Abstract
Extreme droughts, heat waves, frosts, precipitation, wind storms and other climate extremes may impact the structure, composition and functioning of terrestrial ecosystems, and thus carbon cycling and its feedbacks to the climate system. Yet, the interconnected avenues through which climate extremes drive ecological and physiological processes and alter the carbon balance are poorly understood. Here, we review the literature on carbon cycle relevant responses of ecosystems to extreme climatic events. Given that impacts of climate extremes are considered disturbances, we assume the respective general disturbance-induced mechanisms and processes to also operate in an extreme context. The paucity of well-defined studies currently renders a quantitative meta-analysis impossible, but permits us to develop a deductive framework for identifying the main mechanisms (and coupling thereof) through which climate extremes may act on the carbon cycle. We find that ecosystem responses can exceed the duration of the climate impacts via lagged effects on the carbon cycle. The expected regional impacts of future climate extremes will depend on changes in the probability and severity of their occurrence, on the compound effects and timing of different climate extremes, and on the vulnerability of each land-cover type modulated by management. Although processes and sensitivities differ among biomes, based on expert opinion, we expect forests to exhibit the largest net effect of extremes due to their large carbon pools and fluxes, potentially large indirect and lagged impacts, and long recovery time to regain previous stocks. At the global scale, we presume that droughts have the strongest and most widespread effects on terrestrial carbon cycling. Comparing impacts of climate extremes identified via remote sensing vs. ground-based observational case studies reveals that many regions in the (sub-)tropics are understudied. Hence, regional investigations are needed to allow a global upscaling of the impacts of climate extremes on global carbon–climate feedbacks.
Keywords: carbon cycle, climate change, climate extremes, climate variability, disturbance, terrestrial ecosystems
Introduction
There is widespread recognition that climate change is having and will continue to have, fundamental impacts on the natural environment and on human well-being (Parry et al., 2007). Current projections, based upon contrasted emission scenarios, suggest somewhere between 0.3 and 4.8 °C warming by the end of this century (IPCC, 2013). The associated modification of the climate system strongly influences the carbon cycling of the terrestrial biosphere and thus land–atmosphere CO2 fluxes (Fischlin et al., 2007). An important observation is that climate change, and increasing concentrations of atmospheric greenhouse gases, not only lead to gradual mean global warming but may also change the frequency, the severity and even the nature of extreme events (IPCC, 2013). A relatively small change in the mean or variance of a climate variable, inherently leads to disproportionally large changes in the frequency of extremes, that is the infrequent events at the high and low end of the range of values of a particular variable (Nicholls & Alexander, 2007). Furthermore, climate change can fundamentally alter the inherent variability of temperature, precipitation and other weather phenomena (Seneviratne et al., 2012). State-of-the-art climate models project global intensification of heavy precipitation events and heat extremes, and regions with stronger or longer-lasting droughts (Fisher & Knutti, 2014, IPCC, 2013).
Concerns about increasing variability of temperature and precipitation patterns and climate extremes were first articulated over two decades ago by Katz & Brown (1992), and became widely acknowledged after the second IPCC assessment of climate change in 1995 (Nicholls & Alexander, 2007). These concerns were raised because many biological systems (including human societies) are more sensitive to climate extremes than to gradual climate change, due to typically greater response strengths and shorter response times (Hanson et al., 2006).
Key characteristics of the climate, such as heat waves, seem to have already been modified beyond the natural variability within which society and its economic, social and political systems have developed (Schär et al., 2004; Soussana et al., 2010). Both the public media and the scientific community have recognized the widespread consequences of climate extremes such as the European heat wave in 2003 (Ciais et al., 2005; Reichstein et al., 2007; Bastos et al., 2013a), the heat wave and associated forest fires in Greece in 2007 (Founda & Giannakopoulos, 2009), the dry spells in the Amazon basin in 2005 (Phillips et al., 2009) and 2010 (Lewis et al., 2011), in the U.S.A. 2000–2004 (Breshears et al., 2005; Schwalm et al., 2012), the forest fires in Russia in 2010 (Barriopedro et al., 2011; Konovalov et al., 2011; Coumou & Rahmstorf, 2012; Bastos et al., 2013a), the Pakistan Floods in 2010 (Hong et al., 2011; Houze et al., 2011; Trenberth & Fasullo, 2012), the storm Lothar in Europe in 1999 (Lindroth et al., 2009), hurricane Katrina in the U.S. in 2005 (Chambers et al., 2007), or the ice storm in southern and central China in 2008 (Stone, 2008; Sun et al., 2012), and the 2010–2011 La Nina rains over Australia (Boening et al., 2012; Poulter et al., 2014). These documented recent events demonstrate the massive impacts climate extremes can have on harvests, economies and human health, as well as on the carbon balance of terrestrial ecosystems (IPCC, 2012; Reichstein et al., 2013).
Alterations of the biosphere’s carbon balance through changes in the strength of carbon uptake or losses in turn affect the climate system (Friedlingstein et al., 2006; Frank et al., 2010). In addition, extreme drought will often reduce evapotranspiration and its cooling effect and thereby causes a positive local feedback on warming (e.g. Seneviratne et al., 2010; Teuling et al., 2010; Mueller & Seneviratne, 2012; Peng et al., 2014). Regional assessments clearly indicate the relevance of climate extremes on the carbon cycle and potential climate feedbacks (e.g. for drought extreme in Europe, Ciais et al., 2005; Reichstein et al., 2007; and for western North America, Schwalm et al., 2012). Yet a synthesis of the direct and indirect impacts of climate extremes on the carbon cycle and the underlying mechanisms is still lacking. In a recent broad perspective, Reichstein et al. (2013) highlighted the possibility that climate extremes and their impacts on the global carbon cycle may lead to an amplification of positive climate–carbon cycle feedbacks. However, the underlying mechanisms, and how they likely apply to current and future response patterns observed in different biomes and ecosystem types, have not yet been synthesized in detail, especially with respect to possible differences in response time (concurrent/lagged) and direction of impacts (direct/indirect). Such detailed information is needed, given the complexity of carbon cycle responses to climate extremes, and their dependence on background climate and ecosystem conditions (Knapp et al., 2008).
In this review, we aim to (1) develop a coherent conceptual framework based on logically deductive reasoning for integrating direct and indirect effects climate extremes could have on the carbon cycle and to identify the main mechanisms underlying these effects, (2) synthesize how different types of ecosystems are affected by climate extremes based on available well-documented case studies and (3) provide an overview of likely responses of the terrestrial carbon cycle in relation to likely future climate extremes, and the specific role of lagged impacts.
At the outset, we acknowledge that the lack of systematically collected data and the highly nonlinear responses of ecosystems to extreme events makes a quantitative meta-analysis of effects of climate extremes on the carbon cycle across the range of observational and experimental studies virtually impossible (cf. also Vicca et al., 2014). While there is ample information in the literature on specific effects of extreme climatic conditions (experimentally induced or naturally occurring) on specific ecosystems, the severity of these extreme conditions and their consequences has often not been systematically evaluated. This is not only due to a lack of common metrics reported across the various studies (e.g. Vicca et al., 2012), but also complicated by the fact that climate extremes are by definition rare and their effects are highly context dependent, typically threshold based and highly nonlinear (e.g. Knapp et al., 2008; Smith, 2011, Bahn et al., 2014). Thus, in our review, we rely on a qualitative, logically deductive reasoning, supported by multiple case studies, combined with remote sensing-based global analysis to derive hypotheses on potential effects of climate extremes on the terrestrial carbon cycle.
Definitions
Climate extremes and impacts
Terms, such as ‘climate extremes’, ‘weather extremes’ or ‘extreme weather events’, are used in various ways in the scientific literature. Thus, for clarity, we provide and briefly justify the definitions we use in this review:
An ‘extreme’, as stated in Seneviratne et al. (2012), is ‘the occurrence of a value of a weather or climate variable above (or below) a threshold value near the upper (or lower) ends of the range of observed values of the variable’ within a defined climate reference period (e.g. 1981–2010). Thus, ‘climate extreme’ is an aggregate term encompassing both ‘extreme weather’ and ‘extreme climate’ events. The distinction of weather events and climate events is related to the timescale. An extreme climate event occurs on longer timescales than an extreme weather event and can be the accumulation of extreme weather events. This definition follows the IPCC Special Report on Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation (Seneviratne et al., 2012).
However, the above definitions reflecting climatological considerations do not consider potential consequences for the biosphere and the carbon cycle. Smith (2011) suggested that one has to specifically address events where both climates are anomalous and the biosphere experiences a pronounced impact outside the bounds of what is considered normal variability. Along these lines, we use the term ‘extreme impact’ to describe, from a functional perspective, when a resilience threshold (‘extreme response threshold’, sensu Smith, 2011) is passed, placing the ecosystem and associated carbon cycling into an unusual or rare state. Thresholds are typically exceeded when stressor dose (i.e. cumulative amount defined by stress intensity multiplied by stress duration) reaches a critical level (e.g. during flooding, drought and/or extended periods of exceptionally high or low temperatures), or when the intensity of an extreme climatic event is critically high (e.g. during a storm). Thresholds can be passed at organ, plant or community level, and lead to emergent carbon cycle impacts at ecosystem level. We note that the definition of ‘extreme impact’ may partly overlap with the concept of ‘disturbance’ as it is commonly used in ecology (White & Jentsch, 2001). Here, we consider every climate extreme which has an impact on the ecosystem carbon cycle a ‘disturbance’, but note that not every disturbance is caused by climate extremes. A typical example is fire, which can be part of a system intrinsic disturbance cycle. But in this study, we consider those fires which are of rare magnitude or even are unprecedented, and likely facilitated by extreme climate conditions. Given that impacts caused by ‘climate extremes’ can be considered ‘disturbances’, we assume that respective general mechanisms and processes induced by ‘disturbances’ also operate in this specific ‘extreme’ context.
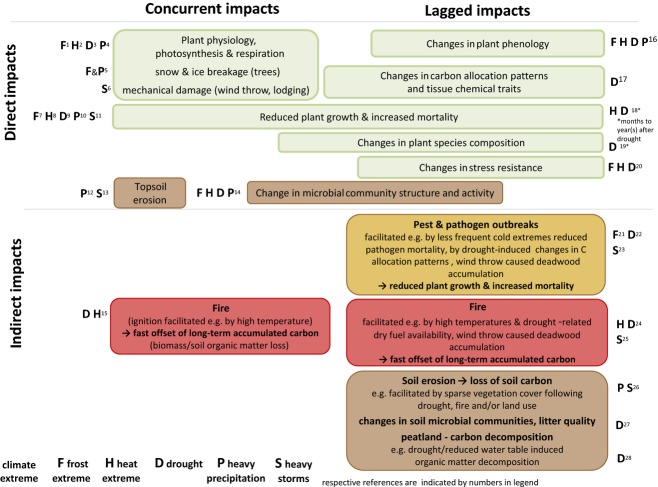
In order to specifically address extreme impacts with repercussions to the carbon cycle, denoted as ‘carbon cycle extreme’ and to entail anomalies in biosphere–atmosphere carbon fluxes or extreme changes in ecosystem carbon pools, it is useful to distinguish ‘concurrent’ vs. ‘lagged’ and ‘direct’ vs. ‘indirect’ impacts (Fig.1). These four categories of impacts indicate how they are related to the stressor. Concurrent impacts begin to occur during the climate extreme, while lagged impacts occur sometime thereafter. Direct impacts are only caused by the climate extreme (either concurrently or lagged) if, and only if, a threshold of the climatic stress dose (dashed line in Fig.1a) is passed. Indirect impacts are facilitated by the climate extreme by increasing the susceptibility of the ecosystem, but directly initiated by another (not necessarily extreme per se) external trigger. Hence, here the likelihood (P) of an extreme system response is a function of both the susceptibility and the characteristics of the external trigger (cf. Fig.1b and d).
Figure 1.
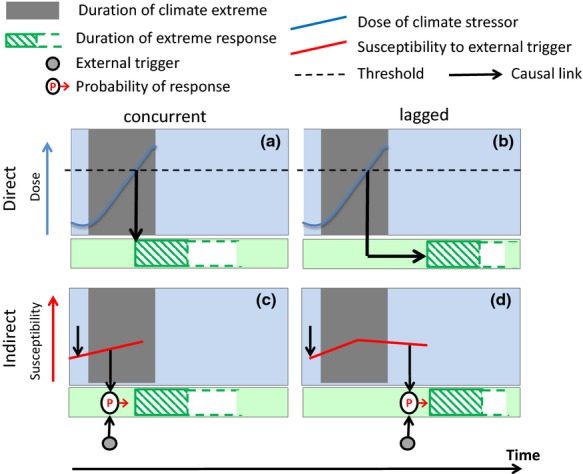
Schematic diagram illustrating direct concurrent and lagged (a, b) and indirect concurrent and lagged (c, d) impacts of climate extremes and corresponding extreme ecosystem responses. In the direct case, the extreme impact occurs if (and only if) a threshold is reached, that is a critical dose (blue line) is passed. In the indirect case, the climate extreme increases the susceptibility (red line) to an external trigger (climatic or nonclimatic, extreme or not extreme). The likelihood as a function of the trigger and the susceptibility is indicated with the symbol ‘P’ in the circle. Concurrent responses start during the climate extreme, but may last longer for indefinite time (dashed extensions of green boxes). Lagged responses only happen after the climate extreme. The responses can be of different nonlinear shapes as indicated in Fig.2.
Examples for these four categories of impacts are as follows:
Direct, concurrent impact: windthrow caused by storm; ice breakage; reduced productivity or increased mortality during drought, thermal stress or flooding (cf. Fig.1a)
Indirect, concurrent impact: loss of biomass or soil organic matter due to fire caused by lightning or human ignition, facilitated by an ongoing extreme dry and/or warm event (cf. Fig.1b)
Direct, lagged impact: reduced productivity/growth in the year(s) following the year of an extreme drought, caused, for example by carbohydrate depletion/reduced bud development/partial mortality during a drought in the previous year (cf. Fig.1c)
Indirect, lagged impact: increased pest- or pathogen-caused mortality following a climate extreme; loss of biomass or soil organic matter due to fire facilitated through deadwood accumulation after a windthrow; loss of soil carbon due to erosion during heavy precipitation or permafrost thawing and carbon losses as indirectly facilitated by reduced vegetation cover and/or changes in soil hydrophobicity following overgrazing, drought or fire (cf. Fig.1d)
Any effect, which can be attributed to a previous climate extreme, is termed here a ‘legacy effect’ and hence per definition time lagged compared to the ‘climate extreme’ [please note that we prefer this terminology compared to the sometimes synonymously used anthropomorphic term ‘memory effect’ (Walter et al., 2013)]. Legacy effects can include both changes in ecosystem states or process rates after the termination of a climate extreme, as well as altered ecosystem responses to environmental conditions, including subsequent extremes, and are often related to changes in species composition and their functional attributes (e.g. Smith, 2011; Sala et al., 2012).
It should be noted that is essential to define the timescale under scrutiny when quantifying the overall effect of a ‘climate extreme’ on the carbon cycle (Fig.2). It is the timescale determining the degree to which concurrent and lagged effects alter the carbon balance of an ecosystem. Negative concurrent effects, often related to the resistance of an ecosystem to an extreme event, may in the long run be balanced by enhanced regrowth during recovery (Fig.2), depending on the resilience of the system. Lagged effects may impair the ability of an ecosystem to recover from an extreme event and may thereby alter the ecosystem carbon balance over a given period (Fig.2).
Figure 2.
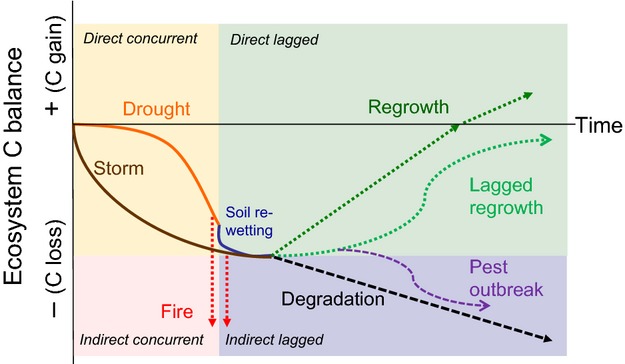
Hypothesized temporal dynamics of direct and indirect concurrent and lagged effects of climate extremes (e.g. drought/heat wave; storm) and of ecosystem recovery on the ecosystem carbon balance. (Note that for simplicity regrowth after fire and pest outbreaks are not shown in this figure). Line colours correspond to the colour of the climate extreme in the figure.
Impacts of climate extremes on the terrestrial carbon cycle: mechanisms and processes
Climate extremes can impact the structure, composition and functioning of terrestrial ecosystems and can thereby severely affect the regional carbon cycle, with the potential of causing a shift from a carbon sink towards a carbon source. During the ‘European 2003 heat wave’, which was an extreme drought event, Western European ecosystems were estimated to have lost an amount of CO2 comparable to that which had been absorbed from the atmosphere during the previous three to five years under normal weather conditions (Ciais et al., 2005; Reichstein et al., 2007; Vetter et al., 2008). Likewise, during the 2000–2004 drought, the strength of the western North American carbon sink declined substantially, with reductions ranging between 30 and 298 Tg C yr-1 (Schwalm et al., 2012). In 2004, heavy precipitation associated with Typhoon Mindulle led to a particulate organic carbon flux of 0.5 Mt over a 96-h period, with subsequent rapid burial of the terrestrial carbon in the ocean (Goldsmith et al., 2008). Also, extreme wind storms and cyclones can severely impact the regional carbon balance: In 1999 storm, Lothar reduced the European C sink by 16 Mt C, which corresponds to 30% of Europe’s net biome production (Lindroth et al., 2009) and, hurricane Katrina in 2005 destroyed an amount equivalent to 50–140% of the net annual U.S. C sink in forest trees (Chambers et al., 2007). Fires, pest and pathogen outbreaks are obviously not climate extremes, but can be facilitated by climate extremes. Extreme fire events release large quantities of carbon to the atmosphere. For example, in Indonesia, people had drained and deforested tropical wetlands which they then ignited to burn the debris awaiting the rain season to extinguish the fires, which failed due to the onset of the strong El-Niño Southern Oscillation in 1997/1998, which instead burnt the duff layers and vegetation releasing between 0.81 and 2.57 Gt C (Page et al., 2002). This amount was equivalent to the estimated annual release (van der Werf et al., 2010) and, together with the extreme fire events occurring in Siberia, produced a signal detected by atmospheric CO2 and CH4 monitoring stations (Simpson et al., 2006). Pest and pathogen outbreaks can have large impacts on forest carbon stocks and fluxes, and may impact the regional carbon cycle (Hicke et al., 2012), as was the case in a mountain pine beetle outbreak in British Columbia of unprecedented extent and severity, which converted the forest from a small net carbon sink to a large net carbon source (during and immediately after the outbreak) with an estimated cumulative regional impact of 270 Mt C for 2000–2020 (Kurz et al., 2008b).
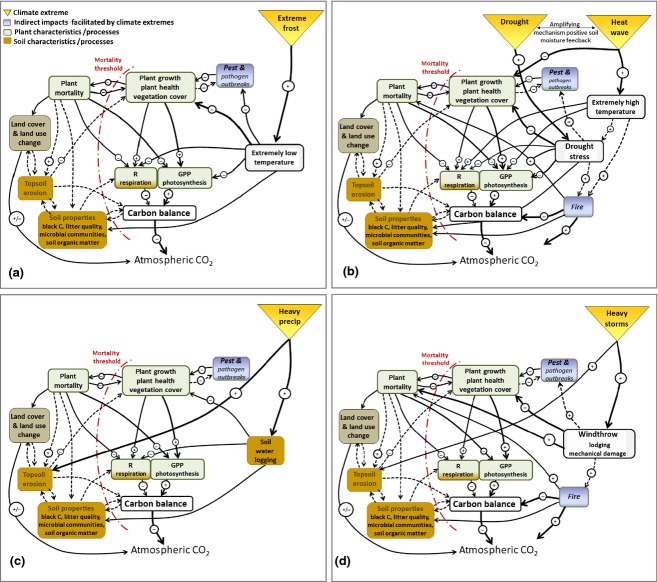
To be able to generalize and project presumable impacts of climate extremes on the carbon cycle, an understanding of the likely mechanisms and processes involved in extreme impacts is crucial. In this section, we review the primary environmental–biological processes according to their hypothesized relevance to different ecosystems, and the cascade of associated consequences. The complex pathways of how climate extremes may act on the major processes and components of the terrestrial CO2 balance are illustrated in Fig.3. We then provide a schematic overview of possible concurrent, lagged, direct and indirect impacts of climate extremes on processes underlying ecosystem carbon dynamics highlighting the importance of lagged impacts (Fig.4).
Figure 3.
Processes and mechanisms underlying impacts of climate extremes on the carbon cycle. Positive/enhancing impacts with a ‘+’ and negative/reducing impacts with a ‘−’sign; predominant (in-)direct impacts (dashed) arrows (for further details please see text); importance of impact/relationship is shown by arrow width (high = thick, low = thin) (modified after Reichstein et al., 2013).
Figure 4.
Schematic overview of concurrent, lagged, direct and indirect impacts of climate extremes on processes underlying ecosystem carbon dynamics. Respective references (selection of examples) are indicated as followed: 1 Larcher (2003) and Mayr et al. (2007); 2 Larcher (2003), Schulze et al. (2005), Lobell et al. (2012), Porter & Semenov (2005) and Niu et al. (2014); 3 Larcher (2003), Bréda et al. (2006), Keenan et al. (2010), Reichstein et al. (2007), Misson et al. (2010), Schwalm et al. (2010) and Eamus et al. (2013); 4 Rosenzweig et al. (2002), Vervuren et al. (2003), Kreuzwieser et al. (2004) and van der Velde et al. (2012); 5 Nykänen et al. (1997), Irland (2000), Changnon (2003), Hao et al. (2011) and Sun et al. (2012); 6 Berry et al. (2003), Fuhrer et al. (2006), MCPFE (2007), Lindroth et al. (2009), Zeng et al. (2009) and Negrón-Juárez et al. (2010b); 7 Larcher (2003), Schulze et al. (2005), Dittmar et al. (2006) and Bokhorst et al. (2009); 8 Larcher (2003), Porter & Semenov (2005), Bréda et al. (2006) and Lobell et al. (2012); 9 Barber et al. (2000), Eilmann et al. (2011), Fuhrer et al. (2006), Phillips et al. (2009), Michaelian et al. (2011), McDowell et al. (2013) and Peñuelas et al. (2013); 10 Vervuren et al. (2003) and Posthumus et al. (2009); 11 MCPFE (2007), Chambers et al. (2007), Zeng et al. (2009) and Negrón-Juárez et al. (2010a,b); 12 Fuhrer et al. (2006), Hilton et al. (2008) and García-Ruiz et al. (2013); 13 Wang et al. (2006) and Shinoda et al. (2011); 14 Jentsch et al. (2011) and Fuchslueger et al. (2014); 15 Moriondo et al. (2006) and Ganteaume et al. (2013); 16 Porter & Semenov (2005), Jentsch et al. (2009), Misson et al. (2011), Nagy et al. (2013) and Peñuelas et al. (2013); 17 Bréda et al. (2006), McDowell et al. (2008, 2011, 2013) and Walter et al. (2012); 18 Bréda et al. (2006), Adams et al. (2009), Allen et al. (2010), Michaelian et al. (2011), McDowell et al. (2008, 2011) and Granda et al. (2013); 19 Kreyling et al. (2011), Suarez & Kitzberger (2008) and Diez et al. (2012); 20 Larcher (2003) and Walter et al. (2013); 21 Virtanen et al. (1998), Stahl et al. (2006), Robinet & Roques (2010) and Kausrud et al. (2012); 22 Bréda et al. (2006), Desprez-Loustau et al. (2006), Rouault et al. (2006), MCPFE (2007), McDowell et al. (2008, 2011), Jactel et al. (2012), Keith et al. (2012), Kausrud et al. (2012) and Walter et al. (2012); 23 Schlyter et al. (2006), MCPFE (2007) and Komonen et al. (2011); 24 Trigo et al. (2006) and Wendler et al. (2011); 25 Kurz et al. (2008a); 26 Øygarden (2003), Valentin et al. (2008) and Thothong et al. (2011); 27 Sheik et al. (2011), Yuste et al. (2011) and, Fuchslueger et al. (2014); 28 Sowerby et al. (2008).
Direct impacts
Temperature extremes can directly and concurrently impact photosynthesis and respiration (cf. Fig.3a and b). Effects differ between species, ecosystem types and biomes, and may change seasonally and even diurnally through hardening responses (Larcher, 2003). Concurrent direct impacts of extremely high temperatures range from disruptions in enzyme activity affecting photosynthesis and respiration, to changes in growth and development (Larcher, 2003; Schulze et al., 2005; Lobell et al., 2012; Niu et al., 2014). Likewise, extremely low temperatures impact physiological functions and developmental processes. Frost damage is perhaps the most important direct concurrent impact of cold climate extremes. In this context, timing is a crucial factor: in temperate ecosystems risk of plant damage is particularly high in spring when temperatures drop below freezing after an early warming event (Bokhorst et al., 2009; Migliavacca et al., 2009), or during cold outbreaks when autumn hardening is insufficient, or when a protective snow cover is absent during extreme frost. In addition to frost damage of needles, xylem embolism in response to freeze-thaw cycles frequently adds to the factors decreasing plant vitality (Fig.3a) (Sperry & Sullivan, 1992; Mayr et al., 2003, 2007).
Unusual warming events at the end of the winter season in temperate and boreal climates can induce plant activity too early, a phenomenon that has been called ‘false spring’ (e.g. Marino et al., 2011). Extreme warm late winters together with the general trend of average warming may lead to earlier onset of the seasonal plant development, unfulfilled chilling requirements, that is the exposure to cool temperatures that is required before dormancy can be broken. A general trend of earlier onset of greening has been observed at local scales, from phenological gardens across Europe and globally from remote sensing NDVI data (Myneni et al., 1997; Menzel et al., 2006; Pilegaard et al., 2011). If plants switch from dormancy to physiological activity earlier, they may become more susceptible to frost events with strong negative consequences, such as tissue mortality (Polle et al., 1996), increased tree crown transparency (Dittmar & Elling, 2007), and reduced tree growth (Dittmar et al., 2006) and plant performance (Kreyling, 2010).
Drought extremes may have manifold impacts on the carbon cycle via direct concurrent impacts (e.g. on plant physiology and soil microbial activity), direct lagged impacts (e.g. on the phenology of plants, reduced growth in the following year due to lower carbohydrate storage in the year of the drought, altered composition of plant species, soil microbial community structure and activity), as well as indirect lagged impacts, for example by drought-facilitated pest and pathogen outbreaks or fire ignition and spread (see Figs3 and 4). Effects of drought on gross ecosystem productivity are typically larger than for ecosystem respiration (Schwalm et al., 2010; cf. Fig.3b).
Drought stress occurs whether the water potential of an organism/tissue drops below a critical threshold. For example, in temperate and Mediterranean forest ecosystems, decreased transpiration, gross photosynthesis and respiration were observed when relative root extractable soil water dropped below 40% (Granier et al., 2007). High temperatures and low relative humidity (often expressed at the vapour pressure deficit) serve to increase evaporative demand, and drought stress of plants occurs when soil water supply can no longer meet the plant evaporative demand (e.g. Sperry, 2000). Plant available water is influenced by soil type and local surface and subsurface characteristics, such as the depth to the groundwater level or bedrock. The amount of water actually available to a plant depends strongly on the distribution of soil water across the profile in relation to root depth and type (Schachtschabel et al., 1992; Tolk, 2003; White, 2006; Vicca et al., 2012).
Droughts and extreme high temperatures (heat waves), both to be considered climate extremes in their own right, cannot be seen as independent phenomena as in many (transitional climate) regions droughts additionally are connected with high temperature extremes (Mueller & Seneviratne, 2012) (Fig.3b). The combination of high temperatures and droughts initiate a positive regional feedback mechanism (e.g. Durre et al., 2000; Seneviratne et al., 2006; Fischer et al., 2007; Vautard et al., 2007; Zampieri et al., 2009; Diffenbaugh & Ashfaq, 2010; Hirschi et al., 2011): the precipitation deficits and enhanced evaporative demand generally associated with warm spells (e.g. atmospheric blockings) triggers soil moisture deficit, thus suppressing evaporative cooling (Teuling et al., 2010) and leading to hotter and drier conditions if soil moisture becomes limiting for evapotranspiration (Seneviratne et al., 2010). Warmer temperatures additionally increase vapour pressure deficit, even without a concurrent reduction in rainfall, and this process alone causes extra drought stress (Williams et al., 2012). In addition, there are likely also nonlocal feedbacks between drought conditions and heat waves, for instance through the advection of dry air or the modification of regional-scale circulation patterns (e.g. Vautard et al., 2007; Haarsma et al., 2009).
Plants may respond to drought stress by structural or physiological adjustments such as decreased leaf area index, changes in the root–shoot ratio, or changes in osmolyte concentration (Larcher, 2003; Bréda et al., 2006). The ability of plants to extract water from deeper layers under soil moisture stress, up to some limit, has been reported (e.g. Nepstad et al., 1994; Canadell et al., 1996; Wan et al., 2002; Teuling et al., 2006). Drought decreases CO2 assimilation rates (according to our definitions, a direct concurrent impact) by reducing stomatal and mesophyll conductance, the activity and concentrations of photosynthetic enzymes (Lawlor, 1995; Chaves et al., 2009; Keenan et al., 2010) and reducing sink strength (Palacio et al., 2014). Generally, direct concurrent drought impacts are larger for plant photosynthesis than for respiration of plants (Atkin & Macherel, 2009) and ecosystems (Schwalm et al., 2010; Shi et al., 2014) (Fig.3b).
In addition to direct concurrent drought impacts like decreased carbon (and nutrient) assimilation (Fig.3b), drought may have lagged impacts on the carbon cycle via the re-allocation of existing stored reserves for repair, maintenance (including that of hydraulic integrity), growth and defence, as well as indirect lagged impacts (Fig.4) by increasing the ecosystems’ vulnerability to additional stressors such as pests and pathogens, or subsequent drought events (Bréda et al., 2006; Desprez-Loustau et al., 2006; McDowell et al., 2011; Sala et al., 2012; Keith et al., 2012).
Water stress has a direct, concurrent impact on microbial activity, which depends on the presence of water films for substrate diffusion and exo-enzyme activity (Davidson & Janssens, 2006), whereas indirect and lagged drought impacts on microbial activity may be initiated by various mechanisms such as a decreased input of labile carbon into the soil due to reduced plant productivity (Araus et al., 2002; Reddy et al., 2004), and altered soil nutrient retention and availability (Muhr et al., 2010; Bloor & Bardgett, 2012). Drought may also alter microbial community structure (Sheik et al., 2011) with consequences for carbon cycling (Fig.4; direct concurrent and (in-)direct lagged impact via changes in species composition) (Fuchslueger et al., 2014). In Mediterranean ecosystems, for example, fungi were less affected by drought than bacteria and controlled soil organic matter decomposition (Curiel-Yuste et al., 2011). While soil and ecosystem respiration are reduced by drought, rewetting by rainfall following drought can strongly stimulate soil CO2 emissions to levels substantially exceeding predrought (or control) rates, with immediate consequences for the carbon cycle (Fig.2, Jarvis et al., 2007; see also reviews by Borken & Matzner, 2009; Kim et al., 2012; Vicca et al., 2014). Different mechanisms act when drying–rewetting cycles become more pronounced. Among others, physical disruption of aggregates (Borken & Matzner, 2009), increased soil water repellency (Goebel et al., 2011) and altered nutrient retention (Borken & Matzner, 2009; Bloor & Bardgett, 2012) can be responsible for legacy effects on microbial activity and respiration, by modifying substrate and nutrient availability (indirect and lagged impact).
The magnitude of the impact on key ecosystem processes from an altered quantity, frequency or intensity of precipitation critically depends on the ecosystems’ (seasonally varying) baseline water limitation (Gerten et al., 2008). In addition to intensity and duration, the timing of droughts is a crucial factor due to the pronounced seasonal cycle of many ecosystems and land uses (Allard et al., 2008; Unger et al., 2009; Misson et al., 2010, 2011; De Boeck et al., 2011).
Extreme precipitation events may alter soil CO2 fluxes and CO2 uptake by plants during water logging phases (direct concurrent impacts on the carbon cycle), may lead to flooding-related tree mortality (Kramer et al., 2007) and may cause topsoil erosion (Fig.3c; see also below and Fig.4) with losses of particulate and dissolved organic carbon from terrestrial to riverine ecosystems (Hilton et al., 2008; Dinsmore et al., 2013). In more water-limited systems, longer intervals between rainfall events may increase the duration and severity of soil drought stress. In contrast, longer intervals between heavy rainfall events may reduce periods of anoxia and be favourable to plant growth in more hydric ecosystems (see also Knapp et al., 2008). The impacts of extreme precipitation events are often exacerbated by their association, in most climatic regions, with extreme wind storms/cyclones.
Ice storms are a form of extreme precipitation that occurs when liquid precipitation (often in a supercooled state) freezes shortly after contact with the terrestrial surface. The growing layer of ice can add substantial weight to vegetation and therefore result in the loss of branches, limbs, or uproot entire trees (Bragg et al., 2003; McCarthy et al., 2006; Sun et al., 2012).
Extreme wind storms and tropical cyclones are often associated with extreme precipitation events, but have the additional potential to cause, depending upon their intensity severe damage and direct concurrent impacts on the carbon cycle (Fig.3d) via defoliation, damage to branches, and windthrow or flooding by (e.g. saltwater) storm surges related tree mortality (Conner & Inabinette, 2003; MCPFE, 2007; Chambers et al., 2007; Imbert & Portecop, 2008; Zeng et al., 2009; Negrón-Juárez et al., 2010a) and lodging in agroecosystems (when crop stems are broken and crops are flattened). In addition, in forests, windthrow can cause long-term indirect lagged impacts on the carbon balance via tree mortality and dry dead wood accumulation that may facilitate lagged insect outbreaks or massive fires (Fig.3d; see also below). Individual extreme storms and cyclones can severely impact the regional carbon balance (e.g. Lindroth et al. (2009) for Europe or Chambers et al. (2007) for the U.S.). For example, in October 2005, Hurricane Wilma made landfall over the Yucatán peninsula with particularly intense winds. Immediate reductions in leaf area and productivity were observed, while in the year following the hurricane, increased carbon emissions from soils were observed that were attributable to the addition of nitrogen-rich organic matter (Vargas, 2012). Depending on the spatial and tempora l scale considered, the frequency and intensity of the storm/cyclone, the characteristics of the impact and the recovery processes involved, the overall carbon balance can vary between a source and a sink (Fig.2; see e.g. Fisk et al., 2013).
Soil erosion can be caused by the extreme precipitation events and extreme wind storms (or a combination of both) and is codetermined by topography, soil characteristics, vegetation cover and human activities (e.g. Lal et al., 2013) with significant on- and off-site impacts. Extreme weather events can result in direct, rapid and substantial local soil carbon losses (Hilton et al., 2008; Jung et al., 2012), and subsequent transport/redistribution and deposition (Goldsmith et al., 2008). Soils are especially susceptible to erosion if vegetation cover is low, for example crop ecosystems at fallow stages or grasslands after drought periods. Soil carbon loss due to erosion can therefore be a direct concurrent as well as an indirect lagged climate extreme impact (see Fig.4). In addition, soil erosion leads to losses of soil nutrient and water retention capacity, and to a generally lower productivity on eroded soils (Lal & Pimentel, 2008), inducing further (indirect) lagged impacts on the ecosystems carbon cycle. Eroded soil and mobilized soil organic matter are often redeposited within the same ecosystem at short-timescales, but soil organic carbon can also be laterally exported from a particular ecosystem (VandenBygaart et al., 2012; Berhe & Kleber, 2013). The deposition and subsequent residence time of carbon removed with eroded soil determines the contribution of soil organic carbon erosion to CO2 fluxes (van Oost et al., 2007; Lal & Pimentel, 2008). Soil erosion processes can also increase the terrestrial carbon sink if eroded carbon is not transformed to CO2, but trapped in deposits with longer residence times than the original soil (van Oost et al., 2007; Hilton et al., 2008). Hence, erosion and subsequent sedimentation affects the overall land carbon budget, but the net effect of erosion on the carbon cycle remains controversial (Lal, 2009) and improved, scientifically rigorous terminology may be needed to describe landscape soil carbon turnover (Berhe & Kleber, 2013).
Indirect impacts
While extreme droughts, heat waves, frosts, precipitation and wind storms are climate extremes, soil erosion can be a direct concurrent impact of extreme precipitation and/or wind storms and, additionally, may be amplified by indirect lagged climate extreme impacts (cf. Fig.4); fires and pest and pathogen outbreaks are impacts facilitated by climate extremes (cf. Fig.4), but initiated by another trigger (not necessarily an extreme event per se) (cf. Fig.1b and d).
Fire-related losses of biomass or soil organic matter generally occur as an indirect, and often lagged, impact of climate extremes (cf. Figs3b, d and 4) and are caused by the interaction between biotic (e.g. fuel load) and abiotic factors (e.g. dry weather, wind velocity, fuel continuity, slope of terrain and landscape fragmentation) and human ignition (Moriondo et al., 2006; Bowman et al., 2009; Aldersley et al., 2011; Pausas & Paula, 2012). Fire frequency and intensity are highly sensitive to climate extremes because fire behaviour responds immediately to fuel moisture, which is affected by the combination of precipitation, relative humidity, air temperature and wind speed (Moriondo et al., 2006). Fires release carbon stored in biomass and organic soils to the atmosphere in form of CO2, CO, CH4 and other climate relevant trace gases and aerosols, but can also serve to prevent land–atmosphere CO2 fluxes when burned organic matter (i.e. charcoal) is formed during the combustion process. Charcoal is typically more resistant to decomposition and is thought to contribute to long-term carbon sequestration in soils (Preston & Schmidt, 2006; Schmidt et al., 2011), although recent advances point to a much faster decomposition rate which depends on thermal conditions during formation and soil conditions afterwards, than previously thought (Major et al., 2010; Singh et al., 2012; Kasin & Ohlson, 2013).
Extreme fire events release large quantities of carbon to the atmosphere (Page et al., 2002) and may have long-lasting consequences on vegetation composition (Bond et al., 2005), soil structure, hydrophobicity and nutrient availability (Certini, 2005) with presumable multiple indirect and lagged impacts on the terrestrial carbon cycle (cf. Figs3b and 4). Carbon stored in litter, and organic soils such as peat, is burned during high-intensity but slow-spreading fires, and can be irreversibly destroyed, particularly during peat fires where carbon accumulated over very long timescales is immediately released, but can be additionally accelerated by another trigger (Page et al., 2002; Turetsky et al., 2011a). Note, however, that not all climate-induced fires are carbon cycle extremes, but are within the range of the particular disturbance regime. For instance, frequent and low-intensity savannah fires (Archibald et al., 2012) may release over a year as much CO2 as would have been decomposed otherwise by microbes (Li et al., 2013).
The occurrence, frequency and magnitude of insect and pathogen outbreaks are often related to natural cycles in population size, driven by predator-prey type dynamics (Jepsen et al., 2009; Kausrud et al., 2012). But there is consensus – despite many uncertainties – that climate conditions influence strength and timing of insect/pathogen outbreaks via changes in dispersal, reproduction, development of host plants, and mortality and distributional range changes of insect herbivores (Netherer & Schopf, 2010; Cornelissen, 2011). Different types of climate extremes may therefore catalyse insect and pathogen outbreaks leading as we hypothesize towards indirect lagged impacts on the carbon cycle (see Figs3 and 4). Warm temperatures appear to favour radical increases in insect populations as a result of reduced mortality during the cold season, accelerated insect development rates and earlier flight periods (Virtanen et al., 1998; Stahl et al., 2006; Robinet & Roques, 2010; Johnson et al., 2010). We regard these patterns as an indirect lagged impact of fewer cold temperature extremes (cf. Figs3a and 4). Mechanisms, associated with indirect lagged impacts of extreme heat and drought (cf. Figs3b and 4), were observed during the European 2003 heat wave. Soil water deficits appeared to lower tree resistance to pest attacks, that is a positive drought – disease association, and defoliators additionally benefitted from increased nitrogen in plant tissues linked to moderate or intermittent drought stress (Desprez-Loustau et al., 2006; Rouault et al., 2006). Multiple examples of how primary productivity and carbon stocks are reduced by insects and pathogens, and changes in carbon sink strength, are given in Hicke et al. (2012).
Impacts of extreme events on different ecosystem types
Ecosystems react differently to climate extremes: therefore, we deduce that a climate extreme of a given magnitude will not have the same impact in a forest, grassland, peatland or cropland. With both large aboveground carbon stocks (standing biomass) and carbon uptake being affected by climate extremes, we expect the largest net effects on the terrestrial carbon balance in forests compared to other ecosystems. Forest carbon stocks may be lost or reduced as CO2 rapidly by fire (as an indirect concurrent or lagged effect due to drought and heat extremes; Fig.4), or more slowly during the decomposition of dead wood after extreme wind and ice storms or forest dieback after an extreme drought, which lead to lagged carbon emissions for a presumable long period after the climate extreme has occurred.
There are notable differences in how individual tree species respond to intra-annual climatic extremes including the timing of maximum sensitivity (Babst et al., 2012), and the complexity of forest ecosystem dynamics makes prediction of the impacts of extreme events on carbon cycling challenging (Rammig et al., 2014). At the same time, we hypothesize the complexity of forest ecosystems contributes to their resilience to climate extreme related impacts as, for example heterogeneous forests are known to be less susceptible to windthrow (Lindroth et al., 2009), insect outbreaks (Drever et al., 2006) and mass movements (Bebi et al., 2009) (see Appendix S1, section A. for biome-specific extremes and related impacts). Forests generally have better access to deeper ground water than grasslands and are reported to be likely less strongly affected by drought and heat waves (Teuling et al., 2010). However, once their mortality thresholds are passed, we suppose forests to be less resilient to extreme events than grasslands, which have evolved to recover rapidly from disturbances. Natural grasslands prevail in regions where climatic constraints limit the occurrence of woody life forms (Suttie et al., 2005). Grasslands are typically characterized by comparatively higher turnover rates compared to woody vegetation, and we therefore assume grasslands to be more resilient to climate extremes than forests (see Appendix S1, section B, for more details). In this context, amongst the climate extremes, drought is expected to have the largest effect on the carbon cycle of grasslands (Zavalloni et al., 2008; Gilgen & Buchmann, 2009; van der Molen et al., 2011), while other extremes (e.g. wind storms) play a smaller if not negligible role (Reichstein et al., 2013). However, degradation feedbacks, as triggered by, for example grazing pressure (Albertson et al., 1957), erosion (Breshears et al., 2003) or fire combined with extreme precipitation events, may amplify effects of extreme drought and lead to substantial soil carbon losses. In comparison with forests, when normalizing for the per cent of bare soil, potential postfire erosion tends to be lower in grassland (Johansen et al., 2001).
Peatlands have characteristics in common with both forests and grasslands, namely large organic carbon stocks and a clear dominance of belowground carbon stocks, respectively. The large carbon stocks stored in peatlands are mainly protected by decomposition-limiting low temperatures and/or high water levels (Freeman et al., 2001). Peatland carbon stocks are highly susceptible to immediate oxidation by fire (van der Werf et al., 2008, 2010; Turetsky et al., 2011a,b) and drought- or drainage-induced processes of microbial decomposition of organic carbon (Jungkunst & Fiedler, 2007; Couwenberg et al., 2010; Frolking et al., 2011). Therefore, we hypothesize peatlands to be highly susceptibility to drought extremes and fire events caused by climate extremes (see Appendix S1, section C for more details).
Croplands are distinct from forests, grasslands and peatlands, in that most crops are planted and harvested on an annual basis. The response of croplands is strongly coupled to the timing of the climate extreme, that is the sensitivity of the growth stage of the impacted crop (e.g. van der Velde et al., 2012) and the management actions taken (e.g. Porter & Semenov, 2005; Ramankutty et al., 2008; van der Velde et al., 2010; Lobell et al., 2012). In croplands, many climate extreme impacts can (theoretically) be mitigated through management, either within the same year (e.g. irrigation, replanting of a failed crop), or through longer term adaptation (e.g. changed rotations, drought- and/or heat-resistant cultivars). Lagged impacts of more than one year are of minor importance in croplands compared with the other ecosystem types.
A quantitative and systematic assessment of the impacts from different types of extreme events is currently limited by the number of observed case studies, a general lack of systematic data, and a lack of common metrics across experimental and impact studies (see Introduction). It is therefore currently only possible to provide a detailed literature survey about how drought, wind storms, temperature and precipitation extremes, may possibly act on carbon cycle processes in forests, grasslands, peatlands and croplands (see Appendix S1).
Future climate extremes and their impact on the carbon cycle
There are inherently few data available to make robust assessments regarding changes in the frequency or intensity of carbon cycle extremes. First of all, climate extremes are hard to predict, as many predictions of climate extremes are either not sufficiently well resolved (e.g. heavy precipitation) or associated with high uncertainties (e.g. drought) in current climate models (Seneviratne et al., 2012). Even in leading sectorial (e.g. agriculture) models, the effects of high temperatures, increased climate variability and several other growth-limiting factors such as soil nutrients, pests and weeds are not yet fully understood, and thus not implemented (Soussana et al., 2010). Hence, it is very difficult to anticipate future impacts of climate extremes on the global carbon cycle. Thus, we here only hypothesize the most important current and future risks of the terrestrial carbon cycle in the face of climate extremes given the available literature.
In those parts of the boreal zone where litter and soil moisture will likely decrease, for example via rising temperatures and decreasing precipitation (Seneviratne et al., 2012) and earlier snowmelt (Grippa et al., 2005), we hypothesize an increased risk that extreme dryness and tree mortality will increase the susceptibility to triggers such as lightning and human ignition, causing fires as an indirect concurrent or lagged effect (c.f. Figs1d and 4; Michaelian et al., 2011).
On the other hand, according to current climate projections, large areas in the boreal zone will likely become wetter (IPCC, 2013). More extreme snow fall has the potential to lead to stronger insulation of the soil in the winter. The higher soil temperatures may favour the thawing of permafrost (Zhang et al., 2001; Gouttevin et al., 2012), but also increase mineralization and growing season productivity (Monson et al., 2006). Assessment of the magnitude and timing of these two opposing effects will require further research. As host–pathogen interactions are strongly influenced by weather and climate, we further hypothesize that decreased frost occurrence and fewer cold extremes will facilitate pest and pathogen outbreaks (e.g. Virtanen et al., 1998; Hicke et al., 2012; Sambaraju et al., 2012; Price et al., 2013) with supposed important indirect and lagged impacts on the carbon cycle.
Temperate regions, being situated between cold boreal and warm, summer-dry Mediterranean regions are susceptible to temperature and precipitation extremes, droughts and storms, and impacts facilitated by them. Storms are considered to be the most important natural disturbance agent in temperate European forests, and even a small increase in storm frequency could potentially lead to a long-term reduction of the carbon stock (Fuhrer et al., 2006; Lindroth et al., 2009). Yet, current predictions of changes in storm intensity and frequency are not very robust (IPCC, 2013), such that no speculation on future impacts of storms on ecosystem is possible.
In contrast, we conjecture that in dry temperate regions, there will be a sizeable negative effect on the carbon cycle through drought extremes, because towards the drier border of temperate regions, there is consensus among climate models that, for example, the number of consecutive dry days will increase (Seneviratne et al., 2012). Droughts, often occurring in concert with heat waves, can extend spatially across subcontinental domains and have a pronounced effect on forests, grasslands and croplands (Reichstein et al., 2007; Schwalm et al., 2010). Yet, the potentially mitigating effect of increased plant water use efficiency through increased CO2 concentrations needs to be scrutinized in future research (e.g. Morgan et al., 2011; Zscheischler et al., 2014c).
Mediterranean and subtropical ecosystems are already shaped by strong seasonality of water availability. Changes in precipitation patterns with longer dry spells and more intense precipitation events are very likely (Seneviratne et al., 2012). We suggest that in forests these changing patterns will contribute to higher tree mortality rates, increased fire activity in forests, and thus more sparse vegetation, and therefore as an indirect lagged effect (cf. Figs1d and 4) enhanced soil erosion, with expected negative consequences for ecosystem productivity (e.g. Allen et al., 2010; Williams et al., 2012). We further hypothesize that such positive feedback loops within the ecosystem triggered and enforced by alternating dry spells and subsequent heavy precipitation are even more likely and rapidly to occur in grasslands and cropland (e.g. with lower thresholds) because the nonwoody vegetation with shorter turnover is likely to respond faster.
In the tropics, susceptibility of the carbon cycle to climate extremes will strongly depend on the interaction with human drivers. For example, fire risk is low in undisturbed Amazonian rainforests, and almost all fires are a consequence of land-use-related burning activities (Aragão & Shimabukuro, 2010). Once burnt, forests are more susceptible to repeated burning, creating a positive feedback, which has the potential to transform large parts of rainforests into degraded forests or even savannah (Barlow & Peres, 2008; Brando et al., 2012, 2014; Morton et al., 2013). Changes in precipitation patterns with longer dry spells might additionally increase fire risk with decreasing canopy closure. While tropical forests and cropping systems are susceptible to long-term droughts, heavy precipitation and wind storms, future projections of these climatic extremes are particularly uncertain. The effect of high temperatures on photosynthesis is the second crucial mechanism that can directly impact tropical forests, where the most intensive CO2-emission scenarios yield temperatures sufficient to damage photosynthesis and growth (Doughty & Goulden, 2008). But the long-term acclimation and adaptation potential of tropical forest ecosystems (e.g. shift to heat-tolerant species) is not well known (Corlett, 2011; Smith & Dukes, 2013). We expect also the susceptibility of tropical peatlands to climate extremes to be strongly dependent on the interaction with human drivers, as peatland carbon stocks are highly susceptible to fires and drought- or drainage-induced microbial decomposition processes of their organic carbon stocks (see section above). Thus, we hypothesize that climate extremes will affect the tropical rainforest and peatland carbon cycle substantially, but the magnitude will strongly depend on the local human influence on these carbon stocks.
Outlook: On improving detection and prediction of global carbon cycle extremes
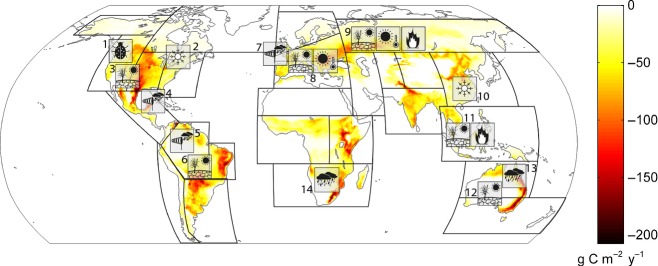
From a mechanistic and process perspective, it is clear that climate extremes can have a profound impact on the carbon cycle, and case studies have reported such impacts (Fig.5). However, great challenges remain for both a rigorous global quantification of carbon cycle extremes and estimation of the future impacts on terrestrial-atmosphere CO2 fluxes, and hence carbon cycle climate feedbacks.
Figure 5.
Global distribution of extreme events in the terrestrial carbon cycle, and approximate geographical locations of published climate extremes with impacts on the carbon cycle. Extreme events in the carbon cycle are defined as contiguous regions of extreme anomalies of GPP during the period 1982–2011 (modified after Zscheischler et al., 2014b). Colour scale indicates the average reduction in gross carbon uptake compared to a normal year due to negative extremes in GPP. Units are gram carbon per square metre per year. The map highlights the IPCC regions with the following references to the published climate extremes. References: 1 pest outbreaks Canada/North America (Soja et al., 2007; Kurz et al., 2008b), 2 ice storm North America (Irland, 2000), 3 drought US (Breshears et al., 2005; Schwalm et al., 2012), 4 heavy storm Southern US (Chambers et al., 2007; Zeng et al., 2009; Negrón-Juárez et al., 2010a), 5 heavy storm Amazon (Negrón-Juárez et al., 2010b), 6 drought Amazon (Tian et al., 1998; Phillips et al., 2009; Lewis et al., 2011), 7 heavy storm Europe (Fuhrer et al., 2006; Lindroth et al., 2009), 8 drought and heat extreme Europe (Ciais et al., 2005; Reichstein et al., 2007), 9 extreme drought, heat and fire in Russia (Barriopedro et al., 2011; Konovalov et al., 2011; Coumou & Rahmstorf, 2012; Bastos et al., 2013a), 10 ice storm China (Stone, 2008; Sun et al., 2012)), 11 fire, drought SE Asia (Page et al., 2002; Schimel & Baker, 2002), 12 drought Australia (Haverd et al., 2013), 13 heavy precipitation Australia (Bastos et al., 2013b; Haverd et al., 2013), 14 heavy precipitation Southern Africa (Bastos et al., 2013b).
Remote sensing of the biosphere from space with a short return interval to identical locations and nearly global coverage offers promising perspectives to detect extreme anomalies in the biosphere in a consistent way (but see below). Land surface states can be estimated by analysing the interaction of electromagnetic radiation (from visible to microwave) with the vegetation or upper centimetres of the soil via relatively well-evaluated radiation transfer models and their inversion. Thus, vegetation states (e.g. leaf area index, biomass) and radiative properties (e.g. fractions of absorbed radiation) can be monitored, albeit they require improvements to correct retrieved signals affected by noise and biases related to atmospheric conditions. Direct methods exist for use on the ground (Pan et al., 2011; Baldocchi et al., 2012; Babst et al., 2014) and can be combined with remote sensing and modelling approaches to infer carbon cycling at the global scale (Jung et al., 2011).
Zscheischler et al. (2013) have taken a first approach to detect extreme changes in fAPAR (fraction of absorbed photosynthetically active radiation) and GPP (Zscheischler et al., 2014a) associated with climate anomalies that occurred during the last three decades and their association with climate anomalies. They presented four major findings: (1) the total effect of negative carbon cycle GPP extremes is of a similar magnitude as the mean terrestrial carbon sink, (2) the spatial distribution of extremes is highly uneven with ‘hotspot’ regions in many semiarid monsoon-affected regions, (3) the distribution of extreme carbon impacts follows a power law and (4) the detected carbon cycle extremes are statistically mostly strongly associated with droughts. The background map in Fig.5 shows the spatial distribution of carbon cycle extremes detected in the Zscheischler et al. studies. Many regions, where case studies have reported carbon cycle extremes, are also detected by the global remote sensing-based approach, but not all. In particular, Amazonian extreme anomalies in the carbon cycle suggested by Phillips et al. (2009) or Negrón-Juárez et al. (2010b) are not evident in the remote sensing-supported analysis of Zscheischler et al. (2013) and are only seen in one model in the analysis of negative extremes in four different data-driven and modelled GPP estimates (Zscheischler et al., 2014a). One reason for this might be the lack of sensitivity of fAPAR in dense evergreen vegetation (data-driven estimates of GPP often rely strongly on fAPAR). Evergreen vegetation often changes its physiology without strong alterations in the leaf or canopy reflective properties. This effect has also been observed outside tropical regions, for instance, during the extreme heat and drought in Europe 2003 (Reichstein et al., 2007). Currently, more direct observations of photosynthetic processes via fluorescence offer the potential to overcome this problem (Frankenberg et al., 2011; Guanter et al., 2014), as well as combined observations of greenness indices and land surface temperature (Mildrexler et al., 2009). However, one striking feature of Fig.5 is the lack of presumably reported extreme impacts on the carbon cycle in some hotspot areas seen by the satellite data analysis. These include North East Brazil, the Indian subcontinent, East Asia, and particularly sub-Saharan Africa. To our understanding, without observations and experiments in those tropical hotspot areas, it will be hard to fully understand climate–carbon cycle feedbacks and the role of carbon cycle extremes therein at a global scale.
According to our understanding, not all climate extremes cause extreme impacts in ecosystems, but they can have in-/direct and/or immediate/lagged effects. Lagged effects can either slow down the carbon cycle, when reduced vegetation productivity and/or wide-spread mortality after an extreme drought are not compensated by regeneration, but they can also accelerate the carbon cycle, when, for example productive tree and shrub seedlings cause rapid regrowth after windthrow or fire. Likewise, not all terrestrial carbon cycle extremes are propagated immediately into the atmosphere. For example, an extreme mortality event increases coarse woody debris, which is then slowly decomposed during the following years. Terrestrial carbon cycle extremes leading to structural changes without immediate fluxes to the atmosphere are currently globally undetectable due to lack of observation capabilities. LiDAR or Radar satellite missions with sufficient spatial and temporal resolution should be encouraged to increase such capabilities in the future. Detection systems need to resolve processes that cause immediate or lagged effects at different spatial and temporal scales, as the resilience of the respective ecosystem differ by ecosystem type.
This review also showed the lack of quantitative and consistent experimental data on the impact of climate extremes on the terrestrial carbon cycle, such that our conclusions are largely based on expert knowledge, scattered case studies and logical reasoning. Future experimental and observational designs should have a clear definition of the extreme conditions at the onset (e.g. by return interval), a consistent classification of resulting (extreme) impacts and should consider testing hypotheses around the conceptual framework presented in Fig.1. In particular, indirect effects (Fig.1b and d) need to receive increased attention in our opinion, given the complexity of the mechanisms involved and the paucity of current studies.
Future experiments should not only strive towards increasing comparability of treatments across case studies, as suggested above; they should also account for increasing severity of future climate extremes and test more explicitly for threshold effects and mortality and recovery responses after extreme events, including those related to changing shifts of ecosystem states (Smith, 2011, Beier et al., 2012; Bahn et al., 2014). Gradient studies that contain at least one very extreme (and possibly unrealistic) treatment would be particularly useful for this (Kreyling et al., 2014). Future experiments should address lagged and legacy effects more consistently, as well as ecosystem responses to multiple subsequent climate extremes, with the aim of elaborating mechanisms, as, for example related to stress physiology, mortality and community assembly, as well as plant–soil interactions and soil processes at large (Backhaus et al., 2014; Kopittke et al., 2014; Vicca et al., 2014). Only through holistic approaches will we be able to fully understand the impacts of climate extremes on ecosystem carbon cycling; information needed to obtain realistic predictions of future carbon cycling and climate feedbacks. For more details and best-practice guidance in climate change experiments that aim to improve our understanding of the impacts of climate extremes, we refer to Beier et al., 2012; Vicca et al., 2012, 2014; Kreyling et al., 2014.
For ecosystems dominated by long-lived species such as forests, a better integration of experimental and modelling studies is needed, with experiments targeting critical hypotheses underlying model assumptions or specific mechanisms (e.g. processes linked to ecosystem transitions). State-of-the-art coupled climate–carbon cycle models (CMIP5) indicate a stronger negative effect of carbon cycle extremes than the above-mentioned observation driven estimates (Reichstein et al., 2013), and an increasing absolute effect in the future. However, a reliable projection of the future impact of climate extremes on the terrestrial carbon cycle must rely on improved earth-system modelling, as well as improved description of the biospheric responses. Higher spatial (both horizontal and vertical) resolution and better representation of convective processes and clouds are prerequisites for the simulation of climate extremes, and particularly hydrometeorological extremes. On the biosphere modelling side, all processes leading to direct/indirect, as well as concurrent/lagged impacts (Fig.4), need to receive attention. In particular, vegetation mortality in response to climate extremes (e.g. drought) and its mechanisms are increasingly well documented. Effort needs to be taken now to include this knowledge into global biosphere models. Including pest and pathogens, their reaction to climate extremes such as cold extremes and their effect on the carbon cycle within an integrated modelling system at global scale is likely still too ambitious and needs landscape-modelling approaches, where lateral interactions are considered. Promising local- to regional-scale approaches do exist here and need to be further developed (Seidl et al., 2011). Representation of these impacts into carbon cycle models will likely increase projected effects of climate extremes on the carbon cycle. On the other hand, we have to note that fundamental adaptive processes, such as acclimation, plasticity, migration, selection and evolution have the potential to mitigate effects of climate extremes. Modelling approaches accounting for these adaptations urgently need to be underpinned with more observational data and further developed (Scheiter et al., 2013).
This study underlines the demand for better structured impacts studies of climate extremes on terrestrial ecosystems and the carbon cycle which follow a standardized protocol and definitions and allow for intercomparison studies. It has also shown the varying depth of analysis for different types of climate extremes, as well as identifying critically understudied regions. The findings underline the importance of biospheric processes in modulating impacts of climate extremes to assess the feedback to the global carbon cycle. In other words, biospheric processes are likely to determine the reaction of the global carbon cycle to climate extremes under global change.
Acknowledgments
This work emerged from the CARBO-Extreme project, funded by the European Community’s 7th framework programme under grant agreement (FP7-ENV-2008-1-226701). We are grateful to the Reviewers and the Subject Editor for helpful guidance. We thank to Silvana Schott for graphic support. Mirco Miglivacca provided helpful comments on the manuscript. Michael Bahn acknowledges support from the Austrian Science Fund (FWF; P22214-B17). Sara Vicca is a postdoctoral research associate of the Fund for Scientific Research – Flanders. Wolfgang Cramer contributes to the Labex OT-Med (no. ANR-11-LABX-0061) funded by the French Government through the A*MIDEX project (no. ANR-11-IDEX-0001-02). Flurin Babst acknowledges support from the Swiss National Science Foundation (P300P2_154543).
Supporting Information
Appendix S1. Provides a detailed literature survey about how climate extremes act on forests, grasslands, peatlands and croplands.
References
- Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7063–7066. doi: 10.1073/pnas.0901438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson FW, Tomanek GW, Andrew Riegel A. Ecology of drought cycles and grazing intensity on grasslands of central great plains. Ecological Monographs. 1957;27:27–44. [Google Scholar]
- Aldersley A, Murray SJ, Cornell SE. Global and regional analysis of climate and human drivers of wildfire. Science of the Total Environment. 2011;409:3472–3481. doi: 10.1016/j.scitotenv.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Allard V, Ourcival J-M, Rambal S, Joffre R, Rocheteau A. Seasonal and annual variation of carbon exchange in an evergreen Mediterranean forest in southern France. Global Change Biology. 2008;14:714–725. [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259:660–684. [Google Scholar]
- Aragão LEOC, Shimabukuro YE. The incidence of fire in Amazonian forests with implications for REDD. Science. 2010;328:1275–1278. doi: 10.1126/science.1186925. [DOI] [PubMed] [Google Scholar]
- Araus JL, Slafer GA, Reynolds MP, Royo C. Plant breeding and drought in C3 cereals: what should we breed for? Annals of Botany. 2002;89:925–940. doi: 10.1093/aob/mcf049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald S, Lehman CER, Gomez-Dans JL, Bradstock RA. Defining pyromes and global syndromes of fire regimes. Proceedings of the National Academy of Sciences of the United States of America. 2012;110:6442–6447. doi: 10.1073/pnas.1211466110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Macherel D. The crucial role of plant mitochondria in orchestrating drought tolerance. Annals of Botany. 2009;103:581–597. doi: 10.1093/aob/mcn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst F, Carrer M, Poulter B, Urbinati C, Neuwirth B, Frank D. 500 years of regional forest growth variability and links to climatic extreme events in Europe. Environmental Research Letters. 2012;7:045705. (045711 pp.) [Google Scholar]
- Babst F, Bouriaud O, Papale D, et al. Aboveground woody carbon sequestration measured from tree rings is coherent with net ecosystem productivity at five eddy covariance sites. New Phytologist. 2014;201:1289–1303. doi: 10.1111/nph.12589. [DOI] [PubMed] [Google Scholar]
- Backhaus S, Kreyling J, Grant K, Beierkuhnlein C, Walter J, Jentsch A. Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems. 2014;17:1068–1081. [Google Scholar]
- Bahn M, Reichstein M, Dukes JS, Smith MD, McDowell NG. Climate-biosphere interactions in a more extreme world. New Phytologist. 2014;202:356–359. doi: 10.1111/nph.12662. [DOI] [PubMed] [Google Scholar]
- Baldocchi D, Reichstein M, Papale D, Koteen L, Vargas R, Agarwal D, Cook R. The role of trace gas flux networks in the biogeosciences. Eos, Transactions American Geophysical Union. 2012;93:217–224. [Google Scholar]
- Barber VA, Juday GP, Finney BP. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature. 2000;405:668–673. doi: 10.1038/35015049. [DOI] [PubMed] [Google Scholar]
- Barlow J, Peres CA. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:1787–1794. doi: 10.1098/rstb.2007.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriopedro D, Fischer EM, Luterbacher J, Trigo RM, García-Herrera R. The hot summer of 2010: redrawing the temperature record map of Europe. Science. 2011;332:220–224. doi: 10.1126/science.1201224. [DOI] [PubMed] [Google Scholar]
- Bastos A, Gouveia CM, Trigo RM, Running SW. Comparing the impacts of 2003 and 2010 heatwaves in NPP over Europe. Biogeosciences Discussions. 2013a;10:15879–15911. [Google Scholar]
- Bastos A, Running SW, Gouveia C, et al. The global NPP dependence on ENSO: La Nina and the extraordinary year of 2011. Journal of Geophysical Research: Biogeosciences. 2013b;118:1247–1255. [Google Scholar]
- Bebi P, Kulakowski D, Rixen C. Snow avalanche disturbances in forest ecosystems - state of research and implications for management. Forest Ecology and Management. 2009;257:1883–1892. [Google Scholar]
- Beier C, Beierkuhnlein C, Wohlgemuth T, et al. Precipitation manipulation experiments–challenges and recommendations for the future. Ecology Letters. 2012;15:899–911. doi: 10.1111/j.1461-0248.2012.01793.x. [DOI] [PubMed] [Google Scholar]
- Berhe AA, Kleber M. Erosion, deposition, and the persistence of soil organic matter: mechanistic considerations and problems with terminology. Earth Surface Processes and Landforms. 2013;38:908–912. [Google Scholar]
- Berry PM, Sterling M, Baker CJ, Spink J, Sparkes DL. A calibrated model of wheat lodging compared with field measurements. Agricultural and Forest Meteorology. 2003;119:167–180. [Google Scholar]
- Bloor JMG, Bardgett RD. Stability of above-ground and below-ground processes to extreme drought in model grassland ecosystems: interactions with plant species diversity and soil nitrogen availability. Perspectives in Plant Ecology Evolution and Systematics. 2012;14:193–204. [Google Scholar]
- Boening C, Willis JK, Landerer FW, Nerem RS, Fasullo J. The 2011 La Niña: so strong, the oceans fell. Geophysical Research Letters. 2012;39:L19602. [Google Scholar]
- Bokhorst SF, Bjerke JW, Tømmervik H, Callaghan TV, Phoenix GK. Winter warming events damage sub-Arctic vegetation: consistent evidence from an experimental manipulation and a natural event. Journal of Ecology. 2009;97:1408–1415. [Google Scholar]
- Bond WJ, Woodward FI, Midgley GF. The global distribution of ecosystems in a world without fire. New Phytologist. 2005;165:525–538. doi: 10.1111/j.1469-8137.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- Borken W, Matzner E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Global Change Biology. 2009;15:808–824. [Google Scholar]
- Bowman DMJS, Balch JK, Artaxo P, et al. Fire in the Earth system. Sciene. 2009;324:481–484. doi: 10.1126/science.1163886. [DOI] [PubMed] [Google Scholar]
- Bragg DC, Shelton MG, Zeide B. Impacts and management implications of ice storms on forests in the southern United States. Forest Ecology and Management. 2003;186:99–123. [Google Scholar]
- Brando PM, Nepstad DC, Balch JK, Bolker B, Christman MC, Coe M, Putz FE. Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density and fire behavior. Global Change Biology. 2012;18:630–641. [Google Scholar]
- Brando PM, Balch JK, Nepstad DC, et al. Abrupt increases in Amazonian tree mortality due to drought-fire interactions. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6347–6352. doi: 10.1073/pnas.1305499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science. 2006;63:625–644. [Google Scholar]
- Breshears DD, Whicker JJ, Johansen MP, Pinder JE. Wind and water erosion and transport in semi-arid shrubland, grassland and forest ecosystems: quantifying dominance of horizontal wind-driven transport. Earth Surface Processes and Landforms. 2003;28:1189–1209. [Google Scholar]
- Breshears DD, Cobb NS, Rich PM, et al. Regional vegetation die-off in response to global-change-type drought. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. Maximum rooting depth of vegetation types at the global scale. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- Certini G. Effects of fire on properties of forest soils: a review. Oecologia. 2005;143:1–10. doi: 10.1007/s00442-004-1788-8. [DOI] [PubMed] [Google Scholar]
- Chambers JQ, Fisher JI, Zeng HC, Chapman EL, Baker DB, Hurtt GC. Hurricane Katrina’s carbon footprint on U.S. Gulf Coast forests. Science. 2007;318:1107. doi: 10.1126/science.1148913. [DOI] [PubMed] [Google Scholar]
- Changnon SA. Characteristics of ice storms in the United States. Journal of Applied Meteorology. 2003;42:630–639. [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciais P, Reichstein M, Viovy N, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- Conner WH, Inabinette LW. Tree growth in three South Carolina (USA) swamps after Hurricane Hugo: 1991–2001. Forest Ecology and Management. 2003;182:371–380. [Google Scholar]
- Corlett RT. Impacts of warming on tropical lowland rainforests. Trends in Ecology and Evolution. 2011;26:606–613. doi: 10.1016/j.tree.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Cornelissen T. Climate change and its effects on terrestrial insects and herbivory patterns. Neotropical Entomology. 2011;40:155–163. doi: 10.1590/s1519-566x2011000200001. [DOI] [PubMed] [Google Scholar]
- Coumou D, Rahmstorf S. A decade of weather extremes. Nature Climate Change. 2012;2:491–496. [Google Scholar]
- Couwenberg J, Dommain R, Joosten H. Greenhouse gas fluxes from tropical peatlands in south-east Asia. Global Change Biology. 2010;16:1715–1732. [Google Scholar]
- Curiel-Yuste J, Peñuelas J, Estiarte M, Garcia-Mas J, Ogaya R, Pujol M, Sardans J. Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Global Change Biology. 2011;17:1475–1486. [Google Scholar]
- Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- De Boeck HJ, Dreesen FE, Janssens IA, Nijs I. Whole-system responses of experimental plant communities to climate extremes imposed in different seasons. New Phytologist. 2011;189:806–817. doi: 10.1111/j.1469-8137.2010.03515.x. [DOI] [PubMed] [Google Scholar]
- Desprez-Loustau M-L, Marçais B, Nageleisen L-M, Piou D, Vannini A. Interactive effects of drought and pathogens in forest trees. Annals of Forest Science. 2006;63:597–612. [Google Scholar]
- Diez JM, D’antonio CM, Dukes JS, et al. Will extreme climatic events facilitate biological invasions? Frontiers in Ecology and the Environment. 2012;10:249–257. [Google Scholar]
- Diffenbaugh NS, Ashfaq M. Intensification of hot extremes in the United States. Geophysical Research Letters. 2010;37:L15701. [Google Scholar]
- Dinsmore KJ, Billett MF, Dyson KE. Temperature and precipitation drive temporal variability in aquatic carbon and GHG concentrations and fluxes in a peatland catchment. Global Change Biology. 2013;19:2133–2148. doi: 10.1111/gcb.12209. [DOI] [PubMed] [Google Scholar]
- Dittmar C, Elling W. Dendroecological investigation of the vitality of Common Beech (Fagus sylvatica L.) in mixed mountain forests of the Northern Alps (South Bavaria) Dendrochronologia. 2007;25:37–56. [Google Scholar]
- Dittmar C, Fricke W, Elling W. Impact of late frost events on radial growth of common beech (Fagus sylvatica L.) in Southern Germany. European Journal of Forest Research. 2006;125:249–259. [Google Scholar]
- Doughty CE, Goulden ML. Are tropical forests near a high temperature threshold? Journal of Geophysical Research: Biogeosciences. 2008;113:G00B07. [Google Scholar]
- Drever C, Messier C, Bergeron Y, Flannigan M. Can forest management based on natural disturbances maintain ecological resilience? Canadian Journal of Forest Research. 2006;36:2285–2299. [Google Scholar]
- Durre I, Wallace JM, Lettenmaier DP. Dependence of extreme daily maximum temperatures on antecedent soil moisture in the contiguous United States during summer. Journal of Climate. 2000;13:2641–2651. [Google Scholar]
- Eamus D, Boulain N, Cleverly J, Breshears DD. Global change-type drought-induced tree mortality: vapor pressure deficit is more important than temperature per se in causing decline in tree health. Ecology and Evolution. 2013;3:2711–2729. doi: 10.1002/ece3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilmann B, Zweifel R, Buchmann N, Pannatier EG, Rigling A. Drought alters timing, quantity, and quality of wood formation in Scots pine. Journal of Experimental Botany. 2011;62:2763–2771. doi: 10.1093/jxb/erq443. [DOI] [PubMed] [Google Scholar]
- Fischer EM, Seneviratne SI, Lüthi D, Schär C. Contribution of land-atmosphere coupling to recent European summer heat waves. Geophysical Research Letters. 2007;34:L06707. [Google Scholar]
- Fischlin A, Midgley GF, Price JT. Ecosystems, their properties, goods, and services. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, et al., editors. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007. pp. 211–272. [Google Scholar]
- Fisher EM, Knutti R. Detection of spatially aggregated changes in temperature and precipitation extremes, Geophys. Geophysical Research Letters. 2014;41:547–554. [Google Scholar]
- Fisk JP, Hurtt GC, Chambers JQ, Zeng H, Dolan KA, Negrón-Juárez RI. The impacts of tropical cyclones on the net carbon balance of eastern US forests (1851–2000) Environmental Research Letters. 2013;8:045017. (6 pp) [Google Scholar]
- Founda D, Giannakopoulos C. The exceptionally hot summer of 2007 in Athens, Greece — A typical summer in the future climate? Global and Planetary Change. 2009;67:227–236. [Google Scholar]
- Frankenberg C, Fisher JB, Worden J, et al. New global observations of the terrestrial carbon cycle from GOSAT: patterns of plant fluorescence with gross primary productivity. Geophysical Research Letters. 2011;38:L17706. [Google Scholar]
- Frank DC, Esper J, Raible CC, Buntgen U, Trouet V, Stocker B, Joos F. Ensemble reconstruction constraints on the global carbon cycle sensitivity to climate. Nature. 2010;463:527–530. doi: 10.1038/nature08769. [DOI] [PubMed] [Google Scholar]
- Freeman C, Ostle N, Kang H. An enzymic ‘latch’ on a global carbon store. Nature. 2001;409:149–150. doi: 10.1038/35051650. [DOI] [PubMed] [Google Scholar]
- Friedlingstein P, Cox P, Betts R, et al. Climate-carbon cycle feedback analysis: Results from the (CMIP)-M-4 model intercomparison. Journal of Climate. 2006;19:3337–3353. [Google Scholar]
- Frolking S, Talbot J, Jones MC, Treat CC, Kauffman JB, Tuittila ES, Roulet N. Peatlands in the Earth’s 21st century climate system. Environmental Reviews. 2011;19:371–396. [Google Scholar]
- Fuchslueger L, Bahn M, Fritz K, Hasibeder R, Richter A. Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytologist. 2014;201:916–927. doi: 10.1111/nph.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer J, Beniston M, Fischlin A, Frei C, Goyette S, Jasper K, Pfister C. Climate risks and their impact on agriculture and forests in Switzerland. Climatic Change. 2006;79:79–102. [Google Scholar]
- Ganteaume A, Camia A, Jappiot M, San-Miguel-Ayanz J, Long-Fournel M, Lampin C. A review of the main driving factors of forest fire ignition over Europe. Environmental Management. 2013;51:651–662. doi: 10.1007/s00267-012-9961-z. [DOI] [PubMed] [Google Scholar]
- García-Ruiz JM, Nadal-Romero E, Lana-Renault N, Beguería S. Erosion in Mediterranean landscapes: changes and future challenges. Geomorphology. 2013;198:20–36. [Google Scholar]
- Gerten D, Luo Y, Le Maire G, et al. Modelled effects of precipitation on ecosystem carbon and water dynamics in different climatic zones. Global Change Biology. 2008;14:2365–2379. [Google Scholar]
- Gilgen AK, Buchmann N. Response of temperate grasslands at different altitudes to simulated summer drought differed but scaled with annual precipitation. Biogeosciences. 2009;6:2525–2539. [Google Scholar]
- Goebel M-O, Bachmann J, Reichstein M, Janssens IA, Guggenberger G. Review: soil water repellency and its implications for organic matter decomposition – is there a link to extreme climatic events? Global Change Biology. 2011;17:2640–2656. [Google Scholar]
- Goldsmith ST, Carey AE, Lyons BW, Kao S-J, Lee T-Y, Chen J. Extreme storm events, landscape denudation, and carbon sequestration: Typhoon Mindulle, Choshui River, Taiwan. Geology. 2008;36:483–486. [Google Scholar]
- Gouttevin I, Menegoz M, Domine F, et al. How the insulating properties of snow affect soil carbon distribution in the continental pan-Arctic area. Journal of Geophysical Research: Biogeosciences. 2012;117:G02020. [Google Scholar]
- Granda E, Camarero JJ, Gimeno TE, Martínez-Fernández J, Valladares F. Intensity and timing of warming and drought differentially affect growth patterns of co-occurring Mediterranean tree species. European Journal of Forest Research. 2013;132:469–480. [Google Scholar]
- Granier A, Reichstein M, Bréda N, et al. Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agricultural and Forest Meteorology. 2007;143:123–145. [Google Scholar]
- Grippa M, Kergoat L, Toan TL, Mognard NM, Delbart N, L′Hermitte J, Vicente-Settano SM. The impact of snow depth and snowmelt on the vegetation variability over central Siberia. Geophysical Research Letters. 2005;32:L21412. [Google Scholar]
- Guanter L, Zhang Y, Jung M, et al. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1327–E1333. doi: 10.1073/pnas.1320008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarsma RJ, Selten F, Hurk BV, Hazeleger W, Wang XL. Drier Mediterranean soils due to greenhouse warming bring easterly winds over summertime central Europe. Geophysical Research Letters. 2009;36:L04705. [Google Scholar]
- Hanson CE, Palutikof JP, Dlugolecki A, Giannakopoulos C. Bridging the gap between science and the stakeholder: the case of climate change research. Climate Research. 2006;31:121–133. [Google Scholar]
- Hao ZX, Zheng JY, Ge QS, Wang W-C. Historical analogues of the 2008 extreme snow event over Central and Southern China. Climate Research. 2011;50:161–170. [Google Scholar]
- Haverd V, Raupach MR, Briggs PR, et al. The Australian terrestrial carbon budget. Biogeosciences. 2013;10:851–869. [Google Scholar]
- Hicke JA, Allen CD, Desai AR, et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Global Change Biology. 2012;18:7–34. [Google Scholar]
- Hilton RG, Galy A, Hovius N, Chen M-C, Horng M-J, Chen H. Tropical-cyclone-driven erosion of the terrestrial biosphere from mountains. Nature Geoscience. 2008;1:759–762. [Google Scholar]
- Hirschi M, Seneviratne SI, Alexandrov V, et al. Observational evidence for soil-moisture impact on hot extremes in southeastern Europe. Nature Geoscience. 2011;4:17–21. [Google Scholar]
- Hong C-C, Hsu H-H, Lin N-H, Chiu H. Roles of European blocking and tropical - extratropical interaction in the 2010 Pakistan flooding. Geophysical Research Letters. 2011;38:L13806. [Google Scholar]
- Houze RA, Rasmussen KL, Medina S, Brodzik SR, Romatschke U. Anomalous Atmospheric Events Leading to the Summer 2010 Floods in Pakistan. Bulletin of the American Meteorological Society. 2011;92:291–298. [Google Scholar]
- Imbert D, Portecop J. Hurricane disturbance and forest resilience: assessing structural vs. functional changes in a Caribbean dry forest. Forest Ecology and Management. 2008;255:3494–3501. [Google Scholar]
- IPCC. Summary for policymakers. In: Field CB, Barros V, Stocker TF, et al., editors. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY: Cambridge University Press; 2012. pp. 1–19. [Google Scholar]
- Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM. IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA: Cambridge University Press; 2013. Summary for policymakers; pp. 3–29. In: (eds ) [Google Scholar]
- Irland LC. Ice storms and forest impacts. Science of the Total Environment. 2000;262:231–242. doi: 10.1016/s0048-9697(00)00525-8. [DOI] [PubMed] [Google Scholar]
- Jactel H, Petit J, Desprez-Loustau M-L, Delzon S, Piou D, Battisti A, Koricheva J. Drought effects on damage by forest insects and pathogens: a meta-analysis. Global Change Biology. 2012;18:267–276. [Google Scholar]
- Jarvis P, Rey A, Petsikos C, et al. Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: the “Birch effect”. Tree Physiology. 2007;27:929–940. doi: 10.1093/treephys/27.7.929. [DOI] [PubMed] [Google Scholar]
- Jentsch A, Kreyling J, Boettcher-Treschkow J, Beierkuhnlein C. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Global Change Biology. 2009;15:837–849. [Google Scholar]
- Jentsch A, Kreyling J, Elmer M, et al. Climate extremes initiate ecosystem-regulating functions while maintaining productivity. Journal of Ecology. 2011;99:689–702. [Google Scholar]
- Jepsen J, Hagen S, Hogda K, Ims R, Karlsen S, Tommervik H, Yoccoz N. Monitoring the spatio-temporal dynamics of geometrid moth outbreaks in birch forest using MODIS-NDVI data. Remote Sensing of Environment. 2009;113:1939–1947. [Google Scholar]
- Johansen MP, Hakonson TE, Breshears DD. Post-fire runoff and erosion from rainfall simulation: contrasting forests with shrublands and grasslands. Hydrological Processes. 2001;15:2953–2965. [Google Scholar]
- Johnson DM, Büntgen U, Frank DC, et al. Climatic warming disrupts recurrent Alpine insect outbreaks. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20576–20581. doi: 10.1073/pnas.1010270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Reichstein M, Margolis HA, et al. Global patterns of land-atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. Journal of Geophysical Research: Biogeosciences. 2011;116:G00J07. [Google Scholar]
- Jung B-J, Lee H-J, Jeong J-J, et al. Storm pulses and varying sources of hydrologic carbon export from a mountainous watershed. Journal of Hydrology. 2012;440–441:90–101. [Google Scholar]
- Jungkunst HF, Fiedler S. Latitudinal differentiated water table control of carbon dioxide, methane and nitrous oxide fluxes from hydromorphic soils: feedbacks to climate change. Global Change Biology. 2007;13:2668–2683. [Google Scholar]
- Kasin I, Ohlson M. An experimental study of charcoal degradation in a boreal forest. Soil Biology & Biochemistry. 2013;65:39–49. [Google Scholar]
- Katz RW, Brown BG. Extreme events in a changing climate: variability is more important than averages. Climatic Change. 1992;21:289–302. [Google Scholar]
- Kausrud K, Okland B, Skarpaas O. Population dynamics in changing environments: the case of an eruptive forest pest species. Biological Reviews. 2012;87:34–51. doi: 10.1111/j.1469-185X.2011.00183.x. [DOI] [PubMed] [Google Scholar]
- Keenan T, Sabate S, Gracia C. The importance of mesophyll conductance in regulating forest ecosystem productivity during drought periods. Global Change Biology. 2010;16:1019–1034. [Google Scholar]
- Keith H, van Gorsel E, Jacobsen KL, Cleugh HA. Dynamics of carbon exchange in a Eucalyptus forest in response to interacting disturbance factors. Agricultural and Forest Meteorology. 2012;153:67–81. [Google Scholar]
- Kim D-G, Vargas R, Bond-Lamberty B, Turetsky MR. Effects of soil rewetting and thawing on soil gas fluxes: a review of current literature and suggestions for future research. Biogeosciences. 2012;9:2459–2483. [Google Scholar]
- Knapp AK, Beier C, Briske DD, et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience. 2008;58:811–821. [Google Scholar]
- Komonen A, Schroeder LM, Weslien J. Ips typographus population development after a severe storm in a nature reserve in southern Sweden. Journal of Applied Entomology. 2011;135:132–141. [Google Scholar]
- Konovalov IB, Beekmann M, Kuznetsova IN, Yurova A, Zvyagintsev AM. Atmospheric impacts of the 2010 Russian wildfires: integrating modelling and measurements of an extreme air pollution episode in the Moscow region. Atmospheric Chemistry and Physics. 2011;11:10031–10056. [Google Scholar]
- Kopittke GR, Tietema A, van Loon EE, Assheman D. Fourteen annually repeated droughts suppressed autotrophic soil respiration and resulted in an ecosystem change. Ecosystems. 2014;17:242–257. [Google Scholar]
- Kramer K, Vreugdenhil SJ, van der Werf DC. Effects of flooding on the recruitment, damage and mortality of riparian tree species: a field and simulation study on the Rhine floodplain. Forest Ecology and Management. 2007;255:3893–3903. [Google Scholar]
- Kreuzwieser J, Papadopoulou E, Rennenberg H. Interaction of flooding with carbon metabolism of forest trees. Plant Biology. 2004;6:299–306. doi: 10.1055/s-2004-817882. [DOI] [PubMed] [Google Scholar]
- Kreyling J. Winter climate change: a critical factor for temperate vegetation performance. Ecology. 2010;91:1939–1948. doi: 10.1890/09-1160.1. [DOI] [PubMed] [Google Scholar]
- Kreyling J, Jentsch A, Beierkuhnlein C. Stochastic trajectories of succession initiated by extreme climatic events. Ecology Letters. 2011;14:758–764. doi: 10.1111/j.1461-0248.2011.01637.x. [DOI] [PubMed] [Google Scholar]
- Kreyling J, Jentsch A, Beier C. Beyond realism in climate change experiments: gradient approaches identify thresholds and tipping points. Ecology Letters. 2014;17:125–e1. doi: 10.1111/ele.12193. [DOI] [PubMed] [Google Scholar]
- Kurz WA, Stinson G, Rampley G. Could increased boreal forest ecosystem productivity offset carbon losses from increased disturbances? Philosophical Transactions of the Royal Society B: Biological Sciences. 2008a;363:2261–2269. doi: 10.1098/rstb.2007.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz WA, Dymond CC, Stinson G, et al. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008b;452:987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- Lal R. Challenges and opportunities in soil organic matter research. European Journal of Soil Science. 2009;60:158–169. [Google Scholar]
- Lal R, Pimentel D. Soil erosion: a carbon sink or source? Science. 2008;319:1040–1042. doi: 10.1126/science.319.5866.1040. [DOI] [PubMed] [Google Scholar]
- Lal R, Lorenz K, Hüttl RF, Schneider BU, von Braun J. Ecosystem Services and Carbon Sequestration in the Biosphere. Dodrecht, Heidelberg, New York, London: Springer; 2013. [Google Scholar]
- Larcher W. Physiological Plant Ecology. Berlin: Springer; 2003. [Google Scholar]
- Lawlor DW. The effects of water deficit on photosynthesis. In: Smirnoff N, editor. Environment and Plant Metabolism – Flexibilty and Acclimation. Oxford: Bios Scientific Publishers; 1995. pp. 129–160. [Google Scholar]
- Lewis SL, Brando PM, Phillips OL, Van Der Heijden GMF, Nepstad D. The 2010 Amazon drought. Science. 2011;331:554. doi: 10.1126/science.1200807. [DOI] [PubMed] [Google Scholar]
- Li F, Bond-Lamberty B, Levis S. Quantifying the role of fire in the Earth system – Part 2: impact on the net carbon balance of global terrestrial ecosystems for the 20th century. Biogeosciences Discussions. 2013;10:17309–17350. [Google Scholar]
- Lindroth A, Lagergren F, Grelle A, Klemedtsson L, Langvall O, Weslien P, Tuulik J. Storms can cause Europe-wide reduction in forest carbon sink. Global Change Biology. 2009;15:346–355. [Google Scholar]
- Lobell DB, Sibley A, Ortiz-Monasterio JI. Extreme heat effects on wheat senescence in India. Nature Climate Change. 2012;2:1–4. [Google Scholar]
- Major J, Lehmann J, Rondon M, Goodale C. Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Global Change Biology. 2010;16:1366–1379. [Google Scholar]
- Marino GP, Kaiser DP, Gu L, Ricciuto DM. Reconstruction of false spring occurrences over the southeastern United States, 1901–2007: an increasing risk of spring freeze damage? Environmental Research Letters. 2011;6:024015. Art. [Google Scholar]
- Mayr S, Gruber A, Bauer H. Repeated freeze-thaw cycles induce embolism in drought stressed conifers (Norway spruce, stone pine) Planta. 2003;217:436–441. doi: 10.1007/s00425-003-0997-4. [DOI] [PubMed] [Google Scholar]
- Mayr S, Cochard H, Améglio T, Kikuta SB. Embolism formation during freezing in the wood of Picea abies. Plant Physiology. 2007;143:60–67. doi: 10.1104/pp.106.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy HR, Oren R, Kim HS, Johnsen KH, Maier C, Pritchard SG, Davis MA. Interaction of ice storms and management practices on current carbon sequestration in forests with potential mitigation under future CO2 atmosphere. Journal of Geophysical Research: Atmospheres. 2006;111:D15103. [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology and Evolution. 2011;26:523–532. doi: 10.1016/j.tree.2011.06.003. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Fisher RA, Xu C, et al. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytologist. 2013;200:304–321. doi: 10.1111/nph.12465. [DOI] [PubMed] [Google Scholar]
- MCPFE. 2007. p. 247. State of Europe’s Forests 2007. The MCPFE Report on Sustainable Forest Management in Europe. Ministerial Conference on the Protection of Forests in Europe (MCPFE) Liaison Unit Warsaw. pp. MCPFE, United Nations Economic Commission for Europe (UNECE), Food and Agricultural Organization of the United Nations (FAO)
- Menzel A, Sparks TH, Estrella N, et al. European phenological response to climate change matches the warming pattern. Global Change Biology. 2006;12:1969–1976. [Google Scholar]
- Michaelian M, Hogg EH, Hall RJ, Arsenault E. Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Global Change Biology. 2011;17:2084–2094. [Google Scholar]
- Migliavacca M, Meroni M, Manca G, et al. Seasonal and interannual patterns of carbon and water fluxes of a poplar plantation under peculiar eco-climatic conditions. Agricultural and Forest Meteorology. 2009;149:1460–1476. [Google Scholar]
- Mildrexler DJ, Zhao MS, Running SW. Testing a MODIS Global Disturbance Index across North America. Remote Sensing of Environment. 2009;113:2103–2117. [Google Scholar]
- Misson L, Limousin J-M, Rodriguez R, Letts MG. Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant, Cell and Environment. 2010;33:1898–1910. doi: 10.1111/j.1365-3040.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- Misson L, Degueldre D, Collin C, Rodriguez R, Rocheteau A, Ourcival J-M, Rambal S. Phenological responses to extreme droughts in a Mediterranean forest. Global Change Biology. 2011;17:1036–1048. [Google Scholar]
- van der Molen MK, Dolman AJ, Ciais P, et al. Drought and ecosystem carbon cycling. Agricultural and Forest Meteorology. 2011;151:765–773. [Google Scholar]
- Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK. Winter forest soil respiration controlled by climate and microbial community composition. Nature. 2006;439:711–714. doi: 10.1038/nature04555. [DOI] [PubMed] [Google Scholar]
- Morgan JA, LeCain DR, Pendall E, et al. C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature. 2011;476:202–205. doi: 10.1038/nature10274. [DOI] [PubMed] [Google Scholar]
- Moriondo M, Good P, Durao R, Bindi M, Giannakopoulos C, Corte-Real J. Potential impact of climate change on fire risk in the Mediterranean area. Climate Research. 2006;31:85–95. [Google Scholar]
- Morton DC, Le Page Y, DeFries R, Collatz GJ, Hurtt GC. Understorey fire frequency and the fate of burned forests in southern Amazonia. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:20120163. doi: 10.1098/rstb.2012.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B, Seneviratne SI. Hot days induced by precipitation deficits at the global scale. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12398–12403. doi: 10.1073/pnas.1204330109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr J, Franke J, Borken W. Drying-rewetting events reduce C and N losses from a Norway spruce forest floor. Soil Biology & Biochemistry. 2010;42:1303–1312. [Google Scholar]
- Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. 1997;386:698–702. [Google Scholar]
- Nagy L, Kreyling J, Gellesch E, Beierkuhnlein C, Jentsch A. Recurring weather extremes alter the flowering phenology of two common temperate shrubs. International Journal of Biometeorology. 2013;57:579–588. doi: 10.1007/s00484-012-0585-z. [DOI] [PubMed] [Google Scholar]
- Negrón-Juárez R, Baker DB, Zeng H, Henkel TK, Chambers JQ. Assessing hurricane-induced tree mortality in U.S. Gulf Coast forest ecosystems. Journal of Geophysical Research: Biogeosciences. 2010a;115:G04030. [Google Scholar]
- Negrón-Juárez RI, Chambers JQ, Guimaraes G, et al. Widespread Amazon forest tree mortality from a single cross-basin squall line event. Geophysical Research Letters. 2010b;37:L16701. [Google Scholar]
- Nepstad DC, Decarvalho CR, Davidson EA, et al. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature. 1994;372:666–669. [Google Scholar]
- Netherer S, Schopf A. Potential effects of climate change on insect herbivores in European forests - General aspects and the pine processionary moth as specific example. Forest Ecology and Management. 2010;259:831–838. [Google Scholar]
- Nicholls N, Alexander L. Has the climate become more variable or extreme? Progress 1992-2006. Progress in Physical Geography. 2007;31:77–87. [Google Scholar]
- Niu S, Luo Y, Li D, Cao S, Xia J, Li J, Smith MD. Plant growth and mortality under climatic extremes: an overview. Environment and Experimental Botany. 2014;98:13–19. [Google Scholar]
- Nykänen M-L, Peltola H, Quine CP, Kellomäki S, Broadgate M. Factors affecting snow damage of trees with particular reference to European conditions. Silva Fennica. 1997;31:193–213. [Google Scholar]
- van Oost K, Quine TA, Govers G, et al. The impact of agricultural soil erosion on the global carbon cycle. Science. 2007;318:626–629. doi: 10.1126/science.1145724. [DOI] [PubMed] [Google Scholar]
- Øygarden L. Rill and gully development during an extreme winter runoff event in Norway. Catena. 2003;50:217–242. [Google Scholar]
- Page SE, Siegert F, Rieley JO, Boehm HDV, Jaya A, Limin S. The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature. 2002;420:61–65. doi: 10.1038/nature01131. [DOI] [PubMed] [Google Scholar]
- Palacio S, Hoch G, Sala A, Körner C, Millard P. Does carbon storage limit tree growth? New Phytologist. 2014;201:1096–1100. doi: 10.1111/nph.12602. [DOI] [PubMed] [Google Scholar]
- Pan Y, Birdsey RA, Fang J, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- Parry ML, Canziani OF, Palutikof JP. Technical summary. In: Parry ML, Canziani OF, Palutikof JP, der van Linden PJ, Hanson CE, et al., editors. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. pp. 23–78. [Google Scholar]
- Pausas JG, Paula S. Fuel shapes the fire-climate relationship: evidence from Mediterranean ecosystems. Global Ecology and Biogeography. 2012;21:1074–1082. [Google Scholar]
- Peng SS, Piao S, Zeng Z, et al. Afforestation in China cools local land surface temperature. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2915–2919. doi: 10.1073/pnas.1315126111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J, Sardans J, Estiarte M, et al. Evidence of current impact of climate change on life: a walk from genes to the biosphere. Global Change Biology. 2013;19:2303–2338. doi: 10.1111/gcb.12143. [DOI] [PubMed] [Google Scholar]
- Phillips OL, Aragão LEOC, Lewis SL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323:1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- Pilegaard K, Ibrom A, Courtney MS, Hummelshøj P, Jensen NO. Increasing net CO2 uptake by a Danish beech forest during the period from 1996 to 2009. Agricultural and Forest Meteorology. 2011;151:934–946. [Google Scholar]
- Polle A, Kröniger W, Rennenberg H. Seasonal fluctuations of ascorbate-related enzymes: acute and delayed effects of late frost in spring on antioxidative systems in needles of Norway spruce (Picea abies L.) Plant and Cell Physiology. 1996;37:717–725. [Google Scholar]
- Porter JR, Semenov MA. Crop responses to climatic variation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:2021–2035. doi: 10.1098/rstb.2005.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthumus H, Morris J, Hess TM, Neville D, Phillips E, Baylis A. Impacts of the summer 2007 floods on agriculture in England. Journal of Flood Risk Management. 2009;2:182–189. [Google Scholar]
- Poulter B, Frank D, Ciais P, et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature. 2014;509:600–603. doi: 10.1038/nature13376. [DOI] [PubMed] [Google Scholar]
- Preston CM, Schmidt MWI. Black (pyrogenic) carbon: a synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences. 2006;3:397–420. [Google Scholar]
- Price DT, Alfaro RI, Brown KJ, Flannigan MD, et al. Anticipating the consequences of climate change for Canada’s boreal forest ecosystems. Environmental Reviews. 2013;21:322–365. [Google Scholar]
- Ramankutty N, Evan AT, Monfreda C, Foley JA. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Global Biogeochemical Cycles. 2008;22:GB1003. [Google Scholar]
- Rammig A, Wiedermann M, Donges J, Babst F, et al. Tree-ring responses to extreme climate events as benchmarks for terrestrial dynamic vegetation models. Biogeosciences Discussions. 2014;11:2537–2568. [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Reichstein M, Ciais P, Papale D, et al. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: a joint flux tower, remote sensing and modelling analysis. Global Change Biology. 2007;13:634–651. [Google Scholar]
- Reichstein M, Bahn M, Ciais P, et al. Climate extremes and the carbon cycle. Nature. 2013;500:287–295. doi: 10.1038/nature12350. [DOI] [PubMed] [Google Scholar]
- Robinet C, Roques A. Direct impacts of recent climate warming on insect populations. Integrative Zoology. 2010;5:132–142. doi: 10.1111/j.1749-4877.2010.00196.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig CE, Tubiello F, Goldberg R, Mills E, Bloomfield J. Increased crop damage in the U.S. from excess precipitation under climate change. Global Environmental Change. 2002;12:197–202. [Google Scholar]
- Rouault G, Candau J-N, Lieutier F, Nageleisen L-M, Martin J-C, Warzee N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Annals of Forest Science. 2006;63:613–624. [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. Carbon dynamics in trees: feast or famine? Tree Physiology. 2012;32:764–775. doi: 10.1093/treephys/tpr143. [DOI] [PubMed] [Google Scholar]
- Sambaraju KR, Carroll AL, Jhu J, Stahl K, Moore RD, Aukema BH. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography. 2012;35:211–223. [Google Scholar]
- Schachtschabel P, Blume H-P, Brümmer G, Hartge K-H, Schwertmann U. Lehrbuch der Bodenkunde. Stuttgart: Enke; 1992. [Google Scholar]
- Schär C, Vidale PL, Lüthi D, Frei C, Häberli C, Liniger MA, Appenzeller C. The role of increasing temperature variability in European summer heatwaves. Nature. 2004;427:332–336. doi: 10.1038/nature02300. [DOI] [PubMed] [Google Scholar]
- Scheiter S, Langgan L, Higgins SI. Next-generation dynamic global vegetation models: learning from community ecology. New Phytologist. 2013;198:957–969. doi: 10.1111/nph.12210. [DOI] [PubMed] [Google Scholar]
- Schimel D, Baker D. The wildfire factor. Nature. 2002;420:29–30. doi: 10.1038/420029a. [DOI] [PubMed] [Google Scholar]
- Schlyter P, Stjernquist I, Bärring L, Jönsson AM, Nilsson C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Climate Research. 2006;31:75–84. [Google Scholar]
- Schmidt MWI, Torn MS, Abiven S, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- Schulze E-D, Beck E, Müller-Hohenstein K. Plant Ecology. Heidelberg: Springer Verlag; 2005. [Google Scholar]
- Schwalm CR, Williams CA, Schaefer K, et al. Assimilation exceeds respiration sensitivity to drought: A FLUXNET synthesis. Global Change Biology. 2010;16:657–670. [Google Scholar]
- Schwalm CR, Williams CA, Schaefer K, et al. Reduction in carbon uptake during turn of the century drought in western North America. Nature Geoscience. 2012;5:551–556. [Google Scholar]
- Seidl R, Fernandes PM, Fonseca TF, et al. Modelling natural disturbances in forest ecosystems: a review. Ecological Modelling. 2011;222:903–924. [Google Scholar]
- Seneviratne SI, Lüthi D, Litschi M, Schär C. Land-atmosphere coupling and climate change in Europe. Nature. 2006;443:205–209. doi: 10.1038/nature05095. [DOI] [PubMed] [Google Scholar]
- Seneviratne SI, Corti T, Davin EL, et al. Investigating soil moisture-climate interactions in a changing climate: a review. Earth-Science Reviews. 2010;99:125–161. [Google Scholar]
- Seneviratne SI, Nicholls N, Easterling D. Changes in climate extremes and their impacts on the natural physical environment. In: Field CB, Barros V, Stocker TF, et al., editors. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY: Cambridge University Press; 2012. pp. 109–230. (IPCC SREX Report). (. In:.) [Google Scholar]
- Sheik CS, Beasley WH, Elshahed MS, Zhou X, Luo Y, Krumholz LR. Effect of warming and drought on grassland microbial communities. The ISME Journal. 2011;5:1692–1700. doi: 10.1038/ismej.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Thomey ML, Mowll W, et al. Differential effects of extreme drought on production and respiration: synthesis and modeling analysis. Biogeosciences. 2014;11:621–633. [Google Scholar]
- Shinoda M, Gillies JA, Mikami M, Shao Y. Temperate grasslands as a dust source: knowledge, uncertainties, and challenges. Aeolian Research. 2011;3:271–293. [Google Scholar]
- Simpson IJ, Rowland FS, Meinardi S, Blake DR. Influence of biomass burning during recent fluctuations in the slow growth of global tropospheric methane. Geophysical Research Letters. 2006;33:L22808. [Google Scholar]
- Singh N, Abiven S, Torn MS, Schmidt MWI. Fire-derived organic carbon in soil turns over on a centennial scale. Biogeosciences. 2012;9:2847–2857. [Google Scholar]
- Smith MD. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. Journal of Ecology. 2011;99:656–663. [Google Scholar]
- Smith NG, Dukes JS. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biology. 2013;19:45–63. doi: 10.1111/j.1365-2486.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- Soja AJ, Tchebakova NM, French NHF, et al. Climate-induced boreal forest change: predictions versus current observations. Global and Planetary Change. 2007;56:274–296. [Google Scholar]
- Soussana J-F, Graux AI, Tubiello F-N. Improving the use of modelling for projections of climate change impacts on crops and pastures. Journal of Experimental Botany. 2010;61:2217–2228. doi: 10.1093/jxb/erq100. [DOI] [PubMed] [Google Scholar]
- Sowerby A, Emmett BA, Tietema A, Beier C. Contrasting effects of repeated summer drought on soil carbon efflux in hydric and mesic heathland soils. Global Change Biology. 2008;14:2388–2404. [Google Scholar]
- Sperry JS. Hydraulic constraints on plant gas exchange. Agricultural and Forest Meteorology. 2000;104:13–23. [Google Scholar]
- Stahl K, Moore RD, Mckendry IG. Climatology of winter cold spells in relation to mountain pine beetle mortality in British Columbia, Canada. Climate Research. 2006;32:13–23. [Google Scholar]
- Stone R. Ecologists report huge storm losses in China’s forests. Science. 2008;319:1318–1319. doi: 10.1126/science.319.5868.1318. [DOI] [PubMed] [Google Scholar]
- Suarez ML, Kitzberger T. Recruitment patterns following a severe drought: long-term compositional shifts in Patagonian forests. Canadian Journal of Forest Research. 2008;38:3002–3010. [Google Scholar]
- Sullivan JEM. Xylem embolism in response to freeze-thaw cycles and water-stress in ring-porous, diffuse-porous, and conifer species. Plant Physiology. 1992;100:605–613. doi: 10.1104/pp.100.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Gu LH, Dickinson RE, Zhou BZ. Forest greenness after the massive 2008 Chinese ice storm: integrated effects of natural processes and human intervention. Environmental Research Letters. 2012;7:035702. Art. [Google Scholar]
- Suttie JM, Reynolds SG, Batello C. Grasslands of the World. Plant Production and Protection Series, 34. Rome: FAO; 2005. [Google Scholar]
- Teuling AJ, Uijlenhoet R, Hupet F, Troch PA. Impact of plant water uptake strategy on soil moisture and evapotranspiration dynamics during drydown. Geophysical Research Letters. 2006;33:L03401. [Google Scholar]
- Teuling AJ, Seneviratne SI, Stöckli R, et al. Contrasting response of European forest and grassland energy exchange to heatwaves. Nature Geoscience. 2010;3:722–727. [Google Scholar]
- Thothong W, Huon S, Janeau JL, et al. Impact of land use change and rainfall on sediment and carbon accumulation in a water reservoir of North Thailand. Agriculture, Ecosystems & Environment. 2011;140:521–533. [Google Scholar]
- Tian H, Melillo JM, Kicklighter DW, McGiure AD, Helfrich J, III, Moore B, III, Vörösmarty CJ. Effect of interannual climate variability on carbon storage in Amazonian ecosystems. Nature. 1998;396:664–667. [Google Scholar]
- Tolk JA. Plant available soil water. In: Stewart BA, Howell TA, editors. Encyclopedia of Water Science. New York, NY: Marcel-Dekker Inc; 2003. pp. 669–672. [Google Scholar]
- Trenberth KE, Fasullo JT. Climate extremes and climate change: the Russian heat wave and other climate extremes of 2010. Journal of Geophysical Research. 2012;117:D17103. [Google Scholar]
- Trigo RM, Pereira JMC, Pereira MRG, et al. Atmospheric conditions associated with the exceptional fire season of 2003 in Portugal. International Journal of Climatology. 2006;26:1741–1757. [Google Scholar]
- Turetsky MR, Donahue WF, Benscoter BW. Experimental drying intensifies burning and carbon losses in a northern peatland. Nature Communications. 2011a;2:514. doi: 10.1038/ncomms1523. Art. [DOI] [PubMed] [Google Scholar]
- Turetsky MR, Kane ES, Harden JW, Ottmar RD, Manies KL, Hoy E, Kasischke ES. Recent acceleration of biomass burning and carbon losses in Alaskan forests and peatlands. Nature Geoscience. 2011b;4:27–31. [Google Scholar]
- Unger S, Máguas C, Pereira JS, Aires LM, David TS, Werner C. Partitioning carbon fluxes in a Mediterranean oak forest to disentangle changes in ecosystem sink strength during drought. Agricultural and Forest Meteorology. 2009;149:949–961. [Google Scholar]
- Valentin C, Agus F, Alamban R, et al. Runoff and sediment losses from 27 upland catchments in Southeast Asia: impact of rapid land use changes and conservation practices. Agriculture, Ecosystems & Environment. 2008;128:225–238. [Google Scholar]
- VandenBygaart AJ, Kroetsch D, Gregorich EG, Lobb DA. Soil C erosion and burial in cropland. Global Change Biology. 2012;18:1441–1452. [Google Scholar]
- Vargas R. How a hurricane disturbance influences extreme CO2 fluxes and variance in a tropical forest. Environmental Research Letters. 2012;7:035704. [Google Scholar]
- Vautard R, Yiou P, D’andrea F, et al. Summertime European heat and drought waves induced by wintertime Mediterranean rainfall deficit. Geophysical Research Letters. 2007;34:L07711. [Google Scholar]
- van der Velde M, Wriedt G, Bouraoui F. Estimating irrigation use and effects on maize yield during the 2003 heatwave in France. Agriculture, Ecosystems & Environment. 2010;135:90–97. [Google Scholar]
- van der Velde M, Tubiello FN, Vrieling A, Bouraoui F. Impacts of extreme weather on wheat and maize in France: evaluating regional crop simulations against observed data. Climatic Change. 2012;113:751–765. [Google Scholar]
- Vervuren PJA, Blom CWPM, De Kroon H. Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. Journal of Ecology. 2003;91:135–146. [Google Scholar]
- Vetter M, Churkina G, Jung M, et al. Analyzing the causes and spatial pattern of the European 2003 carbon flux anomaly using seven models. Biogeosciences. 2008;5:561–583. [Google Scholar]
- Vicca S, Gilgen AK, Camino Serrano M, Dreesen FE, et al. Urgent need for a common metric to make precipitation manipulation experiments comparable. New Phytologist. 2012;195:518–522. doi: 10.1111/j.1469-8137.2012.04224.x. [DOI] [PubMed] [Google Scholar]
- Vicca S, Bahn M, Estiarte M, et al. Can current moisture responses predict soil CO2 efflux under altered precipitation regimes? A synthesis of manipulation experiments. Biogeosciences. 2014;11:2991–3013. [Google Scholar]
- Virtanen T, Neuvonen S, Nikula A. Modelling topoclimatic patterns of egg mortality of Epirrita autumnata (Lepidoptera: Geometridae) with a Geographical Information System: predictions for current climate and warmer climate scenarios. Journal of Applied Ecology. 1998;35:311–322. [Google Scholar]
- Walter J, Hein R, Auge H, et al. How do extreme drought and plant community composition affect host plant metabolites and herbivore performance? Arthropod-Plant Interactions. 2012;6:15–25. [Google Scholar]
- Walter J, Jentsch A, Beierkuhnlein C, Kreyling J. Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environmental and Experimental Botany. 2013;94:3–8. [Google Scholar]
- Wan CG, Yilmaz I, Sosebee RE. Seasonal soil-water availability influences snakeweed root dynamics. Journal of Arid Environments. 2002;51:255–264. [Google Scholar]
- Wang XB, Enema O, Hoogmed WB, Perdok UD, Cai DX. Dust storm erosion and its impact on soil carbon and nitrogen losses in northern China. Catena. 2006;66:221–227. [Google Scholar]
- Wendler G, Conner J, Moor B, Shulski M, Stuefer M. Climatology of Alaskan wildfires with special emphasis on the extreme year of 2004. Theoretical and Applied Climatology. 2011;104:459–472. [Google Scholar]
- van der Werf GR, Dempewolf J, Trigg SN, et al. Climate regulation of fire emissions and deforestation in equatorial Asia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20350–20355. doi: 10.1073/pnas.0803375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf GR, Randerson JT, Giglio L, et al. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009) Atmospheric Chemistry and Physics. 2010;10:11707–11735. [Google Scholar]
- White RE. Hydrology, soil water and temperature. In: White RE, editor. Principles and Practice of Soil Science. 4th edn. Malden, MA, USA, Oxford, UK, Victoria, Australia: Blackwell Publishing; 2006. pp. 103–132. [Google Scholar]
- White PS, Jentsch A. The search for generality in studies of disturbance and ecosystem dynamics. Progress in Botany. 2001;62:399–450. [Google Scholar]
- Williams AP, Allen CD, Macalady AK, et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nature Climate Change. 2012;3:292–297. [Google Scholar]
- Yuste JC, Penuelas J, Estiarte M, et al. Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Global Change Biology. 2011;17:1475–1486. [Google Scholar]
- Zampieri M, D’Andrea F, Vautard R, Ciais P, de Noblet-Ducoudre N, Yiou P. Hot European summers and the role of soil moisture in the propagationof Mediterranean. Journal of Climate. 2009;22:4747–4758. [Google Scholar]
- Zavalloni C, Gielen B, Lemmens CMHM, et al. Does a warmer climate with frequent mild water shortages protect grassland communities against a prolonged drought? Plant and Soil. 2008;308:119–130. [Google Scholar]
- Zeng HC, Chambers JQ, Negrón-Juárez RI, Hurtt GC, Baker DB, Powell MD. Impacts of tropical cyclones on U.S. forest tree mortality and carbon flux from 1851 to 2000. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7888–7892. doi: 10.1073/pnas.0808914106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Barry RG, Haeberli W. Numerical simulations of the influence of the seasonal snow cover on the occurrence of permafrost at high latitudes. Norsk Geografisk Tidsskrift. 2001;55:261–266. [Google Scholar]
- Zscheischler J, Mahecha MD, Harmeling S, Reichstein M. Detection and attribution of large spatiotemporal extreme events in Earth observation data. Ecological Informatics. 2013;15:66–73. [Google Scholar]
- Zscheischler J, Mahecha MD, von Buttlar J, et al. A few extreme events dominate interannual variability in gross primary production. Environmental Research Letters. 2014a;9:035001. [Google Scholar]
- Zscheischler J, Reichstein M, Harmeling S, Rammig A, Tomelleri E, Mahecha MD. Extreme events in gross primary production: a characterization across continents. Biogeosciences. 2014b;11:2909–2924. [Google Scholar]
- Zscheischler J, Reichstein M, von Buttlar J, Mu M, Randerson JT, Mahecha MD. Carbon cycle extremes during the 21st century in CMIP5 models: future evolution and attribution to climatic drivers. Geophysical Research Letters. 2014c;41:8853–8861. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Provides a detailed literature survey about how climate extremes act on forests, grasslands, peatlands and croplands.