Abstract
Background
To determine if there have been contemporary shifts in IE epidemiology in our local population, an analysis of cases from 2007–2013 was conducted.
Methods
Population-based review of all adults (≥18 years) residing in Olmsted County, Minnesota, with definite or possible IE using the Rochester Epidemiology Project from January 1, 2007 through December 31, 2013.
Results
We identified 51 cases of IE in Olmsted County, MN between 2007 and 2013. Median age of IE cases was 68.8 years (IQR 55.6–76.5), and 41% were females. Age- and sex-adjusted incidence of IE was 7.4 (95% CI, 5.3–9.4) cases per 100,000 person-years. From a multivariable Poisson regression model, incidence of IE did not change significantly during the study period (p=0.222) but was significantly higher in males and those of older age (p<0.001). The annual incidences (per 100,000 person years) was 2.5 for Staphylococcus aureus, 1.1 for viridans group streptococci, 1.6 for Enterococcus species, and 0.8 for coagulase-negative staphylococci. Only 19.6% (10/51) of Olmsted County patients underwent valve surgery between 2007–2013 as compared to 44.4% (197/444) of non-Olmsted County patients treated at Mayo Clinic Rochester.
Conclusion
In this population-based study, no significant change in the overall incidence of IE in Olmsted County, MN, between 2007 and 2013 was seen and it was similar to that seen between 1970 and 2006. Male gender and older age were associated with increased IE risk. With a lesser extent of cases attributable to viridans group streptococcal IE compared to previous years, S. aureus was the predominant pathogen in IE cases during 2007–2013. The relatively low valve surgery rate was disparate from that reported from large, tertiary care centers (including our own) with non-population-based cohorts, which are subject to referral bias and can influence the expected characterization of IE.
Keywords: Infective endocarditis, Staphylococcus aureus, viridans group streptococci
Introduction
The epidemiology of infective endocarditis (IE) varies, depending on a number of host factors whose prevalence can be wide-ranging among different populations globally. Moreover, the epidemiology of IE can vary within a population over time; such is the case in the United States over the past half-century1. Several factors have impacted the clinical spectrum of IE in this country, including a decreasing prevalence of rheumatic carditis, increased use of intracardiac devices specifically implantable cardiac defibrillators (ICD)2, an enlarging hemodialysis population, an aging population, and increasing incidence of Staphylococcus aureus bacteremia1, 3, 4. Because IE is both infrequent and management requirements are complex, many reports on its prevalence come from large, tertiary care centers5–7. These reports can be distorted by referral bias and may not reflect true changes in the epidemiology of IE8–10.
Olmsted County, MN, provides a unique opportunity to conduct population-based studies due to the Rochester Epidemiology Project (REP). The REP is a medical records-linkage system established in 1966 providing the capability for population-based studies of disease causes and outcomes that is unique in the United States11. Our group has extensively studied IE cases in Olmsted County, Minnesota between 1970 and 20063, 8. We found no substantial change in the incidence of IE over this time period and no significant temporal trends in the incidence of either Staphylococcus aureus or viridans group streptococcal IE, with viridans group streptococci surpassing S. aureus as the most common causative organism of IE in this population3, 8. In contrast, an increasing incidence and mortality for IE over the past decade have been described in other cohorts1, 10, 12. Due to these differences, we performed a more contemporary temporal trend analysis of the epidemiology of IE in Olmsted County, MN, between 2007 and 2013 to determine whether clinical features of IE have changed in this population.
Methods
The unique features of Olmsted County that make it ideal in conducting population-based studies have been discussed previously8. The Endocarditis Registry of the Division of Infectious Diseases and REP database were our resources for case ascertainment, residential status, and data collection. We identified all Olmsted County residents 18 years or older with a diagnosis of IE between January 1, 2007 and December 31, 2013. For each of these potential cases, their medical record was thoroughly reviewed to verify possible or definite IE, as defined by the modified Duke criteria13. We excluded cases of cardiac implantable electronic device related IE.
Demographic, clinical, laboratory, and outcome data were collected from the medical record using a standardized data abstraction form with detailed definitions of the variables. Death at 6 months was obtained from the electronic medical record in the majority of cases and confirmed by the recording of all notices of deaths by the Mayo Clinic registration office.
Infection site of acquisition was collected from review of the medical records. The definitions of community-acquired, nosocomial, and health care-associated infections were described by Friedman et al.14 Correa de Sa et al,3 discussed the definitions for each site of acquisition and we followed the same definitions. Our study design and data collection of patients were reviewed and approved by the Mayo Clinic Institutional Review Board.
Descriptive statistics were used to summarize the cohort, including counts and percentages for categorical data and medians and interquartile ranges (IQR) for continuous data. For estimating incidence rates, the adult population (≥18 years of age) of Olmsted County between the years 2007 and 2013 was considered to be at risk, with counts of age-, sex-, and calendar time-specific stratum in the denominator enumerated from annual census data. Age- and sex-adjusted incidence rates were computed based on direct standardization against the 2010 US White population. Ninety-five percent confidence intervals (95% CIs) were estimated assuming the Poisson distribution for numbers of cases. Reported rates of incidence are summarized across the entire study period over all ages (or by age group) for descriptive purposes, though formal testing of trends in incidence was analyzed on count data specified in strata per single calendar year and per integer year of age. In particular, the association of sex, age, and calendar time with incidence was evaluated with multivariable Poisson regression. Functional forms of the latter two predictor variables were informed based on graphical impressions of a flexible smoothing curve, estimated by the non-parametric loess method, relating observed incidence to years over entire ranges of age and calendar time. In addition, all two-way interactions among these three main effects were simultaneously tested for significance to avoid inflating the type I error rate. All analyses were performed using the statistical programming language SAS (Version 9.3, SAS Institute Inc., Cary, NC). The level of significance for statistical testing was two-sided, with an alpha of 0.05.
This study was supported by research grants from the Edward C Rosenow III, M. D., Professorship in the Art of Medicine, the Mayo Foundation for Medical Education and Research, and the National Institutes of Health (NIH) grant R01 AG034676 (REP grant).
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
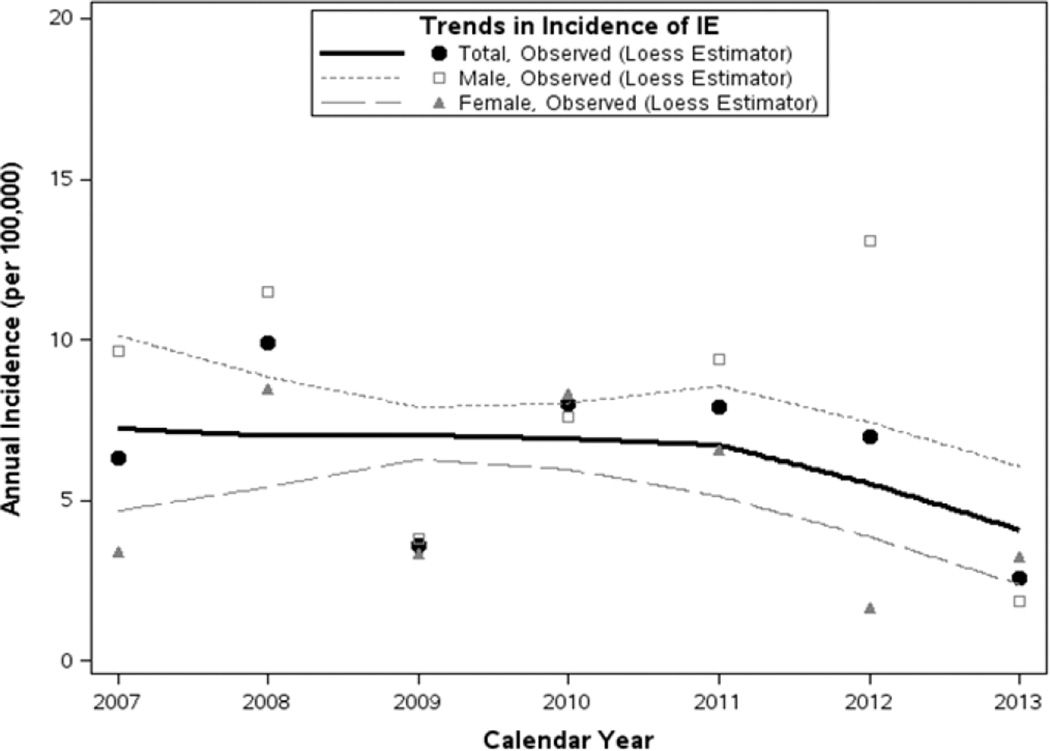
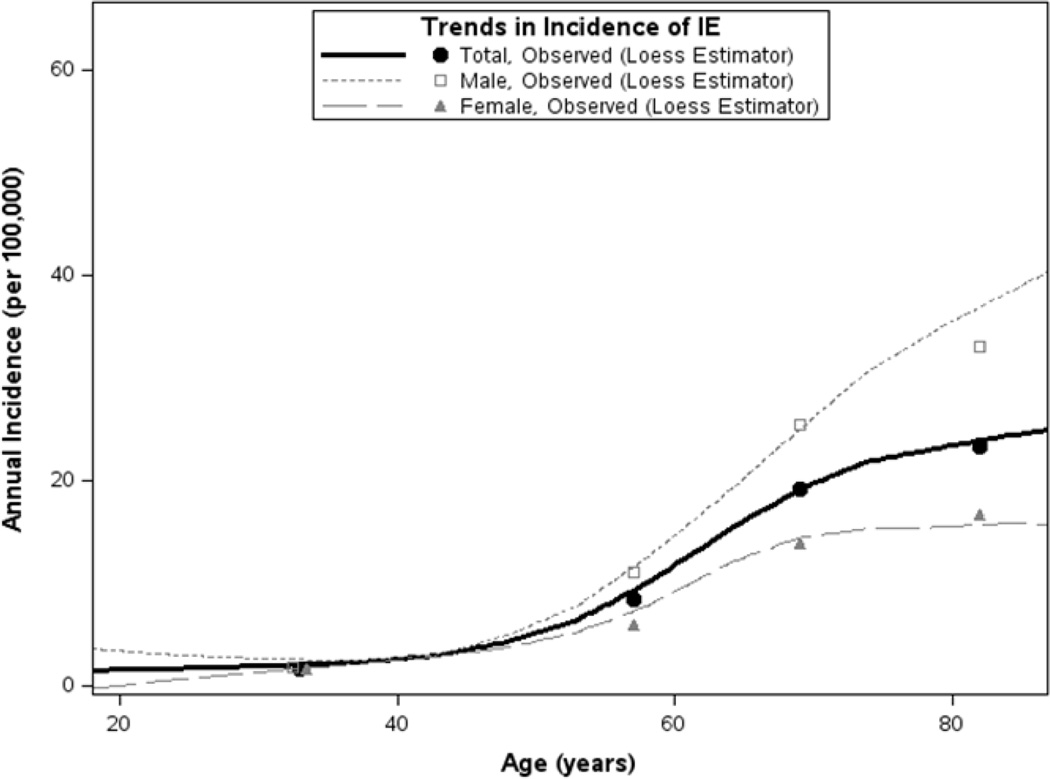
Fifty-one cases of IE were identified in Olmsted County, MN between 2007 and 2013. The age- and sex-adjusted incidence rates of IE (along with 95% CIs) during the study period was 7.4 (5.3 – 9.14) cases per 100,000 person-years (Table 1). For modeling of incidence trends, we assumed linearity in calendar time and age effects since loess-smoothed trends in the observed rates showed no evidence to the contrary (Figures 1 and 2), as well as non-additivity in the model terms since a likelihood ratio test on the group of 2-way interactions was not significant (differences in log-likelihood=5.44, 3 d.f.; p=0.177). From this multivariable model, incidence of IE did not change significantly during the study period (p=0.222). Although, incidence of IE was significantly increased in males (annual incidence [95% CI] per 100,000, 9.5 [6.1 – 13.0] for males compared to 5.7 [3.2 – 8.1] for females; p=0.039) and with older age (p<0.001).
Table 1.
Incidence of IE from 2007–2013 in Olmsted County
| Age | Females | Males | Total |
|---|---|---|---|
| 18–49 | 4 (1.6) | 4 (1.8) | 8 (1.7) |
| 50–64 | 6 (6.0) | 10 (11.1) | 16 (8.4) |
| 65–74 | 5 (13.8) | 8 (25.4) | 13 (19.2) |
| 75+ | 6 (16.8) | 8 (33.1) | 14 (23.3) |
| All Ages | 5.7 (3.2, 8.1) | 9.5 (6.1, 13.0) | 7.4 (5.3, 9.4) |
Age-specific results are presented as number of cases and unadjusted annual rate of incidence (per 100,000), while bolded across-all-age results are represented as incidence rates and 95% confidence intervals that were directly adjusted for age (and gender for “Total” rates) with respect to the 2010 U.S. White population.
Figure 1.
Incidence of infective endocarditis in Olmsted County, Minnesota between 2007 and 2013; incidence did not change significantly over this period (p=0.222), though rates were generally higher in males (p=0.039).
Loess-smoothed curves of total and gender-specific incidence summarize trends from 2007–2013 based on all rates derived per single years of age and calendar time (data points not shown), with corresponding rates of observed incidence (across all ages) shown at each calendar year for reference.
Figure 2.
Incidence of infective endocarditis in Olmsted County, 2007–2013 by age and gender; increasing rates of incidence with older age (p<.001).
Loess-smoothed curves describe total and gender-specific incidence as a function of age using all rates estimated per integer year of age over the entire period; for brevity, observed incidence rates derived on age groups (18–49, 50–64, 65–74, and ≥75 years) are displayed at the midpoint of each respective age interval.
The demographic and clinical characteristics of IE cases over time are summarized in Table 2. The median age of IE cases was 68.8 years (IQR, 55.6–76.5) years, with 41% of the 51 cases females, and 10% intravenous drug abusers (IVDA). Seventy-one percent of cases had native valve IE, with 43% having only aortic valve involvement, 20% only mitral valve involvement, and 8% both aortic and mitral valve involvement. There were 29% of IE cases with prosthetic valve associated IE, 14% with bicuspid aortic valve, 10% with congenital heart disease, 6% with previous IE, and 2% with rheumatic heart disease; and no cases with mitral valve prolapse were seen.
Table 2.
| Variable | IE 2007–2013 (n=51) |
|---|---|
| Patient Characteristics | |
| Age, years (median [IQR]) | 68.8 (55.6, 76.5) |
| Female gender | 21 (41%) |
| Rheumatic heart disease | 1 (2%) |
| Mitral valve prolapse | 0 (0%) |
| Congenital heart disease | 5 (10%) |
| Bicuspid aortic valve | 7 (14%) |
| Prosthetic | 15 (29%) |
| Previous IE | 3 (6%) |
| Native | 35 (69%) |
| Aortic (Only) | 22 (43%) |
| Mitral (Only) | 10 (20%) |
| Aortic and mitral | 4 (8%) |
| Right-sided or bilateral | 4 (8%) |
| IVDA | 5 (10%) |
| Microbiology | |
| Viridans group streptococci | 8 (16%) |
| Staphylococcus aureus | 17 (33%) |
| Enterococcus species | 11 (22%) |
| Coagulase-negative staphylococci | 5 (10%) |
| HACEK | 0 (0%) |
| Outcomes | |
| Valve surgery (within 6 weeks) | 8 (16%) |
| Valve surgery (6 weeks to 1 year) | 2 (4%) |
| 6-month mortality | 15 (29%) |
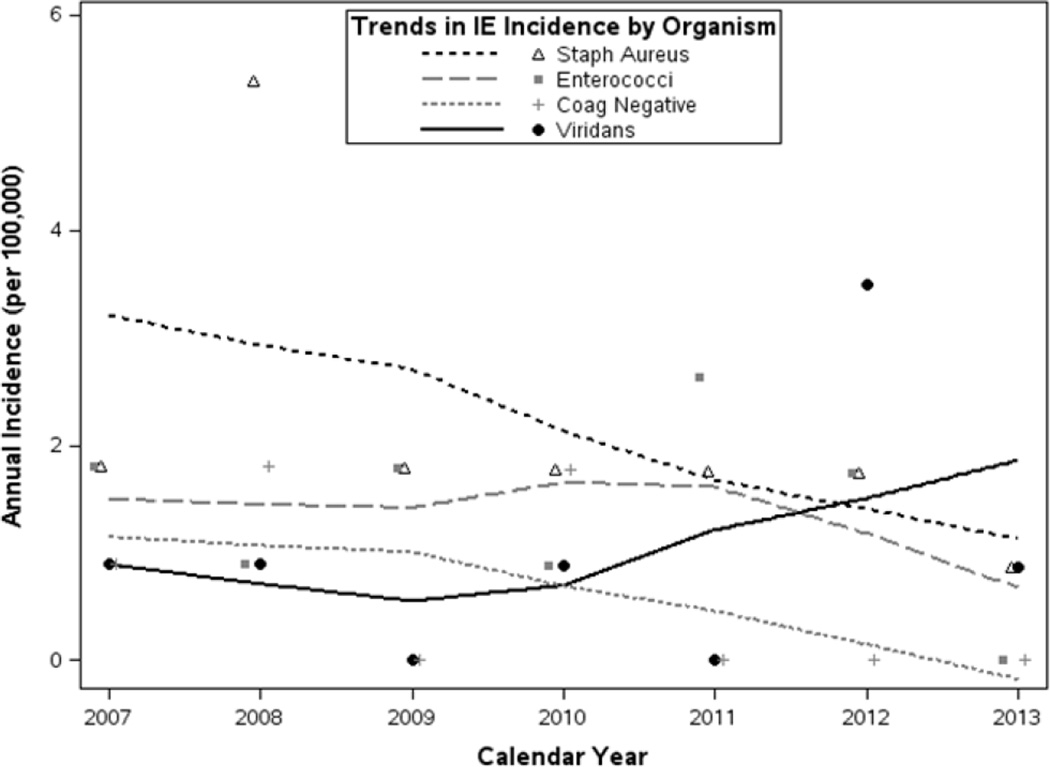
Microbiology and clinical outcomes are also summarized in Table 2. Of the 51 cases, 17 (33%) were due to Staphylococcus aureus, 8 (16%) from viridans group streptococci, 11 (22%) from Enterococcus species, 5 (10%) from coagulase-negative staphylococci, and none were due to the HACEK group. Incidence of microorganism-specific IE is described in Table 3, with higher rates in males noted for viridans group streptococci and enterococcal IE. Similar to overall IE, organism-specific rates tended to increase with age, but no significant changes in incidence were observed between 2007 and 2013 (Figure 3, Table 3). Coagulase-negative staphylococcal IE trended downward over this period though the number of cases was low (n=5) and not statistically significant (p=0.081). Eight (16%) of 51 IE cases underwent cardiac valve surgery within 6 weeks of diagnosis, and 2 (4%) others underwent surgery between 6 weeks and 1 year. Fifteen patients (29%) died within six-months following diagnosis. Infection site of acquisition was obtained in cases between 2007 and 2013, and 55% were health care-associated, 45% were community-acquired, and none were nosocomial infections.
Table 3.
| Organism | Annual Incidence^ (95% CI), per 100,000 | Test for Trend* P- value |
||
|---|---|---|---|---|
| Females | Males | Total | ||
| Viridans group streptococci | 0.0 (0.0, 0.7) | 2.2 (0.7, 3.8)† | 1.1 (0.3, 1.9) | 0.307 |
| Staphylococcus aureus | 2.9 (1.2, 4.7) | 2.0 (0.4, 3.6) | 2.5 (1.3, 3.6)‡ | 0.130 |
| Enterococcus species | 0.3 (0.0, 0.8) | 3.3 (1.2, 5.4)† | 1.6 (0.7, 2.6)‡‡ | 0.508 |
| Coagulase-negative Staphylococcus | 0.8 (0.0, 1.7) | 0.7 (0.0, 1.7) | 0.8 (0.1, 1.4)‡‡ | 0.081 |
| Other | 1.4 (0.2, 2.6) | 0.9 (0.0, 2.0) | 1.2 (0.4, 2.0)‡ | 0.903 |
All rates were directly adjusted for age and gender, and are reported along with 95% confidence intervals
Effect of calendar time assessed in multivariable Poisson regression with age and gender also included in the model
Incidence significantly higher in males than in females (p<0.05 for gender effect in Poisson regression model)
Rates increased with age (p<0.05 for linear age term)
Increasingly higher incidence with older age (p<0.05 for quadratic age term)
Figure 3.
Incidence of infective endocarditis in Olmsted County, 2007–2013 by organism.
Loess-smoothed rates of incidence of organism-specific IE summarize trends from 2007–2013 based on all gender-, single year age- and calendar year-specific estimates; for reference, observed incidence (summarized over all ages and both genders) is displayed annually for each microorganism.
Discussion
Since 1970, the population of Olmsted County, Minnesota, has been extensively studied for a multitude of diseases, including IE. Moreover, the majority of population-based IE studies have come from Olmsted County. Tleyjeh et al.8, evaluated temporal trends in IE between 1970 and 2000 and found no change in the incidence of IE, with viridans group streptococci outnumbering S. aureus as the most common causative agent of IE. More recently, Correa de Sa et al.3, examined this population through 2006 and described an increasing incidence of IE in women with no statistically significant temporal trends in the incidence of IE due to viridans group streptococci or S. aureus. Viridans group streptococci continued to outnumber S. aureus in this population for over three decades. Additional population-based cohort studies have been conducted15,16 in France where they found S. aureus to be the leading cause of IE, highest in elderly men (aged 75–79 years), and no increase in viridans group streptococcal IE incidence following reductions in antibiotic prophylaxis indications.
The present study provides a more contemporary view of the incidence and epidemiology of IE in Olmsted County between 2007 and 2013. Several important changes were identified in this population, including a significantly increased rate of IE in males and those of older age. Our observation of IE predominately affecting older men is a finding that is consistent with other studies1, 8, 17. Furthermore, S. aureus and enterococcal IE have outnumbered viridans group streptococci in many contemporary, large institutional experiences1, 10, 14, 18. IVDA was relatively high (10% of IE cases in this cohort), which represents an increase from 3% seen in a previous study of the Olmsted County population8 and could have impacted the prevalence of S. aureus cases. The proportion of prosthetic valve IE cases was relatively low (29%) compared to large tertiary care referral centers with cardiothoracic specialists, thus limiting referral bias. Between 2007 and 2013, the age- and sex-adjusted incidence rates of IE did not change significantly over time and the incidence for this period was similar to that for 1970 thru 2006. Six-month mortality occurred in approximately 29% (15/51) of cases, which is consistent with previous reports in this population3, 8 and other hospital-based studies18, 19. Interestingly, 14 of 15 patients who died within 6-months did not undergo surgery. Seven of them did not have a surgical indication for valve surgery, and were medically managed. Five patients were deemed “too sick” or “too high-risk” for surgery and therefore not offered surgical intervention. There were 2 patients who were offered surgery, but declined, opting for comfort care measures only. One-year mortality was slightly increased to 37% (19/51). The rejection of these “high-risk” cases for surgery may underlie the relatively high 6-month and 1-year mortality.
Understanding the epidemiological trends in IE is vital in its prevention, diagnosis, and treatment. Globally, significant changes have occurred in the epidemiology of IE over the past half-century, especially in the past decade1. Most of these studies are hospital-based studies and have been performed at large tertiary care referral centers5–7, 10. These studies found an increased mean age at diagnosis and increased male:female ratio, as well as a change in microbiology with an increase in the percentage of cases of staphylococcal and enterococcal IE, while the percentage of viridans group streptococci and culture-negative IE have markedly decreased1. In 2005, the International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) found that S. aureus was the most common cause of IE in many industrialized nations20. A recent 5-year experience at two teaching hospitals in Rio de Janeiro, Brazil10, also found S. aureus as the predominant cause of IE with intravascular catheters as the main source of IE. Interestingly, a significant increase in IVDA-related IE was observed in North America between 2000–2010 compared to 1980–1990, with a strong correlation between IVDA and S. aureus IE1.
The finding of S. aureus as the most common organism of IE in Olmsted County may represent a major shift in the microbiology of IE where previous years viridans group streptococci was the primary pathogen. Despite no linear trend in the incidence of viridans group streptococci during our study period 2007–2013, there was a significant decrease in incidence over the extended timeframe from 1999 to 201321, as well as a reduced proportion of IE cases due to viridans group streptococci (from 37% of all IE cases in 1999–2006 to 16% in 2007–2013). Coinciding with this drop in prevalence of viridans group streptococci IE, there was a compensatory increase in the proportion of cases due to enterococcal IE (8% in 1999–2006, compared to 22% in 2007–2013), although the incidence of enterococcal IE did not increase significantly over the current or extended study periods. These findings from a population-based study mirror the microbiologic trends in prior hospital-based studies1, 14, 18, 20. A possible explanation for the decrease in viridans group streptococcal incidence may be related to the decline in prevalence in rheumatic heart disease in developed countries3. Although speculative, rheumatic carditis may serve as a unique substrate in the development of viridans group streptococci IE22, as studies from some areas where rheumatic fever remain endemic, viridans group streptococci remain the predominant pathogens in IE23, 24. This decline in rheumatic carditis may play a major role in the decline in cases of viridans group streptococcal IE from 1999 to 2013.
Our study period included a major change in the use of antibiotic prophylaxis prior to invasive dental, gastrointestinal, genital-uretal, and respiratory procedures to prevent IE—the 2007 American Heart Association (AHA) IE preventions guidelines25, resulting in a major reduction in the number of patients who would receive antibiotics prior to an invasive procedure. Our group published the first study in the United States examining the incidence of viridans group streptococci following the 2007 AHA guidelines and found no significant increase in incidence between 2007–201022. Recently, there has been an evaluation of a national database using ICD-9 coding that showed a significant increase in the incidence of streptococcal IE when they compared the study periods of 2000–2007 and 2008–201126. However, there is no unique ICD-9 code for the viridans group streptococci. Upon review of the ICD-9 codes used to designate “streptococcal IE” by Pant el al.26 included groups A, B, C, G beta-hemolytic streptococci, enterococcal species, and Streptococcus bovis—which could account for the observed increase in “streptococcal IE” incidence27.
In England, guidelines from the National Institute for Health and Clinical Excellence (NICE) were updated in 2008 where they recommended complete cessation of antibiotic prophylaxis for the prevention of IE27. Dayer et al.28, identified a significant increase in the number of cases of IE above projected historical trends starting approximately 3 months following the 2008 NICE guideline publication. Unfortunately, the microbiology of IE was not available, thus, preventing conclusions about pathogen-specific IE incidence. These studies have fueled the long running debate regarding the role of antibiotic administration for certain dental procedures in the prevention of viridans group streptococcal IE. Our group has recently published an extended evaluation of the Olmsted County, Minnesota population21, and identified a decrease in viridans group streptococcal IE incidence over the study period of 1999 to 2013. The present study between 2007 and 2013 did not identify a significant change in the incidence of IE, regardless of pathogen.
Approximately 20% of patients underwent valve replacement surgery within 1 year of diagnosis, with the majority (80%) of them undergoing surgery within the first 6 weeks from IE diagnosis. The relatively low surgical intervention rate was somewhat unanticipated because non-population based, tertiary care referral studies have reported surgical rates close to 50%18, 29, 30. Over the study period in our investigation, there were 444 non-Olmsted County patients treated at Mayo Clinic Rochester, a large tertiary care referral center. Interestingly, 44.4% (197/444) of these patients underwent cardiac valve surgery within 1 year of diagnosis. This difference in surgical rates between the Olmsted County and non-Olmsted County populations is most likely due to referral bias. Patients admitted to a large center may be sicker with multiple medical comorbidities, and have more indications for surgery. For example, Kanafani et al.31 evaluated the ICE-PCS, a prospective, multinational large cohort study comparing transferred and non-transferred patients with IE, and found that transferred patients underwent surgery for IE more often than non-transferred patients. Given that many existing studies on IE come from large referral centers, population-based studies like ours provide a more accurate and generalizable characterization of IE due to a reduction in referral bias.
Infection site of acquisition is also important in the epidemiology of IE locally. Correa de Sa et al.3 demonstrated similar rates of health care-associated and community-acquired sites of acquisition from 2001–2006 and the present study from 2007–2013, although a slight increase in cases of health care-associated IE was seen in the most recent group. Health care-associated IE in previous studies has been associated with up to 34% of all cases of IE and carries a higher morbidity and mortality when compared to community-acquired IE32.
Our study has limitations. The Olmsted County population is predominantly Caucasian, middle socioeconomic class with low prevalence of IVDA, making our study less generalizable to populations that differ from these demographics. In addition, our population is small and the total numbers of cases are approximately 5 to 7 cases per year. However, complete ascertainment of IE cases in a geographically isolated location limits referral bias. This allows for a thorough assessment of temporal trends of IE in this population.
Conclusion
In this population-based study, no significant change in the overall incidence of IE in Olmsted County, MN, between 2007 and 2013 was seen and it was similar to that seen between 1970 and 2006. Male gender and older age were associated with increased IE risk. With a lesser extent of cases attributable to viridans group streptococcal IE compared to previous years, S. aureus was the predominant pathogen in IE cases during 2007–2013. The relatively low valve surgery rate was disparate from that reported from large, tertiary care centers (including our own) with non-population-based cohorts, which are subject to referral bias and can influence the expected characterization of IE.
ACKNOWLEDGEMENT
Funding/Support
This study was supported by research grants from the Edward C Rosenow III, M. D., Professorship in the Art of Medicine, Mayo Foundation for Medical Education and Research. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Dr. Kevin Greason, Division of Cardiovascular Surgery, for his excellent review of the manuscript.
Role of the Sponsor
The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Dr. DeSimone had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study
DeSimone, Steckelberg, Wilson, Baddour.
Acquisition of data
DeSimone, Steckelberg, Anavekar, Tleyjeh, Correa de Sa, Baddour.
Analysis and interpretation of data
DeSimone, Sohail, Tleyjeh, Correa de Sa, Anavekar, Lahr, Wilson, Baddour.
Critical revisions of the manuscript for important intellectual content
DeSimone, Sohail, Tleyjeh, Correa de Sa, Lahr, Steckelberg, Anavekar, Wilson, Baddour.
Drafting of the manuscript
DeSimone, Wilson, Baddour.
Statistical analysis
DeSimone, Lahr, Baddour.
Obtained funding
DeSimone, Wilson, Baddour.
Administrative, technical, or material support
Wilson, Steckelberg, Baddour.
Study supervision
DeSimone, Steckelberg, Wilson, Baddour.
Financial Disclosures
Sohail: TyRx Inc. (moderate, $ <10,000)
Baddour: Royalty payments-UpToDate, Inc.; Editor-in-Chief payments-Massachusetts Medial Society (Journal Watch Infectious Diseases)
Conflict of Interest
None.
References
- 1.Slipczuk L, Codolosa JN, Davila CD, Romero-Corral A, Yun J, Pressman GS, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One. 2013;8(12):e82665. doi: 10.1371/journal.pone.0082665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Khatib SM, Hellkamp A, Curtis J, Mark D, Peterson E, Sanders GD, et al. Non-evidence-based ICD implantations in the United States. Jama. 2011;305(1):43–49. doi: 10.1001/jama.2010.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa de Sa DD, Tleyjeh IM, Anavekar NS, Schultz JC, Thomas JM, Lahr BD, et al. Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2010;85(5):422–426. doi: 10.4065/mcp.2009.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krecki R, Drozdz J, Ibata G, Lipiec P, Ostrowski S, Kasprzak J, et al. Clinical profile, prognosis and treatment of patients with infective endocarditis--a 14-year follow-up study. Pol Arch Med Wewn. 2007;117(11–12):512–520. [PubMed] [Google Scholar]
- 5.Cabell CH, Jollis JG, Peterson GE, Corey GR, Anderson DJ, Sexton DJ, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162(1):90–94. doi: 10.1001/archinte.162.1.90. [DOI] [PubMed] [Google Scholar]
- 6.McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med. 1987;82(4):681–688. doi: 10.1016/0002-9343(87)90001-5. [DOI] [PubMed] [Google Scholar]
- 7.Tleyjeh IM, Steckelberg JM. Changing epidemiology of infective endocarditis. Curr Infect Dis Rep. 2006;8(4):265–270. doi: 10.1007/s11908-006-0070-0. [DOI] [PubMed] [Google Scholar]
- 8.Tleyjeh IM, Steckelberg JM, Murad HS, Anavekar NS, Ghomrawi HM, Mirzoyev Z, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. Jama. 2005;293(24):3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 9.Steckelberg JM, Melton LJ, 3rd, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88(6):582–588. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 10.Damasco PV, Ramos JN, Correal JC, Potsch MV, Vieira VV, Camello TC, et al. Infective endocarditis in Rio de Janeiro, Brazil: a 5-year experience at two teaching hospitals. Infection. 2014;42(5):835–842. doi: 10.1007/s15010-014-0640-2. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis. 2011;11:48. doi: 10.1186/1471-2334-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 14.Elbey MA, Akdag S, Kalkan ME, Kaya MG, Sayin MR, Karapinar H, et al. A multicenter study on experience of 13 tertiary hospitals in Turkey in patients with infective endocarditis. Anadolu Kardiyol Derg. 2013;13(6):523–527. doi: 10.5152/akd.2013.172. [DOI] [PubMed] [Google Scholar]
- 15.Selton-Suty C, Celard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54(9):1230–1239. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 16.Duval X, Delahaye F, Alla F, Tattevin P, Obadia JF, Le Moing V, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol. 2012;59(22):1968–1976. doi: 10.1016/j.jacc.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS One. 2013;8(3):e60033. doi: 10.1371/journal.pone.0060033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunes MC, Gelape CL, Ferrari TC. Profile of infective endocarditis at a tertiary care center in Brazil during a seven-year period: prognostic factors and in-hospital outcome. Int J Infect Dis. 2010;14(5):e394–e398. doi: 10.1016/j.ijid.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Wu KS, Lee SS, Tsai HC, Wann SR, Chen JK, Sy CL, et al. Non-nosocomial healthcare-associated infective endocarditis in Taiwan: an underrecognized disease with poor outcome. BMC Infect Dis. 2011;11:221. doi: 10.1186/1471-2334-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. Jama. 2005;293(24):3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 21.DeSimone DC, Tleyjeh IM, Correa de Sa DD, Anavekar NS, Lahr BD, Sohail MR, et al. Incidence of Infective Endocarditis Due to Viridans Group Streptococci Before and After the 2007 American Heart Association's Prevention Guidelines: An Extended Evaluation of the Olmsted County, Minnesota, Population and Nationwide Inpatient Sample. Mayo Clin Proc. 2015;90(7):874–881. doi: 10.1016/j.mayocp.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desimone DC, Tleyjeh IM, Correa de Sa DD, Anavekar NS, Lahr BD, Sohail MR, et al. Incidence of infective endocarditis caused by viridans group streptococci before and after publication of the 2007 American Heart Association's endocarditis prevention guidelines. Circulation. 2012;126(1):60–64. doi: 10.1161/CIRCULATIONAHA.112.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trabelsi I, Rekik S, Znazen A, Maaloul I, Abid D, Maalej A, et al. Native valve infective endocarditis in a tertiary care center in a developing country (Tunisia) Am J Cardiol. 2008;102(9):1247–1251. doi: 10.1016/j.amjcard.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 24.Kanafani ZA, Mahfouz TH, Kanj SS. Infective endocarditis at a tertiary care centre in Lebanon: predominance of streptococcal infection. J Infect. 2002;45(3):152–159. doi: 10.1016/s0163-4453(02)91041-8. [DOI] [PubMed] [Google Scholar]
- 25.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 26.Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070–2076. doi: 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- 27.DeSimone DC, Wilson WR, Baddour LM. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011: the devil is in the details. J Am Coll Cardiology. 2015;66(10) doi: 10.1016/j.jacc.2015.05.079. [DOI] [PubMed] [Google Scholar]
- 28.Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time-series analysis. Lancet. 2015;385(9974):1219–1228. doi: 10.1016/S0140-6736(14)62007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simsek-Yavuz S, Sensoy A, Kasikcioglu H, Ceken S, Deniz D, Yavuz A, et al. Infective endocarditis in Turkey: aetiology, clinical features, and analysis of risk factors for mortality in 325 cases. Int J Infect Dis. 2015;30:106–114. doi: 10.1016/j.ijid.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Francischetto O, Silva LA, Senna KM, Vasques MR, Barbosa GF, Weksler C, et al. Healthcare-Associated Infective Endocarditis: a Case Series in a Referral Hospital from 2006 to 2011. Arq Bras Cardiol. 2014;0:0. doi: 10.5935/abc.20140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanafani ZA, Kanj SS, Cabell CH, Cecchi E, de Oliveira Ramos A, Lejko-Zupanc T, et al. Revisiting the effect of referral bias on the clinical spectrum of infective endocarditis in adults. Eur J Clin Microbiol Infect Dis. 2010;29(10):1203–1210. doi: 10.1007/s10096-010-0983-2. [DOI] [PubMed] [Google Scholar]
- 32.Francischetto O, Silva LA, Senna KM, Vasques MR, Barbosa GF, Weksler C, et al. Healthcare-associated infective endocarditis: a case series in a referral hospital from 2006 to 2011. Arq Bras Cardiol. 2014;103(4):292–298. doi: 10.5935/abc.20140126. [DOI] [PMC free article] [PubMed] [Google Scholar]