Abstract
MiRNAs are short, non-coding RNA molecules that regulate gene expression post-transcriptionally. Over the past decade, misregulated miRNA pathways have been associated with various diseases such as cancer, neurodegenerative diseases, and neurodevelopmental disorders. In this article, we aim to discuss the role played by miR-128 in neuropsychiatric disorders, and highlight potential target genes from an in silico analysis of predicted miR-128 targets. We also discuss the differences of target gene determination based on a bioinformatics or empirical approach. Using data from TargetScan and published reports, we narrowed the miR-128 target gene list to those that are known to be associated with neuropsychiatric disorders, and found that these genes can be classified into 29 gene clusters and are mostly enriched in cancer and MAPK signaling pathways. We also highlight some recent studies on several of the miR-128 targets which should be investigated further as potential candidate genes for therapeutic interventions.
Keywords: microRNAs, gene expression, neuropsychiatric disorders, anxiety, fear, movement disorders
Introduction
Mature microRNAs (miRNAs) are endogenous, small (approximately 22 nucleotides long), non-coding RNA molecules which regulate their target genes post-transcriptionally by targeting the 3′ untranslated region (UTR), 5′UTR and coding regions of target messenger RNAs (mRNAs) thus causing translational repression or mRNA degradation (Lewis et al., 2005; Bartel, 2009; Lee et al., 2009; Schmiedel et al., 2015).
MicroRNA-128 (miR-128) is an intronic miRNA and the mature miR-128 form is encoded by the two isoforms; miR-128-1 and miR-128-2 (Megraw et al., 2010). The pri-miR-128-1 gene resides within the R3H domain containing protein 1 gene (R3HDM1) and pri-miR-128-2 lies within the cAMP-regulated phosphoprotein, 21 kDa gene (ARPP21, also known as regulator of calmodulin signaling, RCS). This organization is conserved in human, rat and mouse genomes.
Early studies on miR-128 pointed to its tumor suppressive activity. Loss of MIR-128 was reported in human lung cancers – due to a deletion in chromosome 3p which included the MIR-128-2 and ARPP21 locus (Weiss et al., 2008) and in breast cancer (Qian et al., 2012).
Apart from its anti-cancer activity, miR-128 is of particular interest to neuroscientists as it is a brain-enriched miRNA which is highly expressed in the cortex and cerebellum, with distinct patterns of expression in developing brains and maturing cortical neurons (Krichevsky et al., 2003; Kim et al., 2004; Smirnova et al., 2005). Overexpression of miR-128 promotes neuronal differentiation in P19 cells and in primary embryonic neural stem cells through the suppression of non-sense-mediated decay (Bruno et al., 2011; Karam and Wilkinson, 2012). MiR-128- transduced human induced pluripotent stem cells shows similar characteristics as mature neurons and enhances the expression of beta-tubulin and other neuronal markers (Zare et al., 2015).
Recent studies have begun to relate MIR-128 and ARPP21 with neuropsychiatric disorders such as fear response, anxiety, intellectual disability and in movement disorders (Lin et al., 2011; Davis et al., 2012; Marangi et al., 2013;Tan et al., 2013). Tan et al. (2013) observed early onset fatal epilepsy and increased neuronal activity in mice with miR-128 deficiency. In this study, dopamine-1 receptor neurons with suppressed miR-128 expression showed enhanced dendritic excitability and increased dendritic spine formation. Recently, a family with a deletion of the MIR-128-2 and ARPP21 locus (chromosome 3p22.3p22.2) was reported to present with intellectual disability and epileptic episodes (Marangi et al., 2013). Several family members also had two other chromosomal abnormalities (3p24.3 deletion and 6p22.31 duplication), and suffered from febrile seizures during early childhood and decreased muscle tone. However, no direct link to ARPP21 or MIR-128 was discussed in the paper. It is interesting to note that a deletion in the same locus can also give rise to lung cancer, mentioned above (Weiss et al., 2008). The actual phenotype that manifests may be subject to environmental factors or other uncharacterised genetic factors.
Further evidence of the key role of MIR-128 in cognitive function comes from studies where mice were trained on a fear-conditioning paradigm, and the level of miR-128 expression was observed to increase upon learning the associated tasks (Lin et al., 2011). Interestingly, knockout Arpp21/Rcs mice showed anxiety-like behavior and had decreased motivation in food-rewarded tasks (Davis et al., 2012). However, in these papers there is no data to directly link miR-128 to these phenotypes and it may be that the abnormalities seen could involve other pathways, perhaps through Arpp21/Rcs direct interaction with calmodulin.
Several lines of evidence point to miR-128 and Arpp21 being susceptible to pharmacological modulation in relation to schizophrenia and depression. Rats administered with the antipsychotic drug, haloperidol, exhibited an increase in the expression of miR-128 in the prefrontal cortex, but no differences were seen in human schizophrenic patients when compared to controls (Perkins et al., 2007). MIR-128-1 overexpression has been also reported in the dorsolateral prefrontal cortices of schizophrenic patients (Beveridge et al., 2010) and patients with major depression treated with selective serotonin reuptake inhibitor antidepressant treatments, showed an upregulation of MIR-128 in their blood (Bocchio-Chiavetto et al., 2013). However, these studies do not conclusively show that the MIR-128 upregulation was due to the psychiatric disorders alone, or whether the elevation was in part due to the drug treatment.
Given that there is mounting evidence that MIR-128 is important in neuropsychiatric/ neurological disorders, and the recent Tan et al. (2013) report listing 1061 target genes of miR-128, we aimed to pull together results from numerous reports to determine what were the most likely candidate genes involved in this pathological process. We compared the target gene dataset from Tan et al. (2013) with datasets obtained through TargetScan analysis and searched the literature for reported target genes, to enable further exploration into their potential neuropsychiatric role. Using this approach, we narrowed down the list of potential target genes to 108. These genes can be classified into several functional clusters and share biological pathways that are relevant as possible therapeutic targets for neuropsychiatric disorders.
In Silico Analysis For miR-128 Target Genes
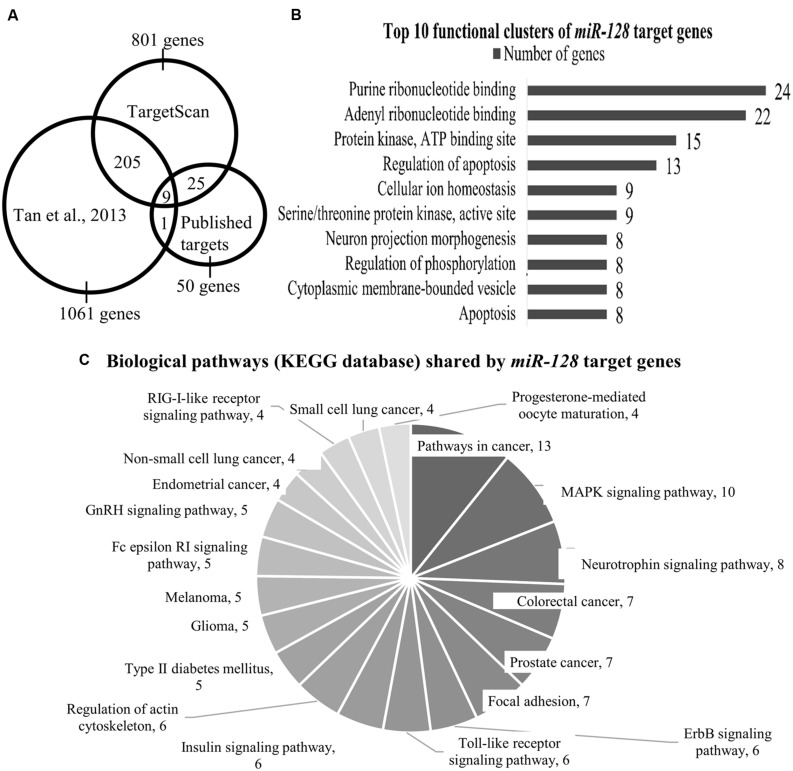
We performed a TargetScan analysis to determine the predicted target genes of miR-128. TargetScan (www.targetscan.org) is an online miRNA target site prediction database which selects possible target genes based on identifying the conserved 7-8mer miRNA sequences on the 3′UTR of these genes that are complementary with the miRNA seed region (Lewis et al., 2005). This analysis identified 801 genes as potential miR-128 targets (TargetScan release 6.2, named as Data TS, Figure 1A).
FIGURE 1.
(A) The Venn diagram shows the number of miR-128 target genes as identified by Tan et al. (2013) TargetScan and published targets. Total number of genes that are shared between Tan et al. (2013) and TargetScan is 214 genes; 34 genes are shared by TargetScan and our list of published miR-128 targets. (B) Top 10 functional clusters of miR-128 target genes as classified by DAVID bioinformatics tool. Using the mouse genome as the background, the threshold was set at the following criteria: classification stringency = highest; maximum EASE score = 0.05. EASE score is a modified Fisher Exact p value which range from 0 to 1, with 0 shows perfect enrichment. (C) Biological pathways (KEGG database) shared by miR-128 target genes as annotated by the KEGG pathway database. Using the mouse genome as background, the threshold was set at the following criteria: minimum number of genes for the corresponding pathway = 2; maximum EASE score = 0.05.
We then used data from Tan et al. (2013) where a HITS-CLIP (high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation) approach identified 1061 genes (Dataset T). This dataset was specific to genes which were in an RNA-induced silencing complex (RISC) with miR-128 in adult Camk2a-neurons. Tan et al. (2013) further defined their dataset by comparing the genes found in the RISC complex with genes that were found to be upregulated in miR-128 knockout D1 neurons, resulting in a smaller pool of 154 genes. The majority of the genes within this smaller dataset were enriched in the ERK pathway and other pathways relevant to neuronal function like ion transport, voltage-gated ion channel activity and G-protein signaling.
Next, we compared the two datasets from TargetScan (801 genes) and Tan et al. (2013; 1061 genes) which indicated 214 genes were commonly shared (Data TS & T, Figure 1A, Supplementary Table S1).
In addition, we performed a literature review for genes that had been empirically identified as direct targets of miR-128. From this, 50 genes (Dataset P) were identified (source: PubMed, Scopus May 2015; Supplementary Table S2). Out of these 50 genes, 9 genes (Arpp21, Bmi1, Csf1, Irs1, Casc3, Mapk14, Rxra, Snap25, and Sp1) were also shared by Dataset TS & T (Figure 1A), meaning that these nine genes were identified by Tan et al. (2013) TargetScan and are confirmed targets of miR-128 (Table 1).
Table 1.
Neuropsychiatric association of several miR-128 target genes from the combined datasets TS, T, and P.
| Gene | Function/association with neuropsychiatric phenotypes |
|---|---|
| Bmi1 polycomb ring finger oncogene (Bmi1) | Promotes cell proliferation and regulates endogenous antioxidant defense pathway (Leung et al., 2004; Zencak et al., 2005). |
| Bmi1-knockout mice exhibit epileptic-like seizures and progressive ataxia (Cao et al., 2012). | |
| Colony stimulating factor 1 (Csf1) | Mediates growth and differentiation of macrophage. Target of p53 tumor suppressor gene (Azzam et al., 2013). |
| Plasma Csf1 level increased in Alzheimer’s disease patients (Laske et al., 2010). | |
| Insulin receptor substrate 1 (Irs1) | Regulates insulin signaling (Zheng and Quirion, 2004). |
| Downregulated in hippocampus of an Alzheimer’s disease model (Han et al., 2012). | |
| Retinoid × receptor, alpha (Rxra) | Regulates cholesterol metabolism (Gentili et al., 2005). |
| The rs3132293 variant is associated with a higher risk of Alzheimer’s disease (Kolsch et al., 2009). | |
| High deletion rate of RXRA in schizophrenic patients (Lee et al., 2010). | |
| Synaptosomal associated protein, 25 kDa (Snap25) | Synaptosome-associated protein (Oyler et al., 1989). Involved in docking and fusion of synaptic vesicles (Sollner et al., 1993; Xu et al., 2013). |
| Association with attention-deficit/ hyperactivity disorder (Faraone et al., 2005). | |
| Downregulated in hippocampi of schizophrenic patients (Thompson et al., 2003). | |
| Sp1 transcription factor (Sp1) | A transcription factor (Dynan and Tjian, 1983). Expressed mainly in glia (Mao et al., 2009, 2014). |
| Upregulates the expression of Akt-induced vascular endothelial growth factor (Pore et al., 2004). | |
| Regulates the expression of Huntingtin (HTT) gene (Wang et al., 2012). | |
We noticed that some of the published miR-128 target genes were not identified by Tan’s study, such as Bcl-2 associated X protein (Bax; Adlakha and Saini, 2011), cAMP responsive element binding protein 1 (Creb1; Lin et al., 2011), Doublecortin (Dcx; Evangelisti et al., 2009) (Egfr; Weiss et al., 2008; Papagiannakopoulos et al., 2012), Neurofibromin 1 (Nf1; Paschou and Doxakis, 2012), Reelin (Reln; Evangelisti et al., 2009; Lin et al., 2011), and Wingless-related MMTV integration site 3a (Wnt3a; Wu et al., 2014). This may indicate several issues when determining target genes using different approaches. In particular, the target genes in Tan et al. (2013) were determined using the HITS-CLIP method with adult Camk2a-neurons and this method may have limited target gene identification to only genes that were expressed in those cells at that particular time. The rationale behind using these cells is justified in the Tan et al. (2013) study as they were most interested in genes that were affected by the miR-128 deletion in dopamine responsive neurons. However, this does impose a limitation to their target gene list. TargetScan and other miRNA database prediction software are likely to produce slightly different lists as these analyses are based on bioinformatics prediction software, irrespective of the tissue expression.
Some of the genes missing from the Tan et al. (2013) dataset included genes that are known to be expressed in the brain at the time points used in the Tan et al. (2013) study. For example, Dcx and Reln are known miR-128 targets and they are expressed in adult mouse forebrains (Evangelisti et al., 2009) including the striatum (Gates et al., 2006; Kang et al., 2011). Furthermore, knockout Dcx and Reln mice also exhibit anxiety and epileptic phenotypes, which are similar to the miR-128 knockout mice in the Tan et al. (2013) study. It is unclear why these genes were not identified. It may be that the level of expression of these particular genes was too low to be detected or the miR-128 regulation is specific to certain time points not included in the Tan et al. (2013) study.
When we grouped together the dataset shared exclusively between TS & T (205 genes) and included the dataset of published target genes (Dataset P, 50 genes), the number of candidate genes was 255 (Data TS and T & P). We then narrowed down the list by screening for associations with mental and behavioral disorders (as outlined by the International Statistical Classification of Diseases and Related Health Problems 10th Revision, ICD-10 version: 2010), first through the Genecard database (http://www.genecards.org/; Stelzer et al., 2011, an integrated human genes compendium), where 95 genes showed an association. The rest of the genes were subjected to systemic review on PubMed. From both of these two approaches, 109 genes out of the 255 genes (42.7%) were found to have neuropsychiatric associations (Data NP; Supplementary Figure S1 and Table S3). We then applied this list through the Database of Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatics tool (version 6.7; Huang et al., 2009) to determine whether any functional clusters were enriched.
Analysis by DAVID yielded 29 functional clusters (under highest classification stringency), the Top 10 of which are shown in Figure 1B (the entire list can be found in Supplementary Table S4). These functional clusters indicated involvement in nucleotide binding, protein kinase activity, apoptosis, neuronal development, ion homeostasis, and maintaining cytoplasmic membrane-bound vesicles (Figure 1B).
The KEGG annotation tool in DAVID further identified 20 shared biological pathways (Figure 1C; Supplementary Table S5). We found several relevant neuropsychiatric pathways, such as the MAPK signaling pathway, neurotrophin signaling and regulation of the actin cytoskeleton which is related to neuronal morphology. The involvement of the MAPK pathway has been demonstrated by Tan et al. (2013), where they found that many genes in their target dataset were associated with the ERK pathway, and Erk2 elevation was seen in the miR-128 knockout mice. Cancer-associated pathways accounted for a large fraction of the genes identified (for example, in colorectal, lung, and prostate cancers), (Figure 1C). The role of miR-128 and its target genes in cancer has been discussed in an excellent review (Li et al., 2013) and we will not discuss this aspect further.
Candidate miR-128 Target Genes With Relevant Associations To Neuropsychiatric Phenotypes
In this section, we aim to review some of the genes that have been identified through the comparative analysis of the different datasets and are relevant to neuropsychiatric disorders. Due to space limitations, we are unable to review all the genes but the ones selected here are those that are involved in the MAPK signaling pathway, neuronal development, and synaptic plasticity.
Mapk10/JNK3
Mitogen-activated protein kinase 10 (Mapk10, or also known as c-Jun N-terminal kinase, JNK3), a known effector of Rho-GTPase kinase, is enriched in the nervous system (Mohit et al., 1995). Although the Mapk10 gene was shown to associate with the miR-128 binding complex in the Tan study, the expression of Mapk10 is not upregulated in miR-128-deficient D1 neurons (Tan et al., 2013). Whilst there was no change in the D1 neurons, MAPK10 is still an important gene to consider as a miR-128 target and in a neuronal context due to its role in phosphorylating and binding PSD-95 in dendritic spines (Kunde et al., 2013). In addition, patients with chromosomal translocations at the MAPK10 locus present with epileptic seizures, motor and cognitive delays (Shoichet et al., 2006; Kunde et al., 2013) and a recent behavioral study by Reinecke et al. (2013) reported that Mapk10 knockout mice showed signs of anxiety as they were less active and did not exhibit normal navigational behavior during the Morris water maze task. The authors hypothesize the behavior could be due to impaired neuronal plasticity as MAPK/JNKs are involved in synaptic development (Tararuk et al., 2006).
Arpp21/Rcs
ARPP21, a cytosolic neuronal phosphoprotein, is highly expressed in the mammalian central nervous system especially within the basal ganglia (Walaas et al., 1983; Ouimet et al., 1989). Arpp21 functions as a calmodulin (CaM) signaling regulator (and it is also known as Regulator of calmodulin signaling, Rcs) (Rakhilin et al., 2004). CaM plays an important role at the synapse as it regulates the release of neurotransmitters from the presynaptic terminal (Ando et al., 2013). Phosphorylation of serine residue 55 (Ser55) by protein kinase A (PKA) in Arpp21 also leads to an increased binding to CaM, which inhibits the CaM activity, and in turn affects calcineurin, a CaM-dependent phosphatase 2B, and CaM kinase I (CaMKI) activities (Williams et al., 1989; Rakhilin et al., 2004). PKA phosphorylates Arpp21 at the Ser55 residue and this phosphorylation can be modulated by D1 and D2 receptor agonists whereby, there was increased phosphorylation upon D1 receptor agonist treatment, but decreased phosphorylation when exposed to D2 receptor agonists (Caporaso et al., 2000). In addition, this phosphorylation is sensitive to methamphetamine and cocaine (Caporaso et al., 2000), which is not surprising, given its expression within the limbic system. Taken together, data from several studies (Megraw et al., 2010; Davis et al., 2012; Tan et al., 2013) and its role in the CAM kinase pathway strongly indicates a potential role for Arpp21 in regulating the development of neuropsychiatric symptoms.
There are six out of seven genes (nine genes in total excluding Mapk19 and Arpp21; Figure 1A) present in Dataset TS, T, and P that have been reported to be associated with neurologically relevant functions (Table 1). For the seventh gene, cancer susceptibility candidate 3 (Casc3, or known as Mln51), most reports link this gene to non-sense-mediated mRNA decay (Chang et al., 2007) and the exon junction complex (Chazal et al., 2013) but there are no reports so far of any association with neurological/neuropsychiatric functions.
In addition to the candidate genes (which were shared by all datasets) reviewed above and in Table 1, we also reviewed other genes with highly suggestive neuropsychiatric associations, which were identified either through the TargetScan prediction and/or through literature reviews.
Reln
Reln is an extracellular glycoprotein secreted by Cajal-Retzius cells during prenatal development (Del Rio et al., 1997) and has an important role in neuronal migration (Frotscher et al., 2009). Reln is a direct target of miR-128 (Evangelisti et al., 2009; Lin et al., 2011).
RELN expression is downregulated in schizophrenia, bipolar disorders (Guidotti et al., 2000), autism (Fatemi et al., 2005), and Alzheimer’s disease (Herring et al., 2012). Polymorphisms in RELN associate with the risk of developing schizophrenia in the Han Chinese female population (Kuang et al., 2011), but this has not been corroborated by Ovadia and Shifman (2011). Rather, what they found was that the shorter isoform of RELN was significantly reduced in bipolar samples and an allelic imbalance of RELN expression in schizophrenia samples. The authors speculate that epigenetic factors or genetic imprinting may be responsible for the abnormal RELN function in these disorders.
Reln homozygous mutant mice have abnormal brain development and an enhanced rate of seizures (Patrylo et al., 2006; Qiu et al., 2006). In addition, Qiu et al. (2006) observed a reduction in freezing behavior (animal stops moving due to fear) which is associated with a defect in associative learning but other anxiety-like behaviors were normal. Similarly, no stress- and anxiety-like behaviors were found in Reln heterozygous mice (Teixeira et al., 2011), but they found that mice with Reln overexpressed were less susceptible to depressive-like behaviors and were resistant to chronic cocaine stimulation.
An interesting study has shown that cleavage at a particular site on the RELN protein (known as N-t), can sustain RELN activity beyond the normal range of the wildtype protein in an in vitro model (Koie et al., 2014), which may point to a potential therapeutic target for certain neuropsychiatric disorders.
Dcx
DCX is a microtubule associated protein which binds to microtubules to enable neuronal migration (Koizumi et al., 2006; Moores et al., 2006). DCX is also involved in the dynamic development of dendrites through regulating their length, branching point and complexity of the dendrites (Cohen et al., 2008). In addition, DCX regulates the distribution of neurofascin, a cell surface adhesion molecule at the outer surface of the cell in a microtubule-independent manner (Yap et al., 2012), which in turn, regulate axonal outgrowth and the formation of GABAergic synapses. DCX mutations are commonly associated with lissencephaly, smooth brain disorder, a neuronal migration disorder which then leads to epilepsy and mental retardation (Jang et al., 2013).
MiR-128 inhibits the expression of Dcx by directly binding on its 3′UTR region and inhibition of miR-128 successfully restores the expression of Dcx (Evangelisti et al., 2009). Dcx mutant mice exhibit a range of abnormal behaviors such as hyperactivity, spontaneous seizures, impaired social interaction, and decreased aggression (Nosten-Bertrand et al., 2008; Germain et al., 2013). Disorganized hippocampal CA3 regions have been observed (Nosten-Bertrand et al., 2008) and may account for the various behaviors, but a recent study indicated that the typical hippocampal-dependent behaviors such as spatial memory were not impaired in Dcx knockout mice (Germain et al., 2013).
Conclusion
In this article, we sought to evaluate the potential role of miR-128 target genes in neuropsychiatric disorders. We noticed that out of the 801 genes predicted to be targets of miR-128 by TargetScan, only a small number of genes have been empirically proven to be regulated by this miRNA. This indicates a large void of knowledge of which genes are true targets of miR-128, and more research is needed to construct a broader picture of the pathways regulated by miR-128.
Based on a systematic review and analysis of predicted and current empirically determined targets of miR-128, we found that miR-128 regulates many pathways within the cell, including pathways involved in cancer or neuronal maturation. With respect to neuropsychiatric phenotypes, we found an association with many miR-128 target genes. As many of these have well characterized functions, we postulate that therapeutic targets can be directed toward these known pathways and the central role miR-128 plays in these pathways may indicate a potential role as a biomarker for selected neuropsychiatric disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank members of the lab for their helpful comments on the manuscript and the Ministry of Higher Education-High Impact Research (MOHE-HIR-E29) and University of Malaya (RG399/12HTM) grants.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fncel.2015.00465
References
- Adlakha Y. K., Saini N. (2011). MicroRNA-128 downregulates Bax and induces apoptosis in human embryonic kidney cells. Cell. Mol. Life Sci. 68 1415–1428. 10.1007/s00018-010-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K., Kudo Y., Aoyagi K., Ishikawa R., Igarashi M., Takahashi M. (2013). Calmodulin-dependent regulation of neurotransmitter release differs in subsets of neuronal cells. Brain Res. 1535 1–13. 10.1016/j.brainres.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Azzam G., Wang X., Bell D., Murphy M. E. (2013). CSF1 is a novel p53 target gene whose protein product functions in a feed-forward manner to suppress apoptosis and enhance p53-mediated growth arrest. PLoS ONE 8:e74297 10.1371/journal.pone.0074297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136 215–233. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge N. J., Gardiner E., Carroll A. P., Tooney P. A., Cairns M. J. (2010). Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry 15 1176–1189. 10.1038/mp.2009.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L., Maffioletti E., Bettinsoli P., Giovannini C., Bignotti S., Tardito D., et al. (2013). Blood microRNA changes in depressed patients during antidepressant treatment. Eur. Neuropsychopharmacol. 23 602–611. 10.1016/j.euroneuro.2012.06.013 [DOI] [PubMed] [Google Scholar]
- Bruno I. G., Karam R., Huang L., Bhardwaj A., Lou C. H., Shum E. Y., et al. (2011). Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Mol. Cell 42 500–510. 10.1016/j.molcel.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G., Gu M., Zhu M., Gao J., Yin Y., Marshall C., et al. (2012). Bmi-1 absence causes premature brain degeneration. PLoS ONE 7:e32015 10.1371/journal.pone.0032015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso G. L., Bibb J. A., Snyder G. L., Valle C., Rakhilin S., Fienberg A. A., et al. (2000). Drugs of abuse modulate the phosphorylation of ARPP-21, a cyclic AMP-regulated phosphoprotein enriched in the basal ganglia. Neuropharmacology 39 1637–1644. 10.1016/S0028-3908(99)00230-0 [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Imam J. S., Wilkinson M. F. (2007). The nonsense-mediated decay RNA surveillance pathway. Annu. Rev. Biochem. 76 51–74. 10.1146/annurev.biochem.76.050106.093909 [DOI] [PubMed] [Google Scholar]
- Chazal P. E., Daguenet E., Wendling C., Ulryck N., Tomasetto C., Sargueil B., et al. (2013). EJC core component MLN51 interacts with eIF3 and activates translation. Proc. Natl. Acad. Sci. U.S.A. 110 5903–5908. 10.1073/pnas.1218732110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Segal M., Reiner O. (2008). Doublecortin supports the development of dendritic arbors in primary hippocampal neurons. Dev. Neurosci. 30 187–199. 10.1159/000109862 [DOI] [PubMed] [Google Scholar]
- Davis M. M., Olausson P., Greengard P., Taylor J. R., Nairn A. C. (2012). Regulator of calmodulin signaling knockout mice display anxiety-like behavior and motivational deficits. Eur. J. Neurosci. 35 300–308. 10.1111/j.1460-9568.2011.07956.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio J. A., Heimrich B., Borrell V., Forster E., Drakew A., Alcantara S., et al. (1997). A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385 70–74. 10.1038/385070a0 [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. (1983). The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell 35 79–87. 10.1016/0092-8674(83)90210-6 [DOI] [PubMed] [Google Scholar]
- Evangelisti C., Florian M. C., Massimi I., Dominici C., Giannini G., Galardi S., et al. (2009). MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J. 23 4276–4287. 10.1096/fj.09-134965 [DOI] [PubMed] [Google Scholar]
- Faraone S. V., Perlis R. H., Doyle A. E., Smoller J. W., Goralnick J. J., Holmgren M. A., et al. (2005). Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 57 1313–1323. 10.1016/j.biopsych.2004.11.024 [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Snow A. V., Stary J. M., Araghi-Niknam M., Reutiman T. J., Lee S., et al. (2005). Reelin signaling is impaired in autism. Biol. Psychiatry 57 777–787. 10.1016/j.biopsych.2004.12.018 [DOI] [PubMed] [Google Scholar]
- Frotscher M., Chai X., Bock H. H., Haas C. A., Forster E., Zhao S. (2009). Role of Reelin in the development and maintenance of cortical lamination. J. Neural Transm. 116 1451–1455. 10.1007/s00702-009-0228-7 [DOI] [PubMed] [Google Scholar]
- Gates M. A., Torres E. M., White A., Fricker-Gates R. A., Dunnett S. B. (2006). Re-examining the ontogeny of substantia nigra dopamine neurons. Eur. J. Neurosci. 23 1384–1390. 10.1111/j.1460-9568.2006.04637.x [DOI] [PubMed] [Google Scholar]
- Gentili C., Tutolo G., Pianezzi A., Cancedda R., Descalzi Cancedda F. (2005). Cholesterol secretion and homeostasis in chondrocytes: a liver X receptor and retinoid X receptor heterodimer mediates apolipoprotein A1 expression. Matrix Biol. 24 35–44. 10.1016/j.matbio.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Germain J., Bruel-Jungerman E., Grannec G., Denis C., Lepousez G., Giros B., et al. (2013). Doublecortin knockout mice show normal hippocampal-dependent memory despite CA3 lamination defects. PLoS ONE 8:e74992 10.1371/journal.pone.0074992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Auta J., Davis J. M., Di-Giorgi-Gerevini V., Dwivedi Y., Grayson D. R., et al. (2000). Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch. Gen. Psychiatry 57 1061–1069. 10.1001/archpsyc.57.11.1061 [DOI] [PubMed] [Google Scholar]
- Han X., Ma Y., Liu X., Wang L., Qi S., Zhang Q., et al. (2012). Changes in insulin-signaling transduction pathway underlie learning/memory deficits in an Alzheimer’s disease rat model. J. Neural Transm. 119 1407–1416. 10.1007/s00702-012-0803-1 [DOI] [PubMed] [Google Scholar]
- Herring A., Donath A., Steiner K. M., Widera M. P., Hamzehian S., Kanakis D., et al. (2012). Reelin depletion is an early phenomenon of Alzheimer’s pathology. J. Alzheimers Dis. 30 963–979. 10.3233/JAD-2012-112069 [DOI] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4 44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Jang M. A., Woo H. I., Kim J. W., Lee J., Ki C. S. (2013). Identification of DCX gene mutation in lissencephaly spectrum with subcortical band heterotopia using whole exome sequencing. Pediatr. Neurol. 48 411–414. 10.1016/j.pediatrneurol.2012.12.033 [DOI] [PubMed] [Google Scholar]
- Kang H. J., Kawasawa Y. I., Cheng F., Zhu Y., Xu X., Li M., et al. (2011). Spatio-temporal transcriptome of the human brain. Nature 478 483–489. 10.1038/nature10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam R., Wilkinson M. (2012). A conserved microRNA/NMD regulatory circuit controls gene expression. RNA Biol. 9 22–26. 10.4161/rna.9.1.18010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Krichevsky A., Grad Y., Hayes G. D., Kosik K. S., Church G. M., et al. (2004). Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc. Natl. Acad. Sci. U.S.A. 101 360–365. 10.1073/pnas.2333854100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koie M., Okumura K., Hisanaga A., Kamei T., Sasaki K., Deng M., et al. (2014). Cleavage within Reelin repeat 3 regulates the duration and range of the signaling activity of Reelin protein. J. Biol. Chem. 289 12922–12930. 10.1074/jbc.M113.536326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H., Tanaka T., Gleeson J. G. (2006). Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron 49 55–66. 10.1016/j.neuron.2005.10.040 [DOI] [PubMed] [Google Scholar]
- Kolsch H., Lutjohann D., Jessen F., Popp J., Hentschel F., Kelemen P., et al. (2009). RXRA gene variations influence Alzheimer’s disease risk and cholesterol metabolism. J. Cell. Mol. Med. 13 589–598. 10.1111/j.1582-4934.2009.00383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky A. M., King K. S., Donahue C. P., Khrapko K., Kosik K. S. (2003). A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9 1274–1281. 10.1261/rna.5980303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang W. J., Sun R. F., Zhu Y. S., Li S. B. (2011). A new single-nucleotide mutation (rs362719) of the reelin (RELN) gene associated with schizophrenia in female Chinese Han. Genet. Mol. Res. 10 1650–1658. 10.4238/vol10-3gmr1343 [DOI] [PubMed] [Google Scholar]
- Kunde S. A., Rademacher N., Tzschach A., Wiedersberg E., Ullmann R., Kalscheuer V. M., et al. (2013). Characterisation of de novo MAPK10/JNK3 truncation mutations associated with cognitive disorders in two unrelated patients. Hum. Genet. 132 461–471. 10.1007/s00439-012-1260-5 [DOI] [PubMed] [Google Scholar]
- Laske C., Stransky E., Hoffmann N., Maetzler W., Straten G., Eschweiler G. W., et al. (2010). Macrophage colony-stimulating factor (M-CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer’s disease. Curr. Alzheimer Res. 7 409–414. 10.2174/156720510791383813 [DOI] [PubMed] [Google Scholar]
- Lee C. H., Liu C. M., Wen C. C., Chang S. M., Hwu H. G. (2010). Genetic copy number variants in sib pairs both affected with schizophrenia. J. Biomed. Sci. 17 2 10.1186/1423-0127-17-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Ajay S. S., Yook J. I., Kim H. S., Hong S. H., Kim N. H., et al. (2009). New class of microRNA targets containing simultaneous 5′-UTR and 3′-UTR interaction sites. Genome Res. 19 1175–1183. 10.1101/gr.089367.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C., Lingbeek M., Shakhova O., Liu J., Tanger E., Saremaslani P., et al. (2004). Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428 337–341. 10.1038/nature02385 [DOI] [PubMed] [Google Scholar]
- Lewis B. P., Burge C. B., Bartel D. P. (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120 15–20. 10.1016/j.cell.2004.12.035 [DOI] [PubMed] [Google Scholar]
- Li M., Fu W., Wo L., Shu X., Liu F., Li C. (2013). miR-128 and its target genes in tumorigenesis and metastasis. Exp. Cell Res. 319 3059–3064. 10.1016/j.yexcr.2013.07.031 [DOI] [PubMed] [Google Scholar]
- Lin Q., Wei W., Coelho C. M., Li X., Baker-Andresen D., Dudley K., et al. (2011). The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat. Neurosci. 14 1115–1117. 10.1038/nn.2891 [DOI] [PubMed] [Google Scholar]
- Mao X. R., Moerman-Herzog A. M., Chen Y., Barger S. W. (2009). Unique aspects of transcriptional regulation in neurons–nuances in NFkappaB and Sp1-related factors. J. Neuroinflammation 6 16 10.1186/1742-2094-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Sun Y., Tang J. (2014). Serum miR-21 is a diagnostic and prognostic marker of primary central nervous system lymphoma. Neurol. Sci. 35 233–238. 10.1007/s10072-013-1491-9 [DOI] [PubMed] [Google Scholar]
- Marangi G., Orteschi D., Milano V., Mancano G., Zollino M. (2013). Interstitial deletion of 3p22.3p22.2 encompassing ARPP21 and CLASP2 is a potential pathogenic factor for a syndromic form of intellectual disability: a co-morbidity model with additional copy number variations in a large family. Am. J. Med. Genet. A 161A, 2890–2893. 10.1002/ajmg.a.36257 [DOI] [PubMed] [Google Scholar]
- Megraw M., Sethupathy P., Gumireddy K., Jensen S., Huang Q., Hatzigeorgiou A. (2010). Isoform specific gene auto-regulation via miRNAs: a case study on miR-128b and ARPP-21. Theor. Chem. Acc. 125 593–598. 10.1007/s00214-009-0647-4 [DOI] [Google Scholar]
- Mohit A. A., Martin J. H., Miller C. A. (1995). p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron 14 67–78. 10.1016/0896-6273(95)90241-4 [DOI] [PubMed] [Google Scholar]
- Moores C. A., Perderiset M., Kappeler C., Kain S., Drummond D., Perkins S. J., et al. (2006). Distinct roles of doublecortin modulating the microtubule cytoskeleton. EMBO J. 25 4448–4457. 10.1038/sj.emboj.7601335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten-Bertrand M., Kappeler C., Dinocourt C., Denis C., Germain J. Phan Dinh,et al. (2008). Epilepsy in Dcx knockout mice associated with discrete lamination defects and enhanced excitability in the hippocampus. PLoS ONE 3:e2473 10.1371/journal.pone.0002473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet C. C., Hemmings H. C., Jr., Greengard P. (1989). ARPP-21, a cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. II. Immunocytochemical localization in rat brain. J. Neurosci. 9 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadia G., Shifman S. (2011). The genetic variation of RELN expression in schizophrenia and bipolar disorder. PLoS ONE 6:e19955 10.1371/journal.pone.0019955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., et al. (1989). The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J. Cell Biol. 109 3039–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiannakopoulos T., Friedmann-Morvinski D., Neveu P., Dugas J. C., Gill R. M., Huillard E., et al. (2012). Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene 31 1884–1895. 10.1038/onc.2011.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschou M., Doxakis E. (2012). Neurofibromin 1 is a miRNA target in neurons. PLoS ONE 7:e46773 10.1371/journal.pone.0046773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo P. R., Browning R. A., Cranick S. (2006). Reeler homozygous mice exhibit enhanced susceptibility to epileptiform activity. Epilepsia 47 257–266. 10.1111/j.1528-1167.2006.00417.x [DOI] [PubMed] [Google Scholar]
- Perkins D. O., Jeffries C. D., Jarskog L. F., Thomson J. M., Woods K., Newman M. A., et al. (2007). microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 8 R27 10.1186/gb-2007-8-2-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pore N., Liu S., Shu H. K., Li B., Haas-Kogan D., Stokoe D., et al. (2004). Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1-independent mechanism. Mol. Biol. Cell 15 4841–4853. 10.1091/mbc.E04-05-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian P., Banerjee A., Wu Z. S., Zhang X., Wang H., Pandey V., et al. (2012). Loss of SNAIL regulated miR-128-2 on chromosome 3p22.3 targets multiple stem cell factors to promote transformation of mammary epithelial cells. Cancer Res. 72 6036–6050. 10.1158/0008-5472.CAN-12-1507 [DOI] [PubMed] [Google Scholar]
- Qiu S., Korwek K. M., Pratt-Davis A. R., Peters M., Bergman M. Y., Weeber E. J. (2006). Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol. Learn. Mem. 85 228–242. 10.1016/j.nlm.2005.11.001 [DOI] [PubMed] [Google Scholar]
- Rakhilin S. V., Olson P. A., Nishi A., Starkova N. N., Fienberg A. A., Nairn A. C., et al. (2004). A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science 306 698–701. 10.1126/science.1099961 [DOI] [PubMed] [Google Scholar]
- Reinecke K., Herdegen T., Eminel S., Aldenhoff J. B., Schiffelholz T. (2013). Knockout of c-Jun N-terminal kinases 1, 2 or 3 isoforms induces behavioural changes. Behav. Brain Res. 245 88–95. 10.1016/j.bbr.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Schmiedel J. M., Klemm S. L., Zheng Y., Sahay A., Bluthgen N., Marks D. S., et al. (2015). Gene expression. MicroRNA control of protein expression noise. Science 348 128–1132. 10.1126/science.aaa1738 [DOI] [PubMed] [Google Scholar]
- Shoichet S. A., Duprez L., Hagens O., Waetzig V., Menzel C., Herdegen T., et al. (2006). Truncation of the CNS-expressed JNK3 in a patient with a severe developmental epileptic encephalopathy. Hum. Genet. 118 559–567. 10.1007/s00439-005-0084-y [DOI] [PubMed] [Google Scholar]
- Smirnova L., Grafe A., Seiler A., Schumacher S., Nitsch R., Wulczyn F. G. (2005). Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 21 1469–1477. 10.1111/j.1460-9568.2005.03978.x [DOI] [PubMed] [Google Scholar]
- Sollner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., et al. (1993). SNAP receptors implicated in vesicle targeting and fusion. Nature 362 318–324. 10.1038/362318a0 [DOI] [PubMed] [Google Scholar]
- Stelzer G., Dalah I., Stein T. I., Satanower Y., Rosen N., Nativ N., et al. (2011). In-silico human genomics with GeneCards. Hum. Genomics 5 709–717. 10.1186/1479-7364-5-6-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. L., Plotkin J. L., Veno M. T., Von Schimmelmann M., Feinberg P., Mann S., et al. (2013). MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 342 1254–1258. 10.1126/science.1244193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tararuk T., Ostman N., Li W., Bjorkblom B., Padzik A., Zdrojewska J., et al. (2006). JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J. Cell Biol. 173 265–277. 10.1083/jcb.200511055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C. M., Martin E. D., Sahun I., Masachs N., Pujadas L., Corvelo A., et al. (2011). Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology 36 2395–2405. 10.1038/npp.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P. M., Egbufoama S., Vawter M. P. (2003). SNAP-25 reduction in the hippocampus of patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 27 411–417. 10.1016/S0278-5846(03)00027-7 [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Nairn A. C., Greengard P. (1983). Regional distribution of calcium– and cyclic adenosine 3’:5’-monophosphate-regulated protein phosphorylation systems in mammalian brain. II. Soluble systems. J. Neurosci. 3 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Luo Y., Ly P. T., Cai F., Zhou W., Zou H., et al. (2012). Sp1 regulates human huntingtin gene expression. J. Mol. Neurosci. 47 311–321. 10.1007/s12031-012-9739-z [DOI] [PubMed] [Google Scholar]
- Weiss G. J., Bemis L. T., Nakajima E., Sugita M., Birks D. K., Robinson W. A., et al. (2008). EGFR regulation by microRNA in lung cancer: correlation with clinical response and survival to gefitinib and EGFR expression in cell lines. Ann. Oncol. 19 1053–1059. 10.1093/annonc/mdn006 [DOI] [PubMed] [Google Scholar]
- Williams K. R., Hemmings H. C., Jr., Lopresti M. B., Greengard P. (1989). ARPP-refvol21, a cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Amino acid sequence of ARPP-21B from bovine caudate nucleus. J. Neurosci. 9 3631–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Tang Y., Zang W., Wang Y., Li M., Du Y., et al. (2014). MicroRNA-128 regulates the differentiation of rat bone mesenchymal stem cells into neuron-like cells by Wnt signaling. Mol. Cell. Biochem. 387 151–158. 10.1007/s11010-013-1880-7 [DOI] [PubMed] [Google Scholar]
- Xu J., Luo F., Zhang Z., Xue L., Wu X. S., Chiang H. C., et al. (2013). SNARE proteins synaptobrevin, SNAP-25, and syntaxin are involvedin rapid and slow endocytosis at synapses. Cell Rep. 3 1414–1421. 10.1016/j.celrep.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap C. C., Vakulenko M., Kruczek K., Motamedi B., Digilio L., Liu J. S., et al. (2012). Doublecortin (DCX) mediates endocytosis of neurofascin independently of microtubule binding. J. Neurosci. 32 7439–7453. 10.1523/JNEUROSCI.5318-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare M., Soleimani M., Akbarzadeh A., Bakhshandeh B., Aghaee-Bakhtiari S. H., Zarghami N. (2015). A novel protocol to differentiate induced pluripotent stem cells by neuronal microRNAs to provide a suitable cellular model. Chem. Biol. Drug Des. 86 232–238. 10.1111/cbdd.12485 [DOI] [PubMed] [Google Scholar]
- Zencak D., Lingbeek M., Kostic C., Tekaya M., Tanger E., Hornfeld D., et al. (2005). Bmi1 loss produces an increase in astroglial cells and a decrease in neural stem cell population and proliferation. J. Neurosci. 25 5774–5783. 10.1523/JNEUROSCI.3452-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W. H., Quirion R. (2004). Comparative signaling pathways of insulin-like growth factor-1 and brain-derived neurotrophic factor in hippocampal neurons and the role of the PI3 kinase pathway in cell survival. J. Neurochem. 89 844–852. 10.1111/j.1471-4159.2004.02350.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.