Abstract
Object
External ventricular drains (EVDs) are commonly used for CSF diversion but pose a risk of ventriculitis, with rates varying in frequency from 2% to 45%. Results of studies examining the utility of prolonged systemic antibiotic therapy for the prevention of EVD-related infection have been contradictory, and no study to date has examined whether this approach confers additional benefit in preventing ventriculitis when used in conjunction with antibiotic-coated EVDs (ac-EVDs).
Methods
A prospective performance analysis was conducted over 4 years to examine the impact of discontinuing systemic antibiotic prophylaxis after insertion of an ac-EVD on rates of catheter-related ventriculitis. Ventriculitis and other nosocomial infections were ascertained by a qualified infection disease nurse using definitions based on published standards from the Centers for Disease Control and Prevention, comparing the period when patients received systemic antibiotic therapy for the duration of EVD treatment (Period 1) compared with only for the peri-insertion period (Period 2). Costs were analyzed and compared across the 2 time periods
Results
Over the 4-year study period, 866 patients were treated with ac-EVDs for a total of 7016 catheter days. There were 8 cases of ventriculitis, for an overall incidence of 0.92%. Rates of ventriculitis did not differ significantly between Period 1 and Period 2 (1.1% vs 0.4%, p = 0.22). The rate of nosocomial infections, however, was significantly higher in Period 1 (2.0% vs 0.0% in Period 2, p = 0.026). Cost savings of $162,516 were realized in Period 2 due to decreased drug costs and savings associated with the reduction in nosocomial infections.
Conclusions
Prolonged systemic antibiotic therapy following placement of ac-EVDs does not seem to reduce the incidence of catheter-related ventriculitis, and was associated with a higher rate of nosocomial infections and increased cost.
Keywords: external ventricular drains, antibiotics, ventriculitis
EXTERNAL ventricular drains (EVDs) are essential neurosurgical devices providing cerebrospinal fluid (CSF) diversion and the ability to monitor intracranial pressure.24 The benefits of EVDs must be balanced against potential complications associated with their use, the most frequent and serious of which is ventriculitis. EVD-associated ventriculitis varies in frequency from 1% to 45% in the literature.1,6,8–11,23,26,30 It has been associated with increased rates of morbidity and mortality as well as increased hospital stays and expenditures.16 Methods proposed to reduce the rate of EVD-associated ventriculitis include chlorhexidene skin preparation,9 antimicrobial coating of EVD catheters,29 various degrees of catheter tunneling,9,24 and intravenous (IV) administration of perioperative (< 24 hr) and prolonged (> 24 hours) systemic antibiotics.4,19
Continuous antibiotic prophylaxis can take the form of prolonged systemic IV antibiotic therapy until removal of the EVD and/or use of antibiotic-coated EVD catheters (ac-EVD catheters). In a previous study, we showed that perioperative administration of antibiotics along with use of ac-EVD catheters decreased the incidence of CSF infection as compared with use of neither perioperative antibiotics nor ac-EVD;11 however, this study did not address the issue of prolonged systemic antibiotics. Perioperative systemic antibiotic therapy is widely regarded to be beneficial,3 but the evidence for prolonged systemic antibiotic therapy is less clear. To date, 3 cohort studies2,25,31,32 and 2 randomized controlled trials4,19 have examined the issue of whether prolonged systemic antibiotic prophylaxis following EVD placement reduces the rate of ventriculitis—the results of which are conflicting. Two cohort studies2 and 1 randomized controlled trial4 suggest prolonged systemic antibiotic prophylaxis after EVD placement does not reduce the rate of ventriculitis, while 1 cohort study32 and 1 randomized controlled trial19 suggest prolonged systemic antibiotic prophylaxis after EVD placement does reduce the rate of ventriculitis. Importantly, none of these studies were performed in patients who were treated with ac-EVDs. Only one of these studies assessed the issue of cost.2
Due to these discrepancies and out of concern for the promotion of drug-resistant nosocomial infections, we discontinued the use of prolonged systemic antibiotic therapy [Proposed edit OK? (underscoring indicates suggested deletion, brackets indicate suggested addition) Explanation: Trying to avoid describing the drugs This is finethemselves as prolonged] following ac-EVD placement in May 2012. This change in practice, along with our prospective recording of infection data,7,12,13 provided a unique opportunity to evaluate whether prolonged antibiotic administration confers additional benefit beyond the use of ac-EVDs alone in the prophylaxis of EVD-associated ventriculitis.
Methods
Study Design
A prospective performance analysis was conducted to examine the impact of discontinuing the use of prolonged antibiotic prophylaxis after insertion of an EVD on rates of catheter-related ventriculitis. Ethics committee approval from the participating center OK was obtained. All patients who underwent intraventricular catheterization and were admitted to our 20-bed neurology-neurosurgical intensive care unit (NNICU) here in Barnes-Jewish Hospital, St Louis, an academic, tertiary care center were included in our study. [Please specify the institution. Trying to avoid implying it could have been any academic tertiary care center with a 20-bed NNICU.] Patients presenting with ventriculitis or a shunt infection were excluded. We included cases from January 2009 (when our management of intraventricular catheters was standardized) to June 2013. After May 2012, we no longer administered prolonged systemic antibiotic prophylaxis following placement of an EVD. Perioperative administration of antibiotics at the time of EVD insertion was continued (≤ 24 hours [does this refer to the duration of antibiotic treatment?]). yes
Data Sources/ Measurements
Microbiological culture data were prospectively identified from a centralized laboratory system utilizing GermWatcher (Washington University St. Louis), an automated surveillance tool that monitors data and identifies those cultures that represent infection and reports them to the infectious disease team. A specially trained infectious diseases nurse rounded evaluated all patients with an EVD in place daily and recorded data. [I know “round” is used with the meaning of “to go on rounds” in practice, but we try to avoid this usage in formal style. Would it be accurate to say that she “visited,” “checked on,” or “evaluated” them?]
We defined ventriculitis based on published standards from the Centers for Disease Control and Prevention (CDC)—1) the presence of organisms in CSF cultures or 2) the presence of fever (temperature > 38 °C), headache, stiff neck, meningeal signs, cranial nerve signs, or irritability with at least one of the following: increased CSF white blood cell count, elevated CSF protein level, or decreased CSF glucose level; organisms seen with Gram staining of CSF sample; organisms cultured from blood; a positive non culture diagnostic laboratory test of CSF, blood, or urine; [positive for what?] The CDC definition is quite long, I will not be able to discuss all aspects of it but I have the reference for them a diagnostic single-antibody titer (IgM) or 4-fold increase in paired sera (IgG) for pathogen. These both refer to blood[Do these last two both refer to blood? Is this paragraph otherwise OK as edited?] this is how the WHO words it so it should be ok
The calculation of institutional savings after discontinuation of prolonged systemic antibiotic prophylaxis was based on the number of patient days per year that EVD catheters were in place. The average cost to the hospital pharmacy for a 1.5-g dose of cefazolin is $1.50; assuming that 3 doses are administered per day, the average cost for routine prophylactic antibiotic therapy is $4.50 per patient per 24 hours. Blood stream infections (BSI) and ventilator-associated pneumonias (VAP) cost analyses for our health care organization have been previously delineated.27,28
For statistical analyses, we compared 2 groups using generalized Fisher exact tests for categorical variables and 2-tailed Student t-tests for continuous variables that were approximately normally distributed. The level of statistical significance was set at p < 0.05.
EVD Protocol
Since January 2009, standard protocols have been used for the management of EVDs. Our protocol for EVD insertion includes hair clipping, use of Betadine scrub, maximal barrier precautions (i.e., use of cap, mask, sterile gown, and sterile gloves), and administration of perioperative antibiotics during the procedure ok. Patients received 1–2 g of IV cefazolin prior to placement of the EVD, followed by 1g IV every 8 hours for 24 hours. Patients allergic to cefazolin were given 1g of IV vancomycin prior to placement of the EVD, followed by 1g IV every 12 hours for 24 hours. Codman Bactiseal EVD catheters (impregnated with 0.15% clindamycin and 0.054% rifampicin, yes [Was this intended as 0.054% rifampicin? I have been unable to confirm the % on the marketing website] DePuy Synthes) were used. Both the inner lumen and the exterior catheter wall are supplied up to 28 days with antibiotic concentrations that protect against colonization with gram-positive bacteria.
The majority of EVDs were inserted at the bedside in the NNICU, with a minority placed in the operating room. Our protocol for post-EVD dressing care [Does this refer to post–EVD placement dressing care? Yes post placement (i.e., while the drain is still in place)] consists of 3 components: use of sterile gauze dressing to cover the EVD site, with adhesive tape to secure borders; routine changing of the EVD site dressing every 48 hours by trained NNICU nurses; and comprehensive documentation of gauze-dressing changes. CSF samples were drawn if the patient had a fever (temperature > 38.5°C) or demonstrated alteration of neurological status with no other apparent cause. Routine cultures of CSF were not performed based on past literature that demonstrates this practice confers no clinical value.22 [Does this mean based on the specific study cited? If so, OK to change to: based on published evidence that this practice confers no yes this change is fine ... Trying to avoid using “literature” to describe a single article.] EVD catheters were replaced at the bedside under the above-described protocol only if EVD malfunction or CSF infection developed.
Before May 2012 (Period 1), patients who underwent EVD placement received systemic antibiotics for infection prophylaxis as long as the catheter was in place. For most patients, 1g IV cefazolin was administered for prophylaxis every 8 hours until the catheter was removed. For those allergic to penicillin, 1g IV vancomycin was administered for prophylaxis every 12 hours until the catheter was removed. In May 2012, this practice was discontinued. Subsequently (Period 2), patients only received perioperative systemic antibiotics (i.e., antibiotics were administered prior to EVD placement, continued for 24 hours, and then discontinued).
Results
Participants
Period 1 refers to the time period during which patients received prolonged systemic antibiotics after EVD placement (> 24 hours) (January 2009–April 2012). Period 2 refers to the time period during which patients received only perioperative antibiotics (≤ 24 hours) after EVD placement (May 2012–June 2013). Four hundred ten patients treated during Period 1 and 135 patients treated during Period 2 qualified for inclusion in the study. [Addition of highlighted sentence OK?] yes Demographic and clinical characteristics for the patients treated during these 2 periods are shown in Table 1. The only statistical difference between period 1 (410 patients) and period 2 (135 patients) was that EVDs were present one day longer (7 vs 6 days median). [Was the statistical test based on comparison of the median values? If so, would the following revision work? “The only statistically significant difference in the variables analyzed involved duration of EVD treatment; the median duration was 1 day longer for the patients treated during Period 1 (7 days vs 6 days for Period 2).” Yes this is fine I suggested moving adding a separate sentence about the numbers of patients and qualifying what was analyzed because the n's seemed quite different and I didn't want to include them in that sentence about lack of difference.] The indications for EVD placement were similar, with no statistically significant difference (Table 2). Neither discharge outcomes nor rates of subsequent shunt placement differed significantly between Period 1 and Period 2 (Table 3 and 4).
TABLE 1.
Comparison of demographic and clinical characteristics of patients treated during Period 1 and Period 2
| Variable | Period 1 | Period 2 | p Value |
|---|---|---|---|
| Sex | 0.57* | ||
| Male | 177 | 62 | |
| Female | 233 | 73 | |
| Age (yrs) | 0.3204 | ||
| Mean | 53.84 | 54.97 | |
| SD | 15.89 | 15.53 | |
| Minimum | 15 | 19 | |
| Median | 54 | 56 | |
| Maximum | 91 | 88 | |
| Length of stay (days) | 0.9902 | ||
| Mean | 18.48 | 19.08 | |
| SD | 13.61 | 17.27 | |
| Minimum | 0 | 0 | |
| Median | 17 | 17 | |
| Maximum | 105 | 141 | |
| Length of EVD duration (days) | 0.0197 | ||
| Mean | 8.11 | 6.84 | |
| SD | 5.66 | 5.01 | |
| Minimum | 1 | 1 | |
| Median | 7 | 6 | |
| Maximum | 31 | 21 |
Chi-square test.
† Kruskal-Wallis test.
TABLE 2.
Indications for EVD placement for the 2 periods
| Indication | Period 1 | Period 2 | Total | p Value |
|---|---|---|---|---|
| Tumor | 84 | 32 | 117 | |
| SAH | 177 | 50 | 227 | |
| Trauma | 23 | 6 | 29 | |
| Stroke | 116 | 44 | 160 | |
| Other | 9 | 3 | 12 | |
| Total | 410 | 135 | 545 | 0.6927 |
* Chi-square test.
TABLE 3.
Discharge disposition after treatment of primary pathology and removal of EVD or shunt placement
| Outcome | Period 1 | Period 2 | Total | p Value |
|---|---|---|---|---|
| Home | 100 | 24 | 124 | |
| Rehab | 174 | 59 | 233 | |
| Nursing | 47 | 15 | 62 | |
| Death | 78 | 31 | 109 | |
| Hospice | 4 | 4 | 8 | |
| LTAC | 7 | 2 | 9 | |
| Total | 410 | 135 | 545 | 0.3522 |
LTAC = long-term acute care
* Chi-square test.
TABLE 4.
Analysis of the numbers of patients who required a shunt after EVD treatment
| Shunt Required | ||||
|---|---|---|---|---|
| Study Period | Yes | No | Total | p Value* |
| Period 1 | 81 | 328 | 409 | |
| Period 2 | 21 | 114 | 135 | |
| Total | 102 | 442 | 544 | 0.2728 |
Chi-square test.
Infection Results
A total of 866 EVDs were placed during this 4-year study period, for a total of 7016 catheter days (Fig. 1). There were 8 cases of ventriculitis, for an overall incidence density of 1.1 infections per 1000 catheter days and a ventriculitis infection rate of 0.92% per catheter insertion. There was no statistically significant difference in rates of ventriculitis between the 2 time periods, with an incidence density of 1.35 cases per 1000 catheter days in Period 1 and 0.54 per 1000 catheter days in Period 2 (p = 0.26, Table 5). [OK as edited?] yes ok
FIG. 1.
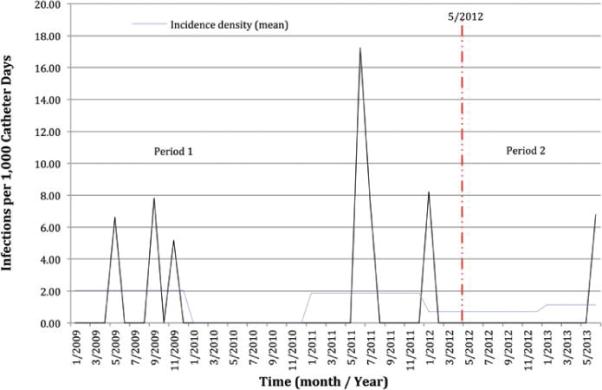
Period 1 Patients who underwent EVD placement received systemic antibiotics for infection prophylaxis as long as the catheter was in place (January 2009–April 2012).Period 2 started after routine iv antibiotics were discontinued upon insertion of the catheter (May 2012–June 2013). [This seems to conflict somewhat with the text. Please clarify. From Results: Before May 2012 (Period 1), patients who underwent EVD placement received systemic antibiotics for infection prophylaxis as long as the catheter was in place. Protocol during Period 2 was described as follows: “perioperative systemic antibiotics (i.e., antibiotics were administered prior to EVD placement, continued for 24 hours, and then discontinued.”] Incidence density is the number of infections per 1000 catheter days. Figure is available in color online only.
TABLE 5.
Number of catheter days, catheters placed, ventriculitis incidence density, rate of ventriculitis as a percentage
| Variable | Period 1 w/ IV Prophylactic ABx | Period 2 w/ IV Prophylactic ABx | p Value* |
|---|---|---|---|
| No. of catheter-days | 5176 | 1840 | |
| No. of catheters placed | 640 | 226 | |
| Incidence density | 1.35 | 0.54 | |
| Rate of ventriculitis | 1.1% | 0.4% |
[The p value was 0.2664. What comparison was it for? Not sure which cell to put it in. Alternative would be footnote.]
Fisher exact test, 2 tailed.
Of these 8 cases, 4 were due to gram-negative bacteria, 2 due to gram-positive bacteria, 1 due to a fungal species of yeast, [OK to change to just “a yeast species”? yes ok The problem with the phrase as written is that can be read as indicating that fungi are a subset of yeast instead of the other way around.] and 1 was culture negative.
In contradistinction, a significantly higher rate of nosocomial infections (BSI and VAP) was noted in Period 1 compared with Period 2 (2.0% vs 0.0%, respectively; p = 0.0261). Specifically, 8 patients in Period 1 contracted a BSI and 5 contracted a VAP, while no patient in Period 2 contracted either (Table 6 and Fig. 2). Central Line related blood stream infection per central line days (CLABSI) were Period 1:2.7 Period 2: 0.9 and VAP rates for our entire NNICU for both both periods were Period 1:3 Period 2: 1.2) [Please clarify. I started to try to clarify this passage myself, but came up with the following for CLABSI: “The rates of central line–related BSI per central line days were 2.7 for Period 1 and 0.9 for Period 2.” This would mean 2.7 cases per day of treatment for Period 1, and that seems awfully high. I am wondering if the rates were intended as rates per a certain number of days -- for example, per 1000 central line days or per 1000 ventilator days. Please check.] yes it was per thousand catheter days
TABLE 6.
Rates of BSI, VAP and BSI and VAP combined for each period
| Variable | Period 1 | Period 2 | p Value |
|---|---|---|---|
| BSI (%) | 8 (1.3) | 0 (0) | 0.12 |
| VAP (%) | 5 (0.8) | 0 (0) | 0.3378 |
| VSI+VAP (%) | 13 (2.0) | 0 (0) | 0.026 |
* Fisher exact test, 2-tailed.
FIG. 2.
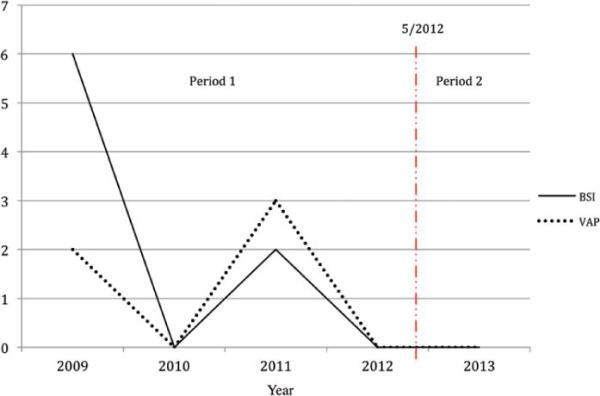
Number of BSI and VAP cases per year. Period 1 and Period 2 are separated by a dotted line indicating the change of practice in May 2012. Figure is available in color online only.
Cost Results
During Period 1, 4824 doses of antibiotics were administered, resulting in a total of $7263 in drug costs not incurred during Period 2. In our health care organization, the cost per case of BSI is $11,97127 and the cost per case of VAP is $11,897.28 During Period 1, $95,768 of BSI-related costs and $59,485 of VAP-related costs were incurred. During Period 2, no BSI- or VAP-related costs were incurred. The total costs saving, therefore, were estimated at $162,516 in the period when no prophylactic systemic antibiotics were used.
Discussion
Our analysis shows that discontinuation of prolonged systemic antibiotic prophylaxis following ac-EVD placement was not associated with higher rates of ventriculitis and was associated with a significant reduction in nosocomial infections, including BSI and VAP. No patient with an ac-EVD contracted a BSI or VAP in Period 2, the period after cessation of prolonged antibiotic prophylaxis. Substantial cost savings related to cessation of prolonged antibiotic administration ($7263) and a reduction in nosocomial infections ($95,768 for BSI and $59,485 for VAP) were also realized. In total, these results argue against a policy of prolonged antibiotic prophylaxis in patients treated with ac-EVDs.
Several studies have examined the utility of prolonged systemic antibiotic prophylaxis in patients treated with EVDs, with conflicting results. Alleyne et al.2 performed a retrospective analysis of a cohort of 308 patients treated with standard EVDs (i.e., not antibiotic coated) and found no relationship between ventriculitis rates and prolonged systemic antibiotic administration (4% with prolonged antibiotics vs 4% without prolonged antibiotics). The authors calculated approximately $80,000 in savings per year related to direct drug costs.2 Stenager et al.25 performed a prospective analysis of a cohort of 87 patients treated with standard EVDs and found no relationship between ventriculitis rates and prolonged systemic antibiotic administration (10% with prolonged antibiotics vs 18% without prolonged antibiotics,). In contradistinction, Wyler et al. performed a retrospective analysis of a cohort of 70 patients with standard EVDs, and noted a significant reduction in the ventriculitis rate of patients who received prolonged systemic antibiotics compared with those who did not (9% vs 27%, respectively; 95% CI 0.11–1.04 In their paper this is how they stated it, I couldn't find a p value[I'm not sure what the confidence interval is for here -- it seems there might be something missing -- p value? RR?]).32 Two randomized controlled trials examining this issue have been published. In the first, Blomstedt et al.4 performed a controlled trial involving 52 patients treated with standard EVDs to assess the effectiveness of prolonged systemic administration of trimethoprim-sulfamethoxazole in reducing the rate of ventriculitis. They found no significant difference in infection rates between those who received prolonged systemic antibiotic therapy and those who did not (4% vs 4%, respectively). In the second trial, Poon et al.19 performed involving 228 patients treated with standard EVDs to assess the effectiveness of prolonged systemic administration of ampicillin-sulbactam and aztreonam in reducing the rate of ventriculitis. They found a significant reduction in the infection rate between those who received prolonged systemic antibiotic therapy and those who did not (3% vs 11%, respectively; p = 0.01). They also reported a higher nosocomial infection rate in the perioperative antibiotic group compared with the prolonged antibiotic group (42% vs 20%, respectively; p = 0.002). A potential weakness of this study, however, is that EVD revision was routinely performed every 5 days—a practice that is now discouraged, as it is associated with an increased risk of infection.19,30 The results of the study therefore may not be applicable to current practice given that very few neurosurgeons electively revise EVDs.30 Finally, Sonabend et al.23 performed a meta-analysis of the aforementioned studies and found that prolonged antibiotic prophylaxis following standard EVD placement appears to provide significant protection against ventriculitis (RR = 0.45, 95% CI 0.27–0.74).
Importantly, none of the above studies used ac-EVDs, which themselves have been shown to reduce the risk of ventriculitis by up to 50%.2,23,31 In fact, to date there is no evidence to suggest that prolonged systemic antibiotic therapy provides additional clinical or microbiological benefit when used in combination with ac-EVDs.31 The possibility that ac-EVDs may obviate the need for prolonged systemic antibiotic prophylaxis was first raised by the recent study of Wright et al.31 However, because their study examined patients treated with standard EVDs in comparison with those treated with ac-EVDs exclusively in the setting of prolonged systemic antibiotic administration, no definitive conclusions could be drawn. Our study, therefore, represents the first direct evidence that prolonged systemic antibiotic prophylaxis in patients treated with ac-EVDs may provide no additional protection against ventriculitis.
Our results show that prolonged systemic antibiotic administration is associated with a statistically significant increase in the rate of systemic nosocomial infections like VAP and BSI. This has important clinical ramifications, as the mortality rate associated with VAP is 10%17 and the mortality rate associated with BSI is as high as 26%.5 The reason for the reduction in nosocomial infections when prolonged systemic antibiotic administration was avoided is less clear. Our ICU yes[OK to change to NNICU?] rates yes [rates, plural? or is singular OK here?] of VAP and BSI in patients without an EVD remained broadly similar throughout both time periods. One potential explanation stems from studies of a related clinical scenario that has been extensively investigated with prospective randomized trials—the use of central venous catheters. VAP and BSI are infections related to the development of biofilm on endotracheal tubes and catheter lines.20,21 [OK to change BSI to CLABSI here? yes they both are provoked to a large degree by biofilms I am concerned that this sentence is implying that VAP and BSI are both limited to the definition given here. It makes sense to me that VAP could be limited to this etiology, but I'm stumbling on the idea that all bloodstream infections occur due to biofilms on catheters. Thinking of awful stories from the Civil War, for instance.] Numerous studies have demonstrated that sub–minimum inhibitory concentrations (MICs) of a variety of chemically distinct antibiotics with different modes of action can significantly induce biofilm formation in phylogenetically diverse gram-negative and gram-positive bacteria in vitro.14,15 To help prevent biofilm formation and reduce the development of antibiotic-resistant organisms in patients treated with central venous catheters, the CDC recommends use of antibiotic-coated catheters and avoidance of systemic antimicrobial prophylaxis.18 Extending this potential mechanism to our study, the prolonged administration of systemic antibiotics administered during Period 1 without MIC monitoring may have provoked the development of biofilms on endotracheal tubes and catheters resulting in the increased cases of VAP and BSI.
There are a number of weaknesses or limitations in our study. It is not a randomized study. Also, our catheter infection rate is significantly lower (1.1 infections per 1000 catheter days) than that reported elsewhere in the literature. We believe bacterial colonization and local inoculation around the intraventricular catheter site likely predisposes a patient to intraventricular catheter–related ventriculitis. Thus, the aseptic insertion and care of an intraventricular catheter are essential to preventing infection. Our incidence of intraventricular catheter–related ventriculitis decreased by more than three-quarters after the implementation of the multiple interventions mentioned in the EVD protocol portion of the text.11 We hypothesize that the implementation of a standardized protocol for dressing an intraventricular catheter site, adapted from guidelines to prevent intravascular catheter–related bloodstream infections, was a key component in reducing the risk of intraventricular catheter–related ventriculitis.11 This may mean that our results are not generalizable; however, other authors have shown similar low rates of infection.9 Our low rates of ventriculitis could also raise the possibility that our study is not powered sufficiently to see a difference. We were also unable to analyze cases in which there was a break in the sterile collection device. Such patients may still benefit from prolonged administration of systemic antibiotics.
Conclusions
Discontinuation of routine prolonged systemic antibiotic prophylaxis had no significant impact on the incidence of intraventricular catheter–related ventriculitis when using ac-EVDs and led to a statistically significant reduction in nosocomial infections. This practice led to substantial cost savings. These data suggest that patients treated with ac-EVDs may not require prolonged systemic antibiotic prophylaxis.
ABBREVIATIONS
- ac
antibiotic-coated
- BSI
blood stream infection
- CDC
Centers for Disease Control and Prevention
- CSF
cerebrospinal fluid
- EVD
external ventricular drain
- IV
intravenous
- MIC
minimum inhibitory concentration
- NNICU
neurology-neurosurgical intensive care unit
- VAP
ventilator-associated pneumonia
Footnotes
Author Contributions
Conception and design: Murphy, B Liu, Reynolds, Craighead. Acquisition of data: Murphy, B Liu, Srinath, Reynolds, Craighead. Analysis and interpretation of data: Zipfel, Murphy, B Liu, Reynolds, Craighead, Camins, Dhar. Drafting the article: Zipfel, Murphy, B Liu, Reynolds, Craighead, Camins. Critically revising the article: Zipfel, Murphy, B Liu, Craighead, Camins, Kummer. Reviewed submitted version of manuscript: Zipfel, Murphy, B Liu, Craighead, Camins, Dhar, Kummer. Statistical analysis: Zipfel, Murphy, B Liu, J Liu, Camins, Kummer.
Administrative/technical/material support: Zipfel, Murphy, Craighead, Camins, Kummer. Study supervision: Zipfel, Murphy, Kummer.
References
- 1.Abla AA, Zabramski JM, Jahnke HK, Fusco D, Nakaji P. Comparison of two antibiotic-impregnated ventricular catheters: a prospective sequential series trial. Neurosurgery. 2011;68:437–442. doi: 10.1227/NEU.0b013e3182039a14. PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Alleyne CH, Jr, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000;47:1124–1129. doi: 10.1097/00006123-200011000-00020. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Arabi Y, Memish ZA, Balkhy HH, Francis C, Ferayan A, Al Shimemeri A, et al. Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Control. 2005;33:137–143. doi: 10.1016/j.ajic.2004.11.008. PubMed. [DOI] [PubMed] [Google Scholar]
- 4.Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. J Neurosurg. 1985;62:694–697. doi: 10.3171/jns.1985.62.5.0694. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Burgmann H, Hiesmayr JM, Savey A, Bauer P, Metnitz B, Metnitz PG. Impact of nosocomial infections on clinical outcome and resource consumption in critically ill patients. Intensive Care Med. 2010;36:1597–1601. doi: 10.1007/s00134-010-1941-2. PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Dasic D, Hanna SJ, Bojanic S, Kerr RS. External ventricular drain infection: the effect of a strict protocol on infection rates and a review of the literature. Br J Neurosurg. 2006;20:296–300. doi: 10.1080/02688690600999901. PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Doherty J, Noirot LA, Mayfield J, Ramiah S, Huang C, Dunagan WC, et al. Implementing GermWatcher, an enterprise infection control application. AMIA Annu Symp Proc. 2006;2006:209–213. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 8.Fichtner J, Güresir E, Seifert V, Raabe A. Efficacy of silver-bearing external ventricular drainage catheters: a retrospective analysis. J Neurosurg. 2010;112:840–846. doi: 10.3171/2009.8.JNS091297. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Flint AC, Rao VA, Renda NC, Faigeles BS, Lasman TE, Sheridan W. A simple protocol to prevent external ventricular drain infections. Neurosurgery. 2013;72:993–999. doi: 10.1227/NEU.0b013e31828e8dfd. PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien) 2008;150:209–214. doi: 10.1007/s00701-007-1458-9. PubMed. [DOI] [PubMed] [Google Scholar]
- 11.Honda H, Jones JC, Craighead MC, Diringer MN, Dacey RG, Warren DK. Reducing the incidence of intraventricular catheter-related ventriculitis in the neurology-neurosurgical intensive care unit at a tertiary care center in St Louis, Missouri: an 8-year follow-up study. Infect Control Hosp Epidemiol. 2010;31:1078–1081. doi: 10.1086/656377. PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Kahn MG, Steib SA, Dunagan WC, Fraser VJ. Monitoring expert system performance using continuous user feedback. J Am Med Inform Assoc. 1996;3:216–223. doi: 10.1136/jamia.1996.96310635. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn MG, Steib SA, Fraser VJ, Dunagan WC. An expert system for culture-based infection control surveillance. Proc Annu Symp Comput Appl Med Care. 1993:171–175. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;34:737–751. doi: 10.5301/ijao.5000027. PubMed. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan JB, Izano EA, Gopal P, Karwacki MT, Kim S, Bose JL, et al. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio. 2012;3:e00198–12. doi: 10.1128/mBio.00198-12. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyke KE, Obasanjo OO, Williams MA, O'Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001;33:2028–2033. doi: 10.1086/324492. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Melsen WG, Rovers MM, Koeman M, Bonten MJ. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit Care Med. 2011;39:2736–2742. doi: 10.1097/CCM.0b013e3182281f33. PubMed. [DOI] [PubMed] [Google Scholar]
- 18.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl 1):S1–S34. doi: 10.1016/j.ajic.2011.01.003. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta Neurochir Suppl. 1998;71:146–148. doi: 10.1007/978-3-7091-6475-4_43. PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–515. doi: 10.1086/501795. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care. 2005;50:725–741. PubMed. [PubMed] [Google Scholar]
- 22.Schade RP, Schinkel J, Roelandse FW, Geskus RB, Visser LG, van Dijk JM, et al. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg. 2006;104:101–108. doi: 10.3171/jns.2006.104.1.101. (Erratum in J Neurosurg 106:941, 2007) PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Sonabend AM, Korenfeld Y, Crisman C, Badjatia N, Mayer SA, Connolly ES., Jr Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: a systematic review. Neurosurgery. 2011;68:996–1005. doi: 10.1227/NEU.0b013e3182096d84. PubMed. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan VM, O'Neill BR, Jho D, Whiting DM, Oh MY. The history of external ventricular drainage. J Neurosurg. 2014;120:228–236. doi: 10.3171/2013.6.JNS121577. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Stenager E, Gerner-Smidt P, Kock-Jensen C. Ventriculostomy-related infections—an epidemiological study. Acta Neurochir (Wien) 1986;83:20–23. doi: 10.1007/BF01420503. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Tse Ts, Cheng K, Wong K, Pang K, Wong C. Ventriculostomy and infection: a 4-year-review in a local hospital. Surg Neurol Int. 2010;1:47. doi: 10.4103/2152-7806.69033. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med. 2006;34:2084–2089. doi: 10.1097/01.CCM.0000227648.15804.2D. PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312–1317. doi: 10.1097/01.CCM.0000063087.93157.06. PubMed. [DOI] [PubMed] [Google Scholar]
- 29.Wong GKC, Ip M, Poon WS, Mak CWK, Ng RYT. Antibiotics-impregnated ventricular catheter versus systemic antibiotics for prevention of nosocomial CSF and non-CSF infections: a prospective randomised clinical trial. J Neurol Neurosurg Psychiatry. 2010;81:1064–1067. doi: 10.1136/jnnp.2009.198523. PubMed. [DOI] [PubMed] [Google Scholar]
- 30.Wong GKC, Poon WS, Wai S, Yu LM, Lyon D, Lam JM. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: result of a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2002;73:759–761. doi: 10.1136/jnnp.73.6.759. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright K, Young P, Brickman C, Sam T, Badjatia N, Pereira M, et al. Rates and determinants of ventriculostomy-related infections during a hospital transition to use of antibiotic-coated external ventricular drains. Neurosurg Focus. 2013;34(5):E12. doi: 10.3171/2013.2.FOCUS12271. PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Wyler AR, Kelly WA. Use of antibiotics with external ventriculostomies. J Neurosurg. 1972;37:185–187. doi: 10.3171/jns.1972.37.2.0185. PubMed. [DOI] [PubMed] [Google Scholar]


