical staining and assessment of the staining scores. EBL, MV and PM provided substantial revisions to first drafts of this manuscript that was further edited by the other co-authors.
References
- 1.Kubben FJ, Sier CF, van Duijn W, Griffioen G, Hanemaaijer R, van de Velde CJ, et al. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br J Cancer. 2006;94(7):1035–1040. doi: 10.1038/sj.bjc.6603041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nomura H, Fujimoto N, Seiki M, Mai M, Okada Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996;69(1):9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Zhu GY, Gao HY, Zhao SP, Xue Y. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric adenocarcinoma. J Surg Oncol. 2010;103(3):243–247. doi: 10.1002/jso.21824. [DOI] [PubMed] [Google Scholar]
- 4.Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem. 2004;279(19):19683–19690. doi: 10.1074/jbc.M313145200. [DOI] [PubMed] [Google Scholar]
- 5.Adachi Y, Li R, Yamamoto H, Min Y, Piao W, Wang Y, et al. Insulin-like growth factor-I receptor blockade reduces the invasiveness of gastrointestinal cancers via blocking production of matrilysin. Carcinogenesis. 2009;30(8):1305–1313. doi: 10.1093/carcin/bgp134. [DOI] [PubMed] [Google Scholar]
- 6.Populo H, Lopes JM, Soares P. The mTOR Signalling Pathway in Human Cancer. Int J Mol Sci. 2012;13(2):1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res. 2009;15(5):1821–1829. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]
- 8.Murayama T, Inokuchi M, Takagi Y, Yamada H, Kojima K, Kumagai J, et al. Relation between outcomes and localisation of p-mTOR expression in gastric cancer. Br J Cancer. 2009;100(5):782–788. doi: 10.1038/sj.bjc.6604915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu DZ, Geng QR, Tian Y, Cai MY, Fang XJ, Zhan YQ, et al. Activated mammalian target of rapamycin is a potential therapeutic target in gastric cancer. BMC Cancer. 2010;10:536. doi: 10.1186/1471-2407-10-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou HY, Wong AS. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology. 2006;147(5):2557–2566. doi: 10.1210/en.2005-1404. [DOI] [PubMed] [Google Scholar]
- 11.Ko HS, Lee HJ, Kim SH, Lee EO. Piceatannol suppresses breast cancer cell invasion through the inhibition of MMP-9: involvement of PI3K/AKT and NF-kappaB pathways. J Agric Food Chem. 2012;60(16):4083–4089. doi: 10.1021/jf205171g. [DOI] [PubMed] [Google Scholar]
- 12.Ji Y, Yang X, Li J, Lu Z, Li X, Yu J, et al. IL-22 promotes the migration and invasion of gastric cancer cells via IL-22R1/AKT/MMP-9 signaling. Int J Clin Exp Pathol. 2014;7(7):3694–3703. [PMC free article] [PubMed] [Google Scholar]
- 13.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8(3):138–140. [PubMed] [Google Scholar]
- 14.Zhao F, Zhang Q, Kang C, Cui X, Wang T, Xu P, et al. Suppression of matrix metalloproteinase-9 expression by RNA interference inhibits SGC7901 gastric adenocarcinoma cell growth and invasion in vitro and in vivo. Med Oncol. 2010;27(3):774–784. doi: 10.1007/s12032-009-9285-x. [DOI] [PubMed] [Google Scholar]
- 15.David L, Nesland JM, Holm R, Sobrinho-Simoes M. Expression of laminin, collagen IV, fibronectin, and type IV collagenase in gastric carcinoma. An immunohistochemical study of 87 patients. Cancer. 1994;73(3):518–527. doi: 10.1002/1097-0142(19940201)73:3<518::AID-CNCR2820730305>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.McCaig C, Duval C, Hemers E, Steele I, Pritchard DM, Przemeck S, et al. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology. 2006;130(6):1754–1763. doi: 10.1053/j.gastro.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Mori N, Sato H, Hayashibara T, Senba M, Geleziunas R, Wada A, et al. Helicobacter pylori induces matrix metalloproteinase-9 through activation of nuclear factor kappaB. Gastroenterology. 2003;124(4):983–992. doi: 10.1053/gast.2003.50152. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wu H, Wu X, Bian Z, Gao Q. Interleukin 17A promotes gastric cancer invasiveness via NF-kappaB mediated matrix metalloproteinases 2 and 9 expression. PLoS One. 2014;9(6):e96678. doi: 10.1371/journal.pone.0096678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2) Cancer Res. 1995;55(12):2548–2555. [PubMed] [Google Scholar]
- 20.Liu J, Liu Q, Wan Y, Zhao Z, Yu H, Luo H, et al. Osteopontin promotes the progression of gastric cancer through the NF-kappaB pathway regulated by the MAPK and PI3K. Int J Oncol. 2014;45(1):282–290. doi: 10.3892/ijo.2014.2393. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto I, Koizumi K, Tatematsu M, Minami T, Cho S, Takeno N, et al. Blocking on the CXCR4/mTOR signalling pathway induces the anti-metastatic properties and autophagic cell death in peritoneal disseminated gastric cancer cells. Eur J Cancer. 2008;44(7):1022–1029. doi: 10.1016/j.ejca.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 22.Das G, Shiras A, Shanmuganandam K, Shastry P. Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. Mol Carcinog. 2011;50(6):412–423. doi: 10.1002/mc.20723. [DOI] [PubMed] [Google Scholar]
- 23.Osman B, el Akool S, Doller A, Muller R, Pfeilschifter J, Eberhardt W. Differential modulation of the cytokine-induced MMP-9/TIMP-1 protease-antiprotease system by the mTOR inhibitor rapamycin. Biochem Pharmacol. 2011;81(1):134–143. doi: 10.1016/j.bcp.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Chung WC, Jung SH, Lee KM, Paik CN, Kawk JW, Jung JH, et al. The detection of Helicobacter pylori cag pathogenicity islands (PAIs) and expression of matrix metalloproteinase-7 (MMP-7) in gastric epithelial dysplasia and intramucosal cancer. Gastric Cancer. 2010;13(3):162–169. doi: 10.1007/s10120-010-0552-5. [DOI] [PubMed] [Google Scholar]
- 25.Wroblewski LE, Noble PJ, Pagliocca A, Pritchard DM, Hart CA, Campbell F, et al. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci. 2003;116(Pt 14):3017–3026. doi: 10.1242/jcs.00518. [DOI] [PubMed] [Google Scholar]
- 26.Bergin PJ, Sicheng W, Qiang PH, Marianne QJ. Secretion of matrix metalloproteinase-9 by macrophages, in vitro, in response to Helicobacter pylori. FEMS Immunol Med Microbiol. 2005;45(2):159–169. doi: 10.1016/j.femsim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Bebb JR, Letley DP, Thomas RJ, Aviles F, Collins HM, Watson SA, et al. Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag dependent manner. Gut. 2003;52(10):1408–1413. doi: 10.1136/gut.52.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long ZW, Wang JL, Wang YN. Matrix metalloproteinase-7 mRNA and protein expression in gastric carcinoma: a meta-analysis. Tumour Biol. 2014;35(11):11415–11426. doi: 10.1007/s13277-014-2441-8. [DOI] [PubMed] [Google Scholar]
- 29.Shen W, Xi H, Wei B, Chen L. The prognostic role of matrix metalloproteinase 2 in gastric cancer: a systematic review with meta-analysis. J Cancer Res Clin Oncol. 2014;140(6):1003–1009. doi: 10.1007/s00432-014-1630-6. [DOI] [PubMed] [Google Scholar]
- 30.Byeon SJ, Han N, Choi J, Kim MA, Kim WH. Prognostic implication of TSC1 and mTOR expression in gastric carcinoma. J Surg Oncol. 2014;109(8):812–817. doi: 10.1002/jso.23585. [DOI] [PubMed] [Google Scholar]
- 31.Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465(1):25–33. doi: 10.1007/s00428-014-1588-4. [DOI] [PubMed] [Google Scholar]


