Abstract
Background
Hormone receptor-positive, human epidermal growth factor receptor-2-negative (HR+/HER2−) is the most common type of metastatic breast cancer (mBC). While mBC patients generally have poor prognosis with limited progression-free survival (PFS) and overall survival (OS), those with multiple metastatic sites may have even worse clinical outcomes due to multiple organ involvement. This study aimed to compare clinical outcomes including PFS, time on treatment (TOT), and OS between HR+/HER2− mBC patients with multiple metastases versus those with a single metastasis in a real-world clinical setting.
Methods
This was a retrospective chart review study of postmenopausal HR+/HER2− mBC women who had failed a non-steroidal aromatase inhibitor in the adjuvant or metastatic setting and initiated a new treatment for mBC between 07/01/2012 and 04/15/2013. Patients were classified to one of two study groups (multiple metastases or single metastasis) based on the number of non-lymph-node metastases at the initiation of the new treatment. PFS, TOT and OS were compared between the two groups using Kaplan–Meier analyses and multivariable Cox proportional hazard models adjusting for patient disease and treatment characteristics. Separate Cox models were conducted including models with an interaction term between line of therapy and study group to assess the impact of multiple metastases on clinical outcomes across different lines of therapy.
Results
A total of 699 patient charts were collected, including 291 patients with multiple metastases and 408 single metastasis patients. Worse performance status and a higher proportion of prior chemotherapy for mBC were observed among patients with multiple metastases. Overall, patients with multiple metastases had significantly shorter PFS [adjusted hazard ratio (HR) = 1.55, 95 % confidence interval (CI) 1.21–1.98], TOT (adjusted HR = 1.33, 95 % CI 1.05–1.67), and OS (adjusted HR = 1.77, 95 % CI 1.15–2.74) than single metastasis patients. Similar outcomes were observed in each line of therapy.
Conclusions
Among HR+/HER2− mBC patients, patients with multiple metastases had significantly shorter PFS, TOT, and OS than single metastasis patients, highlighting the substantial clinical burden and unmet need for more efficacious treatments for the former group of patients.
Keywords: Metastatic breast cancer, HR+/HER2−, Multiple metastases, Overall survival, Progression-free survival, Time on treatment
Background
Breast cancer (BC) is the leading cancer among women [1]. In the US, approximately 230,000 new BC cases were diagnosed in 2014 and 40,000 people died of BC in the same year [2]. The majority of patients are diagnosed at the early stage, for which treatment is generally effective, with a 5-year survival rate nearing 90 % [2]. However, BC also has a high recurrence rate, with up to 40 % of patients eventually progressing to metastatic BC (mBC) [3, 4]. In addition, around 5 % of BC cases are diagnosed at the metastatic stage [2]. Compared to early stage BC, mBC is associated with a much worse prognosis, with a 5-year survival rate of only 25 % [2].
MBC is categorized in several histological subtypes, based on the presence, absence, or overexpression of certain cell receptors [4, 5]. Hormone receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2−) mBC accounts for the majority of mBC cases [5]. Treatment guidelines for HR+/HER2− mBC recommend the use of three consecutive lines of endocrine therapy before the initiation of chemotherapy, or an earlier switch to chemotherapy in patients with visceral symptoms or rapidly progressing/life-threatening disease [6]. Recently, new targeted therapies are emerging and may revolutionize the treatment of mBC [7, 8]. For HR+/HER2− mBC in particular, everolimus in combination with exemestane had superior efficacy in extending progression-free survival (PFS) compared to exemestane alone in the BOLERO-2 trial [9]. Studies also suggest that everolimus is associated with longer PFS and time on treatment (TOT) in postmenopausal women with HR+/HER2− mBC [10, 11]. Palbociclib is another recently approved targeted therapy with demonstrated superior efficacy compared to endocrine therapy alone [12]. In addition, other targeted therapies with various mechanisms of action are under development, and some may become available in the near future.
With the rapid changes in the treatment landscape, the main question faced by physicians and payers is identifying the right patient population for these novel treatments with superior efficacies. Patients who have poor prognosis have a greater unmet need and may benefit more from these treatments. Several real-world studies have examined prognostic factors in mBC; among these, the number of metastases has been shown to be a significant predictor of overall survival (OS) [13–18]. However, these previous studies were all based on a single-center location and have generally focused only on OS [13–18]. While OS is an important outcome in mBC, other outcomes would also be of interest from a clinical perspective, such as PFS and TOT. In addition, a study covering different geographic regions in the US would be more informative and less subject to practices specific to individual centers.
To better understand clinical outcomes associated with multiple metastases, the current study was undertaken to compare PFS, TOT, and OS between HR+/HER2− mBC patients with multiple versus single metastases who were treated in community-based oncology practices in the US. The findings of this study could help us better assess the unmet need in HR+/HER2− mBC among patients with multiple metastases in the US. The study provided updated evidence on mBC populations with greater unmet needs that can inform decision-makers on potential targets for novel treatments in lieu of the rapidly changing landscape of mBC treatment.
Results
Baseline characteristics
A total of 188 physicians contributed 699 patient charts, including 408 patients with a single site and 291 patients with multiple sites of metastasis (Table 1). Physicians were mostly male (>70 %), practicing in a small/intermediate practice setting of 2–9 physicians (>70 %), and had more than 5 years of experience (>90 %). At index treatment initiation, the median patient age was 65 years for the multiple metastases group versus 64 years for the single metastasis group (Table 1). Both patient groups had similar median duration from initiation of the last adjuvant endocrine therapy to mBC diagnosis (19.2 vs. 18.9 months, respectively) (Table 1). The median follow-up duration from index treatment initiation was also similar between the two groups: 16.3 months among patients with multiple metastases and 16.7 months among patients with a single metastasis (p = 0.239) (Table 1).
Table 1.
Patient baseline characteristics
| Baseline characteristicsa | Patients with multiple metastases | Patients with a single metastasis | P-value† |
|---|---|---|---|
| N = 291 | N = 408 | ||
| Age at BC diagnosis, median years (range) | 61.0 (35.0, 83.0) | 61.0 (33.0, 90.0) | 0.785 |
| Age at index treatment initiation, median years (range) | 65.0 (38.0, 86.0) | 64.0 (33.0, 91.0) | 0.878 |
| Menopausal status at the first BC diagnosisb, n (%) | |||
| Postmenopausal | 169 (58.1) | 238 (58.3) | 0.946 |
| Race, n (%) | |||
| White | 155 (53.3) | 254 (62.3) | 0.017* |
| Non-white | 136 (46.7) | 154 (37.7) | |
| Insurance plan type at mBC diagnosis, n (%) | |||
| Commercial/private insurance | 151 (51.9) | 217 (53.2) | 0.460 |
| Medicare only | 115 (39.5) | 166 (40.7) | |
| Others | 25 (8.6) | 25 (6.1) | |
| Type of index treatment, n (%) | |||
| Endocrine therapyc | 196 (67.4) | 356 (87.3) | <0.001* |
| Chemotherapyd | 95 (32.6) | 52 (12.7) | |
| Line of index treatment, n (%) | |||
| First line | 81 (27.8) | 206 (50.5) | <0.001* |
| Second line | 97 (33.3) | 97 (23.8) | |
| Third line and above | 113 (38.8) | 105 (25.7) | |
| mBC type, n (%) | |||
| Recurrent patients with adjuvant endocrine therapy | 184 (63.2) | 290 (71.1) | 0.033* |
| Recurrent patients without adjuvant endocrine therapy | 39 (13.4) | 54 (13.2) | |
| De novo | 68 (23.4) | 64 (15.7) | |
| Number of non-lymph-node metastatic sites at mBC diagnosis, n (%) | |||
| 1 | 58 (19.9) | 228 (55.9) | <0.001* |
| 2 | 75 (25.8) | 132 (32.4) | |
| 3 | 132 (45.4) | 8 (2.0) | |
| 4 | 20 (6.9) | 2 (0.5) | |
| Sites of metastatic disease at mBC diagnosis, n (%) | |||
| Bone | 188 (64.6) | 231 (56.6) | 0.034* |
| Liver | 110 (37.8) | 53 (13.0) | <0.001* |
| Lung | 151 (51.9) | 90 (22.1) | <0.001* |
| Any visceral metastasese | 228 (78.4) | 143 (35.0) | <0.001* |
| Brain | 3 (1.0) | 2 (0.5) | 0.654 |
| Other | 4 (1.4) | 5 (1.2) | 1.000 |
| Number of non-lymph-node metastatic sites at index treatment initiation, n (%) | |||
| 1 | 0 (0.0) | 374 (91.7) | <0.001* |
| 2 | 208 (71.5) | 0 (0.0) | |
| 3 | 79 (27.1) | 0 (0.0) | |
| 4 | 4 (1.4) | 0 (0.0) | |
| Sites of metastatic disease at index treatment initiation, n (%) | |||
| Bone | 224 (77.0) | 231 (56.6) | <0.001* |
| Liver | 177 (60.8) | 47 (11.5) | <0.001* |
| Lung | 215 (73.9) | 87 (21.3) | <0.001* |
| Any visceral metastasese | 288 (99.0) | 139 (34.1) | <0.001* |
| Brain | 10 (3.4) | 2 (0.5) | 0.005* |
| Other | 6 (2.1) | 2 (0.5) | 0.073 |
| Adjusted Charlson Comorbidity Index (CCI)f at index treatment initiation, median (range) | 0.0 (0.0, 8.0) | 0.0 (0.0, 6.0) | 0.002* |
| ECOG performance status at index treatment initiation, n (%) | |||
| 0 | 49 (16.8) | 134 (32.8) | <0.001* |
| 1 | 151 (51.9) | 164 (40.2) | |
| 2 | 45 (15.5) | 24 (5.9) | |
| 3 | 8 (2.7) | 2 (0.5) | |
| Not recorded in medical record | 38 (13.1) | 84 (20.6) | |
| Use of chemotherapy for mBC before index treatment initiation, n (%) | 66 (22.7) | 42 (10.3) | <0.001* |
| Duration from initiation of last adjuvant endocrine therapy to mBC diagnosis, median months (range) | 19.2 (0.0, 216.6) | 18.9 (0.0, 130.8) | 0.879 |
| Duration from initiation of index treatment to available follow-up, median months (range) | 16.3 (1.0, 25.2) | 16.7 (1.0, 24.6) | 0.239 |
BC breast cancer, ECOG Eastern Cooperative Oncology Group, mBC metastatic breast cancer
* p < 0.05
† Statistical comparisons were conducted using Wilcoxon rank-sum tests for continuous variables and Chi square tests for categorical variables
aDisease characteristics described at new treatment initiation include age, treatment type, line of index treatment, number and sites of metastases, adjusted CCI, ECOG performance status, and prior chemotherapy for mBC. Menopausal status, race, insurance plan type, mBC type, number and sites of metastases, and time elapsed (months) from initiation of last adjuvant endocrine therapy to stage IV mBC diagnosis were assessed at mBC diagnosis. Duration from initiation of index treatment to available follow-up was assessed at the end of follow-up
bThe exact variable for menopausal status was not collected in the study but instead imputed based on age (postmenopausal: age ≥60)
cEndocrine therapy includes endocrine monotherapy (anastrozole, exemestane, fluoxymesterone, fulvestrant, letrozole, megestrol acetate, tamoxifen), combination therapy with two endocrine agents (fulvestrant + anastrozole, fulvestrant + exemestane, fulvestrant + exemestane, fulvestrant + tamoxifen), and everolimus-based therapies (everolimus, everolimus + anastrozole, everolimus + exemestane, everolimus + fulvestrant, everolimus + letrozole)
dChemotherapy includes chemotherapy monotherapy (capecitabine, docetaxel, gemcitabine, ixabepilone, paclitaxel, protein-bound paclitaxel, vinorelbine), combinational therapy of two chemotherapy agents (cyclophosphamide + docetaxel, cyclophosphamide + doxorubicin, doxorubicin + docetaxel, paclitaxel + gemcitabine), and combinational therapy of chemotherapy and endocrine therapy (anastrozole + paclitaxel, fulvestrant + capecitabine, fulvestrant + paclitaxel, letrozole + paclitaxel)
eAny visceral metastases refer to liver, lung, and other visceral metastases, including peritoneum, adrenal gland, and kidney
fThe adjusted CCI calculates the comorbidity index excluding metastatic breast cancer (score of 6)
However, patients with multiple metastases had more aggressive mBC than patients with single metastasis. Specifically, at index treatment initiation, 77.0 % patients of the multiple metastases group had bone metastasis versus 56.6 % of the single metastasis group, liver metastasis was present in 60.8 versus 11.5 % of cases, lung metastasis was present in 73.9 versus 21.3 % of cases, and brain metastasis was present in 3.4 versus 0.5 % of cases, respectively (all p < 0.05) (Table 1). Multiple metastases patients also had a worse ECOG performance status than patients with single metastasis (ECOG ≥ 2 was recorded for 18.2 versus 6.4 % of patients, respectively, p < 0.001) (Table 1). Twice as many patients with multiple metastases had used prior chemotherapy for mBC than patients with a single metastasis (22.7 versus 10.3 %, p < 0.001) (Table 1).
Progression-free survival (PFS)
In the overall sample, patients with multiple metastases had significantly shorter PFS than single metastasis patients in both unadjusted (log-rank test p < 0.001, hazard ratio (HR) = 1.98, 95 % confidence interval (CI) 1.59–2.46, Fig. 1 and Table 2) and adjusted analyses (HR = 1.55, 95 % CI 1.21–1.98, Table 2). Results were consistent across different lines of therapy, though any differences between the two groups were not significant in the first-line setting (Table 3). In addition, second-line treatment (versus first-line), bone metastasis (versus no bone metastasis), and worse ECOG performance status were also associated with significantly shorter PFS (Table 2).
Fig. 1.
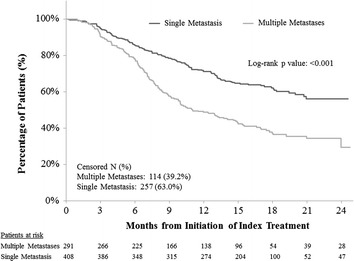
Comparison of progression-free survival between HR+/HER2− mBC patients with multiple metastases versus single metastasis
Table 2.
Comparisons of progression-free survival, time on treatment, and overall survival, between patients with multiple metastases versus single metastasis
| Parameters | Progression-free survival | Time on treatment | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95 % CI) | P-value | HR | (95 % CI) | P-value | HR | (95 % CI) | P-value | |
| Univariate analysis | |||||||||
| Multiple metastases (Ref: single metastasis) | 1.98 | (1.59,2.46) | <0.001* | 1.78 | (1.46, 2.18) | <0.001* | 2.61 | (1.78, 3.83) | <0.001* |
| Multivariable-adjusted analysisa | |||||||||
| Multiple metastases (Ref: single metastasis) | 1.55 | (1.21,1.98) | <0.001 | 1.33 | (1.05, 1.67) | 0.018* | 1.77 | (1.15, 2.74) | 0.010* |
| Age at index therapy initiation | 0.98 | (0.97,1.00) | 0.043* | 0.97 | (0.95, 0.99) | <0.001* | 1.01 | (0.98, 1.04) | 0.646 |
| Race (Ref: non-white) | |||||||||
| White | 0.89 | (0.71,1.12) | 0.331 | 0.83 | (0.67, 1.02) | 0.074 | 0.88 | (0.61, 1.29) | 0.527 |
| Insurance at mBC diagnosis (Ref: other or no insurance) | |||||||||
| Private | 1.35 | (0.85,2.14) | 0.203 | 1.23 | (0.81, 1.87) | 0.326 | 1.12 | (0.50, 2.50) | 0.787 |
| Medicare only | 1.47 | (0.89,2.42) | 0.128 | 1.46 | (0.93, 2.31) | 0.103 | 1.27 | (0.53, 3.04) | 0.584 |
| Type of index treatment (Ref: endocrine therapyb) | |||||||||
| Chemotherapyc | 1.18 | (0.91,1.55) | 0.216 | 2.29 | (1.80, 2.90) | <0.001* | 1.84 | (1.20, 2.81) | 0.005* |
| Line of index treatment (Ref: first-line) | |||||||||
| Second-line | 1.49 | (1.06,2.09) | 0.023* | 1.75 | (1.27, 2.41) | 0.001* | 1.26 | (0.69, 2.29) | 0.448 |
| Third-line and above | 1.34 | (0.93,1.93) | 0.114 | 1.18 | (0.84, 1.65) | 0.337 | 0.85 | (0.44, 1.63) | 0.618 |
| mBC type (Ref: de novo) | |||||||||
| Recurrent with adjuvant endocrine therapy | 1.27 | (0.90,1.78) | 0.175 | 1.53 | (1.11, 2.12) | 0.010* | 1.40 | (0.73, 2.64) | 0.316 |
| Recurrent without adjuvant endocrine therapy | 0.40 | (0.25,0.66) | <0.001* | 0.45 | (0.29, 0.71) | 0.001* | 1.06 | (0.49, 2.31) | 0.882 |
| Adjusted CCI at index treatment initiation | 1.08 | (0.97,1.20) | 0.155 | 1.09 | (0.99, 1.21) | 0.084 | 1.20 | (1.02, 1.40) | 0.024* |
| Bone metastasis at index therapy initiation | |||||||||
| (Ref: no bone metastasis) | 1.56 | (1.20,2.02) | 0.001* | 1.15 | (0.91, 1.45) | 0.233 | 0.85 | (0.57, 1.27) | 0.418 |
| Performance status at index treatment initiation (Ref: ECOG 0) | |||||||||
| ECOG 1 | 1.44 | (1.06,1.97) | 0.021* | 1.04 | (0.79, 1.37) | 0.785 | 1.76 | (0.93, 3.32) | 0.080 |
| ECOG 2 and 3 | 2.60 | (1.70,3.98) | <0.001* | 1.87 | (1.26, 2.78) | 0.002* | 5.04 | (2.45, 10.40) | <0.001* |
| Unknown | 1.77 | (1.20,2.62) | 0.004* | 1.29 | (0.90, 1.83) | 0.166 | 3.15 | (1.47, 6.72) | 0.003* |
| Use of chemotherapy for mBC before index treatment initiation | 0.82 | (0.58,1.17) | 0.283 | 0.89 | (0.65, 1.23) | 0.484 | 2.02 | (1.15, 3.55) | 0.014* |
| Duration from initiation of last adjuvant endocrine therapy to mBC diagnosis | 1.00 | (0.99,1.00) | 0.374 | 1.00 | (0.99, 1.00) | 0.016* | 1.00 | (0.99, 1.01) | 0.671 |
BC breast cancer, CCI Charlson Comorbidity Index, CI confidence interval, ECOG Eastern Cooperative Oncology Group, HR hazard ratio, mBC metastatic breast cancer, Ref reference
* P < 0.05; Ref reference group
aThe model adjusted for age, race, insurance type at mBC diagnosis, treatment type, line of the index treatment, mBC type, adjusted CCI, bone metastasis at index therapy initiation, ECOG performance status, prior chemotherapy for mBC, and months from the initiation of the last adjuvant endocrine therapy to mBC diagnosis
bEndocrine therapy includes endocrine monotherapy, combination therapy with another endocrine agent, and everolimus-based therapies
cChemotherapy includes chemotherapy monotherapy, combinational therapy of chemotherapy agents, and combinational therapy of chemotherapy and endocrine therapy
Table 3.
Comparisons of progression-free survival, time on treatment, and overall survival between patients with multiple metastases versus single metastasis by line of therapy
| Multiple metastases (Ref: single metastasis) | Progression-Free Survival | Time on Treatment | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95 % CI) | P-value | HR | (95 % CI) | P-value | HR | (95 % CI) | P-value | |
| Univariate analysis | |||||||||
| First-line | 1.87 | (1.32, 2.65) | <0.001* | 1.79 | (1.30, 2.47) | <0.001* | 3.32 | (1.88, 5.85) | <0.001* |
| Second-line | 2.01 | (1.33, 3.03) | 0.001* | 1.83 | (1.26, 2.65) | 0.001* | 3.29 | (1.48, 7.33) | 0.004* |
| Third-line and above | 2.33 | (1.54, 3.53) | <0.001* | 2.04 | (1.37, 3.02) | <0.001* | 1.93 | (0.94, 3.95) | 0.074 |
| Multivariate-adjusted analysisa | |||||||||
| First-line | 1.43 | (0.99, 2.08) | 0.059 | 1.20 | (0.85, 1.69) | 0.313 | 1.98 | (1.08, 3.64) | 0.028* |
| Second-line | 1.69 | (1.10, 2.59) | 0.017* | 1.47 | (0.99, 2.17) | 0.055 | 2.41 | (1.05, 5.52) | 0.038* |
| Third-line and above | 1.57 | (1.00, 2.46) | 0.049* | 1.38 | (0.90, 2.11) | 0.143 | 1.09 | (0.50, 2.36) | 0.832 |
CCI Charlson Comorbidity Index, CI confidence interval, ECOG Eastern Cooperative Oncology Group; HR hazard ratio, mBC metastatic breast cancer, Ref reference
* P < 0.05
aThe model adjusted for the following variables: age, race, insurance type at mBC diagnosis, treatment type, mBC type, adjusted CCI, bone metastasis at index therapy initiation, ECOG performance status, prior chemotherapy for mBC, and months from the initiation of the last adjuvant endocrine therapy to mBC diagnosis
Time on treatment (TOT)
Overall, patients with multiple metastases had significantly shorter TOT than single metastasis patients in both unadjusted (log-rank test p < 0.001, HR = 1.78, 95 % CI 1.46–2.18, Fig. 2 and Table 2) and adjusted analyses (HR = 1.33, 95 % CI 1.05–1.67, Table 2). Results across different lines resembled those in the overall sample, but were not significant (Table 3). Use of chemotherapy as index therapy, second-line treatment (versus first-line) and worse ECOG performance status were associated with significantly shorter TOT (Table 2).
Fig. 2.
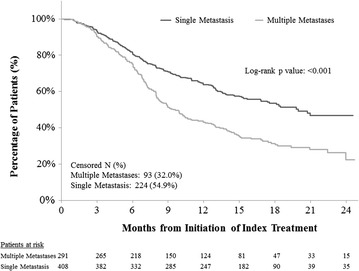
Comparison of time on treatment between HR+/HER2− mBC patients with multiple metastases versus single metastasis
Overall survival (OS)
Overall, patients with multiple metastases had significantly shorter OS than single metastasis patients in both unadjusted (log-rank test p < 0.001, HR = 2.61, 95 % CI 1.78–3.83, Fig. 3 and Table 2) and adjusted analyses (HR = 1.77, 95 % CI 1.15–2.74, Table 2). Results across different lines also resembled those in the overall sample and were significant in the first- and second-line settings (Table 3). Use of chemotherapy as index therapy, higher adjusted Charlson comorbidity index (CCI), worse ECOG performance score, and prior chemotherapy for mBC were associated with significantly shorter OS (Table 2).
Fig. 3.
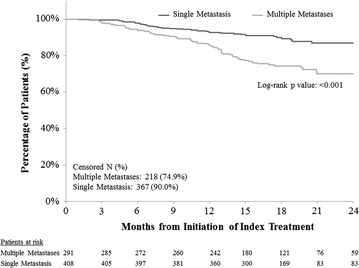
Comparison of overall survival between HR+/HER2− mBC patients with multiple metastases versus single metastasis
Discussion
With advancements in the treatment of HR+/HER2- mBC, it is important to identify the patients who are in greater need of innovative therapies. Previous studies have identified multiple metastases as a risk factor for shorter OS. The current study added to the literature by using a national sample of mBC patients and comparing multiple clinical outcomes (PFS, TOT and OS) between patients with multiple metastases versus those with a single metastasis. The results demonstrate that patients with multiple metastases had significantly worse outcomes, as measured by PFS, TOT and OS, even after controlling for other factors, including bone metastasis. The findings are consistent across different lines of therapy. The current study confirms that the presence of multiple metastases is an independent prognostic factor of worse clinical outcomes among postmenopausal HR+/HER2- mBC patients. Furthermore, consistent with previous literature [14, 19–25], ECOG performance status was independently and significantly associated with all clinical outcomes examined in this study. Bone metastasis was significantly associated with PFS but not with other outcomes.
Due to multiple organ involvement, patients with multiple metastases are likely to be sicker and have more severe mBC than single metastasis patients [23, 26–28]. In the current study, patients with multiple metastases had a higher adjusted CCI score, worse ECOG performance status, and higher use of prior chemotherapy for mBC than single metastasis patients. The differences in clinical outcomes are quite striking between patients with multiple metastases and those with a single metastasis. The former group had a 55 % higher hazard of experiencing progression or death and a 77 % higher hazard of death, even after controlling for other potential prognostic factors of mBC (e.g., ECOG performance status). This impact was evident across different lines of therapy. These findings highlight the substantial burden and unmet need for more efficacious treatments in patients with multiple metastases.
In real-world practice, patients with multiple metastases are more likely to receive chemotherapy, often because their vital organs are affected and the patients are thus perceived to be in “visceral crisis” [29, 30]. However, chemotherapy is associated with serious side effects and can substantially impair patients’ quality of life [31, 32]. More importantly, chemotherapy has limited efficacy and effectiveness [11, 33]. These limitations call for more effective treatments to address the substantial unmet need of patients with multiple metastases.
Recently, a number of novel targeted therapies for the treatment of HR+/HER2− mBC have been approved or are in late-stage clinical development, including mammalian target of rapamycin (mTOR) inhibitors (e.g., everolimus), cyclin dependent kinase (CDK)-4/6 inhibitors (e.g., palbociclib), and phosphoinositide 3-kinase (PI3k) inhibitors (e.g., buparlisib). Large phase III randomized controlled trials (RCTs) have demonstrated the superior efficacy of these treatments compared to conventional endocrine monotherapies. For example, the median PFS associated with everolimus/exemestane combinational therapy was nearly three times longer than the PFS observed with exemestane monotherapy in the BOLERO-2 trial [9], and first-line palbociclib/letrozole combinational therapy doubled the PFS of patients receiving this treatment compared to patients treated with letrozole monotherapy in the PALOMA-1 trial [12]. In addition, everolimus-based therapy was also shown to be associated with significantly longer OS and PFS relative to endocrine therapy and chemotherapy [10, 11]. These innovative treatments may substantially improve outcomes among patients with multiple metastases. However, current literature suggests that individual treatment regimens are similar between first-line and subsequent lines of treatment [30, 33–36], and clinical guidelines have not recommended an optimal treatment sequence for the treatment of HR+/HER2− mBC [6, 37]. With a rapidly evolving treatment landscape, more studies are needed to assess the effects of newer treatments in this population and to shed light on the optimal sequencing of endocrine therapy, targeted therapy, and chemotherapy.
Adding to the previous literature, the current study could provide important evidence for decision-makers. From the physician perspective, identifying the patients who need more advanced treatment could facilitate a targeted approach to mBC management. For payers and health policy makers, understanding the burden of different subgroups of mBC could help in optimizing resource allocation for those in greater need of advanced treatment and could result in the improvement of the overall outcomes of mBC patients through a more cost-effective approach. Finally, the implications of more effective treatment go beyond extending PFS and OS. Such therapies may also improve the quality of life and productivity of patients. Therefore, the benefits of applying advanced treatments in this group with high unmet needs could be more substantial when viewed from the societal perspective.
This study has several limitations inherent to retrospective chart review studies. First, the results might be subject to selection bias if confounding factors were not identified or adjusted for in the analyses [38, 39]. In the current study, we adjusted for important baseline characteristics that were available in patient charts and which were thought to have the potential to affect clinical outcomes. Second, the frequency of follow-up could have differed between the two patient groups. Patients with multiple metastases had more severe mBC and may have been followed up more frequently than single metastasis patients. Thus, they may have been more likely to be identified as experiencing an event (e.g., progression) and this cohort may be biased against in the time-to-event analyses. Third, the current study did not collect any biopsy or histology data, which are important indicators to confirm mBC and inform treatment choice. Fourth, all patients were required to be postmenopausal at index treatment initiation based on eligibility criteria, but this study did not collect detailed menopausal status at BC diagnosis or mBC diagnosis. Despite these limitations, the current study provides important insights about real-world clinical outcomes for HR+/HER2− mBC patients with multiple metastases from a large nationwide sample, and could further help clinical and policy decision-making.
Conclusion
Among HR+/HER2− mBC patients treated in community-based oncology practices in the US, those with multiple metastases had significantly shorter PFS, TOT, and OS than single metastasis patients, highlighting a substantial clinical burden and unmet need for more effective treatments for these high-risk patients.
Methods
Data source and patient selection
Community-based oncologists and hematologists who treated post-menopausal women with HR+/HER2− stage IV mBC (excluding patients with locoregional recurrences) were invited to participate in this chart review study from a nationwide online panel of over 9500 physicians practicing in the US. Participants were asked to randomly select up to ten eligible patients and enter relevant patient chart information into a secure electronic case report form (CRF). The CRF was developed by the study investigators and pilot tested with three physicians. No patient identification information was collected, and the study was approved by the New England Institutional Review Board.
Patients were eligible for this study if they had BC recurrence or progression on or after a non-steroidal aromatase inhibitor in the adjuvant or metastatic setting, and had initiated a new treatment for mBC, defined as the index treatment, between 07/01/2012 and 04/15/2013. Patients enrolled in any clinical trial or with a history of primary non-BC malignancy (with the exception of non-melanoma skin cancer and carcinoma in situ of the cervix uteri) during the 3 years prior to the first mBC diagnosis date were excluded. Patients were classified into two study groups based on the number of non-lymph-node metastatic sites at index treatment initiation: multiple metastases versus single metastases.
Study outcome measures
PFS, TOT, and OS were assessed. PFS was defined as the time from index treatment initiation to disease progression or death, whichever occurred first. Progression was determined by the physician, based on radiographic evidence or tests, physical exams, symptoms, or other methods. TOT was defined as the time from index treatment initiation to discontinuation of the index treatment or death, whichever occurred first. OS was defined as the time from index treatment initiation to death from any cause. For all outcomes, patients who did not have an event at the end of the study were censored at the date of last follow-up.
Statistical analysis
Patient baseline characteristics (i.e., before index treatment initiation) were compared between the two study groups using Wilcoxon rank-sum tests for continuous variables and Chi square tests for categorical variables. Patient characteristics included age, menopausal status (imputed based on age, postmenopausal: age ≥60), race, insurance type, type of index treatment (endocrine or chemotherapy), line of index treatment, mBC type, number of non-lymph-node metastases, sites of metastatic disease, adjusted CCI (excluding a score of six for metastatic cancer), ECOG performance status, prior chemotherapy for mBC, the time elapsed from the initiation of the last adjuvant endocrine therapy to mBC diagnosis, and duration from the initiation of the index treatment to end of follow-up.
PFS, TOT and OS were compared between patients with multiple and single metastases using Kaplan–Meier curves with log-rank tests and univariate Cox regression models. These outcomes were also compared between the two groups using multivariable Cox regression models adjusting for age at index treatment initiation, race, insurance type at mBC diagnosis, treatment type, line of the index treatment, mBC type, adjusted CCI, bone metastasis at index therapy initiation, ECOG performance status, prior chemotherapy for mBC, and time elapsed (months) from the initiation of the last adjuvant endocrine therapy to mBC diagnosis. In addition, separate Cox proportional hazard models were conducted, which included an interaction term between the line of therapy and study group in order to assess the impact of multiple metastases on clinical outcomes across different lines of therapy.
All analyses used a two-sided p value of 0.05 to determine statistical significance. Analyses were performed using SAS 9.3 (Cary, NC, USA).
Authors’ contributions
All authors participated in the design of the study and contributed to the manuscript development. Data were collected by Analysis Group, Inc. and analyzed and interpreted in collaboration with all other authors. Manuscript drafts were prepared by the authors with editorial assistance from a professional medical writer. All the authors vouch for the accuracy and completeness of the data reported and the adherence of the study to the protocol, and all the authors made the decision to submit the manuscript for publication. All authors read and approved the final manuscript.
Acknowledgements
Medical writing assistance was provided by Ana Bozas, PhD, an employee of Analysis Group, Inc. Funding for this research was provided by Novartis Pharmaceuticals Corporation.
A synopsis of the current research was submitted to the San Antonio Breast Cancer Symposium, which will take place in San Antonio, TX during December 8-12, 2015.
Competing interests
YH is an employee of Novartis Pharmaceuticals Corporation and owns stock/stock options. JX, NL, PLL, EO, VK, and EQW are employees of Analysis Group, Inc., which received consultancy fees from Novartis Pharmaceuticals Corporation.
Abbreviations
- BC
breast cancer
- CCI
Charlson comorbidity index
- CI
confidence interval
- CDK
cyclin dependent kinase
- ECOG
Eastern Cooperative Oncology Group
- HR
hazard ratio
- HR+
hormone receptor-positive
- HER2-
human epidermal growth factor receptor-2-negative
- mTOR
mammalian target of rapamycin
- mBC
metastatic breast cancer
- OS
overall survival
- PI3k
phosphoinositide 3-kinase
- PFS
progression-free survival
- RCT
randomized controlled trials
- TOT
time on treatment
Contributor Information
Jipan Xie, Phone: 212-492-8158, Email: jipan.xie@analysisgroup.com.
Yanni Hao, Email: yanni.hao@novartis.com.
Nanxin Li, Email: nick.li@analysisgroup.com.
Peggy L. Lin, Email: peggy.lin@analysisgroup.com
Erika Ohashi, Email: erika.ohashi@analysisgroup.com.
Valerie Koo, Email: valerie.koo@analysisgroup.com.
Eric Q. Wu, Email: eric.wu@analysisgroup.com
References
- 1.International Agency for Research on Cancer, Cancer Research UK. World cancer factsheet. Cancer Research UK, London, UK. 2014. http://publications.cancerresearchuk.org/downloads/Product/CS_REPORT_WORLD.pdf. Accessed 21 May 2015.
- 2.National Cancer Institute. SEER Cancer Statistics Factsheets: Breast Cancer. National Cancer Institute, Bethesda, MD. 2014. http://seer.cancer.gov/statfacts/html/breast.html. Accessed 21 May 2015.
- 3.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 4.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 5.Cadoo KA, Fornier MN, Morris PG. Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging. 2013;57(4):312–321. [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer N. Breast Cancer, Version 2.2015. National Comprehensive Cancer Network (NCCN). 2015. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 21 May 2015.
- 7.Incorvati JA, Shah S, Mu Y, Lu J. Targeted therapy for HER2 positive breast cancer. J Hematol Oncol. 2013;6:38. doi: 10.1186/1756-8722-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto-Ibusuki M, Arnedos M, Andre F. Targeted therapies for ER+/HER2− metastatic breast cancer. BMC Med. 2015;13:137. doi: 10.1186/s12916-015-0369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J, Hao Y, Li N, Lin PL, Ohashi E, Koo V, et al. Comparative effectiveness of everolimus-based therapy versus endocrine monotherapy among postmenopausal women with HR+/HER2− metastatic breast cancer: a retrospective chart review in community oncology practices in the US. Curr Med Res Opin. 2015 doi: 10.1185/03007995.2015.1021906. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Hao Y, Xie J, Lin PL, Koo V, Ohashi E, et al. Everolimus-based therapy versus chemotherapy among patients with HR +/HER2− metastatic breast cancer: comparative effectiveness from a chart review study. Int J Breast Cancer. 2015;2015:1–9. doi: 10.1155/2015/240750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 13.Khadakban D, Gorasia-Khadakban T, Vijaykumar DK, Pavithran K, Anupama R. Factors associated with better survival after surgery in metastatic breast cancer patients. Indian J Surg Oncol. 2013;4(1):52–58. doi: 10.1007/s13193-012-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanfir A, Lahiani F, Bouzguenda R, Ayedi I, Daoud J, Frikha M. Prognostic factors and survival in metastatic breast cancer: a single institution experience. Rep Pract Oncol Radiother. 2013;18(3):127–132. doi: 10.1016/j.rpor.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alba E, Ribelles N, Sevilla I, Rueda A, Alonso L, Marquez A, et al. Adjuvant anthracycline therapy as a prognostic factor in metastatic breast cancer. Breast Cancer Res Treat. 2001;66(1):33–39. doi: 10.1023/A:1010616532332. [DOI] [PubMed] [Google Scholar]
- 16.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23(1):103–112. doi: 10.1007/s10552-011-9859-8. [DOI] [PubMed] [Google Scholar]
- 18.Kwast AB, Voogd AC, Menke-Pluijmers MB, Linn SC, Sonke GS, Kiemeney LA, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status. Breast Cancer Res Treat. 2014;145(2):503–511. doi: 10.1007/s10549-014-2964-0. [DOI] [PubMed] [Google Scholar]
- 19.Seidman AD, Chan S, Wang J, Zhu C, Xu C, Xu B. A pooled analysis of gemcitabine plus docetaxel versus capecitabine plus docetaxel in metastatic breast cancer. Oncologist. 2014;19(5):443–452. doi: 10.1634/theoncologist.2013-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheler J, Tsimberidou AM, Hong D, Naing A, Falchook G, Piha-Paul S, et al. Survival of 1,181 patients in a phase I clinic: the MD Anderson Clinical Center for targeted therapy experience. Clin Cancer Res. 2012;18(10):2922–2929. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;104(8):1742–1750. doi: 10.1002/cncr.21359. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa D, Horiguchi Si, Yamashita T, Kuroi K, Shimizu K. Comparison of outcomes between women with de novo stage IV and relapsed breast cancer. J Nippon Med School Nippon Ika Daigaku zasshi. 2014;81(3):139–147. doi: 10.1272/jnms.81.139. [DOI] [PubMed] [Google Scholar]
- 23.Llombart-Cussac A, Pivot X, Biganzoli L, Cortes-Funes H, Pritchard KI, Pierga JY, et al. A prognostic factor index for overall survival in patients receiving first-line chemotherapy for HER2-negative advanced breast cancer: an analysis of the ATHENA trial. Breast. 2014;23(5):656–662. doi: 10.1016/j.breast.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Nistico C, Cuppone F, Bria E, Fornier M, Giannarelli D, Mottolese M, et al. Ten years of experience with weekly chemotherapy in metastatic breast cancer patients: multivariate analysis of prognostic factors. Anticancer Drugs. 2006;17(10):1193–1200. doi: 10.1097/01.cad.0000231485.17063.d3. [DOI] [PubMed] [Google Scholar]
- 25.Tredan O, Ray-Coquard I, Chvetzoff G, Rebattu P, Bajard A, Chabaud S, et al. Validation of prognostic scores for survival in cancer patients beyond first-line therapy. BMC Cancer. 2011;11:95. doi: 10.1186/1471-2407-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings MC, Simpson PT, Reid LE, Jayanthan J, Skerman J, Song S, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232(1):23–31. doi: 10.1002/path.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Z, Li Y, Hameed O, Siegal GP, Wei S. Prognostic factors in patients with metastatic breast cancer at the time of diagnosis. Pathol Res Pract. 2014;210(5):301–306. doi: 10.1016/j.prp.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Ryberg M, Nielsen D, Osterlind K, Skovsgaard T, Dombernowsky P. Prognostic factors and long-term survival in 585 patients with metastatic breast cancer treated with epirubicin-based chemotherapy. Ann Oncol. 2001;12(1):81–87. doi: 10.1023/A:1008384019411. [DOI] [PubMed] [Google Scholar]
- 29.Cazzaniga M, Pronzato P, Leto di Priolo SL, De Matteis A, Di Costanzo F, Passalacqua R, et al. Patterns of relapse and modalities of treatment of breast cancer: the ‘IRIS’ Project, a multicenter observational study. Oncology. 2004;66(4):260–268. doi: 10.1159/000078325. [DOI] [PubMed] [Google Scholar]
- 30.DeKoven M, Bonthapally V, Jiao X, Ganguli A, Pathak P, Lee WC, et al. Treatment pattern by hormone receptors and HER2 status in patients with metastatic breast cancer in the UK, Germany, France, Spain and Italy (EU-5): results from a physician survey. J Comp Eff Res. 2012;1(5):453–463. doi: 10.2217/cer.12.43. [DOI] [PubMed] [Google Scholar]
- 31.Stockler M, Wilcken NR, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev. 2000;26(3):151–168. doi: 10.1053/ctrv.1999.0161. [DOI] [PubMed] [Google Scholar]
- 32.Bergh J, Jonsson PE, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in breast cancer. Acta Oncol. 2001;40(2–3):253–281. doi: 10.1080/02841860151116349. [DOI] [PubMed] [Google Scholar]
- 33.Macalalad AR, Hao Y, Lin PL, Signorovitch JE, Wu EQ, Ohashi E, et al. Treatment patterns and duration in post-menopausal women with HR+/HER2− metastatic breast cancer in the US: a retrospective chart review in community oncology practices (2004–2010) Curr Med Res Opin. 2015;31(2):263–273. doi: 10.1185/03007995.2014.980885. [DOI] [PubMed] [Google Scholar]
- 34.Donato BM, Burns L, Willey V, Cohenuram M, Oliveria S, Yood MU. Treatment patterns in patients with advanced breast cancer who were exposed to an anthracycline, a taxane, and capecitabine: a descriptive report. Clin Ther. 2010;32(3):546–554. doi: 10.1016/j.clinthera.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Hao Y, Xie J, Lin PL, Zhou Z, Zhong Y, et al. Everolimus use and associated factors among post-menopausal women with HR+/HER2− metastatic breast cancer. Curr Med Res Opin. 2015 doi: 10.1185/03007995.2015.1062358. [DOI] [PubMed] [Google Scholar]
- 36.Ray S, Bonthapally V, McMorrow D, Bonafede M, Landsman-Blumberg P. Patterns of treatment, healthcare utilization and costs by lines of therapy in metastatic breast cancer in a large insured US population. J Comp Eff Res. 2013;2(2):195–206. doi: 10.2217/cer.13.1. [DOI] [PubMed] [Google Scholar]
- 37.Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32(29):3307–3329. doi: 10.1200/JCO.2014.56.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012;30(34):4215–4222. doi: 10.1200/JCO.2012.41.6701. [DOI] [PubMed] [Google Scholar]
- 39.Roche N, Reddel H, Martin R, Brusselle G, Papi A, Thomas M, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014;11(Suppl 2):S99–S104. doi: 10.1513/AnnalsATS.201309-300RM. [DOI] [PubMed] [Google Scholar]


