Abstract
The hippocampus provided the gateway into much of what we have learned about stress and brain structural and functional plasticity, and this initial focus has expanded to other interconnected brain regions, such as the amygdala and prefrontal cortex. Starting with the discovery of adrenal steroid, and later, estrogen receptors in the hippocampal formation, and subsequent discovery of dendritic and spine synapse remodeling and neurogenesis in the dentate gyrus, mechanistic studies have revealed both genomic and rapid non-genomic actions of circulating steroid hormones in the brain. Many of these actions occur epigenetically and result in ever-changing patterns of gene expression, in which there are important sex differences that need further exploration. Moreover, glucocorticoid and estrogen actions occur synergistically with an increasing number of cellular mediators that help determine the qualitative nature of the response. The hippocampus has also been a gateway to understanding lasting epigenetic effects of early-life experiences. These findings in animal models have resulted in translation to the human brain and have helped change thinking about the nature of brain malfunction in psychiatric disorders and during aging, as well as the mechanisms of the effects of early-life adversity on the brain and the body.
INTRODUCTION
The field of neuroendocrinology began with the fundamental discovery of the communication between hypothalamus and pituitary by Harris (1970) and this established the basis for understanding brain–body communication via the neuroendocrine system. The hypothalamus and pituitary gland became the focus and led to the discovery of releasing factors in the hypothalamus for pituitary hormones (eg, see Guillemin, 1978; Schally et al, 1973; Vale et al, 1981) as well as the feedback of hormones on the hypothalamus and pituitary for the purpose of regulating hormone secretion (Meites, 1992). During the same time period, steroid hormones were shown to bind to intracellular receptors that regulate gene expression in tissues such as liver, and the prostate and uterus in the case of sex hormones (Jensen and Jacobson, 1962) and tritium-labeled steroid hormones were used as probes to detect these receptors using cell fractionation (Toft and Gorski, 1966) as well as steroid autoradiography (Pfaff and Keiner, 1973; Stumpf, 1971).
The McEwen laboratory entered this field using tritium-labeled steroids by serendipitously discovering adrenal steroid, and, later, estrogen receptors, in the hippocampal formation of the rat using both steroid autoradiography, as well as cell fractionation methods, and immunocytochemical methods at the electron microscopic and light microscopic levels (Gerlach and McEwen, 1972; Loy et al, 1988; McEwen and Plapinger, 1970; McEwen et al, 1968; Milner et al, 2001; Zigmond and McEwen, 1970). We and others extended these findings to the infrahuman primate brain, as well as to other regions of the brain involved in cognitive and emotional regulation (Gerlach et al, 1976). These findings have catalyzed studies that look at actions of hormonal feedback on the brain, not only to regulate hypothalamic functions, but also to influence neurological, cognitive, and emotional functions throughout the entire brain, with translation to the human brain in relation to aging, mood disorders, and the impact of the social environment.
The hippocampus provided the gateway into much of what we have learned about stress and brain plasticity and the initial focus on hippocampus has expanded to other interconnected brain regions, such as the amygdala and prefrontal cortex (PFC). This article describes research in our and other laboratories on these three brain structures that have led to the discovery of structural remodeling of neurons in response to acute and chronic stressors and then led us and others to uncover both epigenetic and non-genomic mechanisms by which glucocorticoids and estrogens produce their effects, as well as sex differences in these outcomes.
HIPPOCAMPUS AS THE GATEWAY
Glucocorticoid (GR) and Mineralocorticoid (MR) Receptors and Functions
The discovery of stress hormone receptors acting in the hippocampus has been the gateway to the investigation of other brain regions as well as mechanisms of stress and adrenal steroid action. Work by Reul and DeKloet (1985) demonstrated that there are two types of adrenal steroid receptors, MR (Type 1) and GR (Type 2), in the hippocampus and other brain regions. This was further elaborated by immunocytochemical mapping of the receptors (Ahima et al, 1991; Ahima and Harlan, 1990).
Studies in our laboratory and by Diamond et al (1992) and Joels (2006) have shown biphasic effects mediated by MR and GR on long-term potentiation and long-term depression (Pavlides et al, 1995). The biphasic effects on excitability are reflected in memory, such that a biphasic corticosterone dose response is seen on object recognition memory (Okuda et al, 2004). These effects depend on the state of behavioral arousal in a novel environment, which also implies a synergistic role of adrenaline and the fact that the hippocampus and amygdala work together (Roozendaal et al, 1996). The GR is involved in hippocampal-dependent spatial memory via a genomic mechanism as shown in the dimerization-deficient GR mouse, where GR cannot dimerize to bind to glucocorticoid response elements (Oitzl et al, 1997). GRs are also involved in contextual fear memory, mediated by hippocampus and amygdala, based upon the finding that Ru486 is able to block it (Pugh et al, 1997).
Ultradian fluctuations of glucocorticoids drive GR activation and reactivation, whereas MR occupancy for nuclear activation is more constant and promotes excitability (Stavreva et al, 2009) and this has implications for genomic and non-genomic activity of adrenal steroids, as will be discussed below.
Neural Structural Remodeling
Our finding in hippocampus of chronic stress-induced shrinkage of dendrites of hippocampal CA3 and dentate gyrus neurons as well as loss of spines in CA1 neurons (see (McEwen, 1999) revealed aspects of brain structural plasticity (Figure 1). The rediscovery of neurogenesis in the adult as well as developing dentate gyrus of the hippocampal formation (Cameron and Gould, 1994; Gould et al, 1992), which had been strongly suggested (Altman and Das, 1965), but largely ignored (Kaplan, 2001) for a number of decades, helped to establish the concept that the adult brain could show remodeling of neuronal architecture not only after stress but also in other conditions.
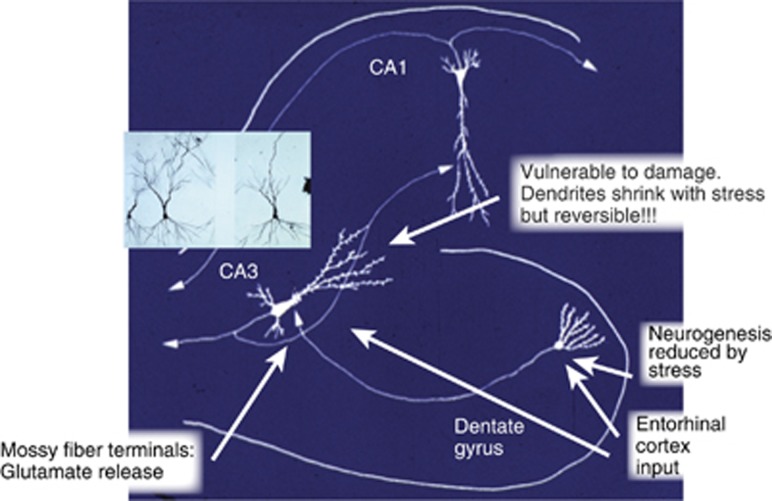
Figure 1.
The trisynaptic organization of the hippocampus showing input from the entorhinal cortex to both CA3 and dentate gyrus (DG), with feed forward and feedback connections between these two regions that promotes memory formation in space and time, but at the same time, makes the CA3 vulnerable to seizure-induced excitation (McEwen, 1999). Chronic stress causes apical dendrites of CA3 neurons to debranch and shorten in a reversible manner, and glutamate release by giant mossy fiber terminals is a driving force. Chronic stress also inhibits neurogenesis in DG and can eventually reduce DG neuron number and DG volume (see text).
Moreover, for both chronic stress effects and the ability of estrogens to induce spine synapse formation, it became apparent that hormones did not work alone but involve other mediators, particularly excitatory amino acids (EAAs) and their receptors (Cameron et al, 1998; Daniel and Dohanich, 2001; Gazzaley et al, 1996; Magarinos and McEwen, 1995; Woolley and McEwen, 1994). For example, for the chronic stress-induced shrinkage of apical dendrites of hippocampal CA3 neurons, the giant mossy fiber terminals (MFTs) have a key role and are a bellwether of the effects of repeated stress (Magarinos et al, 1997; Figure 2). Specifically, MFTs from control rats are fully packed with vesicles containing glutamate, whereas after chronic restraint stress, they become depleted of vesicles, but the remaining vesicles are found at the multiple active synaptic zones in these giant presynaptic terminals, along with increased mitochondria in the stressed MFTs. This suggests not an exhausted but a new steady state of increased activity (Magarinos et al, 1997).
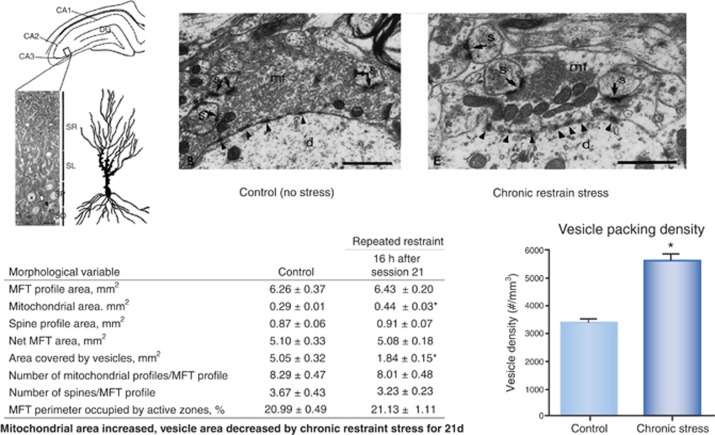
Figure 2.
Giant mossy fiber terminals (MFTs) of DG neurons that synapse in stratum lucidum of CA3 have multiple active sites of glutamate release on the thorny excrescences that penetrate the MFTs. Normally fully packed with synaptic vesicles, the MFTs show depletion of vesicles after 3 weeks of chronic restraint stress (CRS), with the remaining vesicles being near active synaptic zones. As shown at the right, vesicle area is reduced by CRS but packing density of the remaining vesicles is increased and the area occupied by mitochondria is also increased. This suggests that the chronically stressed MFT are not exhausted but rather very active after chronic stress (Magarinos et al, 1997). Interestingly, by microdialysis, glutamate release in hippocampus caused by restraint stress is abolished by adrenalectomy, indicating involvement of adrenal secretions (Lowy et al, 1993). *P<0.001, two-tailed unpaired Student's t-test.
The CA3 region is not the only region of the hippocampus to show dendritic reorganization with chronic stress. Apical dendrites of rat CA1 neurons were reported to be shorter in adulthood after chronic neonatal bedding stress (Brunson et al, 2005), and a comparison of stressors revealed differences within the hippocampus and its functional connectivity to other brain regions (Maras et al, 2014). Comparison of a multimodal stress paradigm (concurrent, hours-long light, loud noise, jostling, and restraint) with restraint or loud noise alone revealed severe deficits in hippocampal-dependent object recognition memory after multimodal stress, but fewer deficits after restraint or loud noise alone. These differences in memory were not explained by differences in plasma corticosterone levels or numbers of Fos-labeled neurons in stress-sensitive hypothalamic neurons. Measures of spine density under these different conditions revealed that synapses in hippocampal CA3 were reduced by both restraint and multimodal stress, whereas multimodal stress alone reduced synapse numbers severely in dorsal CA1. Ventral CA1 synapses were not significantly affected by any of these stressors. Using c-Fos as a marker of neuronal activity, multimodal stress reduced hippocampal connectivity with septum and thalamus compared with restraint and increased connectivity with amygdala and BST more so than restraint stress (Maras et al, 2014).
In C57Bl6 mice, 10 days of chronic immobilization stress (CIS) induced dendritic retraction of CA3 short-shaft pyramidal neurons, but not CA3 long-shaft pyramidal neurons, along with a robust retraction of dendrites in dorsal CA1 pyramidal neurons (Christian et al, 2011). In mice specifically lacking NMDA receptors in CA3 neurons, chronic stress-induced dendritic retraction was not evident in any of the neurons in either CA3 or CA1 and this prevention of dendritic retraction in the mutant mice had a minimal effect on HPA axis activation and behavioral alterations that were induced by chronic stress (Christian et al, 2011). In the hippocampus of hibernating animals, rapid shrinkage of CA3 apical dendrites is seen with onset of hibernation, whereas regrowth of those dendrites occurs within hours of termination of hibernation, suggesting that the cytoskeleton can rapidly depolymerize and repolymerize when needed via a mechanism in which phosphorylation of a soluble form of tau is involved as a factor involved in cytoskeletal dissociation (Arendt et al, 2003; Magarinos et al, 2006; Figure 3).
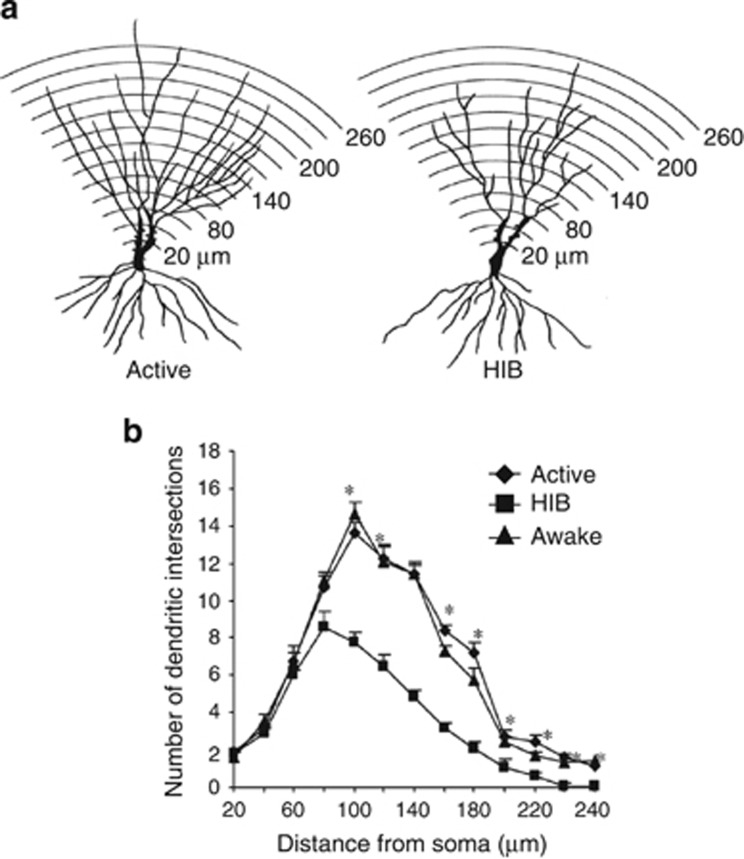
Figure 3.
Effect of deep hibernation and induced awakening on the apical dendritic branching density of CA3 pyramidal neurons. (a) Camera lucida drawings of representative CA3 pyramidal neurons from active and hibernating European hamsters. An overlay of concentric rings centered at the cell body was used for Sholl analysis. (b) One-way ANOVA followed by Tukey post hoc test revealed statistical differences among experimental groups between 100 and 180 μm from the soma (hibernation (HIB) vs active and awaken groups, P<0.001) and also between 200 and 260 μm from the soma (HIB vs active and awaken groups (P<0.005). Taken from reference Magarinos et al (2006).
Besides glucocorticoids and EAA receptors, there is a growing list of mediators implicated in the stress-induced dendritic remodeling (Table 1). These include trophic factors such as brain-derived neurotrophic factor (BDNF) and the secreted signaling molecule and protease tissue plasminogen activator (tPA), another signaling molecule, lipocalin 2, corticotrophin-releasing factor (CRF), and endocannabinoids. Besides these signaling mediators, cell surface molecules have an important role. Expression of the polysialylated form of neural cell adhesion molecule in the hippocampal formation is increased by stress, whereas PSA removal by Endo-neuraminidase-N (Endo-N) is known to cause the mossy fibers to defasciculate and synapse ectopically in their CA3 target area. Enzymatic removal of PSA by Endo-N produced a remarkable expansion of dendritic arbors of CA3 pyramidal neurons, with a lesser effect in CA1. This expansion eclipsed the CIS-induced shortening of CA3 dendrites, with the expanded dendrites of both no-stress-Endo-N and CIS-Endo-N rats being longer than those in no-stress-control rats and much longer than those in CIS-control rats. As predicted by the hypothesis that Endo-N-induced dendritic expansion might increase vulnerability to excitotoxic challenge, systemic injection with kainic acid, showed markedly increased neuronal degeneration, as assessed by fluorojade B histochemistry, in rats that had been treated with Endo-N compared with vehicle-treated rats throughout the entire hippocampal formation. PSA removal also exacerbated the CIS-induced reduction in body weight and abolished effects of CIS on neuropeptide Y and NR2B mRNA levels (McCall et al, 2013; Figure 4).
Table 1. Molecules that are Necessary/Permissive for Remodeling.
| BDNF: brain-derived neurotrophic factor |
| Facilitator of plasticity or growth |
| BDNF overexpression—occludes effects of chronic stress |
| BDNF haploinsufficiency prevents stress-induced plasticity |
| tPA: tissue plasminogen activator |
| Secreted signaling molecule and protease |
| Required for stress-induced spine loss in hippocampus and medial amygdala |
| Required for acute stress-induced increase in anxiety; CRF activates tPA secretion |
| CRF in amygdala regulates tPA release |
| CRF: corticotrophin-releasing factor |
| Secreted in hippocampus by interneurons |
| Downregulates thin spines via RhoA signaling |
| Lipocalin-2: secreted protein; previously unknown function |
| Acute stress induces Lipocalin-2 |
| Lipocalin-2 downregulates mushroom spines |
| Lipocalin-2 KO increases neuronal excitability and anxiety |
| Endocannabinoids |
| Induced via glucocorticoids |
| Regulate emotionality and HPA habituation and shut off |
| CB1 receptor KO increases anxiety and basolateral amygdala dendrite length and causes stress-like retraction of prefrontal cortical dendrites |
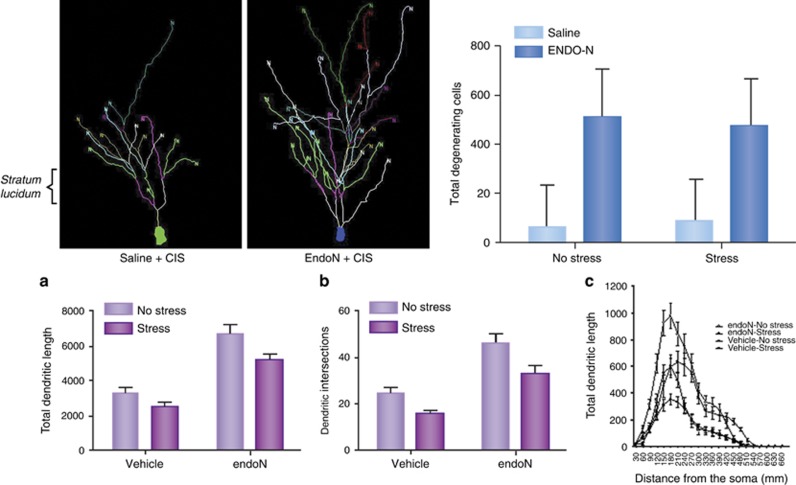
Figure 4.
Polysialylate removal from NCAM neural cell adhesion molecule antagonized the effect of chronic immobilization stress (CIS) to shorten dendritic branches of CA3 neurons. Top: (left) Representative camera lucida traces from vehicle and endo-N-treated rats that were exposed to 10 days of CIS. The dendritic arbors were larger in endo-N-treated animals. (right) Excitotoxic challenge causes greater neuron loss in endo-N treated hippocampus compared to saline control. Bottom: (a) CIS shortened the total length of CA3 dendritic arbor, whereas PSA removal produced an increase in these measures. With combination of CIS and endo-N, the total arbor length was shorter than with endo-N alone but still larger than control values. (b) Interestingly, the effects on numbers of branching points followed a similar pattern. (c) Sholl analysis revealed that PSA removal alone produced an elongation of branches located at all distances from the soma (from 0 to 550 μm approximately), whereas CIS alone specifically shortened branches located near the soma (approximately between 100 and 250 μm). Interestingly, PSA removal antagonized this CIS-induced dendrite atrophy. Data are presented as means (±SEM) N=9–10/group. Taken from reference McCall et al (2013).
Much of the structural remodeling described above is mediated by gene expression changes that results from changes in DNA transcription (genomic effects), principally through binding of activated GRs to glucocorticoid response elements in DNA. However, stress-induced plasticity can also be mediated through non-genomic mechanisms, as described below.
Non-Genomic Effects
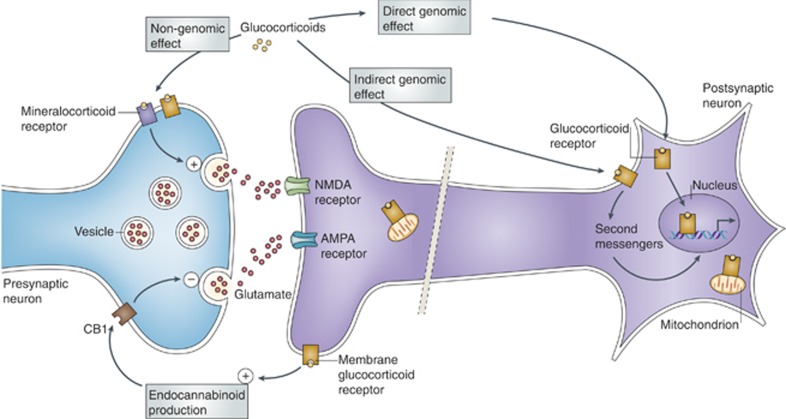
Immunocytochemistry at the electron microscopic level revealed specific immunolabeling for epitopes of glucocorticoid and estrogen receptors near the cell surface, in the mitochondria, dendrites, and presynaptic terminals and post-synaptic densities (Johnson et al, 2005; Liposits and Bohn, 1993; McEwen and Milner, 2007; Milner et al, 2001). Application of steroid autoradiography at the electron microscopic level revealed the putative estrogen receptor sites as having the ability to bind radioactive iodinated estradiol (Milner et al, 2008). Along with evidence that steroid receptors worked via non-genomic, rapid signaling mechanisms along with their epigenetic actions to regulate gene expression (Kelly and Levin, 2001). This led to a new view of estrogen and glucocorticoid action via both direct and indirect genomic stimulation as well as a variety of other rapid signaling mechanisms (Popoli et al, 2012; see Figure 5).
Figure 5.
Basal release of glucocorticoids varies in a diurnal pattern, and release increases several fold after exposure to a stressor. Glucocorticoids can bind with different affinities to glucocorticoid and mineralocorticoid receptors, which are expressed throughout the brain and seem to exist in both membrane-bound form and nuclear form. Adrenal steroids can have both rapid and delayed effects. The effects can result from non-genomic mechanisms (mediated by membrane-associated receptors, see the figure; Karst et al, 2005; Kelly and Levin, 2001), indirect genomic mechanisms (mediated by membrane receptors and second messengers) and genomic mechanisms (mediated by cytoplasmic receptors that move to the nucleus and act as transcription factors; Yamamoto, 1985). Although classical mineralocorticoid and glucocorticoid receptors seem to mediate many of these effects, other membrane-associated receptors, including G-protein-coupled receptors, may also be involved in some of these actions (Orchinik et al, 1992; Tasker et al, 2006). In addition, activated glucocorticoid receptors can translocate to mitochondria and enhance their calcium buffering capacity (Du et al, 2009). Glucocorticoids rapidly induce glutamate release in the hippocampus through a mechanism that is absent when the mineralocorticoid receptor is deleted and that may involve a membrane-associated form of the mineralocorticoid receptor (Karst et al, 2005; Lowy et al, 1993). An indirect way by which glucocorticoids can influence neurotransmission (glutamatergic, as well as GABAergic, cholinergic, noradrenergic, and serotonergic) is through crosstalk with the endocannabinoid system (Katona and Freund, 2008). They rapidly stimulate endocannabinoid production in the brain, whereupon endocannabinoids bind to cannabinoid receptor 1 (CB1) and transient receptor potential cation channel subfamily V member 1 (TRPV1), and inhibit neurotransmitter release (Chavez et al, 2010; Hill and McEwen, 2010). Although a G-protein-coupled receptor is implicated in endocannabinoid production (Di et al, 2009), there is also evidence for a mechanism blocked by Ru486—a selective antagonist of the classical cytoplasmic glucocorticoid receptor—in the rapid actions of glucocorticoids in prefrontal cortex (Hill and McEwen, 2010). Reprinted from reference Popoli et al, 2012 with permission.
A powerful example of non-genomic functions of steroid receptors was the finding that the knockout of the MR abolished the ability of corticosterone to rapidly stimulate EAA release, implying a dual role for MR in both genomic and rapid non-genomic signaling (Karst et al, 2005). Again, this finding began with studies on hippocampus and it helped explain the adrenal dependency of acute restraint stress (ARS)-induced increases in extracellular glutamate in hippocampus (Lowy et al, 1993), which are likely to underlie the ability of chronic restraint stress to cause dendritic remodeling in hippocampus (McEwen, 1999).
Hippocampal involvement in mood disorders and age-related memory loss
The hippocampus has long been implicated in learning and memory; however, it also has an important role in the regulation of mood. In addition to the structural remodeling described earlier, the dentate gyrus is a region that exhibits adult neurogenesis, which is regulated by adrenal steroid levels (Cameron and Gould, 1994; Gould et al, 1992). Subsequent findings have shown that antidepressants increase neurogenesis, and this, in turn, provided a novel mechanism for their action that brought in the hippocampus as a brain structure involved in mood disorders (Duman et al, 2001). Indeed, the dentate gyrus undergoes reduced cell number under chronic stress (Pham et al, 2003) and in response to corticosterone levels (Sousa et al, 1999), whereas physical activity and an enriched environment increase dentate gyrus volume and neuron number (Kempermann et al, 1997; van Praag et al, 1999). Hippocampal neurogenesis has also been linked to BDNF levels, which are highly dynamic in response to chronic stress, where initial decreases have been observed (Smith et al, 1995), but with recovery after stress can return to baseline (Lakshminarasimhan and Chattarji, 2012). And direct infusion of BDNF has been shown to increase hippocampal neurogenesis (Scharfman et al, 2005).
Subsequent work has identified the ventral hippocampus as the major target of these effects (Jayatissa et al, 2006; Sahay and Hen, 2007). Yet, the dentate gyrus and neurogenesis are not the sole explanation for depressive behavior and its treatment by antidepressants because there are behavioral endpoints of antidepressant action that occur when the possibility of neurogenesis is suppressed, eg, by X-irradiation (David et al, 2009); rather, antidepressant actions upon only certain aspects of depressive-like behavior require neurogenesis, whereas other actions involve dendrite remodeling and synapse turnover (Bessa et al, 2008). This fits with human autopsy data on brains from depressed individuals that show no neuronal loss in hippocampus but reduced cell nuclear size of hippocampal pyramidal neurons and reduced glial cell number; glial cell reduction fits with loss of dendritic length and branching (Stockmeier et al, 2004).
The hippocampus is affected by aging. Memory impairment is seen in rats as they age and there is a link between rising glucocorticoid levels, EAAs, and memory impairment (Landfield et al, 1978; Sapolsky et al, 1986). Pharmacological treatment with drugs that affects EAA actions has been shown in several cases to retard the memory loss, using hippocampal-dependent spatial memory as a measure. One study found beneficial effects of a positive allosteric modulator of AMPA receptors (Ampakine) that selectively enhance fast excitatory neurotransmission in the brain and increase overall neuronal excitability and exert some neuroprotective activity (Bloss et al, 2008). Treatment of aging rats by oral administration of the Ampakine S18986 (Servier, France) from 14 to 18 months of age increased locomotor activity and improved performance in a spatial memory task involving the hippocampus. In addition, chronic S18986 treatment retarded the decline of forebrain cholinergic neurons and midbrain dopaminergic neurons by ~40% and attenuated the age-related increase in the expression of a microglial marker indicative of neural inflammation in the hippocampus (Bloss et al, 2008).
In another study involving modulation of excitatory amino-acid actions (Pereira et al, 2014), riluzole was used because it increases glutamate uptake through glial transporters and is thought to decrease glutamate spillover to extrasynaptic NMDA receptors while increasing synaptic glutamatergic activity. Aging rats treated between 10 and 14 months of age were protected against the age-related cognitive decline displayed in non-treated aged animals. Memory performance on tasks involving hippocampal spatial and episodic memory correlated with density of thin spines on apical dendrites in the CA1 region, although not with mushroom spines. Furthermore, riluzole-treated rats had an increase in clustering of thin spines that correlated with memory performance and was specific to the apical, but not the basilar, dendrites of CA1. Clustering of synaptic inputs is thought to allow nonlinear summation of synaptic strength (Pereira et al, 2014).
Hippocampal GRs and Effects of Early-Life Experiences
Another ‘gateway' function of research on the hippocampus was to focus attention on the neural effects of early-life experiences. Meaney and colleagues showed that the effects of ‘neonatal handling' on emotionality (Levine et al, 1967) were due to maternal care after the pups were returned to the nest after a brief separation from the dam (Francis, 1999; Meaney et al, 1988). Rats that received poor maternal care showed deficits in GRs in the hippocampus (Liu et al, 1997) and impaired shut off of the HPA stress response that may result, at least in part, from those deficits (Caldji et al, 2000). Thyroid hormone and serotonin were shown to be involved in maintaining adequate hippocampal GR levels (Meaney et al, 2000), but the methylation of CpG DNA elements in the promotor region of the GR was found to decrease GR expression and, indeed, it was found to be increased in pups receiving poor maternal care (Meaney and Szyf, 2005). Poor HPA shut off in rats that received poor maternal care could be reinstated in adult rats that had received good maternal care as infants by inducing the methylation of adult GR CpG elements in adult animals (Weaver et al, 2005). These seminal findings have catalyzed a huge field of research in the growing field called ‘epigenetics' that now includes translational studies such as the one showing increased GR promotor CpG methylation in individuals who were abused as children (McGowan et al, 2009).
Glucocorticoids and HPA reactivity are also indicators of the quality of maternal care and the response of the offspring to that care. Building upon the importance of maternal care, Tang et al (2014) have developed the ‘maternal modulation' concept in which consistency of maternal care and not absolute quantity are important factors, along with exposure to novelty, leading to better social and cognitive development; one measure of the efficacy of good maternal care is maternal stress self-regulation, referring to low basal CORT and a robust increased CORT secretion in response to a stressor.
Epigenetics, Stress, and Mood-Related Behaviors: Search for Rapidly Acting Treatments
After its original definition, as the emergence of characteristics of an organism during development that were not evident at earlier stages (Waddington, 1942), ‘epigenetics' now refers to events ‘above the genome' that regulate expression of genetic information without altering the DNA sequence. Besides the CpG methylation described above, other mechanisms include histone modifications that repress or activate chromatin unfolding (Allfrey, 1970) and the actions of non-coding RNAs (Mehler, 2008). Again the hippocampus is providing important information. For example, Reul and colleagues have shown that the forced swimming-induced behavioral immobility response requires histone H3 phospho-acetylation and c-Fos induction in distinct dentate granule neurons through recruitment of the NMDA/ERK/MSK 1/2 pathway (Chandramohan et al, 2008).
Another histone mark changed in hippocampus, most prominently in the dentate gyrus, is the dramatic induction by an ARS of trimethylation of lysine 9 on histone H3, which is associated with repression of a number of retrotransposon elements and reduction of the coding and non-coding RNA normally produced by the repressed DNA (Hunter et al, 2009). This repression is lost with repeated stress, suggesting the possibility that those retrotransposon elements may impair genomic stability under conditions of chronic stress (Hunter et al, 2015).
A current practical application is the search for rapidly acting antidepressants, because classical antidepressants work very slowly and are not effective on every depressed individual. Epigenetic processes are likely involved in the chronic relapsing nature of major depression, the strikingly higher incidence of depression in women after puberty, the high discordance rates between monozygotic twins, and the individual responsivity to stress that precipitates mood-related behaviors in susceptible individuals. In the course of these studies, we are learning more about epigenetic mechanisms that connect EAA function with neural remodeling and stress-related behavior. The identification of the fast antidepressant effects of ketamine, an NMDA receptor blocker, has resulted in a paradigm shift toward the discovery of a new generation of rapidly acting antidepressants (Li et al, 2010).
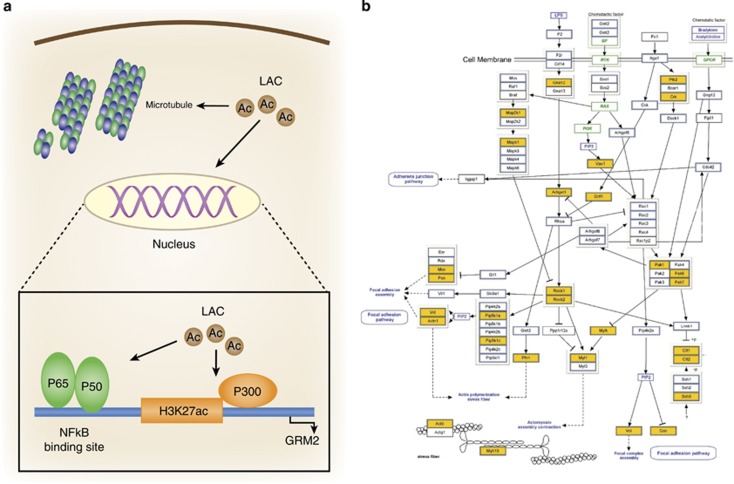
Recently, our laboratory and other groups have found that the naturally occurring compound acetyl-L-carnitine (LAC) shows fast antidepressant efficacy in genetic and environmentally induced animal models of depression through the epigenetic modulation of the metabotropic glutamate receptor, mGlu2, in hippocampus (Cuccurazzu et al, 2013; Nasca et al, 2013). mGlu2 is known to exert an inhibitory tone on glutamate release from synapses and pharmacological modulators for this receptor are under clinical development to treat stress-related mood disorders, such as anxiety and depression (Nicoletti et al., 2015). Using the same animal models, 14 days of treatment with the tricyclic antidepressant clomipramine were needed to promote antidepressant responses, which disappeared when the treatment was stopped. In contrast, LAC antidepressant effects were still evident after 2 weeks of drug withdrawal (Nasca et al, 2013). The persistent effects of LAC suggested the involvement of stable molecular adaptations that may be reflected at the level of histone modifications in controlling mGlu2 transcription in the hippocampus. Indeed, LAC increases levels of mGlu2 receptors by acetylation of the histone H3K27 among other mechanisms (discussed below).
These findings support previous studies that have shown that histone deacetylase inhibitors, given intraperitoneally, normalize gene expression profiles in vulnerable brain regions, such as hippocampus, amygdala, and nucleus accumbens, to promote fast antidepressant responses following stress (Covington et al, 2011; Tsankova et al, 2006). The use of agents like LAC that act on histone remodeling to regulate transcription of the mGlu2 gene offers alternative and complementary strategies to ketamine and histone deacetylase inhibitors with safer profiles and lower potential for drug dependence (Nicoletti et al, 2015).
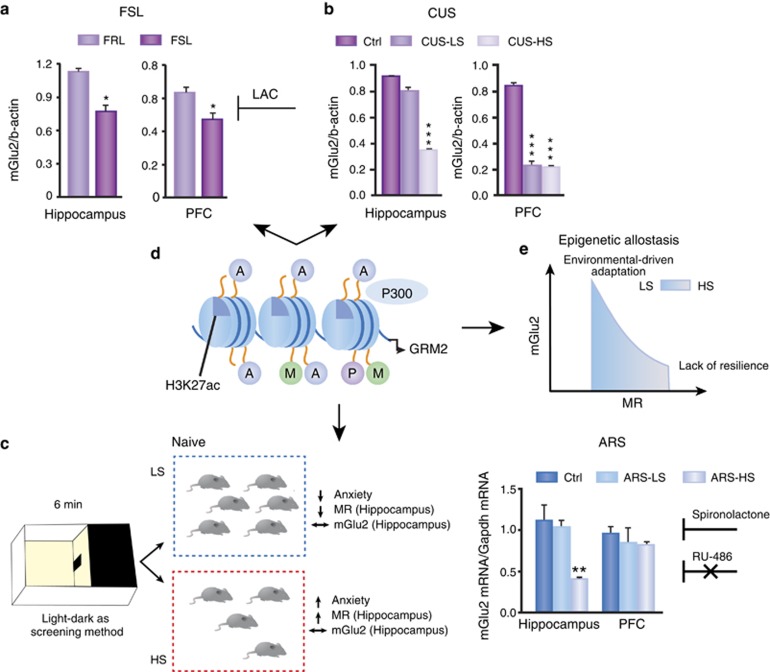
In the course of this work, we have become aware of individual differences among inbred mice and rats (Cavigelli and McClintock, 2003; Freund et al, 2013; Miller et al, 2012). Using a simple light–dark test to rapidly screen naïve mice (Nasca et al, 2014), we found that a subset of mice shows elevated hippocampal MR levels and that this baseline difference makes those mice with higher MR show greater stress-induced reduction in mGlu2 accompanied by more anxiety and depressive-like behaviors. How MR activation does this is not yet clear, but it activates a mechanism that is opposite to that of LAC, which has been shown to use the acetyltransferase P300 to acetylate lysine 27 on histone H3 (Nasca et al, 2013). Likewise, the nature of the experiences of the animals that develop higher MR is also not yet known but may involve maternal care and stressors in the neonatal nesting environment (Francis et al, 1999). The epigenetic allostasis model points to a developmental origin of individual differences in the responses to stress and implies that unknown early-life epigenetic influences program each individual to different trajectories of behavioral and physiological responses to later stressful life events. In line with this model, previous studies have also associated increased hippocampal MR levels in juvenile animals with anxiety-like behavior in adulthood (Brydges et al, 2014; Korte et al, 1995; Figure 6).
Figure 6.
Lower mGlu2 in hippocampus is a biomarker of anxious and depressive-like behaviors and response to rapidly acting antidepressants. (a) mGlu2 receptor expression is reduced in the hippocampus and prefrontal cortex of depressed Flinders Sensitive Line (FSL) rats compared with their controls (Flinders Resistant Line, FRL). These changes are rapidly corrected by acetyl-L-carnitine (LAC), whose effects endure for 2 weeks after drug withdrawal. *P<0.05, two-tailed unpaired Student's t-test. (b) Chronic unpredictable stress (CUS) in susceptible individuals results in depressive-like behaviors that are rapidly corrected by LAC (Nasca et al, 2013). Interestingly, only susceptible individuals show reduced mGlu2 protein levels within the hippocampus. ***P<0.001, one-way analysis of variances followed by Tukey's test for the post hoc analysis. (c) The recently introduced screening method using a light–dark chamber allows identification of high (HS) and low (LS) susceptible individuals, which are characterized by baseline differences in anxiety and in the levels of mineralocorticoid receptors (MRs), but not mGlu2 in the hippocampus. When stressed, HS mice show decreased mGlu2 levels in hippocampus and exacerbation of the baseline anxiety-like behavior compared with LS mice, which cope better with stress. These changes are prevented by a single injection of spironolactone (a MR antagonist), but not RU486 (a GR antagonist). **P<0.01, one-way analysis of variances followed by Tukey's test for the post hoc analysis. (d) Acetylation of histone H3K27, which is regulated by P300, is a key mediator of mGlu2 regulation in response to stress and antidepressant treatment. (e) The MR-driven downregulation of mGlu2 expression is summarized in the epigenetic allostasis model, which suggests that individual differences in stress responsivity may originate from unknown epigenetic influences early in life (Nasca et al, 2014).
Lessons of an Ever-Changing Brain from Gene Expression
The hippocampus has been an important gateway to understanding the effects of glucocorticoids and stress on gene expression. Recent advances in technology have allowed for high-throughput analysis of gene expression changes in response to stress (Rubin et al, 2014). For example, microarray analysis of whole hippocampus after acute and chronic stress, as well as recovery from stress in mice, has revealed a number of insights surrounding stress-induced neuroplasticity (Gray et al, 2014). Although acute and chronic stress modulate a core set of genes, there are numerous expression changes that are exclusive to each condition, highlighting how the duration and intensity of stress alters reactivity. Further, corticosterone injections did not yield the same expression profile as acute stress, suggesting that in vivo stressors are able to activate a diverse set of pathways independent of GR activation. Finally, characterization of expression profiles after an extended recovery from chronic stress (21 days) revealed that, despite a normalization of anxiety-related behaviors, recovery did not represent a return to the stress-naïve baseline, but rather represented a new state in which reactivity to a novel stressor produced a unique expression profile (Gray et al, 2014). Studies in rats have also confirmed that gene expression profiles can vary significantly from the immediate end of stress (1 h) to 24 h after the end of stress (Wang et al, 2010), and that chronic stress can alter the transcriptional response to an acute corticosterone injection in dentate gyrus (Datson et al, 2013). These studies demonstrate that a history of stress exposure can have a lasting impact on future stress reactivity and hippocampal function. Many of the genes identified as changed after chronic stress by Datson and DeKloet are known epigenetic regulators, providing one possible mechanism underlying the persistent alterations in the expression response beyond the end of stress exposure.
Identification of New Gene Candidates
One of the benefits of gene expression studies is the discovery of new gene markers and pathways that contribute to important functions. A recent example is the unexpected role of a cell nuclear pore complex protein, NUP-62, in the stress-induced dendritic remodeling in the CA3 region of hippocampus (Kinoshita et al, 2014). First identified as a gene that was downregulated in the PFC of depressed patients (Tochigi et al, 2008), it was also found to be reduced in response to chronic stress in CA3 neurons of rodents (Kinoshita et al, 2014). Importantly, the levels of other nuclear pore complex genes were unchanged with chronic stress, supporting the specificity of its role in stress remodeling. Subsequent in vitro studies confirmed that the downregulation of NUP-62 is associated with dendritic retraction and this effect is regulated at the molecular level by NUP-62 phosphorylation at a PYK2 site, which results in its retention in the cytoplasm (Kinoshita et al, 2014).
Pathway analysis of high-throughput gene expression data sets allows for a fresh look at known pathways implicated in the stress response. For example, inflammation has been associated with chronic stress and recent microarray data from whole mouse hippocampus have helped to confirm that NF-κB/TNF-α and IL-6 signaling are in fact some of the most affected pathways (Gray et al, 2014). Acetylation of p65, a transcription factor in NF-κB signaling, has also been associated with the rapid antidepressant effects of LAC (Nasca et al, 2013; Wang et al, 2014), which supports a growing literature that underscores an important role for the inflammatory response in the pathophysiology and treatment of depression (Dantzer et al, 2008). Similarly, LAC is a nonspecific acetylating agent and its rapid antidepressant effects may be mediated by acetylation of other proteins outside the nucleus (Russo and Charney, 2013). This raises the possibility that regulation of cytoskeleton genes is presumably necessary for promoting rapid antidepressant effects and for the dendritic remodeling of neurons to occur (Figure 7). High-throughput data sets that can measure the expression levels of the entire transcriptome now allow for the visualization of genes that are changed from the cell surface receptors to the cytoskeleton itself (Figure 7). The data reveal changes in both predictable regulators, such as ROCK1/2, and other signaling intermediates that might not have been anticipated.
Figure 7.
Potential cellular mechanisms for rapid antidepressant action. (a) Acetyl-L-carnitine (LAC) may act inside and outside the nucleus to promote rapid antidepressant responses. Inside the nucleus, LAC increases mGlu2 transcription by enhancing acetylation of the NF-κB and histone H3K27 bound to mGlu2 promoter gene. Outside the nucleus, LAC may control stability of the neuronal cytoskeleton to regulate dendritic remodeling (Nasca et al, 2013). (b) Graphical representation of known intracellular signaling pathways involved in the regulation of the actin cytoskeleton (adapted from WikiPathways) in which genes altered by acute stress, chronic stress, or after recovery from stress are highlighted (yellow) based on microarray data derived from Gray et al. (2014).
Translation to the Human Hippocampus
The hippocampus has also been a gateway to translating animal model findings in order to begin to elucidate the effects of stress and stress-related disorders on the human brain, starting with the hippocampus (McEwen, 2007; McEwen and Gianaros, 2011; McEwen and Morrison, 2013). These include changes in brain structure and functional activity in depression, PTSD, Cushing's disease, and Type 2 diabetes, as well as effects of jet lag and shift work, chronic life stress, perceived stress, and the beneficial effects of physical activity (McEwen and Gianaros, 2011; Sheline, 2003). Circadian disruption, as in shift work, causes dendritic atrophy and impairs cognitive flexibility while also promoting obesity and insulin and leptin resistance (Karatsoreos et al, 2011). Regarding physical activity, previously sedentary older adults who walk 1h a day for 6 months to 1 year show enlargement of the hippocampal formation (Erickson et al, 2011) and this is likely due, at least in part, to the increased dentate gyrus neurogenesis that is stimulated by exercise and by an enriched environment (Kempermann et al, 1997; van Praag et al, 1999). It is also noteworthy that hippocampal volume increases with intense learning (Draganski et al, 2006) but is also decreased in Cushing's disease (Starkman et al, 1992), and chronic jet lag without adequate time for recovery is associated with cognitive impairment, dysregulated cortisol secretion, and a smaller temporal lobe (Cho, 2001). In relation to aging, cortisol levels in aging humans predict memory impairment over 5 years and aged humans with significantly prolonged cortisol elevations showed reduced hippocampal volume and deficits in hippocampus-dependent memory tasks compared with normal cortisol controls. Moreover, the degree of hippocampal atrophy correlated strongly with both the degree of cortisol elevation over time and current basal cortisol levels (Lupien et al, 1998).
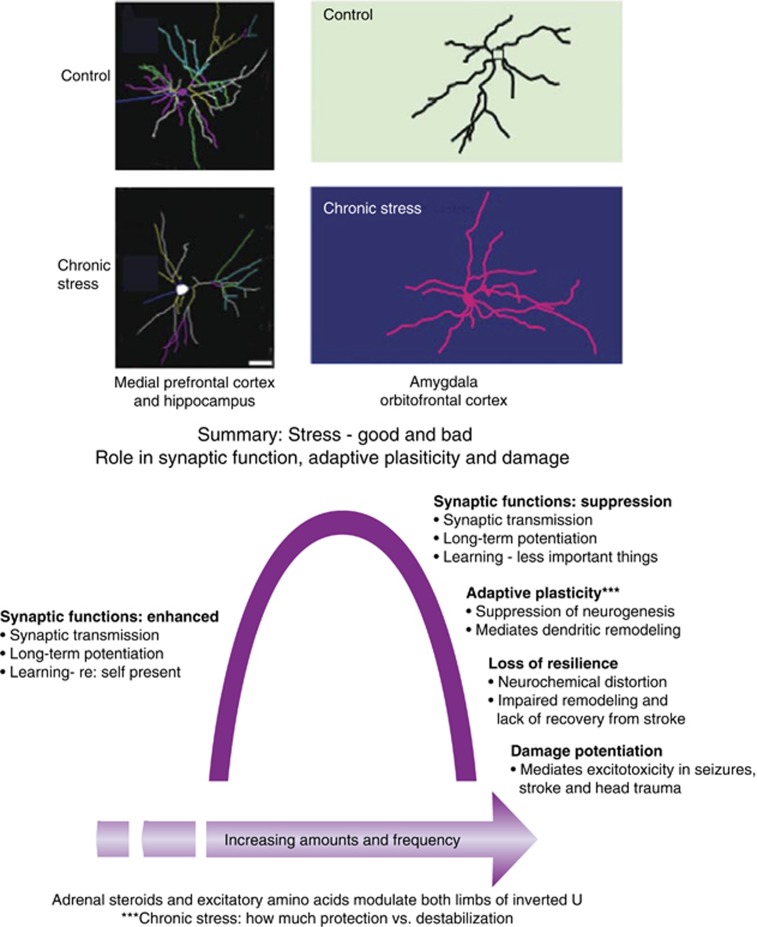
Taken together, information gained from across all subregions of the hippocampus has led to the inverted U shape dose–time response concept summarized in Figure 8. Acute stress, mediated by glucocorticoids and EAAs along with other mediators, increases excitability at moderate physiological levels but can have the opposite effect at higher levels of activity. Chronic stress produces the largely reversible, adaptive plasticity described above in which the retraction of dendrites and reduced synapse density may subserve a protective function against permanent damage, whereas sudden traumatic events such as head trauma, seizures, and ischemia do not give the hippocampus an opportunity to adapt and lead to permanent damage and neuron loss. However, when resilience is lacking after the stressor is over, cognitive impairment and anxiety or depression may persist and require external interventions. In all of this, it should be kept in mind that at each stage of experience, the brain is changing even if there is apparent recovery of morphological and neurochemical changes produced by stressors.
Figure 8.
Chronic stress causes remodeling of dendrites and synaptic connections in many brain regions, including not only hippocampus but also amygdala and medial prefrontal and orbitofrontal cortex (top panel). Effects of acute and chronic stress operate in space and time in an inverted U-shaped manner (bottom panel). Acute stress, mediated by glucocorticoids, and excitatory amino acids and other mediators (see Table 1), can enhance excitability and promote memory over minutes to hours as long as the stressor is not overly intense; intense stress can have the opposite effect. Chronic stress causes neuronal remodeling as depicted in top panel in a largely reversible manner, promoting adaptation (eg, increase vigilance and anxiety in a dangerous environment). Yet, if there is no reversal of the stress-induced changes in neuronal architecture, an outside intervention with pharmaceutical agents and behavioral therapies may be needed to correct the imbalance. Finally, seizures, ischemia, and head trauma can trigger uncontrolled activation of excitatory amino acids leading to free radicals and inflammatory tone potentiated by glucocorticoids.
STRUCTURAL REMODELING IN THE AMYGDALA
The hippocampus provided a gateway into the study of another important brain region involved in stress and stress-related disorders. In 2002, a landmark study showed that while CIS (more severe than restraint and hence effective in 10 vs 21 days) caused shortening of dendrites in the CA3 region of rats, the basolateral amygdala (BLA) responded by an expansion of dendrites (Vyas et al, 2002). At the same time, spine density was downregulated by CIS in medial amygdala, where it was dependent on one of the modulators in Table 1, namely, tPA, as shown in tPA knockout mice; dendrite expansion in the BLA was independent of tPA mediation (Bennur et al, 2007). Increased anxiety after acute stress was also shown to be dependent on tPA (Pawlak et al, 2003) where tPA release was found to be stimulated by CRF via CRF1 receptors (Matys et al, 2004). Interestingly, the ability of chronic stress to reduce spine density on hippocampal CA1 neurons was also dependent on tPA and tPA-KO mice also were not impaired by chronic stress in a spatial memory task in contrast to wild-type mice (Pawlak et al, 2005). Because CRF is also known to regulate spine density in the hippocampus, it is likely that it was activating the tPA release there (Regev and Baram, 2014). A recent study using targeted deletion of GR showed that tPA-BDNF-TrkB signaling altered fear conditioning (Revest et al, 2014)
A Paradoxical Role of BDNF in Acute and Chronic Stress
BDNF also has an important role in dendritic remodeling in both hippocampus and BLA. Overexpression of BDNF in mice increases dendritic length in both CA3 and BLA and occludes the effects of chronic stress to decrease dendritic branching in CA3 and increase it in BLA (Govindarajan et al, 2006). Without such overexpression, chronic stress causes a downregulation of BDNF in CA3 of hippocampus and an upregulation of BDNF in the BLA, and the effect in BLA persists after 21 days post stress, whereas that in CA3 has normalized (Lakshminarasimhan and Chattarji, 2012). Moreover, acute stress with a 10-day delay, which causes increased density of spines in BLA neurons (Mitra et al, 2005), caused BDNF expression to rise and stay elevated for 10 days, whereas levels in CA3 fell after acute stress but did so only transiently (Lakshminarasimhan and Chattarji, 2012). Corticosterone levels increased after both acute and chronic stress and remained elevated after chronic, but not after acute stress. Importantly, Lakshmirnarasimhan and Chattarji (2012) point out how elevated glucocorticoids and glutamate after stress lead to contrasting patterns of BDNF expression and structural plasticity, suggesting there may be a signaling intermediate that could regulate the differential region-specific response. However, the mechanisms regulating this (possibly epigenetic or post-translational) remain to be identified.
Interestingly, beyond the epigenetic action on the acetylated H3K27 to correct glutamate activity in hippocampus, LAC also increases BDNF brain and serum levels to promote antidepressant responses, supporting the existence of an intermediate key mediator between the glutamate system and BDNF signaling in the etiology and treatment of stress-related mood disorders (Nasca et al, 2013).
Traumatic Stress Effects and Paradoxical Actions of Glucocorticoids
Another paradoxical effect of a mediator of stress and adaptation to stressors involves glucocorticoid actions in relation to acute vs chronic stress effects upon the amygdala. Consistent with the elevation of corticosterone after acute and chronic stress accompanying the increase of dendritic length in BLA, as noted above (Lakshminarasimhan and Chattarji, 2012), a single large bolus of corticosterone mimics the ability of 10 consecutive days of CIS to increase anxiety and dendritic length in BLA (Mitra and Sapolsky, 2008). Furthermore, a single traumatic stressor causes a naïve rat to develop anxiety and increased spine density on BLA neurons, but with no increase in BLA dendritic length, with a delay of 10 days as noted above (Mitra et al, 2005).
But a timed elevation of a low to moderate dose of corticosterone at the time of the traumatic stressor prevents the increased anxiety and increased BLA spine density 10 days later (Rao et al, 2012). A similar protective effect of corticosterone has been reported for a different acute traumatic stress paradigm (Zohar et al, 2011). One possibility currently under investigation is that corticosterone stimulation of endocannabinoid production may be involved (Hill et al, 2010a), as endocannabinoids have an important role in the amygdala regulating basal and chronic stress levels of HPA activity (Hill and McEwen, 2009; Hill et al, 2010b) and endocannabinoids are known to modulate amygdala dendritic structure (Hill et al, 2013).
Translation to Human Amygdala: Mood Disorders and PTSD
The protective effects of a timed elevation of glucocorticoids in several animal models of traumatic stress have a human counterpart, as both epidemiologic studies and clinical research on patients undergoing cardiovascular surgery indicate that low glucocorticoid levels at the time of trauma increase probability of PTSD symptoms (Schelling et al, 2004; Yehuda et al, 1998). Moreover, administration of a glucocorticoid within an hour after a traffic accident was reported to reduce subsequent PTSD symptoms (Zohar et al, 2011).
Amygdala overactivity is also associated with mood disorders (Drevets and Raichle, 1992) and amygdala enlargement is reported in children of chronically depressed mothers (Lupien et al, 2011). Therapeutically, individuals with a chronic anxiety disorder have been shown to benefit from mindfulness-based stress reduction and when anxiety is reduced there is a reported decrease in amygdala volume (Holzel et al, 2010).
STRESS INDUCES STRUCTURAL REMODELING IN THE PFC
Findings in the hippocampus have also provided a gateway into another important brain region involved in stress and stress-related behaviors, namely, the PFC, which is important for working memory, executive function, and self-regulatory behaviors and shows sex differences in response to stressors (McEwen and Morrison, 2013).
Stress and Glucocorticoids have Biphasic Effects on PFC Functions
As is the case for the hippocampus, glucocorticoids also have biphasic effects on the PFC, namely, in relation to working memory and recognition memory. Acute stress caused a long-lasting potentiation of NMDAR- and AMPAR-mediated synaptic currents via glucocorticoid receptors in PFC pyramidal neurons, accompanied by increased surface expression of NMDAR and AMPAR subunits. Acute stress enhanced working memory via a GR-dependent mechanism (Yuen et al, 2009). Moreover, acute stress or short-term corticosterone treatment in vitro induced a delayed and sustained potentiation of the synaptic response and surface expression of NMDA and AMPA receptors in PFC pyramidal neurons through a mechanism depending on the induction of serum- and glucocorticoid-inducible kinase and the activation of Rab4, which mediates receptor recycling between early endosomes and the plasma membrane. In vivo, a serum- and glucocorticoid-inducible kinase mechanism is involved in facilitating working memory (Yuen et al, 2011a). Yet, chronic stress significantly reduced AMPA and NMDA receptor-dependent synaptic transmission and cell surface expression. This reduction was related to ubiquitin/proteasome-dependent degradation of GluR1 and NR1 subunits controlled by E3 ubiquitin ligase Nedd4-1 and Fbx2 and inhibition of proteasomes or knockdown of Nedd4-1 or Fbx2 prevented the chronic stress effects on recognition memory and glutamatergic function (Yuen et al, 2012).
Chronic Stress Induced Remodeling of PFC Neural Architecture
As in hippocampus, chronic stress also causes reversible structural remodeling of medial prefrontal cortical neurons that is reversible after the termination of chronic stress in young animals (Cook and Wellman, 2004; Liston et al, 2006; Radley et al, 2004, 2005), but not as readily reversible in middle aged and less so in aged animals (Bloss et al, 2010). However, one aspect of reversibility is that dendrites that shrink are distal to the cell body, whereas those that grow back after termination of stress are more proximal (Goldwater et al, 2009), suggesting that those neurons are different after stress, perhaps in their connectivity and most probably in terms of gene expression as described for hippocampus (Gray et al, 2014). EAAs are mediators of the stress-induced remodeling in PFC as they are in hippocampus (Martin and Wellman, 2011). Likewise, corticosterone administration alone also causes dendritic remodeling in medial PFC (mPFC), just as it does in the CA3 region (Cerqueira et al, 2005; Watanabe et al, 1992; Wellman, 2001). Endocannabinoids can also mediate stress-induced remodeling, as shown by the finding that mice lacking the endocannabinoid CB1 receptor have shorter dendrites in mPFC and respond with more dendritic shrinkage to chronic stress compared with wild-type mice (Hill et al, 2011a). Endocannabinoids in the medial PFC also have a role in the terminaton of the HPA stress response (Hill et al, 2011b).
In contrast to stress effects on the mPFC, the orbitofrontal cortex (OFC) shows expansion of dendrites after the same chronic stress that causes dendrite shrinkage in mPFC and hippocampus and dendrite expansion in the BLA (Liston et al, 2006). Unlike the mPFC under stress where impaired cognitive flexibility after chronic stress resembles lesions to mPFC, but in a manner that is reversible in young animals after termination of the chronic stress (Birrell and Brown, 2000; Liston et al, 2006), the functional significance of neuronal remodeling in OFC is less clear. However, one can speculate that it is a reflection of the role of the OFC in determining the salience of reward or punishment and thus part of an adaptive mechanism to chronic stress to increase vigilance to possible new stressors (McEwen and Morrison, 2013).
Epigenetics for PFC that Complement and Contrast with Hippocampus
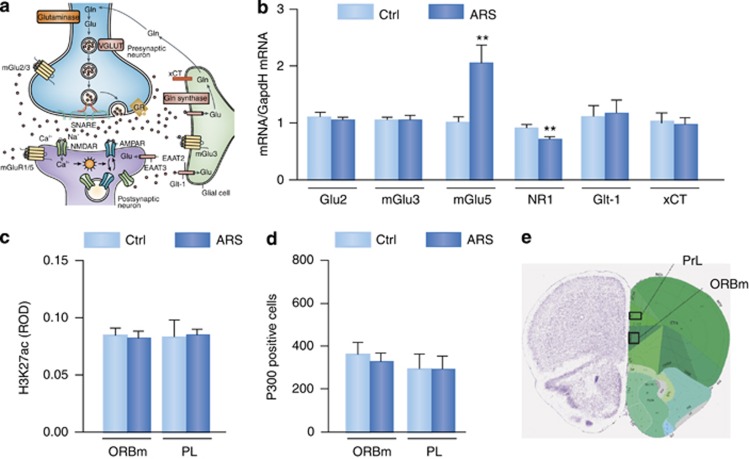
In contrast to stress effects on hippocampus in susceptible individuals, in the PFC, ARS fails to decrease mGlu2 receptors, nor are any changes in the histone H3K27ac observed in the PFC as well as no change in the epigenetic regulator, P300, in the layers I–II and III of the orbitomedial PFC and prelimbic cortex, as determined by immunocytochemistry (Figure 9). Nevertheless, in the PFC, ARS results in strong upregulation of the postsynaptic mGlu5 receptors and decrease of the subunit NR1 of NMDA receptors along with no significant changes in the transcription of the metabotropic mGlu3 receptors and glial transporters xCT and Glt-1 (Figure 9). The ARS-induced decrease in expression of the NR1 subunit that would decrease ionotrophic actions of glutamate and the concomitant increase in postsynaptic mGlu5 receptor expression, which would potentiate overall glutamate signaling, may represent an adaptive, homeostatic response to alterations in neurotransmitter overflow. In contrast, after ARS, the hippocampus shows evidence of an excessive EAA tone along with a decrease of mGlu2 (Nasca et al, 2014). The different responses of the hippocampus and PFC may reflect their respective roles in higher cognitive function, as the hippocampus encodes memories related to spatial orientation and daily events (McEwen, 1999), whereas the PFC has an important role in working memory and executive function as well as in self-regulatory behaviors (McEwen and Morrison, 2013), all of which are affected to some degree by acute and more prolonged stressors.
Figure 9.
Epigenetics coupled to the glutamatergic gene expression profile in the PFC in response to stress. (a) The tripartite glutamatergic synapse. Adapted and modified by Popoli et al (2012) by permission. (b) Glutamatergic gene expression profile in unstressed age-matched mice and in mice subjected to acute restraint stress within the PFC. Glt-1: principal glutamate transporter, which removes glutamate from the neuronal synaptic cleft into neuroglia and neurons; xCT: the cystine–glutamate exchanger that facilitates glutamate exchange from glial cells to the perisynaptic space; mGluRs: metabotropic glutamate receptors, which mainly inhibit the release of glutamate into the synaptic space and generate excitatory responses; NR1, essential subunit of NMDA receptors: ionotropic glutamate receptors, which mainly regulate mechanisms of synaptic plasticity. Bars represent mean+SEM, significant comparisons with corresponding controls, **P<0.01 (t-test). (c) ROD analysis of H3K27ac immunoreactivity showing the lack of ARS effects in the subregions (ORBm and PrL) of the PFC. (d and e) Cell count of P300-positive cells showing the lack of ARS effects in the layers I–II and III of either the ORBm or PrL subregions of the PFC. Image credit: Allen Institute for Brain Science (e).
Translation to the Human PFC
The PFC in humans is affected by stressors and the psychosocial environment. Severe acute stressors impair cognitive function largely via adrenergic mechanisms (Arnsten, 2009). Yet, glucocorticoids facilitate working memory in young people (Lupien et al, 2002), but impair it in older subjects where basal cortisol levels and reactivity are higher (Lupien et al, 1997), which implies a biphasic dose response as well as a possible aging effect. For perceived stress, medical students who had high scores on the 10-item perceived stress scale of Sheldon Cohen, of Carnegie Mellon University, showed impaired functional connectivity by fMRI in a brain circuit involving the PFC as well as impaired performance on a test of mental flexibility; these effects were reversed by a month vacation (Liston et al, 2009). Thus, the young adult human PFC reflects the effects of chronic stress by showing impaired cognitive flexibility and reduced functional connectivity that parallels the effects of stress in the young adult rat brain, including the reversibility after the end of the stressful period as described above.
The long-term psychosocial environment also affects the PFC and its ability to exert control over amygdala activity. Low perceived social standing is associated with reduced PFC gray matter volume as well as with an increased systemic inflammatory tone and altered white matter structure throughout the brain (Gianaros et al, 2007, 2013) and preclinical atherosclerosis is associated with increased amygdala reactivity to stimuli such as sad and angry faces and reduced inhibitory input from the PFC (Gianaros et al, 2009).
The functional connectivity between PFC and amygdala develops first as a positive influence and then as an inhibitory one (Gee et al, 2013b). Under typical conditions, mPFC connections with the amygdala are immature during childhood and become adult-like during adolescence, and rodent models have shown that maternal deprivation accelerates this development. Previously institutionalized youths, who experienced early maternal deprivation, exhibited atypical amygdala–mPFC connectivity, in that they failed to show the positive amygdala–mPFC coupling of children with good maternal attachment. Rather, these children with a history of early adversity showed mature, negative amygdala–mPFC coupling and thus, resembled the adolescent phenotype. Cortisol is implicated as a mediator suggesting a developmental role of the deprivation stress. Despite being age-atypical, negative amygdala–mPFC coupling conferred some degree of reduced anxiety, although anxiety was still significantly higher in the previously institutionalized group indicating only partial compensation for lack of strong maternal attachment, and this suggests that accelerated amygdala–mPFC development is an ontogenetic adaptation in response to early adversity (Gee et al, 2013a).
Plasticity and resilience in the PFC are enhanced by regular moderate aerobic exercise, which increases blood flow to this brain region and improves executive function (Colcombe et al, 2004; Kramer et al, 1999). Cognitive behavioral therapy has been shown to increase gray matter volume in the PFC when it is able to reduce symptoms of chronic fatigue (de Lange et al, 2008).
SEX DIFFERENCES
The hippocampus has also been a gateway for the understanding of the actions of ovarian hormones on neural and cognitive functions over and above the actions that subserve reproduction. The discovery of genomic and non-genomic forms of the estrogen receptor in spines, dendrites, mitochondria, presynaptic terminals, and astrocytes paved the way for discovery of such non-nuclear estrogen receptor localization in other brain regions (McEwen and Milner, 2007). This revelation occurred along with the discovery of ovarian hormone turnover of spine synapses in the CA1 region of the female rat hippocampus. The identification of signaling processes involving estradiol stimulation via PI3 kinase of phosphorylation of LIMK1 and 4E-BP1 leads to actin polymerization and translation of PSD95 mRNA, respectively (Akama and McEwen, 2003; Dumitriu et al, 2010; Yuen et al, 2011b). This is a sexually differentiated response and male rats do not show synapse induction in response to estradiol, but do so in response to testosterone (Leranth et al, 2003).
Females Respond Differently to Chronic Stress
All of the animal model studies of stress effects summarized above were carried out on male rodents, and female rodents do not show the same pattern of neural remodeling after chronic stress as do males. The first recognition of this sex difference was in the hippocampus, in which the remodeling of CA3 dendrites did not occur in females after CRS, even though all the measures of stress hormones indicated that the females were experiencing the stress as much as males (Galea et al, 1997). Females and males also differ in the cognitive consequences of repeated stress, with males showing impairment of hippocampal-dependent memory, whereas females do not (Bowman et al, 2001; Luine et al, 1994, 2007).
In contrast, acute tail shock stress during classical eyeblink conditioning improves performance in males, but suppresses it in females (Wood and Shors, 1998) by mechanisms influenced by gonadal hormones in development and in adult life (Shors and Miesegaes, 2002; Wood et al, 2001). However, giving male and female rats control over the shock abolishes both the stress effects and the sex differences (Leuner et al, 2004). These findings suggest that sex differences involve brain systems that mediate how males and females interpret stressful stimuli and that a sense of control is paramount to coping with those stimuli.
There are sex differences in response to chronic stress in mPFC neurons, in which female rats with intact ovaries or estrogen treatment after ovariectomy showed an expansion of dendrites, whereas males show a chronic stress-induced retraction (Garrett and Wellman, 2009). Refining this in terms of where mPFC neurons project, Shansky showed that female rats fail to show the mPFC dendritic remodeling seen in males after CRS in those neurons that do not project to amygdala. Those neurons that project to amygdala do not change in males with chronic stress but, in females with estrogens, there is a chronic stress-induced expansion of the dendritic tree in the subset of neurons that project to the BLA (Shansky et al, 2010). Moreover, ovariectomy prevented these chronic stress effects on dendritic length and branching. Furthermore, estradiol treatment of ovariectomized females increased spine density in mPFC neurons, irrespective of where they were projecting (Shansky et al, 2010).
Recent behavioral work on male and female rats undergoing fear conditioning and extinction involving, respectively, the prelimbic and infralimbic PFC (Santini et al, 2008) has revealed sex differences based upon individual differences in efficacy of extinction (poor extinction, HF; good extinction, LF) that were evident in both males and females (Gruene et al, 2014). Despite no overall sex differences in freezing behavior, the HF/LF phenotypes emerged in males during extinction, but in females it emerged during fear conditioning, which does not involve infralimbic-BLA neurons. HF vs LF males exhibited neuroanatomical distinctions in dendrites that were not observed in HF vs LF females, namely differences in prefrontal cortical dendritic length that may have either preceded or resulted from the extinction. The authors speculate that, in females, the sex differences in PFC to amygdala circuitry (Shansky et al, 2010) underlie the female HF vs LF difference at the time of conditioning. The fact that estrogen's and androgen's effects are widespread in the central nervous system via both genomic and non-genomic receptors (McEwen and Milner, 2007), there are likely to be many more examples of sex × stress interactions related to many brain regions and multiple functions, as well as developmentally programmed sex differences that affect how the brain responds to stress, eg, in the locus coeruleus (Bangasser et al, 2010, 2011).
Emergence of Sex Differences in Response to Stressors After Puberty
Although the sensitive period for testosterone effects on sexual differentiation is perinatal in rodents, sex differences in response to chronic stressors manifest themselves gradually over the pubertal transition. Demonstration of this was accomplished using a rodent model of chronic restraint stress in juvenile rats daily from postnatal days 20 to 41 (Eiland et al, 2012). Chronic stress produced depressive-like behavior and significant neuronal remodeling of brain regions likely involved in these behavioral alterations: the hippocampus, PFC, and amygdala. Both chronically stressed males and females exhibited anhedonia, increased locomotion when exposed to novelty, and altered coping strategies when exposed to acute stress. Chronic stress produced shrinkage of dendrites in the hippocampus and PFC and concurrent hypertrophy of dendrites in the amygdala, with a trend for males to show more robust responses than females (Eiland et al, 2012).
Even then, in prepubertal female rats there are already sex differences in that young female rats exposed to 1 week of repeated restraint stress show no negative effects on temporal order recognition memory, a cognitive process controlled by the PFC, in contrast to impairment in temporal order recognition memory observed in stressed males (Wei et al, 2014). Chronically stressed females also showed normal glutamatergic transmission and glutamate receptor surface expression in PFC pyramidal neurons, in contrast to impairment seen in stressed males. However, inhibition or knockdown of PFC estrogen receptors revealed chronic stress-induced impairment in females, and estradiol administration to stressed males prevented the inhibitory effects of estradiol. Furthermore, blocking aromatase in females also causes chronic stress to have deleterious effects in these young females. Thus, already at 4–5 weeks of age, before the onset of puberty, estrogens already have protective effects in both chronically stressed females and males (Wei et al, 2014).
Translation to the Human Brain
The impact of sex and sex differences has undergone a revolution and much more is to come (Cahill 2006; McCarthy et al, 2012; McCullough et al, 2014; McEwen and Lasley, 2005), including insights into X and Y chromosome contributions to brain sex differences (Carruth et al, 2002). In men and women, neural activation patterns to the same tasks are quite different between the sexes even when performance is similar (Derntl et al, 2010). This leads to the concept that men and women often use different strategies to approach and deal with issues in their daily lives, in part because of the subtle differences in brain architecture. Nevertheless, from the standpoint of gene expression and epigenetic effects, the principles of what we have learned in animal models regarding plasticity, damage, and resilience are likely to apply to both males and females.
SUMMARY AND CONCLUSIONS
The hippocampus has provided the gateway into much of what we have learned about stress and brain structural and functional plasticity not only in adult life, but also as a result of stressors early in life. The initial focus on hippocampus has expanded to interconnected brain regions such as the amygdala, PFC, and nucleus accumbens. Moreover, the initial focus on gene regulatory effects has broadened with the recognition that steroid hormone receptors also mediate rapid signaling pathways and that both direct and indirect genomic and non-genomic actions of steroids involve non-linear interactions with other intra- and extracelluar mediators (Table 1). The resulting epigenetic effects result in ever-changing patterns of gene expression, in which there are important sex differences that need further exploration. Translation of these findings from animal models to human brain function is changing thinking about the nature of brain malfunction in psychiatric disorders and during aging, as well as the mechanisms of the effects of early-life adversity on the brain and the body.
One outcome of the epigenetic perspective is the new possibility for interventions that help the brain, as the master organ of stress and adaptation to stressors, to change itself. As suggested in the life course developmental perspective (Halfon et al, 2014), the brain changes that occur as a result of adverse experiences may be amenable to intervention that epigenetically changes brain structure and function to remediate those early events, even though true ‘reversal' is not possible. Some of these interventions that have been shown to change brain structure and function are summarized in Table 2. The key may be agents—pharmaceutical or behavioral like physical activity—that facilitate plasticity so that behavioral intervention may be effective, such as the recently reported ability of fluoxetine to enhance recovery from stroke (Chollet et al, 2011). The main goal of this new approach (Castren and Rantamaki, 2010) is to open a ‘window of increased plasticity' that may be capitalized by a positive behavioral intervention, eg, behavioral therapy in the case of depression or the intensive physiotherapy to promote neuroplasticity to counteract the effects of a stroke. This is consistent with animal model work that shows that ocular dominance imbalance from early monocular deprivation can be reversed by patterned light exposure in adulthood that can be facilitated by fluoxetine (Vetencourt et al, 2008), but also by caloric restriction as well as by every-other-day glucocorticoids in the drinking water (Spolidoro et al, 2011). These glucocorticoid actions bring into focus work showing that ultradian pulsatility of glucocorticoids promotes turnover of a subset of synapses throughout the cortex that is involved in motor learning, among other functions (Liston et al, 2013; Liston and Gan, 2011). Investigations of underlying mechanisms for the re-establishment of a new window of plasticity are focusing on the balance between excitatory and inhibitory transmission and removing molecules that put the ‘brakes' on such plasticity (Bavelier et al, 2010).
Table 2. Interventions that help the brain change itself.
| Regular physical activity |
| Increased hippocampal volume and PFC blood flow and improved executive function and memory (Erickson et al, 2011). |
| Mindfulness-based stress reduction |
| Reducing anxiety decreases amygdala volume in those individuals who responded to a mindfulness-based stress reduction therapy (Holzel et al, 2010). |
| Social support and integration |
| Experience Corps for elderly volunteers, who showed improved executive function, increased blood flow in PFC, and better overall health with slower decline. |
| Meaning and purpose (eudaimonia) are a likely component along with social support and increased physical activity (Carlson et al, 2009). |
It is important to reiterate that successful behavioral therapy, which is tailored to individual needs, can produce volumetric changes in both PFC in the case of chronic fatigue (de Lange et al, 2008), and in amygdala, in the case of chronic anxiety (Table 2; Holzel et al, 2010). This reinforces two important messages: (i) that plasticity-facilitating treatments should be given within the framework of a positive behavioral or physical therapy intervention; and (ii) that negative experiences during the window may even make matters worse (Castren and Rantamaki, 2010). In that vain, it should be noted that excess BDNF also has the ability to promote pathophysiology, such as seizures in some instances (Heinrich et al, 2011; Kokaia et al, 1995; Scharfman, 1997).
FUTURE DIRECTIONS
For real progress in diagnosing and treating mental and physical health disorders, it is essential that the life-course developmental perspective on the epigenetic embedding of early-life experience (Halfon et al, 2014) help redefine thinking about the origins of health and disease throughout the life course. The discovery of antibiotics for infectious disease promoted the notion that a pill could cure a disease (‘magic bullets'). Although this may be true for infectious diseases, the recognition of the non-communicable diseases such as diabetes and heart disease has introduced preventative health behaviors such as diet and exercise (Engel 1977) where drugs are not ‘magic bullets' but rather can help correct some physiological imbalances such as high cholesterol and low HDL. The life-course developmental perspective adds the important notion of the biological embedding of early-life experiences that carry over throughout the life course as well as the concept that via epigenetics the physical and social environment is continually changing the brain and the body. This dynamic interaction opens the possibility of redirecting toward a more positive trajectory via behavioral interventions like physical activity and certain pharmaceutical agents that open windows of plasticity for a behavioral intervention to be effective. Nowhere is this more important than for anxiety and depressive disorders as the brain is the central organ of adapting to stressors and is a vulnerable organ that is, however, subject to changes from external behavioral intervention, aided in some cases by pharmaceutical agents that open windows of plasticity.
FUNDING AND DISCLOSURE
Research is supported by RO1 MH41256 from NIH and by the Hope for Depression Research Foundation grant to Bruce McEwen, by the American Foundation for Suicide Prevention to Carla Nasca and NRSA Award F32 MH102065 to Jason Gray. The authors declare no conflict of interest.
References
- Ahima R, Krozowski Z, Harlan R (1991). Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol 313: 522–538. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE (1990). Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39: 579–604. [DOI] [PubMed] [Google Scholar]
- Akama KT, McEwen BS (2003). Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci 23: 2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allfrey VG (1970). Changes in chromosomal proteins at times of gene activation. Fed Proc 29: 1447–1460. [PubMed] [Google Scholar]
- Altman J, Das GD (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–336. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J et al (2003). Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 23: 6972–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I et al (2010). Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ (2011). Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 103: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK (2010). Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 30: 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S (2007). Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 144: 8–16. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ et al (2008). The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiat 14: 764–773. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20: 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Hunter RG, Waters EM, Munoz C, Bernard K, McEwen BS (2008). Behavioral and biological effects of chronic S18986, a positive AMPA receptor modulator, during aging. Exp Neurol 210: 109–117. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH (2010). Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci 30: 6726–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN (2001). Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res 904: 279–289. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL et al (2005). Mechanisms of late-onset cognitive decline after early-like stress. J Neurosci 25: 9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges NM, Jin R, Seckl J, Holmes MC, Drake AJ, Hall J (2014). Juvenile stress enhances anxiety and alters corticosteroid receptor expression in adulthood. Brain Behavior 4: 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L (2006). Why sex matters for neuroscience. Nat Rev Neurosci 7: 477–484. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ (2000). Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiat 48: 1164–1174. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E (1994). Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61: 203–209. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E (1998). Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience 82: 349–354. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N et al (2009). Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J Gerontol A Biolog Sci Med Sci 64: 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP (2002). Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci 5: 933–934. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T (2010). The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70: 289–297. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK (2003). Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA 100: 16131–16136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OFX, Sousa N (2005). Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 25: 7792–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Arthur JS, Reul JM (2008). The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci 27: 2701–2713. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE (2010). TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci 13: 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K (2001). Chronic 'jet lag' produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci 4: 567–568. [DOI] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E et al (2011). Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 10: 123–130. [DOI] [PubMed] [Google Scholar]
- Christian KM, Miracle AD, Wellman CL, Nakazawa K (2011). Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience 174: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E et al (2004). Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 101: 3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL (2004). Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60: 236–248. [DOI] [PubMed] [Google Scholar]
- Covington HE 3rd, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ (2011). Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett 493: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccurazzu B, Bortolotto V, Valente MM, Ubezio F, Koverech A et al (2013). Upregulation of mGlu2 receptors via NF-kappaB p65 acetylation is involved in the Proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology 38: 2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP (2001). Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci 21: 6949–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson NA, van den Oever JM, Korobko OB, Magarinos AM, de Kloet ER, McEwen BS (2013). Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology 154: 3261–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D et al (2009). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P et al (2008). Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain 131: 2172–2180. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI et al (2010). Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology 35: 67–82. [DOI] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG (2009). Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci 29: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM (1992). Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus 2: 421–430. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J et al (2006). Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 26: 6314–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME (1992). Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull 28: 261–274. [PubMed] [Google Scholar]
- Du J, McEwen BS, Manji HK (2009). Glucocorticoid receptors modulate mitochondrial function. Commun. Integr. Biol 2: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J (2001). Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25: 836–844. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH (2010). Estrogen and the aging brain: an elixir for the weary cortical network. Ann NY Acad Sci 1204: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS (2012). Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology 37: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel GL (1977). The need for a new medical model: a challenge for biomedicine. Science 196: 129–136. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A et al (2011). Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286: 1155–1158. [DOI] [PubMed] [Google Scholar]
- Francis D MM (1999). Variations in maternal care form the basis for non-genomic mechanism of inter-generational transmission of individual differences in behavioral and endocrine responses to stress. Abstracts, NY Acad Sci Conf ‘Socioeconomic Status and Health in Industrial Nations' P59.
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M et al (2013). Emergence of individuality in genetically identical mice. Science 340: 756–759. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS (1997). Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81: 689–697. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL (2009). Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH (1996). Differential Regulation of NMDAR1 mRNA and Protein by Estradiol in the RAt Hippocampus. J Neurosci 16: 6830–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL et al (2013. a). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH et al (2013. b). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 33: 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS (1972). Rat brain binds adrenal steroid hormone: radioautography of hippocampus with corticosterone. Science 175: 1133–1136. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS, Pfaff DW, Moskovitz S, Ferin M et al (1976). Cells in regions of rhesus monkey brain and pituitary retain radioactive estradiol, corticosterone and cortisol differently. Brain Res 103: 603–612. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB (2009). Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry 65: 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM et al (2007). Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci 2: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD (2013). Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex 23: 2058–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR et al (2009). Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience 164: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron H, Daniels D, Woolley C, McEwen BS (1992). Adrenal hormones suppress cell division in the adult rat dentate gyrus. J.Neurosci 12: 3642–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Rao BSS, Nair D, Trinh M, Mawjee N et al (2006). Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 103: 13208–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Rubin TG, Hunter RG, McEwen BS (2014). Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry 19: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM (2014). Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry (doi:10.1016/j.biopsych.2014.11.014). [DOI] [PMC free article] [PubMed]
- Guillemin R (1978). Peptides in the brain: the new endocrinology of the neuron. Science 202: 390–402. [DOI] [PubMed] [Google Scholar]
- Halfon N, Larson K, Lu M, Tullis E, Russ S (2014). Lifecourse health development: past, present and future. Matern Child Health J 18: 344–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW (1970). Effects of the nervous system on the pituitary-adrenal activity. Prog Brain Res 32: 86–88. [PubMed] [Google Scholar]
- Heinrich C, Lahteinen S, Suzuki F, Anne-Marie L, Huber S et al (2011). Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol Dis 42: 35–47. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS (2011. a). Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex 21: 2056–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS (2010. a). Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 35: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL et al (2013). Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry 18: 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS (2009). Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuro-Psychopharm Biol Psychiatry 34: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS (2010). Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry 34: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT et al (2010. b). Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci USA 107: 9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ et al (2011. b). Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 31: 10506–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA et al (2010). Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci 5: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Gagnidze K, McEwen BS, Pfaff DW (2015). Stress and the dynamic genome: steroids, epigenetics, and the transposome. Proc Natl Acad Sci USA 112: 6828–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS (2009). Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci USA 106: 20912–20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O (2006). Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 31: 2395–2404. [DOI] [PubMed] [Google Scholar]
- Jensen E, Jacobson H (1962). Basic guides to the mechanism of estrogen action. Rec Prog Horm Res 18: 387–408. [Google Scholar]
- Joels M (2006). Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 27: 244–250. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE (2005). Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience 136: 289–299. [DOI] [PubMed] [Google Scholar]
- Kaplan MS (2001). Environment complexity stimulates visual cortex neurogenesis: death of a dogma and a research career. Trends Neurosci 24: 617–620. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011). Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA 108: 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M (2005). Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102: 19204–19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Freund TF (2008). Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14: 923–930. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER (2001). Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab 12: 152–156. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 586: 493–495. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Hunter RG, Gray JD, Mesias R, McEwen BS et al (2014). Role for NUP62 depletion and PYK2 redistribution in dendritic retraction resulting from chronic stress. Proc Natl Acad Sci USA 111: 16130–16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia M, Ernfors P, Kokaia Z, Elmer E, Jaenisch R, Lindvall O (1995). Suppressed epileptogenesis in BDNF mutant mice. Exp Neurol 133: 215–224. [DOI] [PubMed] [Google Scholar]
- Korte SM, de Boer SF, de Kloet ER, Bohus B (1995). Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneuroendocrinology 20: 385–394. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E et al (1999). Ageing, fitness and neurocognitive function. Nature 400: 418–419. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S (2012). Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One 7: e30481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Waymire JC, Lynch G (1978). Hippocampal aging and adrenocorticoids: quantitative correlations. Science 202: 1098–1102. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ (2003). Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci 23: 1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-loffredo S, Shors TJ (2004). Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry 56: 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Haltmeyer G, Kara G, Denenberg V (1967). Physiological and behavioral effects of infantile stimulation. Physiol Behav 2: 55–59. [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM et al (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liposits Z, Bohn MC (1993). Association of glucocorticoid receptor immunoreactivity with cell membrane and transport vesicles in hippocampal and hypothalamic neurons of the rat. J Neurosci Res 35: 14–19. [DOI] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB (2013). Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 16: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Gan WB (2011). Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA 108: 16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA 106: 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB et al (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26: 7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D et al (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659–1662. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK (1993). Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem 61: 1957–1960. [DOI] [PubMed] [Google Scholar]
- Loy R, Gerlach J, McEwen BS (1988). Autoradiographic localization of estradiol-binding neurons in rat hippocampal formation and entorhinal cortex. Dev Brain Res 39: 245–251. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS (1994). Repeated stress causes reversible impairments of spatial memory performance. Brain Res 639: 167–170. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, MacLusky NJ (2007). Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 19: 743–751. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C et al (1998). Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1: 69–73. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S et al (1997). Stress-induced declarative memory impairment in healthy elderly subjects — relationship to cortisol reactivity. J Clin Endo Metab 82: 2070–2075. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD et al (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 108: 14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Menard C, Kin NMKNY, Nair NPV (2002). The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology 27: 401–416. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS (1995). Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69: 89–98. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Saboureau M, Pevet P (2006). Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci USA 103: 18775–18780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo Garcia JM, McEwen BS (1997). Chronic restraint stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci USA 94: 14002–14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Molet J, Chen Y, Rice C, Ji SG et al (2014). Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry 19: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KP, Wellman CL (2011). NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex 21: 2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys T, Pawlak R, Matys E, Pavlides C, McEwen BS, Strickland S (2004). Tissue plasminogen activator promotes the effects of corticotropin releasing factor on the amygdala and anxiety-like behavior. Proc Natl Acad Sci USA 101: 16345–16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T, Weil ZM, Nacher J, Bloss EB, El Maarouf A et al (2013). Depletion of polysialic acid from neural cell adhesion molecule (PSA-NCAM) increases CA3 dendritic arborization and increases vulnerability to excitotoxicity. Exp Neurol 241: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ (2012). Sex differences in the brain: the not so inconvenient truth. J Neurosci 32: 2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, McCarthy MM, de Vries GJ (2014). NIH policy: status quo is also costly. Nature 510: 340. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1999). Stress and hippocampal plasticity. Annu Rev Neurosci 22: 105–122. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev 87: 873–904. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annu Rev Med 62: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Lasley EN (2005) The End of Sex as We Know It In Cerebrum The Dana Forum on Brain Science. Dana Presshttp://www.dana.org/Cerebrum/2005/The_End_of_Sex_as_We_Know_It/. [Google Scholar]
- McEwen BS, Milner TA (2007). Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev 55: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Plapinger L (1970). Association of corticosterone-1,2 3H with macromolecules extracted from brain cell nuclei. Nature 226: 263–264. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss J, Schwartz L (1968). Selective retention of corticosterone by limbic structures in rat brain. Nature 220: 911–912. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B et al (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12: 241–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M, Aitken D, Berkel H, Bhatnagar S, Sapolsky R (1988). Effect of neonatal handling of age-related impairments associated with the hippocampus. Science 239: 766–768. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K et al (2000). Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: The effects of thyroid hormones and serotonin. J. Neurosci. 20: 3926–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M (2005). Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 7: 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF (2008). Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol 86: 305–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J (1992). Short history of neuroendocrinology and the International Society of Neuroendocrinology. Neuroendocrinology 56: 1–10. [DOI] [PubMed] [Google Scholar]
- Miller MM, Morrison JH, McEwen BS (2012). Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav Brain Res 229: 280–288. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS (2008). Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology 149: 3306–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagen L, Alves SE (2001). Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol 429: 355–371. [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Nat Acad Sci USA 102: 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM (2008). Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 105: 5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS (2014). Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry 20: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S et al (2013). L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 110: 4804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bruno V, Ngomba RT, Gradini R, Battaglia G (2015). Metabotropic glutamate receptors as drug targets: what's new? Curr Opin Pharmacol 20C: 89–94. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, De Kloet ER, Joels M, Schmid W, Cole TJ (1997). Spatial learning deficits in mice with a targeted glucocorticoid receptor gene disruption. Eur J Neurosci 9: 2284–2296. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL (2004). Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA 101: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchinik M, Murray TF, Franklin PH, Moore FL (1992). Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proc Natl Acad Sci USA 89: 3830–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, Magarinos AM, McEwen BS (1995). Opposing role of adrenal steroid Type I and Type II receptors in hippocampal long-term potentiation. Neuroscience 68: 387–394. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S (2003). Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci 6: 168–174. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S (2005). Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA 102: 18201–18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Lambert HK, Grossman YS, Dumitriu D, Waldman R et al (2014). Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci USA 111: 18733–18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Keiner M (1973). Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol 151: 121–158. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS (2003). Repeated, but not acute, restraint stress suppresses proliferation of neural precursor cells and increases PSA-NCAM expression in the adult rat dentate gyrus. J Neurosci 17: 879–886. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW (1997). A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci 111: 503–511. [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH (2005). Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol 196: 199–203. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T et al (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125: 1–6. [DOI] [PubMed] [Google Scholar]
- Rao RP, Anilkumar S, McEwen BS, Chattarji S (2012). Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry 72: 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev L, Baram TZ (2014). Corticotropin releasing factor in neuroplasticity. Front Neuroendocrinol 35: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, DeKloet ER (1985). Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117: 2505–2511. [DOI] [PubMed] [Google Scholar]
- Revest JM, Le Roux A, Roullot-Lacarriere V, Kaouane N, Vallee M et al (2014). BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol Psychiatry 19: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Cahill L, McGaugh JL (1996) Interaction of emotionally activated neuromodulatory systems in regulating memory Storage. In: Ishikawa K, McGaugh JL, Sakata H (eds) Brain Process and Memory. Elsevier: Amsterdam, pp 39–54. [Google Scholar]
- Rubin TG, Gray JD, McEwen BS (2014). Experience and the ever-changing brain: what the transcriptome can reveal. BioEssays 36: 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Charney DS (2013). Next generation antidepressants. Proc Natl Acad Sci U S A 110: 4441–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R (2007). Adult hippocampal neurogenesis in depression. Nat Neurosci 10: 1110–1115. [DOI] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT (2008). Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 28: 4028–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Krey L, McEwen BS (1986). The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev 7: 284–301. [DOI] [PubMed] [Google Scholar]
- Schally AV, Arimura A, Kastin AJ (1973). Hypothalamic regulatory hormones. Science 179: 341–350. [DOI] [PubMed] [Google Scholar]
- Scharfman HE (1997). Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol 78: 1082–1095. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Rigoulot M-A, Berger RE, Walling SG et al (2005). Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol 196: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, De Quervain DJ-F (2004). Can posttraumatic stress disorder be prevented with glucocorticoids? Ann NY Acad Sci 1032: 158–166. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH (2010). Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex 20: 2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI (2003). Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 54: 338–352. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G (2002). Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci USA 99: 13955–13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM (1995). Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 15: 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Paula-Barbosa MM, Almeida OFX (1999). Ligand and subfield specificity of corticoid-induced neuronal loss in the rat hippocampal formation. Neuroscience 89: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Spolidoro M, Baroncelli L, Putignano E, Maya-Vetencourt JF, Viegi A, Maffei L (2011). Food restriction enhances visual cortex plasticity in adulthood. Nat Commun 2: 320. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE (1992). Hippocampal formation volume, memory dysfunction, and cortisol levels in partiens with Cushing's syndrome. Biol Psychiatry 32: 756–765. [DOI] [PubMed] [Google Scholar]
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA et al (2009). Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ et al (2004). Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf W (1971). Autoradiographic techniques and the localization of estrogen, androgen and glucocorticoid in the pituitary and brain. Am Zool 11: 725–739. [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS (2014). On the causes of early life experience effects: evaluating the role of mom. Front Neuroendocrinol 35: 245–251. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R (2006). Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 147: 5549–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochigi M, Iwamoto K, Bundo M, Sasaki T, Kato N, Kato T (2008). Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neuroscience Res 60: 184–191. [DOI] [PubMed] [Google Scholar]
- Toft D, Gorski J (1966). A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci USA 55: 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9: 519–525. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213: 1394–1397. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270. [DOI] [PubMed] [Google Scholar]
- Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R et al (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320: 385–388. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH (1942). The epigenotype. Endeavoour 1: 18–20. [Google Scholar]
- Wang K, Xiang XH, He F, Lin LB, Zhang R et al (2010). Transcriptome profiling analysis reveals region-distinctive changes of gene expression in the CNS in response to different moderate restraint stress. J Neurochem 113: 1436–1446. [DOI] [PubMed] [Google Scholar]
- Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU (2014). A review of current evidence for acetyl-l-carnitine in the treatment of depression. J Psychiatr Res 53: 30–37. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS (1992). Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus 2: 431–436. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Champagne FA, Brown SE, Dymov S, Sharma S et al (2005). Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25: 11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P et al (2014). Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry 19: 588–598. [DOI] [PubMed] [Google Scholar]
- Wellman CL (2001). Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol 49: 245–253. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ (1998). Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA 95: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Shors TJ, Beylin AV (2001). The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci 115: 175–187. [DOI] [PubMed] [Google Scholar]
- Woolley C, McEwen BS (1994). Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor dependent mechanism. J Neurosci 14: 7680–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K (1985). Steroid receptor regulated transcription of specific genes and gene networks. Ann Rev Genet 19: 209–252. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY (1998). Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiat 44: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA 106: 14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J et al (2011. a). Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 16: 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012). Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73: 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen GS, McEwen BS, Akama KT (2011. b). LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res 1379: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R, McEwen BS (1970). Selective retention of oestradiol by cell nuclei in specific brain regions of the ovariectomized rats. J Neurochem 17: 889–899. [DOI] [PubMed] [Google Scholar]
- Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA et al (2011). High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol 21: 796–809. [DOI] [PubMed] [Google Scholar]