Abstract
An imaging cycler microscope (ICM) is a fully automated (epi)fluorescence microscope which overcomes the spectral resolution limit resulting in parameter- and dimension-unlimited fluorescence imaging. This enables the spatial resolution of large molecular systems with their emergent topological properties (toponome) in morphologically intact cells and tissues displaying thousands of multi protein assemblies at a time. The resulting combinatorial geometry of these systems has been shown to be key for in-vivo/in-situ detection of lead proteins controlling protein network topology and (dys)function: If lead proteins are blocked or downregulated the corresponding disease protein network disassembles. Here, correct therapeutic predictions are exemplified for ALS. ICM drug target studies have discovered an 18-dimensional cell surface molecular system in ALS-PBMC with a lead drug target protein, whose therapeutic downregulation is now reported to show statistically significant effect with stop of disease progression in one third of the ALS patients. Together, this clinical and the earlier experimental validations of the ICM approach indicate that ICM readily discovers in vivo robustness nodes of disease with lead proteins controlling them. Breaking in vivo robustness nodes using drugs against their lead proteins is likely to overcome current high drug attrition rates. © 2015 The Author. Published by Wiley Periodicals, Inc, on behalf of ISAC.
Keywords: ALS, functional superresolution, imaging cycler microscopy, toponome, MELC, high content analysis, drug discovery, topology
Definitions
Toponomics is a discipline in systems biology, molecular cell biology, and histology 1. It concerns the study of the toponome of organisms. The toponome is the spatial network code of proteins and other biomolecules in morphologically intact cells and tissues, including fluid tissues such as blood. The term toponome is derived from the ancient Greek nouns topos (τoπoσ), place, and nomos (νoμoζ), law, and thus a descriptive term addressing the fact that the spatial network of biomolecules in cells follows topological rules enabling coordinated actions 1,2. The information contained in a toponome relies on intact tissues.
Microscopic Method For The Study of Molecular Systems
Why Resolve Molecular Systems Spatially (Toponomics)?
Traditionally, tissue analysis is performed by using microscopic methods. It is the daily routine in histology and histopathology labs. Without histopathology clinical medicine would be unthinkable, and as such histopathology is one of the most successful fields in medicine since microscopic analysis methods of tissues have been systematically introduced by Marcello Malpighi (1628–1694). The histopathologist creates histopathological classifications as part of the integral clinical diagnostics by microscopically analysing routinely stained tissue sections in a first step at low magnifications (wide angle of view) to catch the overall staining pattern, and then, if necessary, studies the tissue structures at higher magnification (small angle of view). Mostly, histopathologists can assign a stained pattern to a disease or disease entity. But what is behind these structures? What makes them? Are there disease-specific subtypes expressing specific molecular networks that are not obvious in routine histology stains, and what conclusions could be drawn, if such subtypes could be discovered in situ? These questions address an essential gap in our knowledge suggesting that current routine histology must be extended to become a ‘molecular systems histology‚ disclosing the specific spatial properties of large molecular systems in situ at once. This motivation is substantiated by systems theory indicating that molecular systems such as those driving a disease in a tissue, also termed the disease robustness system, have relational (emergent) topological properties which are essential for the systems’ spatial coding principle: These emergent properties are possessed only by the system as a whole but not by any isolated part of the system 3. Hence, experimental isolation of the corresponding topologically determined molecular components would lead to loss of the systems’ coding information 1,4,5. Answering related questions is not trivial, while the driving force behind, on the other hand, is the scientific and ethical challenge to develop methods enabling us to interrogate tissues in a way that we can obtain the myriad of hitherto unknown structural and functional systems units driving diseases inside tissues. These systems can be considered as having tissue-specific spatial coordinates and collective functional properties of their molecular components. How assess such systems and discover which molecular tissue networks generate normal and abnormal histopathological structures and disease phenotypes. How do they look like? Further, which substructures hold these networks together at specific tissue site? Finally, is it possible to visualize such networks directly in a tissue section thereby aligning “ molecular systems histology” with traditional histopathology and directly derive molecular components whose therapeutic modulation could interfere with the corresponding disease process?
Technological Basis and Proof of Concept
I deal with this problem since 1987 and since that time have collected many tissue types in order to analyse and compare large molecular systems in tissue sections and in peripheral mononuclear blood cells (PMBC) 4. Finally, this led to the discovery of disease specific rules and the general principles of molecular systems organisation in vivo 1,2,4,6. For this purpose an automated device had to be developed 7, today termed imaging cycler microscope (ICM) 8. An ICM is a fully automated (epi)fluorescence microscope which overcomes the spectral resolution limit resulting in parameter- and dimension-unlimited fluorescence imaging (Figs. 1 and 2). The principle and robotic device was described in 1997 7 and eversince has been further developed with a team and with co-workers within the human toponome project 6,8–11.The ICM runs robotically controlled repetitive incubation-imaging-bleaching cycles on a specifically fixed biological sample with dye-conjugated probe libraries (antibodies, lectins, peptides or any specific affinity reagents) on stage of an epifluorescence microscope to co-localize large numbers of different biomolecules in situ. (for technology real time insight see www.huto.toposnomos.com).This results in the transmission of a randomly large number of distinct biological informations by re-using the same fluorescence channel, e.g., for FITC imaging, if FITC is conjugated to each probe, where each probe has its unique specificity. Thereby noise-reduced quasi-multi channel fluorescence images with reproducible physical, geometrical, and biophysical stabilities are generated. The resulting power of combinatorial molecular discrimination (PCMD) per data point is given by, e.g., 65,536k, where 65,536 is the number of grey value levels (output of a 16-bit CCD camera) and k is the number of co-mapped biomolecules and/or many subdomains per biomolecule(s). High PCMD has been shown for k = 100, 6,4,10 with 256100 and in principle can be expanded for much higher numbers of k. In contrast to traditional multi-channel-few parameter fluorescence microscopy (Fig. 1b) high PCMDs in an ICM lead to high functional and spatial resolution (Fig. 1a). Systematic ICM analysis of biological systems reveals the supramolecular segregation law that describes the principle of order of large, hierarchically organized biomolecular networks in situ (toponome) 5. The ICM is the core technology for the systematic mapping of the complete protein network code in tissues (human toponome project) 11. The original ICM method 7 includes any modification of the bleaching step. Corresponding modifications have been reported for antibody retrieval 12 and chemical dye-quenching 13 debated recently 8,14. The toponome imaging systems (TIS) and multi-epitope-ligand cartographs (MELC) represent different stages of the ICM technological development. I have found that the sunlight-similar straylight conditions used in the ICM set up for imaging and bleaching is the method of choice not altering the tissue integrity “on stage” over long periods of time (review in 4). Moreover, repetitive cycling is important for diluting out unspecific binders 15 to yield specific and highly resolved multi molecular biomolecular assemblies (Figs. 1 and 2). Many ICM based studies revealed that molecular networks of membrane associated biomolecules are hierarchically organised with lead proteins controlling protein network topology and function: when the lead protein is blocked or inhibited, the corresponding functional protein network disassembles leading to loss of (dys)function [4–6]. ICM based lead-protein detection and prediction as molecules hierarchically controlling pathogenic molecular networks was confirmed experimentally in mouse models for ALS 16, chronic neuropathic pain and in cultured tumour cells [6,17]. Imaging Cycler Microscopy received the American ISAC best article award in 2008 for the three symbol code of organized proteomes [18], by which large three-dimensional protein networks can be imaged per cell (Fig. 1e) or 2D molecular networks can be detected for diagnostic purposes (Fig. 3). In extension to the latter threshold based code, a non threshold based real time molecular profiling, based on mathematical theorems [19,20], enables the investigator to directly visualise large multimolecular assemblies, e.g., associated with the basal lamina (Fig. 1a), or detect protein profiles that sharply distinguish between normal and diseased tissue at the same time [4] (Fig. 2). Cyclical imaging has been applied to address a variety of biological problems [21–54], and mathematics/informatics approaches to ICM data have been published 19,20,52,55–57. Several editorials 58,59 and a research highlight 60 have featured toponome research.
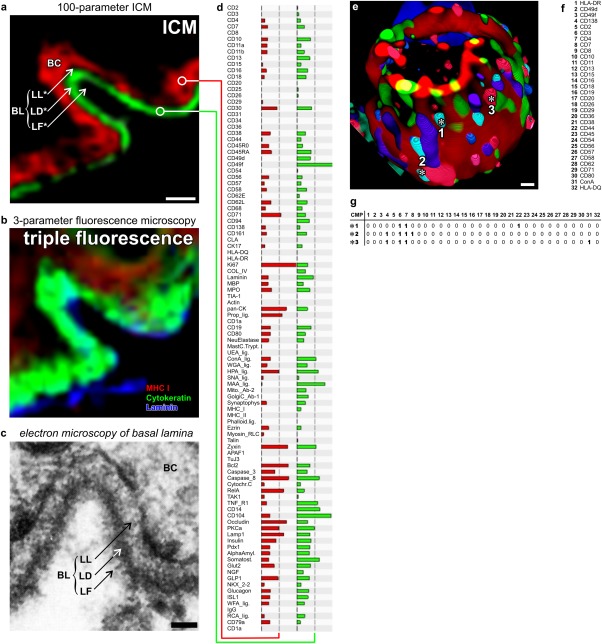
Figure 1.
Functional super resolution of large molecular networks. (a-d) Dermoepithelial junction in human tissue. Imaging cycler microscopy based discovery of molecular networks in situ 4. (a) Direct realtime protein profiling in 100-dimensional ICM data set using an algorithm based on the similarity mapping approach 19,20,22. Each data point has a PCMD of 256100. Note: sharp images at the junctional area discriminating between Lamina Fibroreticularis (LF), Lamina Densa (LD, green profile in d), Lamina Lucida (LL) and the basal ceratinocyte layer (BC, red profile in d), as known from transmission electron microscopy (c). (b) Same area as in (a), displaying traditional triple fluorescence imaging. (e) 3-dimensinal ICM imaging of distinct 32-component multi protein complexes on the cell surface of a blood T-lymphocyte 8. Multi protein complexes are composed of differential combination of 32 proteins/glycotopes listed in (f). (g) Examples are marked with asterisks (number 1 to 3) and detailed as CMPs with proteins present (1) or absent (0) together characterised as individual CMPs. Bars: 10 µm (a, b), 50 nm (c), 1 µm (e). A similar figure is featured in http://www.toposnomos.com/huto/tis.html. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
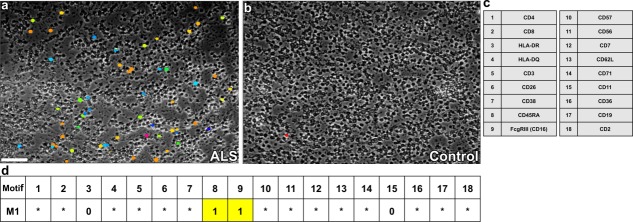
Figure 3.
ALS specific motif as predictive toponome biomarker visualized simultaneously in ALS PBMC (a) as directly compared with healthy control (b). The motif was revealed by fully automated ICM based co-mapping of 18 cell surface proteins (c) on isolated PBMC. The motif as a whole is composed of 200 distinct CMPs. The visual field of (a) shows cells, each of which displays one ALS-specific CMP out of the whole motif (different colours). The motif contains CD16 and CD45RA as lead proteins denoted (1 = lead protein, present in all CMPs) (d), while other proteins are either not associated (0 = anticolocated), or variably associated with the lead proteins (* = wild cards) (d, motif M1) 64–66. Courtesy of HUTO Project. Bar: 100 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
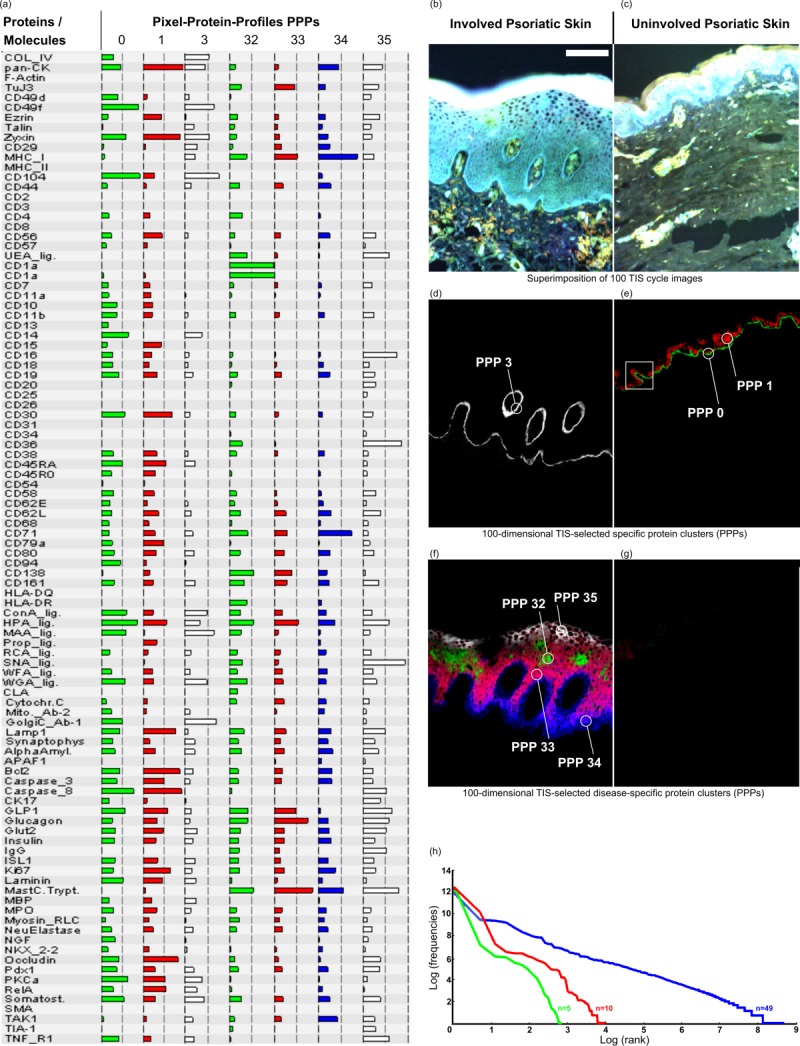
Discovery of disease specific 100-dimansional protein profiles simultaneously and in real time in morphology intact tissue sections at a PCMD of 256100 per pixel, exemplified in human skin. (a) List of 100 co-mapped biomolecules and selected protein profiles (0, 1, 3, 32 – 35) specific for diseased (d, f) and normal skin (e, g). (b,c) Diseased (b) and normal skin (c) are highlighted by pseudo colouring as histological stain for morphological orientation. Note that, by moving the cursor over the pixels, the software directly recognises in realtime which protein profiles are specific for the diseased (d, f) or normal skin (e, g). For example, pixel protein profiles (PPP) with numbers 0 and 1 are specific for the normal skin (e), and PPP 3, as well as PPPs 32 – 35 (d, f, respectively) are specific for the diseased skin. Note: For many similar applications at real time see webpage of the human toponome (HUTO) project (www.huto.toposnomos.com). (h) Power law (Zipf’s law) substantiates highly organised protein systems, as seen in (d-g). If 49 molecules are co-mapped Zipf’s law applies (blue line), but does not apply, if <15 molecules are co-mapped (red and green lines). This is revealed by plotting the log-log-relationship of thousands of distinct protein assemblies in toponome data sets 5,6. Bar: 100 µm (b–g). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
ICM-Based Approach To Disease Robustness
Amyotrophic Lateral Sclerosis: the Predictive ALS Toponome
ALS is a fatal disease of the motor neuron system leading to steadily progressive muscle weakness. Today it is commonly accepted that the clinical phenotype of the disease is the consequence of multiple pathogenic processes, involving motor neurons and surrounding non-neuronal cells, as well as myocytes 61. Over 50 clinical trials have been conducted in ALS over a period of 25 years. With exception of Riluzole trial, all have failed 62. Looking back to the 1990es, a multisystem cause of ALS was not discussed. I have started to address cell surface associated multi-protein clusters in peripheral blood mononuclear cells (PBMC) in ALS between 1990 and 1995 using the technology later termed Imaging Cycler Microscopy (ICM) (see above). I was prompted to analyze the ALS cellular immune system by ICM, because I saw a young patient aged 18 diagnosed as ALS with supranuclear palsy showing a severely altered lymphocyte homeostasis in PBMC with almost complete absence of CD8 T cells. This patient died 6 months after diagnosis of ALS. I interpreted this finding likely to reflect a substantial intrinsic alteration of the cellular immune system rather than merely a reaction to a neurodegeneration, and I took a closer look at the immune cell differentiation. I initiated a first quantitative four-parameter screening study 63. We found highly unusual if not abnormal immune cell subsets in a number of ALS patients expressing CD16+ CD8+ and CD57+ or CD16+ CD8+ CD57− cell surface clusters only in sporadic ALS with supranuclear palsy and in sporadic ALS with predominant involvement of the upper motor neuron. As an association of CD8 and CD16/CD57 in PBMC cell surface membranes is usually not seen in normal blood, the data suggested to look more precisely into the higher order cell surface differentiation, i.e., co-organization of proteins of the cellular immune system in ALS-PBMC using the ICM principle for co-mapping a large number of different cell surface proteins. By overcoming the fluorescence spectral resolution limit using ICM (described above) it was possible to resolve an 18-dimensional molecular system in ALS peripheral blood mononuclear cells in 1999 as described in several international patents 64–66. This molecular system, referred to as the ALS toponome or ALS motif, is hierarchically organized and revealed CD16 (FcγRIII) as lead protein and therapeutic target 64–66. Figure 3a shows presence of this ALS motif only in ALS PBMC, but not in normal control PBMC (Fig. 3b). This functional prediction of CD16 lead protein as therapeutic drug target cluster is now clinically substantiated: well tolerated downregulation of CD16 in a phase I clinical trial 67 is associated with beneficial effects in 27% of ALS patients with stop of disease progression in a phase II clinical trial over a 6 month period, which reached statistical significance when compared to concurrent and historical placebo controls 62. This proof of concept substantiates the predictive power of large molecular systems detected by ICM in toponome-based drug discovery, and shows that parameter-unlimited ICM imaging in intact human tissues is a reliable condition for correct drug target predictions. Imaging the corresponding molecular network as a combinatorial molecular phenotype (CMP) motif (ALS CMP motif) (Fig. 3d) reveals its presence only in ALS PBMC (Fig. 3a). Further, as therapeutic downregulation of the lead-protein CD16 (and dysfunctions associated with this downregulation), as predicted [64–66], results in a beneficial clinical effect [67], the shown ALS motif (Fig. 3a) is a robustness network of ALS, whose stability can be broken by interfering with its lead protein. The wide angle of view is a necessary condition to visualize most of the CMPs of this motif (robustness network) simultaneously. CD16 is present in all cells expressing a given CMP of the ALS motif suggesting that early detection of this pathological motif can be helpful or decisive for diagnosis and early disease management in cases of suspected disease.
Together, ICM correctly identified a molecular disease network of ALS and a lead protein controlling it 64–66. The method of blocking the lead protein to block its pathogenic function can be a direct 64 or an indirect one 65,66. The latter indirect method 65,66 was substantiated experimentally in a CD16 KO mouse (16) having implicated that therapeutically blocking the specific binders of CD16 (the IgG1 and IgG3 proteins) as published earlier 64–66 and thereby preventing them from binding to the corresponding CD16 binding sites is a valid method indirectly inactivating the pathogenic function of CD16 leadprotein and thus is a promising way for clinical therapeutic intervention in ALS. These two direct and indirect principles [64–66, respectively] paved the way and stimulated the use of specific other molecules to downregulate functions of CD16 expressed in cellular systems in ALS, directly or indirectly, and many studies have confirmed CD16 as critical marker in ALS (68). In particular, NP001 was applied and reported to efficiently downregulate CD16 67 as critical biomarker and control element of therapeutic success. Other molecules exerting similar CD16-oriented regulation might be found in the future. The lead-protein principle was confirmed for tumour cells 6 and both, the clinical confirmation in ALS 62,67 and its experimental proof-of-principle in tumour cells 6, are now new drivers in other fields when it comes to tailor toponome-target based therapies, e.g., in cancer. For ALS the impact of ICM has been to find an ALS toponome and a therapy method exerting direct or indirect regulation of the lead-protein CD16 64–66, now confirmed clinically 62,67. The derived immediate advantage of this ICM approach is early diagnosis in order to prevent disease progression: ALS is often difficult to diagnose in early stages, when patients complain, for example, of a clumsiness in their muscles, and the neurologists have no clear clinical proof of ALS at this time point. Instead of waiting for the ALS specific signs (progressed damage of motor neurons), early imaging of PBMC as shown in Figure 3 can be helpful for early therapeutic decisions: If the patient does express the ALS toponome (Fig. 3) therapeutic targeting of CD16 is strongly recommended, since this might prevent ALS progressive neuronal tissue damage, and stop disease progression. This can be expected in cases of development of supranuclear palsy or with predominant involvement of the upper motor neuron system, as suggested by our initial study 63. Corresponding regimes of clinical disease management will have to be worked out. Moreover, the CD16 toponome might show up the road map for complete spatial decoding of the ALS toponome containing many new options for efficient disease treatment and understanding disease mechanisms. For the time being we know, that the cells found in the blood, expressing the CD8+CD16+CD57-/+ phenotype 63 as part of the overall CD16 motif (Fig. 3) 64–66 are critical aberrant cells because they invade and actively damage the neurons in the motor system (to be published). These cells can be interpreted as aberrant T cells (not monocytes) expressing that motif as a biomarker of their agenda. When the cells published in 1995 63 were co-mapped for the ALS toponome (Fig. 3) we have never seen these cellular motifs in other contexts, as revealed by imaging of normal controls (n = 8), and disease controls (stroke, n = 3; polymyositis, n = 6), but have seen them regularly in ALS with supranuclear palsy or predominant involvement of the upper motor neuron (n = 21) 63–66. Together these data suggest presence of an abnormally differentiated cellular immune system driving these cells into aberrant uncontrollable homing to the CNS.
Lessons
Lessons learned from the clinically and experimentally validated ICM based predictions are: Tissues must be intact for successful discovery of topologically intact molecular systems and for this purpose must be fixed as gently as possible 6,9; Isolation of tissue components (cells or molecules) would disrupt the topological properties of the system as a whole; isolated cells do not reveal the system, since disease-driving molecular systems are robust structures involving many cells on a multi-molecular scale in tissues ranging from subcellular to transcellular dimensions (Figs. 1–3). This shows that he best ‘biochip’‚ is the tissue itself (including blood as fluid tissue). Topologically ordered states in health and disease are keys to assess (dys)function with certainty. If relevant tissues are subjected to high-dimensional ICM with a PCMD ranging from 218 to 256100, or 65,536100 per data point, then the key information on the spatial and functional coding of disease-driving and normal networks will be automatically extracted and (most importantly) found in a hypothesis free manner. Such data will often contain relevant lead proteins as we have established by studying cell surface associated molecular systems 6,17,64–66. Lead proteins controlling disease-driving molecular networks can be mathematically described as combinatorial molecular motifs 2,6. They can be interpreted as robustness nodes of disease, because blocking or inhibiting the lead proteins detected by ICM leads to disassembly of the pathogenic network and loss of (dys)function as shown for tumour cells experimentally 6 and now for ALS clinically by successfully downregulating the ICM predicted ALS lead-protein CD16 62. This shows, that specific robustness nodes present in tissue, are built by spatial molecular networks with unique topological coordinates.
Implications for Drug Discovery and Future Strategies in ALS Research
The pharmaceutical industry and the healthcare system are facing major challenges because of high failure rates of drugs, as reported, e.g., for cancer (“High attrition rates-where are we going wrong?” 69. The frequently quoted $1 billion to bring a new drug to market has risen to $4 to 5 billion by some estimates (www.forbes.com/sites/matthewherper/2012/02/10/the-truly-staggering-cost-of-inventing-new-drugs). To address this gap, the U.S. President’s Council of Advisors on Science and Technology (PCAST) published a report in September 2012 on propelling innovation in the pharmaceutical industry, which commented that “the ecosystem for public health is under significant stress” and that research and development (R&D) productivity is declining [commented in Ref. (70]. Inefficient target discovery is likely to be one of the problems 69.The current, above briefly described experience and proof of concept with ICM based drug discovery suggests that future strategies must include the discovery of disease mechanisms in situ on a large scale of in situ protein networks to improve efficiency of drug target prediction, as shown for ALS. Safety assessment is another candidate for the ICM-toponome approach. Technologically, today a complete decoding of all protein networks (the e.g., approx. 6,000 cell surface associated druggable biomolecules in tissues) is feasible by applying a net of ICM robots, for example as a globally accessible platform in appropriate centers. Concerning ALS, the specific advantage of ICM based toponome approach lies in the technical feasibility to monitor PBMCs for presence of the ALS motif (Fig. 3) and thereby improve early diagnosis, explore new toponome motifs in disease subsets, monitor the found motifs over time, and potentially initiate early therapeutic targeting of the lead protein(s) to potentially counteract progressive physical signs of the disease. A key for success can be based on ICM-toponome information content by co-mapping many thousand different multi-protein assemblies at a time, because these data contain the disease specific systems functionalities. Globally accessible ICM platforms will significantly enhance toponome research and drug discovery.
Conclusion
Chronic diseases are robust systems whose emergent molecular properties are hardly understood. High drug attrition rates and failures of treatments 69,70 show that these systems have not yet been therapeutically targeted at their robustness nodes. Direct ICM imaging of disease protein networks inside the diseased tissue provides a new option for therapeutic success, because (i) the hierarchy of molecular disease networks with lead proteins can be directly visualised and quantified inside the relevant tissue 6, (ii) these networks can be disassembled by inhibiting the lead protein controlling the network topology and (dys)function 6, as shown experimentally, and (iii) ICM based prediction and downregulatin of the lead protein and/or functions of it in ALS leads to therapeutic effects, as shown in a clinical trial 62. This may indicate that the molecular disease robustness mechanisms in ALS can be broken, suggesting that the ICM approach is likely to provide similar access to the robustness nodes in other chronic diseases. In ALS, early diagnosis and treatment can now be facilitated by ICM-PBMC screening for the ALS toponome.
Acknowledgments
The author thank ToposNomos Ltd. for providing access to the Imaging Cycler® reference lab and Andreas Krusche and Reyk Hillert for helping with the figures and formatting the manuscript.
Literature Cited
- Schubert W. Toponomics. In: Dubitzky W, Wolkenhauer O, Cho KH, Yokota H, editors. Encyclopedia of Systems Biology. New York: Springer; 2013. pp. 2191–2212. . In:, editors. doi: 10.1007/978-1-4419-9863-7_631. ISBN 978-1-4419-9862-0. [Google Scholar]
- Schubert W. Topological proteomics, toponomics, MELK-technology. Adv Biochem Eng Biotechnol. 2003;83:189–209. doi: 10.1007/3-540-36459-5_8. . doi: 10.1007/3-540-36459-5_8. [DOI] [PubMed] [Google Scholar]
- Van Regenmortel MHVV. Reductionism and complexity in molecular biology. EMBO Rep. 2004;5:1016–1020. doi: 10.1038/sj.embor.7400284. . doi: 10.1038/sj.embor.7400284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W, Gieseler A, Krusche A, Serocka P, Hillert R. Next-generation biomarkers based on 100-parameter functional super-resolution microscopy TIS. Nat Biotechnol. 2012;29:599–610. doi: 10.1016/j.nbt.2011.12.004. . doi: 10.1016/j.nbt.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Schubert W. Systematic, spatial imaging of large multimolecular assemblies and the emerging principles of supramolecular order in biological systems. JMR. 2014;27:3–18. doi: 10.1002/jmr.2326. . doi: 10.1002/jmr.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W, Bonnekoh B, Pommer AJ, Philipsen L, Böckelmann R, Malykh Y, Gollnick H, Friedenberger M, Bode M, Dress AWM. Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nat Biotechnol. 2006;24:1270–1278. doi: 10.1038/nbt1250. . doi: 10.1038/nbt1250. [DOI] [PubMed] [Google Scholar]
- Schubert W. Automated device and method for measuring and identifying molecules or fragments thereof. Eur Pat EP 0810428 B1 1997 [see also Schubert W. US patent US 6,150,173 (2000); Japanese patent 3739528 (1998)]
- Schubert W, Dress A, Ruonala M, Krusche A, Hillert R, Gieseler A, Walden P. Imaging cycler microscopy. Proc Natl Acad Sci U S A. 2014;111:E215–E215. doi: 10.1073/pnas.1319017111. ; doi: 10.1073/pnas.1319017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenberger M, Bode M, Krusche A, Schubert W. Fluorescence detection of protein clusters in individual cells and tissue sections by using toponome imaging system: Sample preparation and measuring procedures. Nat Protoc. 2007;2:2285–2294. doi: 10.1038/nprot.2007.320. . doi: 10.1038/nprot.2007.320. [DOI] [PubMed] [Google Scholar]
- Schubert W. 2013. Direct, spatial imaging of randomly large supermolecules by using parameter unlimited TIS imaging cycler microscopy. International Microscopy Conference, Regensburg, Germany,. Retrieved 2013-09-23.
- Cottingham K. Human toponome project. J Proteome Res. 2009;7:1806. doi: 10.1021/pr083701k. [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: A new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. . doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes MJ, Sevinsky CJ, Sood A, Adak S, Bello MO, Bordwell A, Can A, Corwin A, Dinn S, Filkins RJ, Hollman D, Kamath V, Kaanumalle S, Kenny K, Larsen M, Lazare M, Li Q, Lowes C, McCulloch CC, McDonough E, Montalto MC, Pang Z, Rittscher J, Santamaria-Pang A, Sarachan BD, Seel ML, Seppo A, Shaikh K, Sui Y, Zhang J, Ginty F. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A. 2013;110:11982–11987. doi: 10.1073/pnas.1300136110. . doi: 10.1073/pnas.1300136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes MJ. Reply to schubert et al.: Regarding critique of highly multiplexed technologies. Proc Natl Acad Sci U S A. 2014;111:E216–E216. doi: 10.1073/pnas.1319622111. . doi: 10.1073/pnas.1319622111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel MH. Specificity, polyspecificity, and heterospecificity of antibody-antigen recognition. J Mol Recognit. 2014;27:627–39. doi: 10.1002/jmr.2394. . doi: 10.1002/jmr.2394. [DOI] [PubMed] [Google Scholar]
- Mohamed HA, Mosier DR, Zou LL, Siklós L, Alexianu ME, Engelhardt JI, Beers DR, Le WD, Appel SH. Immunoglobulin Fc gamma receptor promotes immunoglobulin uptake, immunoglobulin-mediated calcium increase, and neurotransmitter release in motor neurons. 2002;69:110–11–6. doi: 10.1002/jnr.10271. J Neurosci Res. doi: 10.1002/jnr.10271. [DOI] [PubMed] [Google Scholar]
- Schubert W. On the origin of cell functions encoded in the toponome. J Biotechnol. 2010;149:252–259. doi: 10.1016/j.jbiotec.2010.03.009. . doi: 10.1016/j.jbiotec.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Schubert W. A three-symbol code for organized proteomes based on cyclical imaging of protein locations. Cytometry A. 2007;71A:352–360. doi: 10.1002/cyto.a.20281. . doi: 10.1002/cyto.a.20281. [DOI] [PubMed] [Google Scholar]
- Dress AWM, Lokot T, Pustyl’nikov LD, Schubert W. Poisson numbers and poisson distributions in subset surprisology. Ann Comb. 2005;8:473–485. . doi: 10.1007/s00026-004-0234-2. [Google Scholar]
- Dress AWM, Lokot T, Schubert W, Serocka P. Two theorems about similarity maps. Ann Comb. 2008;12:279–290. . doi: 10.1007/s00026-008-0351-4. [Google Scholar]
- Ademmer K, Ebert M, Müller-Ostermeyer F, Friess H, Büchler MW, Schubert W, Malfertheiner P. Effector T lymphocyte subsets in human pancreatic cancer: Detection of cd8+ cd18+ cells and cd8+ cd103+ cells by multi-epitope imaging. Clin Exp Immunol. 1998;112:21–26. doi: 10.1046/j.1365-2249.1998.00546.x. . doi: 10.1046/j.1365-2249.1998.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barysenka A, Dress AW, Schubert W. An information theoretic thresholding method for detecting protein colocalizations in stacks of fluorescence images. J Biotechnol. 2010;149:127–131. doi: 10.1016/j.jbiotec.2010.01.009. . doi: 10.1016/j.jbiotec.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Bedner E, Du L, Traganos F, Darzynkiewicz Z. Caffeine dissociates complexes between DNA and intercalating dyes: Application for bleaching fluorochrome-stained cells for their subsequent restaining and analysis by laser scanning cytometry. Cytometry. 2001;43:38–45. . doi: 10.1002/1097-0320(20010101)43:1<38::AID-CYTO1017>3.0.CO;2-S. [PubMed] [Google Scholar]
- Berndt U, Philipsen L, Bartsch S, Hu Y, Röcken C, Bertram W, Hämmerle M, Rösch T, Sturm A. Comparative Multi-Epitope-Ligand-cartography reveals essential immunological alterations in barrett’s metaplasia and esophageal adenocarcinoma. Mol Cancer. 2010;9:177. doi: 10.1186/1476-4598-9-177. . doi: 10.1186/1476-4598- 9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Mathew G, Ruban E, Epstein DB, Krusche A, Hillert R, Schubert W, Khan M. Toponome imaging system: Protein network mapping in normal and cancerous colon from the same patient reveals more than Five-thousand cancer specific protein clusters and their subcellular annotation by using a three symbol code. J Proteome Res. 2010;9:6112–6125. doi: 10.1021/pr100157p. . doi: 10.1021/pr100157p. [DOI] [PubMed] [Google Scholar]
- Bode M, Irmler M, Friedenberger M, May C, Jung K, Stephan C, Meyer HE, Lach C, Hillert R, Krusche A, Beckers J, Marcus K, Schubert W. Interlocking transcriptomics, proteomics and toponomics technologies for brain tissue analysis in murine hippocampus. Proteomics. 2008;8:1170–1178. doi: 10.1002/pmic.200700742. . doi: 10.1002/pmic.200700742. [DOI] [PubMed] [Google Scholar]
- Bonnekoh B, Böckelmann R, Pommer AJ, Malykh Y, Philipsen L, Gollnick H. The CD11a binding site of efalizumab in psoriatic skin tissue as analyzed by Multi-epitope ligand cartography robot technology. Skin Pharmacol Physiol. 2007;20:96–111. doi: 10.1159/000097982. . doi: 10.1159/000097982. [DOI] [PubMed] [Google Scholar]
- Bonnekoh B, Malykh Y, Böckelmann R, Bartsch S, Pommer AJ, Gollnick H. Profiling lymphocyte subpopulations in peripheral blood under efalizumab treatment of psoriasis by multi epitope ligand cartography (MELC) robot microscopy. Eur J Dermatol. 2006;16:623–635. . doi: 10.1684/ejd.2006.0005. [PubMed] [Google Scholar]
- Bonnekoh B, Pommer AJ, Böckelmann R, Hofmeister H, Philipsen L, Gollnick H. Topo-proteomic in situ analysis of psoriatic plaque under efalizumab treatment. Skin Pharmacol Physiol. 2007;20:237–252. doi: 10.1159/000104422. . doi: 10.1159/000104422. [DOI] [PubMed] [Google Scholar]
- Bonnekoh B, Pommer AJ, Böckelmann R, Philipsen L, Hofmeister H, Gollnick H. In-situ-topoproteome analysis of cutaneous lymphomas: Perspectives of assistance for dermatohistologic diagnostics by multi epitope ligand cartography (MELC) J Dtsch Dermatol Ges. 2008;6:1038–1051. doi: 10.1111/j.1610-0387.2007.06754.x. . doi: 10.1111/j.1610-0387.2007.06754.x. [DOI] [PubMed] [Google Scholar]
- Coste O, Brenneis C, Linke B, Pierre S, Maeurer C, Becker W, Schmidt H, Gao W, Geisslinger G, Scholich K. Sphingosine 1-phosphate modulates spinal nociceptive processing. J Biol Chem. 2008;283:32442–32451. doi: 10.1074/jbc.M806410200. . doi: 10.1074/jbc.M806410200. [DOI] [PubMed] [Google Scholar]
- Ebert MP, Ademmer K, Müller-Ostermeyer F, Friess H, Büchler MW, Schubert W, Malfertheiner P. Cd8+cd103+ T cells analogous to intestinal intraepithelial lymphocytes infiltrate the pancreas in chronic pancreatitis. Am J Gastroenterol. 1998;93:2141–2147. doi: 10.1111/j.1572-0241.1998.00610.x. . doi: 10.1111/j.1572-0241.1998.00610.x. [DOI] [PubMed] [Google Scholar]
- Ecker RC, Rogojanu R, Streit M, Oesterreicher K, Steiner GE. An improved method for discrimination of cell populations in tissue sections using microscopy-based multicolor tissue cytometry. Cytometry A. 2006;69A:119–123. doi: 10.1002/cyto.a.20219. . doi: 10.1002/cyto.a.20219. [DOI] [PubMed] [Google Scholar]
- Eckhardt J, Ostalecki C, Kuczera K, Schuler G, Pommer AJ, Lechmann M. Murine Whole-organ immune cell populations revealed by Multi-epitope-ligand cartography. J Histochem Cytochem. 2013;61:125–133. doi: 10.1369/0022155412470140. . doi: 10.1369/0022155412470140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich K, Böckelmann R, Pommer AJ, Foerster S, Hofmeister H, Huss-Marp J, Cavani A, Behrendt H, Ring J, Gollnick H, Bonnekoh B, Traidl-Hoffmann C. Comparative in situ topoproteome analysis reveals differences in patch test-induced eczema: Cytotoxicity-dominated nickel versus pleiotrope pollen reaction. Exp Dermatol. 2010;19:511–517. doi: 10.1111/j.1600-0625.2009.00980.x. . doi: 10.1111/j.1600-0625.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- Gieseler A. Cell Membrane Toponomics. In: Dubitzky W, Wolkenhauer O, Cho KH, Yokota H, editors. Encyclopedia of Systems Biology. New York: Springer; 2013. pp. 364–366. . In:, editors. doi: 10.1007/978-1-4419-9863-7_1568. ISBN 978-1-4419-9862-0. [Google Scholar]
- Gieseler A. Synaptic Toponome. In: Dubitzky W, Wolkenhauer O, Cho KH, Yokota H, editors. Encyclopedia of Systems Biology. New York: Springer; 2013. pp. 2036–2038. . In:, editors. doi: 10.1007/978-1-4419-9863-7_633. ISBN 978-1-4419-9862-0. [Google Scholar]
- Haars R, Schneider A, Bode M, Schubert W. Secretion and differential localization of the proteolytic cleavage products abeta40 and abeta42 of the alzheimer amyloid precursor protein in human fetal myogenic cells. Eur J Cell Biol. 2000;79:400–406. doi: 10.1078/0171-9335-00064. . doi: 10.1078/0171-9335-00064. PMID 10928455. [DOI] [PubMed] [Google Scholar]
- Laffers W, Mittag A, Lenz D, Tárnok A, Gerstner AO. Iterative restaining as a pivotal tool for n-color immunophenotyping by slide-based cytometry. Cytometry A. 2006;69A:127–130. doi: 10.1002/cyto.a.20216. . doi: 10.1002/cyto.a.20216. [DOI] [PubMed] [Google Scholar]
- Mittag A, Lenz D, Gerstner AO, Tárnok A. Hyperchromatic cytometry principles for cytomics using slide based cytometry. Cytometry A. 2006;69A:691–703. doi: 10.1002/cyto.a.20285. . doi: 10.1002/cyto.a.20285. [DOI] [PubMed] [Google Scholar]
- Ostalecki C, Konrad A, Thurau E, Schuler G, Croner RS, Pommer AJ, ael Stürzl M. Combined multi-gene analysis at the RNA and protein levels in single FFPE tissue sections. Exp Mol Pathol. 2013;95:1–6. doi: 10.1016/j.yexmp.2013.03.008. . doi: 10.1016/j.yexmp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Philipsen L, Engels T, Schilling K, Gurbiel S, Fischer KD, Tedford K, Schraven B, Gunzer M, Reichardt P. Multimolecular analysis of stable immunological synapses reveals sustained recruitment and sequential assembly of signaling clusters. Mol Cell Proteomics. 2013;12:2551–2567. doi: 10.1074/mcp.M112.025205. . doi: 10.1074/mcp.M112.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetze M, Gallinat S, Wenck H, Deppert W, Knott A. In situ localization of epidermal stem cells using a novel multi epitope ligand cartography approach. Integr Biol. 2010;2:241. doi: 10.1039/b926147h. doi:10.1039/b926147h. [DOI] [PubMed] [Google Scholar]
- Schubert W. Polymyositis, topological proteomics technology and paradigm for cell invasion dynamics. J Theor Med. 2002;4:75–84. . doi: 10.1080/10273660290015224. [Google Scholar]
- Schubert W. Cytomics in characterizing toponomes: Toward the biological code of the cell. Cytometry A. 2006:209–211. doi: 10.1002/cyto.a.20203. . doi: 10.1002/cyto.a.20203. [DOI] [PubMed] [Google Scholar]
- Schubert W. Exploring molecular networks directly in the cell. Cytometry A. 2006;69A:109–112. doi: 10.1002/cyto.a.20234. . doi: 10.1002/cyto.a.20234. [DOI] [PubMed] [Google Scholar]
- Schubert W. Breaking the biological code. Cytometry A. 2007;71A:771–772. doi: 10.1002/cyto.a.20466. . doi: 10.1002/cyto.a.20466. [DOI] [PubMed] [Google Scholar]
- Schubert W. Toponomanalyse. In: Lottspeich F, Engels J, editors. Bioanalytik. 3rd ed. Heidelberg: Spektrum; 2012. pp. 1139–1151. . In:, editors. ISBN 978-3-8274-2942-1. [Google Scholar]
- Schubert W, Bode M, Hillert R, Krusche A, Friedenberger M. Toponomics and neurotoponomics: A new way to medical systems biology. Exp Rev Proteomics. 2008;5:361–369. doi: 10.1586/14789450.5.2.361. . doi: 10.1586/14789450.5.2.361. [DOI] [PubMed] [Google Scholar]
- Schubert W, Friedenberger M, Bode M, Krusche A, Hillert R. Functional architecture of the cell nucleus: Toward comprehensive toponome reference maps of apoptosis. Biochim Biophys Acta. 2008;1783:2080–2088. doi: 10.1016/j.bbamcr.2008.07.019. . doi: 10.1016/j.bbamcr.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Schubert W, Friedenberger M, Haars R, Bode M, Philipsen L, Nattkemper T, Ritter H. Automatic recognition of muscle-invasive T-lymphocytes expressing dipeptidyl-peptidase IV (cd26) and analysis of the associated cell surface phenotypes. J Theor Med. 2002;4:67–74. . doi: 10.1080/10273660290015189. [Google Scholar]
- Kovacheva VN, Khan AM, Khan M, Epstein DB, Rajpoot NM. DiSWOP: A novel measure for cell-level protein network analysis in localized proteomics image data. Bioinformatics. 2014;30:420–427. doi: 10.1093/bioinformatics/btt676. . doi: 10.1093/bioinformatics/btt676. [DOI] [PubMed] [Google Scholar]
- Schubert W, Gieseler A, Krusche A, Hillert R. Toponome mapping in prostate cancer: Detection of 2000 cell surface protein clusters in a single tissue section and cell type specific annotation by using a three symbol code. J Proteome Res. 2009;8:2696–2707. doi: 10.1021/pr800944f. . doi: 10.1021/pr800944f. [DOI] [PubMed] [Google Scholar]
- Schubert W, de Wit NCJ, Walden P. Systems Biology of Cancer. In: Pelengaris S, Khan M, editors. Molecular Biology of Cancer: A Bridge From Bench to Bedside. 2nd ed. New York: Wiley-Blackwell; 2013. pp. 554–584. . In:, editors. ISBN 978-1-118-02287-0. [Google Scholar]
- Oeltze S, Freiler W, Hillert R, Doleisch H, Preim B, Schubert W. Interactive, Graph-based visual analysis of High-dimensional, Multi-parameter fluorescence microscopy data in toponomics. IEEE Trans Vis Comput Graph. 2011;17:1882–1891. doi: 10.1109/TVCG.2011.217. . doi: 10.1109/TVCG.2011.217. [DOI] [PubMed] [Google Scholar]
- Nattkemper TW, Ritter HJ, Schubert W. A neural classifier enabling high-throughput topological analysis of lymphocytes in tissue sections. IEEE Trans Inf Technol Biomed. 2001;5:138–149. doi: 10.1109/4233.924804. . doi: 10.1109/4233.924804. [DOI] [PubMed] [Google Scholar]
- Nattkemper TW, Twellmann T, Ritter H, Schubert W. Human vs. machine: Evaluation of fluorescence micrographs. Comput Biol Med. 2003;33:31–43. doi: 10.1016/s0010-4825(02)00060-4. . doi: 10.1016/s0010-4825(02)00060-4. [DOI] [PubMed] [Google Scholar]
- Sage L. The molecular face of prostate cancer. J Proteome Res. 2009;8:2616. doi: 10.1021/pr9003129. doi:10.1021/pr9003129. [DOI] [PubMed] [Google Scholar]
- Murphy RF. Putting proteins on the map. Nat Biotechnol. 2006;24:1223–1224. doi: 10.1038/nbt1006-1223. . doi: 10.1038/nbt1006-1223. [DOI] [PubMed] [Google Scholar]
- Abott A . Research highlights. Nature. 2006;443:608–609. . doi: 10.1038/443608a. [Google Scholar]
- Robelin L, Gonzalez De Aguilar JL. Blood biomarkers for amyotrophic lateral sclerosis: Myth or reality? Biomed Res Int. 2014;2014:525097. doi: 10.1155/2014/525097. doi: 10.1155/2014/525097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal NA, Mozaffar T. Experimental trials in amyotrophic lateral sclerosis: A review of recently completed, ongoing and planned trials using existing and novel drugs. Exp Opin Investig Drugs. 2014;23:1541–1551. doi: 10.1517/13543784.2014.933807. . doi: 10.1517/13543784.2014.933807. [DOI] [PubMed] [Google Scholar]
- Schubert W, Schwan H. Detection by 4-parameter microscopic imaging and increase of rare mononuclear blood leukocyte types expressing the fc gamma RIII receptor (cd16) for immunoglobulin G in human sporadic amyotrophic lateral sclerosis (ALS) Neurosci Lett. 1995;198:29–32. doi: 10.1016/0304-3940(95)11956-w. [DOI] [PubMed] [Google Scholar]
- Schubert W. Method of blocking cytotoxic activity in patients with amyotrophic lateral sclerosis using antibodies to FcγRIII. 1999; US patent no. US 6,638,506 (first published as international patent application WO 99/29731, 1999)
- Schubert W . Method of blocking cytotoxic activity in patients with amyotrophic lateral sclerosis using protein V. 2001a; US Pat no. US 6,638,515.
- Schubert W . Method of blocking cytotoxic activity in patients with amyotrophic lateral sclerosis using soluble FcγRIII receptors. 2001b; US Pat no. US 6,649,165.
- Miller RG, Zhang R, Block G, Katz J, Barohn R, Kasarskis E, Forshew D, Gopalakrishnan V, McGrath MS. Np001 regulation of macrophage activation markers in ALS: A phase I clinical and biomarker study. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:601–609. doi: 10.3109/21678421.2014.951940. . doi: 10.3109/21678421.2014.951940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–87. doi: 10.1172/JCI62636. , et al. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L, Kirk R. High drug attrition rates - where are we going wrong? Nat Rev Clin Oncol. 2011;8:189–190. doi: 10.1038/nrclinonc.2011.34. . doi: 10.1038/nrclinonc.2011.34. [DOI] [PubMed] [Google Scholar]
- Staff NP, Runge BK, Windebank AJ. Breaking down translation barriers: Investigator’s perspective. Sci Transl Med. 2014;6:252cm7. doi: 10.1126/scitranslmed.3008252. . doi: 10.1126/scitranslmed.3008252. [DOI] [PubMed] [Google Scholar]