Abstract
Carbon captured by marine organisms helps sequester atmospheric CO2, especially in shallow coastal ecosystems, where rates of primary production and burial of organic carbon (OC) from multiple sources are high. However, linkages between the dynamics of OC derived from multiple sources and carbon sequestration are poorly understood. We investigated the origin (terrestrial, phytobenthos derived, and phytoplankton derived) of particulate OC (POC) and dissolved OC (DOC) in the water column and sedimentary OC using elemental, isotopic, and optical signatures in Furen Lagoon, Japan. Based on these data analysis, we explored how OC from multiple sources contributes to sequestration via storage in sediments, water column sequestration, and air–sea CO2 exchanges, and analyzed how the contributions vary with salinity in a shallow seagrass meadow as well. The relative contribution of terrestrial POC in the water column decreased with increasing salinity, whereas autochthonous POC increased in the salinity range 10–30. Phytoplankton-derived POC dominated the water column POC (65–95%) within this salinity range; however, it was minor in the sediments (3–29%). In contrast, terrestrial and phytobenthos-derived POC were relatively minor contributors in the water column but were major contributors in the sediments (49–78% and 19–36%, respectively), indicating that terrestrial and phytobenthos-derived POC were selectively stored in the sediments. Autochthonous DOC, part of which can contribute to long-term carbon sequestration in the water column, accounted for >25% of the total water column DOC pool in the salinity range 15–30. Autochthonous OC production decreased the concentration of dissolved inorganic carbon in the water column and thereby contributed to atmospheric CO2 uptake, except in the low-salinity zone. Our results indicate that shallow coastal ecosystems function not only as transition zones between land and ocean but also as carbon sequestration filters. They function at different timescales, depending on the salinity, and OC sources.
Keywords: blue carbon, carbon sequestration, estuary, organic carbon dynamics, phytoplankton, seagrass meadow, sediment, stable isotope
Introduction
The ocean functions as an important carbon sink by absorbing atmospheric CO2 at a rate (2.3 ± 0.7 Pg-C yr−1) comparable to the rate of CO2 uptake by terrestrial ecosystems (2.6 ± 1.2 Pg-C yr−1) (IPCC, 2013). One of the contributors to the ocean carbon sink is ‘blue carbon’, which is carbon captured by marine organisms (Nellemann et al., 2009; Duarte et al., 2010, 2013; Macreadie et al., 2014). Processes involved in carbon sequestration include carbon storage in sediments, carbon sequestration in the water column, and air–sea CO2 exchange.
Blue carbon stored in sediment can be sequestered from atmospheric CO2 for geological timescales (Mateo et al., 1997; McLeod et al., 2011; Fourqurean et al., 2012; Macreadie et al., 2012). The burial rate of organic carbon (OC) is estimated to be higher in shallow coastal ecosystems (238 Tg-C yr−1), such as estuaries and seagrass meadows, than in the open ocean (6 Tg-C yr−1) (Nellemann et al., 2009). Furthermore, because shallow coastal ecosystems receive a substantial input of terrestrial carbon (0.9 Pg-C yr−1), they store allochthonous carbon captured on land (Regnier et al., 2013). The most productive marine ecosystems are shallow coastal ecosystems (Bianchi, 2007) in which abundant OC is produced via active photosynthesis by aquatic primary producers such as microalgae, macroalgae, and seagrass. Part of the produced OC is stored in the sediments. For example, seagrass-derived OC is estimated to contribute about 50% of sedimentary OC (Kennedy et al., 2010). However, the variability of the origin of the OC stored in sediments and the mechanisms mediating the composition of stored OC are largely unknown (Macreadie et al., 2012).
Although sequestration of OC in the water column has the potential to sequester atmospheric CO2, a mechanism for sequestering CO2 in the water column of shallow coastal ecosystems has not been identified. In the open ocean, water column sequestration is considered to be an important carbon sink, because refractory dissolved organic carbon (DOC) can be sequestered from atmospheric CO2 for geological timescales (Nagata, 2008; Jiao et al., 2010, 2014). In shallow coastal ecosystems, aquatic primary producers, benefitting from the high nutrient concentrations and sufficient light conditions, generate a substantial amount of autochthonous DOC (Carlson, 2002; Wada et al., 2008; Agustí & Duarte, 2013). Thus, one can expect that refractory OC may be sequestered in the water columns of shallow coastal ecosystems.
Air–sea CO2 exchange is the process that directly determines whether a habitat is a sink or source of atmospheric CO2. Shallow coastal ecosystems are considered to be a net source of atmospheric CO2 due to their high rate of mineralization of terrestrial OC (Borges, 2005; Borges et al., 2005; Cai et al., 2006; Chen & Borges, 2009; Cai, 2011; Regnier et al., 2013). However, recent studies have demonstrated that shallow coastal seagrass meadows in estuaries can be sinks for atmospheric CO2 (Maher & Eyre, 2012; Tokoro et al., 2014). Whether the net ecosystem production (NEP) of seagrass meadows is positive or negative is a key determinant of whether they are sinks or sources, respectively, of atmospheric CO2 (Maher & Eyre, 2012; Tokoro et al., 2014), but the linkages between the gas exchange process and OC dynamics such as inflow/outflow and production/consumption remain unclear.
There is increasing interest in understanding the role of OC derived from multiple sources in coastal carbon dynamics in response to global change (Bauer et al., 2013). Unlike sequestration in terrestrial ecosystems, water column OC dynamics intervene and mediate the above-mentioned key carbon sequestration processes in shallow coastal ecosystems. Furthermore, assessment of the dynamics should take into consideration the salinity gradient, because both freshwater and open ocean water affect shallow coastal ecosystems. In this study, we evaluated how OC derived from multiple sources contributed to the processes of carbon sequestration and how the contribution changed along a salinity gradient in a shallow seagrass meadow. We quantified the origin and composition of the OC [particulate organic carbon (POC), DOC, and sedimentary OC] using elemental, isotopic, and optical signatures. We hypothesized that the composition of sedimentary OC might be related to that of the POC in the water column (e.g., diffusion of terrestrial OC in the water column would occur along the salinity gradient, whereas autochthonous OC would be dominant in the productive zone of the estuary), and differences in the lability of the OC sources could also modify the composition of the sedimentary OC. In addition, we hypothesized that autochthonous OC production decreased the concentration of dissolved inorganic carbon (DIC) in the water column and thereby contributed to air–sea CO2 uptake, except in the low-salinity zone, where CO2 dynamics were dominated by mineralization of terrestrial OC.
Materials and methods
Study area
The Furen Lagoon in Japan is located at the boreal zone (43°19′46.5″N, 145°15′27.8″E) and has a surface area of 57.4 km2 (Fig.1). The lagoon is characterized by an average depth of about 1.0 m, and the lagoon mouth (depth: 13 m) connects the lagoon to the Sea of Okhotsk. The lagoon is brackish (salinity range: ∼31), and the northern part of the lagoon receives freshwater from the Furen, Yausubetsu, and Pon-Yausubetsu Rivers. In the catchment, there are numerous pastures where livestock and fodder crops are grown, and wastes from the livestock cause eutrophication (Montani et al., 2011). Seagrass meadows (dominant species: Zostera marina) occupy 67% of the total area of the lagoon. The aboveground biomass of seagrass ranges from 129 to 2440 g wet weight per m2 (Tokoro et al., 2014).
Figure 1.
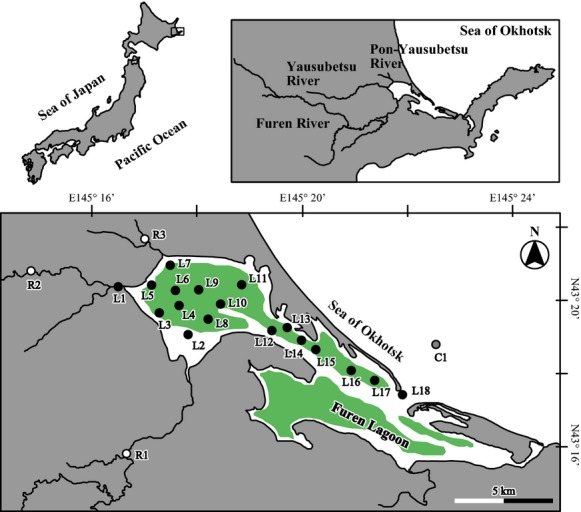
Location of the Furen Lagoon and the catchment. Closed, gray-shaded, and open circles show lagoon stations, a coastal station, and river stations, respectively. The green-shaded area indicates the seagrass meadow area.
The Furen Lagoon is an ideal model site to evaluate the linkage between carbon dynamics and complicated environmental factors because the lagoon is highly enclosed and the OC is derived from multiple sources. Furthermore, the lagoon includes habitats such as estuaries and seagrass meadows, which are hotspots of OC accumulation (Kennedy et al., 2010).
Sample collection
Field surveys were conducted in August and October 2012 and June 2013 at 22 locations from the river mouths to the coastal zone (Fig.1). Surface-water samples for analysis of DIC, nutrients, and organic matter (OM) in the water column were collected in August 2012 at 18 stations (L1–L18) in the lagoon from a research vessel and at stations at each of the three river mouths (R1–R3). Samples of bottom water were collected from the mouth of the lagoon (station L18). An evaluation of temporal variations of the carbon sequestration processes was impossible from this single survey. However, our goal was to understand processes by relating the spatial pattern to salinity.
At each station, the salinity of the water was recorded by using a conductivity–temperature sensor (COMPACT-CT, JFE Advantech, Nishinomiya, Japan). Samples for DIC and the fugacity of CO2 (fCO2) were dispensed into 250-mL Schott Duran bottles, which were poisoned with mercuric chloride (200 μl per bottle) to prevent DIC changed due to biological activity. Water samples for the other analyses were collected in acid-washed polyethylene bottles. Samples for nutrients (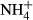 ,
, 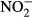 , and
, and 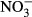 ), total dissolved nitrogen (TDN), DOC, and optical analyses of chromophoric dissolved organic matter (CDOM) were filtered through 0.2-μm polytetrafluoroethylene filters (DISMIC–25HP; Advantec, Durham, NC, USA) into precombusted (450 °C for 2 h) 50- or 100-ml glass vials and frozen at −20 °C until analysis. Samples for chlorophyll a (chl a) concentrations and analyses of POC, particulate nitrogen (PN), and stable isotope analysis (δ13CPOC and δ15NPN) were obtained by filtration (∼1 l) onto precombusted (450 °C for 2 h) glass–fiber filters (GF/F, Whatman, Maidstone, Kent, UK) and stored in the dark at −20 °C until analyses.
), total dissolved nitrogen (TDN), DOC, and optical analyses of chromophoric dissolved organic matter (CDOM) were filtered through 0.2-μm polytetrafluoroethylene filters (DISMIC–25HP; Advantec, Durham, NC, USA) into precombusted (450 °C for 2 h) 50- or 100-ml glass vials and frozen at −20 °C until analysis. Samples for chlorophyll a (chl a) concentrations and analyses of POC, particulate nitrogen (PN), and stable isotope analysis (δ13CPOC and δ15NPN) were obtained by filtration (∼1 l) onto precombusted (450 °C for 2 h) glass–fiber filters (GF/F, Whatman, Maidstone, Kent, UK) and stored in the dark at −20 °C until analyses.
Sediment cores were collected in October 2012 at stations L7, L8, and L17 and in June 2013 at station C1 along a salinity gradient. The surface sediments (depth: 0–20 mm) of triplicate sediment cores (8.6-cm inner diameter) were used for analysis of total organic carbon (TOC) and total nitrogen (TN) concentrations and stable isotope signatures (δ13C and δ15N).
To evaluate the contribution of phytobenthos to particulate organic matter (POM) in the water column and sedimentary OM, the stable isotope signatures and C/N ratio of the phytobenthos were measured. The dominant seagrass (Z. marina) and periphyton of the seagrass were collected in August 2012 at stations L6, L8, L13, and L17. Samples were kept at −20 °C prior to analysis.
Analytical methods
DIC concentrations and fCO2 values were determined on a batch-sample analyzer (ATT-05; Kimoto Electric, Osaka, Japan). DOC was determined via high-temperature catalytic oxidation with a TOC analyzer (TOC5000A; Shimadzu, Kyoto, Japan). Nutrient concentrations in sample filtrates were measured using an AutoAnalyzer (TRAACS 800; Bran+Luebbe, Norderstedt, Germany). TDN was measured via wet oxidation with persulfate (SWAAT; BL-Tec, Osaka, Japan). Concentrations of dissolved organic nitrogen (DON) were calculated as the differences between TDN and total DIN (the sum of 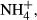
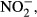
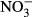 ).
).
For the chl a analyses, the GF/F filters were extracted in the dark for 12 h in 90% acetone, and chl a concentrations were measured by using a UV-visible spectrometer (UV-2450; Shimadzu, Kyoto, Japan) (Lorenzen, 1967).
CDOM absorbance spectra were recorded from 250 to 700 nm at 1-nm increments using a UV-visible spectrometer (UV-2450; Shimadzu, Kyoto, Japan) fitted with a 1-cm quartz flow cell and referenced to ultrapure water (Milli-Q water; Millipore, Billerica, MA, USA). The absorbance values at each wavelength (λ) were transformed into absorption coefficients (aCDOM) using:
where ACDOM is the absorbance value per meter. The absorption value at 375 nm, aCDOM(375), was chosen to quantify CDOM because this wavelength has been commonly used as a metric of DOC absorbance in previous studies (e.g., Bricaud et al., 1981; Astoreca et al., 2009; Para et al., 2013). We also measured aCDOM(254) as a metric of the aromaticity of the DOM (e.g., Weishaar et al., 2003; Zurbrügg et al., 2013). We calculated specific UV absorption at 254 nm (SUVA254) using:
where (DOC) is a DOC concentration. Spectral slopes for the interval of 275–295 nm (S275–295) were calculated using linear regression of the log-transformed aCDOM spectra. Slopes are reported as positive numbers to follow the mathematical convention of fitting to an exponential decay. Thus, higher (or steeper) slopes indicate a more rapid decrease in absorption with increasing wavelength (Helms et al., 2008).
Samples for analyses of TOC and TN content and stable isotope signatures (δ13C and δ15N) were dried in an oven at 60 °C or with a freeze dryer. To remove inorganic carbon, we acidified the samples with 1N HCl and dried them again at 60 °C. TOC and TN concentrations and stable isotope signatures (δ13C and δ15N) were measured with an isotope-ratio mass spectrometer (Delta Plus Advantage, Thermo Electron, Bremen, Germany) coupled with an elemental analyzer (Flash EA 1112; Thermo Electron, Bremen, Germany). Stable isotope ratios are expressed in δ notation as the deviation from standards in parts per thousand (‰) according to the following equation:
where R is 13C/12C or 15N/14N. PeeDee Belemnite and atmospheric nitrogen were used as the isotope standards of carbon and nitrogen, respectively. The analytical precision of the Delta Plus Advantage mass spectrometer system, based on the standard deviation of the internal reference replicates, was <0.2‰ for both δ13C and δ15N.
Data analysis
To estimate biological and/or physicochemical reduction or addition of carbon in the water column, we calculated the difference between the observed concentration of an element (X) and its concentration predicted by conservative mixing (Xmix) as ΔX at each lagoon station: ΔX = X–Xmix. The ΔX concentrations were determined for DIC, DOC, POC, and total carbon (TC = DIC + DOC + POC). Predicted conservative concentrations (Xmix) were estimated using a linear salinity mixing model with two end members. The terrestrial end member was defined as the average concentration in the three rivers weighted by the discharge of the rivers (Furen River: 21.0 m3 s−1; Yausubetsu River: 21.0 m3 s−1; Pon-Yausubetsu River: 0.7 m3 s−1). The concentrations in the bottom water at the lagoon mouth, station L18 with the highest salinity, were used as the marine end member.
The Bayesian isotopic modeling package, Stable Isotope Analysis in R (SIAR) (Parnell et al., 2010), was used to partition the proportional contributions of potential OM sources to the bulk POM and sedimentary OM based on their N/C, δ13C, and δ15N signatures. We chose N/C rather than C/N ratios in the model because the former were statistically more robust; the higher number (TOC concentration) is the denominator and behaves linearly in end-member mixtures (Goñi et al., 2003; Perdue & Koprivnjak, 2007). The SIAR model works by determining probability distributions of the sources that contribute to the observed mixed signal while accounting for the uncertainty in the signatures of the sources and isotopic fractionation. We assumed an isotopic fractionation of 0 and ran the model through 1 × 106 iterations. For each potential source, we report the median and the 95% confidence interval (CI) of the estimate of the proportional contribution of each source to the observed value.
We defined four sources (terrestrial OM, coastal OM, lagoon OM, and phytobenthos-derived OM) as end members for the isotopic and elemental mixing model. To estimate the OM components in sediments, we pooled coastal OM and lagoon OM as phytoplankton-derived OM (PhytoOM). The signature values of terrestrial OM (TerrOM) were determined as the average values of samples taken at the three river mouths (salinity = 0). The values in the bottom layer at the lagoon mouth station (L18) with the highest salinity were used as the signature values of coastal OM (CoastOM). Lagoon OM (LagOM) was defined as autochthonous phytoplankton growing in the brackish area of the lagoon. Because the C/N ratio of fresh phytoplankton is close to 7.0 on a molar basis (Redfield et al., 1963) and a low POC/chl a ratio (<100 on a mass basis) is characteristic of living microalgae (Zeitzschel, 1970; Maksymowska et al., 2000), LagOM from samples collected in the brackish area (salinity range: 5–25), which was characterized by a low POC/chl a ratio (<50) and low C/N ratio (<7.0), was considered to be representative of phytoplankton. Phytobenthos-derived OM (BenthOM) was defined as a mixture of the dominant seagrass (Z. marina) and the epiphyte. The signature values for BenthOM were calculated using a linear interpolation between the values characteristic of seagrass (seagrass; 100%) and of epiphytes (seagrass; 0%). The total range of the calculated signature values revealed that the mixing ratio had only about a 5% effect on the estimation. We therefore determined the BenthOM to be a mixture of 50% seagrass and 50% epiphyte. Because the N/C ratio generally declines while OM is decomposing (Van Mooy et al., 2002), it should be noted that the contribution of OM with a high N/C ratio (i.e., LagOM and CoastOM; Table S1) to POM and sedimentary OM was probably underestimated. In contrast, isotopic fractionation does not occur during the decomposition of organic compounds with a high molecular weight (Fry, 2006).
Results
Characteristics of organic matter in the water column
Concentrations of POM (POC, PN) were higher than predicted by conservative mixing, with the highest values around a salinity of 20 in the lagoon (Fig.2a, b). The distribution of POM concentrations corresponded approximately to the distribution of chl a concentration (Fig.2f). The POC/PN ratio was lower than predicted by the conservative mixing, and there were several high values in the river mouths (Fig.2c). δ13CPOC plotted along the predicted conservative mixing line, but the δ15NPN values were above the predicted line at many stations (Fig.2d, e).
Figure 2.
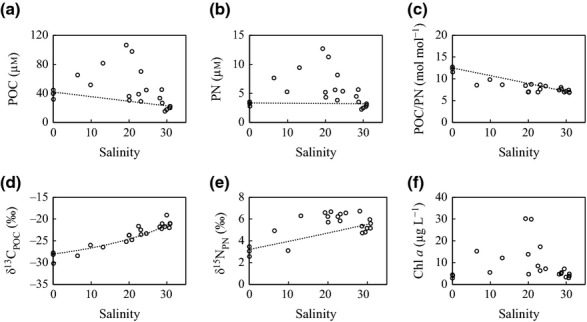
Variations in concentrations of (a) POC and (b) PN, (c) molar POC/PN ratio, (d) δ13CPOC, (e) δ15NPN, and (f) chl a concentration along the salinity gradient in the Furen Lagoon. Dashed lines indicate the predicted conservative mixing relationships.
Dissolved OC and DON concentrations were higher than predicted by the conservative mixing, and the differences were large in the salinity range 5–25 (Fig.3a, b). The DOC/DON ratios were lower than predicted by the conservative mixing (Fig.3c). Although aCDOM(375) values plotted along the conservative mixing line, aCDOM(254) values were higher than predicted by the conservative mixing (Fig.3d, e). SUVA254 values plotted along the conservative mixing line (Fig.3f). S275–295 values were higher than predicted by the conservative mixing, and the highest vales occurred at high salinity (Fig.3g).
Figure 3.
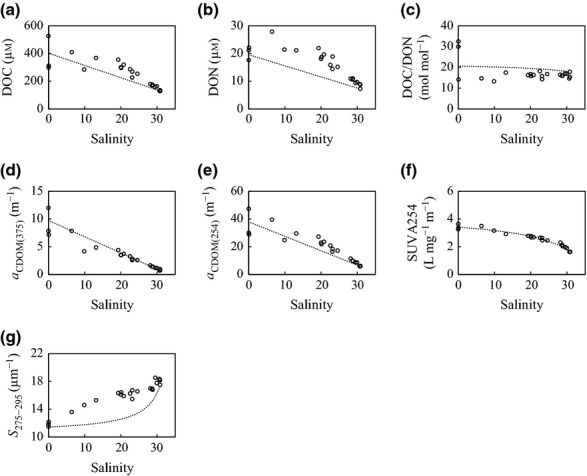
Variations in concentrations of (a) DOC and (b) DON, (c) molar DOC/DON ratio, (d) aCDOM(375), (e) aCDOM(254), (f), SUVA254 and (g) S275−295 along the salinity gradient in the Furen Lagoon. Dashed lines indicate the predicted conservative mixing relationships.
Composition of particulate organic matter
The isotopic and elemental mixing model (Fig.4a, b and Table S1) showed that each OM source made a different contribution to water column POM along the salinity gradient (Fig.5a–d). The relative contribution of TerrOM to POM was high (median value: 89%) at the river mouths and low (<10%) around the lagoon mouth (Fig.5a). LagOM was the largest source of POM in the lagoon (∼83%) in the salinity range 15–25 (Fig.5b). The relative contribution of CoastOM to POM was high (∼92%) around the lagoon mouth and low (<5%) in low-salinity areas (Fig.5c). The relative contribution of BenthOM was low (∼19%) in the lagoon, with the highest contribution (∼20%) around a salinity of 20 (Fig.5d).
Figure 4.
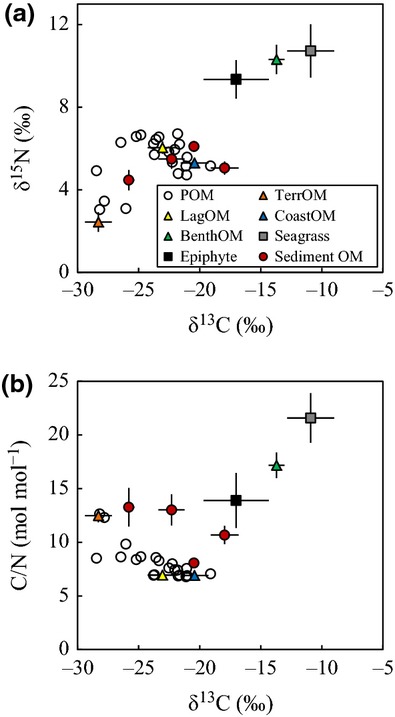
Isotopic and elemental signatures of OM sources, POM, and sedimentary OM sampled in the Furen Lagoon. Error bars show standard deviations of each source.
Figure 5.
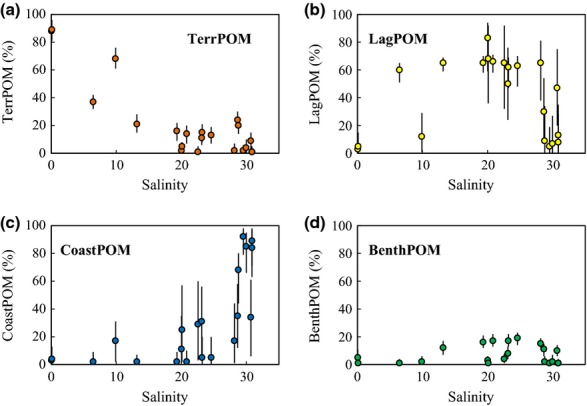
Variations in the relative contributions of different POM sources to water column POM along the salinity gradient. Each plot indicates the median estimated value; error bars show the 95% CIs of the estimated values.
We calculated the concentrations of water column POC derived from each source along the salinity gradient (Fig.6a–d) by multiplying the relative contribution of each source (Fig.5a–d) by the bulk POC concentration (Fig.2a). The terrestrial POC (TerrPOC) concentration was high (median value: 39.1 μmol-C l−1) at the river mouths and low (0.2 μmol-C l−1) around the lagoon mouth (Fig.6a). The lagoon POC (LagPOC) concentration was high in the salinity range 15–25, and the maximum median concentration was 69.6 μmol-C l−1 at station L5 (Fig.6b). The ranges of salinity associated with the highest concentrations were similar for chl a and LagPOC (Figs2f and 6b). Coastal POC (CoastPOC) and Phytobenthos-derived POC (BenthPOC) concentrations were relatively low in the lagoon (Fig.6c, d). The maximum median concentration of CoastPOC was 18.5 μmol-C l−1 at station L18. The BenthPOC concentration was the highest (median value: 17.3 μmol-C l−1) at station L5.
Figure 6.
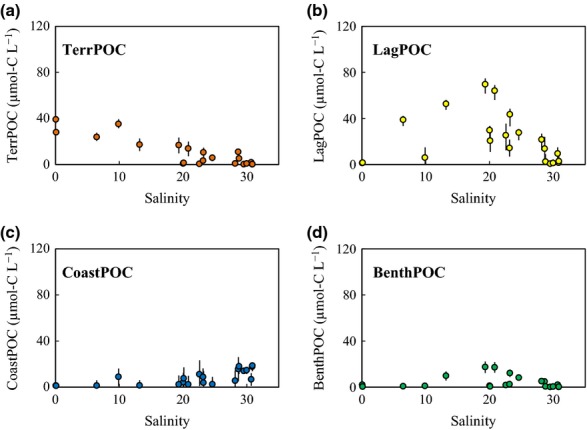
Variations in the calculated carbon concentrations of POC sources along the salinity gradient. The concentrations were calculated by multiplying the relative contribution of each source by the bulk POC concentration. Each plot indicates the median estimated value; error bars show the 95% CIs of the estimated values.
Composition of sedimentary organic matter
The isotopic and elemental mixing model (Fig.4a, b and Table S1) showed that each OM source made a different contribution to sedimentary OM along the salinity gradient (Fig.7a–c). TerrOM made the greatest contribution to OM (49–78%) in the lagoon sediments but contributed only 10% to the sedimentary OM at the coastal station (Fig.7a). The relative contribution of TerrOM was high near the river mouths, where the annual mean salinity at station L7, for example, was 15. PhytoOM was a minor component (3–29%) of the OM in the lagoon sediments (Fig.7b). The relative contributions of PhytoOM were high at the lagoon mouth (station L17) and the coastal station. BenthOM was the second largest OM pool in the lagoon sediments (19–36%; Fig.7c). The relative contribution of BenthOM was lowest (17%) at the coastal station and highest in low-salinity areas, where the annual mean salinity at station L8, for example, was 13.
Figure 7.
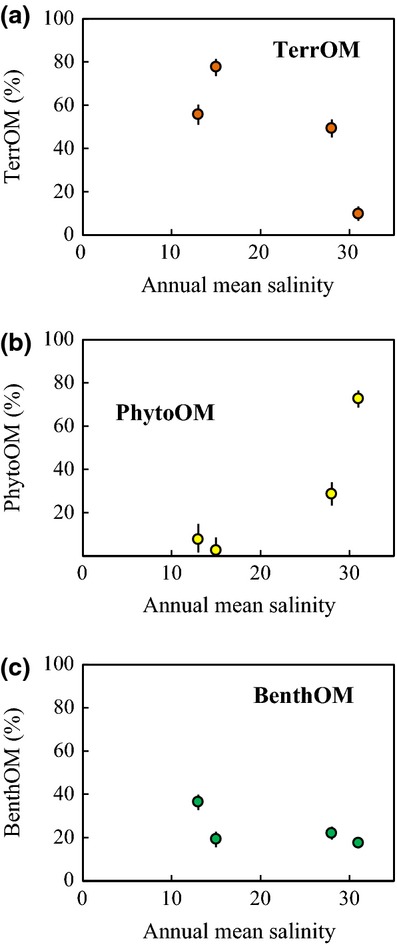
Variations in the relative contributions of different OM sources to sedimentary OM along the salinity gradient. Each plot indicates the median estimated value; error bars show the 95% CIs of the estimated values.
Carbon budget in the water column
ΔTC, ΔDIC, ΔDOC, and ΔPOC values were positive at salinities <15 (Fig.8a, b). At salinities >15, a decrease in DIC corresponded to an increase of OC (e.g., ΔTC near 0; Fig.8a, b). The surface-water fCO2 values were high (∼7332 μatm) at salinities <15 and were close to atmospheric values at salinities >20 (Fig.8c). Surface-water fCO2 values at several stations where salinity was >15 were actually lower than the atmosphere value of 397 μatm (Fig.8c).
Figure 8.
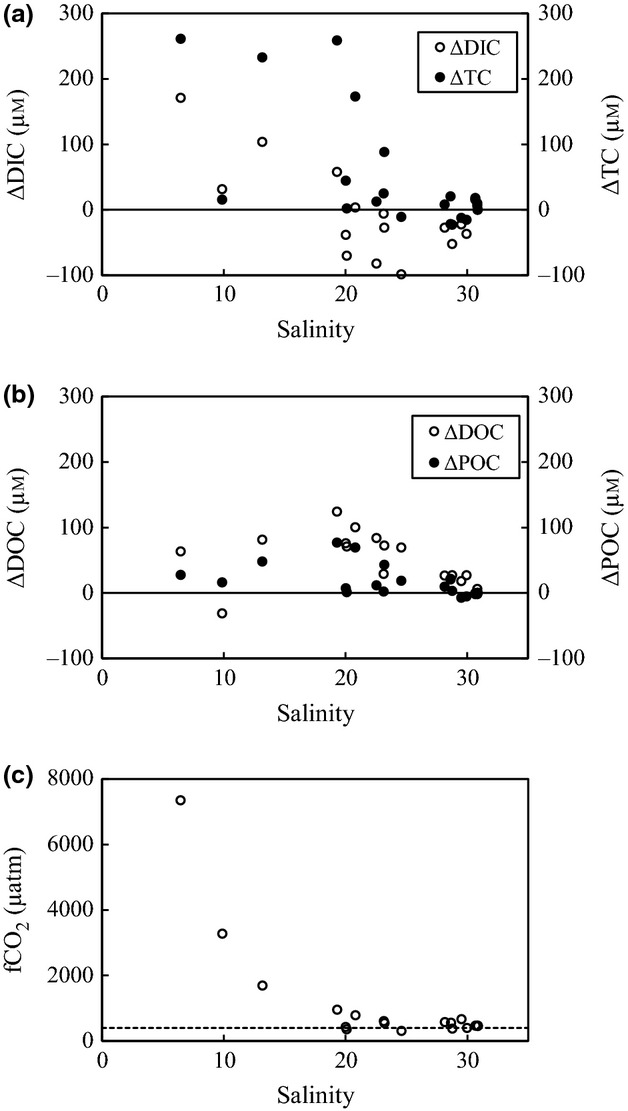
Variations of (a) ΔDIC, ΔTC, (b) ΔDOC, ΔPOC, and (c) fCO2, along the salinity gradient in the Furen Lagoon. Dashed lines indicate the fCO2 of the atmosphere (Tokoro et al., 2014).
Discussion
Selective carbon storage in sediments
To our best knowledge, this study first revealed that the efficiency of OC storage in sediments is dependent on OC derived from multiple sources (Fig.9c, d). The fact that terrestrial OC and phytobenthos-derived OC were more efficiently stored than phytoplankton-derived OC in seagrass meadow sediments suggests that the variability of the composition of OC affects the carbon burial rate.
Figure 9.
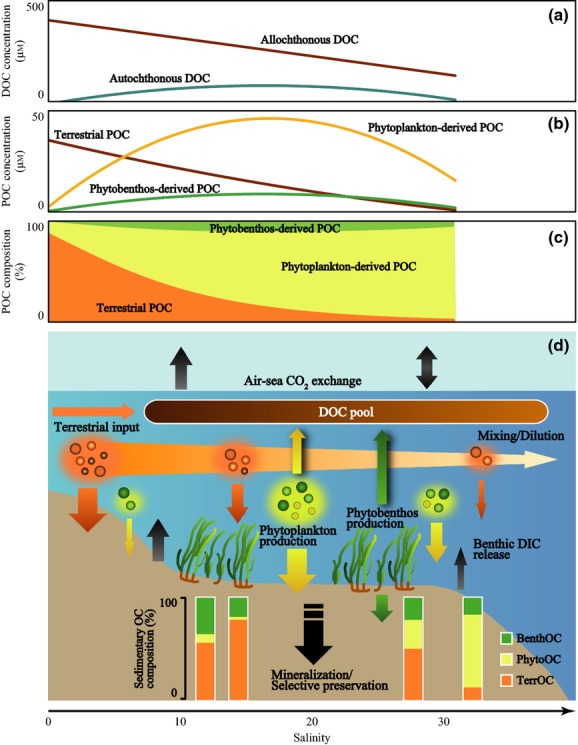
Carbon flow and carbon sequestration processes in shallow coastal ecosystems. Burial of POM into sediments is a major carbon sequestration process. (b, c) Phytoplankton-derived POM is a more dominant component than terrestrial POM in the water column at moderate salinities (>10; Fig.5a, b), indicating that abundant phytoplankton-derived OM is potentially a major source of sedimentary OC. (d) However, terrestrial OM and phytobenthos-derived OM are actually major components of OM in the sediments (Fig.7a–c), indicating that these components are more refractory than phytoplankton-derived OM and selectively preserved in seagrass meadows. (a) Refractory DOM production is another important process. A large fraction of the DOM pool is allochthonous DOM that includes terrestrial and marine origins (Fig.3a, d). Autochthonous DOM is produced by phytoplankton and phytobenthos in seagrass meadows (Fig.3a, c, e, g), part of which would have a long residence time in the ocean. (d) Autochthonous production also affects air–sea CO2 exchanges (Fig.8a–c). DIC is released from a benthic system especially in the low-salinity zone (∼15); thus, this zone would function as a source for atmospheric CO2. In contrast, active primary production (mainly phytoplankton) converts DIC to OC and reduces the DIC concentration in the high-salinity zone (>15). Because this uptake of DIC exceeds the benthic DIC release, this zone can function as a sink for atmospheric CO2. Aquatic primary production can contribute a great deal to atmospheric CO2 uptake on biological timescales. Phytobenthos (mainly seagrass) produces relatively refractory OM, which can be preserved in the ocean on geological timescales. Terrestrial OM is also preserved efficiently in sediments, where it is sequestrated from the atmosphere.
PhytoOM, derived mainly from autochthonous production, was dominant in the water column POM pool (∼95%; Figs5b, c and 9b, c), as has been found in other shallow coastal systems worldwide (Sato et al., 2006; Harmelin-Vivien et al., 2010; Dubois et al., 2012; Savoye et al., 2012; Guerra et al., 2013). Because the composition of POM in the water column fluctuates seasonally, our estimation from a single survey is subject to considerable uncertainty. However, we believe that PhytoOM is the primary component of the water column POM throughout the year, because the chl a concentration during this study (mean value: 10 μg l−1; Fig.2f) was lower than the annual mean value (14 μg l−1; Montani et al., 2011). In addition, the POC/PN ratio in this study (mean value: 7.7 mol mol−1; Fig.2c) was lower than TerrOM (12.5 mol mol−1; Table S1) and similar to the annual mean value (7.7 mol mol−1; S. Montani, S. Shibanuma and Y. Tsuji, unpublished data). Thus, our assessment that PhytoOM accounted for ∼95% of the POM in the water column is basically conservative. Although differences in sinking rates of POM sources affect carbon accumulation rates in sediments, such differences may be of minor importance in very shallow systems like Furen Lagoon. We thus hypothesize that the carbon accumulation rate reflects the composition (concentration) of water column POM and that PhytoOM accounts for the largest flux of POM into the sediments. However, PhytoOM was a relatively minor component (3–29%), and TerrOM and BenthOM were major components (49–78% and 19–36%, respectively) of the sedimentary OM (Figs7a–c and 9d), that is, TerrOM and BenthOM were more efficiently preserved than PhytoOM in the sediments. The ratios of TerrOM to PhytoOM in the sediments were at least 10 times the analogous ratios in the water column of the lagoon (Fig.9c, d). Because TerrOM is derived from terrestrial plants with hard cell walls, it contains refractory OM such as aliphatic (cutan, cutin, suberan, and suberin) and aromatic (lignin, sporopollenin, and dinosporin) cell wall biopolymers (Zonneveld et al., 2010). In addition, the terrestrial OC transported to the ocean has been reported to be relatively refractory and selectively preserved in marine sediments, because much terrestrial OC is highly degraded during the process of being transported through soils (Hedges et al., 1994; Zonneveld et al., 2010).
The contributions of BenthOM in the lagoon sediments were 0.8–6 times the contributions of PhytoOM (Fig.9d). The efficient storage of phytobenthos-derived OM may be partly explained by the fact that seagrass detritus contains a high proportion of refractory OM such as a lignin, especially detritus derived from rhizomes, and roots (Kennedy & Björk, 2009; Kennedy et al., 2010). Like other OM, the flux of BenthOM from the water column to the surface sediment is accounted for by fine particles (<1 mm) derived from plant thalli, because these fine particles are readily resuspended in the water column at our site due to the very shallow, windy conditions. However, it is noteworthy that BenthOM is also supplied from belowground biomass; thus, the actual total flux of BenthOM to the sediments may have been underestimated.
Carbon sequestration in the water column
The dynamics of DOC tend to be overlooked, but they can play a key role in the sequestration of carbon in the water columns of shallow coastal ecosystems, because DOC is generally the dominant fraction of the water column OC pool and includes a large proportion of refractory OC (Nagata, 2008; Jiao et al., 2014). However, the production rates and distribution of refractory DOC in shallow coastal ecosystems are largely unknown (e.g., Wada et al., 2008; Lønborg & Søndergaard, 2009). The release of DOC from seagrass meadows have been reported (Opsahl & Benner, 1993; Barrón & Duarte, 2009); however, using optical analysis, we first showed the possibility of DOC released from seagrass is indeed remained within the whole system.
The production of autochthonous DOC and the dilution of terrestrial DOC regulate the dynamics of DOC in the water column (Figs3a and 9a). To date, aCDOM(254) values have been used as a metric of the concentration of terrestrial aromatic compounds (Weishaar et al., 2003; Zurbrügg et al., 2013) and as a proxy for potential refractory OM (Saadi et al., 2006; Hur et al., 2009). This study revealed that aromatic compounds have an autochthonous origin at our site (Fig.3e), that is, relatively refractory compounds are produced in situ in shallow coastal ecosystems. In a mangrove-dominated estuary located within the Everglades, aromatic DOM is exuded from the mangroves (Bergamaschi et al., 2012). In seagrass meadows, the potential sources of aromatic DOM would be microalgae and seagrasses. Phytoplankton release CDOM with a protein-like fluorescence (Romera-Castillo et al., 2010) that is related to freshly produced aromatic amino acids, such as tyrosine, tryptophan, and phenylalanine (Yamashita et al., 2008). The decrease of DOC/DON ratios and the increase of S275–295 values in the lagoon (Fig.3c, g) support the hypothesis that phytoplankton release labile, protein-rich DOM (Biddanda & Benner, 1997; Fichot & Benner, 2012). In contrast, aquatic vascular plants such as seagrasses contain lignin, which is a refractory biopolymer. The subtropical seagrass Halodule wrightii exudes dissolved, lignin-derived phenols as it decomposes (Opsahl & Benner, 1993). Barrón & Duarte (2009) reported that meadows of the Mediterranean seagrass Posidonia oceanica release DOC that represents ∼71% of the net community production. These findings suggest that seagrasses directly produce refractory DOM that may be preserved in the water column.
The linear decrease with salinity of aCDOM(375), which is also a proxy of potentially terrestrial OM (Astoreca et al., 2009; Para et al., 2013), suggests that conservative mixing of terrestrial DOC with seawater accounted for much of the variability of DOC concentrations in Furen Lagoon (Fig.9a), as has been observed in other estuaries (Chen et al., 2007; Astoreca et al., 2009).
Air–sea CO2 exchange
The uptake of DIC in the surface-water column by aquatic primary producers stimulates an influx of atmospheric CO2, although mixing of high-fCO2 water from land runoff with low-fCO2 ocean water is the major process that mediates air–sea CO2 exchange in shallow coastal ecosystems. The low ΔTC values in the high-salinity zone of Furen Lagoon suggest that the benthic release/adsorption of carbon and biological metabolism (photosynthesis and mineralization) were more or less in balance in the water column. Relevant to this point is the fact that OC increases coincided with DIC decreases (Fig.8a, b), indicating that the aquatic primary producers were converting DIC to OC and thereby decreasing the fCO2 of the water column (Figs8c and 9d). Biological metabolism determines whether a body of water is a sink or a source of atmospheric CO2 (Maher & Eyre, 2012; Tokoro et al., 2014). Our results show that OC sequestration by aquatic primary producers was directly linked to atmospheric CO2 uptake in the high-salinity zone of Furen Lagoon, as expected by previous studies (Maher & Eyre, 2012; Tokoro et al., 2014).
In contrast, the positive values of ΔTC in the low-salinity zone suggest that DIC and/or OC were being released from the benthic system (Fig.8a, b). This fact, combined with the general high-fCO2 of inflowing freshwater (Chen et al., 2012), caused the low-salinity zone to be a source of atmospheric CO2 (Figs8c and 9d).
How does organic carbon derived from multiple sources contribute to carbon sequestration?
Shallow coastal ecosystems function not only as transition zones between the land and ocean but also as carbon sequestration filters that mitigate atmospheric CO2 increases, where planktonic and benthic primary producers sequester OC from atmospheric CO2 and thereby mitigate atmospheric CO2 increases (Fig.9d). We found that (i) phytobenthos-derived and terrestrial OC are stored more efficiently than phytoplankton-derived OC in sediments, (ii) DOC production by aquatic primary producers sequesters carbon in the water column, and (iii) OC sequestration in the water column contributes to the influx of atmospheric CO2 at biological timescales. These three findings are dependent on the salinity gradient. Our findings reveal that the dynamics of OC derived from multiple sources link with carbon sequestration processes at multiple timescales. This discovery suggests that criteria for evaluating the effectiveness of processes for sequestering carbon should be related to the salinity and OC sources. Finally, our methodology inferring the multiple sources and fates of carbon and the results provide a step toward better understanding coastal carbon dynamics in response to global change, in particular, continued human pressures (Bauer et al., 2013).
Acknowledgments
We thank S. Montani, T. Inoue, and anonymous reviewers for helpful comments; S. Shibanuma, E. Miyoshi, T. Tokoro, J. Kyoda, K. Tada, and the students of Hokkaido University for help in observations; K. Sakihara for chemical analysis. This study was supported by a Canon Foundation grant and a Grant-in-Aid for Challenging Exploratory Research (No. 24656316, 26630251) from the Japan Society for the Promotion of Science (JSPS).
Supporting Information
Table S1. Isotopic and elemental signatures of OM sources.
References
- Agustí S, Duarte CM. Phytoplankton lysis predicts dissolved organic carbon release in marine plankton communities. Biogeosciences. 2013;10:1259–1264. [Google Scholar]
- Astoreca R, Rousseau V, Lancelot C. Coloured dissolved organic matter (CDOM) in Southern North Sea waters: optical characterization and possible origin. Estuarine, Coastal and Shelf Science. 2009;85:633–640. [Google Scholar]
- Barrón C, Duarte CM. Dissolved organic matter release in a Posidonia oceanica meadow. Marine Ecology Progress Series. 2009;374:75–84. [Google Scholar]
- Bauer JE, Cai WJ, Raymond PA, Bianchi TS, Hopkinson CS, Regnier PAG. The changing carbon cycle of the coastal ocean. Nature. 2013;504:61–70. doi: 10.1038/nature12857. [DOI] [PubMed] [Google Scholar]
- Bergamaschi BA, Krabbenhoft DP, Aiken GR, Patino E, Rumbold DG, Orem WH. Tidally driven export of dissolved organic carbon, total mercury, and methylmercury from a mangrove-dominated estuary. Environmental Science & Technology. 2012;46:1371–1378. doi: 10.1021/es2029137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi TS. Biogeochemistry of Estuaries. New York, NY, USA: Oxford University Press; 2007. [Google Scholar]
- Biddanda B, Benner R. Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnology and Oceanography. 1997;42:506–518. [Google Scholar]
- Borges AV. Do we have enough pieces of the jigsaw to integrate CO2 fluxes in the coastal ocean? Estuaries. 2005;28:3–27. [Google Scholar]
- Borges AV, Dellile B, Frankignoulle M. Budgeting sinks and sources of CO2 in the coastal ocean: diversity of ecosystems counts. Geophysical Research Letters. 2005;32:L14601. [Google Scholar]
- Bricaud A, Morel A, Prieur L. Absorption by dissolved organic matter of the sea (yellow substance) in the UV and visible domains. Limnology and Oceanography. 1981;26:43–53. [Google Scholar]
- Cai WJ. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annual Review of Marine Science. 2011;3:123–145. doi: 10.1146/annurev-marine-120709-142723. [DOI] [PubMed] [Google Scholar]
- Cai WJ, Dai M, Wang Y. Air-sea exchange of carbon dioxide in ocean margins: a province-based synthesis. Geophysical Research Letters. 2006;33:L12603. [Google Scholar]
- Carlson CA. Production and removal processes. In: Hansell DA, Carlson CA, editors. Biogeochemistry of Marine Dissolved Organic Matter. Waltham, MA, USA: Academic Press; 2002. pp. 91–151. [Google Scholar]
- Chen CTA, Borges AV. Reconciling opposing views on carbon cycling in the coastal ocean: continental shelves as sinks and near-shore ecosystems as sources of atmospheric CO2. Deep-Sea Research II. 2009;56:578–590. [Google Scholar]
- Chen Z, Hu C, Conmy RN, Muller-Karger F, Swarzenski P. Colored dissolved organic matter in Tampa Bay, Florida. Marine Chemistry. 2007;104:98–109. [Google Scholar]
- Chen CTA, Huang TH, Fu YH, Bai Y, He X. Strong sources of CO2 in upper estuaries become sinks of CO2 in large river plumes. Current Opinion in Environmental Sustainability. 2012;4:179–185. [Google Scholar]
- Duarte CM, Marbà N, Gacia E, Fourqurean JW, Beggins J, Barrón C, Apostolaki ET. Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Global Biogeochemical Cycles. 2010;24:GB4032. [Google Scholar]
- Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N. The role of coastal plant communities for climate change mitigation and adaptation. Nature Climate Change. 2013;3:961–968. [Google Scholar]
- Dubois S, Savoye N, Grémare A, Plus M, Charlier K, Beltoise A, Blanchet H. Origin and composition of sediment organic matter in a coastal semi-enclosed ecosystem: an elemental and isotopic study at the ecosystem space scale. Journal of Marine Systems. 2012;94:64–73. [Google Scholar]
- Fichot CG, Benner R. The spectral slope coefficient of chromophoric dissolved organic matter (S275−295) as a tracer of terrigenous dissolved organic carbon in river-influenced ocean margins. Limnology and Oceanography. 2012;57:1453–1466. [Google Scholar]
- Fourqurean JW, Duarte CM, Kennedy H, et al. Seagrass ecosystems as a globally significant carbon stock. Nature Geoscience. 2012;5:505–509. [Google Scholar]
- Fry B. Stable Isotope Ecology. New York, NY, USA: Springer; 2006. [Google Scholar]
- Goñi MA, Teixeira MJ, Perkey DW. Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA) Estuarine, Coastal and Shelf Science. 2003;57:1023–1048. [Google Scholar]
- Guerra R, Pistocchi R, Vanucci S. Dynamics and sources of organic carbon in suspended particulate matter and sediments in Pialassa Baiona lagoon (NW Adriatic Sea, Italy) Estuarine, Coastal and Shelf Science. 2013;135:24–32. [Google Scholar]
- Harmelin-Vivien M, Dierking J, Bănaru D, Fontaine MF, Arlhac D. Seasonal variation in stable C and N isotope ratios of the Rhone River inputs to the Mediterranean Sea (2004–2005) Biogeochemistry. 2010;100:139–150. [Google Scholar]
- Hedges JI, Cowie GL, Richey JE, Quay PD, Brenner R, Strom M, Forsberg B. Origin and processing of organic matter in the Amazon River as indicated by carbohydrates and amino acids. Limnology and Oceanography. 1994;39:743–761. [Google Scholar]
- Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnology and Oceanography. 2008;53:955–969. [Google Scholar]
- Hur J, Park MH, Schlautman MA. Microbial transformation of dissolved leaf litter organic matter and its effects on selected organic matter operational descriptors. Environmental Science & Technology. 2009;43:2315–2321. doi: 10.1021/es802773b. [DOI] [PubMed] [Google Scholar]
- IPCC. Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner GK, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA: Cambridge University Press; 2013. pp. 465–570. [Google Scholar]
- Jiao N, Herndl GJ, Hansell DA, et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nature Reviews Microbiology. 2010;8:593–599. doi: 10.1038/nrmicro2386. [DOI] [PubMed] [Google Scholar]
- Jiao N, Robinson C, Azam F, et al. Mechanisms of microbial carbon sequestration in the ocean – future research directions. Biogeosciences. 2014;11:5285–5306. [Google Scholar]
- Kennedy H, Björk M. Seagrass meadows. In: Laffoley D, Grimsditch G, editors. The Management of Natural Coastal Carbon Sinks. Gland, Switzerland: IUCN; 2009. pp. 23–30. [Google Scholar]
- Kennedy H, Beggins J, Duarte CM, Fourqurean JW, Holmer M, Marbà N, Middelburg JJ. Seagrass sediments as a global carbon sink: isotopic constraints. Global Biogeochemical Cycles. 2010;24:GB4026. [Google Scholar]
- Lønborg C, Søndergaard M. Microbial availability and degradation of dissolved organic carbon and nitrogen in two coastal areas. Estuarine, Coastal and Shelf Science. 2009;81:513–520. [Google Scholar]
- Lorenzen CJ. Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnology and Oceanography. 1967;12:343–346. [Google Scholar]
- Macreadie PI, Allen K, Kelaher BP, Ralph PJ, Skilbeck CG. Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks. Global Change Biology. 2012;18:891–901. [Google Scholar]
- Macreadie PI, Baird ME, Trevathan-Tackett SM, Larkum AWD, Ralph PJ. Quantifying and modelling the carbon sequestration capacity of seagrass meadows – A critical assessment. Marine Pollution Bulletin. 2014;83:430–439. doi: 10.1016/j.marpolbul.2013.07.038. [DOI] [PubMed] [Google Scholar]
- Maher TD, Eyre BD. Carbon budgets for three autotrophic Australian estuaries: implications for global estimates of the coastal air-water CO2 flux. Global Biogeochemical Cycles. 2012;26:GB1032. [Google Scholar]
- Maksymowska D, Richard P, Piekarek-Jankowska H, Riera P. Chemical and isotopic composition of the organic matter sources in the Gulf of Gdansk (southern Baltic Sea) Estuarine, Coastal and Shelf Science. 2000;51:585–598. [Google Scholar]
- Mateo MA, Romero J, Pérez M, Littler MM, Littler DS. Dynamics of millenary organic deposits resulting from the growth of the Mediterranean seagrass Posidonia oceanica. Estuarine, Coastal and Shelf Science. 1997;44:103–110. [Google Scholar]
- McLeod E, Chmura GL, Bouillon S, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment. 2011;9:552–560. [Google Scholar]
- Montani S, Managaki Y, Shibanuma S. The structural changes in biological productivity due to progress of dairy in Lake Furen. Bulletin on Coastal Oceanography. 2011;49:59–67. (in Japanese with English abstract) [Google Scholar]
- Nagata T. Organic matter–bacteria interactions in seawater. In: Kirchman DL, editor. Microbial Ecology of the Oceans. 2nd edn. New York, NY,USA: John Wiley & Sons; 2008. pp. 207–242. [Google Scholar]
- Nellemann C, Corcoran E, Duarte CM, Valdes L, DeYoung C, Fonseca L, Grimsditch G. Blue Carbon. A Rapid Response Assessment. Birkeland: United Nations Environmental Programme, GRID-Arendal, Birkeland Trykkeri AS; 2009. [Google Scholar]
- Opsahl S, Benner R. Decomposition of senescent blades of the seagrass Halodule wrightii in a subtropical lagoon. Marine Ecology Progress Series. 1993;94:191–205. [Google Scholar]
- Para J, Charrière B, Matsuoka A, Miller WL, Rontani JF, Sempéré R. UV/PAR radiation and DOM properties in surface coastal waters of the Canadian shelf of the Beaufort Sea during summer 2009. Biogeosciences. 2013;10:2761–2774. [Google Scholar]
- Parnell AC, Inger R, Bearhop S, Jackson AL. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE. 2010;5:e9672. doi: 10.1371/journal.pone.0009672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue EM, Koprivnjak JF. Using the C/N ratio to estimate terrigenous inputs of organic matter to aquatic environments. Estuarine, Coastal and Shelf Science. 2007;73:65–72. [Google Scholar]
- Redfield AC, Ketchum BH, Richard FA. The influence of organisms on the composition of the sea water. In: Hill MN, editor. The Sea. Chichester, UK: John Wiley; 1963. pp. 26–49. vol. 2 ( ). In: [Google Scholar]
- Regnier PAG, Friedlingstein P, Ciais P, et al. Anthropogenic perturbation of the carbon fluxes from land to ocean. Nature Geoscience. 2013;6:597–607. [Google Scholar]
- Romera-Castillo C, Sarmento H, Alvarez-Salgado XA, Gasol JM, Marrase C. Production of chromophoric dissolved organic matter by marine phytoplankton. Limnology and Oceanography. 2010;55:446–454. [Google Scholar]
- Saadi I, Borisover M, Armon R, Laor Y. Monitoring of effluent DOM biodegradation using fluorescence, UV and DOC measurements. Chemosphere. 2006;63:530–539. doi: 10.1016/j.chemosphere.2005.07.075. [DOI] [PubMed] [Google Scholar]
- Sato T, Miyajima T, Ogawa H, Umezawa Y, Koike I. Temporal variability of stable carbon and nitrogen isotopic composition of size-fractionated particulate organic matter in the hypertrophic Sumida River Estuary of Tokyo Bay, Japan. Estuarine, Coastal and Shelf Science. 2006;68:245–258. [Google Scholar]
- Savoye N, David V, Morisseau F, et al. Origin and composition of particulate organic matter in a macrotidal turbid estuary: the Gironde Estuary, France. Estuarine, Coastal and Shelf Science. 2012;108:16–28. [Google Scholar]
- Tokoro T, Hosokawa S, Miyoshi E, et al. Net uptake of atmospheric CO2 by coastal submerged aquatic vegetation. Global Change Biology. 2014;20:1873–1884. doi: 10.1111/gcb.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mooy BAS, Keil RG, Devol AH. Impact of suboxia on sinking particulate organic carbon: enhanced carbon flux and preferential degradation of amino acids via denitrification. Geochimica et Cosmochimica Acta. 2002;66:457–465. [Google Scholar]
- Wada S, Aoki MN, Mikami A, et al. Bioavailability of macroalgal dissolved organic matter in seawater. Marine Ecology Progress Series. 2008;370:33–44. [Google Scholar]
- Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environmental Science & Technology. 2003;37:4702–4708. doi: 10.1021/es030360x. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Jaffe R, Maie N, Tanoue E. Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC) Limnology and Oceanography. 2008;53:1900–1908. [Google Scholar]
- Zeitzschel B. The quantity, composition and distribution of suspended particulate matter in the Gulf of California. Marine Biology. 1970;7:305–318. [Google Scholar]
- Zonneveld KAF, Versteegh GJM, Kasten S, et al. Selective preservation of organic matter in marine environments; processes and impact on the sedimentary record. Biogeosciences. 2010;7:483–511. [Google Scholar]
- Zurbrügg R, Suter S, Lehmann MF, Wehrli B, Senn DB. Organic carbon and nitrogen export from a tropical dam-impacted floodplain system. Biogeosciences. 2013;10:23–38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Isotopic and elemental signatures of OM sources.


