Abstract
Background
The Val66 to Met polymorphism within the brain-derived neurotrophic factor (BDNF) sequence reduces activity-dependent BDNF release, and is associated with psychiatric disorders in humans. Alcoholism is one of the most prevalent psychiatric diseases. Here, we tested the hypothesis that this polymorphism increases the severity of alcohol abuse disorders.
Methods
We generated transgenic mice carrying the mouse homolog of the human Met66BDNF allele (Met68BDNF), and used alcohol-drinking paradigms in combination with viral-mediated gene delivery and pharmacology.
Results
We found that Met68BDNF mice consumed excessive amounts of alcohol and continued to drink despite negative consequences, a hallmark of addiction. Importantly, compulsive alcohol intake was reversed by overexpression of the wild-type Val68BDNF allele in the ventromedial prefrontal cortex of the Met68BDNF mice, or by systemic administration of the TrkB agonist, LM22A-4.
Conclusions
Our findings suggest that carrying the Met66BDNF allele increases the risk of developing uncontrolled and excessive alcohol drinking that can be reversed by directly activating the BDNF receptor, TrkB. Importantly, this work identifies a potential therapeutic strategy for the treatment of compulsive alcohol drinking in humans carrying the Met66BDNF allele.
Keywords: BDNF, Polymorphism, Alcohol, Ethanol, Addiction, Growth Factor
INTRODUCTION
Addiction is a psychiatric disorder characterized as compulsive drug seeking and taking despite adverse consequences (1–3). Alcohol addiction is the second most impactful psychiatric disorder (4) that results in various somatic diseases (5), and is responsible for 6% of the deaths worldwide (6). Increasing evidence in humans and rodents, suggests an inverse relationship between BDNF and adverse phenotypes associated with harmful alcohol consumption. Similar to other psychiatric disorders (7), alcoholism is associated with low blood levels of BDNF (8, 9). In line with the human data, the development of excessive alcohol drinking in mice is associated with low Bdnf expression within prefrontal cortex (PFC) (10, 11). Alcohol-preferring rats display innate lower levels of BDNF in the bed nucleus of stria terminalis and in the central extended and medial amygdala (CeA and MeA, respectively) as compared to non-alcohol-preferring rats (12). Furthermore, constitutive reduction of Bdnf expression in mice results in increased levels of alcohol intake (13–15) as well as higher sensitivity to the rewarding and sensitizing properties of alcohol (14). Global inhibition of the BDNF pathway (16) and the specific knockdown of BDNF expression in the dorsolateral striatum (DLS) (15, 17) or in the CeA and MeA (18) escalate alcohol-drinking behaviors. Finally, activation of BDNF signaling in the DLS (17, 19), and in the CeA and MeA (18) reduces alcohol intake in rodents. Together, these data indicate that BDNF gates the level of alcohol drinking.
The non-synonymous single-nucleotide polymorphism (SNP) of the BDNF gene (G196A, rs6265) results in a substitution of Valine (Val) 66 residue to Methionine (Met) within the prodomain of BDNF (proBDNF) (20, 21). Val66 to Met substitution substantially alters the intracellular trafficking of the immature form of BDNF and decreased activity-dependent release of the neurotrophic factor (20, 21). The Met66BDNF polymorphism is relatively common in humans (22), and has been associated with increased severity of psychiatric disorders (23–25) including addiction to nicotine, opiates, amphetamine and tetrahydrocannabinol (26–28). We hypothesized that subjects carrying the Met66BDNF allele will be at greater risk of developing “pathological” excessive alcohol drinking behaviors. To this end, we generated knock-in (KI) mice expressing the mouse homolog of the human Met66BDNF allele (Met68BDNF, Supplementary Figs. S1–S2) on a C57BL/6 background, and tested the propensity of mice carrying the polymorphism to develop uncontrolled alcohol drinking.
METHODS AND MATERIALS
Detailed information is available in the Supplemental information. This includes the generation of Met68BDNF KI mice, breeding and housing, preparation of solutions, administration of drugs, and blood alcohol concentration protocols. Behavioral paradigms (saccharin and quinine consumption, compulsivity, anxiety, motor coordination and locomotion tasks) and the construction, preparation, and administration of adeno-associated virus (AAV) expressing BDNF, western blot analysis, immunohistochemistry, and statistical analyses can be found in the Supplemental Information section.
Intermittent access to alcohol and quinine adulteration paradigms
Mice were acclimatized for 1 week to single housing conditions and then given concurrent access to one bottle of 10% or 20% alcohol and one bottle of water for 24 hrs (2-bottle choice), starting at noon on Mondays, Wednesdays, and Fridays, for 4 weeks. Alcohol intake (g/kg of body weight), total fluid intake (ml/kg of body weight) and the preference ratio for alcohol solution (volume of alcohol solution intake/total volume of fluid intake) were recorded after 24 hrs of alcohol access. To test compulsive drinking, the alcohol solution was adulterated with quinine (0.10–0.30 g/l) and intake was monitored as described above. The placement (left or right) of each solution was alternated between each session to control for side preference.
RESULTS
Generation of Met68BDNF KI mice
We generated a transgenic mouse line carrying the Met68BDNF allele that corresponds with the human Met66BDNF homolog (http://weblab.cbi.pku.edu.cn (29), Supplementary Fig. S1 and Supplementary Fig. S2). Litter size of homozygotes Met68BDNF mice was similar to wild-type Val68BDNF mice (Met68BDNF = 6.75 ± 0.52 pups/dam, n=20; Val68BDNF = 7.1 ± 0.53 pups/dam, n=20), we did not observe gross anatomical abnormalities during postnatal development, and growth curves of the two genotypes were also similar (Supplementary Fig. S3A). In addition, Met68BDNF mice did not show changes in basal locomotion in response to a novel environment (Supplementary Fig. S3B), or in the acquisition and execution of motor-dependent tasks (Supplementary Fig. S3C).
Met68BDNF mice exhibit enhanced escalation of excessive alcohol drinking
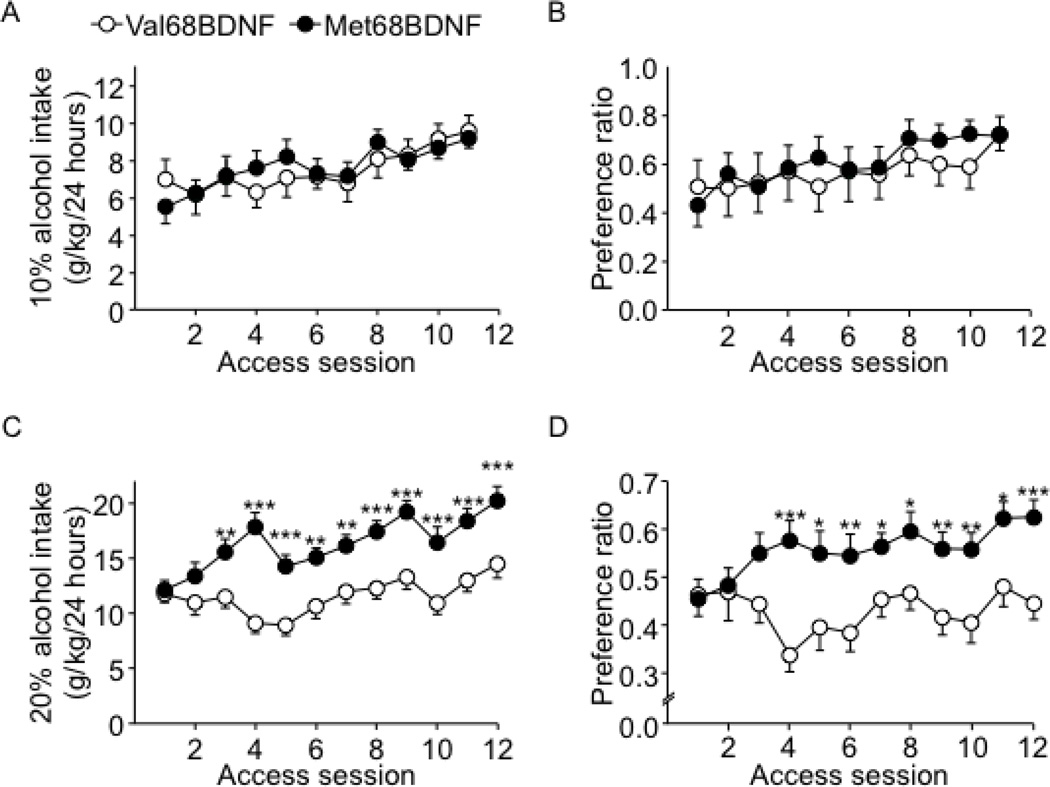
To examine whether the Met68BDNF polymorphism affects levels of alcohol consumption, we used two paradigms, which model human moderate and excessive alcohol drinking in mice. Moderate alcohol drinking is modeled by an intermittent access paradigm of 10% alcohol solution and excessive “pathological” drinking is modeled by an intermittent access paradigm of 20% alcohol solution (30). Specifically, wild-type Val68BDNF mice consumed 9.0±0.5 g/kg/24 hrs of 10% alcohol solution and 14.5±1.2 g/kg/24 hrs of 20% alcohol solution. We found that moderate levels of alcohol intake (Fig. 1A) and preference (Fig. 1B) for alcohol were similar in both genotypes (Fig. 1A; alcohol consumption: two-way RM-ANOVA, no main effect of genotype [F(1,36) = 0.08, P = 0.77], a main effect of session [F(10,360) = 4.18, P < 0.05] and no interaction genotype × session [F(10,360) = 0.34, P = 0.97], Fig. 1B; alcohol preference: two-way RM-ANOVA, no main effect of genotype [F(1,36) = 0.53, P = 0.47], a main effect of session [F(10,360) = 4.78, P < 0.001] and no interaction genotype × session [F(10,360) = 0.85, P = 0.58], Supplementary Fig. S4A). However, access to 20% alcohol solution resulted in a robust escalation of alcohol drinking (Fig. 1C) and preference (Fig. 1D) in Met68BDNF mice (Fig. 1C; alcohol consumption: two-way RM-ANOVA, a main effect of genotype [F(1,35) = 21.5, P < 0.001], a main effect of session [F(11,385) = 8.9, P < 0.001] and a significant interaction genotype × session [F(11,385) = 2.9, P = 0.001], Fig. 1D; preference across sessions: two-way RM-ANOVA, a main effect of genotype [F(1,35) = 11.44, P < 0.01], no main effect of session [F(11,385) = 1.99, P < 0.05] and a significant interaction genotype × session [F(11,385) = 2.26, P < 0.05], Supplementary Fig. S4B). These data suggest that the Met68BDNF polymorphism enhances excessive alcohol drinking.
Figure 1. Excessive, but not moderate, alcohol intake is exacerbated in Met68BDNF mice.
Met68BDNF and Val68BDNF mice underwent intermittent access to 10% (A–B) or 20% (C–D) alcohol 2-bottle choice paradigm for 24 hrs starting at noon on Mondays, Wednesdays, and Fridays for 4 weeks. Intake (g/kg/24 hrs) of a 10% (A) or 20% (C) alcohol solution. Preference for a 10% (B) or 20% (D) alcohol solution. Alcohol preference was calculated as a ratio of alcohol consumed relative to total fluid intake (alcohol + water). Results are expressed as mean ± SEM; *P < 0.05, **P < 0.01 and ***P < 0.001 compared to Val68BDNF mice during the same session of alcohol access, LSD post hoc test; (A–B) n=19 per genotype, (C–D) n=18–19 per genotype.
Met68BDNF mice exhibit compulsive alcohol drinking
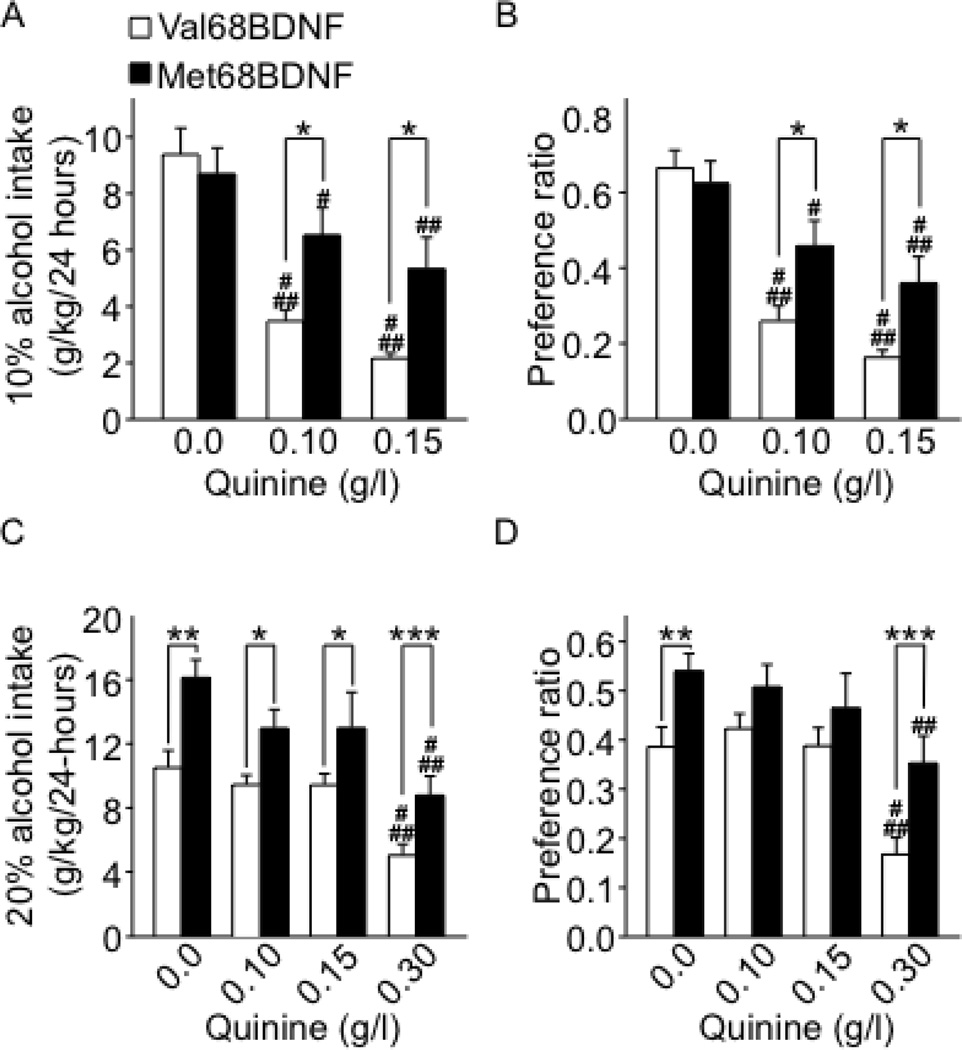
One major characteristic of alcohol abuse disorder is compulsive alcohol consumption, in which individuals are not able to control their drinking despite serious health and social consequences (2, 3, 5). To determine whether carrying the Met68BDNF polymorphism promotes compulsive alcohol drinking, we used an aversion-resistance paradigm in which alcohol is adulterated with the bitter-tasting substance, quinine, which is aversive to mice (31). If Met68BDNF mice continued to drink and prefer alcohol, despite quinine adulteration, this would indicate greater compulsive alcohol consumption despite the negative consequence of consuming an aversive substance (31). To test this hypothesis, 2 cohorts of mice were given 10% or 20% alcohol solution with the addition of quinine (Fig. 2 and Supplementary Figs. S5–S7). We found that intake and preference of quinine-adulterated 10% alcohol solution were significantly decreased in the wild-type Val68BDNF mice (Fig. 2A–B and Supplementary Fig. S5, LSD post hoc, Ps <0.05). In contrast, quinine was much less aversive to the Met68BDNF mice, which continued to consume large amounts of 10% alcohol solution despite quinine adulteration (Fig. 2A; quinine-adulterated 10% alcohol intake: two-way RM-ANOVA, no main effect of genotype [F(1,16) = 3.13, P = 0.09], a main effect of quinine concentration [F(2,32) = 35.9, P < 0.001], and a significant interaction genotype × quinine concentration [F(2,32) = 5.63, P < 0.01], Fig. 2B; quinine-adulterated alcohol preference: two-way RM-ANOVA, no main effect of genotype [F(1,16) = 3.65, P = 0.07], a main effect of quinine concentration [F(2,32) = 39.2, P < 0.001] and a significant interaction genotype × quinine concentration [F(2,32) = 4.62, P < 0.05]). Next, we tested the consequences of quinine adulteration on the consumption of and preference to a solution of 20% alcohol (Fig. 2C–D, and Supplementary Figs. S6–S7). We found that high dose of quinine (0.3g/L) reduced both intake and preference for 20% alcohol (P < 0.001) in a genotype-independent manner and that Met68BDNF mice showed higher 20% alcohol intake and preference than wild-type Val68BDNF mice (P < 0.001), independently of quinine adulteration (Fig. 2C; quinine-adulterated 20% alcohol intake: two-way RM-ANOVA, a main effect of genotype [F(1,20) = 18.05, P < 0.001], a main effect of quinine concentration [F(3,60) = 22.9, P < 0.001]. Fig. 2D; quinine-adulterated alcohol preference: two-way RM-ANOVA, a main effect of genotype [F(1,20) = 12.61, P <0.01], a main effect of quinine concentration [F(3,60) = 16.55, P < 0.001]). We further found that the reduction in quinine (0.3g/L) adulterated-20% alcohol preference (Supplementary Fig. S7B, method of contrasts, P<0.05) but not intake (Supplementary Fig. S7A, method of contrasts, P=0.34) was greater in the Val68BDNF than the Met68BDNF groups, indicating an aversion-resistant preference for 20% alcohol. Finally, the addition of quinine to the 10% or 20% alcohol solution did not affect the level of total fluid intake (alcohol, quinine and water) in either genotype (Supplementary Figs. S5C and S6C). Together, these findings suggest that the Val68 to Met68 substitution within the BDNF sequence produces greater levels of intake of and/or preference to alcohol despite negative consequences.
Figure 2. Met68BDNF mice consume high levels of alcohol despite the addition of quinine.
After 4 weeks of intermittent access to 10% (A–B) or 20% (C–D) alcohol, quinine was added to the alcohol solution in increasing concentrations (0.0–0.30 g/l; each concentration was maintained for 3 weeks before being increased). Intake (g/kg/24 hrs) of a 10% (A) or 20% (C) alcohol solution in the absence and presence of quinine. Preference for a 10% (B) or 20% (D) alcohol solution in the absence or presence of quinine was calculated as described in Figure 1. See also Supplementary Figure S7. Results are expressed as mean ± SEM, #P < 0.05, ##P < 0.01 and ###P < 0.001 compared to 0.0 g/l quinine in the same genotype; *P < 0.05 Met68BDNF mice compared to Val68BDNF at the same dose of quinine, method of contrasts; n=9–11 per genotype.
Compulsive alcohol drinking in Met68BDNF mice is specific to alcohol
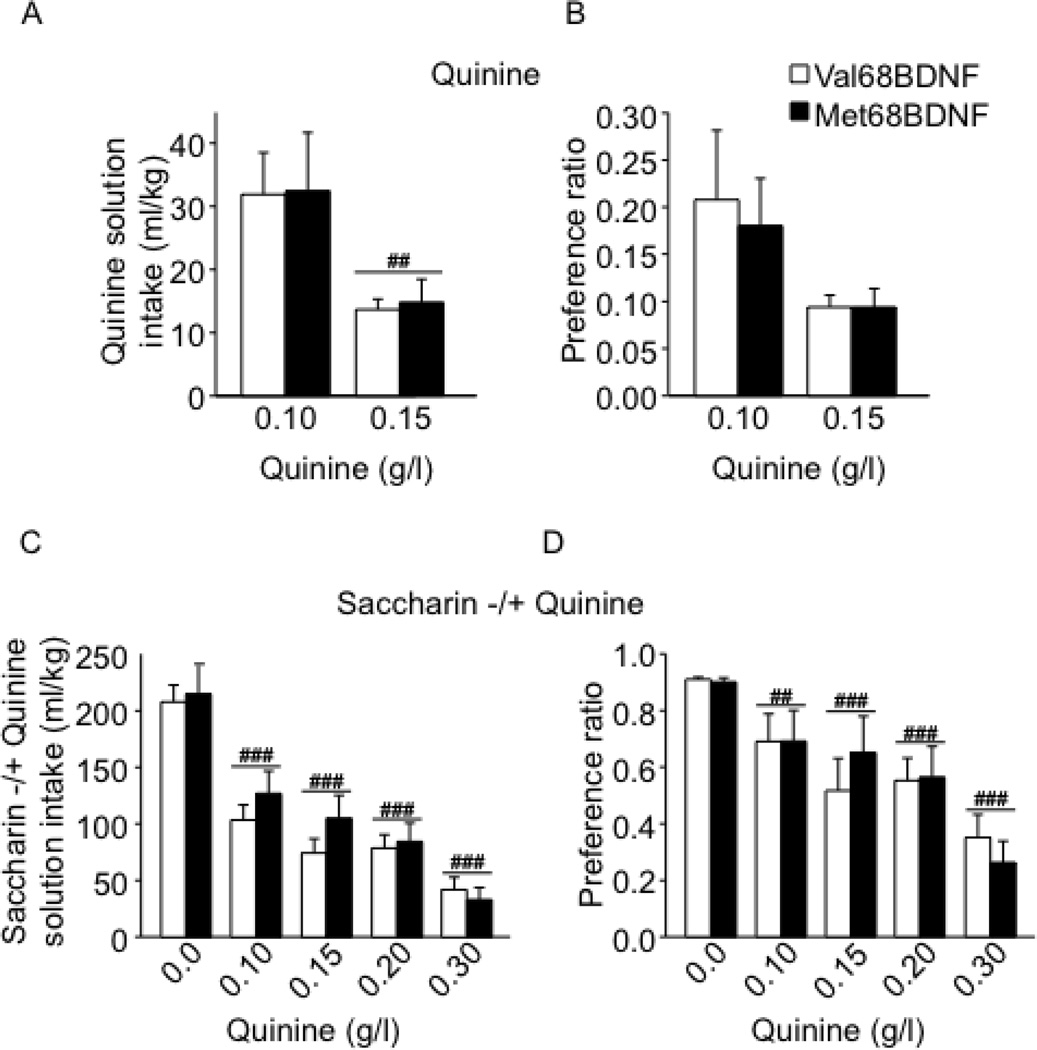
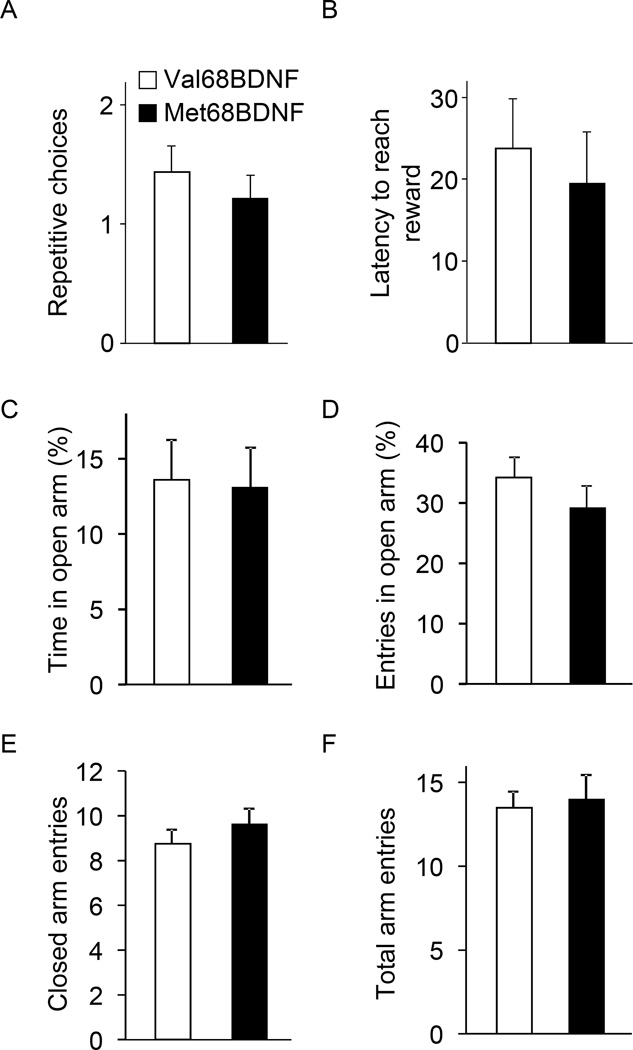
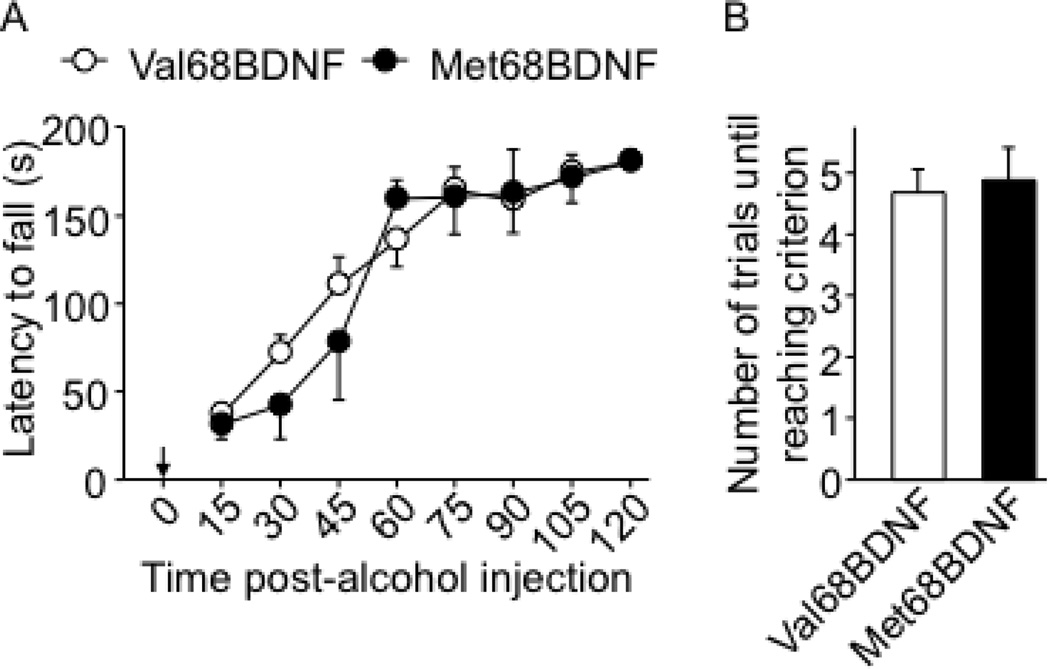
The aversion-resistant alcohol-drinking phenotype observed in the Met68BDNF mice could have multiple behavioral explanations. It could reflect differences in quinine taste reactivity, or reward sensitivity between groups, differences in compulsive behavior, differences in alcohol metabolism or differences in basal levels of anxiety. As shown in Fig. 3A–B and Supplementary Fig. S8, aversion to quinine did not differ between genotypes, suggesting that the two genotypes have similar quinine taste reactivity (Fig. 3A; quinine intake: two-way RM-ANOVA showed no main effect of the genotype on quinine solution drinking (F(1,12) = 0.01, P = 0.90), a significant main effect of concentration on the level of quinine intake (F(1,12) = 11.16, P = 0.05) and no significant interaction genotype × quinine concentration (intake, F(1,12) = 0.002, P = 0.96); and Fig. 3B; quinine preference: no main effect of the genotype F(1,12) = 0.09, P = 0.77). Furthermore, both genotypes exhibited the same levels of intake (Fig. 3C) preference (Fig. 3D) for a saccharin solution in the absence or presence of quinine, (Fig. 3C; saccharin consumption: two-way RM-ANOVA showed no main effect of the genotype on drinking of (F(1,12) = 0.49, P = 0.49), a significant main effect of quinine adulteration on saccharin intake (F(4.48) = 52.5, P < 0.001) and Fig. 3D; saccharin preference: no main effect of the genotype (F(1,12) = 0.01, P = 0.91), a significant main effect of quinine adulteration (F(4.48) = 16.2, P < 0.001) and no significant interaction genotype × quinine concentration (F(4.48) = 0.56, P = 0.69) and Supplementary Fig. S8B). To examine whether the Met68BDNF polymorphism promotes perseverance of general compulsive behavior, mice were tested in a T-maze paradigm using a paradigm in which the development of persistent entries into the same arm across multiple consecutive trials is considered to reflect inflexibility and a behavioral demonstration of compulsivity (32, 33). Notably, no differences in general compulsive-like behaviors were observed in Met68BDNF and Val68BDNF (Fig. 4A; repetitive choices: t-tests, T(16)= 0.67, P = 0.51, Fig. 4B; latency: T(16)= 0.44, P = 0.66). Basal anxiety levels measured by the elevated plus maze paradigm were also similar between groups (Fig. 4C; open arm time (%): t-tests, T(18)= 0.13, P = 0.59, Fig. 4D; open arm entries (%): T(18)= 0.99, P = 0.76; Fig. 4E; closed arm entries: T(18)= 0.89, P = 0.80, Fig. 4F; total arm entries: T(18)= 0.30, P = 0.50), and Met68BDNF and Val68BDNF exhibited the same level of ataxia in response to alcohol (Fig. 5A; latency to fall: two-way RM-ANOVA, no genotype effect [F(1,26) = 0.33, P = 0.57], a main effect of time [F(7,182) = 75.8, P < 0.001] and no interaction genotype × time [F(7,182) = 1.9, P = 0.07]), Fig. 5B; numbers of trials to reach criterion: t-tests, T(26)= −0.31, P = 0.76). Finally, blood alcohol concentrations (BAC) were 111.0 ± 5.5 mg/% and 109.8 ± 3.5 mg/% in Met68BDNF and Val68BDNF mice respectively, 90 min after systemic administration of 2.5 g/kg of alcohol (t-test, t(8)= −0.2, P = 0.85; n=5). Together, these results indicate that the enhanced compulsive drinking in Met68BDNF mice is specific for alcohol.
Figure 3. The Met68BDNF polymorphism does not alter quinine or quinine-adulterated saccharin intake.
(A–B) Met68BDNF and Val68BDNF mice underwent an intermittent access to quinine paradigm (0.10 and 0.15 g/kg, 2 bottle choice) for 24 hrs starting at 12:00 PM (Mondays, Wednesdays, and Fridays). Each quinine concentration was maintained for 2 weeks. Intake of (A) and preference for (B) for quinine. Results are expressed as mean ± SEM, ##P < 0.01 compared to 0.10 g/l quinine, LSD post hoc test; n=7 per genotype. (C–D) Mice had intermittent access to 0.03% saccharin (2 bottle choice) for 24 hrs starting at 12:00 PM (Mondays, Wednesdays, and Fridays). After 2 weeks, the saccharin solution was adulterated with quinine (0.10, 0.15, 0.20 and 0.30 g/l; concentrations were increased every 2 weeks). Intake of (A), and preference for (B) saccharin in the absence and presence of quinine. Results are expressed as mean ± SEM, ##P < 0.01 and ###P < 0.001 compared to 0.0 g/l quinine, LSD post hoc test; n=7 per genotype.
Figure 4. The Met68BDNF polymorphism does not alter general compulsive-like behavior and anxiety levels in mice.
To assess compulsive-like behavior, the inclination of mice to make an alternative arm-entry choice in a T-Maze was measured (32, 33). In this procedure, animals learn to associate each goal arm with access to a reward (chocolate milk solution). A persistent entry into the same arm across several consecutive trials reflects an inflexible strategy to obtain the reward and represents a behavioral demonstration of compulsivity. During the test, the alternation between the two goal arms and the latency to reach the reward for each trial were measured. (A) Number of repeated arm entries before alternation designated as the first entry into the opposite goal arm). (B) Latency to reach the reward. (C–F) Anxiety-like behaviors were measured in the elevated plus maze (EPM). (C) Percentage of time spent in the open arms and (D) number of visits to the open arms. (E–F) Locomotor activity was measured by the number of entries to closed arm (E) and the total arm entries (F). Results are expressed as mean ± SEM; (A–B) n = 9 per genotype, (C–F) n=8–12 per genotype.
Figure 5. Met68BDNF polymorphism does not affect the sensitivity to ataxic effects of alcohol.
Mice were trained for 2 consecutive days to perform the rotarod task (Supplementary Figure S3C), and on the third day mice were given a systematic administration of 1.5 g/kg alcohol and placed on the rod every 15 minutes for 2 hrs. Arrow indicates the time point of the alcohol injection. (A) Latency to fall. (B) Number of trials necessary to reach the criterion of 180 seconds on the rod without falling. Results are expressed as mean ± SEM; n = 11–17 per genotype.
Overexpression of Val68BDNF in the ventral medial prefrontal cortex of Met68BDNF mice reduces compulsive alcohol drinking
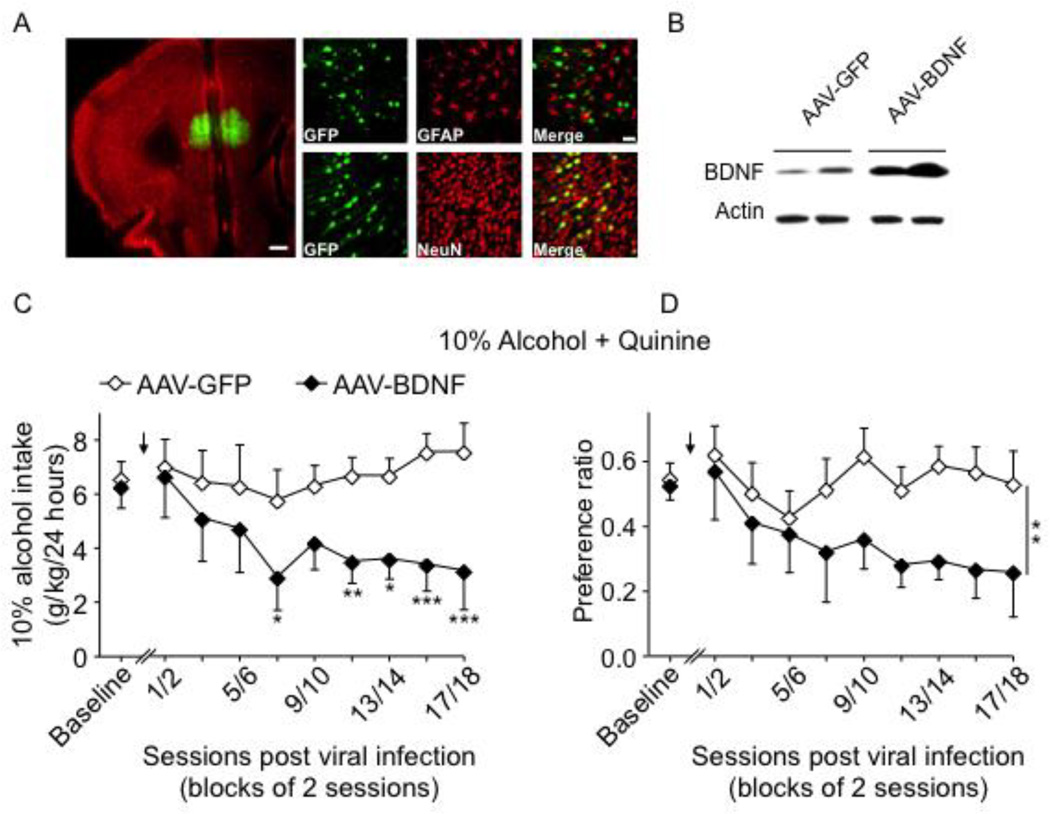
We previously demonstrated that the BDNF/TrkB signaling pathway in the dorsal striatum of rodents controls alcohol intake (14, 16, 17, 19, 34). The major source of BDNF in the dorsal striatum is the PFC (35, 36), and we found that the consumption of high levels of alcohol is associated with low Bdnf expression in the PFC of mice (10, 11). Met68BDNF polymorphism reduces the activity dependent release of BDNF leading to a deficit in TrkB signaling (20, 21). The ventromedial part of the PFC (vmPFC) is one of the major brain regions involved in compulsive drug and alcohol drinking and seeking (37–39). Therefore, if the deficit in BDNF function in the PFC of Met68BDNF mice is responsible for the development of quinine-resistant drinking, then overexpressing wild-type Val68BDNF in this brain region will decrease quinine-resistant drinking in the Met68BDNF KI mice. Adeno-associated virus (AAV) expressing the Val68BDNF protein and a green fluoresence reporter (GFP) (AAV-BDNF) infected vmPFC neurons of Met68BDNF mice (Fig. 6A), which produced high levels of BDNF expression as compared with animals infected with the GFP control virus (AAV-GFP; Fig. 6B). vmPFC of Met68BDNF mice was then infected with AAV-BDNF or AAV-GFP and consumption of quinine-adultarated 10% alcohol solution was measured (Supplementary Fig. S9A: Timeline). As shown in Fig.6C–D, compulsive alcohol intake despite quinine-adulteration was observed in Met68BDNF mice infected with the control AAV-GFP. In contrast, overexpression of the wild-type Val68BDNF in the vmPFC of Met68BDNF mice dramatically reduced intake of and preference for the quinine-adulterated alcohol solution (Fig. 6C; quinine-adulterated alcohol consumption: (two-way RM-ANOVA, main effect of BDNF overexpression [F(1,19) = 10.75, P < 0.01], no main effect of session block [F(9,171) = 1.81, P = 0.07] and a significant interaction BDNF overexpression × session [F(9,171) = 1.93, P = 0.05]), Fig. 6D; quinine-adulterated alcohol preference: two-way RM-ANOVA, main effect of BDNF overexpression [F(1,19) = 10.85, P < 0.01], no main effect of session block [F(9,171) = 1.99, P < 0.05] and no significant interaction BDNF overexpression × session [F(9,171) = 1.36, P = 0.21], LSD post hoc, Ps <0.05), without affecting total fluid intake (Supplementary Fig. S10). As there are no compensatory alterations in TrkB levels in the mPFC and striatal regions of Met68BDNF mice (Supplementary Fig. S11), our findings strongly suggest that restoring BDNF function in the vmPFC of the Met68BDNF mice adjusts alcohol-drinking behaviors to non-compulsive, moderate levels.
Figure 6. Overexpression of Val68BDNF in the vmPFC reduces consumption of quinine-adulterated alcohol by Met68BDNF mice.
(A–B) Infection of vmPFC of the Met68BDNF mice with AAV overexpressing the wildtype Val68BDNF gene (AAV-BDNF). (A) AAV-BDNF infected neurons. AAV-BDNF (1 × 1012 TU/ml) was bilaterally infused and the brains were fixed for the immunochemistry 3 weeks after the viral infusion. The left-hand panel depicts the specific, regionally constrained injection site. Slices were co-stained with anti-GFP (green) and anti-NeuN (red) antibodies. Scale bar: 500µm. Right-hand panels are representative images of GFP (green) co-stained with either GFAP (red; top panels) or NeuN (red; bottom panels), showing localization of viral infection within neurons but not GFAP-positive astrocytes. Scale bar: 50µm. (B) AAV-BDNF increases BDNF expression in the vmPFC of Met68BDNF mice. Western blot analysis was conducted 3 weeks after the viral infusion. The levels of BDNF were assessed using anti-BDNF antibody. Actin immunoreactivity was used as an internal loading control. (C–D) Met68BDNF mice undergoing an intermittent access to 10% alcohol containing 0.10 g/l quinine were bilaterally infused into the vmPFC with either AAV-BDNF or the AAV-GFP control. After 5 days of recovery, access to the alcohol+quinine solution was resumed. Arrows indicates the time point of the intra-vmPFC infusion of the viruses. Alcohol intake (g/kg/24 hrs) (C), and preference for the alcohol solution (D) were recorded for 6 weeks. Results are expressed as mean ± SEM; *P < 0.05, **P < 0.01 and ***P < 0.001 compared to AAV-GFP, LSD post hoc test; n=10–11 per group.
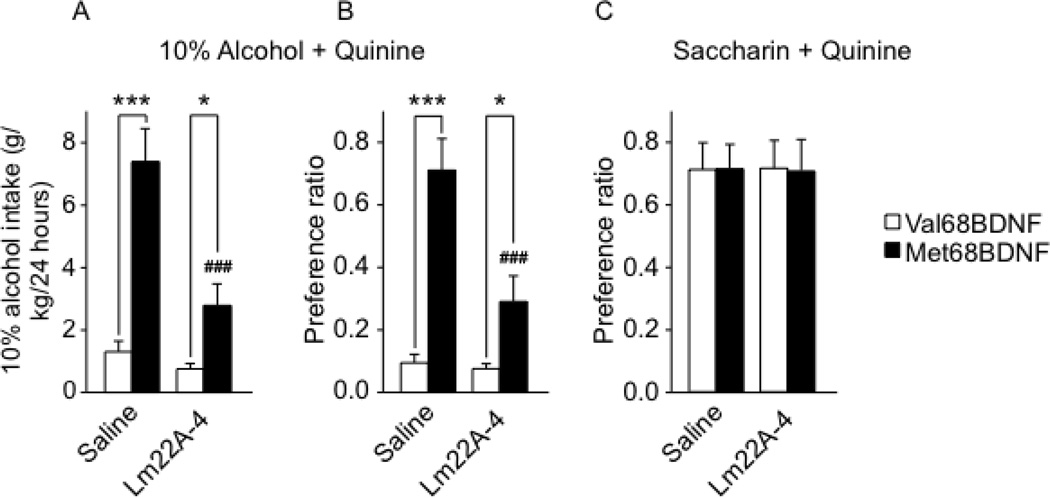
Administration of a TrkB agonist, LM22A-4, reduces compulsive alcohol drinking in Met68BDNF mice
Since restoring BDNF function in the vmPFC of Met68BDNF mice successfully suppressed compulsive alcohol consumption, we sought to determine whether a translational tractable method to enhance BDNF signaling would also suppress compulsive drinking. We examined whether intake of quinine-adulterated alcohol solution can be reduced by systemic administration of the TrkB activator, LM22A-4 (40). In Met68BDNF and Val68BDNF mice undergoing intermittent access to 10% alcohol adulterated with 0.10 g/l quinine, LM22A-4 (100 mg/kg) or saline were administered systemically, and intake was recorded at the end of a 24-hr drinking session (Supplementary Figs. S9B; S12A–B). As shown in Fig. 7A–B, and similar to the results shown in Fig. 2A–B, saline-treated Val68BDNF mice consumed a low level of quinine-adulterated alcohol solution, whereas saline-treated Met68BDNF mice exhibited compulsive alcohol drinking behavior (Fig. 7A; alcohol consumption: two-way RM-ANOVA, main effect of genotype [F(1,14) = 30.0, P < 0.001], of LM22A-4 treatment [F(1,14) = 20.4, P < 0.001] and a significant interaction genotype × LM22A-4 treatment [F(1,14) = 12.6, P < 0.01]), Fig. 7B; alcohol preference: two-way RM-ANOVA, main effect of genotype [F(1,14) = 29.6, P < 0.001], of LM22A-4 treatment [F(1,14) = 15.7, P < 0.001] and a significant interaction genotype × LM22A-4 treatment [F(1,14) = 13.1, P < 0.01). Systemic administration of LM22A-4 significantly reduced both intake and preference of quinine-adulterated alcohol solution in Met68BDNF mice (Fig. 7A–B, LSD post hoc, Ps <0.05), without affecting total fluid intake (Supplementary Fig. S13A). Importantly, this effect was specific to alcohol, as LM22A-4 did not alter the consumption of saccharin/quinine solution tested in an independent cohort of naive mice (Fig. 7C; two-way RM-ANOVA, [F(1,12) = 0.001, P = 0.97]), Supplementary Fig. S9C, S12C–D and S13B). Together, our results demonstrate that activation of TrkB prevents compulsive drinking in Met68BDNF mice.
Figure 7. Activating the TrkB signaling pathway reduces consumption of quinine-adulterated alcohol by Met68BDNF mice.
Mice undergoing an intermittent access (2-bottle choice) to 10% alcohol/0.10 g/l quinine solution (see Supplementary Figure S12 A–B for basal level of alcohol drinking with or without quinine and Supplementary Fig. S12 C–D for basal level of saccharin drinking with our without quinine) received i.p. administration of 100 mg/kg LM22A-4 or saline immediately before the beginning of the drinking session. Intake of alcohol (g/kg/24 hrs) (A) or saccharin (ml/kg/24 hrs) (C). Preference for the alcohol+quinine (B) or saccharin+quinine (D) solution. Results are expressed as mean ± SEM; ###P < 0.001 compared to Met68BDNF mice receiving saline, *P < 0.05 and ***P < 0.001; LSD post hoc test; (A–B) n=8 per genotype, (C) n=7 per genotype.
DISCUSSION
In this study we demonstrate that the mouse homolog of the human Met66BDNF polymorphism (Met68BDNF) is associated with the development of excessive “pathological” and compulsive alcohol drinking. Specifically, we show that compared to Val68BDNF wild-type mice, Met68BDNF mice consumed higher levels of alcohol in an intermittent access to 20% alcohol, a model of uncontrolled drinking. Furthermore, Met68BDNF mice consumed a solution of 10% and 20% alcohol even when the solution was adulterated with quinine, indicating the Met68BDNF carriers compulsively drink alcohol despite aversive consequences. We further demonstrate that overexpression of the wild-type Val68BDNF allele in the vmPFC or systemic administration of the TrkB agonist LM22A-4 reduced compulsive (quinine-adulterated) alcohol drinking in Met68BDNF mice.
Alcohol intake that persists despite adverse consequences in rats and mice is considered to model some key aspects of compulsive alcohol drinking in humans (31), and key finding from this study is that alcohol intake and preference of 10% alcohol in an intermittent access paradigm is maintained in the Met68BDNF mice even after the addition of aversive, bitter-tasting quinine, which produces a strong aversive reaction in rodents (41). The aversion-resistant alcohol consumption exhibited by the Met68BDNF mice was less evident when 20% alcohol was adulterated with quinine. In particular, preference but not intake of quinine-adulterated 20% alcohol was higher in the Met68BDNF than the wild-type Val68BDNF mice. Nevertheless, the latter finding brings additional evidence supporting the association between Met68BDNF polymorphism and compulsive alcohol use (e.g. consumption despite negative consequences). In addition to the compulsive drinking aspect, it is possible that the increase in non-adulterated 20% alcohol consumption associated with Met68BDNF polymorphism may be due to an enhanced sensitivity to the rewarding properties of alcohol. Another hypothesis would be that Met68BDNF polymorphism reduces the sensitivity to the negative emotional-like state of alcohol withdrawal. Indeed, perpetuation of drug abuse is thought to be driven by positive and negative sources of reinforcement, with the positive reinforcement being related to the rewarding properties of the drug it-self and the negative reinforcement referring to drug intake alleviating the negative emotional state of withdrawal (42). Additional experiments are needed to disentangle the involvement of the positive and negative reinforcements in the enhanced consumption of 20% alcohol displayed by the Met68BDNF mice.
Further studies are needed to address the mechanism(s) that underlie compulsive alcohol drinking in subjects carrying the Met66BDNF allele. An intriguing possibility is that a reduction in BDNF signaling in corticostriatal circuitries underlies the excessive uncontrolled drinking observed in the Met68BDNF mice. This possibility is supported by the current finding, showing that compulsive alcohol drinking phenotypes can be rescued by overexpression of the Val68BDNF allele in the vmPFC of the Met68BDNF mice. The vmPFC controls the transition from moderate to excessive, uncontrolled alcohol drinking (31, 38, 41, 43), and low neuronal activity in the vmPFC is also associated with compulsive craving and relapse to alcohol in humans (39). Importantly, the Met66/68BDNF polymorphism in both humans and mice is associated with abnormalities in the vmPFC, including morphological alterations (44–46) and reduced neuronal activity (46–48). The possibility that BDNF disregulation in the mPFC promotes the development of excessive alcohol drinking is also supported by recent data showing that prolonged alcohol intake in mice is associated with low cortical Bdnf expression (10) and including the mPFC (11), which is mediated by microRNA-206 and microRNA-30a-5p to promote excessive alcohol intake (11, 49).
Another intriguing possibility stems from a recent human study showing that the Met66BDNF carriers exhibit higher neural activity in the mesocorticolimbic pathway than Val66BDNF homozygotes in response to an anticipated reward (50). The authors further reported that the Met66BDNF carriers have a high D2/D3 dopamine receptor ratio dopamine release in the nucleus accumbens (50). Thus, it is plausible that the excessive and compulsive alcohol dinking observed in Met68BDNF mice could be the result of alterations in the dopaminergic mesocorticolimbic pathway.
Secreted ProBDNF (51) and BDNF may produce opposite biological functions by signaling through p75NTR and TrkB, respectively (52–55). BDNF/TrkB signaling mediates neuronal proliferation, differentiation and survival (52), and induces late long-term potentiation (L-LTP) in the hippocampus (53), whereas proBDNF/p75NTR signaling induces neuronal death (54) and enhances hippocampal long-term depression (LTD) (55). Recently, Anastasia et al. demonstrated that the Met66proBDNF binds more effectively to the p75NTR/sortilin complex receptor than Val66proBDNF (56). Since BDNF/TrkB signaling reduces alcohol intake (12–14, 16–19, 34), it is tempting to speculate that activation of proBDNF/p75NTR pathway may have an opposite effect and could induce alcohol drinking, and that the Met68BDNF polymorphism may promote excessive and compulsive alcohol drinking through the activation of p75NTR signaling.
Our data showing that Met68BDNF mice continue to drink quinine-adulterated alcohol solution despite its bitter taste differs from previous studies indicating that Met68BDNF polymorphism promotes a deficit in extinction of aversive memories (45, 48). However, compulsive alcohol drinking is likely to be driven by other processes such as the devaluation of an adverse consequence over the desire to consume alcohol (31) and/or to habitual learning (57, 58). Finally, it is important to note that the Met68BDNF mice used in Yu et al., and Soliman et al., (45, 48) and our mice were generated separately. More studies need to be conducted to confirm that all phenotypes can be observed in both lines of transgenic knock-in mice.
The Met66BDNF allele is a common polymorphism in the human population (22). Therefore, a large group of individuals could be susceptible to developing harmful alcohol drinking. Other SNPs have previously been the vulnerability and severity of alcohol abuse disorder (59–64). Together, these data suggest that different SNPs in humans could lead to the development of different patterns of alcohol abuse disorders. This study, in conjunction with a recent study in mice carrying the respective human OPRM1 A118G alleles (65), demonstrates the potential of a mouse knock-in approach towards alcohol research.
We used a translational approach to restore BDNF signaling and adjust the level of alcohol intake in mice carrying the SNP by systemically administrating, LM22A-4, a new compound developed to mimic the action of BDNF. This compound selectively binds and activates the BDNF receptor, TrkB, and initiates its intracellular signaling pathways in vitro and in vivo (40, 66–68). Previous studies showed that LM22A-4 activates ERK signaling via TrkB (67, 69, 70) and that BDNF reduced alcohol drinking via the activation of ERK signaling (19). Thus, it is highly likely that the TrkB agonist, bypassing BDNF, restores BDNF signaling in the Met68BDNF mice. Our lab has previously showed that downstream effector genes of BDNF, prodynorphin (34) and dopamine D3 receptor (16) in the dorsal striatum contribute to mechanisms by which BDNF reduces alcohol drinking. Thus, it is plausible that LM22A-4 decreases compulsive alcohol drinking by promoting the expression of these and/or other yet unidentified downstream effectors.
LM22A-4 treatment reduces neuronal death (40) and promotes neurogenesis (71). Furthermore, LM22A-4 shows beneficial effects in rodent models of traumatic brain injury (40), stroke (71), Rett syndrome (69), and Huntington's disease (70). Importantly, from a therapeutic perspective, we demonstrated that LM22A-4 does not affect quinine-adulterated saccharin drinking, suggesting that LM22A-4 effects are specific to alcohol and do not produce a general effect on motivation. This contrasts with current FDA-approved drugs (Acamprosate, Naltrexone) used to treat alcohol addiction, which reduce water, food, and sucrose intake (72–74) resulting in compliance issues due to anhedonia associated with these drugs (75).
In summary, our results suggest that the Met68BDNF polymorphism is associated with increased propensity to develop excessive and compulsive alcohol drinking. Reduced BDNF function may represent a risk factor that may promote the development of severe alcohol abuse phenotypes, including compulsive drinking. Our results further demonstrate that overcoming the deficits in BDNF function via the administration of the TrkB agonist, LM22A-4, is a plausible therapeutic approach to treat the disease. Furthermore, our studies emphasize the crucial role for genetic predisposition in the mechanisms leading to harmful excessive alcohol drinking, and highlight the importance of a patient’s genotype when developing strategies for the treatment and prevention of alcohol use and abuse disorders.
Supplementary Material
ACKNOWLEDGMENTS
We thank Wenheng Zhu, Stuart L. Gibb, Segev Barak, Quinn Yowell, Feng Liu, Rachel Jurd and Marian Logrip for technical assistance, and Samuel Sakhai, Somayeh Ahmadiantehrani, Virginia Long and Frederic W. Hopf for reading the manuscript. This work was supported by NIH-NIAAA RO1 AA016848 (D.R.), NIAAA R37 AA016848 (D.R.), NIH-NIAAA P50 AA017072 (D.R.), by the State of California (D.R.), the Jean Perkins Foundation (F.L.), and VA Merit award I01BX000267 (S.M.M).
Drs. Longo and Massa are listed as inventors on patents relating to a compound in this report, which are assigned to the University of North Carolina, University of California, San Francisco and the Dept. of Veterans Affairs. Drs. Longo and Massa are entitled to royalties distributed by the assigned universities per their standard agreements. Dr. Longo is a principal of, and has financial interest in PharmatrophiX, a company focused on the development of small molecule ligands for neurotrophin receptors, which has licensed several of these patents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTION
D.R. conceived the project. V.W. E.D. and D.R. designed the experiments. V.W., E.D., K.P. and N.M. performed the experiments. V.W. and E.D. analyzed the data. S.M.M. and F.M.L. provided the TrkB agonist. V.W, E.D and D.R. wrote the paper. All authors approved the final manuscript.
CONFLICT OF INTEREST
Drs. Ron, Darcq, Morisot, Wilbrecht and Warnault and Mr. Phamluong report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature reviews Neuroscience. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Arlington, VA: American Psychiatric Association; 2013. American Psychiatric Association. DSM-5 Task Force. [Google Scholar]
- 4.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedmann PD. Alcohol use in adults. The New England journal of medicine. 2013;368:1655–1656. doi: 10.1056/NEJMc1302445. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Global status report on alcohol and health. Geneva, Switzerland: World Health Organization; 2014. Management of Substance Abuse Team. [Google Scholar]
- 7.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, et al. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcoholism, clinical and experimental research. 2007;31:1833–1838. doi: 10.1111/j.1530-0277.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 9.Zanardini R, Fontana A, Pagano R, Mazzaro E, Bergamasco F, Romagnosi G, et al. Alterations of brain-derived neurotrophic factor serum levels in patients with alcohol dependence. Alcoholism, clinical and experimental research. 2011;35:1529–1533. doi: 10.1111/j.1530-0277.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 10.Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009;109:1459–1468. doi: 10.1111/j.1471-4159.2009.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darcq E, Warnault V, Phamluong K, Mercado Besserer G, Feng L, Ron D. MicroRNA-30a-5p in the Prefrontal Cortex Controls the Transition from Moderate to Excessive Alcohol Consumption. Mol Psychiatry. 2014 [Google Scholar]
- 12.Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcoholism, clinical and experimental research. 2008;32:909–920. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- 13.Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J Neurochem. 2003;85:1139–1147. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- 14.McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain research. 2015 doi: 10.1016/j.brainres.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, et al. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26:1457–1464. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeanblanc J, Logrip ML, Janak PH, Ron D. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. The European journal of neuroscience. 2013;37:607–612. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 2010;15:810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- 24.Rakofsky JJ, Ressler KJ, Dunlop BW. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol Psychiatry. 2012;17:22–35. doi: 10.1038/mp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol Psychiatry. 2014;19:8–10. doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]
- 26.Duncan JR. Current perspectives on the neurobiology of drug addiction: a focus on genetics and factors regulating gene expression. ISRN neurology. 2012;2012:972607. doi: 10.5402/2012/972607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwald MK, Steinmiller CL, Sliwerska E, Lundahl L, Burmeister M. BDNF Val(66)Met genotype is associated with drug-seeking phenotypes in heroin-dependent individuals: a pilot study. Addiction biology. 2013;18:836–845. doi: 10.1111/j.1369-1600.2011.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biskupska J, Borowiak KS, Karlin-Grazewicz K, Janus T, Waloszczyk P, Potocka-Banas B, et al. Estimation of BDNF gene polymorphism and predisposition to dependence development for selected psychoactive compounds: genetic aspects of addiction with the selected drugs, amphetamine, tetrahydrocannabinol and opiates. Human & experimental toxicology. 2013;32:236–240. doi: 10.1177/0960327112459203. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Wu J, Wang J, Zhao S, Li Z, Kong L, et al. WebLab: a data-centric, knowledge-sharing bioinformatic platform. Nucleic acids research. 2009;37:W33–W39. doi: 10.1093/nar/gkp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol. 2014;48:253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadin E, Friedman E, Bridger WH. Spontaneous alternation behavior: an animal model for obsessive-compulsive disorder? Pharmacology, biochemistry, and behavior. 1991;40:311–315. doi: 10.1016/0091-3057(91)90559-k. [DOI] [PubMed] [Google Scholar]
- 33.Albelda N, Joel D. Animal models of obsessive-compulsive disorder: exploring pharmacology and neural substrates. Neuroscience and biobehavioral reviews. 2012;36:47–63. doi: 10.1016/j.neubiorev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. Faseb J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- 35.Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 38.George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcoholism, clinical and experimental research. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biological psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012;32:2410–2421. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, et al. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci. 2014;34:4581–4588. doi: 10.1523/JNEUROSCI.0445-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecina M, Martinez-Jauand M, Love T, Heffernan J, Montoya P, Hodgkinson C, et al. Valence-specific effects of BDNF Val66Met polymorphism on dopaminergic stress and reward processing in humans. J Neurosci. 2014;34:5874–5881. doi: 10.1523/JNEUROSCI.2152-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, et al. Neuronal release of proBDNF. Nature neuroscience. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andero R, Choi DC, Ressler KJ. BDNF-TrkB Receptor Regulation of Distributed Adult Neural Plasticity, Memory Formation, and Psychiatric Disorders. Progress in molecular biology and translational science. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- 53.Panja D, Bramham CR. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology. 2014;76(Pt C):664–676. doi: 10.1016/j.neuropharm.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 54.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature neuroscience. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 56.Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nature communications. 2013;4:2490. doi: 10.1038/ncomms3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behavioural brain research. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 58.Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Current opinion in neurobiology. 2013;23:564–572. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 59.Munoz X, Amiano P, Celorrio D, Dorronsoro M, Sanchez MJ, Huerta JM, et al. Association of alcohol dehydrogenase polymorphisms and life-style factors with excessive alcohol intake within the Spanish population (EPIC-Spain) Addiction. 2012;107:2117–2127. doi: 10.1111/j.1360-0443.2012.03970.x. [DOI] [PubMed] [Google Scholar]
- 60.Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 61.Mottagui-Tabar S, Prince JA, Wahlestedt C, Zhu G, Goldman D, Heilig M. A novel single nucleotide polymorphism of the neuropeptide Y (NPY) gene associated with alcohol dependence. Alcoholism, clinical and experimental research. 2005;29:702–707. doi: 10.1097/01.alc.0000164365.04961.b1. [DOI] [PubMed] [Google Scholar]
- 62.Frances F, Guillen M, Verdu F, Portoles O, Castello A, Sorli JV, et al. The 1258 G>A polymorphism in the neuropeptide Y gene is associated with greater alcohol consumption in a Mediterranean population. Alcohol. 2011;45:131–136. doi: 10.1016/j.alcohol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Clarke TK, Dempster E, Docherty SJ, Desrivieres S, Lourdsamy A, Wodarz N, et al. Multiple polymorphisms in genes of the adrenergic stress system confer vulnerability to alcohol abuse. Addiction biology. 2012;17:202–208. doi: 10.1111/j.1369-1600.2010.00263.x. [DOI] [PubMed] [Google Scholar]
- 64.Ray LA, Bujarski S, MacKillop J, Courtney KE, Monti PM, Miotto K. Subjective response to alcohol among alcohol-dependent individuals: effects of the mu-opioid receptor (OPRM1) gene and alcoholism severity. Alcoholism, clinical and experimental research. 2013;37(Suppl 1):E116–E124. doi: 10.1111/j.1530-0277.2012.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH, et al. A Pharmacogenetic Determinant of Mu-Opioid Receptor Antagonist Effects on Alcohol Reward and Consumption: Evidence from Humanized Mice. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 66.Al-Qudah M, Anderson CD, Mahavadi S, Bradley ZL, Akbarali HI, Murthy KS, et al. Brain-derived neurotrophic factor enhances cholinergic contraction of longitudinal muscle of rabbit intestine via activation of phospholipase C. American journal of physiology Gastrointestinal and liver physiology. 2014;306:G328–G337. doi: 10.1152/ajpgi.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kajiya M, Takeshita K, Kittaka M, Matsuda S, Ouhara K, Takeda K, et al. BDNF mimetic compound LM22A-4 regulates cementoblast differentiation via the TrkB-ERK/Akt signaling cascade. International immunopharmacology. 2014;19:245–252. doi: 10.1016/j.intimp.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 68.Todd D, Gowers I, Dowler SJ, Wall MD, McAllister G, Fischer DF, et al. A monoclonal antibody TrkB receptor agonist as a potential therapeutic for Huntington's disease. PloS one. 2014;9:e87923. doi: 10.1371/journal.pone.0087923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmid DA, Yang T, Ogier M, Adams I, Mirakhur Y, Wang Q, et al. A TrkB Small Molecule Partial Agonist Rescues TrkB Phosphorylation Deficits and Improves Respiratory Function in a Mouse Model of Rett Syndrome. J Neurosci. 2012;32:1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simmons DA, Belichenko NP, Yang T, Condon C, Monbureau M, Shamloo M, et al. A small molecule TrkB ligand reduces motor impairment and neuropathology in R6/2 and BACHD mouse models of Huntington's disease. J Neurosci. 2013;33:18712–18727. doi: 10.1523/JNEUROSCI.1310-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han J, Pollak J, Yang T, Siddiqui MR, Doyle KP, Taravosh-Lahn K, et al. Delayed administration of a small molecule tropomyosin-related kinase B ligand promotes recovery after hypoxic-ischemic stroke. Stroke; a journal of cerebral circulation. 2012;43:1918–1924. doi: 10.1161/STROKEAHA.111.641878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Escher T, Mittleman G. Schedule-induced alcohol drinking: non-selective effects of acamprosate and naltrexone. Addiction biology. 2006;11:55–63. doi: 10.1111/j.1369-1600.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 73.Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.