Abstract
Background: Islet autoantibody testing provides the basis for assessment of risk of progression to type 1 diabetes. We set out to determine the feasibility and acceptability of dried capillary blood spot–based screening to identify islet autoantibody–positive relatives potentially eligible for inclusion in prevention trials.
Materials and Methods: Dried blood spot (DBS) and venous samples were collected from 229 relatives participating in the TrialNet Pathway to Prevention Study. Both samples were tested for glutamic acid decarboxylase, islet antigen 2, and zinc transporter 8 autoantibodies, and venous samples were additionally tested for insulin autoantibodies and islet cell antibodies. We defined multiple autoantibody positive as two or more autoantibodies in venous serum and DBS screen positive if one or more autoantibodies were detected. Participant questionnaires compared the sample collection methods.
Results: Of 44 relatives who were multiple autoantibody positive in venous samples, 42 (95.5%) were DBS screen positive, and DBS accurately detected 145 of 147 autoantibody-negative relatives (98.6%). Capillary blood sampling was perceived as more painful than venous blood draw, but 60% of participants would prefer initial screening using home fingerstick with clinic visits only required if autoantibodies were found.
Conclusions: Capillary blood sampling could facilitate screening for type 1 diabetes prevention studies.
Introduction
Islet autoantibody testing provides the basis for assessment of risk of progression to type 1 diabetes, but screening generally requires venous blood sampling, which can be traumatic for children.1 Collecting capillary blood samples offers a potential alternative2–4 and could also give additional flexibility for staff. If samples can ultimately be collected at home, it could mean that families recruited for screening would not need to come to a clinic, hospital, or laboratory for venipuncture and could therefore enhance recruitment. We set out to determine the feasibility and acceptability of sample collection using dried capillary blood spots (DBS) and to evaluate its performance in identifying multiple autoantibody-positive relatives at increased risk of type 1 diabetes who would be potentially eligible for inclusion in TrialNet prevention trials. We envisaged DBS-based testing being used for first-line screening with confirmation in a venous sample if an individual screened autoantibody positive.
Research Design and Methods
We recruited relatives of people with type 1 diabetes participating in the TrialNet Pathway to Prevention (PTP) Study at 15 TrialNet Clinical Centers in North America and Europe.5 Recruitment was stratified by age to ensure that adequate numbers of young children were enrolled, and participants attending for semiannual monitoring visits were preferentially selected to ensure inclusion of individuals positive for two or more islet autoantibodies.6 Participants were asked to provide both DBS and venous samples at a screening or follow-up visit. All samples were collected by research nurses using standard procedures. Staff were trained to collect capillary blood samples using BD Microtainer® contact-activated lancets (Becton Dickinson, Franklin Lakes, NJ) and were asked to fill five circles (diameter, 1 cm) on filter paper (Whatman 903 Protein Saver card; GE Healthcare Bio-Sciences, Pittsburgh, PA), which was air-dried before sealing in a plastic envelope and mailing to the laboratory. Venous samples were handled in accordance with PTP operating procedures.
Venous samples were tested using the established TrialNet strategy: screening for autoantibodies to glutamic acid decarboxylase (GADA), islet antigen 2 (IA-2A), and insulin (IAA) with supplementary testing for zinc transporter 8 autoantibodies (ZnT8A) and islet cell antibodies (ICA) if any autoantibody was positive on initial screen.6 DBS samples were tested for GADA, IA-2A, and ZnT8A after overnight soaking and elution at 40°C in 60 μL of 20 mM Tris-HCl (pH 7.4) buffer containing 150 mM NaCl, 0.1% bovine serum albumin, 0.15% Tween-20, and 0.1% NaN3, and assays were performed on 20 μL of retrieved eluate. GADA, IA-2A, ZnT8A, and IAA were determined by radioimmunoassay, and ICA was assessed by indirect immunofluorescence as previously described.7,8 The same GADA, IA-2A, and ZnT8A assays and thresholds were used for venous serum and eluted DBS samples.
Participant questionnaires were used to compare the sample collection methods (Supplementary Data are available online at www.liebertonline.com/dia). The quality of DBS samples was reported by the laboratory as “optimal” (sufficient to allow all three autoantibodies to be measured in duplicate with confirmation in autoantibody-positive samples if required; three or more circles filled), “borderline” (DBS circles had blank sections but were insufficient to allow confirmatory testing), and “poor” (individual DBS circles were unevenly filled and blotchy, leading to potentially unreliable results).
Multiple autoantibody-positive (high-risk) status was defined as detection of two or more of the five autoantibodies tested in the venous sample, and DBS screening was considered positive if one or more of the three autoantibodies tested were detected. We calculated the sensitivity and specificity of DBS screening for detection of high-risk, multiple autoantibody-positive individuals and determined 95% exact confidence intervals. Differences in sample quality by age and reported level of discomfort were analyzed by χ2 testing. Antibody levels in DBS and venous blood were compared using linear regression.
Results
DBS and venous samples were collected from 229 individuals: 130 at screening visits, 97 at semiannual monitoring visits, and two at annual re-screening visits. The median age of participants was 20 years (interquartile range, 12–38 years); 28 were 8 years of age or less, 83 were 9–18 years of age, and 118 were more than 19 years of age. One hundred thirty-one were female. Of the 229 venous samples, 72 (31%) were positive for GADA, 22 (10%) for IA-2A, nine (3.9%) for IAA, 27 (12%) for ZnT8A, and 37 (16.2%) for ICA. Questionnaires were completed by 212 participants. There was no difference in age between questionnaire responders and nonresponders (P = 0.55).
Levels of GADA, IA-2A, and ZnT8A in DBS eluate correlated well with levels in venous serum (Fig. 1). The sensitivity and specificity of DBS compared with serum assays for GADA, IA2A, and ZnT8A are given in Supplementary Table S1.
FIG. 1.
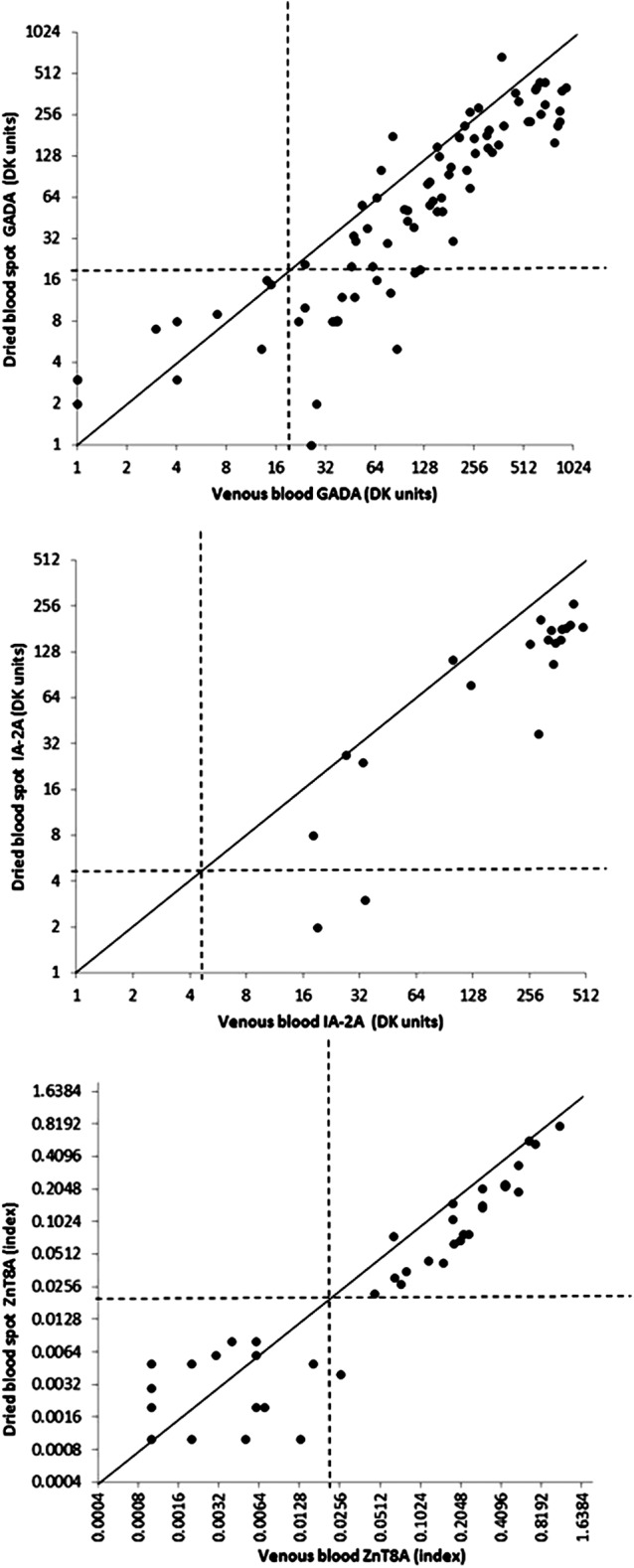
Assay concordance in venous serum and dried blood spots. Solid lines equate to identical autoantibody levels in venous and dried blood spot samples. The correlation coefficients (r) for (upper panel) glutamic acid decarboxylase autoantibodies (GADA), (middle panel) islet antigen 2 autoantibodies (IA-2A), and (lower panel) zinc transporter 8 autoantibodies (ZnT8A) were 0.866, 0.960, and 0.894, respectively (all P < 0.001). Dotted lines indicate the thresholds used to define autoantibody-positive status. GADA and IA-2A results are expressed in digestive and kidney (DK) units.7
The number of autoantibodies detected in paired DBS and venous blood samples, as well as the sensitivity and specificity of DBS screening for detection of multiple autoantibody-positive relatives, are shown in Tables 1 and 2. Of 44 relatives found to be multiple autoantibody positive using the TrialNet strategy in venous samples, 42 (95.5%) were positive on DBS screening. DBS accurately detected 145 of 147 autoantibody-negative relatives (98.6%). The two individuals positive on DBS screening but autoantibody negative in venous samples were weakly positive for ZnT8A or GADA and had insufficient DBS sample to allow confirmation. Of the 15 participants who were single autoantibody positive in venous but not DBS samples, five were positive for IAA, one for ICA, eight for GADA, and one for IA-2A.
Table 1.
Number of Autoantibodies Detected in Paired Dried Blood Spot and Venous Blood Samples
| Number of positive antibodies in DBS sample | |||||
|---|---|---|---|---|---|
| Number of positive antibodies in venous sample | 0 | 1 | 2 | 3 | Total |
| 0 | 145 | 2 | 0 | 0 | 147 |
| 1 | 15 | 23 | 0 | 0 | 38 |
| 2 | 2 | 12 | 2 | 0 | 16 |
| 3 | 0 | 6 | 9 | 1 | 16 |
| 4 | 0 | 1 | 4 | 6 | 11 |
| 5 | 0 | 0 | 0 | 1 | 1 |
| Total | 162 | 44 | 15 | 8 | 229 |
DBS, dried blood spot.
Table 2.
Accuracy of Dried Blood Spot Screening for Detection of Multiple Autoantibody-Positive Individuals
| DBS-positive | DBS-negative | Total | |
|---|---|---|---|
| ≥2 antibodies in venous sample | |||
| n | 42 | 2 | 44 |
| Percentage | 95.45% | 4.55% | |
| 95% CIa | 84.5, 99.4 | 0.6, 15.5 | |
| Definition/use | Sensitivity | Screen “false negatives” | |
| ≤1 antibody positive in venous sample | 25b | 160 | 185 |
| n | 13.5% | 86.5% | |
| 95% CIa | 8.9, 19.3 | 80.7, 91.1 | |
| Definition/use | Screen “false positives” | Specificity | |
| Total | 67 | 162 | 229 |
Exact binomial confidence intervals (CIs).
Twenty-three of these were positive for a single autoantibody.
DBS, dried blood spot.
It was possible to report results for GADA, IA-2A, and ZnT8A in all the 229 DBS samples. DBS sample quality was optimal with at least three circles filled in 55% of participants, but this varied from 20 to 100% among centers. The frequency of suboptimal samples was highest in adults. The median age of relatives with optimal samples was 16 years (interquartile range, 11–34 years) compared with 30 years (interquartile range, 14–39 years) in those with suboptimal samples (P = 0.002). DBS sample quality was optimal in 64% of participants 8 years of age or less, 65% of those 9–18 years of age, and 46% of those more than 19 years of age (P = 0.013).
Capillary blood sample collection was perceived as more painful than venous blood draw by 51% of questionnaire respondents, whereas 28% reported the blood draw hurt more; 34% of respondents thought they would be more worried about a blood draw versus 16% about a DBS test. For future testing, 38% would choose a blood draw, whereas 41% would prefer a DBS test as carried out in the study. However, 63% of participants/families felt they would prefer the option of initial screening using a home fingerprick with clinic visits only required if autoantibodies were detected, including 59% of relatives at screening visits and 68% of those attending for monitoring visits.
There was a linear-by-linear association between the reported level of discomfort associated with DBS collection and the quality of the sample (P = 0.013); of 88 individuals whose samples were categorized as “poor” quality, 18 (20%) reported that DBS sample collection was associated with “a lot” of discomfort, compared with only 10 of 114 individuals (9%) whose sample was “optimal.” This association was observed among 108 adults 19 years of age or above (P = 0.005), but not in children (n = 104; P = 0.249).
Discussion
Our findings demonstrate that collecting capillary blood on filter paper provides a feasible and acceptable alternative to venous blood draw for obtaining samples for islet autoantibody testing. The DBS-based screening strategy achieved high sensitivity for identifying multiple autoantibody-positive relatives at high risk of developing type 1 diabetes but, as expected from earlier studies,2 was less sensitive for detection of single autoantibody-positive individuals. We also found that obtaining DBS samples can be difficult, even for healthcare professionals, and 45% of samples were not of sufficient quality to confirm positive results. It is important that the questionnaires showed that the potential to avoid clinic visits was very important to families. Even though participants found the fingerprick test more painful than the venous blood draw and they might be asked to come to the clinic for a confirmatory blood draw, families expressed a preference for collecting capillary blood samples at home.
By including participants with a range of antibody levels, we were able to assess both the sensitivity and specificity of our screening strategy. We showed that DBS screening is very sensitive for detection of high-risk relatives, and although some individuals invited back to give a venous blood sample would not be confirmed as multiple autoantibody positive, the majority of these would be single autoantibody positive and thus potentially eligible for follow-up in the PTP and future prevention studies. DBS-based screening therefore offers a suitable alternative for initial testing in situations when venipuncture is difficult, for example, at camps and community events.
A further strength is our evaluation of the acceptability of the two blood sampling techniques, although these data have some limitations in informing more widespread use of capillary blood–based screening. First, samples were collected by healthcare professionals rather than participants or family members as would be necessary for home screening. Second, parts of the questionnaire were necessarily theoretical; for example, “Do you think that you could do this at home?” Also, our study design meant that we were only able to obtain the preferences of individuals who had experienced both venipuncture and capillary sampling. It is possible that people recruited for screening without prior experience of either method may have different views. Our study therefore represents only one step in process of developing a strategy for self-collection of screening samples.
The use of DBS in islet autoantibody screening has some drawbacks. Previous studies have found low sensitivity for detection of IAA.2 We therefore elected not to test for these, but rather to substitute ZnT8A in the initial screen. As IAA generally provide the first indication of autoimmunity in infancy,9 there may be problems with using DBS for screening young children. We also need to overcome the technical problems associated with collection of DBS samples and reduce the variability and proportion of suboptimal samples obtained. This could perhaps be accomplished by better training and/or collecting capillary whole blood samples from which serum can be obtained. We were interested to find age-related differences in the quality of DBS sample collected but do not have an adequate explanation. The observation that poor sample quality is associated with higher reported levels of discomfort in adults but not in children may be relevant, and it will be interesting to see whether these differences are observed in future studies. In this study we used the same thresholds to define autoantibody positivity in venous and DBS samples. It is possible that the performance of DBS-based screening—particularly the sensitivity for identifying single autoantibody-positive individuals—could be enhanced by optimizing these thresholds. The number of samples in this study did not allow us to do this, but it should be considered if DBS testing is to be applied on a larger scale.
We conclude that, with samples collected by research staff as in this study, capillary blood–based screening using DBS could provide a useful recruitment tool for prevention trials. In the future, this approach could also be suitable for general population screening for risk of type 1 diabetes and other autoimmune conditions. Taken together with acceptability and the families' enthusiasm for the possibility of collecting samples at home, these results clearly justify further exploration to enhance the feasibility and/or acceptability of home-based sample collection techniques, which have proved successful in other settings.10
Supplementary Material
Contributor Information
Collaborators: the TrialNet Study Group
Acknowledgments
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the following: the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through cooperative agreements U01 DK061010, U01 DK061016, U01 DK061034, U01 DK061036, U01 DK061040, U01 DK061041, U01 DK061042, U01 DK061055, U01 DK061058, U01 DK084565, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085505, and U01 DK085509, and contract HHSN267200800019C, as well as the National Center for Research Resources, through Clinical Translational Science Awards UL1 RR024131, UL1 RR024139, UL1 RR024153, UL1 RR024975, UL1 RR024982, UL1 RR025744, UL1 RR025761, UL1 RR025780, UL1 RR029890, and UL1 RR031986 and General Clinical Research Center Award M01 RR00400; the JDRF; and the American Diabetes Association.
Author Disclosure Statement
No competing financial interests exist.
P.J.B. wrote the manuscript. L.E.R., D.M., L.Y., A.K.S., and C.H. conducted the study, assisted in writing the manuscript, and reviewed the manuscript. C.A.B. and D.C.B. provided statistical support, analyzed the data, assisted in writing the manuscript, and reviewed the manuscript. C.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Hummel M, Ziegler AG, Roth R: Psychological impact of childhood islet autoantibody testing in families participating in the BABYDIAB study. Diabet Med 2004;21:324–328 [DOI] [PubMed] [Google Scholar]
- 2.Bazzigaluppi E, Bonfanti R, Bingley PJ, et al. : Capillary whole blood measurement of islet autoantibodies. Diabetes Care 1999;22:275–279 [DOI] [PubMed] [Google Scholar]
- 3.Costa A, Conget I, Casamitjana R: Response to Bazzigaluppi et al.: Capillary whole-blood measurement of islet autoantibodies. Diabetes Care 2000;23:874. [DOI] [PubMed] [Google Scholar]
- 4.Siraj ES, Rogers DG, Gupta MK, et al. : A simple screening method for individuals at risk of developing type 1 diabetes: measurement of islet cell autoantibodies (GADA, IA-2A, and IAA) on dried capillary blood spots collected on a filter paper. Horm Metab Res 2013;44:855–860 [DOI] [PubMed] [Google Scholar]
- 5.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. : The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 6.Type 1 Diabetes-TrialNet Study Group: Natural History Study of the Development of Type 1 Diabetes (Protocol TN-01). https://www.diabetestrialnet.org/documents/ancillary/PTPProtocolSynopsis&SpecimenCollectionSchedule_ProtocolV15Aug11.pdf (accessed April13, 2015)
- 7.Bonifacio E, Yu L, Williams AJK, et al. : Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases Consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu L, Boulware DC, Beam CA, et al. : Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care. 2012;35:1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams AJK, Bingley PJ: Worth the wait: type 1 diabetes prospective birth cohort studies enter adolescence. Diabetologia 2012;55:1873–1876 [DOI] [PubMed] [Google Scholar]
- 10.Bethel MA, Price HC, Sourij H, et al. : Evaluation of a self-administered oral glucose tolerance test. Diabetes Care 2013,36:1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


