INTRODUCTION
Sepsis is defined as an infection associated with systemic manifestations of inflammation, which has become the most common complication in the perioperative period caused by severe burn/trauma and major surgical operation.[1,2] The incidence of sepsis in adults is estimated to be 149–240/100 000 per year, and that of severe sepsis and septic shock is 56–91/100 000 per year. In the last decade, the short-term mortality of sepsis has declined to around 20% in developed countries partly due to the international Surviving Sepsis Campaign (SSC), while the mortality remains very high (50%–80%) in 1–5 years after discharge from hospitals.[3–7] Thus, sepsis and its subsequent severe sepsis and septic shock are currently major issues in the field of medical and health care.
Control of infection, involving removal of infected and necrotic tissue, surgical drainage of abscess combined with early antimicrobial therapy, is essential to the successful treatment of sepsis.[8] Imbalance of pro-inflammation and anti-inflammation in septic patients is usually caused by the failure of infection control or the perforation of hollow organs. Fast exacerbation of sepsis constantly leads to circulatory and respiratory insufficiency and/or other viscera damage. Hence the risk of septic patients is very high during anesthesia. To improve the quality of clinical anesthesia and reduce the incidence of perioperative death and organ dysfunction in patients with sepsis, the Chinese Association of Anesthesiologists (CAA) set up a consensus committee consisting of 22 well-known Chinese anesthesiologists to draft a consensus on the perioperative management of patients with sepsis.
CLASSIFICATION OF RECOMMENDATIONS
Recommendations were classified according to the principles of ASA membership.[9] Firstly, the recommendations were designed as a questionnaire by the expert anesthesiologists. A questionnaire survey was conducted among the members of the CAA and the Youth Committee of the CAA (YCCAA) at the annual meetings. The members should independently make a single choice to each recommendation from the options including 1 – strongly supported, 2 – supported, 3 – equivocal, 4 – opposed, 5 – strongly opposed.
Responses to the questionnaire survey from the members were analyzed, and were used to grade the recommendations into class I to V:
Class I: at least 50% of the responses are 1 – strongly supported;
Class II: at least 50% of the responses are 2 – strongly supported, or 1 – strongly supported and 2 – supported;
Class III: at least 50% of the responses are 3 – equivocal, or no other option contains at least 50% of the responses;
Class IV: at least 50% of the responses are 4 – opposed, or 4 – opposed and 5 – strongly opposed;
Class V: at least 50% of the responses are 5 – strongly opposed.
Finally, the grade was signed at the back of recommen-dations to help the clinical anesthesiologists to use them.
DIAGNOSIS AND DEFINITION OF SEPSIS
Recommendations
1. The definitions of sepsis, severe sepsis and septic shock were based on the principles of definition and diagnosis proposed by the International Sepsis Definitions Conference (class I).
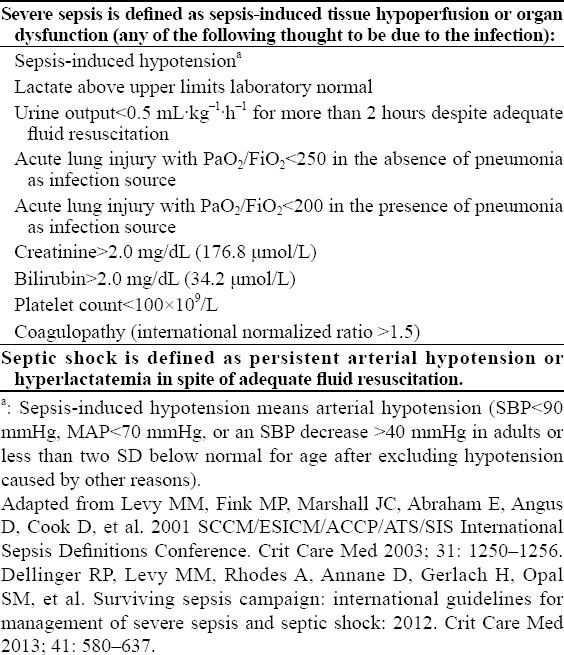
In 2001, an international sepsis definitions conference was held jointly by the Society of Critical Care Medicine (SCCM), European Society of Intensive Care Medicine, ESICM), American College of Chest Physicians (ACCP), American Thoracic Society (ATS) and Surgical Infection Society (SIS). The proposed definition and diagnosis of sepsis, severe sepsis and septic shock have been applied in clinics and research institutions for decades (Tables 1 and 2).[1,2] A recent retrospective study showed that about 12% of patients with severe sepsis did not meet two or more criteria of Systemic Inflammatory Response Syndrome (SIRS). Moreover, adjusted analysis revealed that the mortality of SIRS increased linearly with each additional criterion, without any transitional increase in risk at a threshold of two SIRS criteria.[9,10] Thus, we do not hold that the diagnosis of sepsis, severe sepsis and septic shock was strictly adherent to two or more SIRS criteria. It is necessary to get a detailed medical history, clinical symptoms, signs and laboratory examinations (Table 1), then to make a clinical diagnosis after comprehensive evaluation.
Table 1.
Diagnostic criteria for sepsis
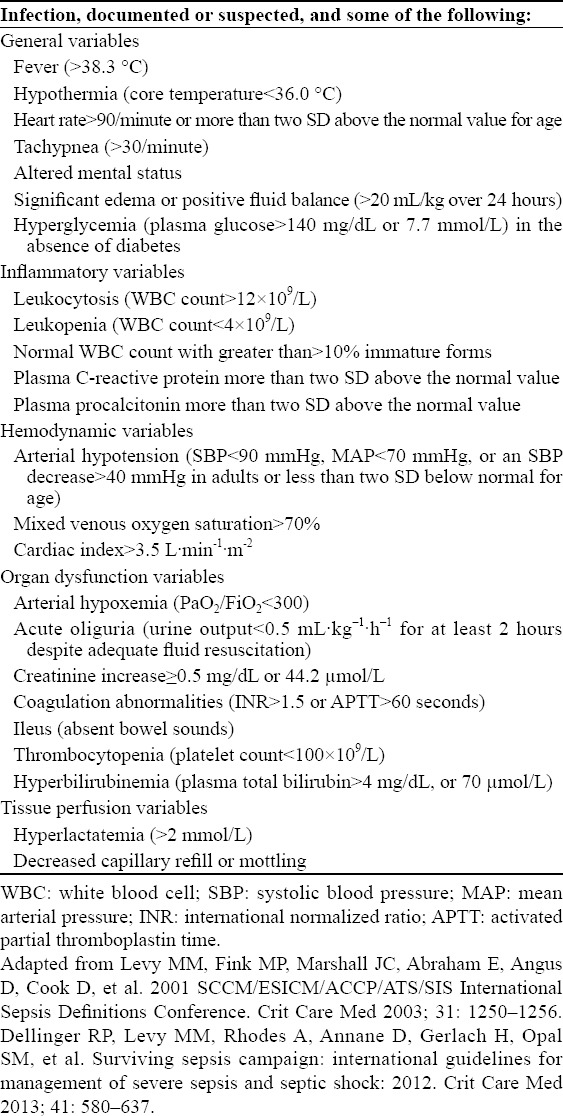
Table 2.
Severe sepsis and septic shock
PATHOPHYSIOLOGICAL CHARACTERISTICS OF SEPSIS
Sepsis and subsequent sepsis-related diseases are recognized as a group of clinical syndromes induced by trauma, pneumonia, suppurative cholangitis, suppurative peritonitis and severe acute pancreatitis. Clinical manifestations of sepsis vary among different patients, but the pathophysiologic process of sepsis is similar.
The occurrence and development of sepsis are mediated by various immune cells and inflammatory factors. In the early stage of sepsis, the immune system is over activated and mainly manifests as the uncontrolled systemic inflammatory response. The Toll-like receptors (TLRs) and the non-toll-like receptors (non-TLRs) expressed on host immune cells including monocyte/macrophages and neutrophils mediated the recognition of pathogen-associated molecular patterns (PAMPs) and/or danger-associated molecular patterns (DAMPs). In the late stage of sepsis, however, host gradually progresses to immunosuppressive state, showing that the response of immune cells to the pathogen is abate, primary focal infection is difficult to remove, and the secondary double infection and latent viruses replicate actively.[11,12]
The imbalance of immune and inflammatory response in the development of sepsis could inevitably cause the disruption of vascular endothelial barrier, tissue edema, hypotension, decrease of oxygen-carrying function of red blood cells, and thrombosis of microcirculation. Essentially, it causes tissue hypoperfusion, decrease of tissue oxygen supply, dysfunction of cellular oxygen metabolism, which leads to dysfunction of the nervous system, circulatory failure, respiratory dysfunction, hepatic/renal insufficiency, coagulation dysfunction, etc.[13–19]
PREOPERATIVE EVALUATION AND TREATMENT
Sepsis is lack of specific criteria of diagnosis in early stage, and it is likely to develop to severe sepsis or septic shock. Patients with sepsis often undergo emergency surgery, but anesthesiologists usually have inadequate time to evaluate these patients before the operation. Hence, preoperative analysis of organ function, medical history and surgical conditions of septic patients should be emphasized for the precise assessement of the risk of anesthesia and the effective therapy.
Assessment of organ function
The cardiovascular system
The impairment of the cardiovascular system is common in septic patients, which can subsequently develop to septic shock in severe cases. It has been reported that the incidence of cardiovascular dysfunction within 24 hours in septic patients is about 30%.[20] Moreover, the risk of anesthesia is related to the severity of cardiovascular dysfunction. Therefore, it is meaningful to assess the function of the cardiovascular system before surgery so as to identify early septic shock patients and provide early therapy.
Recommendations
Body temperature, skin color, urine volume, heart rate and blood pressure of septic patients should be observed closely to assess cardiovascular condition and determine septic shock early (class I).
At least two venous accesses should be implanted for fluid infusion in addition to the preparation of some micro-infusion pumps and vasoactive drugs including noradrenaline, adrenaline, vasopressin, dopamine, dobutamine and phenylephrine (class I).
Invasive artery blood pressure (IABP), central venous pressure (CVP) and blood lactate should be monitored to evaluate the effect of fluid management (class I).
Central or mixed venous oxygen saturation should be monitored to assess tissue perfusion of the patients (class III).
Cardiac ultrasound and test of FloTrac or PiCCO should be done to guide fluid management (class II).
The volume status, cardiac function, tissue perfusion and vascular tension of the patients are evaluated to determine whether they have cardiac dysfunction or septic shock. In the early stage of shock, the patients often demonstrate clammy and pale skin, hypourocrinia, increased heart rate and normal or increased blood pressure, etc. Hence, anesthesiologists must observe these symptoms so as to identify early septic shock before surgery. Once septic shock occurs, early goal directed therapy (EGDT) must be initiated. Referring to the international guidelines for the treatment of severe sepsis and septic shock proposed by the international surviving sepsis campaign (SSC guideline), targets for fluid management within 6 hours in patients with septic shock included: mean arterial pressure (MAP) ≥65 mmHg, CVP 8–12 mmHg, urine volume≥0.5 mL/kg per hour, ScvO2>70% and SvO2>65%.[2] It has been confirmed that EGDT could significantly decrease the mortality of septic shock patients.[21–23] IABP and CVP monitoring is necessary to evaluate the volume status and the effect of fluid management. Clinical studies[24–26] demonstrated that blood lactate was a standard marker for tissue infusion and the effect of fluid therapy, and the clearance rate of lactate was significantly related to the mortality of the patients. Recent multi-center clinical studies[2,27] found that fluid therapy targeted ScvO2>70% had no beneficial effect on the survival rate of patients with severe sepsis, which is consistent with the finding of River et al, a single center clinical study, indicating that fluid therapy targeted ScvO2>70% is beneficial for the patients with severe sepsis. Therefore, we still do not define the role of continuous monitoring ScvO2 in septic patients. As sepsis-induced cardiomyopathy and chronic heart disease are commonly associated with septic patients who are vulnerable to cardiac dysfunction, we suggest that cardiac ultrasound and test of FloTrac or PiCCO are helpful to assess cardiac function, fluid therapy and use of vasoactive drugs.[28]
The respiratory system
It has been reported that 30%–50% of septic patients have respiratory dysfunction, and that acute respiratory distress syndrome (ARDS) is regarded as one of the most common complications in patients with sepsis.[3,4,20] Thus it is necessary to determine whether sepsis induced ARDS has a pivotal role in intraoperative mechanical ventilation and postoperative clinical treatment.
Recommendations
Respiratory features, respiratory rate and SpO2 should be closely observed to evaluate respiratory function (class I).
Clinicians should perform arterial blood gas analysis and assess oxygenation status via oxygen index (PaO2/FiO2) (class I).
Imaging examinations including chest radiography, chest CT, and even lung ultrasonography could be used to assess lung injury (class II).
Berlin definition is used as the principle for the assessment of severity of ARDS (class II).
Oxygen inhalation with ordinary or oxygen masks, noninvasive positive pressure ventilation, and even emergency tracheal intubation with mechanical ventilation are used as a respiratory treatment according to the severity of the patients (class I).
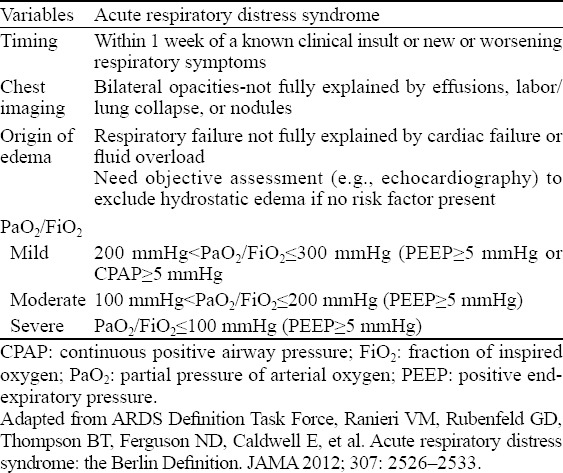
In 1994, the American-European Consensus Meeting first proposed the definition for ALI and ARDS (Table 3).[29] In 2011, a panel of experts convened a conference and modified the definition of ARDS, which was published later in JAMA as the Berlin Definition given by the European Critical Care Medicine (ECCM).[30] The updated definition of ARDS specified the acute time frame of ARDS (within 7 days of a known clinical insult or new or worsening respiratory symptoms), and erased the definition of ALI. In addition, the conference proposed 3 mutually exclusive severity categories of ARDS: mild (200 mmHg<PaO2/FiO2≤300 mmHg), moderate (100 mmHg<PaO2/FiO2≤200 mmHg) and severe (PaO2/FiO2≤100 mmHg). The standard of pulmonary artery occlusion pressure (PAOP) ≤18 mmHg was eliminated, while emphasizing differential diagnosis from heart failure and liquid overload. Moreover, meta-analyses suggested that the updated Berlin definition was strongly associated with the mortality of patients and could more accurately predict the outcome of ARDS as compared with the previous definition. Imaging examinations including chest radiography, chest CT and lung ultrasonography are beneficial for the assessment of lung injury and helpful to identify the type of lung injury (hydrothorax, lung consolidation and alveolar interstitial syndrome), which could enhance the sensitivity and specificity of diagnosis and guide the performance of mechanical ventilation.[31] Moreover, lung ultrasonography is an imaging tool for detecting the occurrence of “spared area” in lung tissue (at least one intercostal space of normal lung tissue exists in the areas of alveolar-interstitial syndrome) that can differentiate ARDS from cardiogenic pulmonary edema.[32–34]
Table 3.
The Berlin definition of acute respiratory distress syndrome
Renal function
Acute kidney injury (AKI) is due to inflammation reaction, endothelial injury and microcirculation disturbance, which were induced by sepsis. Studies[35,36] revealed that 50% of patients with AKI were induced by sepsis in the ICU, suggesting that sepsis is the main cause of AKI. AKI is regarded as an independent risk factor of death in septic patients.
Recommendations
Urine volume, serum creatinine and urea nitrogen are routinely tested to evaluate the renal function before surgery (class I).
Preoperative hyperkalemia (>5.5 mmol/L) should be adjusted using glucose combined with insulin, calcium supplement, sodium bicarbonate and furosemide (class II).
Continuous renal replacement therapy (CRRT) is given to the patients with severe renal function injury or renal failure while monitoring intermittent coagulation function to evaluate the risk of bleeding (class II).
Renal dysfunction, a clinical syndrome that often occurs in septic patients, is characterized by oliguria, increased serum creatinine and urea nitrogen, and even acid-base imbalance and hyperkalemia. Patients with abnormal electrolyte especially hyperkalemia (>5.5 mmol/L) should be treated before operation. Glucose combined with insulin can promote the transfer of potassium to reduce the concentration of serous potassium by enhancing the utility of glucose. Calcium supplements including calcium chloride and calcium gluconate which are able to stabilize cardiomyocytes, and can be used for the treatment of patients with hyperkalemia who are accompanied with abnormal electrical activity of myocardial cells and even fatal arrhythmia. Severe hyperkalemia combined with acidosis (pH<7.15) can be treated with sodium bicarbonate to promote the transfer of potassium into cells because of decreased concentration of serous potassium. Furosemide, loop diuretics, can be applied to patients with hyperkalemia who are combined with oliguria and fluid overload.[37–39] In patients with severe renal function injury or renal failure, CRRT should be considered. More importantly, invasive operation is not recommended within 24 hours after heparinization. Activated clotting time (ACT) should be monitored if an operation is inevitable and protamine should be used to antagonize heparin.[37]
Liver function
Liver injury is often ignored in the treatment of sepsis. Epidemiological investigation revealed that 20%–30% of ICU patients with sepsis had liver dysfunction within 24 hours that can be seen after discharge from hospitals.[40] Moreover, severe patients with sepsis may present with liver-kidney syndrome, liver failure and liver-kidney encephalopathy. As sepsis associated with liver function injury can affect the outcome of septic patients, clinicians should assess liver function of the patients to avoid exacerbation of liver injury during anesthesia and surgery.
Recommendations
1. The assessment of liver function should depend on the levels of bilirubin, alkaline phosphatase, ALT, AST, γ-GGTP, and serum protein in addition to coagulation function (class I).
The underlying mechanisms of sepsis-induced liver injury include liver microcirculation dysfunction, inflammation reaction and bile metabolic abnormalities along with mild liver cell injury. Therefore, septic patients with liver injury are mostly characterized by increased levels of alkaline phosphatase and bilirubin in contrast to the slightly incrased levels of ALT and AST.[41–43] Moreover, severe liver injury can lead to decreased level of protein as well as coagulation dysfunction. Thus, these results should be reviewed by anesthesiologists and the severity of liver injury be assessed before the operation.
The blood system
Sepsis may lead to anemia, dysfunction of the coagulation-fibrinolysis system, and even disseminated intravascular coagulation (DIC).[44,45] Sepsis-induced DIC severely threatens the life of patients, and increases the mortality.
Recommendations
Skin color, mucosal color, petechiae, purpura and ecchymoses should be closely monitored to predict the occurrence of severe anemia and coagulation dysfunction (class I).
Test of routine blood and coagulation function should be done before surgery. Thrombelastography is recommended as a method for dynamical monitoring of coagulation function if necessary (class I).
The dysfunction of blood system in septic patients is mainly characterized by a declined hemoglobin level, platelet depletion, coagulation dysfunction and even DIC. Patients with severe anemia commonly present wtih pale skin and mucosa. The patients who have a bleeding trend, coagulation dysfunction or DIC always present with petechiae, purpura and ecchymoses. Sepsis with explicit/implicit bleeding and intravascular thrombosis may lead to declined levels of hemoglobin and platelet. PT and APTT are prolonged in patients with coagulation dysfunction, even plasma protamine paracoagulation (3P) test is positive.[44–46] Thromboelastography is a method for dynamic monitoring of coagulation function while evaluating clot formation to stability.[47,48] If necessary, thromboelastography should be used to monitor the coagulation function of septic patients.
The nervous system
Septic patients are viable to develop dysfunction of the nervous system. It has been reported that 70% of patients with sepsis have symptoms of neurological dysfunction, and about 50% have sepsis-associated encephalopathy (SAE).[15] Sepsis induced brain dysfunction is associated with the overall outcome of the patients.
Recommendations
Whether the patients have sepsis-induced dysfunction of the nervous system should be determined according to patients’ consciousness, cognition, response status and compliance with the instruction (class II).
Sepsis induced brain dysfunction is due to general inflammatory response, imbalance of oxygen delivery, and consumption of brain tissue. SAE is characterized by acute changes in mental status, impaired attention, irritation, disorientation, alteration of sleep/wake cycle, lethargy and even coma. In septic patients, pathological changes in the brain were seen on imaging, including cytotoxic edema, vasogenic edema, ischemic lesions, white matter disruption and brain atrophy.[15–17] Sepsis induced dysfunction of the nervous system not only increased the mortality but also affected the long-term prognosis of patients.[18] Iwashyna et al[19] found that 17% of septic patients who survived for 3 years suffered from moderate to severe cognitive dysfunction. Therefore, it is necessary to evaluate whether patients have sepsis-induced dysfunction of the nervous system according to their consciousness, cognition, response status, and compliance with the instruction.
Other assessment and preparation
Sepsis induces abnormality of glucose metabolism by affecting the endocrine system under inflammatory stress as well as water and electrolyte disorder as a result of liver and kidney dysfunction. Abdominal trauma, infection and intestinal twist and obstruction lead to intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS), which disturb the cardiovascular and respiratory function of septic patients. Thus the relevant symptoms, signs of the patients as well as the results of laboratory examinations should be monitored carefully, and appropriate treatments should be given to correct the abnormality before surgery.
Recommendations
Blood glucose, water-electrolyte and acid-base balance should be assessed, and appropriate treatments are necessary to correct the abnormality before surgery (class I).
To assess the occurrence of IAH and ACS, abdominal pressure should be monitored according to the IAH and ACS Consensus on the Definitions and Clinical Guidelines published by the World Society of Abdominal Compartment Syndrome (WSACS) (class II).
Sepsis can lead to dysfunction of the neuro-endocrine system and increase the excitability of the sympathetic nervous system, resulting in the abnormalities of glucose and lipid metabolism. Blood glucose testing should be done to find the patients with hyperglycemia, and venous insulin should be used reasonably before surgery. RCTs[2,49,50] revealed that intensive insulin therapy targeting blood glucose to 80–110 mg/dL did not significantly decrease the mortality of adult patients in the ICU, whereas a large international randomized trial (NICE-SUGAR trial) demonstrated an increased mortality. Additionally, Brunkhorst et al[51] reported that septic patients did not benefit from intensive insulin therapy, whereas strikingly increased the risk of hypoglycemia. Clinical studies[49–51] also showed that blood glucose levels>180 mg/dL as the threshold to start an intravenous insulin might be more reasonable for critically ill patients or septic patients.
More studies[52,53] have been focused on intra-abdominal hypertension. About 60% of septic patients had IAH, which could further develop to ACS if the treatment was inappropriate. In 2013, the WSACS consensus on definition and management of IAH and ACS has been revised.[54] The updated consensus or guidelines recommend that a sustained or repeated intra-abdominal pressure (IAP)>12 mmHg is defined for the occurrence of IAH, whereas a sustained IAP>20 mmHg [with or without an abdominal perfusion pressure (APP)>60 mmHg] for ACS, that is associated with organ dysfunction/failure. Lots of factors are associated with IAH and ACS, including diminished abdominal wall compliance, increased intra-luminal content, increased intra-abdominal content, capillary leak/fluid resuscitation, etc (Table 4).[54,55] Therefore, it is necessary to evaluate the occurrence of IAH and ACS according to the factors and related examinations, and to monitor IAP if necessary.
Table 4.
Risk factors for intra-abdominal hypertension and abdominal compartment syndrome

Medical history
Septic patients are often complicated with chronic diseases such as tumor, coronary heart disease, diabetes and chronic obstructive pulmonary disease.[56] These patients are in a poor physical capacity and immune status, and easily develop complications and organ dysfunction, even severe sepsis and septic shock.
Recommendations
Medical history of the patients should be assessed in detail. If necessary, experts from other departments should be called for consultation in evaluating the risk of anesthesia and surgery and making a plan for perioperative management (Class II).
In the medical history, tumor, coronary heart disease, diabetes and chronic obstructive pulmonary disease are susceptible factors of sepsis that can increase the risk of death. Such patients are always under poor basic conditions resulted from cardiovascular, renal and liver dysfunction before surgery, sepsis can further exacerbate functional injury of related organs and even cause death of the patients.[57,58] Therefore, the detailed medical history should be assessed including history of chronic diseases, current physical capacity, and whether there are organ dysfunction and the severity of sepsis in the preoperative period. In addition, to ensure the safety of patients in the perioperative period, other related departments should be called for consultation in evaluating the risk of anesthesia and surgery and making a plan of perioperative management if necessary.
Screening and control of infection source
Control of infection source is the most important treatment of sepsis. Patients with trauma, burn injury and intestinal obstruction/perforation have a definite infectious site, whereas there are also patients with masked focal infection. Therefore, communication between anesthesiologists and surgeons is of great importance on the detailed medical history and tests for assisting to identify the infection source and pathogenic bacteria for early application of antibiotic therapy.
Recommendations
Communication between anesthesiologists and surgeons is required to obtain samples of suspicious focal infection for culture before application of first-line anti-microbial agents (class II).
Bacteriological examination should be done for the patients who are suspicious of catheter-related infection (arteriovenous catheter, urethral catheter and pleural or abdominal drainage tube) before surgery (class I).
“SSC guidelines” recommend that antibiotics should be prescribed within 1 hour after the diagnosis of sepsis, and bacterial culture should be done before antibiotic therapy.[2] A clinical study demonstrated that early application of antibiotic therapy was associated with decreased mortality of patients with severe sepsis and septic shock.[59] However, because of the emergency surgery of septic patients surgeons may ignore bacterial culture and antibiotic therapy before surgery. Hence, communication is necessary between anesthesiologists and surgeons to ensure the early rational application of antibiotics. Studies showed that 80% of the patients had sepsis caused by hospital acquired infection, and that in these patients catheter-related infection is the main causal factor.[3–5] Thus check tubes should be retained in patients, including arteriovenous catheter, urethral catheter, and pleural or abdominal drainage tube. Moreover, bacteriological examination needs to be performed to exclude catheter-related infection.
Preoperative preparation
Perioperative transportation, an important proceure in the management of anesthesia, is associated with the safety of patients.[60] Although septic patients have received basic cardiovascular and respiratory supportive treatment in the early preoperative period, the condition of patients is still vulnerable, especially in the process of transportation to the operating room. Thus, every step in the treatment should be well prepared to guarantee the safety of the patients in the process of transportation.
Recommendations
Continuous monitoring is needed for the patients during the process of transportation, and attention should be paid to the handover of personnel, facilities and drugs (class I).
When septic patients are transported to operation room they should be subjected to persistent monitoring of oxygenation status, blood pressure, heart rate, etc.[61] Communication between surgeons and anesthesiologists is beneficial for the preoperative preparation. The individuals responsible for transportation, persistent monitoring and handover of personnel, facilities and drugs are important for the safety of patients during the process of transportation.
INTRAOPERATIVE MONITORING AND MANAGEMENT
Septic patients are usually complicated with tumor, chronic pulmonary diseases, cardiovascular and cerebrovascular diseases and intake of immunosuppressive drugs. Since septic patients usually undergo emergency surgery and frequently have circulatory failure, respiratory dysfunction or other organ dysfunction, more attention should be paid to anesthetic methods and agents.
Anesthetic methods
Patients with sepsis always have a poor physical condition at the time of operation, and exhibite complicated pathophysiological changes. Hence anesthesia should be given carefully to these patients.
Recommendations
Local infiltration anesthesia, regional blocking anesthesia, and general anesthesia are feasible in septic patients, but they should be selected individually after preoperative evaluation (class I).
Intravertebral anesthesia is not suitable for septic patients if their hemodynamics is not stable (class II).
Reflux and aspiration must be avoided in patients with full stomach (class II).
Almost all the expert anesthesiologists agreed that local infiltration anesthesia, regional blocking anesthesia and general anesthesia are available for septic patients. The most appropriate anesthetic method was selected for the patient according to surgical site, surgical type, scope of surgery and operative time.[8] In 1992, Carp et al[62] reported that spinal block increased the risk of infection of the central nervous system in septic rats. Furthermore, intravertebral anesthesia, especially spinal block, greatly influenced the hemodynamics because of the blocking of the sympathetic nervous system, which could result in deterioration of circulatory failure of septic patients. Thus, intravertebral anesthesia is not recommended for septic patients with instable hemodynamic. Septic patients with gastrointestinal perforation, intestinal obstruction or infection of the biliary tract are subjected to emergency surgery and in whom attention should be paid to the prevention of reflux and aspiration.
Anesthetic drugs
Septic patients are often complicated with circulatory instability and damage to the liver and kidney. Hence in the process of anesthetic induction and maintenance, the effect of anesthetics on circulatory function as well as the liver and kidney burden should be considered.
Recommendations
Titration method of drug delivery is recommended in the process of anesthetic induction to avoid hemodynamic fluctuation (class I).
Midazolam, propofol, ketamine and etomidate as intravenous anesthetics are suitable for septic patients, but etomidate is not indicated for patients with adrenal insufficiency (class II).
Fentanyl, sufentanil and remifentanil as opioid analgesics are suitable for septic patients (class I).
Cisatracurium, atracurium, rocuronium and vecuronium as muscle relaxants are suitable for septic patients (class II).
N2O, isoflurane, sevoflurane and desflurane as volatile anesthetics are feasible for septic patients, but N2O is not indicated for patients with pneumothorax or intestinal obstruction (class II).
Dexmedetomidine as an adjuvant is indicated for septic patients during anesthetic induction and maintenance (class II).
Anesthetics including sedative drugs and opioid analgesics are effective in inhibiting cardiac function and relaxing vessels. Titration method of drug delivery is can reduce the disturbance of hemodynamic.[8] Midazolam is a member of benzodiazepines which has the effect of sedation, hypnosis and anterograde amnesia, and it is often used to prevent intraoperative awareness. Based on the slight effect on the circulation, ketamine and etomidate are more suitable for septic patients.[63] Although etomidate can inhibit the function of adrenal cortex, clinical studies demonstrated that single-dose etomidate could not influence the outcome of septic patients.[64,65] Ketamine, another safe and valuable agent for endotracheal intubation, does not increase the risk of side-effect in septic patients.[66,67] Although animal studies showed that propofol could significantly inhibit myocardial and circulatory function,[68] Tang et al[69–71] found that propofol has a beneficial effect on the regulation of inflammatory response. Thus we must be careful when propofol is used in septic patients. It is necessary to adopt the titration method, starting from a low dosage while adjusting it slowly according to the response of the patients. At present, there is no clinical evidence indicating that inhalation anesthesia is beneficial for patients with sepsis. Schilling et al[72] found that volatile anesthetics could improve the outcome of patients with ischemia-reperfusion injury who had undergone cardiac surgery. Dexmedetomidine has the effect of sedation and inflammatory regulation, which could reduce the prevalence of delirium in critical ill patients.[73] It is feasible as an adjuvant for septic patients to decrease the dosage of other anesthetics.
Intraoperative monitoring
Besides routine monitoring, it is necessary to monitor the organ function, tissue perfusion and depth of anesthesia for septic patients in the perioperative period.
Recommendations
Routine monitoring including electrocardiogram, blood pressure, SpO2, PETCO2, pressure of airway, respiratory waveform, body temperature and urine output should be used for septic patients (class I).
Invasive blood pressure and central venous pressure should be monitored for patients with hemodynamic instability (class I).
FloTrac, PiCCO, Swan-Ganz and cardiac ultrasound could be used for septic patients, and the parameters of stroke volume variation (SVV), pulse pressure variation (PPV), cardiac index (CI), and corrected flow time (FTc) could be used to guide fluid therapy during the operation (class II).
Blood glucose, arterial blood gas, blood lactate, blood routine, blood electrolyte, coagulation function, BNP and troponin should be monitored during the operation (class I).
The depth of anesthesia and muscle relaxation should be monitored during the operation (class II).
Routine monitoring including electrocardiogram, blood pressure, SpO2, PETCO2, pressure of airway, respiratory waveform, body temperature and urine output is applicable for septic patients during the operation.[74] For patients with hemodynamic instability, advanced monitoring including invasive blood pressure and central venous pressure monitoring should also be performed. New techniques of monitoring such as FloTrac, PiCCO, Swan-Ganz and cardiac ultrasound could be used in evaluating myocardial contractility, which is useful to guide fluid therapy using the parameters of SVV, PPV, CI, FTc.[28] Blood glucose, arterial blood gas, blood lactate, blood routine, blood electrolyte, coagulation function, BNP and troponin should be monitored, and the depth of anesthesia and muscle relaxation also need to be monitored during the operation.
Management of the circulatory system
The evaluation of volume status and cardiac function is essential to the management of the circulatory system in septic patients during the operation. The strategy of EGDT including fluid challenge, blood transfusion and use of vasoactive agents would benefit septic patients.
Recommendations
The first objective of fluid management: MAP≥65 mmHg, CVP 8–12 mmHg, urine output≥0.5 mL/kg per hour, arterial blood lactate<4.0 mmol/L (class I).
The second objective of fluid management: ScvO2 ≥70% or SvO2≥65% (class III).
Crystalloid solution such as acetated Ringer’s solution or lactated Ringer’s solution was recommended as the first-choice fluid for septic patients, whereas hydroxyethyl starch was not recommended as a treatment for patients with severe sepsis during the operation. If it is inevitable to use colloid solution, albumin is suggested as the first choice (class II).
Norepinephrine is used as the first-choice vasopressor, then epinephrine, vasopressin, dopamine, dobutamine and phenylephrine could be used according to the condition of septic patients (class I).
Fresh frozen plasma (FFP) is not applicable for the increase of blood volume in septic patients (class II).
Platelet count should be beyond 50×109/L during the operation (class II).
The level of hemoglobin should be beyond 70 g/L during the operation (class II).
Intravenous hydrocortisone at a dose of 200 mg per day is prescribled as a treatment for septic patients when adequate fluid resuscitation and vasopressor therapy fail to restore stable hemodynamics (class II).
Clinical studies[27,75,76] found that EGDT did not reduce the mortality of patients with severe sepsis. However, since EGDT was incorporated into 2004 “SSC guidelines”, the mortality of patients with sepsis has decreased in the recent decades. Prospective retrospective studies also showed that adherence of “SSC guidelines” could improve the outcome of sepsis. Thus, EGDT is recommended as a fluid management for septic patients during the operation. The optimal target to evaluate the effect of fluid management is still worth investigating. Clinical studies[24–26] demonstrated that lactate clearance is an effective parameter to evaluate the fluid management of septic patients. The “SSC guidelines” also recommended that the recovery of blood lactate serves as a target of fluid management of septic patients.[2,77,78] Recently, Asfar et al[79] found that high blood pressure (MAP 80–85 mmHg) did not improve the outcome of septic patients, but reduced the rate of the renal replacement therapy in the patients who had hypertension before surgery. It is still difficult to decide which one is the best fluid for septic patients. Clinically, crystalloid solution such as acetated Ringer’s solution or lactated Ringer’s solution was recommended as the first-choice fluid, whereas hydroxyethyl starch was not used for the treatment of patients with severe sepsis.[2,77,78] A large dose of crystalloid solution was detrimental to the recovery of patients after the operation.[2] Therefore 5% albumin is recommended as the first-choice for the treatment of patients who have to use colloid solution. The 2012 “SSC guidelines” suggest norepinephrine as the first-choice vasopressor. Epinephrine, vasopressin, dopamine, dobutamine and phenylephrine could be prescribed according to the condition of patients when an additional agent is needed to maintain adequate blood pressure. FFP is recommended as a treatment for abnormal coagulation function, but not for regular fluid management. During the operation, platelet count should be maintained beyond 50×109/L. Up to present, there is no investigation specifically on the optimum concentration of hemoglobin in patients with severe sepsis. However, a clinical trial[2] suggested that a hemoglobin level of 70–90 g/L, compared with 100–120 g/L, was not associated with increased mortality in critically ill adult patients. Another randomized trial in patients undergoing cardiac surgery with cardiopulmonary bypass also showed a restrictive transfusion using a threshold of hemoglobin 80 g/L as equivalent to a transfusion threshold of hemoglobin 100 g/L. Several RCTs showed that the response of septic shock patients to fluid and vasopressor therapy seems to be an important factor in selecting patients for optional hydrocortisone therapy. Intravenous hydrocortisone at a dose of 200 mg/day could improve the outcome of septic patients who have failed to restore hemodynamic stability after adequate fluid resuscitation and vasopressor therapy.
Management of the respiratory system
Lung protective ventilation (LPV) could improve the outcome of patients with ARDS, and this bentilation is also suitable for septic patients during operation.
Recommendations
LPV is a treatment for septic patients with ARDS (including low tidal volume, PEEP and lung recruitment maneuver, etc.) (class I).
Sepsis commonly induces the dysfunction of the respiratory system. Clinical studies showed that LPV such as low tidal volume (6 mL/kg) and plateau pressure ≤30 cmH2O can significantly reduce the mortality of septic patients with ARDS.[80] Studies[80,81] also showed that low tidal volume (7 mL/kg), PEEP (10 cmH2O) and lung recruitment maneuver could significantly improve respiratory function, shorten the length of hospital stay, and improve the outcome of paitents after a major abdominal surgery.
Regulation of the immune system
Sepsis is a complex syndrome caused by a variety of diseases. The imbalance of immune and inflammatory response is the main pathophysiological mechanism of sepsis. The treatment of regulating immunity and inflammation would be beneficial to septic patients during operation.
Recommendations
Intravenous injection of immunoglobulin is not suitbale for septic patients during operation (class II).
Intravenous injection of ulinastain is suitable for the treatment of septic patients during operation (class II).
Subcutaneous injection of thymosin and intravenous drip of Xuebijing could be used as a treatment for septic patients during operation (class III).
Large multicenter RCTs in septic patients demonstrated that there is no benefit for intravenous injection of immunoglobulin (IVIG).[82] Ulinastain is a kind of protease inhibitor, which can stabilize the lysosome membrane, inhibit the release of lysosomal enzyme, eliminate oxygen radicals, and reduce the inflammatory response. Animal and clinical studies showed that ulinastain improved the outcome of surgical patients and septic patients via regulating oxidative stress and inflammatory response.[83–85] A meta-analysis[86] also found that the intraoperative use of ulinastatin could shorten mechanical ventilation time in the patients with extracorporeal circulation surgery. Recently, Karnad et al[87] have confirmed that ulinastatin can reduce the incidence of organ dysfunction, shorten mechanical ventilation time, and improve the outcome of septic patients. Thymosin is a group of small molecular peptides secreted by the thymus tissue, which can strengthen the immune function of the host, and has been used as a treatment for patients with viral hepatitis, immune deficiency disorders, and chemotherapy. In the treatment of sepsis, Wu et al[88] found that subcutaneous injection of thymosin alpha 1 reduced the mortality of septic patients. Additionally, a meta-analysis also revealed that thymosin obviously improved the outcome of patients with sepsis.[89] Xuebijing, a traditional Chinese medicine preparation, has functions of anti-inflammation, neutralizing endotoxin and regulating immune cells, etc. Animal experiments[90,91] showed that Xuebijing reduced the mortality of septic mice. A single center clinical study found that Xuebijing could promote the stabilization of hemodynamics and improve the outcome of patients with sepsis complicated with DIC.[92] Furthermore, Zhang et al[93] and Li et al[94] found that immunomodulatory therapy that combines thymosin alpha 1 and ulinastatin improved the survival rate of patients infected with carbapenem-resistant bacteria. However, these studies are done by single centers and the sample size is small, hance large RCTs are needed to elucidate the effect of immunomodulatory therapy combined with two or more agents in the treatment of septic patients.
TREATMENT IN INTENSIVE CARE UNIT
Postoperative management is very important for septic patients. If hemodynamics was stable without organ dysfunction, it is advisable to transfer the patients to the post anesthesia care unit (PACU) after surgery. However, if the patients are associated with severe chronic diseases, unstable breathing and circulation or other vital organ dysfunction, it is imperative to transfer the patients to the ICU for advanced treatment. The “SSC guidelines” have been revised twice based on large RCTs since 2004.[2,77,78] The present consensus focuses on the anesthesia-associated management during the perioperative period. Thus we suggest the 2012 “SSC guidelines” for the treatment in the ICU.
Recommendations
Patients with severe sepsis and septic shock should be transferred to the ICU for advanced treatment after surgery (class I).
List of recommendations
(Class I-V: class I is the strongest recommendation, class V is the weakest recommendation.)

Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: The authors declare that there are no conflicts of interest relevant to the content of the article.
Contributors: All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med 2012. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35:2538–2546. doi: 10.1097/01.CCM.0000284492.30800.00. [DOI] [PubMed] [Google Scholar]
- 5.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 6.Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. 2012;2:010404. doi: 10.7189/jogh.02.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 8.Eissa D, Carton EG, Buggy DJ. Anaesthetic management of patients with severe sepsis. Br J Anaesth. 2010;105:734–743. doi: 10.1093/bja/aeq305. [DOI] [PubMed] [Google Scholar]
- 9.American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120:268–286. doi: 10.1097/ALN.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 10.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 11.van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8:32–43. doi: 10.1016/S1473-3099(07)70265-7. [DOI] [PubMed] [Google Scholar]
- 12.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2014;370:583. doi: 10.1056/NEJMc1314999. [DOI] [PubMed] [Google Scholar]
- 15.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8:557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 16.Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13:630–636. doi: 10.1016/S1474-4422(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 17.Hosokawa K, Gaspard N, Su F, Oddo M, Vincent JL, Taccone FS. Clinical neurophysiological assessment of sepsis-associated brain dysfunction: a systematic review. Crit Care. 2014;18:674. doi: 10.1186/s13054-014-0674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, et al. Understanding brain dysfunction in sepsis. Ann Intensive Care. 2013;3:15. doi: 10.1186/2110-5820-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K, et al. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31:2332–2338. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 21.van Zanten AR, Brinkman S, Arbous MS, Abu-Hanna A, Levy MM, de Keizer NF, et al. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med. 2014;42:1890–1898. doi: 10.1097/CCM.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 22.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43:3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 23.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 24.Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the surviving sepsis campaign database. Crit Care Med. 2015;43:567–573. doi: 10.1097/CCM.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 25.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 27.Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. AmJ Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 30.Ranieri V, Rubenfeld GD, Thompson B, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative Diagnostic Performances of Auscultation,Chest Radiography, and Lung Ultrasonography in Acute Respiratory Distress Syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Stefanidis K, Dimopoulos S, Tripodaki ES, Vitzilaios K, Politis P, Piperopoulos P, et al. Lung sonography and recruitment in patients with early acute respiratory distress syndrome: a pilot study. Crit Care. 2011;15:R185. doi: 10.1186/cc10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leblanc D, Bouvet C, Degiovanni F, Nedelcu C, Bouhours G, Rineau E, et al. Early lung ultrasonography predicts the occurrence of acute respiratory distress syndrome in blunt trauma patients. Intensive Care Med. 2014;40:1468–1474. doi: 10.1007/s00134-014-3382-9. [DOI] [PubMed] [Google Scholar]
- 34.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagshaw SM, Langenberg C, Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48:695–705. doi: 10.1053/j.ajkd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Ghimire M, Pahari B, Sharma SK, Thapa L, Das G, Das GC. Outcome of sepsis-associated acute kidney injury in an intensive care unit: an experience from a tertiary care center of central Nepal. Saudi J Kidney Dis Transpl. 2014;25:912–917. doi: 10.4103/1319-2442.135229. [DOI] [PubMed] [Google Scholar]
- 37.Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol. 2010;6:521–529. doi: 10.1038/nrneph.2010.100. [DOI] [PubMed] [Google Scholar]
- 38.Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20:588–595. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oannidis M, Druml W, Forni LG, Groeneveld AB, Honore P, Oudemans-van Straaten HM, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit. Expert opinion of the Working Group for Nephrology, ESICM. Intensive Care Med. 2010;36:392–411. doi: 10.1007/s00134-009-1678-y. [DOI] [PubMed] [Google Scholar]
- 40.Kramer L, Jordan B, Druml W, Bauer P, Metnitz PG. Incidence and prognosis of early hepatic dysfunction in critically ill patients-a prospective multicenter study. Crit Care Med. 2007;35:1099–1104. doi: 10.1097/01.CCM.0000259462.97164.A0. [DOI] [PubMed] [Google Scholar]
- 41.Gilroy RK, Mailliard ME, Gollan JL. Gastrointestinal disorders of the critically ill. Cholestasis of sepsis. Best Pract Res Clin Gastroenterol. 2003;17:357–367. doi: 10.1016/s1521-6918(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 42.Nesseler N, Launey Y, Aninat C, Morel F, Mallédant Y, Seguin P. Clinical review: The liver in sepsis. Crit Care. 2012;16:235. doi: 10.1186/cc11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lescot T, Karvellas C, Beaussier M, Magder S. Acquired liver injury in the intensive care unit. Anesthesiology. 2012;117:898–904. doi: 10.1097/ALN.0b013e318266c6df. [DOI] [PubMed] [Google Scholar]
- 44.Zimmerman JL. Use of blood products in sepsis: an evidence-based review. Crit Care Med. 2004;32:S542–S547. doi: 10.1097/01.ccm.0000145906.63859.1a. [DOI] [PubMed] [Google Scholar]
- 45.Fourrier F. Severe sepsis, coagulation, and fibrinolysis: dead end or one way? Crit Care Med. 2012;40:2704–2708. doi: 10.1097/CCM.0b013e318258ff30. [DOI] [PubMed] [Google Scholar]
- 46.Yamakawa K, Ogura H, Fujimi S, Morikawa M, Ogawa Y, Mohri T, et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med. 2013;39:644–652. doi: 10.1007/s00134-013-2822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nair SC, Dargaud Y, Chitlur M, Srivastava A. Tests of global haemostasis and their applications in bleeding disorders. Haemophilia. 2010;16:85–92. doi: 10.1111/j.1365-2516.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 48.Holli Halset J, Hanssen SW, Espinosa A, Klepstad P. Tromboelastography: variability and relation to conventional coagulation test in non-bleeding intensive care unit patients. BMC Anesthesiol. 2015;15:28. doi: 10.1186/s12871-015-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, et al. Intensive versus conventional insulin therapy: A randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 50.The NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 51.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 52.Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822–829. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 53.Vidal MG, Ruiz Weisser J, Gonzalez F, Toro MA, Loudet C, Balasini C, et al. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med. 2008;36:1823–1831. doi: 10.1097/CCM.0b013e31817c7a4d. [DOI] [PubMed] [Google Scholar]
- 54.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holodinsky JK, Roberts DJ, Ball CG, Blaser AR, Starkopf J, Zygun DA, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care. 2013;17:R249. doi: 10.1186/cc13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubulotta F, Marshall JC, Ramsay G, Nelson D, Levy M, Williams M. Predisposition, insult/infection, response, and organ dysfunction: A new model for staging severe sepsis. Crit Care Med. 2009;37:1329–1335. doi: 10.1097/CCM.0b013e31819d5db1. [DOI] [PubMed] [Google Scholar]
- 57.Howell MD, Talmor D, Schuetz P, Hunziker S, Jones AE, Shapiro NI. Proof of principle: the predisposition, infection, response, organ failure sepsis staging system. Crit Care Med. 2011;39:322–327. doi: 10.1097/CCM.0b013e3182037a8e. [DOI] [PubMed] [Google Scholar]
- 58.Chen YX, Li CS. Risk stratification and prognostic performance of the predisposition, infection, response, and organ dysfunction (PIRO) scoring system in septic patients in the emergency department: a cohort study. Crit Care. 2014;18:R74. doi: 10.1186/cc13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Zheng H, Dong HL, Wang DX. Expert consensus on the transportation of surgical patients during perioperation (2014) Chinese Expert Consensus and Guidelines of Anesthesiology. Chinese Society of Anesthesiology. 2014 [Google Scholar]
- 61.Fanara B, Manzon C, Barbot O, Desmettre T, Capellier G. Recommendations for the intra-hospital transport of critically ill patients. Crit Care. 2010;14:R87. doi: 10.1186/cc9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carp H, Bailey S. The association between meningitis and dural puncture in bacteremic rats. Anesthesiology. 1992;76:739–742. doi: 10.1097/00000542-199205000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Yoon SH. Concerns of the anesthesiologist: anesthetic induction in severe sepsis or septic shock patients. Korean J Anesthesiol. 2012;63:3–10. doi: 10.4097/kjae.2012.63.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heinrich S, Schmidt J, Ackermann A, Moritz A, Harig F, Castellanos I. Comparison of clinical outcome variables in patients with and without etomidate-facilitated anesthesia induction ahead of major cardiac surgery: a retrospective analysis. Crit Care. 2014;18:R150. doi: 10.1186/cc13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McPhee LC, Badawi O, Fraser GL, Lerwick PA, Riker RR, Zuckerman IH, et al. Single-dose etomidate is not associated with increased mortality in ICU patients with sepsis: analysis of a large electronic ICU database. Crit Care Med. 2013;41:774–783. doi: 10.1097/CCM.0b013e318274190d. [DOI] [PubMed] [Google Scholar]
- 66.Tarquinio KM, Howell JD, Montgomery V, Turner DA, Hsing DD, Parker MM, et al. Current medication practice and tracheal intubation safety outcomes from a prospective multicenter observational cohort study. Pediatr Crit Care Med. 2015;16:210–218. doi: 10.1097/PCC.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 67.Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300. doi: 10.1016/S0140-6736(09)60949-1. [DOI] [PubMed] [Google Scholar]
- 68.Zausig YA, Busse H, Lunz D, Sinner B, Zink W, Graf BM. Cardiac effects of induction agents in the septic rat heart. Crit Care. 2009;13:R144. doi: 10.1186/cc8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang J, Hu JJ, Lu CH, Liang JN, Xiao JF, Liu YT, et al. Propofol inhibits lipopolysaccharide-induced tumor necrosis factor-alpha expression and myocardial depression through decreasing the generation of superoxide anion in cardiomyocytes. Oxid Med Cell Longev 2014. 2014:157376. doi: 10.1155/2014/157376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsing CH, Chen YH, Chen CL, Huang WC, Lin MC, Tseng PC, et al. Anesthetic propofol causes glycogen synthase kinase-3β-regulated lysosomal/mitochondrial apoptosis in macrophages. Anesthesiology. 2012;116:868–881. doi: 10.1097/ALN.0b013e31824af68a. [DOI] [PubMed] [Google Scholar]
- 71.Hsing CH, Lin MC, Choi PC, Huang WC, Kai JI, Tsai CC, et al. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKβ/NF-κB signaling. PLoS One. 2011;6:e17598. doi: 10.1371/journal.pone.0017598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schilling T, Kozian A, Senturk M, Huth C, Reinhold A, Hedenstierna G, et al. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology. 2011;115:65–74. doi: 10.1097/ALN.0b013e318214b9de. [DOI] [PubMed] [Google Scholar]
- 73.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 74.Yu BW, Wang GL, Wang QY, Liu J, Deng XM, Zuo MZ, et al. Guidelines for Clinical Anesthesia Monitoring (2014). Chinese Expert Consensus and Guidelines of Anesthesiology. Chinese Society of Anesthesiology. 2014 [Google Scholar]
- 75.Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 76.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 77.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 78.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med 2008. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 79.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 80.Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–1321. doi: 10.1097/ALN.0b013e31829102de. [DOI] [PubMed] [Google Scholar]
- 81.Coppola S, Froio S, Chiumello D. Protective lung ventilation during general anesthesia: is there any evidence? Crit Care. 2014;18:210. doi: 10.1186/cc13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Futier E, Marret E, Jaber S. Perioperative positive pressure ventilation: an integrated approach to improve pulmonary care. Anesthesiology. 2014;121:400–408. doi: 10.1097/ALN.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 83.Wang N, Liu X, Zheng X, Cao H, Wei G, Zhu Y, et al. Ulinastatin is a novel candidate drug for sepsis and secondary acute lung injury, evidence from an optimized CLP rat model. Int Immunopharmacol. 2013;17:799–807. doi: 10.1016/j.intimp.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 84.Chen X, Wang Y, Luo H, Luo Z, Liu L, Xu W, et al. Ulinastatin reduces urinary sepsis-related inflammation by upregulating IL-10 and downregulating TNF-α levels. Mol Med Rep. 2013;8:29–34. doi: 10.3892/mmr.2013.1480. [DOI] [PubMed] [Google Scholar]
- 85.Yang H, Mao Y, Lu X, Sang X, Du S, Zhao H, et al. The effects of urinary trypsin inhibitor on liver function and inflammatory factors in patients undergoing hepatectomy: a prospective, randomized, controlled clinical study. Am J Surg. 2011;202:151–157. doi: 10.1016/j.amjsurg.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 86.He QL, Zhong F, Ye F, Wei M, Liu WF, Li MN, et al. Does intraoperative ulinastatin improve postoperative clinical outcomes in patients undergoing cardiac surgery: a meta-analysis of randomized controlled trials. Biomed Res Int 2014. 2014:630835. doi: 10.1155/2014/630835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karnad DR, Bhadade R, Verma PK, Moulick ND, Daga MK, Chafekar ND, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 2014;40:830–838. doi: 10.1007/s00134-014-3278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J, Zhou L, Liu J, Ma G, Kou Q, He Z, et al. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit Care. 2013;17:R8. doi: 10.1186/cc11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li C, Bo L, Liu Q, Jin F. Thymosin alpha1 based immunomodulatory therapy for sepsis: a systematic review and meta-analysis. Int J Infect Dis. 2014;33:90–96. doi: 10.1016/j.ijid.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 90.Liu MW, Wang YH, Qian CY, Li H. Xuebijing exerts protective effects on lung permeability leakage and lung injury by upregulating Toll-interacting protein expression in rats with sepsis. Int J Mol Med. 2014;34:1492–1504. doi: 10.3892/ijmm.2014.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He XD, Wang Y, Wu Q, Wang HX, Chen ZD, Zheng RS, et al. Xuebijing Protects Rats from Sepsis Challenged with Acinetobacter baumannii by Promoting Annexin A1 Expression and Inhibiting Proinflammatory Cytokines Secretion. Evid Based Complement Alternat Med 2013. 2013:804940. doi: 10.1155/2013/804940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin Q, Li C. Treatment effects of xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evid Based Complement Alternat Med 2014. 2014:949254. doi: 10.1155/2014/949254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Chen H, Li YM, Zheng SS, Chen YG, Li LJ, et al. Thymosin alpha1- and ulinastatin-based immunomodulatory strategy for sepsis arising from intra-abdominal infection due to carbapenem-resistant bacteria. J Infect Dis. 2008;198:723–730. doi: 10.1086/590500. [DOI] [PubMed] [Google Scholar]
- 94.Li Yumin, Chen Hao, Li Xun, Zhou Wence, He Minyan, Chiriva-Internati M, et al. A new immunomodulatory therapy for severe sepsis: Ulinastatin Plus Thymosin alpha-1. J Intensive Care Med. 2009;24:47–53. doi: 10.1177/0885066608326970. [DOI] [PubMed] [Google Scholar]


