Abstract
Corneas with severe pathologies have a high risk of rejection when conventionally grafted with human donor tissues. In this early observational study, we grafted bioengineered corneal implants made from recombinant human collagen and synthetic phosphorylcholine polymer into three patients for whom donor cornea transplantation carried a high risk of transplant failure. These patients suffered from corneal ulcers and recurrent erosions preoperatively. The implants provided relief from pain and discomfort, restored corneal integrity by promoting endogenous regeneration of corneal tissues, and improved vision in two of three patients. Such implants could in the future be alternatives to donor corneas for high‐risk patients, and therefore, merits further testing in a clinical trial.
Keywords: collagen, epithelium, grafting, patients, remodeling, transplantation
Introduction
The human cornea is the main refractive surface focusing light into the eye, so its transparency is critical for vision. Irreversible transparency loss from injury or disease can, therefore, lead to blindness. Globally, approximately 4.9 million individuals have bilateral cornea blindness, while 23 million are unilaterally cornea blind.1 Donor tissue transplantation is the only widely accepted treatment but the need for donor corneas exceeds the supply and the gap only increases as the population ages.
Recent advances in corneal transplantation techniques with a shift from penetrating to safer lamellar procedures and wide application of limbal epithelial stem cell transplantation, have resulted in improved outcomes, and have expanded the number of cases of corneal blindness that can now be treated successfully.2 However, patients with severe pathologies (e.g., chemical burns, previously rejected grafts, autoimmune disease, and infections) still have a high risk of rejection and failure, often needing multiple surgeries.3, 4, 5, 6, 7, 8 Corneal prostheses are available, but they remain mainly for end‐stage disease and even if implanted as primary procedure they often are accompanied by vision‐threatening complications,1, 9 leaving an unmet need for a de novo solution for high‐risk patients.
In Ukraine, as in many countries, there is a severe shortage of donor corneas. The annual need in 2010 was 4000 grafts, but only 511 corneal transplantations were performed due to the lack of donated tissues. Fifty‐four percent of these transplantations were performed for tectonic purposes due to the increasing number of infections and injuries.10 To solve the problems of lack of donor tissues as well as the high risk of rejection of allografted corneas, we had developed corneal implants made from interpenetrating networks of cross‐linked recombinant human collagen type III (RHCIII) and 2‐methacryloyloxyethyl phosphorylcholine (MPC), a synthetic phospholipid11 (Figure 1). MPC‐polymer has antifouling properties and has been FDA approved as a polymer coating in vascular stents.12 Here, it was incorporated into RHCIII to prevent neovascularization.
Figure 1.
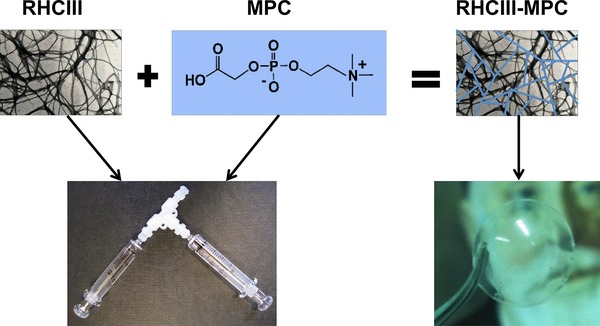
Schematic diagram showing the combination of recombinant human collagen type III (RHCIII) and 2‐methacryloyloxyethyl phosphorylcholine (MPC) to form interpenetrating networks of RHCIII‐MPC. The RHCIII and MPC were mixed together in a syringe, while the final hydrogel was molded into an implant. An image of Vladimir Filatov, pioneer of human donor cornea grafting, can be seen through the transparent implant.
We previously tested implants comprising RHCIII alone as substitutes for donor corneas in a Phase 1 clinical study, where they promoted good tissue integration with stable regeneration of corneal epithelium, stroma and nerves, restoration of corneal reflex better than allograft (p = 0.04), maintenance of transparency in cornea and no tissue rejection reported on 4‐year follow‐up, in the absence of long‐term steroid immunosuppression beyond Week 7 postimplantation, in patients with keratoconus or central scarring, i.e., low‐risk patients,13 Like donor corneas, however, RHCIII only implants became neovascularized when grafted into a model of severe pathology, the alkali‐burned rabbit cornea.14 Incorporation of MPC into RHCIII, on the other hand, yielded implants that repelled blood vessels14 while allowing regeneration.15, 16
Here, we report our initial experience with RHCIII‐MPC implants into three patients for whom donor cornea grafting carried a high risk of rejection. The primary aim of this early investigation was to assess the safety of such an approach. The secondary aim was to test the feasibility of restoring the integrity of the cornea.
Methods
This study was performed in accordance to the Declaration of Helsinki Convention of the Council of Europe on Human Rights and Biomedicine, relevant Laws of Ukraine, and after approval by the Bioethics Commission of the Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine and trial registration (registered EudraCT No. 2013‐002442‐37). After providing written informed consent, each patient was grafted with a RHCIII‐MPC implant consisting of RHCIII (8% wt/vol; from FibroGen, Inc, San Francisco, USA),17 MPC (4% wt/vol) and poly(ethylene glycol) diacrylate (1.37% wt/vol)11 by anterior lamellar corneal transplantation.
Patient 1, an 80‐year‐old male, suffered an alkali burn to his right eye resulting in a persistent corneal ulcer resistant to conventional medical treatment and bandage contact lens wear. He suffered from pain, tearing, and photophobia due to the inability of the corneal epithelium to stably adhere to the underlying damaged and vascularized stroma. Patient 1's best corrected visual acuity (BCVA) was 6/600, i.e., near blindness. Patient 2 was a 72‐year‐old female with a previously rejected penetrating human cornea graft in her left eye, which was combined with cataract extraction and intraocular lens (IOL) implantation. She suffered from corneal ulceration, which was unresponsive to medical treatment and bandage contact lens wear, and had the same symptoms as Patient 1, with only light perception, i.e., effectively blind. She had a very dense stromal opacification in the ulcerated area. Patient 3 was a 52‐year‐old male who suffered from recurrent corneal erosions following an acid burn. The erosions were resistant both to medical treatment and bandage contact lens wear. The underlying stroma was scarred and vascularized. His symptoms were similar to those of the other two patients. During the chronic stage of the burn, he received excimer laser phototherapeutic keratectomy and a human amniotic membrane (HAM) graft for ocular surface healing. He has also had a cataract phacoemulsification and was implanted with an IOL one year after the injury. His BCVA was 6/600, i.e., near blindness.
All three patients needed surgery to treat the ulceration, restore corneal integrity, alleviate the associated pain and discomfort, and to improve vision. Based on literature review all were considered high‐risk patients for limbal epithelial graft rejection as well as penetrating or lamellar cornea graft rejection and failure.
Each patient's pathologic cornea was initially cut with a 4–5‐mm‐diameter trephine (depending on lesion diameter) to remove the lesioned area with a small epithelialized margin around it. The incision was then deepened with a diamond knife set to a depth of 250 or 350 μm (depending on lesion depth). Manual lamellar dissection was then used to remove the pathologic corneal tissue. A trephine of the same diameter was used to cut the biosynthetic implant of corresponding thickness. Once in place, the implant was anchored with three to four overlying 10–0 nylon mattress sutures to avoid puncturing the implant material. After surgery, they received a topical antibiotic (moxifloxacin hydrochloride 0.5%, s.a. Alcon‐Couvreur n.v., Puurs, Belgium), a short‐term mydriatic (cyclopentolate 1%, Sentiss Pharma Pvt. Ltd., Gurgaon, India) and a nonsteroidal anti‐inflammatory drug (indomethacin 0.1%, Bausch + Lomb GmbH, Dr. Gerhard Mann chem.‐pharm. Fabrik GmbH, Berlin, Germany) for 2 weeks. This was followed by a topical antiseptic (chlorhexidine bigluconate 0.02%, Farmacia, Lugansk, Ukraine) and a steroid (dexamethasone 0.1%, s.a. Alcon‐Couvreur n.v., Puurs, Belgium) for 4 weeks. The patients wore 14‐mm bandage contact lenses containing 36% water (Bausch & Lomb PureVision) until epithelial regeneration was complete.
Patients were assessed at 1, 3, 6, 9, and 12 months postoperatively or as determined required by the physician. The patients’ observations of their own pain, irritation and photophobia were recorded. Clinical assessment performed included slit‐lamp biomicroscopy to check for signs of inflammation or infection, a fluorescein staining test to confirm epithelial integrity, corneal surface sensitivity assessment (Cochet–Bonnet esthesiometer, Luneau Ophthalmologie, France), best corrected visual acuity measurement, transpalpebral tonometry to measure intraocular pressure without damaging the epithelium (Diaton, Ryazan State Instrument‐Making Enterprise, Russia), ultrasound pachymetry (SP‐100, Tomey, Japan) and in vivo confocal microscopy (ConfoScan4, Nidek, Japan).
Results
Table 1 shows the optical and physical properties of the RHCIII‐MPC implants. All implants had similar optical properties to healthy human corneas. While they were mechanically weaker than human donor corneas, they were nevertheless robust enough to withstand the handling and grafting procedure.
Table 1.
Properties of RHCIII‐MPC hydrogels used as corneal implants
| Properties | Transmission at 500 nm (%) | Backscatter (%) | Refractive index | Tensile strength (MPa) | Elongation (%) | Modulus (MPa) | Denaturation temperature (°C) |
|---|---|---|---|---|---|---|---|
| Implants | 92.1 ± 0.1 | 1.66 ± 0.55 | 1.334 ± 0.000 | 0.26 ± 0.06 | 12.15 ± 0.84 | 3.63 ± 0.84 | 56.96 ± 1.05 |
| Human cornea | 87.1 ± 2.01 | <3+ | 1.373–1.380‡ | 3.81 ± 0.40§ | – | 3–13**,++ | 65.1‡‡ |
1Doutch J, Quantock AJ, Smith VA, Meek KM. Light transmission in the human cornea as a function of position across the ocular surface: theoretical and experimental aspects. Biophys J. 2008; 95: 5092–5099.
+van den Berg TJ, Tan KE. Light transmittance of the human cornea from 320 to 700 nm for different ages. Vision Res. 1994; 34: 1453–1456.
‡Patel S, Marshall J, Fitzke FW, 3rd. Refractive index of the human corneal epithelium and stroma. J Refract Surg. 1995; 11: 100–105.
§Zeng Y, Yang J, Huang K, Lee Z, Lee X. A comparison of biomechanical properties between human and porcine cornea. J Biomech. 2001; 34: 533–537.
**Crabb RA, Chau EP, Evans MC, Barocas VH, Hubel A. Biomechanical and microstructural characteristics of a collagen film‐based corneal stroma equivalent. Tissue Eng. 2006; 12: 1565–1575.
++Jue B, Maurice DM. The mechanical properties of the rabbit and human cornea. J Biomech. 1986; 19: 847–853.
‡‡Merrett K, Fagerholm P, McLaughlin CR, Dravida S, Lagali N, Shinozaki N, Watsky MA, Munger R, Kato Y, Li F, Marmo CJ, Griffith M. Tissue‐engineered recombinant human collagen‐based corneal substitutes for implantation: performance of type I versus type III collagen. Invest Ophthalmol Vis Sci. 2008; 49: 3887–3894.
Epithelial coverage of the initially cell‐free implants took on average 6 weeks (Table 2, Figure 2). All three implants remained free of neovascularization or epithelial erosions over the 9–12 months postoperation follow‐up period. However, conjunctival epithelium invaded the implant surface in Patient 3, due to limbal stem cell deficiency (Figure 2). There was no stromal edema, no prolonged inflammation nor any infection in any patient. Touch sensitivity increased after surgery in all patients although not to the level of normal human corneas (Figure 3). After the mild deturgescence of the edema that always accompanies corneal ulceration, the corneal thickness remained stable throughout the follow‐ups. Intraocular pressure remained within normal ranges. Tear production was comparable to that in the nonoperated contralateral eyes. Signs of punctate precipitate and haze appeared at the posterior surface of the implant during the third postoperative week in all cases, but resolved after two weeks of treatment with topical steroids. Visual acuity improved in Patients 1 and 2 from near blindness (6/600 and LP) to moderate (6/38) and severe (6/75) vision loss, respectively, allowing for restricted function with enhancing aids. Patient 3, who had conjunctivalization of corneal surface, showed no change in vision but no longer had recurrent painful erosions seen preoperatively, suggesting that RHCIII‐MPC implants could stabilize the corneal surface pending further treatment, e.g., limbal stem cell grafting to improve vision. Implants remained stably incorporated without immunosuppression beyond the 6‐week course of prophylactic anti‐inflammatory medication.
Table 2.
Details of implanted patients showing their biometrics, diagnosis, treatment, and results
| No. | Age (years) | Sex | Diagnosis | Time after disease start (months) | Previous surgeries | Graft diameter (mm) | Graft thickness (μm) | Suture removal (weeks after surgery) | Full epithelial coverage (weeks after surgery) | BCVA* before | BCVA at last follow‐up | Corneal pachymetry of operated eye (μm) before surgery/at last follow‐up | Schirmer test I at last follow‐up operated/fellow nonoperated control (mm/5 min) | IOP of operated eye before surgery/at last follow‐up (mm Hg) | Follow‐up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80 | M | CU/AlkB | 2 | – | 4 | 250 | 3 | 8 | 6/200 | 6/38 | 550/459 | 12/11 | 21/19 | 12 |
| 2 | 72 | F | CU/PK | 14 | PK + CE + IOL | 4 | 250 | 11 | 8 | LP | 6/75 | 512/447 | 7/6 | 17/19 | 12 |
| 3 | 52 | M | RCE/AcB | 36 | PTK,HAM, Phaco + IOL | 5 | 350 | 2 | 2 | 6/600 | 6/200 | 532/512 | 15+/15+ | 16/18 | 9 |
CU = corneal ulcer; RCE = recurrent corneal erosion; AcB = acid burn; AlkB = alkali burn; PTK = phototherapeutic keratectomy; CE = cataract extraction; IOL = intraocular lens implantation; Phaco = cataract phacoemulsification; HAM = human amniotic membrane transplantation; PK = penetrating keratoplasty; LP = light perception.
*BCVA = best corrected visual acuity (LP, 6/600–6/375: near blindness; 6/300–6/150: profound vision loss; 6/120–6/60: severe vision loss: 6/48–6/24: moderate vision loss; 6/19–6/9.5: mild vision loss; 6/7.5–6/3.8: normal range of vision).
Figure 2.
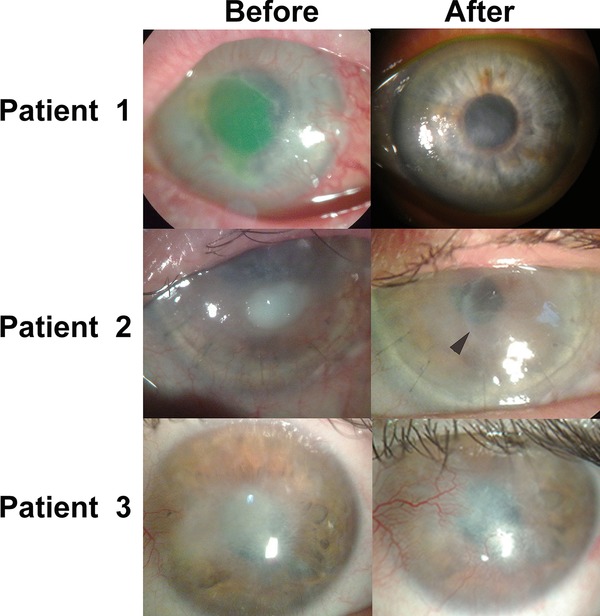
Corneas of all three patients before and after implantation with tectonic grafts of RHCIII‐MPC. Patient 1's cornea had an ulcer overlying a vascularized stroma. The green fluorescein staining delineates the large area of eroded epithelium. At 12 months postoperation, the cornea is intact and relatively clear. Patient 2 had a failed 8.5‐mm‐diameter graft with a persistent ulcer and dense stromal opacification in the visual zone prior to surgery. A small 4‐mm implant was grafted into the ulcerated area of the failed graft (arrowhead) and has remained relatively clear after 12 months. Patient 3 had an opacity with an unstable epithelial surface and vascularized stroma prior to surgery. At 9 months postoperation, while the implant remained clear, the ingrowing conjunctiva has left the surface hazy.
Figure 3.
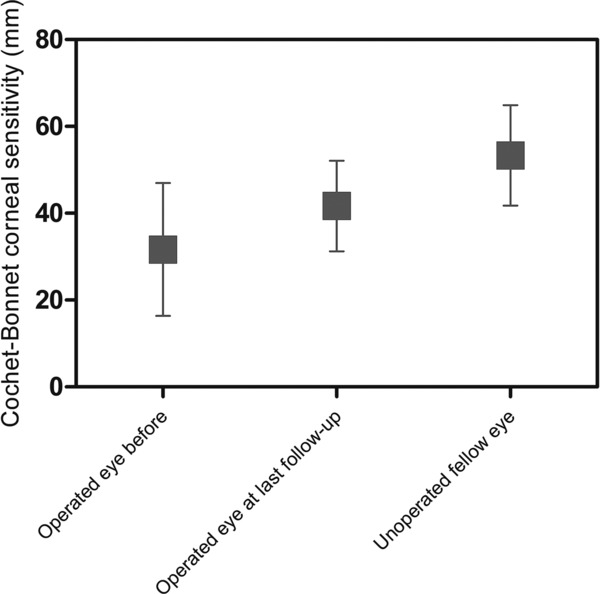
Restoration of touch sensitivity after grafting with RHCIII‐MPC implants as an indication of nerve function restoration. Central corneal touch sensitivity was assessed by contact esthesiometry in the patients’ corneas before and after implantation, with the nonoperated, contralateral eyes serving as controls. Measurements were obtained using a Cochet–Bonnet esthesiometer with a monofilament, where an increase in filament length (mm) corresponds to an increase in touch sensitivity (n = 3 patients).
In vivo confocal microscopy of the regenerated neo‐cornea of Patient 1 showed a regenerated epithelium (Figure 4A), which resembled that of a healthy cornea (Figure 4B). Stromal cells were not clearly seen due to corneal haze, but they appear to have started populating the initially cell‐free implant (Figure 4C) but have not reached the steady state seen in a healthy cornea (Figure 4D). The patient's preserved endothelium remained intact (Figure 4E).
Figure 4.
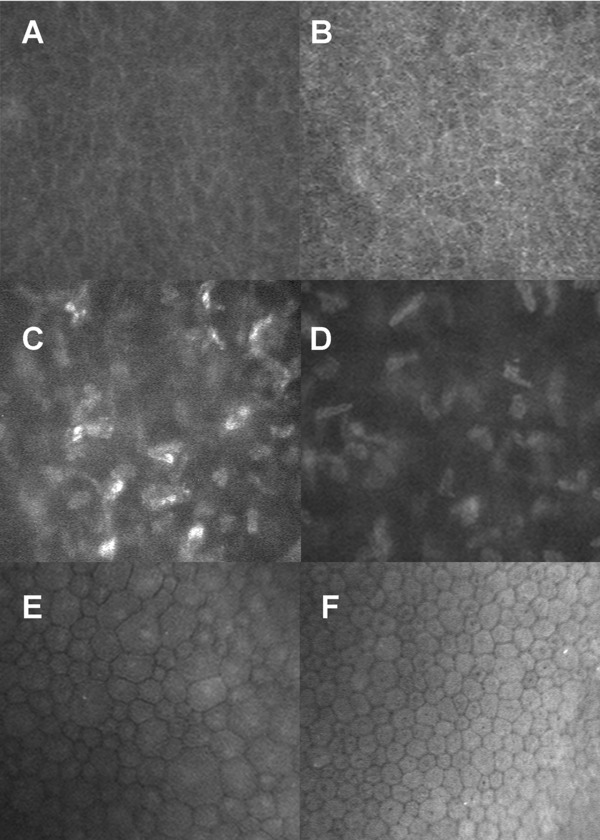
In vivo confocal microscopy through an implanted cornea at 12 months postoperation showing regenerated patient epithelium (A) that resembles that of a healthy cornea (B). Stromal cells have grown into the initially cell‐free graft, although partially obscured by haze (C), unlike the healthy cornea where the cells are clearly visible (D). The preserved patient endothelium (E) has a number of larger sized cells but also endothelial cells that resemble those of a healthy cornea (F).
Discussion
In many countries, such as Ukraine, China, South East Asia, and India, corneal blindness is a huge problem. In addition to the severe shortage in donated corneas,10 many patients suffer from severe pathologies that have a high risk of graft rejection or failure. Poor outcomes of corneal transplantation are likely due to graft rejection and stem cell deficiency.
Graft rejection is directed at allogenic cells from donor human corneas.18 This is circumvented by cell‐free RHCIII‐based corneal implants. The RHCIII replaces the largely collagenous stroma of the native cornea allowing for repopulation by endogenous host corneal cells, as we had previously reported in low‐risk patients.13 We have previously shown that the RHCIII‐MPC implants are composed of lamellae‐like layers that are interconnected with fiber‐like structures that crudely mimics the structure of the human cornea.11 Furthermore, the coefficients of glucose and albumin diffusion through these hydrogels were comparable to that of the human cornea.15 Additionally, MPC polymer has reported anti‐inflammatory properties,19, 20 possibly accounting for the capacity of RHCIII‐MPC implants to quiesce the immunopathologic corneas, allowing stable restoration of the ocular surface.
Corneal epithelial limbal stem cell transplantation, which is available in large urban centers in the developed world, is too cost prohibitive for the healthcare systems in many countries such as Ukraine, as it requires specialized, certified clean rooms and staffing to expand the cultures, plus time for harvesting through cultivation of cells. In addition, where the damage to the cornea extends below the epithelium, the patients require a subsequent donor graft. In this case, a grafted RHCIII‐MPC implant can allow for healing of the epithelium and stroma, providing relief from pain and discomfort for patients and allowing for a future limbal epithelial graft as the second step in the treatment. In this initial observational study, small grafts of 4–5 mm were used to replace the lesioned area with a minimal margin of healthy tissue. The rationale for this was patient safety, i.e., in case of a serious adverse device effect, the implant can be removed and replaced with a larger human donor cornea graft by deep lamellar keratoplasty, the current conventional treatment.
Following corneal wounding, overexpression of unaligned, mainly type III collagen occurs to form a scar.21 Bridging the wound gap with RHCIII‐MPC implants that have a regular lamellar structure,11 however, appears to provide a template for more controlled in‐growth of stromal cells that in turn provides for an optically clear regenerated cornea. Nonetheless, in patients with limbal epithelial stem cell deficiency, transplantation with corneal epithelial stem cells in conjunction with an implant will be needed to prevent conjunctival in‐growth that decreases optical clarity.
We have shown that under conditions that render standard of care suboptimal, e.g., Ukraine due to its on‐going crisis, making the situation approach that in much of the developing world,22 RHCIII implants have the potential to overcome the cornea supply shortage in various regions in the world, and avoid social and religious stigma some may have with allograft corneas. Furthermore, RHCIII‐MPC implants may be an alternative to donor corneas and possibly a superior substitute in high‐risk cases for restoring corneal integrity.
Conclusion
Despite of the limitations of this preliminary case study such as the very small sample population and relatively short followup of 9–12 months, our initial results nevertheless suggest that bioengineered RHCIII‐MPC implants may potentially be more efficacious alternatives to donor tissues in repairing corneas with severe pathologies. Therefore, further clinical evaluation in form of a clinical study with high‐risk patients is merited to determine their safety and full potential as alternatives to donor human cornea transplantation.
Acknowledgments
We thank Dr. Sally Hayes, School of Optometry and Vision Science, Cardiff University for implant optical characterization help; former PDFs and staff at Linköping University (LiU), Sweden and the Ottawa Hospital Research Inst., Canada who contributed to earlier versions of the current implants: W. Liu, T. Ekblad, C. Deng, C. He, F. Li, J‐I. Ahn, and K. Merrett. We also thank M. Rafat, (LiU), for technical assistance with RHCIII filtration and processing; L. Kuffova, J.V. Forrester (University of Aberdeen) and M. Ljunggren (LiU) for pre‐clinical testing of implants; and Illya Nasinnyk (Filatov Inst.) for performing IVCM on the patients.
This study was supported by the grants from the Swedish Research Council (EU Nanomedicine project “I‐CARE”; grant dnr 521‐2012‐5706), Integrative Regenerative Medicine Centre, Linköping, Sweden (to M.G. and P.F.), and a Swedish Institute fellowship (to O.B.).
References
- 1. Avadhanam VS, Liu CS. A brief review of Boston type‐1 and osteo‐odontokeratoprostheses. Br J Ophthalmol. 2014. Oct 27. pii: bjophthalmol‐2014‐305359. [Google Scholar]
- 2. Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012; 379: 1749–1761. [DOI] [PubMed] [Google Scholar]
- 3. Hicks CR, Fitton JH, Chirila TV, Crawford GJ, Constable IJ. Keratoprostheses: advancing toward a true artificial cornea. Surv Ophthalmol. 1997; 42: 175–189. [DOI] [PubMed] [Google Scholar]
- 4. Lyall DA, Tarafdar S, Gilhooly MJ, Roberts F, Ramaesh K. Long term visual outcomes, graft survival and complications of deep anterior lamellar keratoplasty in patients with herpes simplex related corneal scarring. Br J Ophthalmol. 2012; 96: 1200–1203. [DOI] [PubMed] [Google Scholar]
- 5. Yu AL, Kaiser M, Schaumberger M, Messmer E, Kook D. Welge‐Lussen U: donor‐related risk factors and preoperative recipient‐related risk factors for graft failure. Cornea. 2014; 33: 1149–1156. [DOI] [PubMed] [Google Scholar]
- 6. Arenas E, Esquenazi S, Anwar M, Terry M. Lamellar corneal transplantation. Surv Ophthalmol. 2012; 57: 510–529. [DOI] [PubMed] [Google Scholar]
- 7. Shortt AJ, Bunce C, Levis HJ, Blows P, Doré CJ, Vernon A, Secker GA, Tuft SJ, Daniels JT. Three‐year outcomes of cultured limbal epithelial allografts in aniridia and Stevens–Johnson syndrome evaluated using the clinical outcome assessment in surgical Trials assessment tool. Stem Cells Transl Med. 2014; 3: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramachandran C, Basu S, Sangwan VS, Balasubramanian D. Concise review: the coming of age of stem cell treatment for corneal surface damage. Stem Cells Transl Med. 2014; 3: 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang HY, Luo ZK, Chodosh J, Dohlman CH, Colby KA. Primary implantation of type I Boston keratoprosthesis in nonautoimmune corneal diseases. Cornea. 2015; 34: 264–270. [DOI] [PubMed] [Google Scholar]
- 10. Pasyechnikova NV, Drozhzhyna GI, Ostashevskiy VL, Gaydamaka TB. The modern problems of corneal transplantation in Ukraine. Medytsyna siogodni i zavtra. 2011; 50‐51: 218–222.
- 11. Islam MM, Cepla V, He C, Edin J, Rakickas T, Kobuch K, Ružel˙e Ž, Jackson BW, Rafat M, Lohmann CP, Valiokas R, Griffith M. Functional fabrication of recombinant human collagen‐phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater. 2015; 12: 70–80. [DOI] [PubMed] [Google Scholar]
- 12. Stefanini GG, Holmes DR, Jr . Drug‐eluting coronary–artery stents. N Engl J Med. 2013; 368: 254–265. [DOI] [PubMed] [Google Scholar]
- 13. Fagerholm P, Lagali NS, Ong JA, Merrett K, Jackson WB, Polarek JW, Suuronen EJ, Liu Y, Brunette I, Griffith M. Stable corneal regeneration four years after implantation of a cell‐free recombinant human collagen scaffold. Biomaterials. 2014; 35: 2420–2427. [DOI] [PubMed] [Google Scholar]
- 14. Hackett JM, Lagali N, Merrett K, Edelhauser H, Sun Y, Gan L, Griffith M, Fagerholm P. Biosynthetic corneal implants for replacement of pathologic corneal tissue: performance in a controlled rabbit alkali burn model. Invest Ophthalmol Vis Sci. 2011; 52: 651–657. [DOI] [PubMed] [Google Scholar]
- 15. Liu W, Deng C, McLaughlin CR, Fagerholm P, Lagali NS, Heyne B, Scaiano JC, Watsky MA, Kato Y, Munger R, Shinozaki N, Li F, Griffith M. Collagen‐phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials. 2009; 30:1551–1559. [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin CR, Acosta MC, Luna C, Fagerholm P, Lagali NS, Heyne B, Scaiano JC, Watsky MA, Kato Y, Munger R, Shinozaki N, Li F, Griffith M. Regeneration of functional nerves within full thickness collagen‐phosphorylcholine corneal substitute implants in guinea pigs. Biomaterials. 2010; 31: 2770–2778. [DOI] [PubMed] [Google Scholar]
- 17. Yang C, Hillas PJ, Báez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004; 18: 103–119. [DOI] [PubMed] [Google Scholar]
- 18. Koizumi N, Kinoshita S. The surgical treatment for corneal epithelial stem cell deficiency, corneal epithelial defect, and peripheral corneal ulcer In Dartt DA. ed. Encyclopedia of the Eye. Oxford, United Kingdom: Academic Press; 2010: pp. 239–246. [Google Scholar]
- 19. Yumoto H, Hirota K, Hirao K, Miyazaki T, Yamamoto N, Miyamoto K, Murakami K, Fujiwara N, Matsuo T, Miyake Y. Anti‐inflammatory and protective effects of 2‐methacryloyloxyethyl phosphorylcholine polymer on oral epithelial cells. J Biomed Mater Res A. 2015; 103: 555–563. [DOI] [PubMed] [Google Scholar]
- 20. Ehashi T, Takemura T, Hanagata N, Minowa T, Kobayashi H, Ishihara K, Yamaoka T. Comprehensive genetic analysis of early host body reactions to the bioactive and bio‐inert porous scaffolds. PLoS ONE. 2014; 9: e85132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janin‐Manificat H, Rovere MR, Galiacy SD, Malecaze F, Hulmes DJ, Moali C, Damour O. Development of ex vivo organ culture models to mimic human corneal scarring. Mol Vis. 2012; 18: 2896–2908. [PMC free article] [PubMed] [Google Scholar]
- 22. Oliva MS, Schottman T, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol. 2012; 60: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]


