Abstract
Adversity experienced during gestation is a predictor of lifetime neuropsychiatric disease susceptibility. Specifically, maternal stress during pregnancy predisposes offspring to sex-biased neurodevelopmental disorders, including schizophrenia, attention deficit/hyperactivity disorder, and autism spectrum disorders. Animal models have demonstrated disease-relevant endophenotypes in prenatally stressed offspring and have provided unique insight into potential programmatic mechanisms. The placenta has a critical role in the deleterious and sex-specific effects of maternal stress and other fetal exposures on the developing brain. Stress-induced perturbations of the maternal milieu are conveyed to the embryo via the placenta, the maternal–fetal intermediary responsible for maintaining intrauterine homeostasis. Disruption of vital placental functions can have a significant impact on fetal development, including the brain, outcomes that are largely sex-specific. Here we review the novel involvement of the placenta in the transmission of the maternal adverse environment and effects on the developing brain.
INTRODUCTION
Prenatal adversity is a risk factor for lifetime neuropsychiatric disease susceptibility. During gestation, rapid growth and plasticity render the brain sensitive to the effects of environmental factors that can confer adaptive advantages or lasting vulnerability. As the fetal origins of disease hypothesis was pioneered four decades ago by Barker (1997) and Dörner (1973), substantial advances have been achieved in understanding the neurodevelopmental consequences of intrauterine challenges, including maternal psychosocial stress, infection, and metabolic dysfunction (Bock et al, 2014, 2015; Brown and Derkits, 2010; Markham and Koenig, 2011; Weinstock, 2008). Maternal stress during pregnancy, in particular, has been identified in autism spectrum disorders (ASDs), attention deficit/hyperactivity disorder (ADHD), and schizophrenia risk (Beversdorf et al, 2005; Khashan et al, 2008; Kinney et al, 2008a; Li et al, 2010; Ronald et al, 2010). These neurodevelopmental disorders exhibit a strong sex bias, where boys are more likely to develop ASD, ADHD, and earlier-onset schizophrenia (Baio, 2012; Bloom et al, 2011; Froehlich et al, 2007; Zhang et al, 2012). Consistent with the male predominance of these disorders, recent epidemiological and preclinical studies suggest that males are uniquely vulnerable to prenatal adversity (Clifton, 2010; Davis and Pfaff, 2014). Although studies have revealed sex-specific changes in the developing brain (Bale, 2011; Kapoor and Matthews, 2005, 2008; Kapoor et al, 2009; Weinstock, 2011), the specific mechanisms by which perturbations in the maternal environment promote sex-biased fetal reprogramming remain unclear.
The programmatic effects of prenatal stress likely involve a complex interaction between the fetal genetic background, sex, and gestational age at the time of exposure (Bale, 2011). As the placenta resides at the interface between mother and fetus, it is uniquely positioned to modulate interactions within an adverse intrauterine environment. The placenta actively maintains intrauterine homeostasis through vital functions including exchange of nutrients, oxygen, and waste, immunoprotection of the semi-allogenic fetus, buffering from deleterious maternal factors, and secretion of hormones and growth factors into both maternal and fetal compartments (Jansson and Powell, 2007). Impairment of placental organogenesis and function can broadly have an impact on fetal development, conferring lasting effects on the brain (Myatt, 2006). The placenta comprises specialized cells derived from the embryo and thereby expresses the fetal genetic sex (Rossant and Cross, 2001). In uncomplicated pregnancies, sex differences in placental size and gene expression are present throughout gestation (Buckberry et al, 2014; Gabory et al, 2013; Mao et al, 2010; O'Connell et al, 2013; Sood et al, 2006). Such basal placental sex differences likely facilitate sex-specific responses to both normal and pathologic environments. Supporting this, sex differences in placental inflammatory responses, vascular remodeling, and placental size and efficiency have been reported in human pregnancies complicated by asthma, preeclampsia, and malnutrition (Clifton, 2010; Gabory et al, 2013). Such complications are also associated with altered neurodevelopment (Bale et al, 2010; Walker et al, 2015). In addition, prospective studies have found abnormal placental histology associated with an autism diagnosis (Anderson et al, 2007; Walker et al, 2013). Together, these data support the importance of the placenta, and changes in this tissue in response to perturbations across gestation, in the etiology of neurodevelopmental disorders.
In this review we will discuss the programmatic role of the placenta in the transmission of maternal adversity to the developing brain. We will broadly review the effects of maternal stress on offspring neurodevelopment and long-term changes in stress responsivity and behavior, discussing the placenta as a key orchestrator of this sex-specific programming.
LONG-TERM IMPACT OF MATERNAL STRESS
Prenatal Stress and Neuropsychiatric Vulnerability
Epidemiological evidence implicates maternal stress during pregnancy as a factor in poor offspring neuropsychiatric outcome across the lifespan. Children prenatally exposed to maternal stressors such as anxiety, depression, bereavement, and natural or manmade disasters are more likely to present with neurodevelopmental disorders and subclinical psychosocial problems (Beversdorf et al, 2005; King and Laplante, 2005; Kinney et al, 2008a, 2008b; Li et al, 2010; Rodriguez and Bohlin, 2005). For example, a higher prevalence of autism was associated with in utero exposure to severe tropical storms and ADHD risk was significantly increased following prenatal maternal bereavement (Kinney et al, 2008a; Li et al, 2010). In addition, children whose mothers experienced high stress levels during the 1998 Quebec Ice Storm exhibited reduced cognitive and language abilities and increased autistic-like traits (Laplante et al, 2008; Walder et al, 2014). Studies suggest susceptibility is greatest in the first half of gestation, a period of development where males and females appear differentially affected (Gerardin et al, 2011; Huttunen and Niskanen, 1978; Khashan et al, 2008; van Os and Selten, 1998; Walder et al, 2014). Although males present more frequently with childhood behavioral disorders and intellectual impairment associated with prenatal stress, females acquire more subtle later-onset anxiety and affective disorders (Davis and Pfaff, 2014). Such studies predict important sex differences in underlying mechanisms originating early in gestation.
Animal models of prenatal stress have demonstrated disease-relevant endophenotypes that recapitulate aspects of these clinical findings. In nonhuman primates, chronic maternal stress leads to deficits in attention, object permanence, and motor function in offspring, in particular when the stress was experienced early in gestation (Schneider and Coe, 1993; Schneider, 1992; Schneider et al, 1999). In rodent models, prenatal stress induces hypothalamic–pituitary–adrenal (HPA) stress axis dysregulation, heightened behavioral stress reactivity, depression-like behaviors, and cognitive deficits (Bock et al, 2015; Brunton and Russell, 2010; Cottrell and Seckl, 2009; Darnaudéry and Maccari, 2008; Kapoor and Matthews, 2005; Kapoor et al, 2009; Lemaire et al, 2000; Mueller and Bale, 2007, 2008; Weinstock, 2008). Similar to human studies, the gestational timing of the stress and fetal sex are key determinants in offspring outcome, where studies in both mice and guinea pigs found that prenatal stress produced HPA axis dysregulation and cognitive effects only in male offspring exposed during early or mid-gestation (Kapoor and Matthews, 2005, 2008; Kapoor et al, 2009; Mueller and Bale, 2007, 2008).
Reprogramming in the Prenatally Stressed Brain
Although the long-term effects of maternal stress in humans on disease outcomes have been studied, earlier assessment of the perinatal brain has been limited to noninvasive measures of growth and macrostructure (Beversdorf et al, 2005; Khashan et al, 2008; Kinney et al, 2008b; Li et al, 2010; Ronald et al, 2010). These studies reported delayed overall brain growth, limbic system-specific volumetric changes, and white matter abnormalities (Li et al, 2012; Lou et al, 1994; Qiu et al, 2013, 2015; Rifkin-Graboi et al, 2013). Such early observations appear to persist into childhood and adolescence, are correlated with affective problems, and may contribute to symptoms in patients with schizophrenia and autism (Buss et al, 2010, 2012; Davis et al, 2013; Du et al, 2013; Sarkar et al, 2014; Zikopoulos and Barbas, 2010).
Results from animal models of maternal stress support these data and indicate that volumetric abnormalities in prenatally stressed offspring reflect reduced numbers of both neurons and glia, in particular in the hippocampus and amygdala due, in part, to decreased neurogenesis (Coe et al, 2003; Fujioka et al, 2006; Kawamura et al, 2006; Kraszpulski et al, 2006; Lemaire et al, 2000; Rayen et al, 2011). Further, reduced synaptogenesis and hypo-myelination in limbic regions was found in male, but not in female, juvenile rats exposed to maternal stress (Murmu et al, 2006; Xu et al, 2013). Such changes appear to persist into adulthood, where, eg, decreased hippocampal cell proliferation and reduced dendritic length and complexity were detected in adult prenatally stressed male rats (Mandyam et al, 2008; Suenaga et al, 2012). These data suggest potential primary impairment of cell proliferation, growth, survival, and connectivity in the prenatally stressed brain, and more specifically in the male brain.
PLACENTAL ORCHESTRATION OF FETAL BRAIN PROGRAMMING
The Programmatic Capacity of the Placenta
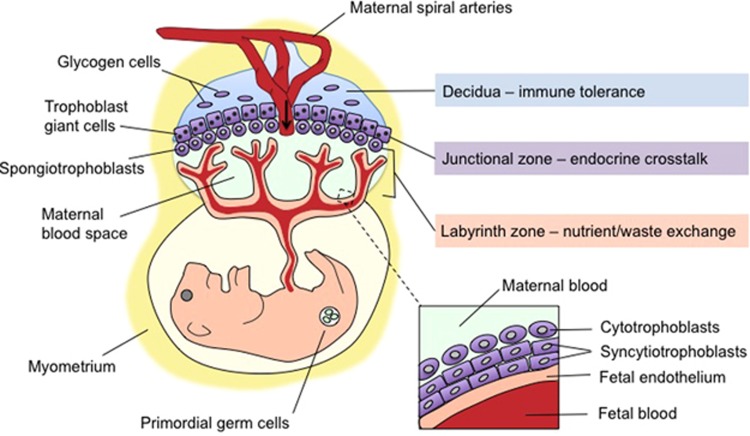
The mechanisms by which maternal stress confers lasting sex-specific neurobehavioral dysfunction likely initiate within the placenta. Stress-mediated perturbations of the maternal milieu must be conveyed to the embryo via interactions with the placenta. This transient organ is not simply a passive thoroughfare, but is an active maternal–fetal intermediary within which distinct functional zones collectively maintain intrauterine homeostasis (depicted in Figure 1). Although human and rodent placentas differ somewhat in their microstructure and developmental trajectory, the gross organization and functions of these zones are conserved (Georgiades et al, 2002). The decidua, comprising maternal uterine and immune cells, mediates immunological tolerance of the embryo (Arck and Hecher, 2013). Fetally derived trophoblast cells predominate in the basal plate (junctional zone in rodents), where they synthesize and secrete endocrine factors into both maternal and fetal circulations. Finally, in the chorionic villous (labyrinth zone in rodents), trophoblasts residing between the maternal and fetal vasculature control exchange of nutrients, oxygen, and waste via facilitated diffusion and macro- and micronutrient transporters (Georgiades et al, 2002; Rossant and Cross, 2001; Watson and Cross, 2005). Disruption of these critical functions can have an impact on fetal development, including the brain and primordial germ cells. Importantly, sex-specific reprogramming in response to maternal stress likely arises, owing to sex differences in trophoblasts derived from male (XY) and female (XX) embryos. Sex differences in placental size and gene expression have been identified in normal human and rodent placentas, and sex-specific placental abnormalities predict offspring outcome in pregnancies complicated by maternal asthma and preeclampsia (Buckberry et al, 2014; Clifton, 2010; Gabory et al, 2013; Howerton et al, 2013; Mao et al, 2010; O'Connell et al, 2013; Sood et al, 2006).
Figure 1.
Morphology of the maternal–fetal interface: navigating a complex interaction between maternal and fetal compartments. Mid-sagittal schematic depicting the intricate arrangement of maternal tissue (blue), fetally derived trophoblasts (all five subtypes in purple), and fetal endothelial cells (tan) within the three major functional zones of the mature mouse placenta. The decidua, the most superficial layer from the maternal side (top), comprises maternal uterine and immune cells, as well as specialized glycogen-storing cells from the trophoblast lineage. The decidua is traversed by the maternal spiral arteries, veins, and the fetally derived endovascular trophoblasts that line it. The arrow indicates the direction of maternal blood flow. Bordering the decidua is the junctional zone (basal plate in humans), where maternal vasculature penetrates a layer of trophoblast giant cells and spongiotrophoblasts (extravillous cytotrophoblasts in humans) that secrete hormones to modulate maternal–fetal cross-talk, angiogenesis, and tissue remodeling. Finally, in the labyrinth zone (chorionic villous in humans), cytotrophoblasts and syncytiotrophoblasts residing between maternal blood spaces and fetal endothelial cells (inset) prevent direct blood contact while facilitating selective and essential nutrient/waste exchange. Environmental stimuli such as maternal stress can disrupt vital aspects of placental organogenesis and function, including decidual immune tolerance, vascularization and utero-placental blood flow, trophoblast hormone secretion, and nutrient exchange within the labyrinth zone. The neurodevelopmental consequences of stress depend on the maturational state of the entire maternal–placental–fetal unit at the time of exposure. Although implantation occurs early in gestation (embryonic day 4.5 in mice and the second week in humans), placental maturation and expansion continues throughout gestation, leaving this fetal lifeline and the somatic and germ cells it sustains continuously vulnerable to maternal stress signals.
Stress at the Dynamic Maternal–Fetal Interface
Placental function is regulated by the collective responses of maternal decidual cells, trophoblastic cells, and fetal endothelial cells to the local environment (Fowden et al, 2008). Thus, disruption of the maternal milieu by stress and other stimuli can influence vital aspects of placental structure and function, including integrity of the protective transplacental barrier, nutrient and oxygen exchange, and placental endocrine action (Jansson and Powell, 2007; Myatt, 2006). The specific consequences likely depend on the timing of exposure, as stress signals interact with dynamic events ongoing in maternal, fetal, and placental compartments. Environmental stimuli may modulate the onset, offset, or duration of these sequential events, resulting in distinct programmatic outcomes.
For example, perturbations in early pregnancy are more likely to produce prolonged effects on placental function via interference with organizational processes including trophoblast differentiation and vascular remodeling (Watson and Cross, 2005). Such changes would then have the potential to impact neurodevelopment across the duration of pregnancy, likely initiating broad programmatic consequences. Epidemiological studies support this unique early gestational vulnerability, as increased schizophrenia and autism risk were specific to first-trimester maternal stress and viral infection exposure, respectively (Atladóttir et al, 2010; Khashan et al, 2008). In contrast, late-pregnancy perturbations may have a more transient impact on placental actions, by impairing nutrient delivery during periods of high fetal demand or disrupting processes such as neurogenesis, synaptogenesis, and early myelination in the fetal brain. This timing would likely introduce region and cell-type-specific effects on the developing brain that vary depending on species-specific neurodevelopmental trajectories (Andersen, 2003; Avishai-Eliner et al, 2002). The capacity for reprogramming, however, is not limited to fetal somatic cells. Beginning in the second week of gestation in mice (3–10 weeks post conception in humans), the newly specified primordial germ cells migrate and undergo a wave of epigenetic remodeling, permitting a unique window during which stress effects may be transmitted to subsequent generations via new epigenetic marks (Bale, 2015; De Felici, 2013; Gapp et al, 2014; Saitou and Yamaji, 2012). Supporting this, male mice exposed to early prenatal stress and presenting with disease-relevant endophenotypes, including HPA axis dysfunction and hypothalamic reprogramming, transmitted this phenotype to their offspring in the absence of direct stress re-exposure (Morgan and Bale, 2011).
Human and animal studies have demonstrated timing-dependent and sex-specific effects of maternal stress on placental size, efficiency, and gene expression (summarized in Table 1). Recent studies using transgenic mouse lines to selectively target stress-sensitive placental genes, including O-GlcNAc transferase, were able to recapitulate the effects of prenatal stress on hypothalamic programming and function, and thereby provide strong evidence for the importance of placental function in brain development (Howerton and Bale, 2014; Howerton et al, 2013). In the next sections, we describe the effects of maternal stress on aspects of placental structure and function in more detail, and discuss potential mechanisms by which these changes may subsequently reprogram the developing brain.
Table 1. Summary of Maternal Stress Effects on the Placenta.
| Species | Stress type | Stress timing | Sex | Placenta phenotype | Reference |
|---|---|---|---|---|---|
| Human | Anxiety | 36 wpc | ? | ↑ Umbilical artery resistance, redistribution of fetal blood flow | Sjöström et al, 1997 |
| Human | Anxiety | 28–36 wpc | ? | ↑ Uterine artery resistance | Teixeira et al, 1999 |
| Human | Anxiety | 20 wpc | ? | No association with uterine artery resistance | Kent et al, 2002 |
| Human | Life stress | 30 wpc | ♂ and ♀ | ↑ Placenta weight | Tegethoff et al, 2010 |
| Human | Anxiety and depression | Second to third trimester | ♂ and ♀ | ↑ SLC6A4, trending↑SLC6A2 and 11βHSD2 | Ponder et al, 2011 |
| Human | Anxiety | Term | ♂ and ♀ | ↓ 11βHSD2 | O'Donnell et al, 2012 |
| Human | Depression | Term | ♂ and ♀ | Trending↓11βHSD2 | O'Donnell et al, 2012 |
| Human | Depression | Term | ♂ and ♀ | ↓ MAOA in villous trophoblasts | Blakeley et al, 2013 |
| Human | Anxiety | Term | ♂ and ♀ | Trending↓MAOA in villous trophoblasts | Blakeley et al, 2013 |
| Human | Depression | Pregnancy | ♂ and ♀ | ↑ Methylation of glucocorticoid receptor | Conradt et al, 2013 |
| Human | Anxiety | Pregnancy | ♂ and ♀ | ↑ Methylation of 11βHSD2 | Conradt et al, 2013 |
| Mouse | Chronic variable (1/day) | 1–7 dpc | ♂ and ♀ | ↑ IGF2 in ♂ and ♀,↓11βHSD in ♀ | Pankevich et al, 2009 |
| Mouse | Chronic variable (1/day) | 1–7 dpc | ♂ and ♀ | ♂:↑PPARα, IGFBP1, GLUT4, HIF3α ♀:↓PPARα,↑DNMT1 | Mueller and Bale, 2008 |
| Mouse | Chronic variable (1/day) | 1–7 dpc | ♂ and ♀ | ↓ OGT in ♂ and ♀ | Howerton et al, 2013 |
| Mouse | Chronic variable (1/day) | 1–7 dpc | ♂ and ♀ | ♂:↑FASL, pro-inflammatory cytokines (IL6, IL1B, IL12RA, PTGS2), chemokines (CCR7, CCL5, CXCL10), cell surface antigens (H2-EB1, PTPRC), and endothelial molecules (EDN1, SELP). ♀:↑FASL,↓CCL2. | Bronson and Bale, 2014 |
| Rat | Restraint (3/day) | 10–21 dpc | ♂ | ↓ GLUT1 and 11βHSD2,↑GLUT3 and GLUT4 | Mairesse et al, 2007 |
| Rat | Social defeat (1/day)+restraint (1/day) | 5–20 dpc | ? | ↑ 11βHSD2 in low, but not high anxiety rat strains | Lucassen et al, 2009 |
| Rat | Restraint (1/day) | 14–20 dpc | ? | ↓ 11βHSD2,↑DNMT3a,↑11βHSD2 methylation | Jensen Peña et al, 2012 |
Abbreviations: 11βHSD, 11β-hydroxysteroid dehydrogenase 2; DNMT, DNA methyltransferase; dpc, days post conception; GLUT, glucose transporter; HIF, hypoxia-inducible factor; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; IL, interleukin; MAOA, monoamine oxidase A; OGT, O-GlcNAc transferase; PPAR, peroxisome proliferator-activated receptor; PTGS, prostaglandin; SLC6A4, serotonin transporter; SLC6A2, norepinephrine transporter; wpc, weeks post conception.
In human studies, stress timing refers to the gestational age of maternal stress assessment (wpc). In rodent studies, stress timing indicates the gestational age at maternal stress administration (dpc),
Transplacental Barrier Permeability
One prominent hypothesis posits that stress compromises the transplacental barrier and, in turn, increases fetal exposure to selectively permeable factors such as steroid hormones and exogenous teratogens (Aye and Keelan, 2013; Seckl and Holmes, 2007). In addition to the structural separation within the labyrinth zone that prevents direct contact between maternal and fetal blood supplies (depicted in Figure 1, inset), metabolizing enzymes localized within trophoblasts facilitate fetal protection from excess glucocorticoids and amines (Brown et al, 1996; Nguyen et al, 1999). These barrier enzymes can be sensitive to maternal stress, where, eg, reduced placental expression of the glucocorticoid-inactivating enzyme, 11β-hydroxysteroid dehydrogenase type-2 (11βHSD2), has been associated with maternal anxiety and depressed mood in humans, as well as with chronic maternal stress in rodents (Blakeley et al, 2013; Jensen Peña et al, 2012; Mairesse et al, 2007; O'Donnell et al, 2012; Pankevich et al, 2009; Ponder et al, 2011). The result is for a potential fetal glucocorticoid overexposure to impact ongoing developmental events including a restriction of fetal growth, premature maturation of proliferative neural precursors, and altered HPA axis development (Seckl and Holmes, 2007). Notably, these predicted effects are consistent with the reduced perinatal brain volumetric findings in association with maternal stress described earlier (Li et al, 2012; Lou et al, 1994; Qiu et al, 2013). In addition to the potential for actions of excess glucocorticoids on the developing fetal brain, excess glucocorticoids also act within the placenta to have an impact on endocrine functions, thus compromising placental growth, vascularization, and nutrient transport (Hewitt et al, 2006; Wyrwoll et al, 2009). Further, known sex differences in placental 11βHSD2 expression, both at baseline and in response to environmental stimuli including stress, may contribute to sex-biased neurodevelopmental outcomes (Cuffe et al, 2011; Pankevich et al, 2009).
In addition to glucocorticoid release, stress increases levels of stress-related neurotransmitters, including serotonin, norepinephrine, and dopamine within both the maternal brain and circulation (Joëls and Baram, 2009). However, less is known about maternal stress effects on placental barrier proteins that may moderate passage of these amines to the fetus. Their fate within the placenta is controlled by their cell-surface transporters and intracellular metabolizing enzymes, such as monoamine oxidase (MAO) and catechol-O-methyl transferase, which regulate delivery to the fetus as well as local processes within the placenta (Nguyen et al, 1999). Recent human studies have associated maternal stress with increased levels of serotonin and norepinephine transporters, and a downregulation of MAO in villous trophoblasts at term (Blakeley et al, 2013; Ponder et al, 2011). As these cells reside between the maternal and fetal vasculature, such changes in transport would increase their intrauterine availability, especially that of serotonin, which is synthesized in maternal, placental, and fetal compartments (Bonnin and Levitt, 2011; Nguyen et al, 1999; Verhaagh et al, 2001). Subsequent fetal overexposure may have deleterious effects on brain development, where, eg, serotonin excess impaired embryonic cortical interneuron migration in mice (Riccio et al, 2009; Velasquez et al, 2013). Serotonin, a potent vasoconstrictor, also elevates vascular resistance and reduces utero-placental blood flow, a mechanism thought to underlie hypertension in preeclampsia and gestational diabetes (Bolte et al, 2001; Li et al, 2014). Consistent with these vascular effects, maternal stress has been correlated with Doppler ultrasound indicators of restricted umbilical artery blood flow (Sjöström et al, 1997; Teixeira et al, 1999). Taken together, maternal stress-induced increased permeability of the transplacental barrier can influence fetal brain development via both direct and indirect actions, and aspects of placental function, such as nutrient transfer and endocrine actions.
Nutrient Exchange and Energy Homeostasis
The fetus relies on placental transfer of nutrients from the maternal circulation, which is achieved via diffusion or active transport across trophoblastic and endothelial cell membranes. The transfer capacity is determined by multiple factors including placenta size, vascularization, metabolism, and the availability of maternal-facing and fetal-facing transmembrane transporters. These factors are sensitive to the forces of maternal supply and fetal demand, and to adverse intrauterine conditions (Fowden et al, 2006; Jansson and Powell, 2006). Clinical studies suggest that placental transport capacity may be perturbed by maternal stress, owing to the utero-placental vascular dysfunction described in the previous section, which can impair bidirectional exchange of flow-limited substrates including oxygen and carbon dioxide (Myatt, 2006). The link between vascular function and nutrient delivery has been well established in uterine artery ligation models of placental insufficiency, where fetal hypoxia and oxidative stress are associated with reduced hippocampal size, abnormal neural migration, and hypomyelination in the offspring (Basilious et al, 2014; Lane et al, 2001; Reid et al, 2012). In addition, uterine artery ligation in rats disrupted striatal amino acid metabolism at term (Thordstein et al, 1992). Thus, fetal hypoxia, oxidative stress, and altered amino acid availability subsequent to stress-induced vascular dysfunction can adversely impact cell survival and differentiation within the fetal brain.
Although not yet investigated in humans, dysregulation of placental nutrient transporters has been demonstrated in animal models of prenatal stress (Mairesse et al, 2007; Mueller and Bale, 2008). Transporters are necessary for the delivery of maternally derived glucose, amino acids, fatty acids, and cholesterol-containing lipoproteins, macronutrients essential for fetal development (Brett et al, 2014; Lager and Powell, 2012). Placental expression of the glucose transporter (GLUT) family is particularly sensitive to adverse conditions, such as psychosocial stress, diabetes, and malnutrition in both humans and rodents (Das et al, 1998; Illsley, 2000; Mairesse et al, 2007; Mueller and Bale, 2008). Further, maternal stress decreased GLUT1 in rat placentas at term (Mairesse et al, 2007; Mueller and Bale, 2008). As GLUT1 is the predominant isoform in late pregnancy, these data suggest that stress may reduce glucose transfer to the fetus (Brett et al, 2014; Illsley, 2000). In addition, the effects of stress on nutrient exchange are also sex dependent, where stress increased expression of GLUT4 as well as genes regulating fatty acid and oxygen availability in male, but not in female, placentas in rodents (Mairesse et al, 2007; Mueller and Bale, 2008). These changes, indicative of disrupted intrauterine energy homeostasis, were also associated with markers of delayed neurodevelopment in male neonates (Mueller and Bale, 2008).
Although changes in nutrient exchange in response to environmental stimuli promote adaptive advantages and maintenance of maternal–fetal homeostasis in utero, dysregulated availability of glucose, amino acids, and fatty acids initiates broad programmatic effects on the fetal brain (Innis, 2005; Morgane et al, 1993). For example, the fetus relies on fatty acids derived from the maternal circulation (Lager and Powell, 2012). Deficiency in long-chain polyunsaturated fatty acids during gestation, such as docosahexaenoic acid and arachidonic acid, which comprise 40%–50% of neuronal membrane phospholipids in the developing brain, resulted in altered neuronal membrane composition, increased neural inflammation, impaired microglia motility, and programmed long-term impairment in sensorimotor gating in offspring (Fedorova et al, 2009; Madore et al, 2014; Tam and Innis, 2006). Reduced fatty acid transport can also directly compromise placental function, owing to excess accumulation and subsequent oxidative stress (Jarvie et al, 2010). The high fetal demand for cholesterol in late gestation is met by both placental transfer from the maternal circulation and by fetal de novo synthesis (Lindegaard et al, 2008; Yoshida and Wada, 2005). Therefore, changes in placental cholesterol uptake and transfer are more likely to affect the fetal brain via further impact on placental function. For example, impaired trophoblast uptake of cholesterol may disrupt maternal–fetal cross-talk by reducing substrates for steroidogenesis as well as for synthesis of cholesterol-containing exosomes, non-hormonal communicators that regulate immune homeostasis (Beninson and Fleshner, 2014; Ouyang et al, 2014).
Endocrine Action
Bidirectional communication is achieved via hormone secretion by maternal-facing and fetal-facing trophoblasts. These cells synthesize and secrete growth factors, immunomodulators, sex steroids, metabolic mediators such as leptin and lactogen, and neuromodulators such as corticotropin-releasing factor (CRF) and serotonin (reviewed in Bonnin and Levitt, 2011; Bowen et al, 2002; Fowden et al, 2014; Reis et al, 2001; Sagawa et al, 2002; Sandman, 2015). Placental hormones act as endocrine, paracrine, and autocrine modulators of maternal and fetal physiology throughout pregnancy, in particular during implantation, at parturition, and in response to intrauterine conditions including stress signals. Their synthesis is regulated, in part, by gestational age and fetal sex, and some placental hormones are species specific (Carter, 2012; Fowden et al, 2014).
Perturbation of placental endocrine actions by stress can have profound effects on fetal neurodevelopment and subsequent disease risk. The role of placental-derived CRF in this reprogramming has been thoroughly investigated (reviewed in Avishai-Eliner et al, 2002; Sandman, 2015). Although placental synthesis of CRF in rodents has not yet been demonstrated, the human placenta produces CRF beginning in the seventh gestational week, the levels of which exponentially increase in the maternal circulation as pregnancy progresses (Emanuel et al, 1994; Goland et al, 1988; Petraglia et al, 1987; Robinson et al, 1989). Placental CRF is also released into the fetal circulation, where it has influence on nervous system development including regulation of proliferation and survival of neural progenitors (Koutmani et al, 2013). The bioavailability of CRF during pregnancy is determined by levels of a circulating binding protein (CRF-BP) (Bowman et al, 2001). Stress-related changes in CRF and CRF-BP can lead to neurotoxic effects, in particular in HPA and limbic circuits (Avishai-Eliner et al, 2002; Fujioka et al, 1999). Supporting this, negative correlations between placental CRF levels and fetal startle responses suggest that CRF overexposure delays maturation in humans (Sandman, 2015).
PLACENTAL INTEGRATION OF STRESS SIGNALS
Signal Detection and Response Coordination
Information about adverse intrauterine status is communicated to the placenta by maternally and fetally derived endocrine factors, which together determine placental adaptive responses and resource re-allocation. The placenta expresses receptors for numerous hormones including glucocorticoids, insulin, insulin-like growth factors (IGFs), leptin, gonadal hormones, cytokines, and prostaglandins (Bodner et al, 1999; Fowden et al, 2014; Hiden et al, 2006; Keelan and Mitchell, 2007; McCormick et al, 1981; Unlugedik et al, 2010). These signals are integrated by downstream cascades that ultimately influence placental growth, energy homeostasis, and endocrine action. For example, Jansson and Powell (2006) proposed that the mammalian target of rapamycin (mTOR) pathway serves as a nutrient sensor that regulates transporter expression in order to facilitate compatibility between maternal supply and fetal demand. Circulating insulin and IGF1 activate the protein kinase activity of mTOR, leading to increased expression of genes promoting cell growth and metabolism (Roos et al, 2009).
Stress during pregnancy compromises the maternal hormonal milieu in humans and in animal models, where elevations in CRF, pro-inflammatory cytokines, and glucocorticoids have been reported (Parker and Douglas, 2010). The placenta integrates these signals and facilitates a coordinated, sex-specific response involving immune function, nutrient metabolism/transport, or its own endocrine actions (Fowden et al, 2014). For example, in mice, prenatal stress increased pro-inflammatory cytokines, including tumor necrosis factor-α and interleukin-6 in male, but not in female, placentas and the neurodevelopmental programming effects in this model were ameliorated by maternal anti-inflammatory treatment during stress exposure (Bronson and Bale, 2014; Mueller and Bale, 2008). The effects of immune dysregulation on the developing brain program endophenotypes of neurodevelopmental disorders, including autism and schizophrenia, and have been reviewed previously (Hsiao and Patterson, 2012).
A Common Programmatic Pathway
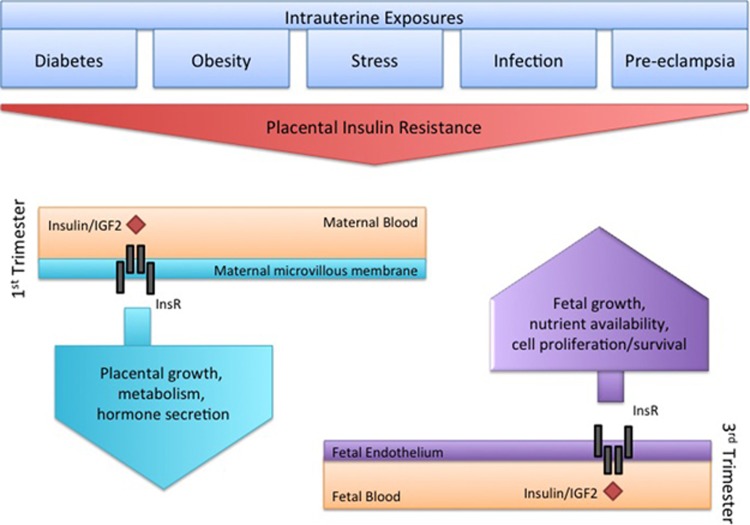
In addition to maternal psychosocial stress, fetal exposures involving stressful metabolic challenges are also associated with increased neuropsychiatric disease risk, including maternal diabetes, obesity, infection, and pre-eclampsia (Bale et al, 2010; Brown and Derkits, 2010; Walker et al, 2015). It is well established that maternal insulin is dysregulated in pregnancies complicated by diabetes, obesity, undernutrition, and preeclampsia, and insulin resistance also occurs in the placentas from diabetic, preeclamptic, and growth-restricted pregnancies (Colomiere et al, 2009; Rademacher et al, 2007; Scioscia et al, 2006; Street et al, 2011). Maternal infection during pregnancy increases cytokines within the placenta and is predicted to disrupt insulin signaling due to the inhibitory effects of cytokines on insulin action (Aguirre et al, 2002; Sykiotis and Papavassiliou, 2001; Tanti and Jager, 2009). This same mechanism is proposed to impair placental insulin signaling in pregnancies complicated by maternal psychosocial stress, as pro-inflammatory cytokines were increased in stress-exposed placentas and were associated with the male-biased endophenotype (Bronson and Bale, 2014). Programmatic effects in these conditions likely depend on the gestational age at which placental insulin signaling is affected, as insulin receptors are expressed in mammalian placental trophoblasts and fetal endothelial cells in a spatiotemporal pattern (Desoye et al, 1994). Functional analysis of insulin-responsive genes suggests that maternal insulin regulates placental metabolism (predominately of lipids and fatty acids) in early pregnancy, whereas fetal insulin communicates demand for growth and cell proliferation/survival near term (Hiden et al, 2006). Therefore, insulin perturbation can influence placental function, nutrient availability, and intrauterine homeostasis throughout gestation (depicted in Figure 2) and vastly have an impact on neurodevelopmental programming.
Figure 2.
Insulin signaling as potential common programmatic placental pathway. A simplified schematic depicting the predicted impairment of insulin signaling within placentas complicated by diverse fetal exposures. In diabetes, obesity, and pre-eclampsia, changes in insulin receptor (InsR) localization, kinase activity, and substrate availability lead to placenta insulin resistance. Maternal stress and infection are predicted to also elicit insulin resistance, owing to the inhibitory effects of cytokines on insulin action. Predicted programmatic effects of placenta insulin resistance depend on exposure timing. In early pregnancy, InsRs are localized to maternal-facing trophoblasts and predominately regulate expression of genes related to metabolism of lipids and fatty acids. Such changes may have an impact on placental growth, trophoblast survival, and hormone secretion in early pregnancy. Later in gestation, their expression is restricted to the fetal endothelial cells where insulin communicates fetal demand for growth, cell proliferation, and cell survival.
Encoding of Stress Memories in the Epigenome
Epigenomic remodeling is increasingly recognized as a molecular bridge linking placental adaptive responses to adversity with long-term phenotypic outcomes. Epigenetic processes including DNA methylation, histone modifications, and changes in small noncoding RNA expression are dynamic mechanisms by which the environment can shape gene expression and placental function, and when maintained within the fetal germ cells can influence the phenotype of future generations (reviewed in Bale, 2015; Franklin et al, 2010; Maccani and Marsit, 2009; Marsit, 2015; Monk et al, 2012). Tight epigenetic regulation of gene expression is essential for normal developmental processes, including placentation, cell fate determination, genomic imprinting, and X-inactivation, in particular throughout critical developmental periods during which imprinted epigenetic marks are erased and re-established (Gabory et al, 2011; Rugg-Gunn, 2012).
Intrauterine conditions, especially perturbations in nutrient availability and metabolism, have dramatic effects on epigenetic machinery (Tarrade et al, 2015). For example, maternally derived micronutrients such as folate and choline serve as substrates for DNA methyltransferases (DNMT) and their deficiency decreases placental DNA methylation (Kim et al, 2009). In calorie-restricted as well as high-fat diet-exposed mice, distinct hypomethylation patterns were detected in male and female placentas, including at imprinted loci, suggesting sex-specific placental adaptive responses to nutritional state (Chen et al, 2013; Gallou-Kabani et al, 2010). Similarly, maternal stress in humans and rodents elicits sex-specific placental epigenetic adaptations, including changes in DNA methylation and DNMT expression that were associated with neurodevelopmental outcomes (Table 1, (Conradt et al, 2013; Jensen Peña et al, 2012; Mueller and Bale, 2008). Differential methylation of the placental 11βHSD2 promoter has been the epigenetic mark most consistently correlated with gene expression levels and maternal stress (Conradt et al, 2013; Jensen Peña et al, 2012). Although the effects of stress on histone modifications and microRNA expression within the placenta have yet to be fully elucidated, it is clear that placental epigenetic processes maintain important sex differences in homeostatic strategies at the maternal–fetal interface. Sex-dependent molecular memories of the prenatal environment can then be encoded by the fetal epigenome and, when incorporated into the germline, communicated to future generations.
CONCLUSIONS
Nearly 20%–40% of pregnancies are complicated by adverse intrauterine conditions, including maternal mental health disorders, diabetes, obesity, and preeclampsia (Ananth et al, 2013; Bennett et al, 2004; Dawson et al, 2015; DeSisto et al, 2014; Goodman et al, 2014; Rubertsson et al, 2014). These fetal exposures are significant risk factors for neuropsychiatric disease predisposition, in particular in male offspring. Growing evidence supports a critical role for the placenta in the deleterious and sex-specific effects of these fetal exposures on the developing brain. Although a great deal more investigation is necessary to elucidate the specific mechanisms by which the placenta imparts these critical signals to the developing embryo, the potential importance of the placenta as a biomarker of maternal stress and its potential for determining neurodevelopmental and neuropsychiatric disease risk is enormous.
FUNDING AND DISCLOSURE
The work described was funded by grants from NIH MH073030, MH087597, MH091258, MH099910 and MH104184. The authors declare no conflict of interest.
Acknowledgments
We thank Ali Rodgers and Katie Morrison for reading of the manuscript.
References
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF (2002). Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537. [DOI] [PubMed] [Google Scholar]
- Ananth C V, Keyes KM, Wapner RJ (2013). Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 347: f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27: 3–18. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Jacobs-Stannard A, Chawarska K, Volkmar FR, Kliman HJ (2007). Placental trophoblast inclusions in autism spectrum disorder. Biol Psychiatry 61: 487–491. [DOI] [PubMed] [Google Scholar]
- Arck PC, Hecher K (2013). Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med 19: 548–556. [DOI] [PubMed] [Google Scholar]
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M et al (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40: 1423–1430. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ (2002). Stressed-out, or in (utero)? Trends Neurosci 25: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye ILMH, Keelan JA (2013). Placental ABC transporters, cellular toxicity and stress in pregnancy. Chem Biol Interact 203: 456–466. [DOI] [PubMed] [Google Scholar]
- Baio J (2012). Prevalence of autism spectrum disorders: autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Sum Volume 61(Number 3): 1–19 Centers Dis Control Prev at 〈http://eric.ed.gov/?id=ED530639〉. [PubMed] [Google Scholar]
- Bale TL (2011). Sex differences in prenatal epigenetic programming of stress pathways. Stress 14: 348–356. [DOI] [PubMed] [Google Scholar]
- Bale TL (2015). Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 16: 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM et al (2010). Early life programming and neurodevelopmental disorders. Biol Psychiatry 68: 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ (1997). Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 13: 807–813. [DOI] [PubMed] [Google Scholar]
- Basilious A, Yager J, Fehlings MG (2014). Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: a systematic review. Dev Med Child Neurol 57: 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninson LA, Fleshner M (2014). Exosomes: an emerging factor in stress-induced immunomodulation. Semin Immunol 26: 394–401. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR (2004). Prevalence of depression during pregnancy: systematic review. Obstet Gynecol 103: 698–709. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE et al (2005). Timing of prenatal stressors and autism. J Autism Dev Disord 35: 471–478. [DOI] [PubMed] [Google Scholar]
- Blakeley PM, Capron LE, Jensen AB, O'Donnell KJ, Glover V (2013). Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J Psychosom Res 75: 341–345. [DOI] [PubMed] [Google Scholar]
- Bloom B, Cohen RA, Freeman G (2011). Summary health statistics for U.S. children: National Health Interview Survey, 2010. Vital Health Stat 10: 1–80 at 〈http://www.ncbi.nlm.nih.gov/pubmed/22338334〉. [PubMed] [Google Scholar]
- Bock J, Rether K, Gröger N, Xie L, Braun K (2014). Perinatal programming of emotional brain circuits: an integrative view from systems to molecules. Front Neurosci 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Wainstock T, Braun K, Segal M (2015). Stress in utero: prenatal programming of brain plasticity and cognition. Biol Psychiatry 78: 315–326. [DOI] [PubMed] [Google Scholar]
- Bodner J, Ebenbichler CF, Wolf HJ, Müller-Holzner E, Stanzl U, Gander R et al (1999). Leptin receptor in human term placenta: in situ hybridization and immunohistochemical localization. Placenta 20: 677–682. [DOI] [PubMed] [Google Scholar]
- Bolte AC, Geijn HP, van, Dekker GA (2001). Pathophysiology of preeclampsia and the role of serotonin. Eur J Obstet Gynecol Reprod Biol 95: 12–21. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Levitt P (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen JM, Chamley L, Keelan JA, Mitchell MD (2002). Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta 23: 257–273. [DOI] [PubMed] [Google Scholar]
- Bowman ME, Lopata A, Jaffe RB, Golos TG, Wickings J, Smith R (2001). Corticotropin-releasing hormone-binding protein in primates. Am J Primatol 53: 123–130. [DOI] [PubMed] [Google Scholar]
- Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB (2014). Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 15: 16153–16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Bale TL (2014). Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155: 2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ (2010). Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167: 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotelevtsev Y V, Mullins JJ, Kaufman MH et al (1996). The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology 137: 794–797. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA (2010). Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol 22: 258–271. [DOI] [PubMed] [Google Scholar]
- Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT (2014). Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Mol Hum Reprod 20: 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA (2010). High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9-year-old children. Psychoneuroendocrinology 35: 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA 109: E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM (2012). Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol Rev 92: 1543–1576. [DOI] [PubMed] [Google Scholar]
- Chen P-Y, Ganguly A, Rubbi L, Orozco LD, Morselli M, Ashraf D et al (2013). Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol Genomics 45: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL (2010). Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 31 Suppl: S33–S39. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C et al (2003). Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 54: 1025–1034. [DOI] [PubMed] [Google Scholar]
- Colomiere M, Permezel M, Riley C, Desoye G, Lappas M (2009). Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol 160: 567–578. [DOI] [PubMed] [Google Scholar]
- Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ (2013). The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 8: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR (2009). Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffe JSM, Dickinson H, Simmons DG, Moritz KM (2011). Sex specific changes in placental growth and MAPK following short term maternal dexamethasone exposure in the mouse. Placenta 32: 981–989. [DOI] [PubMed] [Google Scholar]
- Darnaudéry M, Maccari S (2008). Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57: 571–585. [DOI] [PubMed] [Google Scholar]
- Das UG, Sadiq HF, Soares MJ, Hay WW, Devaskar SU (1998). Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol 274: R339–R347. [DOI] [PubMed] [Google Scholar]
- Davis EP, Pfaff D (2014). Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology 49: 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K (2013). Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry 74: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson AL, Razzaghi H, Arth A, Canfield MA, Parker SE, Reefhuis J (2015). Maternal exposures in the National Birth Defects Prevention Study: time trends of selected exposures. Birth Defects Res A Clin Mol Teratol 103: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felici M (2013). Origin, migration and proliferation of human primoridal germ cells. Oogenesis 19–37 (doi:10.1007/978-0-85729-826-3).
- DeSisto CL, Kim SY, Sharma AJ (2014). Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis 11: E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoye G, Hartmann M, Blaschitz A, Dohr G, Hahn T, Kohnen G et al (1994). Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern. Histochemistry 101: 277–285. [DOI] [PubMed] [Google Scholar]
- Du F, Cooper AJ, Thida T, Shinn AK, Cohen BM, Ongür D (2013). Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biol Psychiatry 74: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörner G (1973). [Possible significance of prenatal and-or perinatal nutrition for the pathogenesis of obesity]. Acta Biol Med Ger 30: K19–K22. [PubMed] [Google Scholar]
- Emanuel RL, Robinson BG, Seely EW, Graves SW, Kohane I, Saltzman D et al (1994). Corticotrophin releasing hormone levels in human plasma and amniotic fluid during gestation. Clin Endocrinol (Oxf) 40: 257–262. [DOI] [PubMed] [Google Scholar]
- Fedorova I, Alvheim AR, Hussein N, Salem N (2009). Deficit in prepulse inhibition in mice caused by dietary n-3 fatty acid deficiency. Behav Neurosci 123: 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ, Coan PM, Burton GJ (2008). The placenta and intrauterine programming. J Neuroendocrinol 20: 439–450. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ, Sferruzzi-Perri AN, Burton GJ, Vaughan OR (2014). Review: endocrine regulation of placental phenotype. Placenta 36 Suppl 1: S50–S59. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Wooding FPB, Forhead AJ, Constancia M (2006). Programming placental nutrient transport capacity. J Physiol 572: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A et al (2010). Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 68: 408–415. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS (2007). Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med 161: 857–864. [DOI] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S (2006). Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience 141: 907–915. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Sakata Y, Yamaguchi K, Shibasaki T, Kato H, Nakamura S (1999). The effects of prenatal stress on the development of hypothalamic paraventricular neurons in fetal rats. Neuroscience 92: 1079–1088. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C (2011). Developmental programming and epigenetics. Am J Clin Nutr 94: 1943S–1952S. [DOI] [PubMed] [Google Scholar]
- Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C (2013). Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallou-Kabani C, Gabory A, Tost J, Karimi M, Mayeur S, Lesage J et al (2010). Sex- and diet-specific changes of imprinted gene expression and DNA methylation in mouse placenta under a high-fat diet. PLoS One 5: e14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Ziegler L, von, Tweedie-Cullen RY, Mansuy IM (2014). Early life epigenetic programming and transmission of stress-induced traits in mammals: how and when can environmental factors influence traits and their transgenerational inheritance? Bioessays 36: 491–502. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ (2002). Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23: 3–19. [DOI] [PubMed] [Google Scholar]
- Gerardin P, Wendland J, Bodeau N, Galin A, Bialobos S, Tordjman S et al (2011). Depression during pregnancy: is the developmental impact earlier in boys? A prospective case-control study. J Clin Psychiatry 72: 378–387. [DOI] [PubMed] [Google Scholar]
- Goland RS, Wardlaw SL, Blum M, Tropper PJ, Stark RI (1988). Biologically active corticotropin-releasing hormone in maternal and fetal plasma during pregnancy. Am J Obstet Gynecol 159: 884–890. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Chenausky KL, Freeman MP (2014). Anxiety disorders during pregnancy: a systematic review. J Clin Psychiatry 75: e1153–e1184. [DOI] [PubMed] [Google Scholar]
- Hewitt DP, Mark PJ, Waddell BJ (2006). Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology 147: 5568–5574. [DOI] [PubMed] [Google Scholar]
- Hiden U, Maier A, Bilban M, Ghaffari-Tabrizi N, Wadsack C, Lang I et al (2006). Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia 49: 123–131. [DOI] [PubMed] [Google Scholar]
- Howerton CL, Bale TL (2014). Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc Natl Acad Sci USA 111: 9639–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, Bale TL (2013). O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci USA 110: 5169–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH (2012). Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol 72: 1317–1326. [DOI] [PubMed] [Google Scholar]
- Huttunen MO, Niskanen P (1978). Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry 35: 429–431. [DOI] [PubMed] [Google Scholar]
- Illsley NP (2000). Glucose transporters in the human placenta. Placenta 21: 14–22. [DOI] [PubMed] [Google Scholar]
- Innis SM (2005). Essential fatty acid transfer and fetal development. Placenta 26(Suppl A): S70–S75. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL (2006). IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? — a review. Placenta 27(Suppl A): S91–S97. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL (2007). Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 113: 1–13. [DOI] [PubMed] [Google Scholar]
- Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ (2010). Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 119: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen Peña C, Monk C, Champagne FA (2012). Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One 7: e39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Baram TZ (2009). The neuro-symphony of stress. Nat Rev Neurosci 10: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Kostaki A, Janus C, Matthews SG (2009). The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res 197: 144–149. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG (2005). Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol 566: 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG (2008). Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology 149: 6406–6415. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Chen J, Takahashi T, Ichitani Y, Nakahara D (2006). Prenatal stress suppresses cell proliferation in the early developing brain. Neuroreport 17: 1515–1518. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Mitchell MD (2007). Placental cytokines and preeclampsia. Front Biosci 12: 2706–2727. [DOI] [PubMed] [Google Scholar]
- Kent A, Hughes P, Ormerod L, Jones G, Thilaganathan B (2002). Uterine artery resistance and anxiety in the second trimester of pregnancy. Ultrasound Obstet Gynecol 19: 177–179. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN et al (2008). Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry 65: 146–152. [DOI] [PubMed] [Google Scholar]
- Kim J-M, Hong K, Lee JH, Lee S, Chang N (2009). Effect of folate deficiency on placental DNA methylation in hyperhomocysteinemic rats. J Nutr Biochem 20: 172–176. [DOI] [PubMed] [Google Scholar]
- King S, Laplante DP (2005). The effects of prenatal maternal stress on children's cognitive development: Project Ice Storm. Stress 8: 35–45. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E (2008. a). Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord 38: 481–488. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Miller AM (2008. b). Prenatal stress and risk for autism. Neurosci Biobehav Rev 32: 1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutmani Y, Politis PK, Elkouris M, Agrogiannis G, Kemerli M, Patsouris E et al (2013). Corticotropin-releasing hormone exerts direct effects on neuronal progenitor cells: implications for neuroprotection. Mol Psychiatry 18: 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszpulski M, Dickerson PA, Salm AK (2006). Prenatal stress affects the developmental trajectory of the rat amygdala. Stress 9: 85–95. [DOI] [PubMed] [Google Scholar]
- Lager S, Powell TL (2012). Regulation of nutrient transport across the placenta. J Pregnancy 2012: 179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RH, Ramirez RJ, Tsirka AE, Kloesz JL, McLaughlin MK, Gruetzmacher EM et al (2001). Uteroplacental insufficiency lowers the threshold towards hypoxia-induced cerebral apoptosis in growth-retarded fetal rats. Brain Res 895: 186–193. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S (2008). Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5½-year-old children. J Am Acad Child Adolesc Psychiatry 47: 1063–1072. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN (2000). Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 97: 11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C (2010). Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry 19: 747–753. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Z-N, Chen Y-P, Dong Y-P, Shuai H-L, Xiao X-M et al (2012). Late gestational maternal serum cortisol is inversely associated with fetal brain growth. Neurosci Biobehav Rev 36: 1085–1092. [DOI] [PubMed] [Google Scholar]
- Li Y, Hadden C, Singh P, Mercado CP, Murphy P, Dajani NK et al (2014). GDM-associated insulin deficiency hinders the dissociation of SERT from ERp44 and down-regulates placental 5-HT uptake. Proc Natl Acad Sci USA 111: E5697–E5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard ML, Wassif CA, Vaisman B, Amar M, Wasmuth E V, Shamburek R et al (2008). Characterization of placental cholesterol transport: ABCA1 is a potential target for in utero therapy of Smith-Lemli-Opitz syndrome. Hum Mol Genet 17: 3806–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Hansen D, Nordentoft M, Pryds O, Jensen F, Nim J et al (1994). Prenatal stressors of human life affect fetal brain development. Dev Med Child Neurol 36: 826–832. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Bosch OJ, Jousma E, Krömer SA, Andrew R, Seckl JR (2009). Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: possible key role of placental 11beta-hydroxysteroid dehydrogenase type 2. Eur J Neurosci 29: 97–103. [DOI] [PubMed] [Google Scholar]
- Maccani MA, Marsit CJ (2009). Epigenetics in the placenta. Am J Reprod Immunol 62: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore C, Nadjar A, Delpech J-C, Sere A, Aubert A, Portal C et al (2014). Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav Immun 41: 22–31. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Bréant B, Hahn T, Darnaudéry M et al (2007). Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab 292: E1526–E1533. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN (2008). Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol 68: 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS (2010). Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA 107: 5557–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Koenig JI (2011). Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology (Berl) 214: 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsit CJ (2015). Influence of environmental exposure on human epigenetic regulation. J Exp Biol 218: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick PD, Razel AJ, Spelsberg TC, Coulam CB (1981). Evidence for an androgen receptor in the human placenta. Am J Obstet Gynecol 140: 8–13. [DOI] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA (2012). Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev Psychopathol 24: 1361–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CP, Bale TL (2011). Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci 31: 11748–11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Díaz-Cintra S, Cintra L et al (1993). Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev 17: 91–128. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL (2007). Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav 91: 55–65. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 28: 9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J (2006). Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci 24: 1477–1487. [DOI] [PubMed] [Google Scholar]
- Myatt L (2006). Placental adaptive responses and fetal programming. J Physiol 572: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Tseng YT, McGonnigal B, Stabila JP, Worrell LA, Saha S et al (1999). Placental biogenic amine transporters: in vivo function, regulation and pathobiological significance. Placenta 20: 3–11. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten JP (1998). Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry 172: 324–326. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Mouillet J-F, Coyne CB, Sadovsky Y (2014). Review: placenta-specific microRNAs in exosomes - good things come in nano-packages. Placenta 35 Suppl: S69–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell BA, Moritz KM, Walker DW, Dickinson H (2013). Sexually dimorphic placental development throughout gestation in the spiny mouse (Acomys cahirinus. Placenta 34: 119–126. [DOI] [PubMed] [Google Scholar]
- O'Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O'Connor TG, Glover V (2012). Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology 37: 818–826. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Mueller BR, Brockel B, Bale TL (2009). Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiol Behav 98: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker VJ, Douglas AJ (2010). Stress in early pregnancy: maternal neuro-endocrine-immune responses and effects. J Reprod Immunol 85: 86–92. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Sawchenko PE, Rivier J, Vale W (1987). Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature 328: 717–719. [DOI] [PubMed] [Google Scholar]
- Ponder KL, Salisbury A, McGonnigal B, Laliberte A, Lester B, Padbury JF (2011). Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: implications for fetal programming. Dev Psychobiol 53: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP et al (2015). Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 5: e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Rifkin-Graboi A, Chen H, Chong Y-S, Kwek K, Gluckman PD et al (2013). Maternal anxiety and infants' hippocampal development: timing matters. Transl Psychiatry 3: e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher TW, Gumaa K, Scioscia M (2007). Preeclampsia, insulin signalling and immunological dysfunction: a fetal, maternal or placental disorder? J Reprod Immunol 76: 78–84. [DOI] [PubMed] [Google Scholar]
- Rayen I, Hove DL, van den, Prickaerts J, Steinbusch HW, Pawluski JL (2011). Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS One 6: e24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA, Grinspan JB (2012). Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol 71: 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis FM, Florio P, Cobellis L, Luisi S, Severi FM, Bocchi C et al (2001). Human placenta as a source of neuroendocrine factors. Biol Neonate 79: 150–156. [DOI] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabó G, Vutskits L et al (2009). Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry 14: 280–290. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Bai J, Chen H, Hameed WB, Sim LW, Tint MT et al (2013). Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry 74: 837–844. [DOI] [PubMed] [Google Scholar]
- Robinson BG, Arbiser JL, Emanuel RL, Majzoub JA (1989). Species-specific placental corticotropin releasing hormone messenger RNA and peptide expression. Mol Cell Endocrinol 62: 337–341. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bohlin G (2005). Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry 46: 246–254. [DOI] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJO (2010). Prenatal maternal stress associated with adhd and autistic traits in early childhood. Front Psychol 1: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos S, Powell TL, Jansson T (2009). Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans 37: 295–298. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC (2001). Placental development: lessons from mouse mutants. Nat Rev Genet 2: 538–548. [DOI] [PubMed] [Google Scholar]
- Rubertsson C, Hellström J, Cross M, Sydsjö G (2014). Anxiety in early pregnancy: prevalence and contributing factors. Arch Womens Ment Health 17: 221–228. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn PJ (2012). Epigenetic features of the mouse trophoblast. Reprod Biomed Online 25: 21–30. [DOI] [PubMed] [Google Scholar]
- Sagawa N, Yura S, Itoh H, Kakui K, Takemura M, Nuamah MA et al (2002). Possible role of placental leptin in pregnancy: a review. Endocrine 19: 65–71. [DOI] [PubMed] [Google Scholar]
- Saitou M, Yamaji M (2012). Primordial germ cells in mice. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA (2015). Fetal exposure to placental corticotropin-releasing hormone (pCRH) programs developmental trajectories. Peptides doi:10.1016/j.peptides.2015.03.020 (in press). [DOI] [PMC free article] [PubMed]
- Sarkar S, Craig MC, Dell'Acqua F, O'Connor TG, Catani M, Deeley Q et al (2014). Prenatal stress and limbic-prefrontal white matter microstructure in children aged 6-9 years: a preliminary diffusion tensor imaging study. World J Biol Psychiatry 15: 346–352. [DOI] [PubMed] [Google Scholar]
- Schneider ML (1992). Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Dev Psychobiol 25: 529–540. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Coe CL (1993). Repeated social stress during pregnancy impairs neuromotor development of the primate infant. J Dev Behav Pediatr 14: 81–87. [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR (1999). Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev 70: 263–274. [DOI] [PubMed] [Google Scholar]
- Scioscia M, Gumaa K, Kunjara S, Paine MA, Selvaggi LE, Rodeck CH et al (2006). Insulin resistance in human preeclamptic placenta is mediated by serine phosphorylation of insulin receptor substrate-1 and -2. J Clin Endocrinol Metab 91: 709–717. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC (2007). Mechanisms of disease: glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nat Clin Pract Endocrinol Metab 3: 479–488. [DOI] [PubMed] [Google Scholar]
- Sjöström K, Valentin L, Thelin T, Marsál K (1997). Maternal anxiety in late pregnancy and fetal hemodynamics. Eur J Obstet Gynecol Reprod Biol 74: 149–155. [DOI] [PubMed] [Google Scholar]
- Sood R, Zehnder JL, Druzin ML, Brown PO (2006). Gene expression patterns in human placenta. Proc Natl Acad Sci USA 103: 5478–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street ME, Viani I, Ziveri MA, Volta C, Smerieri A, Bernasconi S (2011). Impairment of insulin receptor signal transduction in placentas of intra-uterine growth-restricted newborns and its relationship with fetal growth. Eur J Endocrinol 164: 45–52. [DOI] [PubMed] [Google Scholar]
- Suenaga T, Yukie M, Gao S, Nakahara D (2012). Sex-specific effects of prenatal stress on neuronal development in the medial prefrontal cortex and the hippocampus. Neuroreport 23: 430–435. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Papavassiliou AG (2001). Serine phosphorylation of insulin receptor substrate-1: a novel target for the reversal of insulin resistance. Mol Endocrinol 15: 1864–1869. [DOI] [PubMed] [Google Scholar]
- Tam O, Innis SM (2006). Dietary polyunsaturated fatty acids in gestation alter fetal cortical phospholipids, fatty acids and phosphatidylserine synthesis. Dev Neurosci 28: 222–229. [DOI] [PubMed] [Google Scholar]
- Tanti J-F, Jager J (2009). Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol 9: 753–762. [DOI] [PubMed] [Google Scholar]
- Tarrade A, Panchenko P, Junien C, Gabory A (2015). Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol 218: 50–58. [DOI] [PubMed] [Google Scholar]
- Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G (2010). Maternal psychosocial stress during pregnancy and placenta weight: evidence from a national cohort study. PLoS One 5: e14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira JM, Fisk NM, Glover V (1999). Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 318: 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordstein M, Andiné P, Lehmann A, Hagberg H (1992). Cerebral amino acids and energy metabolites in the growth retarded rat fetus under normoxia and hypoxia. J Dev Physiol 18: 59–65. [PubMed] [Google Scholar]
- Unlugedik E, Alfaidy N, Holloway A, Lye S, Bocking A, Challis J et al (2010). Expression and regulation of prostaglandin receptors in the human placenta and fetal membranes at term and preterm. Reprod Fertil Dev 22: 796–807. [DOI] [PubMed] [Google Scholar]
- Velasquez JC, Goeden N, Bonnin A (2013). Placental serotonin: implications for the developmental effects of SSRIs and maternal depression. Front Cell Neurosci 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaagh S, Barlow DP, Zwart R (2001). The extraneuronal monoamine transporter Slc22a3/Orct3 co-localizes with the Maoa metabolizing enzyme in mouse placenta. Mech Dev 100: 127–130. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Laplante DP, Sousa-Pires A, Veru F, Brunet A, King S (2014). Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res 219: 353–360. [DOI] [PubMed] [Google Scholar]
- Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN et al (2013). Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biol Psychiatry 74: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I (2015). Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr 169: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ED, Cross JC (2005). Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20: 180–193. [DOI] [PubMed] [Google Scholar]
- Weinstock M (2008). The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32: 1073–1086. [DOI] [PubMed] [Google Scholar]
- Weinstock M (2011). Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update. Stress 14: 604–613. [DOI] [PubMed] [Google Scholar]
- Wyrwoll CS, Seckl JR, Holmes MC (2009). Altered placental function of 11beta-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology 150: 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang B, Yan C, Hu H, Cai S, Liu J et al (2013). Effects of duration and timing of prenatal stress on hippocampal myelination and synaptophysin expression. Brain Res 1527: 57–66. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Wada Y (2005). Transfer of maternal cholesterol to embryo and fetus in pregnant mice. J Lipid Res 46: 2168–2174. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chen DC, Xiu MH, De Yang F, Haile CN, Kosten TA et al (2012). Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients. J Clin Psychiatry 73: 1025–1033. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H (2010). Changes in prefrontal axons may disrupt the network in autism. J Neurosci 30: 14595–14609. [DOI] [PMC free article] [PubMed] [Google Scholar]