Abstract
Background
The Boston Criteria are the basis for a non-invasive diagnosis of cerebral amyloid angiopathy(CAA) in the setting of lobar intracerebral hemorrhage(ICH). We assessed the accuracy of these Criteria in individuals with lobar microbleeds(MBs) without ICH.
Methods
We identified individuals aged>55 having brain MRI and pathological assessment of CAA in a single academic hospital and a community-based population (Framingham Heart Study [FHS]). We determined the positive predictive value (PPV) of the Boston Criteria for CAA in both cohorts, using lobar MBs as the only hemorrhagic lesion to fulfill the Criteria.
Results
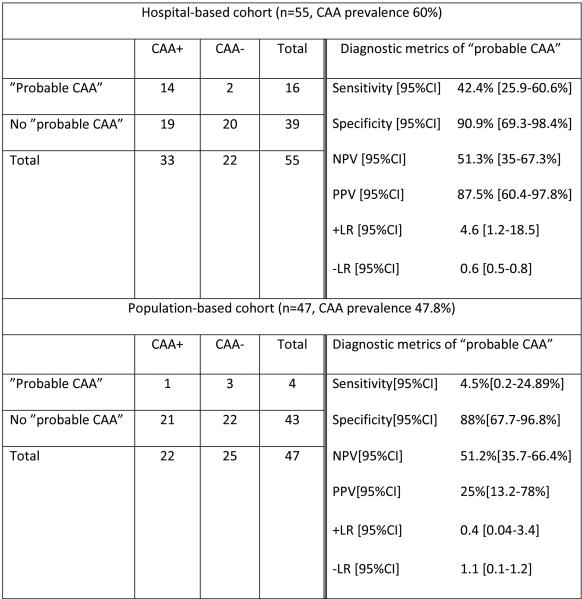
We included 102 individuals: 55 from the hospital-based cohort and 47 from FHS(mean age at MRI 74.7±8.5 and 83.4±10.9 years; CAA prevalence 60% and 46.8%; cases with any lobar MB 49% and 21.3%; cases with ≥2 strictly lobar MBs 29.1% and 8.5%, respectively). PPV of “probable CAA” (≥2 strictly lobar MBs) was 87.5%[95%CI 60.4-97.8%] and 25%[95%CI 13.2-78%], in hospital and general populations, respectively.
Conclusions
Strictly lobar MB strongly predict CAA in non-ICH individuals when found in a hospital context. However, their diagnostic accuracy in general population appears limited.
Keywords: Cerebral amyloid angiopathy, microbleed, intracerebral hemorrhage, Boston Criteria, sensitivity, specificity, predictive value, likelihood ratio
1. Introduction
Cerebral amyloid angiopathy(CAA) is caused by the accumulation of β-amyloid protein in the walls of cortical and leptomeningeal arteries[1-3]. It represents a common etiology of lobar intracerebral hemorrhage (ICH) in the elderly[4, 5]. Lobar microbleeds(MBs) are a hallmark of the disease when seen in patients with lobar ICH[6]. Although frequently associated with Alzheimer’s disease (AD), CAA also independently contributes to cognitive impairment[7]. Indeed, lobar MBs are often identified in patients followed in memory clinics[8-10], where they may potentially serve as markers of CAA. However, lobar MBs are also found in the absence of any of the known above-described clinical features of the disease. Up to 19% of community – based healthy elderly subjects exhibit lobar MBs, and they are strictly lobar in 58.4% of these cases[11]. Understanding the true diagnostic value of lobar MBs, both as clinical and incidental findings, could help improve our ability to detect CAA in its early stages, prior to dementia or devastating ICH.
The Boston Criteria are a set of clinical-radiological criteria that were developed as a means of diagnosing CAA in a non-invasive way[12]. According to these Criteria, the detection of multiple (≥2) strictly lobar hemorrhages (large or small, symptomatic or asymptomatic) without known underlying cause in individuals aged >55 is highly specific of the disease[4]. However, validation studies of these Criteria have been mostly based on patients admitted due to lobar ICH[4, 13, 14], precluding the study of the diagnostic value of lobar MBs without symptomatic ICH. At a community level, the Rotterdam and Framingham studies have shown that healthy elderly individuals with strictly lobar MBs have an increased frequency of the APOE-ε4 allele (compared to patients with MBs not strictly confined to lobar regions)[11, 15], which is in agreement with increased APOE- ε4 frequencies seen in patients with “probable CAA”[16]. Also, strictly lobar MBs do not correlate with classic vascular risk factors[11, 15], which further reinforces their possible association with CAA. However, pathological data supporting the suspected link between lobar MBs and CAA in individuals without lobar ICH are currently lacking.
Using pathological assessment of CAA as a gold-standard, we aimed to determine the positive predictive value (PPV) and negative predictive value (NPV) of the Boston Criteria for CAA applied to MB-only subjects, in two highly different settings: a hospital-based cohort and a population-based cohort from the Framingham Heart Study (FHS).
2. Methods
2.1 Study Cohorts
Hospital-based cohort
We searched across datasets of the Massachusetts General Hospital (MGH) for patients age >55 having both brain MRI and either brain biopsy or brain autopsy. Data search covered patients seen at the hospital during the period 1997-2012. On more than 3200 cases initially retrieved, we applied a multistep exclusion algorithm (Figure). We excluded all individuals with lobar ICH at, or prior to, baseline MRI as lobar ICH has been shown to be strongly associated with CAA[4] and could confound the interpretation of the results.
Figure 1.
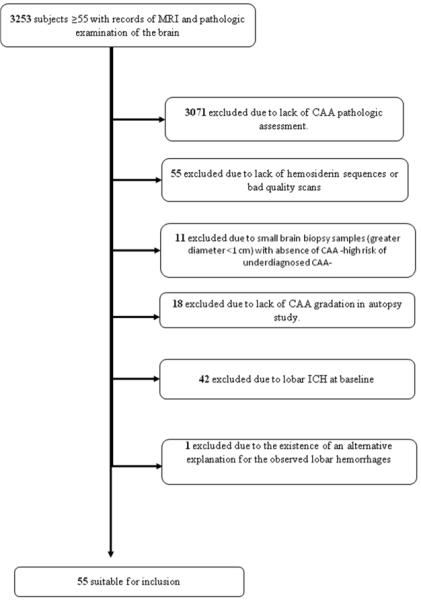
Inclusion/exclusion workflow for hospital-based cases
Population-based cohort
This second cohort of individuals came from the FHS. Original[17] and Offspring Cohort[18] participants were invited to undergo MRI brain imaging beginning in March 1999. T2*-weighted MRI protocols were added in December 2000. Details on MRI protocol and subject enrollment have been described elsewehere[19]. In 1997 the FHS began a postmortem brain tissue donation program in collaboration with the Boston University Alzheimer’s Disease Center’s Neuropathology Core. Details of the enrollment process are available elsewhere[20]. As of June 2009, 16% of surviving Original cohort participants (n=35) and 11% of surviving Offspring cohort participants (n=398) were enrolled as potential brain donors. After 12 years since the program began, a total of 1804 Original and Offspring cohort participants had died, 10% of whom (n=186) were registered brain donors. Of the latter, 139 brains (74%) were received and detailed neuropathology reports were available for all cases. Fifty-eight percent of the brains analyzed were deemed pathologically normal. The present study sample was obtained from the Original and Offspring cohort participants with available brain MRI and neuropathological data, including explicit CAA assessment. All these individuals were aged >55.
2.2 Standard Protocol Approvals, Registrations, and Patient Consents
The local Institutional Review Boards at Massachusetts General Hospital and Boston University Medical Center approved the study protocol. Informed consent was obtained from all subjects in the FHS.
2.3 Imaging
MGH and FHS protocols were similar regarding the acquisition parameters of T2*-gradient echo sequences (GRE). Some MGH cases had susceptibility-weighted image (SWI) studies, which are recognized as more sensitive for hemosiderin detection than GRE[21]. Table 1 shows the MRI protocols used in each institution.
Table 1.
Comparison of MRI protocols for hemosiderin detection
| MGH | FHS | ||
|---|---|---|---|
| Type of sequence | SWI | GRE | GRE |
| MRI equipment | Siemens Trio | GE Signa | Siemens Magnetom |
| Magnet (Teslas) | 3 | 1.5 | 1.5 |
| Slice thickness (mm) | 1.2 | 5 | 5 |
| Interslice gap (mm) | 0 | 1 | 2 |
| TR (msec) | 27 | 750 | 656 |
| TE (msec) | 21 | 24 | 26 |
| Flip angle (degrees) | 15 | 30 | 12 |
| Acquisition matrix | 448 × 299 | 256 × 144 | 256 × 144 |
| Field of view (mm) | 224 | 230 | 220 |
SWI= susceptibility-weighted imaging; GRE= T2*-gradient echo; TR=repetition time; TE= echo time.
Detection of MBs was performed on either GRE or SWI sequences as previously described[21, 22]. Briefly, MBs were defined as focal round or ovoid areas of marked signal-loss, different from vascular flow voids, calcifications, cavernous malformations and basal ganglia mineralization.
MBs were rated and labeled by a stroke neurologist (M.E.G.) for the MGH cohort and a trained neurologist (J.R.R.) for the FHS cohort, both blind to the subjects’ demographics, clinical characteristics and neuropathological findings. MB rating was assisted by image-processing softwares: MRIcron(www.cabiatl.com/mricro/mricron/) at MGH; and a custom-designed image analysis package (QUANTA 2), written for the Linux operating system, at FHS. The MGH group has previously reported a high inter-rater reliability for MB[23]. Inter-rater reliability was also high (Kappa 0.78) in FHS when comparing one independent reader from MGH (SMR) and one from FHS (JRR) in a subset of 200 scans not related to the present study. Also, the intra-rater reliability based on blinded reading of 200 scans on two separate occasions was high (Kappa 0.78).
The number and location of MBs were recorded. Lobar location referred to cortico-subcortical regions of brain lobes, whereas non-lobar location referred to deep white matter, basal ganglia, thalamus and brainstem. Cerebellar MBs were only considered as lobar in nature when found in the presence of strictly lobar MBs in the brain lobes.
2.4 Classification of cases based on MB profile
Operationally, we defined the following 2 subgroups: 1) cases with “probable CAA”, as per Boston Criteria definition (≥2 strictly lobar MB) and 2) cases without “probable CAA”, which included cases without MB and those with alternative MB patterns: single lobar MB (“possible CAA”); mixed lobar and non-lobar MB; and non-lobar MB only.
2.5 Demographic and clinical variables
We recorded data on sex, age at the time of MRI study, age at the time of pathological examination, time between MRI and pathology, type of hemosiderin sequence, type of pathological study, presence of hypertension, chronic use of antithrombotic drugs (antiplatelet agents, anticoagulants) previous to MRI, presence of dementia at the time of MRI, development of lobar ICH after baseline MRI, MB patterns, lobar MB count, and presence of pathologically-confirmed moderate/severe CAA.
2.6 Pathological data
Methodology used to evaluate presence and severity of CAA was similar in MGH and FHS cohorts (see details below). Additionally, the presence and severity of hypertensive vasculopathy (HV), defined as segmentally occurring hyalinization and fibrinoid changes in the vessel wall of small penetrating arteries (< 200 mm in diameter)[24], was documented in all autopsy studies. While pathologic examinations were blinded and prospectively evaluated in FHS, this could not be ensured in all MGH cases due to the clinical and retrospective nature of this cohort.
In FHS, neuropathological evaluation of the autopsied brains was done by a single neuropathologist (A.C.M.), blinded to all demographic, clinical and brain MRI information. Once the brains were received fresh, macroscopic neuropathological findings were recorded. The median postmortem delay was 6 hours (range 1.5 –72 hours, interquartile range 4 – 14.8 hours). Detailed description of the processing of the brain tissue and histological assessments is provided elsewhere[20]. In the MGH cohort, pathology reports were reviewed and the diagnoses were recorded by a neurologist blind to radiologic and clinical data (SMR). In both cohorts, specific studies for CAA detection consisted in Congo red staining and/or ß-amyloid immunostaining (DAKO) of paraffin-embedded sections of neocortex and leptomeninges[25]. Since mild CAA is a common finding in elderly individuals and may not be responsible for any clinical manifestations, we defined CAA as the presence of at least a moderate degree of vascular amyloid deposition (≥ 2 in Vonsattel’s severity scale for CAA)[26]. This definition was not applied to non-autopsy studies, where only limited tissue is available for examination; in these cases, any vascular amyloid was considered as diagnostic of the disease.
2.7 Data analysis
Sensitivity, specificity, predictive values and likelihood ratios
In both hospital and community-based cohorts, we determined sensitivity, specificity, PPV, NPV, positive likelihood ratio (+LR) and negative likelihood ratio (−LR) of MB-only “probable CAA”. Due to the small number of “possible CAA” cases, diagnostic metrics of “possible CAA” alone could not be determined. As a complementary analysis, we also calculated sensitivity, specificity, PPV, NPV, +LR and −LR of mixed lobar and non-lobar MB for CAA diagnosis in the hospital cohort.
Statistics
Univariate tests were performed to compare characteristics between individuals from both cohorts. Chi-square test, median test and Student’s T-test were used as appropriate. Sensitivity, specificity, PPV, NPV, +LR and −LR values were calculated using 2×2 contingency tables, and reported along with their95% CI. We used logistic regression models to identify variables, other than the presence of strictly lobar MB, independently associated with pathological diagnosis of CAA. The p value for significance was set at 0.05 in all analyses. JMP Pro-9 and SAS were used for analyses (SAS Corporation, Cary, NC).
3. Results
A total of 102 cases were included in the study: 55 in the hospital-based cohort (mean age at MRI 74.7±8.5 years, 40% women) and 47 in the population-based cohort (mean age at MRI 83.4±10.9 years, 48.9% women). Hospital and community populations significantly differed in most of the demographic and clinical characteristics (Table 2). Notably, dementia at the time of MRI and development of lobar ICH were significantly more frequent in hospital-based individuals (p<0.001 and p=0.03, respectively). Also, median lobar MB count in strictly lobar MB carriers was significantly higher in the hospital cohort (p<0.001). Autopsy represented the source of pathological data in 100% of community-based individuals and 56.4% of hospital-based individuals; the remaining cases underwent brain biopsy. Cause of death/indication for autopsy in hospital-based individuals was as follows: primary cardio-respiratory disease in 4(12.9%); massive lobar ICH in 3(9.7%); neurodegenerative processes (other than AD) in 3(9.7%); systemic diseases in 2(6.5%); brain tumor in 1(3.2%); multiple ischemic strokes in 1 (3.2%); and not available in 17(54.8%). Indications for brain biopsy were: encephalopathy/rapid cognitive decline (with or without accompanying seizures) and related MRI abnormalities in 13(54.2%); clinical and radiographical suspicion of CNS vasculitis in 5(20.8%); brain tumor in 2(8.4%); ICH (in subjects with a previous MRI study) in 2(8.4%); excision of brain aneurysm in 1(4.1%); and hemicraniectomy secondary to large ischemic stroke in 1(4.1%). CAA prevalence was higher in the hospital cohort (60% vs 46.8%), though this difference did not reach statistical significance. Cases with “probable CAA” accounted for 29.1% of the hospital cohort and 8.5% of the community cohort. Table 3 displays the distribution of all MB patterns across both study cohorts. Mixed MB cases were all predominantly lobar (lobar MB outnumbered non-lobar MB) in the hospital cohort.
Table 2.
Comparison of characteristics between hospital and community-based cohorts.
| Hospital-based cases (n = 55) |
Community- based cases (n = 47) |
p-value | |
|---|---|---|---|
| Female sex, n(%) | 22(40) | 23(48.9) | 0.36 |
| Age at MRI study, years (mean ± SD) | 74.7±8.5 | 83.4±10.9 | <0.001 |
| Age at pathological study, years (mean ± SD) | 75.9±9 | 87.2±10.7 | <0.001 |
| Time MRI-pathology, years (mean ± SD) | 1.2±2.8 | 3.8±2.4 | <0.001 |
| T2*-gradient-echo study, n(%) | 51(92.7)a | 47(100) | 0.06 |
| Autopsy study, n(%) | 31(56.4)b | 47(100) | <0.001 |
| Hypertension, n(%) | 35(63.6) | 37(80.4) | 0.09 |
| Antiplatelet drug users, n(%) | 22(40) | 11(28.2) | 0.07 |
| Anticoagulant users, n(%) | 6(10.9) | 3(10.7) | 0.42 |
| Pre-existing dementia, n(%) | 34(61.8) | 12(25.5) | <0.001 |
| Lobar ICH after baseline MRI, n(%) | 5(9) | 0(0) | 0.03 |
| Presence of any lobar MB, n(%) | 27(49) | 10(21.3) | 0.003 |
| Lobar MB count, median(range)c | 20(1-129) | 2(1-7) | <0.001 |
| CAA at pathological examination, n(%) | 33(60) | 22(46.8) | 0.18 |
MB=microbleed; CAA= cerebral amyloid angiopathy (defined as Vonsattel grade ≥2)
The remaining cases underwent susceptibility-weighted imaging
The remaining cases underwent brain biopsy
Only in individuals with strictly lobar MB
Table 3.
Distribution of MB patterns across cases from the hospital (n=55) and community-based (n=47) cohorts.
| “Probable CAA” | No “probable CAA” | |||||
|---|---|---|---|---|---|---|
| Total | “Possible CAA” | Mixed MB | Non-lobar MB only | No MB | ||
| Hospital | 16 | 39 | 2 | 9 | 0 | 28 |
| Community | 4 | 43 | 2 | 4 | 5 | 32 |
Sensitivity, specificity, PPV, NPV and likelihood ratios of MB-only “probable CAA” for CAA diagnosis in both populations are displayed in Table 4. Two MB-only “probable CAA” cases in the hospital cohort, and 3 in the community cohort, did not have any evidence of moderate/severe CAA at pathological examination (false positives); moderate or severe degree of HV was the only alternative explanation for the presence of lobar MBs in 4 out of these 5 cases.
Table 4.
Sensitivity, specificity, predictive values and likelihood ratios of MB-only “probable CAA” (≥2 strictly lobar MB) for pathologically-confirmed CAA in hospital and population-based cohorts.
In the hospital cohort, mixed MBs yielded the following diagnostic parameters: specificity 70% [95%CI 45.7 – 88.1], sensitivity 22.2% [95%CI 6.4 – 47.6], PPV 44.4% [95%CI 15.3% - 77.3%], NPV 50% [95%CI 31% - 68.9%], +LR 0.84 [95%CI 0.3 - 2.7] and −LR 1 [95%CI 0.8 – 1.4].
In the hospital-based cohort, logistic regression analysis showed that the presence of multiple strictly lobar MB was the only predictor of moderate/severe CAA at pathology (p=0.01), irrespective of age, dementia and development of lobar ICH. Within the subgroup of individuals with multiple strictly lobar MB (“probable CAA”), increasing lobar MB count was significantly associated with CAA (p=0.03). Logistic regression was not performed in the community-based cohort given the low number of cases with strictly lobar MB.
4. Discussion
The main finding of our study is that, in individuals without lobar ICH, the presence of multiple strictly lobar MBs on MRI does not predict CAA consistently across different populations. We found that almost 90% of non-ICH individuals with “probable CAA” studied in a hospital setting harbored moderate or severe CAA; however, in community elderly individuals, only 25% of those with “probable CAA” had the disease. This suggests that strictly lobar MB on MRI might have a higher diagnostic accuracy for CAA in hospital populations than in the community. Although these results arise from cohorts of limited sample size, they might provide guidance for appropriate selection of subjects in observational studies and intervention trials on CAA.
The importance of studying the diagnostic value of lobar MBs per se has been previously recognized[14, 27, 28], though never addressed in a radiological-pathological correlation study. The original description of the Boston Criteria emphasized the lobar topography of brain hemorrhages as the key feature for CAA diagnosis[12]. As used in the Criteria, the term “lobar hemorrhage” is inclusive, and implicitly attributes the same diagnostic weight to lobar ICH (large, symptomatic hemorrhage) and lobar MB (small, usually asymptomatic hemorrhage). However, pathologic studies conducted to validate the Boston Criteria have focused almost exclusively in lobar ICH patients[4, 13, 14]. Concerning lobar MBs found in the absence of ICH, little insight has been provided by small pathological series and case reports[29, 30]. It is indirect evidence from population-based studies and memory clinic cohorts that has mainly supported the potential role of lobar MBs alone as markers of CAA. In these studies, lobar MBs were found associated with APOE-ε4 allele and they showed a predominantly posterior distribution across the brain lobes[11, 31, 32], resembling findings from “probable CAA” cases in ICH cohorts [16, 33]. Furthermore, recent work has shown that older patients with lobar MBs in the absence of ICH, brain tumor or dementia were very similar to “probable CAA” patients presenting with a lobar ICH in terms of baseline clinical, genetic and radiologic characteristics[34]. Our study provides pathological insight into these previous observations and suggests that the Boston Criteria may be applied reliably to individuals without large symptomatic hemorrhages when a supporting clinical context exists (i.e. old individuals with cognitive decline or other syndromes potentially related to CAA[35, 36]). Furthermore, the results of our regression analysis imply that increasing number of strictly lobar MB, beyond the classic 2-hemorrhage cut-off set by the Boston Criteria, may increase our ability to identify CAA.
Specificity and sensitivity are fixed characteristics of a diagnostic test (in our study, the presence/absence of strictly lobar MB on MRI). Thus, it is not surprising that specificity of “probable CAA” was similarly high in both cohorts. However, sensitivity was much lower in the community-based cohort compared to the hospital-based cohort. This can be partially explained by the higher time lapse between MRI and pathological studies in the community-based cohort, which may have artificially increased the number of false negative cases. In other words, some CAA+ individuals who did not have lobar MB at the time of MRI could have been classified as false negatives but may have developed lobar MB in the years preceding death. Additionally, the use of SWI imaging in some hospital-based cases may have resulted in an overall increased MB detection[21] in the hospital-based cohort and, possibly, higher sensitivity values. In the community cohort, the aforementioned inflated rate of false negative cases may have also decreased the NPV. Nevertheless, a similarly low NPV was observed in the hospital cohort, which argues against interpreting the absence of strictly lobar MB as the absence of CAA. This is consistent with the fact that CAA prevalence was greater than the prevalence of strictly lobar hemorrhages in both sets of study subjects.
There may be several factors responsible for the higher PPV of “probable CAA” in the hospital-based cohort compared to the community-cohort. First, the higher prevalence of CAA in hospital-based individuals likely contributes to the observed increased PPV. Second, logistic regression on the “probable CAA” subgroup from the hospital-based cohort showed that increasing lobar MB burden was associated with CAA diagnosis. Although we could not explore such association in the community-based cohort, the fact that lobar MB counts were significantly higher in hospital individuals suggests that higher lobar MB burden may have contributed to the observed increased PPV in the hospital-based cohort. Thirdly, older age, cognitive decline and development of lobar ICH are known to be associated with CAA[6, 7, 37], and all these characteristics were more frequent in the hospital-based cohort. We could not identify an independent association between these conditions and CAA, but they still may have contributed to the observed increased PPV.
Paradoxically, we found that in the community-based cohort, the PPV estimate was lower than the actual CAA prevalence, implying that the presence of strictly lobar MB in this cohort lowers the baseline likelihood of CAA. Although the factors mentioned above could have favored such a low PPV estimate in the community cohort, the wide confidence intervals may call into question the reliability of this estimate. However, while the true PPV in the community-based cohort may be higher than our estimate, nevertheless our results suggest that strictly lobar MB may be less informative of the CAA status in the general population. Better characterization of clinical contexts that favor CAA diagnosis may be warranted.
Our study focused on the diagnostic value of multiple strictly lobar MB. However, the finding of a single lobar MB is not infrequent in healthy elderly individuals[11, 15, 38], and poses important diagnostic challenges. Although what is identified as a single MB may represent an early sign of a widespread vasculopathy, misidentification of normal vessel structures as a single MB may reduce diagnostic accuracy in these cases. Unfortunately, we could not determine the diagnostic value of a single lobar MB, as this scenario was rarely identified in either of the study cohorts. Future, larger studies will need to address the significance of this particular diagnostic category.
We also investigated the accuracy of lobar MBs for CAA when found in conjunction with non-lobar MB. This so-called mixed MB pattern can be found in as much as 5.5% of healthy elderly individuals[11] and 25% of patients with ICH[33]. Classically, this pattern has been attributed to either the coexistence of CAA and HV or the solely presence of HV. In our hospital cohort, all mixed cases were predominantly lobar, and yet the PPV of mixed MBs for CAA was more than 40% lower than the PPV of strictly lobar MBs. It is conceivable that this difference could even be greater if predominantly non-lobar patterns were also present. This argues against using mixed MB profile to target individuals for CAA-related research or interventions.
The major strength of our study is the use of two highly distinct populations to test the accuracy of the Boston Criteria for CAA in individuals with only MBs. Particularly valuable is the contribution from the FHS, which allowed for the first-ever pathological correlation study between lobar MB and CAA in a general elderly population. The prevalence of “probable CAA” and pathologically-confirmed moderate/severe CAA in our study are generally in line with previous reports[11, 13, 37, 39] may thus support the generalizability of our results. We intentionally did not investigate other neuroimaging markers, such as superficial siderosis, dilated perivascular spaces in the white matter, antero-posterior distribution of white matter disease or cortical PiB retention on PET imaging, which have previously shown to have potential utility in the diagnosis of CAA[13, 40-42]. This was in order to better focus the study on the particular significance of lobar MBs, a widely accepted neuroimaging marker easily accessed in clinical practice and known to many clinicians. Indeed, with this approach, our results have highlighted a real need for ancillary markers to increase current sensitivity of the Boston Criteria for CAA diagnosis.
The main limitations of this study are found in the hospital-based cohort, mainly due to its retrospective nature. In this cohort, several factors may have contributed to a bias towards a higher likelihood of CAA: 1) lack of a systematic pathological assessment of CAA in all individuals; 2) inability to ensure that this assessment was blind to clinical and radiological data; 3) high number of brain biopsies, which were often performed due to a high clinical suspicion of CAA; 3) our decision to accept small pathological specimens with evidence of CAA, while excluding those lacking CAA (due to a suspected insufficient amount of material for conclusive diagnosis). The latter represented a strategy conceived to reduce false positive cases with the goal of approaching the true specificity of lobar MBs for CAA. While these limitations are important, they reflect the reality of clinical practice in that individuals assessed with hemosiderin-sensitive MR imaging in a hospital, especially in the context of cognitive decline, are more likely to harbor a larger number of lobar MB and have pathologic manifestations of moderate to severe CAA. Finally, it is necessary to emphasize that our results should be interpreted with caution due to the small sample sizes and wide confidence intervals.
In conclusion, our study provides further evidence for the role of strictly lobar MB as CAA markers in different contexts. Strictly lobar MB could be highly accurate markers of CAA in individuals with features that support the diagnosis. From this point of view, lobar MB can be used as a tool to identify patients with CAA that have not developed lobar ICH. Conversely, indiscriminate screening for the presence of lobar MB in unselected elderly population may be problematic and lead to an unacceptable rate of false positive cases. Future work should aim to identify novel markers to increase our ability to capture non-hemorrhagic or pre-hemorrhagic forms of CAA. A reliable identification of individuals with CAA in the absence of lobar ICH represents a critical step towards a better clinical management, and the eventual design of therapeutic trials to prevent CAA progression and its associated clinical consequences.
Systematic review.
Lobar microbleeds (MB) are frequently found in elderly individuals, even asymptomatic. In the specific context of lobar intracerebral hemorrhage (ICH), lobar MB have shown to be highly indicative of cerebral amyloid angiopathy (CAA); however, pathological evidence supporting their role as more general, widely reliable markers of CAA is largely lacking.
Interpretation.
In the absence of ICH, strictly lobar MB are highly predictive of CAA in hospital-based individuals, but not in community-based ageing populations. These results may provide an objective basis to guide appropriate selection of individuals without ICH for CAA therapeutic trials.
Future directions.
Further investigations on the natural history of CAA are needed to ascertain whether lobar MB generally represent an early stage of the disease, or the manifestation of a particular phenotype not prone to ICH.
7. Acknowledgements/funding sources
We would like to thank Teresa Gomez-Isla (MD, PhD) and Jamary Oliveira-Filho (MD, PhD,) for their highly valuable comments and suggestions on the study design and execution.
This work (design and conduct of the study, collection and management of the data) was financially supported by:
- Grants from the National Institute of Neurological Disorders and Stroke (R01NS070834) and the National Institute on Aging (5P50AG005134-30) – Massachusetts General Hospital
- The Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195) and grants from the National Institute of Neurological Disorders and Stroke (R01 NS17950), the National Institute on Aging (R01 AG16495; AG08122; AG033193; AG031287; K23AG038444; P30AG13846); NIH grant (1RO1 HL64753; R01 HL076784; 1 R01 AG028321) and the Department of Veterans Affairs - Framingham Heart Study.
Abbreviations
- CAA
Cerebral amyloid angiopathy
- ICH
Intracerebral hemorrhage
- MB
Microbleed
- AD
Alzheimer’s disease
- FHS
Framingham Heart Study
- PPV
Positive predictive value
- NPV
Negative predictive value
- MGH
Massachusetts General Hospital
- GRE
T2*-gradient echo
- SWI
Susceptibility-weighted image
- HV
Hypertensive vasculopathy
- +LR
Positive likelihood ratio
- −LR
Negative likelihood ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
conflicts
All authors declare no conflicts of interest related to the present work.
6. References
- 1.Pantelakis S. [A particular type of senile angiopathy of the central nervous system: congophilic angiopathy, topography and frequency] Monatsschr Psychiatr Neurol. 1954;128:219–256. [PubMed] [Google Scholar]
- 2.Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54:22–31. [PubMed] [Google Scholar]
- 3.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke; a journal of cerebral circulation. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara T, Takahashi M, Yokota T, et al. The significance of cerebrovascular amyloid in the aetiology of superficial (lobar) cerebral haemorrhage and its incidence in the elderly population. J Pathol. 1991;165:229–234. doi: 10.1002/path.1711650306. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke; a journal of cerebral circulation. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 7.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Annals of neurology. 2011;69:320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996;46:1751–1754. doi: 10.1212/wnl.46.6.1751. [DOI] [PubMed] [Google Scholar]
- 9.Offenbacher H, Fazekas F, Schmidt R, Koch M, Fazekas G, Kapeller P. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. AJNR American journal of neuroradiology. 1996;17:573–578. [PMC free article] [PubMed] [Google Scholar]
- 10.Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–1360. doi: 10.1212/01.wnl.0000210535.20297.ae. [DOI] [PubMed] [Google Scholar]
- 11.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg SM. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology. 1998;51:690–694. doi: 10.1212/wnl.51.3.690. [DOI] [PubMed] [Google Scholar]
- 13.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rooden S, van der Grond J, van den Boom R, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke; a journal of cerebral circulation. 2009;40:3022–3027. doi: 10.1161/STROKEAHA.109.554378. [DOI] [PubMed] [Google Scholar]
- 15.Romero JRPS, Beiser A, et al. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. doi: 10.1161/STROKEAHA.114.004130. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 17.Kiely DK, Wolf PA, Cupples LA, Beiser AS, Myers RH. Familial aggregation of stroke. The Framingham Study. Stroke; a journal of cerebral circulation. 1993;24:1366–1371. doi: 10.1161/01.str.24.9.1366. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. American journal of epidemiology. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 19.Romero JR, Preis SR, Beiser A, et al. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke; a journal of cerebral circulation. 2014;45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au R, Seshadri S, Knox K, et al. The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Current Alzheimer research. 2012;9:673–686. doi: 10.2174/156720512801322609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR American journal of neuroradiology. 2009;30:338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg SM, O'Donnell HC, Schaefer PW, Kraft E. MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology. 1999;53:1135–1138. doi: 10.1212/wnl.53.5.1135. [DOI] [PubMed] [Google Scholar]
- 24.Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. Journal of neuropathology and experimental neurology. 1971;30:536–550. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke; a journal of cerebral circulation. 2009;40:2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke; a journal of cerebral circulation. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. The New England journal of medicine. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 28.Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain : a journal of neurology. 2011;134:335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- 29.Tatsumi S, Shinohara M, Yamamoto T. Direct comparison of histology of microbleeds with postmortem MR images: a case report. Cerebrovascular diseases. 2008;26:142–146. doi: 10.1159/000139661. [DOI] [PubMed] [Google Scholar]
- 30.Schrag M, McAuley G, Pomakian J, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta neuropathologica. 2010;119:291–302. doi: 10.1007/s00401-009-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesker DJ, Poels MM, Ikram MA, et al. Lobar distribution of cerebral microbleeds: the Rotterdam Scan Study. Archives of neurology. 2011;68:656–659. doi: 10.1001/archneurol.2011.93. [DOI] [PubMed] [Google Scholar]
- 32.Pettersen JA, Sathiyamoorthy G, Gao FQ, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Archives of neurology. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 33.Smith EE, Nandigam KR, Chen YW, et al. MRI markers of small vessel disease in lobar and deep hemispheric intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2010;41:1933–1938. doi: 10.1161/STROKEAHA.110.579078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Etten ESAE, Haley KE, et al. Incidence of Symptomatic Hemorrhage in Patients with Lobar Microbleeds. Stroke. 2014 doi: 10.1161/STROKEAHA.114.005151. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke; a journal of cerebral circulation. 2012;43:2324–2330. doi: 10.1161/STROKEAHA.112.657759. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg SM, Vonsattel JP, Stakes JW, Gruber M, Finklestein SP. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology. 1993;43:2073–2079. doi: 10.1212/wnl.43.10.2073. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 38.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. Journal of neurology, neurosurgery, and psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanskanen M, Lindsberg PJ, Tienari PJ, et al. Cerebral amyloid angiopathy in a 95+ cohort: complement activation and apolipoprotein E (ApoE) genotype. Neuropathology and applied neurobiology. 2005;31:589–599. doi: 10.1111/j.1365-2990.2005.00652.x. [DOI] [PubMed] [Google Scholar]
- 40.Charidimou A, Jaunmuktane Z, Baron JC, et al. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82:57–62. doi: 10.1212/01.wnl.0000438225.02729.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, et al. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology. 2014 doi: 10.1212/WNL.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurol ME, Dierksen G, Betensky R, et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. 2012;79:320–326. doi: 10.1212/WNL.0b013e31826043a9. [DOI] [PMC free article] [PubMed] [Google Scholar]