Abstract
Objective
The incidence of gastric cancer is high in Chinese Tibetan. This study aimed to identify the differentially expressed microRNAs (miRNAs) and further explore their potential roles in Tibetan with gastric cancer so as to predict potential therapeutic targets.
Methods
A total of 10 Tibetan patients (male:female = 6:4) with gastric cancer were enrolled for isolation of matched gastric cancer and adjacent non-cancerous tissue samples. Affymetrix GeneChip microRNA 3.0 Array was employed for detection of miRNA expression in samples. Differential expression analysis between two sample groups was analyzed using Limma package. Then, MultiMiR package was used to predict targets for miRNAs. Following, the target genes were put into DAVID (Database for Annotation, Visualization and Integrated Discovery) to identify the significant pathways of miRNAs.
Results
Using Limma package in R, a total of 27 differentially expressed miRNAs were screened out in gastric cancer, including 25 down-regulated (e.g. hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p) and 2 up-regulated miRNAs. According to multiMiR package, a number of 1445 target genes (e.g. Wnt1, KLF4 and S1PR1) of 13 differentially expressed miRNAs were screened out. Among those miRNAs, hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p were identified with the most target genes. Furthermore, three miRNAs were significantly enriched in numerous common cancer-related pathways, including “Wnt signaling pathway”, “MAPK signaling pathway” and “Jak-STAT signaling pathway”.
Conclusions
The present study identified a downregulation and enrichment in cancer-related pathways of hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p in Tibetan with gastric cancer, which can be suggested as therapeutic targets.
Electronic supplementary material
The online version of this article (doi:10.1186/s12935-015-0266-1) contains supplementary material, which is available to authorized users.
Keywords: Gastric cancer, miRNA microarray, Differentially expressed miRNAs, Enrichment analysis, Network of miRNAs-targets
Background
Gastric cancer is one of the most common malignancies worldwide. Although incidence and mortality of gastric cancer has been dramatically decreased over the last decades worldwide, the declines are becoming less remarkable in some countries [1]. The main reason may be that there are limited treatment options and patients in advanced stages could not be cured by surgical removal [2]. Moreover, 5-year survival rates of gastric cancer are decreased with gastric cancer progression [3] and metastatic stage could lead to poor outcomes [4]. In Chinese Qinghai, the Tibetan ethnic group has a higher incidence of gastric cancer than the Han ethnic group [5]. Thus, there is an urgent need to develop effective treatments to improve diagnosis and reduce burden of gastric cancer in gastric cancer-infected Tibetan.
A number of key genes with abnormal expression in the progression of gastric cancer have been screened out. For example, the over-expression of SPAG9 (sperm associated antigen 9) correlates with poor prognosis and leads to gastric cancer invasion and chemo-resistance [6]. In terms of Tibetan with gastric cancer, the expression pattern of tumor-associated antigen MG(7)-Ag is abnormal and it can be used as a reliable marker to predict gastric cancer at early stage [7]. The polymorphisms of prostate stem cell antigen gene are also associated with gastric cancer in Tibetans [8]. Therefore, the identification of key genes can improve diagnosis and management of gastric cancer in Tibetan.
MicroRNAs (miRNAs) are a group of small non-coding RNAs that have important roles in the development of numerous cancer types, through down-regulation of the target genes [9, 10]. Multiple miRNAs are expressed aberrantly and are involved in the progression and prognosis of gastric cancer [11]. Therefore, investigating role of miRNAs in gastric cancer could provide new insight into the biological mechanism of this disease. Reportedly, miR-21 is up-regulated in gastric cancer and its dysregulation can enhance cell proliferation, invasion and migration through down-regulating a set of tumor suppressor genes, such as RECK (reversion-inducing-cysteine-rich protein with kazal motifs) [12]. In addition, miR-544a could activate the Wnt signaling pathway by stabilizing the β-catenin in nucleus and its inhibition may be a therapeutic method for metastatic gastric cancer [13]. However, the research on miRNAs in gastric cancer in Tibetan is really rare and therefore, the exploration of miRNAs in the progression of gastric cancer in Tibetan is of great significance.
In the present study, the paired cancerous and adjacent non-cancerous tissue samples were collected from 10 patients with gastric cancer, and further conducted for miRNA expression profiling analysis. Differentially expressed miRNAs (DE-miRs) were screened out between two sample groups, followed by identification of target genes based on bioinformatics methods. Furthermore, functional enrichment analysis was performed for the DE-miRs so as to reveal their potential roles in progression of gastric cancer.
Methods
Sample collections
A total of 10 Tibetan patients (male:female = 6:4) with gastric cancer were enrolled in this study. They were aged between 33 and 77 years old, and the median age was 51.1. The tumor node metastasis stages (TNM) were determined basing on the International Union Against Cancer and the American Joint Committee on Cancer pathological staging systems. The patients were identified with clinical stages at T2N0M0(1/10), TisN0M0, TisN0M0IIc, TisN0M0IIc, T3N2M0, T3N0M0, T4aN1M0, T2N1M0, T3N2M0 or T3N2M0 (Table 1). Matched gastric cancer and adjacent non-cancerous tissue samples (n = 10 in each group) were obtained during surgical operation and immediately stored at −80 °C for microarray analysis. All the enrolled patients have given written informed consent and the present study was approved by Ethics Committee of Qinghai University Affiliated Hospital.
Table 1.
Information on sample cases
| Number | Age | Sex | Elevation (m) | Staging | HP infection | Pathological description |
|---|---|---|---|---|---|---|
| B1 299295 | 46 | Male | 2260 | T2N0M0(1/10) | High-differentiated adenocarcinoma invaded submucosa, lymph node 0/10 | |
| B2 302629 | 38 | Female | 2850 | TisN0M0 | Intramucosal carcinoma in gastric antrum (0/11) | |
| B3 WJ | 57 | Male | 2840 | T4aN1M0 | Distal gastric carcinoma, tumor invaded full thickness and vascular nerves (+) 2/15, low and median differentiated adenocarcinoma | |
| B5 211409 | 56 | Male | 2850 | TisN0M0IIc | ||
| B6 | 49 | Male | 2050 | TisN0M0IIc | Intramucosal carcinoma | |
| A1 303135 | 43 | Male | 3280 | T3N2M0 | Low differentiated tubular adenocarcinoma invaded deep muscle layer (6/9), vascular and nerve (+) | |
| A2 302628 | 33 | Male | 3860 | T3N0M0 | Ulcer tubular well-differentiated adenocarcinoma invaded the deep muscularis serosa, vascular nerve (+), 0/13 | |
| A3 WJ | 56 | Female | 3800 | T2N1M0 | HP−, gastroscope | Distal gastric cancer |
| A4 WJ | 77 | Female | 4800 | T3N2M0 | HP+, gastroscope | Gastric carcinoma |
| A6 300804 | 56 | Male | 3100 | T3N2M0 | Differentiated tubular adenocarcinoma invaded deep muscularis, vascular+, nerve+ |
HP Helicobacter pylori
Microarray profiling of miRNAs
Total RNA was extracted from the matched cancerous and adjacent non-cancerous tissues according to the manufacture’ s instructions using RNAiso Plus purchased from Treasure Biological Engineering (Dalian, China). Reverse transcription-quantitative polymerase chain reaction was conducted according to the manufacture’ s instructions using a PrimeScript® First Strand cDNA Synthesis kit and miRNA qPCR primer mix (Takara Bio, Inc, CA, USA). Affymetrix GeneChip microRNA 3.0 Array (Affymetrix, Inc, Santa, CA, USA) was employed for detection of miRNA expression in samples, which provides for 100 % miRBase v17 coverage (http://www.mirbase.org) by a one-color approach.
Differential expression analysis
Raw data of miRNA expression profile from cancerous and adjacent non-cancerous tissues were converted into recognizable miRNA expression data by RMA (robust multi-array analysis) method, followed by median normalization and log2 transformation using Affy package (http://www.bioconductor.org/packages/release/bioc/html/affy.html) in R [14]. During the expression conversion from probe level to miRNA level, the expression values of probes corresponding to each miRNA were averaged as the miRNA value. Differential expression analysis between two groups was analyzed using Limma package of R language (http://www.bioconductor.org/packages/release/bioc/html/limma.html) [15] based on the criteria of |log2 FC (fold change)| ≥1 and P value <0.05.
Prediction of targets for differentially expressed miRNAs
MultiMiR package (http://multimir.ucdenver.edu/) [16] was previously established to predict targets of miRNAs, which covered 14 databases including miRecords, miRTarBase, TarBase, DIANA-microT, ElMMo, MicroCosm, miRanda, miRDB, PicTar, PITA, TargetScan, miR2Disease, PharmacomiR and PhenomiR. In the present study, multiMiR package was employed to predict targets of DE-miRs with the criterion of primary score listed in top 35. Meanwhile, only the target genes predicted in at least three databases were selected for following analysis. Accordingly, the network between targets and DE-miRs was constructed using Cytoscape v3.2.1 (http://www.cytoscape.org/) [17] software.
Functional enrichment analysis for DE-miRs
Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/home.jsp) [18] is a powerful tool to mine functions of interested genes. As miRNAs function through down-regulating target genes, to further reveal the potential biological functions or pathways that may be changed by the DE-miRs, the target genes were put into DAVID to screen out significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.kegg.jp/) [19] pathways and Gene Ontology (GO, http://www.geneontology.org) [20] biological processes. The selection criterion for significant GO and KEGG pathway terms was P value <0.05.
Results
DE-miRs between gastric cancer and adjacent non-cancerous samples
By using Limma package in R with the criteria of |log2 FC| ≥1 and P value <0.05, a total of 27 DE-miRs in gastric cancer were screened out, including 25 down-regulated miRNAs (e.g. hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p) and 2 up-regulated miRNAs (hsa-miR-196b-3p and hsa-miR-138-1-3p) (Table 2). The heap map of DE-miRs was shown in Fig. 1.
Table 2.
The 27 differentially expressed microRNAs in gastric cancer
| Accession | MicroRNAs | Log fold change | P value |
|---|---|---|---|
| MIMAT0000243 | hsa-miR-148a-3p | −1.8142778 | 0.006134223 |
| MIMAT0027583 | hsa-miR-6840-3p | −1.47970911 | 0.015759083 |
| MIMAT0027464 | hsa-miR-6782-5p | −1.45651738 | 0.018393456 |
| MIMAT0000759 | hsa-miR-148b-3p | −1.43506791 | 0.010053602 |
| MIMAT0028117 | hsa-miR-7110-5p | −1.43359191 | 0.038837699 |
| MIMAT0000707 | hsa-miR-363-3p | −1.3662862 | 0.049770472 |
| MIMAT0018352 | hsa-miR-3937 | −1.32835296 | 0.023596283 |
| MIMAT0004694 | hsa-miR-342-5p | −1.27792593 | 0.044159324 |
| MIMAT0022721 | hsa-miR-1247-3p | −1.17878373 | 0.020578891 |
| MIMAT0019077 | hsa-miR-1587 | −1.1697371 | 0.048251207 |
| MIMAT0016882 | hsa-miR-4253 | −1.15418525 | 0.017333874 |
| MIMAT0027474 | hsa-miR-6787-5p | −1.14471871 | 0.040401132 |
| MIMAT0015070 | hsa-miR-3188 | −1.10659872 | 0.042107841 |
| MIMAT0025844 | hsa-miR-6716-5p | −1.08787366 | 0.019653906 |
| MIMAT0019033 | hsa-miR-4498 | −1.08655973 | 0.018678343 |
| MIMAT0004948 | hsa-miR-885-3p | −1.0692305 | 0.044870796 |
| MIMAT0018986 | hsa-miR-4462 | −1.0690462 | 0.004846197 |
| MIMAT0028211 | hsa-miR-7150 | −1.06220727 | 0.040895785 |
| MIMAT0027408 | hsa-miR-6754-5p | −1.03580421 | 0.022410575 |
| MIMAT0021128 | hsa-miR-5196-5p | −1.0324015 | 0.035802131 |
| MIMAT0020601 | hsa-miR-1273f | −1.02885192 | 0.031420972 |
| MIMAT0022260 | hsa-miR-5572 | −1.02726895 | 0.033720698 |
| MIMAT0021120 | hsa-miR-5189-5p | −1.01814356 | 0.020114063 |
| MIMAT0027548 | hsa-miR-6824-5p | −1.01710815 | 0.020467938 |
| MIMAT0031016 | hsa-miR-8089 | −1.01329602 | 0.021980497 |
| MIMAT0009201 | hsa-miR-196b-3p | 1.33573282 | 0.048038696 |
| MIMAT0004607 | hsa-miR-138-1-3p | 1.405280355 | 0.023694469 |
Log fold change >0, up-regulated; log fold change <0, down-regulated
Fig. 1.
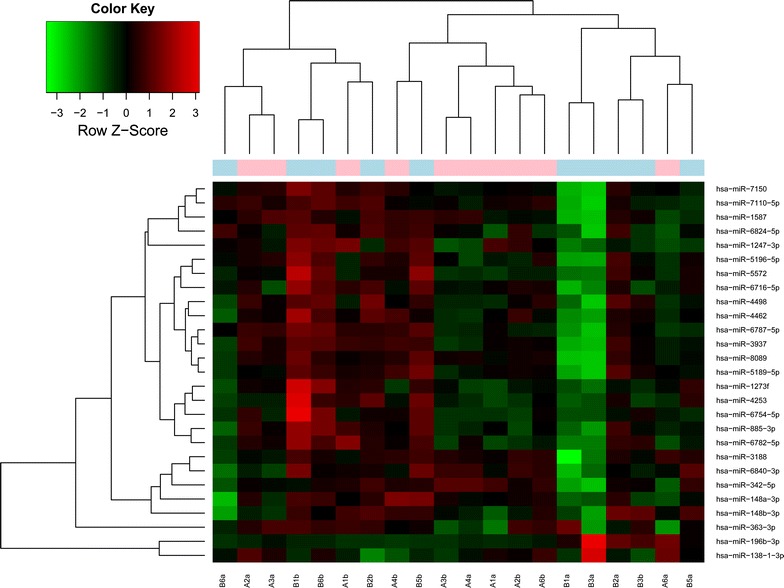
Heat map of differentially expressed microRNAs. Red represents up-regulation and green represents down-regulation
Analysis of network of miRNAs-targets
According to multiMiR package, a number of 1445 target genes of 13 DE-miRs were screened out. Based on the aforementioned criterion, the network of miRNA-target was constructed using Cytoscape (Fig. 2). In the network, hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p were identified with the most target genes, and furthermore, the three miRNAs targeted numerous common genes such as CAND1 (cullin-associated and neddylation-dissociated 1), KLF4 (Kruppel-like factor 4), S1PR1 (sphingosine-1-phosphate receptor 1), Wnt1 (wingless-type MMTV integration site family, member 1), CNTN4 (contactin 4) and BCL2L11 [BCL2-like 11 (apoptosis facilitator)].
Fig. 2.
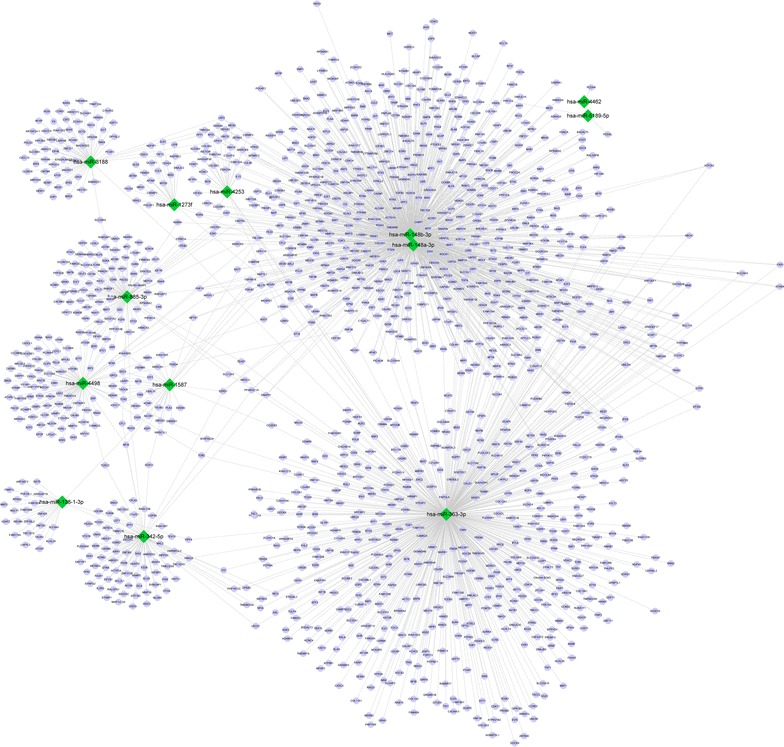
Network of differentially expressed microRNAs-target genes. Circle represents target genes and diamond represents microRNAs. Red represents up-regulation and green represents down-regulation
Functional and pathways of DE-miRs
KEGG pathway and GO functional enrichment analysis indicated that only three miRNAs (hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p) were significantly enriched in numerous common KEGG pathways and GO biological processes (P < 0.05, Additional file 1: Table S1). Among those significant pathways, some known cancer-related pathways were screened out, including “pathway in cancer”, “Wnt signaling pathway”, “MAPK signaling pathway” and “Jak-STAT signaling pathway” (Fig. 3).
Fig. 3.
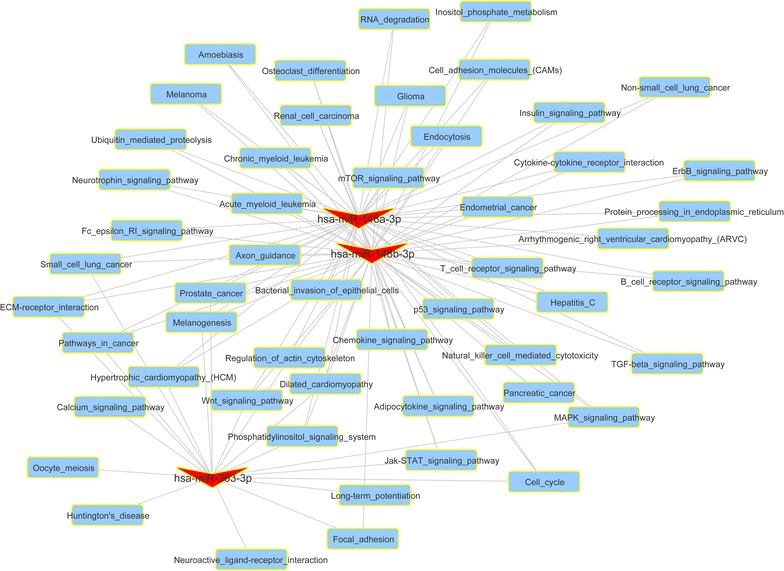
Network of functional enrichment results of differentially expressed microRNAs. V-shape represents microRNA and rectangle represents significant pathways enriched by target genes of microRNAs
Discussion
MiRNAs exert regulatory effects on gene expression in humans, resulting in cell growth, differentiation and apoptosis via down-regulating target genes in cancer [21]. In the present study, a total of 27 DE-miRs were screened out, including hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p, which targeted the most genes. Furthermore, the three miRNAs were significantly enriched in cancer-related pathways, including Wnt signaling pathway, MAPK (mitogen-activated protein kinase) signaling pathway and Jak-STAT (Janus kinase-signal transducer and activator of transcription) signaling pathway.
Wnt signaling pathway is implicated in cancer development and its hyperactivation can lead to enhanced tumorigenicity and increased metastatic potential [22]. In gastric cancer, overexpressed miR-544a is demonstrated to activate WNT signaling pathway which further contributes to the disease progression [13]. In the present study, we identified that hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p were remarkably enriched in Wnt signaling pathway. Reportedly, miR-148a inhibits the metastasis of hepatocellular carcinoma via acting on Wnt signaling pathway [23]. MiR-363 down-regulates the expression of myeloid cell leukemia-1 [24] whose expression correlates with phosphorylated glycogen synthase kinase-3beta, the key component of Wnt signaling pathway in breast cancer [25]. Notably, the above miRNA targets, such as Wnt1, were significantly enriched in Wnt signaling pathway. We can speculate that the miR-148a, miR-148b and miR-363 may play significant roles in gastric cancer progression via regulating the Wnt signaling pathway.
Besides, MAPK signaling pathway is dysregulated in gastric cancer, leading to abnormal cell proliferation and metastasis [26]. Moreover, the signaling pathway is implicated in drug resistance in gastric cancer by regulating the expression of apoptotic proteins Bax (BCL2-associated X protein)/Bcl-2 (B-cell CLL/lymphoma 2) [27]. Our enrichment analysis showed that hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p were also significantly enriched in MAPK signaling pathway. In breast cancer, miR-148a acts as a tumor suppressor via targeting IGF-IR (insulin-like growth factor-I receptor) and IRS1 (insulin receptor substrate 1) and further suppressing the downstream MAPK signaling pathway [28]. Besides, miR-363 is found to be down-regulated in gastric cancer and its down-regulation is associated with tumor differentiation, TNM stage and lymph-node metastasis [29]. Notably, the suppression of their common target KLF4 could inhibit the expression of various Erk5 (mitogen-activated protein kinase 7) targets and further affect the MAPK cascade in the regulation of endothelial integrity in cancer [30]. The enrichment of miR-148a, miR-148b and miR-363 in MAPK signaling pathway may suggest a joint contribution to gastric cancer development via involving MAPK signaling pathway.
Additionally, hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p were found to be dramatically enriched in Jak-STAT signaling pathway. Jak-STAT serves as a straightforward mechanism whereby cells sense environmental cures and further regulate cell growth and differentiation in cancer [31]. The inhibition of Jak-STAT signaling pathway can lead to decreased cell proliferation and enhanced cell apoptosis in gastric cancer cells [32]. Exogenous miR-363 promotes cell growth, progression and tumorsphere formation of SC-M1 gastric cancer cells, and the knockdown of miR-363 suppresses tumorigenesis of SC-M1 cells [33]. MiR-148a/b is dysregulated and their haplotype is significantly correlated with gastric cancer [34]. S1PR1, one of the common targets of miR-148a-3p, -148b-3p and -363-3p, is implicated in NFκB/IL-6/STAT3/S1PR1 amplification loop that is important for chronic colitis-related cancer and can be suggested as therapeutic option [35]. The enrichment of the three miRNAs in Jak-STAT signaling pathway implies their involvement in gastric cancer progression via Jak-STAT signaling pathway.
We should note that there were some limitations in the present study. Herein, although we demonstrated a significant enrichment of dysregulated hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p in cancer-related pathways in patients with gastric cancer, we did not further validate their expressions, nor demonstrate their roles in cancer-related pathways using systematically functional experiments. Moreover, as there are only 10 samples enrolled in this study, we did not further consider the DE-miRs among different stages. Besides, the present results were just obtained based on microarray analysis and bioinformatics prediction, and needed to be further validated in future. However, this study can be regarded as a preliminary study and to an extent provide some valuable directions for future studies, especially for researches on gastric cancer in Tibetan.
In summary, the present study identified a downregulation of hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p in gastric cancer in Tibetan using microarray analysis. What is more, we demonstrated their significant enrichment in cancer-related pathways, including Wnt signaling pathway, MAPK signaling pathway and Jak-STAT signaling pathway. These findings suggested the potential usage of hsa-miR-148a-3p, hsa-miR-148b-3p and hsa-miR-363-3p as diagnostic and therapeutic biomarkers for gastric cancer-infected Tibetan. However, further experimental validations are in urgent need to confirm these results.
Authors’ contributions
YL and CZ carried out the molecular studies, FT participated in the sequence alignment, JZ, CS and CW drafted the manuscript. PY carried out the study. YL participated in the sequence alignment. MW and JD participated in the design of the study and performed the statistical analysis. RC, GR, YL and CZ conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by Scientific research project funds of Qinghai department (2014-ZJ-737) and Qinghai-Utah Joint Research Key Lab for High Altitude Medicine (No.2014-ZJ-Y39). We wish to express our warm thanks to CaptialBio Corporation. Their ideas and help gave a valuable added dimension to our research.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- Bax
BCL2-associated X protein
- Bcl-2
B-cell CLL/lymphoma 2
- CAND1
cullin-associated and neddylation-dissociated 1
- KLF4
Kruppel-like factor 4
- S1PR1
sphingosine-1-phosphate receptor 1
- Wnt1
wingless-type MMTV integration site family, member 1
- CNTN4
contactin 4
- BCL2L11
BCL2-like 11 (apoptosis facilitator)
- GO
Gene Ontology
- MicroRNAs
miRNAs
- SPAG9
sperm associated antigen 9
- RECK
reversion-inducing-cysteine-rich protein with kazal motifs
- TNM
tumor node metastasis stages
Additional file
10.1186/s12935-015-0266-1 The significant KEGG and GO_BP terms of differentially expressed microRNAs. KEGG, Kyoto Encyclopedia of Genes and Genomes; GO Gene Ontology; BP, biological processes.
Footnotes
Yushuang Luo and Chengwu Zhang are regarded as co-first author
Contributor Information
Yushuang Luo, Email: qfyluoyushuang@163.com.
Chengwu Zhang, Email: xtoof@sina.com.
Feng Tang, Email: leileitang1984@163.com.
Junhui Zhao, Email: zhao699@126.com.
Cunfang Shen, Email: qfyshengcunfang@163.com.
Cheng Wang, Email: wanggh-8@163.com.
Pengjie Yu, Email: Hnypi768@126.com.
Miaozhou Wang, Email: wangmiaozhou@163.com.
Yan Li, Email: 15202562528@163.com.
J. I. Di, Email: luosangdj@126.com
Rong Chen, Email: sorrykitty-333@163.com.
Ge Rili, Phone: 13997016441, Phone: 09716142063, Email: geriligao@hotmail.com.
References
- 1.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Gu Y, Chen T, Li G, Yu X, Lu Y, Wang H, Teng L. LncRNAs: emerging biomarkers in gastric cancer. Future Oncol (London, England). 2015;11:2427–2441. doi: 10.2217/fon.15.175. [DOI] [PubMed] [Google Scholar]
- 3.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, Ono H, Tanabe S, Kaminishi M. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thrumurthy SG, Chaudry MA, Chau I, Allum W. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol. 2015;12:676–682. doi: 10.1038/nrclinonc.2015.132. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Zhao JH, Wang XL, Di JI, Liu ZB, Li GY, Wang MZ, Li Y, Chen R, Ge RL. Analysis of mitochondrial DNA in Tibetan gastric cancer patients at high altitude. Mol Clin Oncol. 2015;3:875–879. doi: 10.3892/mco.2015.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao ZF, Wang ZN, Zhao TT, Xu YY, Wu JH, Liu XY, Xu H, You Y, Xu HM. Overexpression of SPAG9 in human gastric cancer is correlated with poor prognosis. Virchows Arch. 2015;467:525–533. doi: 10.1007/s00428-015-1826-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhaxi CM, Wu KC, Qiao TD, Shen HQ, Du AC, Suolang DJ, Huang Y, Fan DM. Expression pattern of tumor-associated antigen MG7-Ag in gastric cancer: difference between Tibetans and Hans. Zhonghua nei ke za zhi. 2004;43:265–268. [PubMed] [Google Scholar]
- 8.Ou J, Li K, Ren H, Bai H, Zeng D, Zhang C. Association and haplotype analysis of prostate stem cell antigen with gastric cancer in Tibetans. DNA Cell Biol. 2010;29:319–323. doi: 10.1089/dna.2009.0960. [DOI] [PubMed] [Google Scholar]
- 9.Gayral M, Jo S, Hanoun N, Vignolle-Vidoni A, Lulka H, Delpu Y, Meulle A, Dufresne M, Humeau M, Du Rieu MC. MicroRNAs as emerging biomarkers and therapeutic targets for pancreatic cancer. World J Gastroenterol. 2014;20:11199. doi: 10.3748/wjg.v20.i32.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174–1179. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu C-G, Oue N, Yasui W. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi Kurdistani Z, Saberi S, Tsai KW, Mohammadi M. MicroRNA-21: mechanisms of oncogenesis and its application in diagnosis and prognosis of gastric cancer. Arch Iran Med. 2015;18:524–536. [PubMed] [Google Scholar]
- 13.Yanaka Y, Muramatsu T, Uetake H, Kozaki KI, Inazawa J. miR-544a induces epithelial-mesenchymal transition through the activation of WNT signaling pathway in gastric cancer. Carcinogenesis. 2015;36:1363–1371. doi: 10.1093/carcin/bgv106. [DOI] [PubMed] [Google Scholar]
- 14.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 15.Smyth G. Limma: linear models for microarray data Bioinformatics and Computational Biology Solutions using R and Bioconductor 2005. New York: Springer; 2005. [Google Scholar]
- 16.Ru Y, Kechris KJ, Tabakoff B, Hoffman P, Radcliffe RA, Bowler R, Mahaffey S, Rossi S, Calin GA, Bemis L. The multiMiR R package and database: integration of microRNA–target interactions along with their disease and drug associations. Nucleic Acids Res. 2014;42:e133-2–e133-10. doi: 10.1093/nar/gku631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smoot ME, Ono K, Ruscheinski J, Wang P-L, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 19.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2015;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consortium GO Gene Ontology annotations and resources. Nucleic Acids Res. 2013;41:D530–D535. doi: 10.1093/nar/gks1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juzenas S, Salteniene V, Kupcinskas J, Link A, Kiudelis G, Jonaitis L, Jarmalaite S, Kupcinskas L, Malfertheiner P, Skieceviciene J. Correction: analysis of deregulated microRNAs and their target genes in gastric cancer. PLoS One. 2015;10:e0135762. doi: 10.1371/journal.pone.0135762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai D, Wells K, Arcaroli J, Vanderbilt C, Aisner DL, Messersmith WA, Lieu CH. Targeting the WNT signaling pathway in cancer therapeutics. Oncol. 2015;20:1189–1198. doi: 10.1634/theoncologist.2015-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan H, Dong X, Zhong X, Ye J, Zhou Y, Yang X, Shen J, Zhang J. Inhibitions of epithelial to mesenchymal transition and cancer stem cells-like properties are involved in miR-148a-mediated anti-metastasis of hepatocellular carcinoma. Mol Carcinog. 2014;53:960–969. doi: 10.1002/mc.22064. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, Li Y, Dong X, Peng L, Nie X. MiR-363 sensitizes cisplatin-induced apoptosis targeting in Mcl-1 in breast cancer. Med Oncol. 2014;31:347. doi: 10.1007/s12032-014-0347-3. [DOI] [PubMed] [Google Scholar]
- 25.Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, Lee DF, Yang JY, Xie X, Liu JC, Hung MC. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67:4564–4571. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 26.Luo G, Zhou Y, Yi W, Yi H. Lactotransferrin expression is downregulated and affects the mitogen-activated protein kinase pathway in gastric cancer. Oncol Lett. 2015;9:2409–2413. doi: 10.3892/ol.2015.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan W, Yu HG, Luo HS. Inhibition of the p38 MAPK pathway sensitizes human gastric cells to doxorubicin treatment in vitro and in vivo. Mol Med Report. 2014;10:3275–3281. doi: 10.3892/mmr.2014.2598. [DOI] [PubMed] [Google Scholar]
- 28.Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y, Qi YT, Xu Q, Li W, Lu B, Peiper SS, Jiang BH, Liu LZ. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J Mol Cell Biol. 2013;5:3–13. doi: 10.1093/jmcb/mjs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Liu X, Hu Z, Wang Y, Liu M, Liu X, Li H, Ji R, Guo Q, Zhou Y. Identification and characterization of tumor suppressor and oncogenic miRNAs in gastric cancer. Oncol Lett. 2015;10:329–336. doi: 10.3892/ol.2015.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohnesorge N, Viemann D, Schmidt N, Czymai T, Spiering D, Schmolke M, Ludwig S, Roth J, Goebeler M, Schmidt M. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4) J Biol Chem. 2010;285:26199–26210. doi: 10.1074/jbc.M110.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’shea JJ, Schwartz DM, Villarino AV, Gadina M, Mcinnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim TY, Oh DY, Bang YJ. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 2013;335:145–152. doi: 10.1016/j.canlet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Hsu KW, Wang AM, Ping YH, Huang KH, Huang TT, Lee HC, Lo SS, Chi CW, Yeh TS. Downregulation of tumor suppressor MBP-1 by microRNA-363 in gastric carcinogenesis. Carcinogenesis. 2014;35:208–217. doi: 10.1093/carcin/bgt285. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Wang G, Lu X, Gao P, Song Y, Sun J, Li A, Xu Y, Xu H, Wang Z. Polymorphisms and haplotypes of the miR-148/152 family are associated with the risk and clinicopathological features of gastric cancer in a Northern Chinese population. Mutagenesis. 2014;29:401–407. doi: 10.1093/mutage/geu050. [DOI] [PubMed] [Google Scholar]
- 35.Theiss AL. Sphingosine-1-phosphate: driver of NFkappaB and STAT3 persistent activation in chronic intestinal inflammation and colitis-associated cancer. Jak-stat. 2013;2:e24150. doi: 10.4161/jkst.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]


