Abstract
Traumatic brain injury (TBI) is a significant clinical problem with few therapeutic interventions successfully translated to the clinic. Increased importance on the progressive, long-term consequences of TBI have been emphasized, both in the experimental and clinical literature. Thus, there is a need for a better understanding of the chronic consequences of TBI, with the ultimate goal of developing novel therapeutic interventions to treat the devastating consequences of brain injury. In models of mild, moderate, and severe TBI, histopathological and behavioral studies have emphasized the progressive nature of the initial traumatic insult and the involvement of multiple pathophysiological mechanisms, including sustained injury cascades leading to prolonged motor and cognitive deficits. Recently, the increased incidence in age-dependent neurodegenerative diseases in this patient population has also been emphasized. Pathomechanisms felt to be active in the acute and long-term consequences of TBI include excitotoxicity, apoptosis, inflammatory events, seizures, demyelination, white matter pathology, as well as decreased neurogenesis. The current article will review many of these pathophysiological mechanisms that may be important targets for limiting the chronic consequences of TBI.
Key words: : atrophy, inflammation, neurogenesis, progressive damage, TBI, white matter
Introduction
Traumatic brain injury (TBI) produces both acute and more chronic consequences that lead to permanent disabilities that increase long-term mortality and reduced life expectation.1–4 The direct consequences of a single TBI or repetitive insults can result in various secondary pathological conditions, including seizures, sleep disorders, neurodegenerative diseases, neuroendocrine dysregulation, and psychiatric problems.5–13 Changes initiated by TBI can persist for months or years after injury and significantly affect quality-of-life issues in these patients. Our current understanding of the pathophysiology of TBI has emphasized the multi-factorial nature of injury events that are activated by TBI. Indeed, many pre-clinical, as well as some clinical, studies have evaluated various therapeutic interventions to target both the acute and more chronic consequences of TBI with some degree of success.14–22 In this regard, neuroprotective, as well as treatment, strategies that target abnormally sustained elevations in intracranial pressure have been attempted. However, currently, there are limited therapeutic interventions that have been shown to improve the long-term consequences of TBI.20,23 This is a growing problem because of the increased incidence of TBI in many countries, including a growing incidence of concussion in sport- and military-related activities.24–26
The fact that acute injury can lead to long-term consequences, including progressive brain atrophy and an increased vulnerability to neurodegenerative disorders, is a critical problem. In some models of TBI, immunocytochemical evidence for chronic traumatic encephalopathy (CTE) has been presented.11,25,27 Indeed, several studies have emphasized that months to years after injury, evidence for progressive gray and white matter (GM/WM) atrophy is observed after TBI (Fig. 1).14,28–34 Similar observations have been shown in TBI patients using computed tomography and magnetic resonance imaging (MRI) modalities (Fig. 2).2,35–41 Underlying mechanisms for these progressive injuries emphasize the complex nature of this disease process and require a multi-disciplinary approach to clarifying and targeting multiple injury cascades associated with different injury severities.1 Original articles and review articles have summarized the histopathological and behavioral consequences of TBI that have been described in various animal models. Recently, the importance of repetitive episodes of mild TBI have also been emphasized in the trauma literature. Various publications have demonstrated the potential for repetitive insults producing cumulative effects and leading to long-term consequences, including age-related neurodegerative disorders (i.e., CTE and Alzheimer's disease [AD]). The aim of this article is to review the pathomechanisms that have been attributed to the progressive nature of TBI. The wide range of pathophysiological processes that have been implicated clearly emphasize the complexity of TBI in terms of patterns of cell vulnerability, sustained secondary injury processes, as well as endogenous reparative processes after injury (Fig. 3).42 Below, injury processes are discussed in terms of current research knowledge and potential therapeutic interventions targeting these processes.
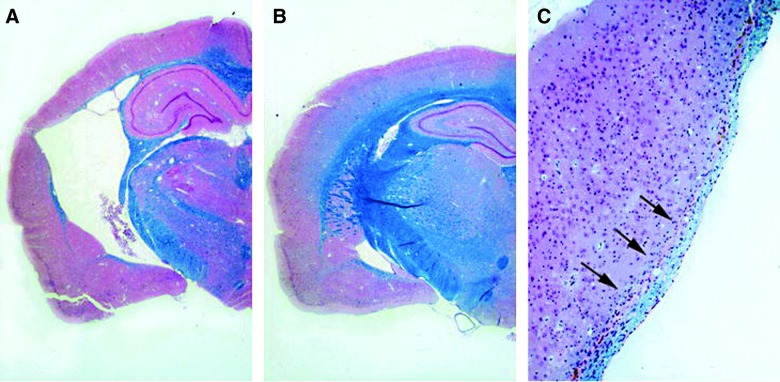
FIG. 1.
Double-stained hematoxylin-eosin and Luxol-fast blue sections 1 year after traumatic brain injury (TBI) or sham procedure. (A) TBI animal showing gross atrophy with marked expansion of the ipsilateral lateral ventricle. (B) Sham-operated animal appearing unremarkable. (C) Higher magnification of external capsule thinning (arrows) after TBI. Reprinted from Bramlett and Dietrich (2002), with kind permission of Springer Science and Business Media.
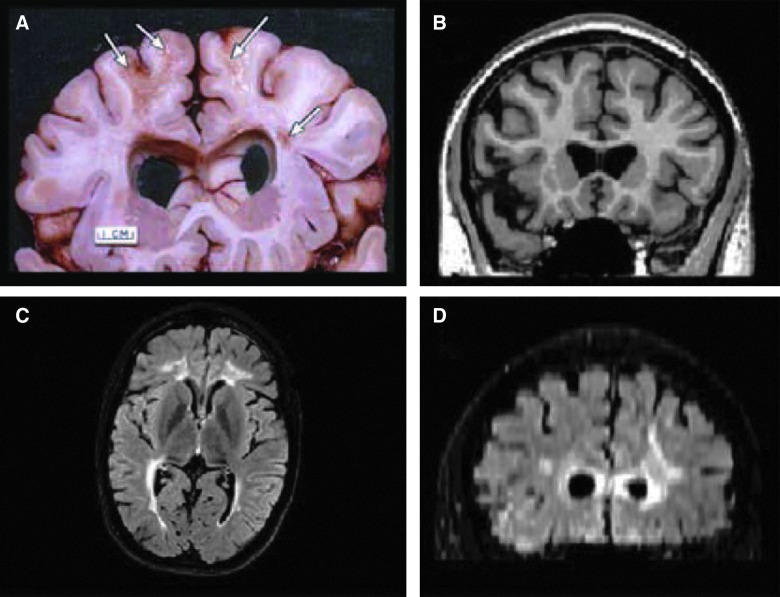
FIG. 2.
(A) Postmortem from a patient who survived 7 years after severe TBI. Note the dilated ventricles, focal areas of white matter (WM) degeneration (arrows), atrophic corpus callosum, and prominence of the interhemispheric and Sylvian fissures reflective of generalized cortical atrophy. (B) Similar-level T1 MRI coronal section depicting comparable ventricular dilation, and also note the prominence of the cortical sulci and Sylvian fissure as well. (C) Axial fluid-attenuated inversion recovery (FLAIR) sequence depicting bilateral WM signal abnormality. (D) Resliced from the axial acquisition of the FLAIR sequence, this coronal FLAIR image shows extensive WM changes in the periventricular and deep WM of the frontal lobes, in association with the dilated ventricles. The focal areas of WM degeneration that can be seen in (A) will display as signal abnormality in the WM as shown in (D). Reprinted from Bigler and Maxwell (2011), with kind permission of IOS Press.
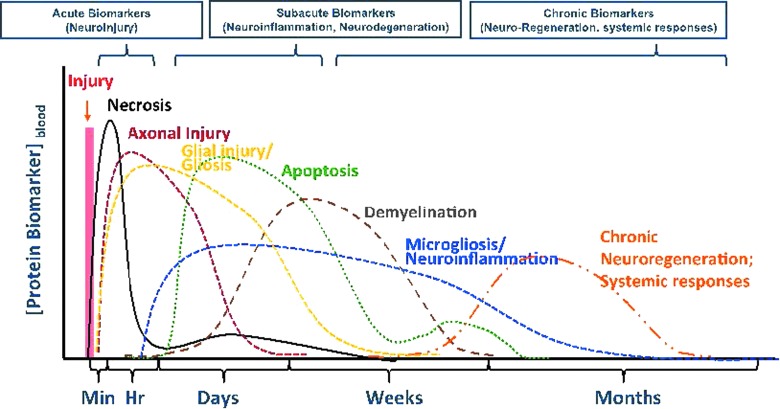
FIG. 3.
Continuum of biomarkers for TBI pathophysiology and its manifestation over time. Reprinted from Wang and colleagues (2013), with kind permission of the Society of Photo-Optical Instrumentation Engineers (SPIE).
Excitotoxicity
One of the initial pathomechanisms that was investigated to target neuronal vulnerability after cerebral ischemia or trauma was neuronal excitotoxicity.43 Early studies showed that neurons as well as oligodendrocytes were highly vulnerable to glutamate receptor activation and that selective inhibitors of specific glutamatergic receptors could provide protection in terms of cell death.44–46 Subsequent to TBI, many studies have emphasized that excitotoxic processes participate in the pathophysiology and have reported neuroprotection with treatments that block these specific receptors.47–54 Regional microdialysis studies have reported on early elevations in the extracellular levels of excitatory amino acids, including glutamate, that appear to be dependent on both injury severity as well as a number of physiological variables.53,55–57 In the 1990s, numerous studies reported that treatments directed toward N-methyl-d-aspartate receptor (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation were protective in models of TBI.58–60
Studies have also assessed whether receptor plasticity in vulnerable brain regions may increase the vulnerability of cells at chronic periods after brain injury.61 Indeed, recent evidence indicates that oligodendrocyte vulnerability may be delayed after brain or spinal cord injury (SCI) and could involve excitotoxic cell death mechanisms leading to vulnerability of myelin-producing cells and progressive demyelination.39,62 Demyelination has been documented in models of TBI as well as a variety of other types of central nervous system (CNS) disorders including multiple sclerosis, stroke, and schizophrenia.28,63 More recently, a new approach to targeting NMDA receptors has been advanced, where stimulation of the receptor in the subacute period after TBI has been reported to be beneficial.64 Strategies that target these acute and more chronic excitotoxic processes continue to represent potential strategies for reducing the long-term consequences of circuit dysfunction after TBI.
Reactive Oxygen Generation
Experimental and clinical studies have emphasized the importance of generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) as occurring in the early post-traumatic stages of the injury process.65–67 Oxidative stress (OS) leads to irreversible neuronal membrane damage, resulting in a pattern of secondary injury mechanisms ultimately leading to dysfunction and cell death. A variety of OS markers have been evaluated in models of TBI, including carbonylated proteins, lipoperoxides, ROS, and RNS, whereas antioxidant defense enzymes decrease after trauma.68,69 Thus, the imbalance produced by the injury environment between oxidant and antioxidant agents is an important target in the development of new therapeutic interventions that could improve outcome and antioxidant strategies have been developed and tested.70–73 Selective poly (ADP-ribose) polymerase 1 inhibition decreases the generation of ROS and proinflammatory cytokines.74 In terms of injury severity, generation of ROS indicates that severity plays a major role in oxidant and antioxidant balance.75 Ongoing studies using sensitive indicators of OS continue to be developed and analyzed to determine the importance of these ongoing injury cascades in progressive damage after TBI.76,77
In terms of molecular mechanisms underlying OS, recent investigations have concentrated on the transcription factor, nuclear factor erythoid-related factor 2 (NRF2), that mediates antioxidant and cytoprotective genes by binding two antioxidant response elements (AREs).77 In one study, NRF2 messenger RNA was reported to increase after focal TBI. In another study, administration of the NRF2-ARE activators, sulforaphane and carnosic acid, protected mitochondria in vivo from toxic exposure in the subacute phase after TBI.76 Using a model of cortical impact injury, treatment with phenelzine scavenger lipid pro-oxidation prevented respiratory depression effects in cortical mitochondria. Hughes and colleagues72 recently reported protection in a TBI model by genepin, a fruit extract. The continued development of novel antioxidant therapeutic strategies is an active area of neurotrauma research, with new studies suggesting that combination treatments including complimentary antioxidants may be required to protect against the long-term consequences of TBI.65
Apoptosis
Apoptosis is a mechanism underlying programmed cell death that has been characterized in models of brain injury and SCI. Molecular indicators of apoptosis, including activation of intrinsic and extrinsic apoptotic pathways, have been reported in several models of TBI.78,79 More recently, biomarker studies have reported that proapoptotic proteins are up-regulated in plasma and cerebrospinal fluid (CSF) samples from TBI patients.80,81 In contrast to necrosis, apoptotic cell death is commonly felt to be delayed and more prolonged.82 Thus, apoptotic cell death of neuronal or myelin-producing cells is a potential mechanism for the progressive nature of TBI. Indeed, evidence of delayed apoptosis of oligodendrocytes has been reported using immunocytochemical (ICC) analysis of brain tissues weeks to months after injury.83,84 These data indicate that delayed apoptotic cell death, as well as other programmed cell death mechanisms, currently being investigated in the laboratory could underlie some of these long-term consequences of brain injury.
Various strategies have been utilized to target apoptotic cell death after brain injury. Nonspecific apoptotic inhibitors have been reported, in some studies, to be protective after TBI and to be associated with improved behavioral outcome.21,85–87 In contrast, other studies have reported only transient protection with these inhibitors, and, currently, more-selective antiapoptotic molecules are being developed and tested.88–90 Because of the importance of apoptotic cell death in injury models, this is an important therapeutic target for limiting the long-term consequences of TBI.
Inflammation
Inflammatory processes have long been viewed as important secondary injury mechanisms after TBI.91 Numerous studies have reported, in models of TBI, evidence for acute and more chronic inflammatory events that, in specific cases, are felt to lead to secondary damage and long-term neuronal disorders.27,92–95 Evidence for activation of intrinsic inflammatory cells, including microglia, as well as recruitment of circulating inflammatory cells and macrophages into injured tissue, have been described in both experimental and clinical tissues.34,92,96–98 These inflammatory cells are associated with increased synthesis of a variety of chemokines and cytokines that can be proinflammatory and thus potentially neurotoxic. Biochemical analyses have indicated that increased elevations in tissue levels of interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), IL-6, and transforming growth factor as well as many others are observed at various times after TBI.93,99 Recent evidence has also been provided for increased inflammasome activation after TBI, an important innate immune regulator of inflammatory events active after several neurological conditions.100,101
In clinical studies, biomarker analysis has also provided direct evidence for inflammatory processes being active in various TBI patients weeks to months after injury.102,103 Elevated levels of IL-1 have been reported weeks after TBI, indicating that active inflammatory events are still apparent in the later TBI stages.104 Histopathological evaluation of human tissues after TBI has also provided direct evidence for prolonged inflammatory events.96,105 For example, evidence for activated microglia in WM tracts years after brain trauma has been emphasized in human brain specimens.106,107 Although inflammatory processes can have both damaging, as well as reparative, consequences, abnormal inflammation remains an important target for reducing the long-term consequences of TBI.
Experimental and clinical studies evaluating receptor blockers against IL-1β are showing some encouraging results.108–114 Other published studies using progesterone or anti-inflammatory steroids appear to show some promise in the clinical literature.115 In the area of therapeutic hypothermia, a number of published studies have reported that the beneficial effects of early cooling and temperature management strategies appear to be relevant to targeting inflammatory events after TBI.116 Several studies, for example, have shown that therapeutic hypothermia in models of TBI reduce levels of the TNF-α and IL-1β families of inflammatory genes as well as abnormal inflammasome activation.117–122 Future studies are required to determine factors associated with prolonged inflammatory events as well as appropriate therapeutic targets for reducing the detrimental consequences of inflammation.
A relatively new development in the area of inflammatory cascades after TBI is the discovery that inflammatory cells are relatively plastic and may participate in both neurodegenerative, as well as reparative, processes.123–125 Distinct microglia/macrophage subsets can be classified in four main phenotypes: classically activated M1 phenotype with cytotoxic properties; alternative activated M2A phenotype, with proregenerative functions; M2B immunoregulatory phenotype; and the M2C deactivated phenotype surface antigens that recognize specific functions.126,127 The importance of microglia/macrophage polarization patterns has been reported in several experimental conditions with the heterogeneity of the inflammatory cell responses to trauma emphasized.123,128 Currently, novel approaches to reduce excitotoxic and growth-limiting inflammatory phenotypes while promoting those that promote reparative processes are currently being evaluated. In this regard, pharmacological strategies that limit proinflammatory M1 phenotype while promoting the anti-inflammatory M2 phenotype and thereby modifying the M1/M2 macrophage ratio have implications for brain protection and repair. Recently, specific molecules, including pattern receptor recognition receptors, integrins, cytokine/chemokines, nuclear receptors, and galectins, have been shown to be important signaling pathways affecting both immune and neuronal cells.123 Markers of neuroinflammation can persist in the brain parenchyma up to 16 years after TBI, and recent evidence for chronic immune activation associated with behavioral deficits, including chronic depression, has been documented.96,129
Post-Traumatic Epilepsy and Cortical Spreading Depolarizations
Post-traumatic epilepsy (PTE) is a major consequence of TBI occurring in approximately 25% of patients with severe TBI.5 Clinical studies have documented the incidence of PTE showing that these periods of abnormal neuronal excitation can occur weeks, months, or years after injury.130–132 Importantly, PTE is an important secondary injury mechanism that can aggravate damage or impede endogenous recovery mechanisms.133–139 In one experimental study, induced periods of PTE were shown to increase the vulnerability of cortical and subcortical structures after experimental TBI.135 This study first emphasized the potential for a brief episode of PTE to have a significant impact on the vulnerability of the post-traumatic brain. Because recent clinical data have emphasized the high incidence of PTE and episodes of cortical spreading depolarization in TBI patients, these post-traumatic events require more investigation.140 Currently, various strategies are being evaluated, including single or combinatorial drug therapies, as well as therapeutic hypothermia to target these episodes of circuit excitation.15,141,142
More recent data have emphasized the incidence of subclinical (nonconvulsive) seizures after TBI that, although not routinely documented in the human population, can be recorded using monitoring procedures, including long-term electroencephalography surface recordings.131,143 Investigators have described a high incidence of subclinical seizures that appear in patients at variable periods after TBI.131 This is an important finding given that subclinical seizures in the TBI population could represent an important secondary injury mechanism leading to long-term consequences, including circuit dysfunction and neuronal cell death.144 In studies being conducted in pre-clinical models, evidence for subclinical seizures has also been documented.143,145 Using noninvasive long-term monitoring in awake rats after TBI, for example, evidence for patterns of abnormal EEG activity is observed at various post-traumatic periods.
In addition to PTE, episodes of cortical spreading depolarization (CSD), as mentioned previously, are also observed in models of brain injury.146–149 There is a rich history of evidence showing that repetitive episodes of CSD can represent a secondary injury mechanism in models of focal cerebral ischemia and trauma that may increase the vulnerability of neuronal populations surrounding areas of contusive brain injury.148 Most recently, evidence for repetitive episodes of CSD has been reported in patients after stroke and TBI.146,150 Thus, repetitive episodes of CSDs are another potential mechanism of secondary injury that can continue to affect the manner in which the brain responds to trauma long after the traumatic insult. Episodes of CSD have been shown to be sensitive to selective pharmacological treatments, including NMDA receptor and calcium antagonists.151–153 Also, previous data have indicated that CSD episodes are sensitive to brain temperature.154,155 Additional experimental and clinical data are therefore required to determine the incidence of CSD in various TBI patient populations and the testing of novel treatment strategies targeting episodes of PTE and CSD in patients who could benefit in terms of long-term outcomes.
Subsequent to TBI, bleeding within the neuropil leads to hemolysis and the deposition of hemoglobin.156,157 A recently proposed mechanism for development of PTE is that iron compounds deposited in brains of TBI patients form reactive free radical oxidants, leading to release of glutamate and subsequent abnormal neuronal excitation.157,158 Comprehensive gene expression and gene network analyses have also clarified molecular events associated with PTE and reported up-regulation of phosphatase A2 and lipid metabolism, which may be related to seizure propagation.139,158 Importantly, administration of antioxidants appears to cause an interruption of the generation of chronic seizures induced by hemorrhage.157
Calpain-Mediated Proteolysis
TBI produces a prolonged activation of calpains resulting in the proteolysis of numerous cellular substrates, including cytoskeletal components and membrane receptors.159–163 Using both in vitro and in vivo models, calpain-specific breakdown products, including α-spectrin, are observed within axonal, dendritic, and somatic regions after injury, leading to transport disruption and secondary axotomy.164 Recently, specific spectrin breakdown products have been reported to be present in CSF from traumatized animals and humans, representing a useful biomarker to assess injury severity and pathophysiological events.160,165 Indeed, direct relationships between calpain-mediated proteolysis and injury severity have been associated with neurodegenerative and restorative responses.162
Multiple strategies targeting calpain breakdown have been used to target the acute and more chronic consequences of CNS injury. Intravenous administration of calpain inhibitors, such as MDL-28170, protect against axonal pathology, as well as neuronal damage, in some experimental models.166,167 More recently, transgenic mice overexpressing calpastatin demonstrate reduced calpain-mediated spectrin proteolysis after TBI with some benefits in functional outcome.159,168 Based on current literature, future research should include the continued development and testing of specific, long-lasting inhibitors of calpain-mediated proteolysis.
Diffuse axonal injury
WM injury is a hallmark of TBI, with pre-clinical and human autopsy data demonstrating axonal injury in conditions of both focal as well as diffuse brain injury.169 Experimental and clinical studies have used a variety of ICC markers, including β-amyloid precursor protein, to demonstrate evidence of early and progressive axonal injury, subsequent deafferentation of cellular targets, Wallerian degeneration, as well as circuit remodeling.14,27,28,30–33,35,37–40,170–173 Also, using special silver stain, several studies have emphasized the widespread degree of damage to neuronal processes, connections, and the microenvironment produced by TBI.174,175 One question that is sometimes difficult to answer is whether these progressive axonal changes are the result of primarily Wallerian degeneration or more-active secondary injury mechanisms.31,33,176–179 In human specimens, evidence for diffuse axonal injury throughout the corpus callosum fibers was first shown to be a common indicator of TBI.37 The degree of axonal injury appears to be injury-severity dependent and a potentially important cellular event that underlies many of the functional consequences of TBI. Progressive WM damage is also a hallmark of TBI, with MRI approaches showing reduced WM volumes over time as well as other indicators of axonal damage. State-of-the-art imaging approaches clarifying WM damage using diffusion tension imaging and anisotropy are also providing evidence for subtle changes in the microenvironment and axonal structure, which could underlie some of the long-term progressive changes and prolonged functional disturbances reported in current animal models and in humans.39,40,172
In addition to axonal damage, evidence for abnormal axonal sprouting of specific neural populations is also reported in models of TBI.180–183 Subsequent to axonal damage, multiple imaging approaches have shown evidence for fiber sprouting that could represent reparative events associated with recovery mechanisms.184–186 In recent studies using genetic mouse models to identify vulnerable cortical neurons after TBI, researchers have described evidence for progressive axonal sprouting extending into deeper cortical regions.184 The long-term functional consequences of these potentially reparative processes are unknown. It is important to note that in the area of post-traumatic epilepsy, evidence for axonal sprouting has also been demonstrated in the hippocampal mossy fiber pathway.15,136,183,187 In animal and human studies, where repetitive episodes of seizure activity occur, mossy fiber sprouting has been reported as evidence for abnormal axonal growth to specific targets.15,181,183 In this particular case, aberrant hippocampal sprouting may participate in some of the mechanisms associated with abnormal excitation and seizure activity.
Several investigations have evaluated therapeutic interventions that may target axonal pathology. These include strategies targeting excitotoxicity, free radical generation, or combination approaches that may be directed at multiple pathomechanisms. Recent evidence has also indicated that therapeutic hypothermia may prevent axonal pathology in several models of TBI.188–191 A greater understanding of the pathophysiology of axonal damage, both in the acute and more progressive state, is required to clarify how best to treat WM vulnerability after brain injury.
Demyelination and White Matter Loss
As previously described, a variety of WM changes are observed after TBI in both experimental and clinical investigations. Subsequent to TBI, evidence for local demyelination associated with these WM changes has also been emphasized.29,32,39,84,192 In animals where survival has been allowed up to 1 year after TBI, significant evidence of WM atrophy and demyelination has been reported.28,30,31,33 Demyelination can occur as a result of several mechanisms, including primary axonal damage and subsequent Wallerian degeneration or death of myelinating cells. Indeed, in a recent publication, oligodendrocyte cell death by apoptotic-dependent mechanisms was demonstrated after a moderate TBI injury.84 These and other studies emphasize that, in addition to primary axonal damage, axonal demyelination may also underlie some of the more progressive consequences of TBI. Importantly, because myelination is critical to the normal role of axons in terms of the complex function of neuronal circuits, demyelination could underlie many of the long-term functional consequences of brain injury.193 Strategies to protect against oligodendrocyte cell death, as well as progressive demyelination, include therapeutic interventions targeting excitotoxicity, apoptosis, as well as inflammation. Emphasis needs to be placed on demonstrating evidence for demyelination in our TBI population using state-of-the-art imaging approaches determining the importance of this cellular response in terms of acute and more long-term TBI outcomes.
Adult Neurogenesis
Previous studies have emphasized the occurrence of ongoing neurogenesis in specific areas of the adult brain.194,195 These regions include the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampus. Current research efforts are directed to evaluating the importance of adult neurogenesis on normal cognitive function and integration of new memories into our neural circuits.196–198 In a recent investigation, direct evidence for adult neurogenesis occurring in humans was obtained from human specimens, where above-ground nuclear bomb testing allowed for determination of neuronal cell birth.199 Importantly, this study emphasized that sustained neurogenesis occurs in the hippocampus, thus supporting a role for adult neurogenesis participating in hippocampal-dependent learning and memory both during normal aging and after brain injury.
In this regard, several laboratories have reported that after TBI, there is a significant increase in cell proliferation and neurogenesis in the hippocampus using a variety of ICC approaches.200–203 Several studies have reported increased bromodeoxyuridine (BrdU) cell staining in the SVZ and SGZ after TBI.15,204,205 Single- and double-label ICC procedures using BrdU and doublecortin staining, along with quantitative NeuN counts, have reported that a population of these proliferating cells turn into immature neurons and, over several weeks, differentiate into mature neurons that integrate into the granular cell circuitry.201,203,206 In terms of the significance of this cellular response to TBI, investigators have concluded that hippocampal neurogenesis may participate in cognitive recovery mechanisms after TBI using experimental strategies that inhibit neurogenesis response to injury.202,207 However, another recent observation is that this altered neurogenic response to TBI may be short-lived and that, with chronic survival, numbers of newly formed neurons are reduced compared to control levels.205,208 Recent studies have also indicated that the state of microglia activation could potentially play a major role in maintaining homeostasis of baseline neurogenesis in areas such as the SGZ of the hippocampus.209 In the area of angiogenesis, data are also available indicating that anti-inflammatory M2 phenotype promotes angiogenesis whereas the proinflammatory M1 phenotype has antiangiogenic effects.128 A greater understanding of the mechanisms controlling neurogenesis and angiogenesis after TBI and strategies to promote long-term repair mechanisms represent an important strategy for protecting the brain from progressive injury and long-term cognitive problems. In this regard, specific strategies, including exercise, motor rehabilitation, growth factor or hormonal, or other proneurogenic treatments, as well as therapeutic hypothermia, may enhance neurogenesis after brain injury.206,210–213 Studies are critically evaluating mechanisms by which these various interventions can promote and sustain neurogenesis and whether these cellular events lead to chronic improvements in cognitive function after brain injury. The fact that neurogenesis occurs in the adult brain and can be negatively impacted by TBI emphasizes that these reparative processes represent extremely important targets for treating the progressive nature of TBI.
Cerebral Blood Flow Alterations
Early alterations in regional cerebral blood flow (rCBF) have been demonstrated in experimental and clinical models of TBI.214–221 Immediately following TBI, significant changes in cerebral blood flow, as well as glucose metabolism, have been reported.214,222–224 Reductions in blood flow are usually highly correlated with severity of injury, with trauma models showing reductions in rCBF that do not reach ischemic levels.225,226 However, after TBI, metabolic/hemodynamic requirements of the tissue may differ from normal tissues and could potentially participate in both the acute and more chronic consequences of neurotrauma.227 In addition to alterations in blood flow, changes in vascular reactivity to various stimuli, including vasodilators, have also been emphasized in the literature.228–231 Using a pial window model to evaluate vascular reactivity after TBI, researchers have reported that the vasodilatory characteristics of injured vessels are severely affected and may therefore participate in secondary injury mechanisms that include reduced hemodynamic needs to injured tissue.228, 229 In human studies, stimulation-induced increases in blood flow have also been shown to be perturbed after TBI.232–234
Data are also available showing that, in addition to the acute changes in CBF, long-term alterations in rCBF are also observed in experimental and clinical models (Fig. 4).233–241 Using MRI imaging or other methodologies, evidence for depressed blood flow throughout the injured and contralateral hemisphere have been documented. Moderate reductions in chronic blood flow can augment slow, evolving injury processes or aggravate events that were initiated by the acute insult. For example, inflammatory or excitotoxic processes could be aggravated by reduced blood flow. Strategies to improve blood flow include use of modifiers of vascular reactivity, such as nitric oxide or other strategies. The potential for cerebral emboli produced by the primary injury to occlude microvessels, abnormalities in coagulopathy, or whether microvascular vasospasm participates in reduced CBF needs to be addressed in future studies.214,242,243
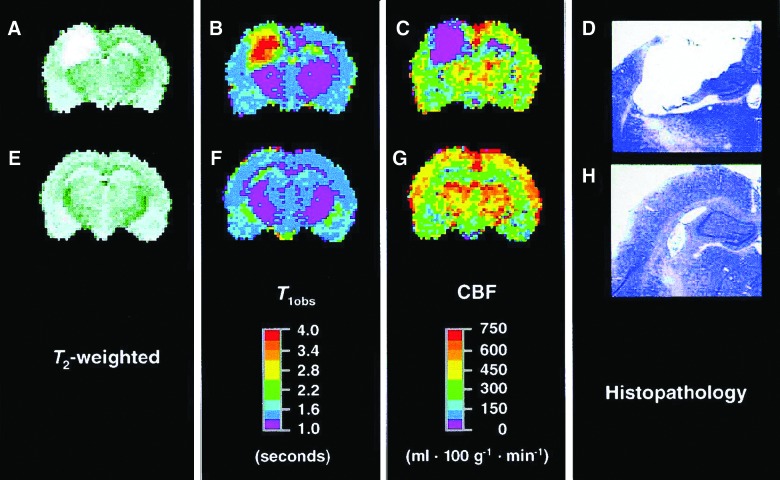
FIG. 4.
Coronal brain magnetic resonance images and histopathology from rats at 1 year after controlled cortical impact (CCI; A, B, C, and D) and 1 year after sham surgery (E, F, G, and H). In the CCI example shown, a large cystic lesion was observed encompassing both the cortex and hippocampus ipsilateral to the impact at 1 year after injury. That lesion is identified as a region of hyperintensity on both the T2-weighted image (A) and the T1obs map (B) and as a region lacking perfusion (C). The corresponding histopathology (cresyl-violet–stained section through the imaging plane) confirms the location of the cystic cavity after CCI (D), but not after sham surgery (H). For figure presentation purposes, pixels outside the brain were assigned to zero intensity. Although the perfusion slice shown for injured rat (C) represents the best example of average for the overall effect of injury on CBF, it does not necessarily reflect the mean CBF for each individual region of interest. Reprinted from Kochanek and colleagues (2002), with kind permission of Mary Ann Liebert, Inc. CBF, cerebral blood flow.
Neurodegenerative Processes
Over the last several years, there has been an increased awareness that TBI can be an important risk factor for development of age-related neurodegenerative diseases, including AD and Parkinson's disease.1,244 Clinical studies have emphasized that people with evidence of early TBI can show an increased incidence of neurodegenerative diseases.10,11,245,246 Currently, the reason for this post-traumatic outcome is unknown, but recent data have implicated genetic factors and environmental as well as a variety of injury cascades, including prolonged inflammatory events, that may promote neurodegenerative processes. In several studies, evidence for focal protein aggregation, as well as abnormal accumulation of beta-amyloid precursor or tau protein, has been demonstrated in experimental as well as clinical specimens.170,171,247–252 These patterns of abnormal protein accumulation occur in both GM and WM structures.170,252,253 In a series of human brains obtained from individuals with repetitive mild concussions, increase tau ICC has been indicated as an ICC signature for CTE.254 Whether similar pathomechanisms involved in the progressive nature of TBI include CTE and other neurodegenerative disorders remain to be determined. However, it is important to emphasize that a spectrum of TBI severities as well as repetitive mild concussions appear to produce similar cellular responses to injury and therefore may include common pathomechanisms occurring in the more chronic stages of TBI.
Experimental and clinical research programs are targeting the devastating consequences of neurodegenerative diseases, including AD. Vaccine and antibody treatments are being tested to target abnormal protein aggregation and other neurodegenerative processes.255–257 Whether or not therapeutic strategies that seem viable in TBI can also be used to reduce the more delayed and progressive pathophysiological cascades associated with neurodegenerative disorders is an area that needs to be emphasized in future research.
Summary
Both experimental and clinical evidence indicates the multi-factorial nature of pathophysiological mechanisms that may underlie the progressive nature of TBI. Because the chronic consequences of TBI, including histopathological, physiological, and behavioral abnormalities, can be observed after mild, moderate, or severe injury, it may be difficult in the future to clarify whether any one dominant pathomechanism underlies the progressive nature of TBI. In addition, the acknowledgement of TBI being a possible risk factor for age-dependent neurodegenerative disease is also complicating the trauma field in terms of understanding pathophysiological events and treatment options. Hopefully, through genetic modeling approaches, combined with innovative cellular and functional approaches to evaluate novel pathomechanisms, a better understanding of how to best treat the chronic consequences of TBI will be achieved. Importantly, a greater understanding of the pathophysiological mechanisms associated with chronic TBI and the testing of novel therapeutic interventions using clinically relevant animal models will enhance the translation of new discoveries to the clinic.
Past attempts to translate postive pre-clinical findings targeting acute neuroprotection have been unsuccessful in terms of improving functional outcome.258 However, lessons have been learned and strategies proposed on how best to approach the translation of pre-clinical data to the clinic. Specifically, several conditions, including independent data replication, the critical evaluation of the quality of research data, and clinical relevance are now emphasized in the neurotrauma literature.259–261 Also, strategies that allow for data to be more easily shared among the scientific community have recently been published.262 An increased emphasis of better communication between the laboratory and clinic in terms of designing trials and selecting treatments is also encouraged. It is anticipated that, in the future, pre-clinical findings will lead to successful clinical investigations potentially using novel combinatorial approaches, including neuroprotection, reparative approaches, and neurorehabilitation, to ultimately improve function in people living with the devastating consequences of TBI.
Acknowledgments
This work was funded by the National Institutes of Health (NS030291) and Veterans Affairs (1 I01 BX000521). The authors thank Jeremy Lytle for editorial support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Masel B.E., and DeWitt D.S. (2010). Traumatic brain injury: a disease process, not an event. J. Neurotrauma 27, 1529–1540 [DOI] [PubMed] [Google Scholar]
- 2.Bergeson A.G., Lundin R., Parkinson R.B., Tate D.F., Victoroff J., Hopkins R.O., and Bigler E.D. (2004). Clinical rating of cortical atrophy and cognitive correlates following traumatic brain injury. Clin. Neuropsychol. 18, 509–520 [DOI] [PubMed] [Google Scholar]
- 3.Levin H.S., Amparo E., Eisenberg H.M., Williams D.H., High W.M., Jr., McArdle C.B., and Weiner R.L. (1987). Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J. Neurosurg. 66, 706–713 [DOI] [PubMed] [Google Scholar]
- 4.Corrigan J.D., and Hammond F.M. (2013). Traumatic brain injury as a chronic health condition. Arch. Phys. Med. Rehabil. 94, 1199–1201 [DOI] [PubMed] [Google Scholar]
- 5.Annegers J.F., Hauser W.A., Coan S.P., and Rocca W.A. (1998). A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 338, 20–24 [DOI] [PubMed] [Google Scholar]
- 6.McLean A., Jr., Dikmen S., Temkin N., Wyler A.R., and Gale J.L. (1984). Psychosocial functioning at 1 month after head injury. Neurosurgery 14, 393–399 [DOI] [PubMed] [Google Scholar]
- 7.Masel B.E., Scheibel R.S., Kimbark T., and Kuna S.T. (2001). Excessive daytime sleepiness in adults with brain injuries. Arch. Phys. Med. Rehabil. 82, 1526–1532 [DOI] [PubMed] [Google Scholar]
- 8.Lye T.C., and Shores E.A. (2000). Traumatic brain injury as a risk factor for Alzheimer's disease: a review. Neuropsychol. Rev. 10, 115–129 [DOI] [PubMed] [Google Scholar]
- 9.Schneider H.J., Kreitschmann-Andermahr I., Ghigo E., Stalla G.K., and Agha A. (2007). Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA 298, 1429–1438 [DOI] [PubMed] [Google Scholar]
- 10.Goldman S.M., Tanner C.M., Oakes D., Bhudhikanok G.S., Gupta A., and Langston J.W. (2006). Head injury and Parkinson's disease risk in twins. Ann. Neurol. 60, 65–72 [DOI] [PubMed] [Google Scholar]
- 11.DeKosky S.T., Blennow K., Ikonomovic M.D., and Gandy S. (2013). Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 9, 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine. (2009). Longterm consequences of traumatic brain injury, in: Gulf War and Health. The National Academies Press: Washington, D.C. [Google Scholar]
- 13.Vespa P.M. (2013). Hormonal dysfunction in neurocritical patients. Curr. Opin. Crit. Care 19, 107–112 [DOI] [PubMed] [Google Scholar]
- 14.Bramlett H.M., Dietrich W.D., Green E.J., and Busto R. (1997). Chronic histopathological consequences of fluid-percussion brain injury in rats: effects of post-traumatic hypothermia. Acta Neuropathol. (Berl.) 93, 190–199 [DOI] [PubMed] [Google Scholar]
- 15.Atkins C.M., Truettner J.S., Lotocki G., Sanchez-Molano J., Kang Y., Alonso O.F., Sick T.J., Dietrich W.D., and Bramlett H.M. (2010). Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur. J. Neurosci. 32, 1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R., Choi S.C., Clifton G.L., Contant C.F., Coplin W.M., Dietrich W.D., Ghajar J., Grady S.M., Grossman R.G., Hall E.D., Heetderks W., Hovda D.A., Jallo J., Katz R.L., Knoller N., Kochanek P.M., Maas A.I., Majde J., Marion D.W., Marmarou A., Marshall L.F., McIntosh T.K., Miller E., Mohberg N., Muizelaar J.P., Pitts L.H., Quinn P., Riesenfeld G., Robertson C.S., Strauss K.I., Teasdale G., Temkin N., Tuma R., Wade C., Walker M.D., Weinrich M., Whyte J., Wilberger J., Young A.B., and Yurkewicz L. (2002). Clinical trials in head injury. J. Neurotrauma 19, 503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belur P.K., Chang J.J., He S., Emanuel B.A., and Mack W.J. (2013). Emerging experimental therapies for intracerebral hemorrhage: targeting mechanisms of secondary brain injury. Neurosurg. Focus 34, E9. [DOI] [PubMed] [Google Scholar]
- 18.Lu J., Gary K.W., Neimeier J.P., Ward J., and Lapane K.L. (2012). Randomized controlled trials in adult traumatic brain injury. Brain Inj. 26, 1523–1548 [DOI] [PubMed] [Google Scholar]
- 19.Uchino H., Hatakeyama K., Morota S., Tanoue T., Nishiyama T., Usui D., Taguchi C., Suzuki M., Hansson M.J., and Elmer E. (2013). Cyclophilin-D inhibition in neuroprotection: dawn of a new era of mitochondrial medicine. Acta Neurochir. Suppl. 118, 311–315 [DOI] [PubMed] [Google Scholar]
- 20.Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loane D.J., and Faden A.I. (2010). Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 31, 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Y., Xiong Y., Mahmood A., Zhang Y., Qu C., and Chopp M. (2011). Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J. Neurosurg. 115, 550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marklund N., and Hillered L. (2011). Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 164, 1207–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dashnaw M.L., Petraglia A.L., and Bailes J.E. (2012). An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg. Focus 33, E5. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld J.V., McFarlane A.C., Bragge P., Armonda R.A., Grimes J.B., and Ling G.S. (2013). Blast-related traumatic brain injury. Lancet Neurol. 12, 882–893 [DOI] [PubMed] [Google Scholar]
- 26.McCrory P., Meeuwisse W.H., Aubry M., Cantu R.C., Dvořák J., Echemendia R.J., Engebretsen L., Johnston K.M., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R., Guskiewicz K.M., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D.L., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on concussion in sport—the 4th International Conference on Concussion in Sport held in Zurich, November 2012. PM R 5, 255–279 [DOI] [PubMed] [Google Scholar]
- 27.Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2013). Chronic neuropathological and neurobehavioral changes in a repetitive mTBI model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 28.Bramlett H.M., and Dietrich W.D. (2002). Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. (Berl.) 103, 607–614 [DOI] [PubMed] [Google Scholar]
- 29.Bramlett H.M., and Dietrich W.D. (2007). Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141 [DOI] [PubMed] [Google Scholar]
- 30.Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 31.Pierce J.E., Smith D.H., Trojanowski J.Q., and McIntosh T.K. (1998). Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience 87, 359–369 [DOI] [PubMed] [Google Scholar]
- 32.Smith D.H., Chen X.H., Pierce J.E., Wolf J.A., Trojanowski J.Q., Graham D.I., and McIntosh T.K. (1997). Progressive atrophy and neuron death for one year following brain trauma in the rat. J. Neurotrauma 14, 715–727 [DOI] [PubMed] [Google Scholar]
- 33.Dixon C.E., Kochanek P.M., Yan H.Q., Schiding J.K., Griffith R.G., Baum E., Marion D.W., and DeKosky S.T. (1999). One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma 16, 109–122 [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Paez A.C., Brunschwig J.P., and Bramlett H.M. (2005). Light and electron microscopic assessment of progressive atrophy following moderate traumatic brain injury in the rat. Acta Neuropathol. 109, 603–616 [DOI] [PubMed] [Google Scholar]
- 35.van der Naalt J., Hew J.M., van Zomeren A.H., Sluiter W.J., and Minderhoud J.M. (1999). Computed tomography and magnetic resonance imaging in mild to moderate head injury: early and late imaging related to outcome. Ann. Neurol. 46, 70–78 [DOI] [PubMed] [Google Scholar]
- 36.Hayasaki K., Marmarou A., Barzo P., Fatouros P., and Corwin F. (1997). Detection of brain atrophy following traumatic brain injury using gravimetric techniques. Acta Neurochir. Suppl. 70, 75–77 [DOI] [PubMed] [Google Scholar]
- 37.Anderson C.V., and Bigler E.D. (1995). Ventricular dilation, cortical atrophy, and neuropsychological outcome following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 7, 42–48 [DOI] [PubMed] [Google Scholar]
- 38.Cullum C.M., and Bigler E.D. (1986). Ventricle size, cortical atrophy and the relationship with neuropsychological status in closed head injury: a quantitative analysis. J. Clin. Exp. Neuropsychol. 8, 437–452 [DOI] [PubMed] [Google Scholar]
- 39.Adnan A., Crawley A., Mikulis D., Moscovitch M., Colella B., and Green R. (2013). Moderate-severe traumatic brain injury causes delayed loss of white matter integrity: evidence of fornix deterioration in the chronic stage of injury. Brain Inj. 27, 1415–1422 [DOI] [PubMed] [Google Scholar]
- 40.Palacios E.M., Sala-Llonch R., Junque C., Roig T., Tormos J.M., Bargallo N., and Vendrell P. (2013). White matter/gray matter contrast changes in chronic and diffuse traumatic brain injury. J. Neurotrauma 30, 1991–1994 [DOI] [PubMed] [Google Scholar]
- 41.Bigler E.D., and Maxwell W.L. (2011). Neuroimaging and neuropathology of TBI. NeuroRehabilitation 28, 63–74 [DOI] [PubMed] [Google Scholar]
- 42.Wang K.K.W., Moghieb A., Yang Z., and Zhang Z. (2013). Systems biomarkers as acute diagnostics and chronic monitoring tools for traumatic brain injury, pps. 87230O-87230O-87215
- 43.Bramlett H.M. and Dietrich W.D. (2004). Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J. Cereb. Blood Flow Metab. 24, 133–150 [DOI] [PubMed] [Google Scholar]
- 44.Grossman S.D., Wolfe B.B., Yasuda R.P., and Wrathall J.R. (1999). Alterations in AMPA receptor subunit expression after experimental spinal cord contusion injury. J. Neurosci. 19, 5711–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald J.W., Althomsons S.P., Hyrc K.L., Choi D.W., and Goldberg M.P. (1998). Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat. Med. 4, 291–297 [DOI] [PubMed] [Google Scholar]
- 46.Globus M.Y., Alonso O., Dietrich W.D., Busto R., and Ginsberg M.D. (1995). Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J. Neurochem. 65, 1704–1711 [DOI] [PubMed] [Google Scholar]
- 47.Algattas H., and Huang J.H. (2013). Traumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 15, 309–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katayama Y., Becker D.P., Tamura T., and Hovda D.A. (1990). Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 73, 889–900 [DOI] [PubMed] [Google Scholar]
- 49.Nilsson P., Hillered L., Ponten U., and Ungerstedt U. (1990). Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 10, 631–637 [DOI] [PubMed] [Google Scholar]
- 50.Zauner A., Bullock R., Kuta A.J., Woodward J., and Young H.F. (1996). Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir. Suppl. 67, 40–44 [DOI] [PubMed] [Google Scholar]
- 51.Faden A.I., Demediuk P., Panter S.S., and Vink R. (1989). The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244, 798–800 [DOI] [PubMed] [Google Scholar]
- 52.Hayes R.L., Jenkins L.W. and Lyeth B.G. (1992). Neurotransmitter-mediated mechanisms of traumatic brain injury: acetylcholine and excitatory amino acids. J. Neurotrauma 9, Suppl. 1, S173–S187 [PubMed] [Google Scholar]
- 53.Palmer A.M., Marion D.W., Botscheller M.L., Swedlow P.E., Styren S.D., and DeKosky S.T. (1993). Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 61, 2015–2024 [DOI] [PubMed] [Google Scholar]
- 54.Miller L.P., Lyeth B.G., Jenkins L.W., Oleniak L., Panchision D., Hamm R.J., Phillips L.L., Dixon C.E., Clifton G.L., and Hayes R.L. (1990). Excitatory amino acid receptor subtype binding following traumatic brain injury. Brain Res/ 526, 103–107 [DOI] [PubMed] [Google Scholar]
- 55.Bullock R., Zauner A., Myseros J.S., Marmarou A., Woodward J.J. and Young H.F. (1995). Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann. N. Y. Acad. Sci. 765, 290–297; discussion, 298 [DOI] [PubMed] [Google Scholar]
- 56.Vespa P., Prins M., Ronne-Engstrom E., Caron M., Shalmon E., Hovda D.A., Martin N.A. and Becker D.P. (1998). Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J. Neurosurg. 89, 971–982 [DOI] [PubMed] [Google Scholar]
- 57.Hutchinson P.J. (2005). Microdialysis in traumatic brain injury–methodology and pathophysiology. Acta Neurochir. Suppl. 95, 441–445 [DOI] [PubMed] [Google Scholar]
- 58.Hamm R.J., O'Dell D.M., Pike B.R., and Lyeth B.G. (1993). Cognitive impairment following traumatic brain injury: the effect of pre- and post-injury administration of scopolamine and MK-801. Brain Res. Cogn. Brain Res. 1, 223–226 [DOI] [PubMed] [Google Scholar]
- 59.Bullock R., Kuroda Y., Teasdale G.M., and McCulloch J. (1992). Prevention of post-traumatic excitotoxic brain damage with NMDA antagonist drugs: a new strategy for the nineties. Acta Neurochir. Suppl. (Wien) 55, 49–55 [DOI] [PubMed] [Google Scholar]
- 60.Shapira Y., Yadid G., Cotev S., Niska A., and Shohami E. (1990). Protective effect of MK801 in experimental brain injury. J. Neurotrauma 7, 131–139 [DOI] [PubMed] [Google Scholar]
- 61.Avramescu S., Nita D.A., and Timofeev I. (2009). Neocortical post-traumatic epileptogenesis is associated with loss of GABAergic neurons. J. Neurotrauma 26, 799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waxman S.G. (1989). Demyelination in spinal cord injury. J. Neurol. Sci. 91, 1–14 [DOI] [PubMed] [Google Scholar]
- 63.Matute C., and Ransom B.R. (2012). Roles of white matter in central nervous system pathophysiologies. ASN Neuro 4 pii: . doi: 10.1042/AN20110060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shohami E., and Biegon A. (2014). Novel approach to the role of NMDA receptors in traumatic brain injury. CNS Neurol. Disord. Drug Targets 13, 567–573 [DOI] [PubMed] [Google Scholar]
- 65.Bains M., and Hall E.D. (2012). Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 1822, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornelius C., Crupi R., Calabrese V., Graziano A., Milone P., Pennisi G., Radak Z., Calabrese E.J., and Cuzzocrea S. (2013). Traumatic brain injury: oxidative stress and neuroprotection. Antioxid. Redox Signal. 19, 836–853 [DOI] [PubMed] [Google Scholar]
- 67.Kontos H.A., Wei E.P., Ellis E.F., Dietrich W.D., and Povlishock J.T. (1981). Prostaglandins in physiological and in certain pathological responses of the cerebral circulation. Fed. Proc. 40, 2326–2330 [PubMed] [Google Scholar]
- 68.Rodriguez-Rodriguez A., Egea-Guerrero J.J., Murillo-Cabezas F., and Carrillo-Vico A. (2014). Oxidative stress in traumatic brain injury. Curr. Med. Chem. 21, 1201–1211 [DOI] [PubMed] [Google Scholar]
- 69.Hall E.D., Wang J.A., and Miller D.M. (2012). Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp. Neurol. 238, 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tewari A., Mahendru V., Sinha A., and Bilotta F. (2014). Antioxidants: the new frontier for translational research in cerebroprotection. J. Anaesthesiol. Clin. Pharmacol. 30, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh I.N., Gilmer L.K., Miller D.M., Cebak J.E., Wang J.A., and Hall E.D. (2013). Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J. Cereb. Blood Flow Metab. 33, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes R.H., Silva V.A., Ahmed I., Shreiber D.I., and Morrison B., III. (2014). Neuroprotection by genipin against reactive oxygen and reactive nitrogen species-mediated injury in organotypic hippocampal slice cultures. Brain Res. 1543, 308–314 [DOI] [PubMed] [Google Scholar]
- 73.Clond M.A., Lee B.S., Yu J.J., Singer M.B., Amano T., Lamb A.W., Drazin D., Kateb B., Ley E.J., and Yu J.S. (2013). Reactive oxygen species-activated nanoprodrug of Ibuprofen for targeting traumatic brain injury in mice. PloS One 8, e61819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoica B.A., Loane D.J., Zhao Z., Kabadi S.V., Hanscom M., Byrnes K.R., and Faden A.I. (2014). PARP-1 inhibition attenuates neuronal loss, microglia activation and neurological deficits after traumatic brain injury. J. Neurotrauma 31, 758–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Pietro V., Lazzarino G., Amorini A.M., Tavazzi B., D'Urso S., Longo S., Vagnozzi R., Signoretti S., Clementi E., Giardina B., Lazzarino G., and Belli A. (2014). Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic. Biol. Med. 69, 258–264 [DOI] [PubMed] [Google Scholar]
- 76.Miller D.M., Singh I.N., Wang J.A., and Hall E.D. (2013). Administration of the Nrf2-ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic. Biol. Med. 57, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller D.M., Wang J.A., Buchanan A.K., and Hall E.D. (2014). Temporal and spatial dynamics of nrf2-antioxidant response elements mediated gene targets in cortex and hippocampus after controlled cortical impact traumatic brain injury in mice. J. Neurotrauma 31, 1194–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keane R.W., Kraydieh S., Lotocki G., Alonso O.F., Aldana P., and Dietrich W.D. (2001). Apoptotic and antiapoptotic mechanisms after traumatic brain injury. J. Cereb. Blood Flow Metab. 21, 1189–1198 [DOI] [PubMed] [Google Scholar]
- 79.Eldadah B.A., and Faden A.I. (2000). Caspase pathways, neuronal apoptosis, and CNS injury. J. Neurotrauma 17, 811–829 [DOI] [PubMed] [Google Scholar]
- 80.Uzan M., Erman H., Tanriverdi T., Sanus G.Z., Kafadar A., and Uzun H. (2006). Evaluation of apoptosis in cerebrospinal fluid of patients with severe head injury. Acta Neurochir. (Wien) 148, 1157–1164; discussion [DOI] [PubMed] [Google Scholar]
- 81.Wagner A.K., Amin K.B., Niyonkuru C., Postal B.A., McCullough E.H., Ozawa H., Dixon C.E., Bayir H., Clark R.S., Kochanek P.M., and Fabio A. (2011). CSF Bcl-2 and cytochrome C temporal profiles in outcome prediction for adults with severe TBI. J. Cereb. Blood Flow Metab. 31, 1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raghupathi R. (2004). Cell death mechanisms following traumatic brain injury. Brain Pathol. 14, 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flygt J., Djupsjo A., Lenne F., and Marklund N. (2013). Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat. Eur. J. Neurosci. 38, 2153–2165 [DOI] [PubMed] [Google Scholar]
- 84.Lotocki G., de Rivero Vaccari J.P., Alonso O., Molano J.S., Nixon R., Safavi P., Dietrich W.D., and Bramlett H.M. (2011). Oligodendrocyte vulnerability following traumatic brain injury in rats. Neurosci. Lett. 499, 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yakovlev A.G., and Faden A.I. (2001). Caspase-dependent apoptotic pathways in CNS injury. Mol. Neurobiol. 24, 131–144 [DOI] [PubMed] [Google Scholar]
- 86.Zhang X., Chen Y., Jenkins L.W., Kochanek P.M., and Clark R.S. (2005). Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Critical Care (Lond.) 9, 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knoblach S.M., Nikolaeva M., Huang X., Fan L., Krajewski S., Reed J.C., and Faden A.I. (2002). Multiple caspases are activated after traumatic brain injury: evidence for involvement in functional outcome. J. Neurotrauma 19, 1155–1170 [DOI] [PubMed] [Google Scholar]
- 88.Clark R.S., Kochanek P.M., Watkins S.C., Chen M., Dixon C.E., Seidberg N.A., Melick J., Loeffert J.E., Nathaniel P.D., Jin K.L., and Graham S.H. (2000). Caspase-3 mediated neuronal death after traumatic brain injury in rats. J. Neurochem 74, 740–753 [DOI] [PubMed] [Google Scholar]
- 89.Satchell M.A., Zhang X., Kochanek P.M., Dixon C.E., Jenkins L.W., Melick J., Szabo C., and Clark R.S. (2003). A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD+ depletion and ribosylation of 14-3-3gamma. J. Neurochem. 85, 697–708 [DOI] [PubMed] [Google Scholar]
- 90.Wong J., Hoe N.W., Zhiwei F., and Ng I. (2005). Apoptosis and traumatic brain injury. Neurocrit. Care 3, 177–182 [DOI] [PubMed] [Google Scholar]
- 91.Das M., Mohapatra S., and Mohapatra S.S. (2012). New perspectives on central and peripheral immune responses to acute traumatic brain injury. J. Neuroinflammation 9, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., and Faden A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ziebell J.M., and Morganti-Kossmann M.C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith C., Gentleman S.M., Leclercq P.D., Murray L.S., Griffin W.S., Graham D.I., and Nicoll J.A. (2013). The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mayer C.L., Huber B.R., and Peskind E. (2013). Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache 53, 1523–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gentleman S.M., Leclercq P.D., Moyes L., Graham D.I., Smith C., Griffin W.S., and Nicoll J.A. (2004). Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci. Int. 146, 97–104 [DOI] [PubMed] [Google Scholar]
- 97.Nonaka M., Chen X.H., Pierce J.E., Leoni M.J., McIntosh T.K., Wolf J.A., and Smith D.H. (1999). Prolonged activation of NF-kappaB following traumatic brain injury in rats. J. Neurotrauma 16, 1023–1034 [DOI] [PubMed] [Google Scholar]
- 98.Acosta S.A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D., Sanberg P.R., Bickford P.C., Kaneko Y., and Borlongan C.V. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PloS One 8, e53376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morganti-Kossmann M.C., Rancan M., Stahel P.F., and Kossmann T. (2002). Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care 8, 101–105 [DOI] [PubMed] [Google Scholar]
- 100.de Rivero Vaccari J.P., Dietrich W.D., and Keane R.W. (2014). Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 34, 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Rivero Vaccari J.P., Lotocki G., Marcillo A.E., Dietrich W.D., and Keane R.W. (2008). A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 28, 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adamczak S., Dale G., de Rivero Vaccari J.P., Bullock M.R., Dietrich W.D., and Keane R.W. (2012). Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J. Neurosurg. 117, 1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siman R., Giovannone N., Hanten G., Wilde E.A., McCauley S.R., Hunter J.V., Li X., Levin H.S., and Smith D.H. (2013). Evidence that the blood biomarker SNTF predicts brain imaging changes and persistent cognitive dysfunction in mild TBI patients. Front. Neurol. 4, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McClain C.J., Cohen D., Ott L., Dinarello C.A., and Young B. (1987). Ventricular fluid interleukin-1 activity in patients with head injury. J. Lab. Clin. Med. 110, 48–54 [PubMed] [Google Scholar]
- 105.Frugier T., Morganti-Kossmann M.C., O'Reilly D., and McLean C.A. (2010). In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J. Neurotrauma 27, 497–507 [DOI] [PubMed] [Google Scholar]
- 106.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., and Sharp D.J. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 70, 374–383 [DOI] [PubMed] [Google Scholar]
- 108.Singh N., Hopkins S.J., Hulme S., Galea J.P., Hoadley M., Vail A., Hutchinson P.J., Grainger S., Rothwell N.J., King A.T., and Tyrrell P.J. (2014). The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J. Neuroinflammation 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tehranian R., Andell-Jonsson S., Beni S.M., Yatsiv I., Shohami E., Bartfai T., Lundkvist J., and Iverfeldt K. (2002). Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J. Neurotrauma 19, 939–951 [DOI] [PubMed] [Google Scholar]
- 110.Lucas S.M., Rothwell N.J., and Gibson R.M. (2006). The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 147, Suppl. 1, S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jones N.C., Prior M.J., Burden-Teh E., Marsden C.A., Morris P.G., and Murphy S. (2005). Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2-positive cells and improves anatomical and functional outcomes. Eur. J. Neurosci. 22, 72–78 [DOI] [PubMed] [Google Scholar]
- 112.Adamczak S.E., de Rivero Vaccari J.P., Dale G., Brand Iii F.J., Nonner D., Bullock M., Dahl G.P., Dietrich W., and Keane R.W. (2014). Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J. Cereb. Blood Flow Metab. 34, 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clausen F., Hanell A., Bjork M., Hillered L., Mir A.K., Gram H., and Marklund N. (2009). Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 30, 385–396 [DOI] [PubMed] [Google Scholar]
- 114.Clausen F., Hanell A., Israelsson C., Hedin J., Ebendal T., Mir A.K., Gram H., and Marklund N. (2011). Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 34, 110–123 [DOI] [PubMed] [Google Scholar]
- 115.Xiao G., Wei J., Yan W., Wang W., and Lu Z. (2008). Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit. Care (Lond.) 12, R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dietrich W.D., Atkins C.M., and Bramlett H.M. (2009). Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J. Neurotrauma 26, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chatzipanteli K., Alonso O.F., Kraydieh S., and Dietrich W.D. (2000). Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J. Cereb. Blood Flow Metab. 20, 531–542 [DOI] [PubMed] [Google Scholar]
- 118.Chatzipanteli K., Yanagawa Y., Marcillo A.E., Kraydieh S., Yezierski R.P., and Dietrich W.D. (2000). Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. J. Neurotrauma 17, 321–332 [DOI] [PubMed] [Google Scholar]
- 119.Kinoshita K., Chatzipanteli K., Vitarbo E., Truettner J.S., Alonso O.F., and Dietrich W.D. (2002). Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery 51, 195–203; discussion, 203 [DOI] [PubMed] [Google Scholar]
- 120.Vitarbo E.A., Chatzipanteli K., Kinoshita K., Truettner J.S., Alonso O.F., and Dietrich W.D. (2004). Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. Neurosurgery 55, 416–424; discussion, 424–415 [DOI] [PubMed] [Google Scholar]
- 121.Tomura S., de Rivero Vaccari J.P., Keane R.W., Bramlett H.M., and Dietrich W.D. (2012). Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J. Cereb. Blood Flow Metab. 32, 1939–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dietrich W.D. (1992). The importance of brain temperature in cerebral injury. J. Neurotrauma 9, Suppl. 2, S475–S485 [PubMed] [Google Scholar]
- 123.Gensel J.C., Kigerl K.A., Mandrekar-Colucci S.S., Gaudet A.D., and Popovich P.G. (2012). Achieving CNS axon regeneration by manipulating convergent neuro-immune signaling. Cell Tissue Res. 349, 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donnelly D.J., Gensel J.C., Ankeny D.P., van Rooijen N., and Popovich P.G. (2009). An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J. Neurosci. Methods 181, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chhor V., Le Charpentier T., Lebon S., Ore M.V., Celador I.L., Josserand J., Degos V., Jacotot E., Hagberg H., Savman K., Mallard C., Gressens P., and Fleiss B. (2013). Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 32, 70–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu X., Li P., Guo Y., Wang H., Leak R.K., Chen S., Gao Y., and Chen J. (2012). Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070 [DOI] [PubMed] [Google Scholar]
- 128.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P., and Donners M.M. (2014). Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17, 109–118 [DOI] [PubMed] [Google Scholar]
- 129.Fenn A.M., Gensel J.C., Huang Y., Popovich P.G., Lifshitz J., and Godbout J.P. (2013). Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol. Psychiatry 76, 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lowenstein D.H. (2009). Epilepsy after head injury: an overview. Epilepsia 50, Suppl. 2, 4–9 [DOI] [PubMed] [Google Scholar]
- 131.Vespa P.M., Nuwer M.R., Nenov V., Ronne-Engstrom E., Hovda D.A., Bergsneider M., Kelly D.F., Martin N.A., and Becker D.P. (1999). Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J. Neurosurg. 91, 750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bushnik T., Englander J., Wright J., and Kolakowsky-Hayner S.A. (2012). Traumatic brain injury with and without late posttraumatic seizures: what are the impacts in the post-acute phase: a NIDRR Traumatic Brain Injury Model Systems study. J. Head Trauma Rehabil. 27, E36–E44 [DOI] [PubMed] [Google Scholar]
- 133.Asikainen I., Kaste M., and Sarna S. (1999). Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia 40, 584–589 [DOI] [PubMed] [Google Scholar]
- 134.Garga N., and Lowenstein D.H. (2006). Posttraumatic epilepsy: a major problem in desperate need of major advances. Epilepsy Curr. 6, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bao Y.H., Bramlett H.M., Atkins C.M., Truettner J.S., Lotocki G., Alonso O.F., and Dietrich W.D. (2011). Post-traumatic seizures exacerbate histopathological damage after fluid-percussion brain injury. J. Neurotrauma 28, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kharatishvili I., Nissinen J.P., McIntosh T.K., and Pitkanen A. (2006). A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140, 685–697 [DOI] [PubMed] [Google Scholar]
- 137.Santhakumar V., Ratzliff A.D., Jeng J., Toth Z., and Soltesz I. (2001). Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 50, 708–717 [DOI] [PubMed] [Google Scholar]
- 138.Stewart T.H., Eastman C.L., Groblewski P.A., Fender J.S., Verley D.R., Cook D.G., and D'Ambrosio R. (2010). Chronic dysfunction of astrocytic inwardly rectifying K+ channels specific to the neocortical epileptic focus after fluid percussion injury in the rat. J. Neurophysiol. 104, 3345–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ueda Y., Kitamoto A., Willmore L.J., and Kojima T. (2011). Hippocampal gene network analysis in an experimental model of posttraumatic epilepsy. Neurochem. Res. 36, 1323–1328 [DOI] [PubMed] [Google Scholar]
- 140.Arndt D.H., Lerner J.T., Matsumoto J.H., Madikians A., Yudovin S., Valino H., McArthur D.L., Wu J.Y., Leung M., Buxey F., Szeliga C., Van Hirtum-Das M., Sankar R., Brooks-Kayal A., and Giza C.C. (2013). Subclinical early posttraumatic seizures detected by continuous EEG monitoring in a consecutive pediatric cohort. Epilepsia 54, 1780–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Temkin N.R. (2009). Preventing and treating posttraumatic seizures: the human experience. Epilepsia 50, Suppl. 2, 10–13 [DOI] [PubMed] [Google Scholar]
- 142.Pearl P.L., McCarter R., McGavin C.L., Yu Y., Sandoval F., Trzcinski S., Atabaki S.M., Tsuchida T., van den Anker J., He J., and Klein P. (2013). Results of phase II levetiracetam trial following acute head injury in children at risk for posttraumatic epilepsy. Epilepsia 54, e135–e137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sick J., Bray E., Bregy A., Dietrich W.D., Bramlett H.M., and Sick T. (2013). EEGgui: a program used to detect electroencephalogram anomalies after traumatic brain injury. Source Code Biol. Med. 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vespa P.M., McArthur D.L., Xu Y., Eliseo M., Etchepare M., Dinov I., Alger J., Glenn T.P., and Hovda D. (2010). Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology 75, 792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu X.C., Mountney A., Chen Z., Wei G., Cao Y., Leung L.Y., Khatri V., Cunningham T., and Tortella F.C. (2013). Similarities and differences of acute nonconvulsive seizures and other epileptic activities following penetrating and ischemic brain injuries in rats. J. Neurotrauma 30, 580–590 [DOI] [PubMed] [Google Scholar]
- 146.Hartings J.A., Strong A.J., Fabricius M., Manning A., Bhatia R., Dreier J.P., Mazzeo A.T., Tortella F.C., and Bullock M.R.; Co-Operative Study of Brain Injury Depolarizations. (2009). Spreading depolarizations and late secondary insults after traumatic brain injury. J. Neurotrauma 26, 1857–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alessandri B., Tretzel J.S., Heimann A., and Kempski O. (2012). Spontaneous cortical spreading depression and intracranial pressure following acute subdural hematoma in a rat. Acta Neurochir. Suppl. 114, 373–376 [DOI] [PubMed] [Google Scholar]
- 148.Dreier J.P. (2011). The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 17, 439–447 [DOI] [PubMed] [Google Scholar]
- 149.Dreier J.P., Major S., Pannek H.W., Woitzik J., Scheel M., Wiesenthal D., Martus P., Winkler M.K., Hartings J.A., Fabricius M., Speckmann E.J., and Gorji A.; COSBID Study Group. (2012). Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 135, 259–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dohmen C., Sakowitz O.W., Fabricius M., Bosche B., Reithmeier T., Ernestus R.I., Brinker G., Dreier J.P., Woitzik J., Strong A.J., and Graf R.; Co-Operative Study of Brain Injury Depolarizations (COSBID). (2008). Spreading depolarizations occur in human ischemic stroke with high incidence. Ann. Neurol. 63, 720–728 [DOI] [PubMed] [Google Scholar]
- 151.Menniti F.S., Pagnozzi M.J., Butler P., Chenard B.L., Jaw-Tsai S.S., and Frost White W. (2000). CP-101,606, an NR2B subunit selective NMDA receptor antagonist, inhibits NMDA and injury induced c-fos expression and cortical spreading depression in rodents. Neuropharmacology 39, 1147–1155 [DOI] [PubMed] [Google Scholar]
- 152.Hertle D.N., Dreier J.P., Woitzik J., Hartings J.A., Bullock R., Okonkwo D.O., Shutter L.A., Vidgeon S., Strong A.J., Kowoll C., Dohmen C., Diedler J., Veltkamp R., Bruckner T., Unterberg A.W., Sakowitz O.W., and Cooperative Study of Brain Injury D. (2012). Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain 135, 2390–2398 [DOI] [PubMed] [Google Scholar]
- 153.Piilgaard H., Witgen B.M., Rasmussen P., and Lauritzen M. (2011). Cyclosporine A, FK506, and NIM811 ameliorate prolonged CBF reduction and impaired neurovascular coupling after cortical spreading depression. J. Cereb. Blood Flow Metab. 31, 1588–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yenari M.A., Onley D., Hedehus M., deCrespigny A., Sun G.H., Moseley M.E., and Steinberg G.K. (2000). Diffusion- and perfusion-weighted magnetic resonance imaging of focal cerebral ischemia and cortical spreading depression under conditions of mild hypothermia. Brain Res. 885, 208–219 [DOI] [PubMed] [Google Scholar]
- 155.Katsura K., Minamisawa H., Ekholm A., Folbergrova J., and Siesjo B.K. (1992). Changes of labile metabolites during anoxia in moderately hypo- and hyperthermic rats: correlation to membrane fluxes of K+. Brain Res. 590, 6–12 [DOI] [PubMed] [Google Scholar]
- 156.Willmore L.J. (2012). Posttraumatic epilepsy: what's contusion got to do with it? Epilepsy Curr. 12, 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Willmore L.J., and Ueda Y. (2009). Posttraumatic epilepsy: hemorrhage, free radicals and the molecular regulation of glutamate. Neurochem. Res. 34, 688–697 [DOI] [PubMed] [Google Scholar]
- 158.Ueda Y., Kitamoto A., Willmore L.J., and Kojima T. (2013). Hippocampal gene expression profiling in a rat model of posttraumatic epilepsy reveals temporal upregulation of lipid metabolism-related genes. Neurochem. Res. 38, 1399–1406 [DOI] [PubMed] [Google Scholar]
- 159.Schoch K.M., von Reyn C.R., Bian J., Telling G.C., Meaney D.F., and Saatman K.E. (2013). Brain injury-induced proteolysis is reduced in a novel calpastatin-overexpressing transgenic mouse. J. Neurochem. 125, 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ottens A.K., Golden E.C., Bustamante L., Hayes R.L., Denslow N.D., and Wang K.K. (2008). Proteolysis of multiple myelin basic protein isoforms after neurotrauma: characterization by mass spectrometry. J. Neurochem. 104, 1404–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reeves T.M., Greer J.E., Vanderveer A.S., and Phillips L.L. (2010). Proteolysis of submembrane cytoskeletal proteins ankyrin-G and alphaII-spectrin following diffuse brain injury: a role in white matter vulnerability at Nodes of Ranvier. Brain. Pathol. 20, 1055–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thompson S.N., Gibson T.R., Thompson B.M., Deng Y., and Hall E.D. (2006). Relationship of calpain-mediated proteolysis to the expression of axonal and synaptic plasticity markers following traumatic brain injury in mice. Exp. Neurol. 201, 253–265 [DOI] [PubMed] [Google Scholar]
- 163.Liu M.C., Akle V., Zheng W., Dave J.R., Tortella F.C., Hayes R.L., and Wang K.K. (2006). Comparing calpain- and caspase-3-mediated degradation patterns in traumatic brain injury by differential proteome analysis. Biochem. J. 394, 715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma M., Shofer F.S., and Neumar R.W. (2012). Calpastatin overexpression protects axonal transport in an in vivo model of traumatic axonal injury. J. Neurotrauma 29, 2555–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brophy G.M., Pineda J.A., Papa L., Lewis S.B., Valadka A.B., Hannay H.J., Heaton S.C., Demery J.A., Liu M.C., Tepas J.J., III, Gabrielli A., Robicsek S., Wang K.K., Robertson C.S., and Hayes R.L. (2009). alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma 26, 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ma M., Li L., Wang X., Bull D.L., Shofer F.S., Meaney D.F., and Neumar R.W. (2012). Short-duration treatment with the calpain inhibitor MDL-28170 does not protect axonal transport in an in vivo model of traumatic axonal injury. J. Neurotrauma 29, 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Czeiter E., Buki A., Bukovics P., Farkas O., Pal J., Kovesdi E., Doczi T., and Sandor J. (2009). Calpain inhibition reduces axolemmal leakage in traumatic axonal injury. Molecules 14, 5115–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schoch K.M., Evans H.N., Brelsfoard J.M., Madathil S.K., Takano J., Saido T.C., and Saatman K.E. (2012). Calpastatin overexpression limits calpain-mediated proteolysis and behavioral deficits following traumatic brain injury. Exp. Neurol. 236, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Povlishock J.T., and Christman C.W. (1995). The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J. Neurotrauma 12, 555–564 [DOI] [PubMed] [Google Scholar]
- 170.Bramlett H.M., Kraydieh S., Green E.J., and Dietrich W.D. (1997). Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J. Neuropathol. Exp. Neurol. 56, 1132–1141 [DOI] [PubMed] [Google Scholar]
- 171.Pierce J.E., Trojanowski J.Q., Graham D.I., Smith D.H., and McIntosh T.K. (1996). Immunohistochemical characterization of alterations in the distribution of amyloid precursor proteins and beta-amyloid peptide after experimental brain injury in the rat. J. Neurosci. 16, 1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson V.E., Stewart W., and Smith D.H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Greenberg G., Mikulis D.J., Ng K., DeSouza D., and Green R.E. (2008). Use of diffusion tensor imaging to examine subacute white matter injury progression in moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 89, S45–S50 [DOI] [PubMed] [Google Scholar]
- 174.Hall E.D., Bryant Y.D., Cho W. and Sullivan P.G. (2008). Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 25, 235–247 [DOI] [PubMed] [Google Scholar]
- 175.Hemerka J.N., Wu X., Dixon C.E., Garman R.H., Exo J.L., Shellington D.K., Blasiole B., Vagni V.A., Janesko-Feldman K., Xu M., Wisniewski S.R., Bayir H., Jenkins L.W., Clark R.S., Tisherman S.A., and Kochanek P.M. (2012). Severe brief pressure-controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice. J. Neurotrauma 29, 2192–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Adams J.H., Graham D.I., and Jennett B. (2000). The neuropathology of the vegetative state after an acute brain insult. Brain 123, Pt. 7, 1327–1338 [DOI] [PubMed] [Google Scholar]
- 177.Graham D.I., Adams J.H., Nicoll J.A., Maxwell W.L., and Gennarelli T.A. (1995). The nature, distribution and causes of traumatic brain injury. Brain Pathol. 5, 397–406 [DOI] [PubMed] [Google Scholar]
- 178.Povlishock J.T. (1992). Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 2, 1–12 [PubMed] [Google Scholar]
- 179.Conti A.C., Raghupathi R., Trojanowski J.Q., and McIntosh T.K. (1998). Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 18, 5663–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sutula T., He X.X., Cavazos J., and Scott G. (1988). Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science 239, 1147–1150 [DOI] [PubMed] [Google Scholar]
- 181.Swartz B.E., Houser C.R., Tomiyasu U., Walsh G.O., DeSalles A., Rich J.R., and Delgado-Escueta A. (2006). Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia 47, 1373–1382 [DOI] [PubMed] [Google Scholar]
- 182.Santhakumar V., Bender R., Frotscher M., Ross S.T., Hollrigel G.S., Toth Z., and Soltesz I. (2000). Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J. Physiol. 524, Pt. 1, 117–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Golarai G., Greenwood A.C., Feeney D.M., and Connor J.A. (2001). Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci. 21, 8523–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Greer J.E., McGinn M.J., and Povlishock J.T. (2011). Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J. Neurosci. 31, 5089–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kharatishvili I., Immonen R., Grohn O., and Pitkanen A. (2007). Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain 130, 3155–3168 [DOI] [PubMed] [Google Scholar]
- 186.Dickson T.C., Chung R.S., McCormack G.H., Staal J.A., and Vickers J.C. (2007). Acute reactive and regenerative changes in mature cortical axons following injury. Neuroreport 18, 283–288 [DOI] [PubMed] [Google Scholar]
- 187.Babb T.L., Kupfer W.R., Pretorius J.K., Crandall P.H., and Levesque M.F. (1991). Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience 42, 351–363 [DOI] [PubMed] [Google Scholar]
- 188.Fujita M., Oda Y., Wei E.P., and Povlishock J.T. (2011). The combination of either tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J. Neurotrauma 28, 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Buki A., Koizumi H., and Povlishock J.T. (1999). Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp. Neurol. 159, 319–328 [DOI] [PubMed] [Google Scholar]
- 190.Ma M., Matthews B.T., Lampe J.W., Meaney D.F., Shofer F.S., and Neumar R.W. (2009). Immediate short-duration hypothermia provides long-term protection in an in vivo model of traumatic axonal injury. Exp. Neurol. 215, 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maxwell W.L., Watson A., Queen R., Conway B., Russell D., Neilson M., and Graham D.I. (2005). Slow, medium, or fast re-warming following post-traumatic hypothermia therapy? An ultrastructural perspective. J. Neurotrauma 22, 873–884 [DOI] [PubMed] [Google Scholar]
- 192.Kumar R., Husain M., Gupta R.K., Hasan K.M., Haris M., Agarwal A.K., Pandey C.M., and Narayana P.A. (2009). Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J. Neurotrauma 26, 481–495 [DOI] [PubMed] [Google Scholar]
- 193.Fields R.D. (2008). White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhao C., Deng W., and Gage F.H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 [DOI] [PubMed] [Google Scholar]
- 195.Zhao C., Teng E.M., Summers R.G., Jr., Ming G.L., and Gage F.H. (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Deng W., Aimone J.B., and Gage F.H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dupret D., Revest J.M., Koehl M., Ichas F., De Giorgi F., Costet P., Abrous D.N., and Piazza P.V. (2008). Spatial relational memory requires hippocampal adult neurogenesis. PloS One 3, e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gould E., Beylin A., Tanapat P., Reeves A., and Shors T.J. (1999). Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 [DOI] [PubMed] [Google Scholar]
- 199.Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Bostrom E., Westerlund I., Vial C., Buchholz B.A., Possnert G., Mash D.C., Druid H., and Frisen J. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chirumamilla S., Sun D., Bullock M.R., and Colello R.J. (2002). Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma 19, 693–703 [DOI] [PubMed] [Google Scholar]
- 201.Dash P.K., Mach S.A., and Moore A.N. (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 63, 313–319 [DOI] [PubMed] [Google Scholar]
- 202.Sun D., McGinn M.J., Zhou Z., Harvey H.B., Bullock M.R., and Colello R.J. (2007). Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 204, 264–272 [DOI] [PubMed] [Google Scholar]
- 203.Gao X., Enikolopov G., and Chen J. (2009). Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp. Neurol. 219, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Urrea C., Castellanos D.A., Sagen J., Tsoulfas P., Bramlett H.M., and Dietrich W.D. (2007). Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor. Neurol. Neurosci. 25, 65–76 [PubMed] [Google Scholar]
- 205.Chen X.H., Iwata A., Nonaka M., Browne K.D., and Smith D.H. (2003). Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J. Neurotrauma 20, 623–631 [DOI] [PubMed] [Google Scholar]
- 206.Bregy A., Nixon R., Lotocki G., Alonso O.F., Atkins C.M., Tsoulfas P., Bramlett H.M., and Dietrich W.D. (2012). Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp. Neurol. 233, 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Blaiss C.A., Yu T.S., Zhang G., Chen J., Dimchev G., Parada L.F., Powell C.M., and Kernie S.G. (2011). Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 31, 4906–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gao X., Deng-Bryant Y., Cho W., Carrico K.M., Hall E.D., and Chen J. (2008). Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 86, 2258–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sierra A., Encinas J.M., Deudero J.J., Chancey J.H., Enikolopov G., Overstreet-Wadiche L.S., Tsirka S.E., and Maletic-Savatic M. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Barha C.K., Ishrat T., Epp J.R., Galea L.A., and Stein D.G. (2011). Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp. Neurol. 231, 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Blaya M.O., Bramlett H., Nadoo J., Pieper A.A., and Dietrich W.D., III. (2014). Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J. Neurotrauma 31, 476–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang Y., Chopp M., Mahmood A., Meng Y., Qu C., and Xiong Y. (2012). Impact of inhibition of erythropoietin treatment-mediated neurogenesis in the dentate gyrus of the hippocampus on restoration of spatial learning after traumatic brain injury. Exp. Neurol. 235, 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gaulke L.J., Horner P.J., Fink A.J., McNamara C.L., and Hicks R.R. (2005). Environmental enrichment increases progenitor cell survival in the dentate gyrus following lateral fluid percussion injury. Brain Res. Mol. Brain Res. 141, 138–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dietrich W.D., Alonso O., Busto R., Prado R., Dewanjee S., Dewanjee M.K., and Ginsberg M.D. (1996). Widespread hemodynamic depression and focal platelet accumulation after fluid percussion brain injury: a double-label autoradiographic study in rats. J. Cereb. Blood Flow Metab. 16, 481–489 [DOI] [PubMed] [Google Scholar]
- 215.Dietrich W.D., Alonso O., Busto R., Prado R., Zhao W., Dewanjee M.K., and Ginsberg M.D. (1998). Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats. Neurosurgery 43, 585–593; discussion, 593–584 [DOI] [PubMed] [Google Scholar]
- 216.DeWitt D.S., Jenkins L.W., Wei E.P., Lutz H., Becker D.P., and Kontos H.A. (1986). Effects of fluid-percussion brain injury on regional cerebral blood flow and pial arteriolar diameter. J. Neurosurg. 64, 787–794 [DOI] [PubMed] [Google Scholar]
- 217.Bouma G.J., Muizelaar J.P., Choi S.C., Newlon P.G., and Young H.F. (1991). Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J. Neurosurg. 75, 685–693 [DOI] [PubMed] [Google Scholar]
- 218.Marion D.W., Darby J., and Yonas H. (1991). Acute regional cerebral blood flow changes caused by severe head injuries. J. Neurosurg. 74, 407–414 [DOI] [PubMed] [Google Scholar]
- 219.Forbes M.L., Hendrich K.S., Kochanek P.M., Williams D.S., Schiding J.K., Wisniewski S.R., Kelsey S.F., DeKosky S.T., Graham S.H., Marion D.W., and Ho C. (1997). Assessment of cerebral blood flow and CO2 reactivity after controlled cortical impact by perfusion magnetic resonance imaging using arterial spin-labeling in rats. J. Cereb. Blood Flow Metab. 17, 865–874 [DOI] [PubMed] [Google Scholar]
- 220.Bryan R.M., Jr., Cherian L., and Robertson C. (1995). Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth. Analg. 80, 687–695 [DOI] [PubMed] [Google Scholar]
- 221.Len T.K., and Neary J.P. (2011). Cerebrovascular pathophysiology following mild traumatic brain injury. Clin. Physiol. Funct. Imaging 31, 85–93 [DOI] [PubMed] [Google Scholar]
- 222.Ginsberg M.D., Zhao W., Alonso O.F., Loor-Estades J.Y., Dietrich W.D., and Busto R. (1997). Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am. J. Physiol. 272, H2859–H2868 [DOI] [PubMed] [Google Scholar]
- 223.Bartnik B.L., Hovda D.A., and Lee P.W. (2007). Glucose metabolism after traumatic brain injury: estimation of pyruvate carboxylase and pyruvate dehydrogenase flux by mass isotopomer analysis. J. Neurotrauma 24, 181–194 [DOI] [PubMed] [Google Scholar]
- 224.Wu H.M., Huang S.C., Vespa P., Hovda D.A., and Bergsneider M. (2013). Redefining the pericontusional penumbra following traumatic brain injury: evidence of deteriorating metabolic derangements based on positron emission tomography. J. Neurotrauma 30, 352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Honda M., Sase S., Yokota K., Ichibayashi R., Yoshihara K., Masuda H., Uekusa H., Nomoto J., Sugo N., Kishi T., and Seiki Y. (2013). Early cerebral circulation disturbance in patients suffering from different types of severe traumatic brain injury: a xenon CT and perfusion CT study. Acta Neurochir. Suppl. 118, 259–263 [DOI] [PubMed] [Google Scholar]
- 226.Liu W., Wang B., Wolfowitz R., Yeh P.H., Nathan D.E., Graner J., Tang H., Pan H., Harper J., Pham D., Oakes T.R., French L.M., and Riedy G. (2013). Perfusion deficits in patients with mild traumatic brain injury characterized by dynamic susceptibility contrast MRI. NMR Biomed. 26, 651–663 [DOI] [PubMed] [Google Scholar]
- 227.Rangel-Castilla L., Gasco J., Nauta H.J., Okonkwo D.O., and Robertson C.S. (2008). Cerebral pressure autoregulation in traumatic brain injury. Neurosurg. Focus 25, E7. [DOI] [PubMed] [Google Scholar]
- 228.Oda Y., Gao G., Wei E.P., and Povlishock J.T. (2011). Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J. Cereb. Blood Flow Metab. 31, 1143–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Baranova A.I., Wei E.P., Ueda Y., Sholley M.M., Kontos H.A., and Povlishock J.T. (2008). Cerebral vascular responsiveness after experimental traumatic brain injury: the beneficial effects of delayed hypothermia combined with superoxide dismutase administration. J. Neurosurg. 109, 502–509 [DOI] [PubMed] [Google Scholar]
- 230.Danton G.H., Prado R., Truettner J., Watson B.D., and Dietrich W.D. (2002). Endothelial nitric oxide synthase pathophysiology after nonocclusive common carotid artery thrombosis in rats. J. Cereb. Blood Flow Metab. 22, 612–619 [DOI] [PubMed] [Google Scholar]
- 231.Wei E.P., Dietrich W.D., Povlishock J.T., Navari R.M., and Kontos H.A. (1980). Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ. Res. 46, 37–47 [DOI] [PubMed] [Google Scholar]
- 232.Len T.K., Neary J.P., Asmundson G.J., Candow D.G., Goodman D.G., Bjornson B., and Bhambhani Y.N. (2013). Serial monitoring of CO2 reactivity following sport concussion using hypocapnia and hypercapnia. Brain Inj. 27, 346–353 [DOI] [PubMed] [Google Scholar]
- 233.Bailey D.M., Jones D.W., Sinnott A., Brugniaux J.V., New K.J., Hodson D., Marley C.J., Smirl J.D., Ogoh S., and Ainslie P.N. (2013). Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin. Sci. 124, 177–189 [DOI] [PubMed] [Google Scholar]
- 234.Kim J., Whyte J., Patel S., Europa E., Slattery J., Coslett H.B., and Detre J.A. (2012). A perfusion fMRI study of the neural correlates of sustained-attention and working-memory deficits in chronic traumatic brain injury. Neurorehabil. Neural Repair 26, 870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hayward N.M., Immonen R., Tuunanen P.I., Ndode-Ekane X.E., Grohn O., and Pitkanen A. (2010). Association of chronic vascular changes with functional outcome after traumatic brain injury in rats. J. Neurotrauma 27, 2203–2219 [DOI] [PubMed] [Google Scholar]
- 236.Hayward N.M., Tuunanen P.I., Immonen R., Ndode-Ekane X.E., Pitkanen A., and Grohn O. (2011). Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. J. Cereb. Blood Flow Metab. 31, 166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Abdel-Dayem H.M., Abu-Judeh H., Kumar M., Atay S., Naddaf S., El-Zeftawy H., and Luo J.Q. (1998). SPECT brain perfusion abnormalities in mild or moderate traumatic brain injury. Clin. Nucl. Med. 23, 309–317 [DOI] [PubMed] [Google Scholar]
- 238.Goldenberg G., Oder W., Spatt J., and Podreka I. (1992). Cerebral correlates of disturbed executive function and memory in survivors of severe closed head injury: a SPECT study. J. Neurol. Neurosurg. Psychiatry 55, 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gray B.G., Ichise M., Chung D.G., Kirsh J.C., and Franks W. (1992). Technetium-99m-HMPAO SPECT in the evaluation of patients with a remote history of traumatic brain injury: a comparison with x-ray computed tomography. J. Nucl. Med. 33, 52–58 [PubMed] [Google Scholar]
- 240.Jacobs A., Put E., Ingels M., Put T., and Bossuyt A. (1996). One-year follow-up of technetium-99m-HMPAO SPECT in mild head injury. J. Nucl. Med. 37, 1605–1609 [PubMed] [Google Scholar]
- 241.Kochanek P.M., Hendrich K.S., Dixon C.E., Schiding J.K., Williams D.S., and Ho C. (2002). Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J. Neurotrauma 19, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 242.Stein S.C., Graham D.I., Chen X.H., and Smith D.H. (2004). Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery 54, 687–691; discussion, 691 [DOI] [PubMed] [Google Scholar]
- 243.Stein S.C., and Smith D.H. (2004). Coagulopathy in traumatic brain injury. Neurocrit. Care 1, 479–488 [DOI] [PubMed] [Google Scholar]
- 244.Daneshvar D.H., Riley D.O., Nowinski C.J., McKee A.C., Stern R.A., and Cantu R.C. (2011). Long-term consequences: effects on normal development profile after concussion. Phys. Med. Rehabil. Clin. N. Am. 22, 683–700, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsh-Bohmer K.A., Burke J.R., Guralnik J.M., and Breitner J.C. (2000). Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 246.Jellinger K.A., Paulus W., Wrocklage C., and Litvan I. (2001). Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Blumbergs P.C., Scott G., Manavis J., Wainwright H., Simpson D.A., and McLean A.J. (1994). Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 344, 1055–1056 [DOI] [PubMed] [Google Scholar]
- 248.Graham D.I., Gentleman S.M., Lynch A., and Roberts G.W. (1995). Distribution of beta-amyloid protein in the brain following severe head injury. Neuropathol. Appl. Neurobiol. 21, 27–34 [DOI] [PubMed] [Google Scholar]
- 249.Lewen A., Li G.L., Nilsson P., Olsson Y., and Hillered L. (1995). Traumatic brain injury in rat produces changes of beta-amyloid precursor protein immunoreactivity. Neuroreport 6, 357–360 [DOI] [PubMed] [Google Scholar]
- 250.Hamberger A., Huang Y.L., Zhu H., Bao F., Ding M., Blennow K., Olsson A., Hansson H.A., Viano D., and Haglid K.G. (2003). Redistribution of neurofilaments and accumulation of beta-amyloid protein after brain injury by rotational acceleration of the head. J. Neurotrauma 20, 169–178 [DOI] [PubMed] [Google Scholar]
- 251.Bramlett H.M., and Dietrich W.D. (2003). Synuclein aggregation: possible role in traumatic brain injury. Exp. Neurol. 184, 27–30 [DOI] [PubMed] [Google Scholar]
- 252.Irving E.A., Nicoll J., Graham D.I., and Dewar D. (1996). Increased tau immunoreactivity in oligodendrocytes following human stroke and head injury. Neurosci. Lett. 213, 189–192 [DOI] [PubMed] [Google Scholar]
- 253.Sherriff F.E., Bridges L.R., and Sivaloganathan S. (1994). Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathol. (Berl.) 87, 55–62 [DOI] [PubMed] [Google Scholar]
- 254.Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A., Nowinski C.J., Cantu R.C., McKee A.C., and Stern R.A. (2012). Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 6, 244–254 [DOI] [PubMed] [Google Scholar]
- 255.Castillo-Carranza D.L., Lasagna-Reeves C.A., and Kayed R. (2013). Tau aggregates as immunotherapeutic targets. Front. Biosci. 5, 426–438 [DOI] [PubMed] [Google Scholar]
- 256.Schenk D., Basi G.S., and Pangalos M.N. (2012). Treatment strategies targeting amyloid beta-protein. Cold Spring Harb. Perspect. Med. 2, a006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schneeberger A., Mandler M., Mattner F., and Schmidt W. (2012). Vaccination for Parkinson's disease. Parkinsonism Relat. Disord. 18, Suppl. 1, S11–S13 [DOI] [PubMed] [Google Scholar]
- 258.McConeghy K.W., Hatton J., Hughes L., and Cook A.M. (2012). A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 26, 613–636 [DOI] [PubMed] [Google Scholar]
- 259.Steward O., Popovich P.G., Dietrich W.D., and Kleitman N. (2012). Replication and reproducibility in spinal cord injury research. Exp. Neurol. 233, 597–605 [DOI] [PubMed] [Google Scholar]
- 260.Nielson J.L., Guandique C.F., Liu A.W., Burke D.A., Lash A.T., Moseanko R., Hawbecker S., Strand S.C., Zdunowski S., Irvine K.A., Brock J.H., Nout-Lomas Y.S., Gensel J.C., Anderson K.D., Segal M.R., Rosenzweig E.S., Magnuson D.S., Whittemore S.R., McTigue D.M., Popovich P.G., Rabchevsky A.G., Scheff S.W., Steward O., Courtine G., Edgerton V.R., Tuszynski M.H., Beattie M.S., Bresnahan J.C., and Ferguson A.R. (2014). Development of a database for translational spinal cord injury research. J. Neurotrauma 31, 1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kochanek P.M., Bramlett H., Dietrich W.D., Dixon C.E., Hayes R.L., Povlishock J., Tortella F.C., and Wang K.K. (2011). A novel multicenter preclinical drug screening and biomarker consortium for experimental traumatic brain injury: operation brain trauma therapy. J. Trauma 71, S15–S24 [DOI] [PubMed] [Google Scholar]
- 262.Lemmon V.P., Ferguson A.R., Popovich P.G., Xu X.M., Snow D.M., Igarashi M., Beattie C.E., and Bixby J.L. (2014). Minimum information about a spinal cord injury experiment: a proposed reporting standard for spinal cord injury experiments. J. Neurotrauma 31, 1354–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]