Abstract
Introduction
Cancer anorexia-cachexia syndrome (CACS) is associated with increased morbidity and mortality. Anamorelin is a novel, orally active ghrelin receptor agonist in clinical development for the treatment of CACS in non-small cell lung cancer (NSCLC). The aim of this review is to summarize preclinical and clinical studies evaluating anamorelin as a potential promising treatment for CACS in NSCLC.
Area covered
Pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety, and tolerability of anamorelin for the treatment of CACS in NSCLC were reviewed. Anamorelin administration may lead to increases in food intake, body weight and lean body mass, and a stimulatory effect on GH secretion in NSCLC patients. Anamorelin is well tolerated with no dose-limiting toxicities identified to date.
Expert opinion
Targeting ghrelin receptors presents the advantage of potentially addressing multiple mechanisms of CACS simultaneously including appetite, muscle protein balance, adipose tissue metabolism, energy expenditure and inflammation. Clinical data suggest that anamorelin is well tolerated and it effectively increases appetite, body weight and lean mass in patients with advanced NSCLC. Long-term safety remains unknown at this time. The potential synergistic effects of anamorelin with nutritional support or exercise as well as its efficacy/safety in other tumor types are also unknown.
Keywords: Anamorelin, cancer-anorexia-cachexia syndrome, ghrelin, non-small cell lung cancer
1. Introduction
Cancer anorexia-cachexia syndrome (CACS) has been recognized as an adverse consequence of cancer and its treatments and remains a challenging clinical syndrome. CACS is defined as a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (+/− fat loss) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment1. The weight loss criteria recommended is >5% over the previous six months or >2% in individuals already showing depletion according to current body-mass index (<20 kg/m2) or skeletal muscle mass (sarcopenia)1.
The incidence of CACS depends on the tumor type and ranges from 16% to over 50%2, 3 being responsible for more than 30% of cancer-related deaths3. CACS is associated with poor quality of life (QoL), tolerance and response to anticancer therapy, and survival4, 5. Lung cancer is a leading cause of cancer death worldwide and non-small cell lung cancer (NSCLC) is the most common type of lung cancer. Despite recent advance in the treatment of NSCLC, the 5-year survival rate for patients with metastatic disease remains less than 20%6, 7. Approximately 60% of lung cancer patients show significant weight loss at the time of diagnosis, and more than 10% of patients die from CACS itself3. CACS and skeletal muscle wasting are commonly seen in NSCLC patients at baseline and are strongly associated with poor survival8, 9. Currently, the exact mechanisms underlying death due to cachexia has not been well-studied; however, these may include diaphragmatic muscle dysfunction10 and poor nutritional status11. Diaphragmatic muscle weakness is associated with respiratory failure. Poor nutrition can lead to reduced immunity and increased susceptibility to infection.
The pathophysiology of CACS is characterized by a negative protein and energy balance driven by a variable combination of reduced food intake and hypercatabolism caused by systemic inflammation, tumor metabolism directly, and/or other tumor-mediated effects. Insulin resistance, prolonged high dose-corticosteroid therapy and hypogonadism may also contribute to catabolism12.
The European Palliative Care Research Collaboration (EPCRC) treatment guidelines13 recommend the treatment goal for cachexia should be the reversal of the loss of body weight and muscle mass through a multimodal approach. This includes detailed assessment and repeated monitoring, nutritional support, anti-inflammatory treatment, treatment of secondary gastrointestinal symptoms and other causes for decreased oral intake as well as evaluation of anti-neoplastic options to reduce the catabolic drive of the cancer. However, current treatment approaches for CACS are limited as there are no standard effective treatments for this condition.
1.1 Overview of current treatments
Treatment goals in CACS include improvements in appetite, lean body mass, resting energy expenditure, quality of life (QoL), performance status and inflammation14, 15. Adequate nutrition is essential in the treatment of these patients to ensure that malnutrition is not contributing to CACS even though patients do not appear to benefit from nutritional supplementation alone16, 17. Corticosteroids and progestins, such as megestrol acetate, are the most widely used off-label treatment options and appear to stimulate appetite and increase fat mass, only partially alleviating CACS. Corticosteroids use is recommended for periods of only up to 2 weeks due to side effects, which include deterioration of muscle strength3. Recent evidence has also suggested a role for insulin resistance in CACS; insulin treatment has been found to potentially play a palliative role in CACS18. Drugs with a strong rationale that have not demonstrated consistent and convincing efficacy in clinical trials include melatonin19, eicosapentaenoic acid20, cannabinoids21, bortezomib22 and anti-cytokine therapies including thalidomide23, and an anti-TNF-alpha monoclonal antibody (infliximab)22. The selective androgen receptor modulator (SARM) enobosarm showed good results in a phase 2 study24 but failed to consistently improve the primary endpoints (lean body mass and stair climbing power) in phase 3 studies25. Several other targeted therapies are in clinical development. These include anti-IL-6 antibodies, cytokine antagonists, myostatin inhibitors and ghrelin/ghrelin mimetics.
1.2 Ghrelin and GHSR: Mechanisms of Action
Ghrelin is a 28 amino acid peptide that is the natural ligand for the growth hormone secretagogue receptor-1a26, 27(GHS-R1a). Ghrelin plays an important role in several physiological processes including stimulation of appetite by stimulating the production of orexigenic mediators such as neuropeptide Y28, 29; by stimulating GH secretion and by regulating energy balance through GH-independent mechanisms26, 30. Ghrelin is produced primarily by the stomach and increases during periods of fasting or under negative energy balance. In contrast, ghrelin levels are low post-prandially and in obesity. Also, animal studies suggest that ghrelin may increase food reward via the mesolimbic dopamine system31.
Ghrelin promotes adiposity through the activation of lipogenic pathways in the central nervous system (CNS)32. Ghrelin also activates white adipocytes, while inactivating brown adipocytes, resulting in decreased energy expenditure33. It also promoted lipogenesis and decreases lipolysis and lipid oxidation in white adipose tissue in an animal model of cisplatin-induced cachexia34. Ghrelin also stimulates gastric contraction and gastric emptying and its agonists are being developed for the treatment of gastroparesis and postoperative ileus35, 36. As nausea and constipation are common symptoms in cancer patients, these effects may be of further benefit in this setting.
Another important role of ghrelin in CACS treatment involves its anti-inflammatory actions37. Ghrelin exerted anti-inflammatory effects and attenuates endotoxin-induced anorexia in mice38 and more recently in a murine model of cancer cachexia39. Ghrelin also inhibits the activation of nuclear factor-kB (NFkB), a transcription factor that stimulates the production of proinflammatory cytokines and increases muscle proteolysis40. A randomized clinical trial of the ghrelin mimetic MK-677 suggested that activation if GHSR-1a can also decrease energy expenditure as subjects receiving the active drug lost less weight upon caloric restriction than those receiving placebo41.
The ghrelin receptor (growth hormone secretagogue, GHS-R1a) is a G protein-coupled receptor (GPCR) that is expressed in the central nervous system and in peripheral tissues. In the CNS, its expression is highest in several discrete hypothalamic nuclei, including the anterior and lateral hypothalamic areas, and the ventromedial hypothalamus (VMH) and arcuate nuclei42, 43. GHS-R1a is co-expressed with both Neuropeptide Y (NPY) and growth hormone releasing hormone (GHRH), and ghrelin is thereby able to increase food intake and GH secretion44, 45. In peripheral tissues GHS-R1a is expressed in the anterior pituitary, pancreas46, thyroid, spleen, myocardium and adrenal glands47. Notably, GHSR-1a receptor is not expressed in liver, skeletal muscle or adipose tissue48. Recent evidence suggest that ghrelin may have effects directly in adipocytes49 and skeletal muscle37, 39, 50, and an alternative, not yet discovered receptor has been proposed.
1.3 Clinical Trials of Ghrelin in Cancer Cachexia
In the setting of cachexia, data suggest that pharmacological doses of ghrelin may alleviate CACS and also are well-tolerated and safe in patients with advanced cancer. An acute, randomized, placebo-controlled, cross-over single dose clinical trial51 performed in seven cancer patients showed a marked increase in energy intake (31±7%; P=.005) with a ghrelin infusion compared with saline. The meal appreciation score increased by 28±8% (P=.02) with ghrelin treatment and no side effects were observed. Another single-center randomized, double-blind, placebo-controlled, two-arm, double-crossover study52 was carried out in 21 adult patients with advanced incurable cancer. Intravenous ghrelin infusion for 60 min at 2 or 8 mg/kg of body weight was well-tolerated and safe in these patients. More recently, a prospective, randomized phase 2 trial evaluated the effects of ghrelin during cisplatin-based chemotherapy in patients with advanced esophageal cancer53. Patients received either intravenous infusions of synthetic human ghrelin (3 μg/kg) or saline twice daily for 1 week with cisplatin administration. Food intake and appetite visual analog scale (VAS) scores were significantly higher in the ghrelin group than in the placebo group. Patients in the ghrelin group had fewer adverse events during chemotherapy related to anorexia and nausea than patients in the control group. The benefits from long-term provision of ghrelin for the treatment of CACS was also evaluated in a randomized, double-blind, phase 2 study54. Weight-losing cancer patients with solid gastrointestinal tumors were randomized to receive either high-dose ghrelin treatment (13 microg/kg daily; n=17) or low-dose ghrelin treatment (0.7 microg/kg daily; n=14) for 8 weeks as once-daily, subcutaneous injections. Appetite scores were increased significantly by high-dose ghrelin. High-dose ghrelin also reduced fat loss (P<.04) and increased serum GH (P<.05). There was a trend for high-dose ghrelin to improve energy balance (P<.07). Adverse effects were not observed by high-dose ghrelin in this study.
Taken together, data suggest that ghrelin may play an important role in stimulating appetite and food intake in CACS; however, the short half-life (<30 min), and the parenteral administration requirement of ghrelin has limited its clinical usefulness. Therefore, interest has switched to the development of orally-available ghrelin mimetics55–57. One of these mimetics, anamorelin (ONO-7643, formerly known as RC-1291), is a ghrelin receptor agonist currently in development for the treatment of non-small-cell lung cancer (NSCLC)-related cachexia.
2.1 Anamorelin: Chemistry
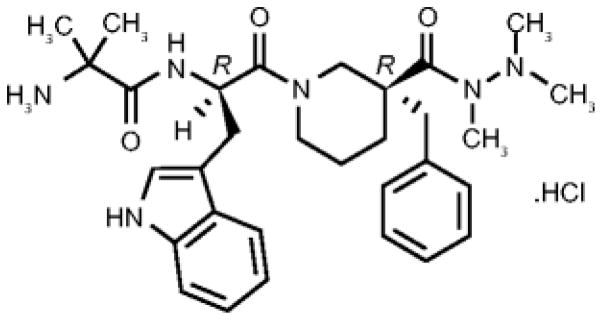
Anamorelin HCl (ANAM) is a potent and selective novel GHSR-1a agonist that is orally active and that mimics the N terminal active core of ghrelin. It has a longer half-life than ghrelin (7h vs. 0.5h)58 and is currently undergoing evaluation as a potential treatment of CACS in Phase III clinical trials in NSCLC. The chemical name for anamorelin HCl is (3R)-1-(2-methylalanyl-D-tryptophyl)-3-(phenylmethyl)-3-piperidine-carboxylic acid 1,2,2-trimethylhydrazide hydrochloride (Figure 1)58.
Figure 1.
Chemical structure of Anamorelin.
Reproduced with permission from [58]
2.2 Pharmacodynamics
Anamorelin shows significant agonist activity on the ghrelin receptor with half-maximal effective concentration (EC50) values of 0.74 nM58, and no significant antagonist activity at concentrations of up to 1,000 nM. Anamorelin binds to the ghrelin receptor with a binding affinity constant (Ki) of 0.70 nM. In rat pituitary cells incubated with anamorelin, there was a dose-dependent stimulatory effect on GH release and the potency (EC50) was 1.5 nM58. In vivo, when rats were treated with anamorelin 3, 10, or 30 mg/kg or vehicle orally, daily for 6 days, anamorelin significantly and dose-dependently increased food intake at all dose levels compared with controls. Administration of anamorelin at a single oral dose of 3, 10, or 30 mg/kg induced a dose-dependent increase in plasma GH levels. The maximum plasma GH concentration was reached at 0.5–2h postdose. Anamorelin stimulated a maximum increase in GH concentration ranging from 2.3-fold for the 3 mg/kg dose to 4.1-fold for the 30 mg/kg dose. Increases in GH and IGF-1 levels were also observed following anamorelin administration in pigs and dogs58. The implications of this GH increase from a safety perspective are discussed in section 2.5 Safety and Tolerability below.
2.3 Pharmacokinetics and metabolism
Pharmacokinetics of anamorelin was evaluated in phase I trials in healthy volunteers. Anamorelin administered in single doses of 10mg (n=6), 25mg (n=6), and 50mg (n=6) achieved plasma concentrations that peaked 0.5–2.0h post-dose59. Plasma half-life was approximately 7 hrs. Plasma was cleared of radio-labeled anamorelin by 18h post-dose with 99.8% of the drug recovered in feces (92%) and urine (8%). In order to assess the effects of food and CYP3A4 inhibition, subjects (n=13) received one 25-mg oral dose of anamorelin when fasted, fed (high-fat meal), and with ketoconazole. Plasma AUC0-24h values showed a 4-fold decrease with food and a 3-fold increase with ketoconazole.
2.4 Clinical Efficacy
2.4.1 Phase I studies
In a phase I randomized, double-blind, placebo-controlled single dose-rising study, 9 healthy male volunteers received 10, 25, and 50 mg oral doses of anamorelin. The 10-mg dose showed no effect. Pooled active doses (25 and 50 mg) indicated a rapid onset of response with significant appetite stimulating effect compared with placebo at 30 minutes; effect remained significant for 4 hours, the last time point assessed before eating. Spontaneous food intake increased 18.4% and was significantly greater than placebo. Dose-related increases in serum GH were recorded following placebo (5.38 ng/ml) and anamorelin 10 mg (23.68 ng/ml), 25mg (61.79 ng/ml), and 50 mg (88.15 ng/ml, p=.00009). Adverse events reported were: anamorelin–headache (n=4) and stomachache (n=1); placebo–lightheadedness (n=1). All adverse events (AEs) were mild/moderate and resolved spontaneously. No dose-limiting toxicities were reported and no subjects discontinued anamorelin due to AEs60.
In another randomized, double-blind, placebo-controlled, multiple-dose, dose-escalation phase I study in 29 healthy volunteers, anamorelin was well tolerated with no dose-limiting AEs. Anamorelin 50 or 75 mg induced significant dose-related weight gain after 6 days versus placebo, with the greatest increases seen with daily dosing. The mean increase in weight from baseline after 50 mg daily was 1.25±0.73 kg (p=.0022 versus placebo), and after 75 mg daily it was 1.16±0.65 kg (p=.0022 versus placebo)55. In the same study, single 25-mg, 50-mg, and 75-mg doses of anamorelin significantly increased circulating GH. The magnitude of this effect was attenuated with continued dosing. A robust increase in IGF-1 occurred after administration of the 50 mg and 75 mg doses of anamorelin61. One subject had moderate elevations in AST and ALT levels that normalized upon discontinuation of the drug. Other adverse events considered possibly related to study drug were: anamorelin-nausea, feeling hot, stomach discomfort, diarrhea (n=1 for each symptom), and headache (n=2).
2.4.2 Phase II studies in CACS
A phase II multicenter, double-blind, placebo-controlled, crossover study evaluated the acute effects of anamorelin in 16 patients with CACS. Patients were randomly assigned to anamorelin 50 mg/day or placebo for 3 days. A 3- to 7-day washout period followed and then treatments were switched. Anamorelin significantly increased body weight compared with placebo (0.77kg vs. −0.33kg, p value= 0.016). GH, IGF-1 and insulin growth factor binding protein-3 (IGFBP-3) significantly increased with anamorelin compared with placebo although levels remained within the normal range. Patient-reported symptoms as measured by the Anderson Symptom Assessment Scale (ASAS), significantly improved. AEs in four patients were possibly or probably related to anamorelin: hyperglycemia (n=2), nausea (n=1), and dizziness (n=1). Most AEs were mild62.
More recently, two phase II multicenter, randomized, double-blind, placebo-controlled trials in 82 patients with incurable cancer were reported. The design of these trials was identical except that in one of them, patients enrolled in the 3-day cross-over trial described above were allowed after a wash-out period of at least 5 half-lives; data from these two trials were pooled a priori63 Patients with different solid tumors were included in these studies. Pooled analyses showed that over 12 weeks, LBM measured by Dual-energy X-ray absorptiometry (DEXA) increased 1.89±0.53kg in the anamorelin group compared with a decrease −0.20±0.52kg in the placebo group (treatment difference of 2.09±0.58kg, p=0·0006). Increases in total body mass (TBM), appendicular LBM, handgrip strength and QOL were also noted. Scale weight was also higher and inflammatory cytokines lower in the anamorelin group, although the differences did not reach statistical significance. IGF-1 and IGFBP-3 also significantly increased at wks 4, 8, and 12, but remained within normal ranges. In this study, anamorelin was well tolerated, and AEs were similar between treatment arms64. Another international, randomized, placebo-controlled, multicenter, phase II trial evaluated the effect of anamorelin 50 mg (n=76) and 100 mg (n=73) vs. placebo (n=77) on body weight, HGS and quality of life as assessed by the MDASI score over 12 weeks. Patients in the ANA 100 mg group gained 0.14 kg, compared to mean losses of 0.3 kg and 1.32 kg for the 50 mg and placebo groups, respectively (mean treatment difference between 100 mg ANA and placebo was 1.47 kg; p = 0.0005). There were also improvements in HGS and MDASI scores in the ANA 100 mg dose group although these did not reach statistical significance. ANA was safe and well-tolerated in this study, and AEs of anorexia, nausea, and fatigue were reported in fewer ANA-treated than placebo-treated patients65, 66.
2.4.3 Phase III studies
Two double-blind, Phase III trials (ROMANA 1, NCT01387269, n=484; ROMANA 2, NCT01387282, n=495) assessed the efficacy and safety of anamorelin 100mg in patients with incurable stage III/IV NSCLC and cachexia defined as ≥ 5% weight loss within prior 6 months or BMI <20 kg/m2. Patients were randomized (2:1) to receive daily anamorelin or placebo for 12 weeks. Co-primary endpoints were change from baseline over 12 weeks in LBM (measured by DXA) and in hand-grip strength (HGS). Secondary endpoints included the anorexia-cachexia domain of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) and the fatigue domain of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). In both studies, anamorelin increased LBM vs placebo (ROMANA 1: 1.10 kg vs −0.44 kg; ROMANA 2: 0.75 kg vs −0.96 kg; p<.0001 for both), but failed to show significant improvements in HGS67. Both studies also significantly improved FAACT anorexia/cachexia scores vs placebo, and increased body weight. In ROMANA 1, FACIT-F fatigue scores reached statistically significant differences at week 9 and week 12 only, but did not reach statistical significance over the entire 12 week treatment period. The most frequent drug-related AEs in both trials included hyperglycemia, nausea, and diabetes although their incidence were low (~2–5%). In ROMANA 3, over 500 patients who completed dosing in the ROMANA 1 or ROMANA 2 trials continued to receive their original treatment assigned for another 12 weeks to further evaluate efficacy and safety of anamorelin68. The independent data monitoring committee allowed the ROMANA 1–3 trials to continue as planned to completion.
2.5 Safety and Tolerability
In a phase I single dose study in healthy volunteers, AEs were mild and transient60. In a multiple-dose phase I study, all AEs were mild or moderate in intensity except for one event of severe headache reported that was not considered related to study drug. Effects on heart rate and blood pressure were modest and without clinical consequence. One subject had moderate elevations in AST and ALT levels that normalized upon discontinuation of the drug. Other adverse events considered possibly related to the study drug were: anamorelin-nausea, feeling hot, stomach discomfort, diarrhea (n=1 for each symptom), and headache (n=2). In the phase 3 studies, most frequent drug-related AEs were hyperglycemia (5.3%) and nausea (3.8%) for ROMANA 1, and hyperglycemia (4.2%) and diabetes (2.1%) for ROMANA 2. Both studies had few drug-related Grade ≥ 3 AEs (0.9%, 2.7%)69. The glucose-related AEs in Phase 3 trials are believed to be consistent with the mechanism of action of ghrelin, as ghrelin has been shown to regulate glucose metabolism through multiple pathways70.
Concerns have been raised that administration of GHSR-1a agonists to cancer patients may potentially stimulate tumor growth. The effects of anamorelin and ghrelin on tumor growth were evaluated on A549 NSCLC xenographs in mice administered ghrelin (2mg/kg) or anamorelin (3–30mg/kg) for 28 days. Specifically, anamorelin increased GH concentrations by up to 2.5-fold, while repeated doses of ghrelin resulted in a maximum increase in GH concentrations of approximately 50-fold. For IGF-1, the mean concentrations were increased up to 122% and 109% in anamorelin and ghrelin groups respectively71. Neither ghrelin nor anamorelin treatment for 28 days affected tumor growth, as measured by tumor volumes, despite the significantly increased GH and IGF-1 levels. Also, clinical studies with 50 mg and 100 mg of anamorelin have shown no significant effect on long-term overall survival compared with placebo65 although none of these studies have been adequately powered for this endpoint.
2.6 Long Term Effects
Long-term benefits of increasing LBM have not been well-established as effective treatments for cachexia are currently unavailable. Nevertheless there is an association between lean body mass loss and increased mortality in this setting72. Whether preventing or ameliorating cancer cachexia will lead to improved survival in clinical trials remain to be determined.
The effects of anamorelin on long-term prognosis and longevity have not been established. However, treatment with Rikkunshito, a ghrelin potentiator, was observed to prolong survival in an animal model of cachexia73, and in a rodent model of cancer cachexia induced by Lewis Lung Carcinoma (LLC) tumor implantation, ghrelin administration prevented the development of cachexia (lean and fat mass loss) and this was associated with improved survival39.
3 Conclusion
Given that CACS is a multifactorial syndrome and the complexity of this debilitating condition, therapeutic interventions for CACS are likely to require a multimodal treatment approach including a combination of proper nutrition, agents with orexigenic, anabolic, anti-catabolic and anti-inflammatory effects and also non-pharmacologic interventions (i.e. exercise). Ghrelin appears to play an important role in stimulating appetite and food intake, having anti-inflammatory actions and preventing losses in muscle mass and adiposity in the setting of cancer-related cachexia. Results to date indicate anamorelin is effective at stimulating appetite and increasing body weight and lean body mass with acceptable tolerability. However, there is a need to generate more clinical data to further explore its safety, efficacy and tolerability. Completion of the ROMANA 3 study and publication of the results of all phase III clinical trials are eagerly awaited.
4 Expert Opinion
CACS is an unmet clinical need that affects a large number of cancer patients. Although significant advances in this field have been made over the last decade increasing our understanding of how appetite, muscle and fat mass are regulated in this setting, these efforts have fell short of delivering a drug to the market. Several molecular pathways are currently being targeted in clinical trials for this indication including some that are focused almost exclusively on muscle (i.e. myostatin, androgens), on inflammation (IL-1α) or through not well-characterized mechanisms (β-blockers, angiotensin-converting-enzyme inhibitors). Targeting ghrelin presents the advantage of potentially addressing multiple mechanisms simultaneously including appetite, muscle protein balance, adipose tissue metabolism, energy expenditure and inflammation. The orally available ghrelin receptor agonist anamorelin is the agent most advanced in its class for this indication. Clinical data published to this date suggest that anamorelin is well tolerated and it effectively increases appetite, body weight and lean mass in patients with advanced NSCLC. Long-term safety remains unknown at this time although more data will be available once ROMANA 3 is completed and presented. The functional impact of anamorelin also remains to be determined. Although it failed to show significant increases in HGS compared to placebo in phase III studies, this could represent lack of sensitivity of this test used to assess this outcome (handgrip), confounding due to other co-morbid conditions in a population with advanced cancer, or that longer exposure is required to see an effect in this domain. The potential synergistic effects of anamorelin with nutritional support or exercise as well as its efficacy/safety in other tumor types are also unknown. Future studies will be needed in order to address these questions.
5 Drug Summary box
| Drug name (generic) | Anamorelin |
| Phase (for indication under discussion) | |
| Indication (specific to discussion) | NSCLC cachexia |
| Pharmacology description/mechanism of action | Anamorelin is a novel, potent and selective GHSR-1a agonist; it is orally active and effective at stimulating appetite and increasing body weight and lean body mass with acceptable tolerability. |
| Route of administration | Orally |
| Chemical structure | Figure 1 |
| Pivotal trials: | Phase I, [55], [60], [61]. Phase II [62], [64], [65], and [66]. Phase III: [67] and [68]. |
Footnotes
Financial and competing interests disclosure:
The authors have received funding from Department of Veterans Affairs (MERIT grants: BX000507 and CX000174) and the NIA (AG040583), Aeterna Zentaris Inc., and Helsinn Therapeutics (US), Inc.J Garcia has received consulting or advisory role’s fees from Aeterna Zentaris Inc. and Helsinn Therapeutics (US), Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
*=of important,
**=of considerable important
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011 May;12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Buchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg. 2008 Jul;12(7):1193–201. doi: 10.1007/s11605-008-0505-z. [DOI] [PubMed] [Google Scholar]
- 3.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010 Sep;1(1):1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980 Oct;69(4):491–7. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 5.Kumar NB, Kazi A, Smith T, Crocker T, Yu D, Reich RR, et al. Cancer cachexia: traditional therapies and novel molecular mechanism-based approaches to treatment. Curr Treat Options Oncol. 2010 Dec;11(3–4):107–17. doi: 10.1007/s11864-010-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008 Jul 20;26(21):3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 8.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010 Apr;91(4):1133S–37S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 9.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013 Apr 20;31(12):1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 10.Roberts BM, Ahn B, Smuder AJ, Al-Rajhi M, Gill LC, Beharry AW, et al. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J. 2013 Jul;27(7):2600–10. doi: 10.1096/fj.12-222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laviano A, Koverech A, Mari A. Cachexia: clinical features when inflammation drives malnutrition. The Proceedings of the Nutrition Society. 2015 Mar;26:1–7. doi: 10.1017/S0029665115000117. [DOI] [PubMed] [Google Scholar]
- 12.Argiles JM, Stemmler B. The potential of ghrelin in the treatment of cancer cachexia. Expert Opin Biol Ther. 2013 Jan;13(1):67–76. doi: 10.1517/14712598.2013.727390. [DOI] [PubMed] [Google Scholar]
- 13.European Palliative Care Research Collaborative. European Clinical Guidelines: Clincal practice guidelines on cancer cachexia in advancer cancer patients with a focus on refractory cachexia. 2010. [Google Scholar]
- 14.Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterology research and practice. 2011;2011:601434. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nature reviews Clinical oncology. 2013 Feb;10(2):90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 16.Bozzetti F, Gavazzi C, Mariani L, Crippa F. Artificial nutrition in cancer patients: which route, what composition? World J Surg. 1999 Jun;23(6):577–83. doi: 10.1007/pl00012350. [DOI] [PubMed] [Google Scholar]
- 17.Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. J Clin Oncol. 1993 Oct;11(10):2043–9. doi: 10.1200/JCO.1993.11.10.2043. [DOI] [PubMed] [Google Scholar]
- 18.Lundholm K, Korner U, Gunnebo L, Sixt-Ammilon P, Fouladiun M, Daneryd P, et al. Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res. 2007 May 1;13(9):2699–706. doi: 10.1158/1078-0432.CCR-06-2720. [DOI] [PubMed] [Google Scholar]
- 19.Del Fabbro E, Dev R, Hui D, Palmer L, Bruera E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol. 2013 Apr 1;31(10):1271–6. doi: 10.1200/JCO.2012.43.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy RA, Yeung E, Mazurak VC, Mourtzakis M. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br J Cancer. 2011 Nov 8;105(10):1469–73. doi: 10.1038/bjc.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorter RW. Experiences with dronabinol (delta-tetrahydrocannabinol) in oncological patients with anorexia-cachexia syndrome. Illustration of clinical problems and therapy based on 2 case reports. Schmerz. 2004 Dec;18(Suppl 2):S31–3. [PubMed] [Google Scholar]
- 22.Tazi E, Errihani H. Treatment of cachexia in oncology. Indian J Palliat Care. 2010 Sep;16(3):129–37. doi: 10.4103/0973-1075.73644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yennurajalingam S, Willey JS, Palmer JL, Allo J, Del Fabbro E, Cohen EN, et al. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: results of a double-blind placebo-controlled randomized study. J Palliat Med. 2012 Oct;15(10):1059–64. doi: 10.1089/jpm.2012.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013 Apr;14(4):335–45. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford J, Dalton JT, Hancock ML. Results from two Phase 3 randomized trials of enobosarm, selective androgen receptor modulator (SARM), for the prevention and treatment of muscle wasting in NSCLC. Eur J Cancer. 2013;49(Suppl 2):LBA21. [Google Scholar]
- 26.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 27.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin--a hormone with multiple functions. Front Neuroendocrinol. 2004 Apr;25(1):27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005 Apr;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 29.van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004 Jun;25(3):426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 30.Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85(12):4908–11. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- 31.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008 Sep;13(3–4):358–63. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 32.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003 Feb 20;37(4):649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 33.Mano-Otagiri A, Iwasaki-Sekino A, Nemoto T, Ohata H, Shuto Y, Nakabayashi H, et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul Pept. 2010 Feb 25;160(1–3):81–90. doi: 10.1016/j.regpep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Garcia JM, Scherer T, Chen JA, Guillory B, Nassif A, Papusha V, et al. Inhibition of Cisplatin-induced lipid catabolism and weight loss by ghrelin in male mice. Endocrinology. 2013 Sep;154(9):3118–29. doi: 10.1210/en.2013-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck DE, Sweeney WB, McCarter MD Ipamorelin 201 Study G. Prospective, randomized, controlled, proof-of-concept study of the Ghrelin mimetic ipamorelin for the management of postoperative ileus in bowel resection patients. Int J Colorectal Dis. 2014 Dec;29(12):1527–34. doi: 10.1007/s00384-014-2030-8. [DOI] [PubMed] [Google Scholar]
- 36.Van der Ploeg L, Laken H, Sharma S, Datta R, Halem H, Dong J, et al. Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131. Life Sci. 2014 Jul 25;109(1):20–9. doi: 10.1016/j.lfs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle. 2010 Dec;1(2):169–76. doi: 10.1007/s13539-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004 Jul;114(1):57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.chen J, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, et al. Ghrelin prevents tumor- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. Journal of cachexia, sacropenia and muscle. 2015 doi: 10.1002/jcsm.12023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004 May 11;109(18):2221–6. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 41.Chapman IM, Pescovitz OH, Murphy G, Treep T, Cerchio KA, Krupa D, et al. Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adults. J Clin Endocrinol Metab. 1997 Oct;82(10):3455–63. doi: 10.1210/jcem.82.10.4297. [DOI] [PubMed] [Google Scholar]
- 42.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997 Aug;48(1):23–9. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 43.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006 Jan 20;494(3):528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, et al. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept. 2005 Mar 15;126(1–2):55–9. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–16. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 46.Yada T, Damdindorj B, Rita RS, Kurashina T, Ando A, Taguchi M, et al. Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Diabetes, obesity & metabolism. 2014 Sep;16(Suppl 1):111–7. doi: 10.1111/dom.12344. [DOI] [PubMed] [Google Scholar]
- 47.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002 Jun;87(6):2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008 Feb;149(2):843–50. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitahara A, Takahashi K, Moriya R, Onuma H, Handa K, Sumitani Y, et al. Ghrelin augments the expressions and secretions of proinflammatory adipokines, VEGF120 and MCP-1, in differentiated 3T3-L1 adipocytes. J Cell Physiol. 2015 Jan;230(1):199–209. doi: 10.1002/jcp.24699. [DOI] [PubMed] [Google Scholar]
- 50.Porporato PE, Filigheddu N, Reano S, Ferrara M, Angelino E, Gnocchi VF, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J Clin Invest. 2013 Feb 1;123(2):611–22. doi: 10.1172/JCI39920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004 Jun;89(6):2832–6. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 52.Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschop M, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008 Jan 29;98(2):300–8. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer. 2012 Oct 1;118(19):4785–94. doi: 10.1002/cncr.27430. [DOI] [PubMed] [Google Scholar]
- 54.Lundholm K, Gunnebo L, Korner U, Iresjo BM, Engstrom C, Hyltander A, et al. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer. 2010 Apr 15;116(8):2044–52. doi: 10.1002/cncr.24917. [DOI] [PubMed] [Google Scholar]
- 55**.Garcia JM, Polvino WJ. Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncologist. 2007 May;12(5):594–600. doi: 10.1634/theoncologist.12-5-594. This randomized, double-blinded, placebo-controlled, multiple-dose, dose-escalation phase I study showed significant dose-related weight gain after 6 days of anamorelin treatment. [DOI] [PubMed] [Google Scholar]
- 56.Khan AS, Smith LC, Anscombe IW, Cummings KK, Pope MA, Draghia-Akli R. Growth hormone releasing hormone plasmid supplementation, a potential treatment for cancer cachexia, does not increase tumor growth in nude mice. Cancer Gene Ther. 2005 Jan;12(1):54–60. doi: 10.1038/sj.cgt.7700767. [DOI] [PubMed] [Google Scholar]
- 57.Kim HJ, Kim HJ, Yun J, Kim KH, Kim SH, Lee SC, et al. Pathophysiological role of hormones and cytokines in cancer cachexia. J Korean Med Sci. 2012 Feb;27(2):128–34. doi: 10.3346/jkms.2012.27.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pietra C, Takeda Y, Tazawa-Ogata N, Minami M, Yuanfeng X, Duus EM, et al. Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: preclinical profile. Journal of cachexia, sarcopenia and muscle. 2014 Dec;5(4):329–37. doi: 10.1007/s13539-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leese PT, Trang JM, Blum RA, Groot E. An open-label clinical trial of the effects of age and gender on the pharmacodynamics, pharmacokinetics and safety of the ghrelin receptor agonist anamorelin. Clinical Pharmacology in Drug Development. 2015;4(2):112–20. doi: 10.1002/cpdd.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Kumor K, Polvino W. Biologic activity of RC-1291, a novel oral ghrelin mimetic for cancer anorexia/cachexia: results from a phase I randomized, double-blind, placebo-controlled trial in healthy volunteer. Supportive care cancer. 2006;14:583–687. (abstract 03-009). Pooled active doses (25 and 50mg) of Anamorelin indicated a rapid onset of response with significant appetite stimulating effect. Spontaneous food intake increased 18.4%. [Google Scholar]
- 61.Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res. 2009 Jun;19(3):267–73. doi: 10.1016/j.ghir.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 62*.Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013 Jan;21(1):129–37. doi: 10.1007/s00520-012-1500-1. Anamorelin significantly increased body weight compared with placebo (0.77kg vs. −0.33kg, p value 0.016). GH, IGF-1 and insulin growth factor binding protein-3 (IGFBP-3) significantly increased. [DOI] [PubMed] [Google Scholar]
- 63**.Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015 Jan;16(1):108–16. doi: 10.1016/S1470-2045(14)71154-4. Pooled analyses showed that over 12 weeks, LBM measured by DEXA increased 1.89±0.53kg in the anamorelin group compared with a decrease −0.20±0.52kg in the placebo group (treatment difference of 2.09±0.58kg, p=0·0006). Increases in total body mass (TBM), appendicular LBM, handgrip strength and QOL were also noted. [DOI] [PubMed] [Google Scholar]
- 64.Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. The Lancet Oncology. 2014 Dec 15; doi: 10.1016/S1470-2045(14)71154-4. [DOI] [PubMed] [Google Scholar]
- 65.Temel J, Bondarde S, Jain M, Allen S, Mann W. Efficacy and safety of anamorelin HCl in NSCLC patients: results from a randomized, double-blind, placebo-controlled, multicenter Phase II study. European Cancer Congress; Amsterdam, The Netherlands. 2013. [Accessed 14 November 2013]. www.poster-submissioncom/board/ [Google Scholar]
- 66*.Temel JS, Bondarde SA, Jain MM, Yan Y, Duus EM, Allen S, et al. Evaluation of quality of life from a phase II study of anamorelin HCl in NSCLC patients. J Clin Oncol. 2013;31(suppl 31):abstr 42. Patient in the ANA 100mg group gained 0.14kg, compared to mean losses of 0.3kg and 1.32kg for the 50mg and placebo groups respectively. [Google Scholar]
- 67*.Bonomi P, Temel J, Currow D, Fearon K, Gleich L, Yan Y, et al. Anamorelin for the Treatment of Cancer Anorexia-Cachexia in Advanced NSCLC Patients: Results From ROMANA 1, a Pivotal Phase 3 Study. Int J Radiat Oncol Biol Phys. 2014;90(5S Supplement):abstr 5. Anamorelin increased LBM vs placebo (ROMANA 1: 1.10 kg vs −0.44 kg; ROMANA 2: 0.75 kg vs −0.96 kg; p<.0001 for both) in patients with incurable stage III/IV NSCLC. [Google Scholar]
- 68**.Abernethy AP, Currow D, Fearon K, Gleich LL, Friend J, Temel JS. Anamorelin for the treatment of cancer anorexia-cachexia: Baseline characteristics from three phase III clinical trials (the ROMANA program) J Clin Oncol. 2014;32(suppl):abstr e20694. This study will further evaluate efficacy and safety of anamorelin. [Google Scholar]
- 69.Temel J, Currow D, Fearon K, Gleich L, Yan Y, Friend J, et al. Anamorelin for the treatment of cancer anorexia-cachexia in NSCLC: Results from the phase 3 studies ROMANA 1 and 2. Annals of Oncology. 2014;25(5):1–41. doi:101093/annonc/mdu438. [Google Scholar]
- 70.Heppner KM, Tong J. Mechanisms in endocrinology: regulation of glucose metabolism by the ghrelin system: multiple players and multiple actions. European journal of endocrinology / European Federation of Endocrine Societies. 2014 Jul;171(1):R21–32. doi: 10.1530/EJE-14-0183. [DOI] [PubMed] [Google Scholar]
- 71.Northrup R, Kuroda K, Duus EM, Barnes SR, Cheatham L, Wiley T, et al. Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support Care Cancer. 2013 Sep;21(9):2409–15. doi: 10.1007/s00520-013-1800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Utech AE, Tadros EM, Hayes TG, Garcia JM. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle. 2012 Dec;3(4):245–51. doi: 10.1007/s13539-012-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujitsuka N, Uezono Y. Rikkunshito, a ghrelin potentiator, ameliorates anorexia-cachexia syndrome. Frontiers in pharmacology. 2014;5:271. doi: 10.3389/fphar.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]