Abstract
Fear conditioning has been commonly used as a model of emotional learning in animals and, with the introduction of functional neuroimaging techniques, has proven useful in establishing the neurocircuitry of emotional learning in humans. Studies of fear acquisition suggest that regions such as amygdala, insula, anterior cingulate cortex, and hippocampus play an important role in acquisition of fear, whereas studies of fear extinction suggest that the amygdala is also crucial for safety learning. Extinction retention testing points to the ventromedial prefrontal cortex as an essential region in the recall of the safety trace, and explicit learning of fear and safety associations recruits additional cortical and subcortical regions. Importantly, many of these findings have implications in our understanding of the pathophysiology of psychiatric disease. Recent studies using clinical populations have lent insight into the changes in regional activity in specific disorders, and treatment studies have shown how pharmaceutical and other therapeutic interventions modulate brain activation during emotional learning. Finally, research investigating individual differences in neurotransmitter receptor genotypes has highlighted the contribution of these systems in fear-associated learning.
INTRODUCTION
Fear-associated learning (fear conditioning and extinction) has been used for decades to study the neurocircuitry that underlies emotional learning. Animal studies of fear conditioning have established the regions and systems responsible for emotional processing, and these findings have provided a basis for our understanding of the corresponding neurocircuitry in humans. However, it was with the development of functional neuroimaging techniques in the 1990s that researchers have been able to examine whether the regions found to be involved in emotional learning in rodents are also responsible for similar processes in humans. Fear-associated learning has been used extensively in both animals and humans because it is a convenient, although simplistic, model of the acquisition and maintenance of fear responses, and altered fear learning has been hypothesized to play an important role in the development of anxiety disorders such as posttraumatic stress disorder (PTSD) and specific phobia. The goal of this review is to systematically present the key findings of recent human neuroimaging studies of fear learning and extinction, highlight the emerging neural circuitry involved, and describe the contributions of important modulators of fear-associated learning such as genetic variability and hormones. In addition, the more significant results are highlighted and discussed in light of findings available from animal fear-associated learning studies, as well as other experiments from the human literature.
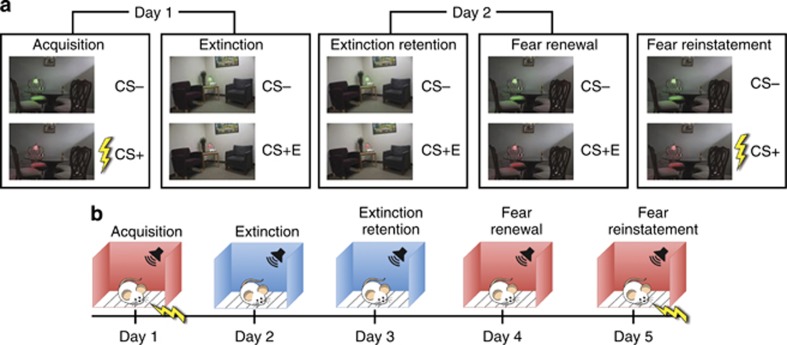
At its most basic level, fear conditioning involves the association of a neutral conditioned stimulus (CS, often a tone or image) with an aversive unconditioned stimulus (US, often electrical shock or a loud noise). After repetitive presentations of the CS with the US, the CS begins to elicit a conditioned response (CR) that occurs independently of the US. Fear conditioning protocols are similar in animal models and human subjects, and the two paradigms are compared visually in Figure 1. In rats, the CR is typically assessed by measuring freezing or fear-enhanced startle response, and in humans the CR is often assessed via psychophysiological measures such as skin conductance response (SCR), electromyography (EMG), or changes in heart rate. During fear extinction, the previously fear conditioned CS is presented repeatedly without the US, usually in a different environment (context). Over time, the CS is no longer associated with the conditioned response when presented in the extinction context. Fear extinction is thus an effective model of learning, establishing that previously ‘dangerous' stimuli are no longer threatening and are rather ‘safe'. Furthermore, exposure therapy commonly used in anxiety disorders involves components of fear extinction. Finally, during extinction retention (or recall) and fear renewal, the appropriate, context-specific retrieval of previously acquired fear and safety memory traces is tested. Studying extinction retention and fear renewal in humans elucidates how people use contextual processing to disambiguate how conditioned stimuli indicate threat in the 'danger' context but not in the 'safe' context.
Figure 1.
A comparison of typical fear-associated learning paradigms in human neuroimaging (a) and rodents (b).
Animal studies of fear-associated learning have provided an understanding of the brain circuits that govern fear conditioning and extinction and, in general, these findings have been corroborated by human neuroimaging studies. Other articles in this issue review findings from animal studies in detail, and describe how these results have shaped our understanding of emotional learning. Briefly, the neurocircuitry conserved across species involves the amygdala as the key region involved in the acquisition of the fear response, receiving inputs from somatosensory cortex, thalamus, and hippocampus that encodes contextual information and compares current contextual cues to previously encoded memories (Maren and Quirk, 2004). Basolateral amygdala (BLA) receives these various sensory and contextual inputs, and the CS–US trace is formed. The centromedial amygdala receives information from BLA, as well as other direct inputs, and projects to the hypothalamus, periaqueductal gray, and other brain stem nuclei, causing the behavioral and physiological changes associated with the CR. Interestingly, it appears that acquisition of the extinction memory also occurs in BLA, whereas hippocampus plays an important role in consolidation of extinction (Quirk and Mueller, 2008), and mPFC seems to be important in the retrieval of the extinction memory (Davis, 1992). In addition, numerous studies suggest that the central amygdala also plays a critical role in fear learning (Li et al, 2013; Penzo et al, 2014), and optogenetic studies suggest that different projections to the central amygdala enhance or inhibit fear learning (Tye et al, 2011). These complementary pathways could explain how the central amygdala appears to be involved in both fear acquisition and extinction, as separate inputs to this region both increase and decrease the fear response.
NEUROCIRCUITRY OF FEAR CONDITIONING AND EXTINCTION IN HUMANS
To date, the majority of human neuroimaging studies of fear conditioning and extinction have been performed using functional magnetic resonance imaging (fMRI). The fMRI allows researchers to identify the neuronal circuitry involved in performing various tasks by measuring blood oxygen-level dependent (BOLD) effects. More recent work has used functional connectivity measures that analyze the time courses from the BOLD fMRI signal to glean information about how various regions in the brain communicate with one another. A number of studies have used positron emission tomography (PET)-based methodology to assess local changes in metabolism in association with fear conditioning and extinction, and electroencephalography (EEG) and magnetoencephalography (MEG) have occasionally been used to examine changes in cortical electrical activity, both directly and indirectly. These various imaging modalities are usually combined with psychophysiological measures such as skin conductance response (SCR), fear-potentiated startle response, or changes in heart rate in order to assess the CR in humans.
NEUROIMAGING OF FEAR ACQUISITION IN HUMANS
A variety of stimuli have been used in human studies of fear conditioning, with the CS+ typically a distinct visual cue or sound, and the US a mild electrical shock, an aversive loud noise or, less frequently, an unpleasant smell or an uncomfortable visceral stimulus (Gramsch et al, 2014; Kattoor et al, 2013, 2014). Human fear conditioning studies typically include a second cue (CS−) that is not associated with the US, allowing for a comparison of the CS+ and CS− responses to isolate the CR using psychophysiological measures and the neural correlates of fear conditioning using neuroimaging techniques.
Early studies using fMRI found differential responses (to CS+ versus CS−) in ACC, anterior insula, hippocampus, and amygdala using both trace and delay conditioning paradigms (Buchel et al, 1998, 1999; LaBar et al, 1998). These findings have been consistently replicated by other research using fMRI (Andreatta et al, 2012; Armony and Dolan, 2002; Bach et al, 2011; Critchley et al, 2002; Knight et al, 2005, 2009; Marschner et al, 2008; Pohlack et al, 2012; Reinhardt et al, 2010; van Well et al, 2012), PET (Furmark et al, 1997), and MEG (Balderston et al, 2014; Moses et al, 2007). Activation in amygdala, anterior insula, ACC, and left PFC have also been observed while subjects viewed a movie of someone else undergoing fear conditioning (Ma et al, 2013; Olsson et al, 2007), suggesting similar processes of fear learning can be elicited without directly experiencing the US. Over the course of fear conditioning, amygdala activation typically decreases, as does hippocampal activation during trace conditioning (Buchel et al, 1998, 1999; LaBar et al, 1998; Reinhardt et al, 2010). Meanwhile, ACC and insula activation remain more consistent (Buchel et al, 1999), suggesting that amygdala and hippocampus may play a key role during acquisition of the CR, whereas ACC and insula are more involved in CR expression. Furthermore, these results closely resemble findings observed in animal models of fear conditioning (Maren et al, 2013) with additional cortical regions implicated mainly in human neuroimaging experiments. One recent large meta-analysis of fear conditioning experiments (Fullana et al, 2015) involving 677 subjects further confirmed large-scale activations in ACC/mPFC and the anterior insula, as well as additional cortical regions (supplementary motor area, dorsolateral PFC, and precuneus), ventral striatum, and midbrain. These were interpreted as representing a ‘central autonomic-interoceptive' network. Interestingly, amygdala signal was not found in this analysis, and the authors interpret these negative findings (and the strong ACC and anterior insula signals) in light of the fact that human studies primarily involve conscious fear processing. However, the authors also acknowledge that the meta-analytical approach is subject to false negative errors, as well as specific technical difficulties in imaging small structures.
Among the regions implicated by neuroimaging studies of fear-associated learning, the roles of mPFC and, to some degree, the dACC in fear conditioning have been debated, with some studies suggesting that these regions are involved specifically in instructed fear learning. However, a meta-analysis of previous work (Mechias et al, 2010), as well as a study that used both instructed fear and Pavlovian conditioning (Maier et al, 2012), demonstrated that these regions are activated across a variety of different fear conditioning paradigms. DACC thickness also directly correlates with SCR during conditioning (Milad et al, 2007). Thus, dACC and dmPFC appear to play a role in general threat appraisal across a variety of fear conditioning paradigms. The mPFC also appears to be involved in fear acquisition in rodents, but it is not required for fear learning (Burgos-Robles et al, 2009; Herry et al, 1999; Morgan and LeDoux, 1995). Ventral infralimbic (IL) and dorsal prelimbic (PL) regions that are analogous to the mPFC in humans project to neurons in both central (Quirk et al, 2003) and basolateral (Rosenkranz et al, 2003) amygdala, with IL seemingly more important in inhibitory innervation to central amygdala (Vertes, 2004). These findings, and in particular the direct connections between mPFC and amygdala, support the notion that mPFC might play a modulatory or supportive role during fear acquisition. In addition, human studies that varied the contingency rate between the CS and US have implicated additional cortical regions in fear conditioning. Studies using high contingency rates show that dlPFC is commonly activated when the threat is highly predictable (Eippert et al, 2012; Wheelock et al, 2014). This raises the possibility that working memory processes in dlPFC become involved once the CS–US pairing has been explicitly learned. The use of partial reinforcement schedule eliminates this cognitive expectancy effect and limits the role of explicit, declarative memory in the process. Amygdala activation has been observed independent of contingency awareness (Tabbert et al, 2011), whereas interestingly, increased CS–US contingency and US expectancy have been associated with lower activation in regions implicated in fear learning, including amygdala, insula, ACC, and vmPFC (Dunsmoor et al, 2008; Knight et al, 2010; Wood et al, 2012, 2013). The aforementioned meta-analysis (Fullana et al, 2015) also found that higher reinforcement rates were associated with lower activation in rostral dACC/ dmPFC, ventral anterior insula, and right secondary somatosensory cortex. This potentially suggests that when implicit learning has taken place, there is less robust activation of the basic fear-associated learning circuitry described above.
Striatum has also been consistently implicated in fear conditioning studies in humans (Fullana et al, 2015). Specifically, one meta-analysis reported that ventral striatum was activated more in subjects who consciously learned the CS–US contingency during conditioning (Klucken et al, 2009). These results, as well as results from other neuroimaging studies of decision making (Balleine et al, 2007; Hsu et al, 2005; Tom et al, 2007), are consistent with the hypothesis that the striatum plays a role in assessing the probability of various outcomes. In addition, striatal–amygdala interactions have been observed in avoidance learning during fear conditioning (Delgado et al, 2009), suggesting that the striatum accesses information from the amygdala when conscious decision making is occurring in a fearful environment. Furthermore, increased differential ventral striatum activation has also been observed in late acquisition, after explicit representation of the CS–US link had likely been formed (Pohlack et al, 2012). Finally, striatal activation has been observed in a study using monetary loss as a US (Delgado et al, 2011). All of these results are in line with previous findings and suggest that the striatum's role in emotional learning might be to assess the probabilities of experiencing the US once the CS–US contingency has been established.
In addition to the ACC, amygdala, insula, hippocampus, mPFC, and striatum that consistently appear in human fear conditioning studies, another region occasionally implicated by neuroimaging studies of conditioning in healthy humans is lateral orbitofrontal cortex (lOFC). Differential lOFC activation has been observed during both instructed and uninstructed fear conditioning tasks (Armony and Dolan, 2002; Gottfried et al, 2002; Tabbert et al, 2011). The increased activation in lOFC following the CS+ matches findings in various other neuroimaging studies using aversive stimuli that suggest that lOFC responds specifically to negatively valenced stimuli (Milad and Rauch, 2007). Thus, lOFC may play a role in identifying stimuli as negative, but the region's role has not been replicated reliably in fear learning specifically, and in fact deactivations in lOFC have been found in the meta-analysis performed by Fullana et al (2015).
Structural studies have also linked increased SCR during fear acquisition to some of the same key regions implicated by functional neuroimaging studies, particularly amygdala (Cacciaglia et al, 2014; Winkelmann et al, 2015). One study also found that greater posterior insula thickness was associated with a greater CR during conditioning (Hartley et al, 2011). However, positive correlations between CR to CS+ during conditioning and regional thickness found in the amygdala and insula have not been universally observed when the association between hippocampal volume and fear acquisition has been examined. Larger hippocampal volumes have been associated with greater ability to discriminate between contexts (Pohlack et al, 2012), and smaller hippocampal volumes have been associated with lower conscious cue discrimination but not differences in SCR (Cacciaglia et al, 2014).
In agreement with animal studies, contextual fear conditioning studies in humans have also implicated the amygdala, hippocampus, and mPFC as key regions in this process, although the number of studies that have used contextual conditioning in humans is quite limited. One study analyzing differential responses during contextual conditioning found that activation in medial amygdala was coupled with activation in several important fear learning regions, including hippocampus, anterior insula, parahippocampal gyrus, subgenual ACC, and OFC (Alvarez et al, 2008). This analysis fits with findings from cued conditioning that identified these regions as areas involved in either fear learning or the recognition of fearful stimuli. Importantly, the hippocampus plays a central role during contextual conditioning, as several studies have established (Alvarez et al, 2008; Andreatta et al, 2015). One recent study also reported that initial activity in contextual conditioning was found in dorsomedial PFC, dorsolateral PFC, and OFC (Andreatta et al, 2015). These results corroborate the current understanding of prefrontal–hippocampal circuitry and connectivity as the key network responsible for contextual processing (Maren et al, 2013; Preston and Eichenbaum, 2013).
Functional connectivity analyses have been used more recently to examine the connectivity between brain regions during fear conditioning tasks. Analyzing the time courses of data collected with BOLD fMRI allows for the identification of regions that are activated and deactivated simultaneously, suggesting that these areas are functionally connected. In addition, resting-state fMRI has been used before and after fear conditioning in an attempt to measure the connections between brain regions at rest and how differences in this connectivity might correlate with changes in fear conditioning (Feng et al, 2014; Schultz et al, 2012; Tzschoppe et al, 2014). Studies during or immediately following fear acquisition have found that several regions implicated by neuroimaging studies of cued fear conditioning also show functional connectivity with each other. During conditioning, greater connectivity between amygdala and regions, including hippocampus, vmPFC, dlPFC, and ACC, was associated with higher conditionability (Tzschoppe et al, 2014). Immediately following fear acquisition, subjects have shown enhanced amygdala–dACC and hippocampus–insula functional connectivity, as well as less amygdala–mPFC functional coupling (Feng et al, 2014), although a different study reported greater amygdala–dmPFC functional connectivity (Schultz et al, 2012). In another study, resting dACC metabolism positively predicted differential SCR response and SCR performance was positively correlated with the amygdala–ACC connectivity (Linnman et al, 2012). These results support the hypothesis that fear conditioning involves communication and modification of the connections between the same regions implicated in previous neuroimaging studies and studies using rodent models, but the specific mechanism and the exact changes have to be further elucidated.
NEUROIMAGING OF FEAR EXTINCTION IN HUMANS
Neuroimaging studies of fear extinction have provided insight into how humans associate previously aversive stimuli with safety. In concert with the animal literature, amygdala activation has also been observed during extinction learning in humans, and similarly to the observation in the amygdala during fear acquisition, this effect is often graded through the course of extinction (Gottfried and Dolan, 2004; LaBar et al, 1998; Phelps et al, 2004). BLA is activated when CS–US associations become more predictable (Boll et al, 2013), suggesting that BLA encodes information about the relationship between the CS and the US. This is in concert with our understanding of the role of BLA in extinction learning from animal studies (Quirk and Mueller, 2008). Interestingly, a meta-analysis that combined fear extinction with additional emotional regulation experiments confirmed amygdala activation across these types of studies (Diekhof et al, 2011) with the vmPFC activated specifically in fear extinction studies.
The role of vmPFC in extinction learning has been discussed extensively in recent years. Although these findings have been reported by fewer studies than findings in the amygdala, several studies have replicated findings in rodents suggesting that vmPFC plays a role in extinction learning (Gottfried and Dolan, 2004; Phelps et al, 2004). vmPFC activation is also observed in fear reversal tasks (a modified acquisition task that flips the CS+ and CS− halfway through the run) when a CS that used to be associated with fear is now safe (Schiller et al, 2008). However, as more neuroimaging studies have included the fear extinction retention phase, evidence is accumulating that vmPFC is particularly important to consolidation of the extinction memory, and the region is especially involved in the recall of extinction in subsequent testing. Evidence from the animal literature (Quirk and Mueller, 2008) also points toward a similar role of vmPFC in extinction. Nonetheless, it is also possible that vmPFC might have an important role in inhibiting the conditioned fear response at the beginning of the extinction phase (Gottfried and Dolan, 2004; Phelps et al, 2004).
One commonly studied aspect of fear extinction is prediction error (PE) that occurs when a subject expects a CS–US pairing that does not occur, or expects a CS without US, when they are actually paired. As confirmed by meta-analysis (Garrison et al, 2013), PEs are commonly associated with striatal activation (Robinson et al, 2013; Schiller et al, 2008), and greater striatal activation is seen following PEs that occur in a threatening context versus a safe context (Robinson et al, 2013). These results are in line with our understanding of the role of striatum in assessing probabilities of aversive outcomes during fear conditioning. SCR response to an omitted US is also associated with striatal activation, as well as activation in ACC, parietal cortex, and insula (Dunsmoor and LaBar, 2012). As extinction consists of repeated CS trials without the US, and the initial omissions of the US are clearly unexpected, it is not surprising that PE-associated regions are activated at least transiently at the beginning of extinction. One study has also reported greater differential activation in amygdala and midbrain while subjects committed PEs (Boll et al, 2013) that may represent activation of the expected fear response.
Extinction of trace conditioning appears to also involve dorsolateral PFC (dlPFC), possibly due to the extra time that the trace interval must be held in short-term memory (Ewald et al, 2014). Safety learning in humans, a task that associates cue with sense of safety (no US is delivered) rather than with the sense of danger, also seems to involve the dlPFC, and DTI suggests that there may be a connection between dlPFC and amygdala (Pollak et al, 2010). These limited studies further support our interpretation of the role of dlPFC as the site of explicit working memory processes in humans that maintain the trace interval, in concert with results from studies of fear acquisition.
NEUROIMAGING OF EXTINCTION RETENTION, FEAR REINSTATEMENT, AND RENEWAL IN HUMANS
More recently, studies have started to examine the extinction retention (or recall) and the fear renewal phases that are usually tested 24 h following the extinction phase. Both examine responses to the extinguished cue (CS+E); however, in extinction retention this cue is presented in the extinction (safe) context, and in fear renewal it is presented in the original fear acquisition (danger) context or a novel context. It has been reported that vmPFC plays an important role in extinction retention (Kalisch et al, 2006), corroborating animal work implicating the region in the retrieval of the extinction memory. Studies have also found that the degree of fear extinction retention is positively correlated with vmPFC thickness (Hartley et al, 2011; Milad et al, 2005; Winkelmann et al, 2015). The role of vmPFC in modulating response during extinction retention has also been observed using EEG (Mueller et al, 2014), although the limited spatial resolution of EEG makes it difficult to pinpoint activity specifically to the vmPFC. The hypothesis that vmPFC modulates amygdala activation during this process has been recently supported by fMRI findings in patients with vmPFC damage (Motzkin et al, 2015), although studies of Vietnam veterans with penetrating brain injuries found that mPFC lesions were associated with decreased prevalence of PTSD (Koenigs et al, 2008). Yet, baseline amygdala metabolism has been negatively correlated with activation in vmPFC during extinction retention (Linnman et al, 2012). Sleep seems to play an important role in extinction retention, both in terms of SCR and activation of specific brain regions. Subjects who had REM sleep between extinction and extinction retention showed reduced SCR during extinction retention, as well as greater activation of left vmPFC and bilateral lingual gyrus (Spoormaker et al, 2010). Deprivation of REM sleep leads to increased SCR during early extinction retention, as well as changes in activation in left middle temporal gyrus (Spoormaker et al, 2012).
During fear renewal, a previously extinguished CS is presented either in the fear context (the context that was used for fear acquisition) or a novel context, and no US is presented. Similar to extinction retention testing, the context triggers recall of CS memory; however, here it triggers the fear trace rather than the safety trace. Fear renewal has not been studied using neuroimaging until very recently; however, our lab recently reported greater differential activation of bilateral insula, as well as the cingulate gyrus and precuneus, during fear renewal, suggesting that renewal may recruit this key region of fear acquisition (Garfinkel et al, 2014). In an associative learning task, renewal of extinguished associations was correlated with activation in hippocampus and parahippocampal gyrus (Lissek et al, 2013), affirming the role of these regions in contextual processing. As expected from the difference in context, these results suggest that in comparison with extinction retention, fear renewal activates more regions in the fear acquisition circuitry.
Fear reinstatement is a process where a previously extinguished CS is reassociated with a US in the same context as conditioning (danger context). Like renewal, reinstatement is not studied as often as acquisition and extinction, but a number of experiments have been reported in the literature. During reinstatement using visceral stimuli, greater differential activation was observed in parahippocampal cortex and posterior cingulate cortex (Gramsch et al, 2014; Kattoor et al, 2013). In addition, greater activation in response to the CS+ has been observed in hippocampus and thalamus for females versus males (Benson et al, 2014). Research in patients with hippocampal atrophy (LaBar and Phelps, 2005) corroborates these limited results from neuroimaging studies suggesting that hippocampus might be involved in human fear reinstatement, most likely when subjects are accessing the previously consolidated fear memory trace.
NEUROIMAGING STUDIES OF FEAR CONDITIONING AND EXTINCTION IN PTSD AND ANXIETY DISORDERS
Posttraumatic Stress Disorder
Among various psychiatric disorders, fear conditioning and extinction research has been particularly salient to our understanding of PTSD, phobias, and other anxiety disorders. The major findings from neuroimaging studies of fear-associated learning in patients with psychiatric disorders are outlined in Table 1. With respect to PTSD, fear-associated learning has been utilized both in studies of PTSD patients and in studies using animal models of PTSD with remarkably converging findings. Although alterations during fear acquisition (Linnman et al, 2011) or extinction (Fani et al, 2012, 2015; Sripada et al, 2013) in PTSD patients have been occasionally reported, in general the preponderance of the findings suggest that fear acquisition and extinction are overall preserved in PTSD patients (Garfinkel et al, 2014; Milad et al, 2009; Shvil et al, 2014) as well as in animal models of PTSD (Knox et al, 2012).
Table 1. Summary of Results from Studies of Fear-Associative Learning in Patients with Psychiatric Disorders.
| Disorder | Phase of fear learning | Reference | Subjects | Key brain regions implicated |
|---|---|---|---|---|
| Posttraumatic stress disorder (PTSD) | Acquisition | Linnman et al (2011) | 19 PTSD patients, 24 trauma-exposed controls | Amygdala, dACC, HC, insula |
| Acquisition and extinction | Sripada et al (2013) | 15 PTSD patients | Amygdala, HC, insula, OFG, superior MFG | |
| Acquisition and extinctiona | Fani et al (2015) | 48 African-American females | ACC, cingulum, HC | |
| Acquisition, extinction, recall | Milad et al (2009) | 16 PTSD patients, 15 trauma-exposed controls | Amygdala, cerebellum, dACC, HC, vmPFC | |
| Acquisition, extinction, recall | Rougemont-Bucking et al (2011) | 18 PTSD patients, 16 trauma-exposed controls | dACC, MFG, PCC, vmPFC | |
| Acquisition, extinction, recallb | Shvil et al (2014) | 31 PTSD patients, 25 trauma-exposed controls | dACC, insula, vmPFC | |
| Acquisition, extinction, recall, renewal | Garfinkel et al (2014) | 14 PTSD patients, 14 combat-exposed controls | Amygdala, insula, thalamus, vmPFC | |
| Specific phobia | Acquisitionc | Schweckendiek et al (2011) | 15 Spider phobics, 14 healthy controls | ACC, amygdala, insula, mPFC, thalamus |
| Acquisitionc | Wiemer et al (2014) | 18 Female spider phobics, 18 healthy controls | Amygdala, dlPFC, insula | |
| Panic disorder (PD) | Acquisition | Tuescher et al (2011) | 8 PD patients, 8 PTSD patients, 8 healthy controls | ACC, amygdala, dlPFC, HC, insula, PAG, thalamus, ventral striatum |
| Panic disorder with agoraphobia (PD/A) | Acquisitiond | Kircher et al (2013) | 42 PD/A patients, 42 healthy controls | ACC, amygdala, IFG, insula, MFG |
| Acquisition | Lueken et al (2014) | 60 PD/A patients, 60 healthy controls | IFG, insula, midbrain | |
| Acquisitiond | Straube et al (2014a) | 42 PD/A patients, 42 healthy controls | Hippocampus, IFG | |
| Acquisitiond, e | Straube et al (2014b) | 42 PD/A patients | ACC, amygdala, hippocampus, insula, MFG | |
| Acquisition and extinctiond | Lueken et al (2013) | 49 PD/A patients | ACC, amygdala, HC | |
| Acquisition and extinctiond, e | Lueken et al (2015) | 41 PD/A patients | ACC, amygdala | |
| Generalized anxiety disorder (GAD) | Acquisition | Britton et al (2013) | 14 Anxious youth, 15 anxious adults, 25 healthy youth, 28 healthy adults | sgACC, vmPFC |
| Acquisition | Greenberg et al (2013b) | 32 Female GAD patients, 25 healthy controls | ACC, amygdala, insula, SMA, vmPFC | |
| Acquisitiona | Cha et al (2014) | 32 Female GAD patients, 25 healthy controls | Amygdala, dlPFC, PHG, MFG, thalamus, vmPFC | |
| Schizophrenia | Acquisition, extinction, recall, renewal | Holt et al (2012) | 20 Schizophrenia patients, 17 healthy controls | HC, insula, PCC, thalamus, vmPFC |
| Obsessive–compulsive disorder (OCD) | Acquisition, extinction, recall | Milad et al (2013) | 21 OCD patients, 21 healthy controls | Cerebellum, dACC, HC, insula, PAG, PCC, vmPFC |
| Attention deficit hyperactivity disorder (ADHD) | Acquisition | Maier et al (2014) | 17 ADHD patients, 17 healthy controls | Amygdala, dACC, dmPFC |
Abbreviations: ACC, anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; HC, hippocampus; MFG, medial frontal gyrus; OFG, orbital frontal gyrus; PAG, periaqueductal gray; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; sgACC, subgenual anterior cingulate cortex; SMA, supplementary motor area; vmPFC, ventromedial prefrontal cortex.
Examined structural connectivity.
Examined sex differences.
Used phobia-related stimuli.
Examined the effect of cognitive behavioral therapy.
Examined genotypes.
In contrast, abnormalities in extinction retention and fear renewal have been consistently demonstrated, both in an animal model of PTSD (Knox et al, 2012) and in PTSD patients, with associated changes in regional brain activation and volume. PTSD patients have demonstrated lower activation in hippocampus and vmPFC, and greater activation in dACC during extinction recall (Milad et al, 2009; Rougemont-Bucking et al, 2011), changes that have also been associated with higher SCR to CS+E, signifying impaired recall (Milad et al, 2009). Our laboratory demonstrated higher SCR and greater amygdala activity to the CS+E during extinction recall in PTSD patients (Garfinkel et al, 2014). We also observed altered fear renewal in PTSD patients, with lower SCR to the CS+E, and lower activity in amygdala and vmPFC as compared with combat controls (Garfinkel et al, 2014). As both extinction recall and fear renewal are dependent on contextual disambiguation of the CS, the deficits in both processes strongly implicate abnormalities in contextual processing during fear-associated learning in PTSD. This is also very consistent with the abnormalities observed in hippocampal–mPFC circuitry, as these structures play a key role in the contextualization of memory (Maren et al, 2013; Preston and Eichenbaum, 2013). In concert, in an animal model of PTSD, single prolonged stress (Liberzon et al, 1997), both abnormal fear renewal (Knox et al, 2012), and changes in hippocampus and mPFC (Knox et al, 2012) have been consistently demonstrated. Volumetric findings of reduced hippocampal volume, reduced vmPFC volume, and reduced gray matter density in dACC are also associated with PTSD symptomology (Bonne et al, 2008; Gilbertson et al, 2007; Kuhn and Gallinat, 2013; Rogers et al, 2009), further implicating hippocampal–prefrontal involvement. It should be noted that one study of men and women with PTSD found that only men showed deficient extinction recall, and men also showed greater activation in rACC compared with women during recall (Shvil et al, 2014). These results reflect findings in studies performed in healthy subjects that demonstrate gender differences in fear-associated learning. In sum, patients with PTSD consistently show impairments in the contextual modulation of both fear extinction and enhancement, and vmPFC seems to be particularly deficient.
Studies investigating the effect of treatment on fear learning and extinction in PTSD patients have been limited thus far, but represent a field with a great opportunity to advance our understanding of the disorder and how to effectively treat patients. Repeated exposure to traumatic memory (RETM) is a commonly used therapy for PTSD based upon fear extinction. One study using functional connectivity measures showed that RETM is associated with strengthened connectivity of the amygdala with regions such as hippocampus and insula, as well as mPFC with insula, and hippocampus with striatum, dorsal cingulate cortex, and OFC (Cisler et al, 2014). These results suggest that exposure therapy may change the way that several key regions involved in fear learning communicate with one another, helping to establish the proper associations between cues, context, and the fear response.
Specific Phobia
Generally, functional imaging studies of patients with specific phobia suggest that phobic patients have greater activation in response to phobia-related stimuli in regions such as amygdala, insula, and thalamus (Ipser et al, 2013). Specifically, fear conditioning using aversive pictures as unconditioned stimuli has been used to assess differences in emotional learning in patients with specific phobia. One such study found that patients with spider phobia showed enhanced activation in amygdala to phobia-related conditioned stimuli versus nonphobia-related conditioned stimuli (Schweckendiek et al, 2011). This greater activation in the amygdala most likely suggests that these patients show an exaggerated fear response to the more individually salient phobia-related stimuli. However, spider phobics also overestimate the correlation between CS and US when the CS is a picture of a spider, and this is associated with greater activation in dorsolateral PFC (Wiemer et al, 2014). The larger dlPFC signal here might be reflective of various processes like increased access of working memory (Curtis and D'Esposito, 2003) as subjects process the association between the CS and the US, and volitional control of emotional responses to phobic stimuli (MacDonald et al, 2000). Although these changes in amygdala and dlPFC are probably not alterations in the fear learning process per se, they may enhance fear acquisition by increasing the reactivity of the fear neurocircuitry. Additional studies of fear conditioning in specific phobia are necessary to replicate these results and further investigate emotional learning differences in this patient population.
Exposure therapy, which uses elements of fear extinction, is the most widely used treatment for specific phobia and has been studied often with neuroimaging. Exposure therapy has been shown to increase activation in prefrontal cortex during the presentation of phobia-related images, whereas it decreases activation in amygdala, insula, and cingulate cortex (Hauner et al, 2012). At 6 months after completion of exposure therapy, prefrontal cortex activation was lower as well. A small meta-analysis of three neuroimaging studies in patients with specific phobia (Goossens et al, 2007; Paquette et al, 2003; Straube et al, 2006) found that exposure therapy leads to lower activation in right frontal cortex, limbic cortex, and basal ganglia (Ipser et al, 2013). This was interpreted to suggest that, over time, exposure therapy reduces the reactivity in several key regions of fear neurocircuitry. These changes are probably not indicative of an alteration in the process of fear learning, but rather a reduction of the salience of fearful stimuli over the course of treatment.
Panic Disorder
A relatively small number of studies have investigated fear conditioning in patients with panic disorder (PD), as well as patients with panic disorder with agoraphobia (PD/A). Interestingly, inferior frontal gyrus (IFG) has been implicated in fear conditioning in PD/A patients. During acquisition, greater differential activation of IFG has been seen in PD/A patients versus controls (Lueken et al, 2014), and cognitive behavioral therapy (CBT) reduced this activation in PD/A patients (Kircher et al, 2013). Further studies found that PD/A patients receiving CBT treatment also showed greater activation of the hippocampus during fear acquisition, and this was positively correlated with treatment outcome (Lueken et al, 2013; Straube et al, 2014a). These studies also found that CBT treatment is associated with lowered connectivity between the left IFG and the left hippocampus across time. These results are interesting, as previous work in healthy subjects typically does not implicate IFG in fear-associated learning and may suggest that patients with panic disorder recruit additional regions of frontal cortex to modulate fear acquisition. IFG is a crucial region in the ventral attention network and appears to be activated during cue detection (Hampshire et al, 2010). Another study in patients with PD demonstrated greater activity to CS− during conditioning in ventral striatum, amygdala, subgenual cingulate, and periaqueductal gray (Tuescher et al, 2011). Taken together, these results might indicate that panic disorder may be associated with altered attention to cues, as well as inappropriate fear responses to safe cues, but more research is clearly required, and these interpretations have to be tested directly.
Generalized Anxiety Disorder and Social Anxiety Disorder
Generalization of fear conditioning has been postulated to play a key role in the development of generalized anxiety disorder (GAD), and fear-associated learning has been examined in GAD using neuroimaging. The studies often used a modified conditioning task (generalization of conditioned threat) where subjects were presented with a variety of cues that were similar in various degrees to the CS (ie, rectangles of varying widths), but only the CS was associated with the US. Several studies investigating the generalization of conditioned fear have shown that insula reactivity increases as stimuli become more similar to the US (Dunsmoor et al, 2011; Greenberg et al, 2013a; Lissek et al, 2014). Greater SCR associated with generalized fear has also been correlated with amygdala activity (Dunsmoor et al, 2011). In another generalization task, greater differential activation was observed in insula, ACC, and striatum when subjects expected a US that was not delivered (Dunsmoor and LaBar, 2012). These results consistently implicate insula and other key areas of the fear learning circuitry in the generalization process, reinforcing the role of these regions in cue discrimination. On the other hand, it was reported that hippocampus and vmPFC reactivity increases as stimuli become less similar (Lissek et al, 2014). Furthermore, connectivity analyses suggest that these changes are modulated by the hippocampus.
Several experiments using the generalization of conditioned threat task have examined potential neural deficits in patients with GAD. Patients with GAD showed less discrimination between the fear and safety cues, especially in vmPFC (Greenberg et al, 2013b). This decrement has also been correlated with lower vmPFC thickness as well as with lower connectivity between vmPFC and thalamus and IFG (Cha et al, 2014). If vmPFC is indeed involved in discrimination between safe and danger signals, this is consistent with the reports of increased trait anxiety association with lower ventral PFC activation during fear acquisition, as well as lower connectivity between vPFC and hippocampus (Indovina et al, 2011). Consistent with this, patients with GAD (and social phobia) showed lower vmPFC and subgenual ACC activation during cognitive appraisal of previously conditioned CS+ as compared with healthy subjects (Britton et al, 2013). These results are interesting, as the role of vmPFC in fear responses is generally seen as inhibitory; therefore, these deficits might be indicative of broad deficits in the inhibition of the fear response.
Only one study of fear-associated learning has focused on the effect of social anxiety on fear learning and extinction. In a population of healthy subjects, there was a slight negative correlation between social anxiety and vmPFC activation during extinction recall, similar to deficits observed in other anxiety or fear disorders (Pejic et al, 2013). More work needs to be done to understand whether patients with social anxiety disorder actually show impairments in fear-associated learning, and whether these impairments differ from those observed in other anxiety disorders and PTSD.
Neuroimaging Studies of Fear Learning and Extinction in Other Psychiatric Disorders
Fear conditioning and extinction experiments have also been used to study emotional learning in other psychiatric disorders. Although some findings point toward changes in fear-associated learning and in regions implicated in fear and anxiety regulation like vmPFC, dACC, and amygdala, overall the very small number of studies in these disorders clearly render these findings as preliminary and require replication. Two studies have reported deficiencies in extinction recall, similar to changes observed in PTSD, in patients with schizophrenia, with lower differential vmPFC activation versus controls (Holt et al, 2009, 2012). Schizophrenic patients also showed less activation in hippocampus, thalamus, and posterior cingulate gyrus during acquisition, but it is unclear whether these differences are indicative of a fear learning deficit or differences in fear reactivity that are associated with schizophrenia symptomatology. Other studies in animal models and human patients suggest that hippocampal deficits play a major role in schizophrenia pathophysiology (Harrison, 2004). These changes in hippocampal functions can clearly affect contextual processing and fear-associated learning downstream. Impaired extinction recall has also been reported recently in a single study of patients with obsessive–compulsive disorder (OCD) (Milad et al, 2013). Patients with OCD showed reduced vmPFC activation during recall, but they also exhibited reduced activation in caudate and hippocampus during fear conditioning. However, symptom severity was positively correlated with vmPFC response, and inversely correlated with dACC response. These results are opposite of observations in PTSD, and the experimenters speculate that these findings may be indicative of a coping mechanism that severe OCD patients are using to avoid aversive stimuli. Further studies are needed to ascertain the significance of these differences in regional activations.
Fear learning has also been studied in attention deficit hyperactivity disorder (ADHD). No difference in response was exhibited in an uninstructed fear learning paradigm, but ADHD patients showed lower SCR and lower dACC activation in response to the CS+ (Maier et al, 2014). In addition, ADHD patients showed higher amygdala activation to the CS− versus controls. These differences could indicate reduced attention to aversive stimuli that healthy subjects find more salient, making the response to CS+ and CS− more similar. Finally, recent work has investigated changes in fear conditioning and extinction in irritable bowel syndrome (IBS), a gastrointestinal disorder with psychological factors. Greater activation of prefrontal cortex and amygdala in response to the CS+ (Icenhour et al, 2015) was reported in IBS, as well as differential hippocampal activation during reinstatement. These differences may be indicative of the increased salience of specific aversive cues to IBS patients, as this study used rectal distension as the US. Using visceral pain as the US in subjects who typically have increased gastrointestinal discomfort should enhance the response in the fear learning neurocircuitry, but may not signify an actual change in fear learning. As only a single neuroimaging study of fear learning in patients with each of these disorders (OCD, ADHD, and IBS) has been reported, further research is needed to replicate these results.
NEUROBIOLOGICAL FACTORS AND INDIVIDUAL DIFFERENCES IN FEAR-ASSOCIATED LEARNING
The studies described above have examined the contribution of various brain regions and networks to different phases of fear-associated learning in both healthy controls and patients with psychiatric disorders. However, it is also clear from animal studies, work in other emotional tasks, and the neuropathology seen in psychiatric patients that substantial individual differences are present in fear-associated learning. One of the potential contributors to these differences is the individual level of various hormones and neurotransmitters that have an important role in regulating fear-associated learning. Although the formation and consolidation of fear and extinction traces are believed to involve cellular and system plasticity, ie, synaptic reorganization, the relevant hormonal and neurotransmitter level can have a critical role in expression or modulation of this synaptic plasticity. In this context, key molecules involved in the stress response and its regulation have been studied extensively. For example, one study focused on NR3C1 and CRHR1 genotypes containing greater numbers of minor alleles that have been previously associated with hypersensitivity to glucocorticoids, decreased cortisol levels, and increased PTSD symptomology (Bachmann et al, 2005; Hauer et al, 2011). Researchers found that these genotypes controlling expression of the glucocorticoid and corticotropin-releasing hormone receptors, respectively, were associated with greater activation in amygdala during conditioning, reduced activation in prefrontal cortex during extinction, and changes in coupling between the two regions (Ridder et al, 2012). Thus, increased sensitivity to glucocorticoids may lead to impairments in fear learning and extinction that manifest in psychiatric disorders.
Cortisol and other LHPA hormones appear to modulate fear learning, but their effect (at least in some regions) may vary based upon gender and differences in levels of sex hormones. Numerous studies suggest that there might be an interaction between stress and sex hormones in fear-associated learning, with hydrocortisone administration enhancing fear acquisition in women and inhibiting fear acquisition in men (Merz et al, 2010; Tabbert et al, 2010). In men, cortisol has been shown to reduce CS+/CS− differentiation in hippocampus, thalamus, and insula but appears to enhance this differentiation in women (Tabbert et al, 2010). A similar pattern was observed when stress was induced by the Trier Social Stress Test. Across gender, stress enhanced CS+/CS− differentiation in the hippocampus and inhibited activation in the medial frontal cortex (Merz et al, 2013). However, stress reduced CS+/CS− differentiation in the amygdala and nucleus accumbens in men but enhanced differentiation in women taking oral contraceptives (low estrogen and progesterone levels) (Merz et al, 2013). In men, hydrocortisone administered 45 min before fear extinction also led to greater SCR and diminished differential CS+/CS− activation in amygdala, MFC, and nucleus accumbens (Merz et al, 2014), suggesting that cortisol might impair both extinction and conditioning, specifically in men.
Several experiments have examined the direct effects of gender and/or sex hormones on fear learning and extinction. One neuroimaging study of fear learning in women found that women with high levels of estrogen showed greater activation of vmPFC during fear extinction than women with low levels of estrogen, as well as greater activation of vmPFC and amygdala during extinction recall (Zeidan et al, 2011). These results are in concert with rodent studies (Chang et al, 2009; Zeidan, et al, 2011) demonstrating that high levels of estrogen enhance extinction learning and retention. Similarly, human studies have associated low levels of estradiol in women with impaired fear inhibition (Glover et al, 2013) and impaired extinction learning (Glover et al, 2012; Milad et al, 2010; Wegerer et al, 2014). However, neuroimaging studies of the differences between genders have not yielded consistent results. One study reported similar SCR responses in men and women during fear acquisition but greater BOLD response in right amygdala, rACC, and dACC in women (Lebron-Milad et al, 2012). During extinction recall, on the other hand, men showed greater differential activation in bilateral rACC as compared with women (Lebron-Milad et al, 2012). This complexity is further highlighted by findings from a different study that showed that women with low estrogen and progesterone levels have shown greater CS+/CS− differentiation during fear extinction in amygdala, ACC, vmPFC, and thalamus, compared with both women with high estrogen and progesterone levels, and as compared with men (Merz et al, 2012). The emerging picture is thus quite complex, with gender differences that cannot simply be understood in terms of circulating levels of gonadal steroids. The preponderance of animal and human findings suggest that, in females, higher levels of estrogen enhance extinction learning and retention and affect activation in brain regions commonly involved in fear-associated learning. Nonetheless, further research is required to address gender differences and the various effects of sex hormones.
Among the neurotransmitter systems, variability in the serotoninergic system has most often been reported to affect fear-associated learning. The short allele 5-HTTLPR (a serotonin transporter) genotype has been associated with greater response to CS+ during fear conditioning in amygdala, insula, thalamus, and occipital cortex (Klucken et al, 2013), and subjects with the short allele 5-HTTLPR genotype and history of stressful life events have shown greater reactivity in insula in response to CS+ during fear conditioning (Hermann et al, 2012; Klucken et al, 2013). 5-HTTLPR polymorphisms have also been shown to affect connections between amygdala and the perigenual ACC (Pezawas et al, 2005). Concerns have been raised, however, about the reproducibility of genetic association studies (Munafò et al, 2009), and thus further research is necessary to confirm the results from these experiments. However, in support of serotonin's role in fear learning, serotonin-depleted subjects have shown lower activation in amygdala and OFC in response to CS+ during fear acquisition (Hindi Attar et al, 2012). Together, these reports raise the possibility that serotoninergic transmission might play an important role in fear learning, such that lower serotonin response impairs activation in critical emotional learning regions, particularly the insula and amygdala.
Limited data have also implicated genetic variability in dopaminergic, glutamatergic, and BDNF systems in fear-associated learning. Subjects with a variant of an NMDA receptor gene (GRIN2A) have shown lower amygdala response during fear learning (Cacciaglia et al, 2013). In a different study, subjects with the 9R allele of DAT1, a dopamine transporter gene predominantly expressed in the striatum, had significantly higher extinction rates, as well as stronger activation following prediction errors in the ventral striatum (Raczka et al, 2011). The Val66Met single-nucleotide polymorphism (SNP) of the brain-derived neurotrophin (BDNF) gene has been associated with impaired fear extinction in mice and humans (Soliman et al, 2010), but neuroimaging studies of Val66Met carriers only found activation changes in amygdala and ACC during fear acquisition (Lonsdorf et al, 2014). No changes were seen in fear extinction. These studies have not been replicated, and (as stated earlier) results from genetic association experiments are often variable. However, these limited findings do suggest that, like serotonin, individual differences in NMDA and BDNF may affect fear learning processes in regions like the amygdala. Differences in the dopaminergic system, on the other hand, may affect fear learning by altering associated processes in striatum that assess the probability of aversive outcomes, in concert with the role of dopamine transmission in risk and reward assessment.
More recently, evidence of the role of cannabinoids in the modulation of fear-associated learning has been accumulating, particularly with respect to fear extinction. Genetically, variants in the human FAAH gene (that controls expression of an endocannabinoid-degrading enzyme) have been associated with habituation to threat in the human amygdala (Gunduz-Cinar et al, 2013), and participants who received synthetic tetrahydrocannibinol (THC) showed greater vmPFC and hippocampal activation during extinction recall (Rabinak et al, 2014). In addition, synthetic THC administration has since been associated with enhanced basolateral and superficial amygdala connectivity to rostral ACC and mPFC (Gorka et al, 2015). These results, observed in a number of laboratories, suggest that cannabinoids might serve as interesting modulators of fear learning and extinction, as well as potential therapeutic agents for some disorders involving the same processes.
Studies of individual differences in patients with psychiatric disorders have the potential to elucidate how hormones and neurotransmitter systems contribute to emotional learning deficits. Currently, only a handful of neuroimaging studies have investigated genotype associations with differences in the fear learning neurocircuitry in patient populations. Studies in PD/A patients, for example, reported that inhibitory ACC–amygdala coupling during fear conditioning was seen specifically in CBT responders with the 5-HTTPLR L/L genotype (Lueken et al, 2015). In contrast, a serotonin receptor 1A gene (HTR1A) ‘risk' genotype was associated with reduced effects of CBT on differential activation in ACC and insula during conditioning (Straube et al, 2014b). These reports support the idea that individual variance in serotonin signaling modulates fear learning, but more work is needed to elucidate the relationship between serotonin receptor genotypes and psychiatric symptomology. In the same vein, the effects of gender/sex hormones on fear extinction was examined in patients with PTSD, and increased fear-potentiated startle was linked to low estrogen levels (Glover et al, 2012). Thus, future research combining neuroimaging, fear learning tasks, and measures of individual differences in hormone levels in patient populations may be useful in uncovering the complex mechanisms responsible for emotional learning deficits in psychiatric disorders.
SUMMARY AND FUTURE RESEARCH DIRECTIONS
Functional neuroimaging studies of fear-associated learning have been very useful in delineating the neural circuits involved in human emotional learning by: (1) confirming the roles of key brain regions identified by prior animal studies in the support of fear and safety learning in humans; (2) further extending our understanding of the neural circuitry involved in explicit emotional learning in humans by delineating the unique role of brain regions involved in processes like anticipation, working memory, probability assessment, and prediction error; and (3) starting to identify the key biological systems (genes, neurotransmitters, and hormones) that modulate emotional learning and contribute to individual differences in conditioning, extinction, and extinction retention. Beyond confirming the key roles of ACC, amygdala, insula, hippocampus, and vmPFC in fear conditioning, extinction, and extinction retention, these studies highlight the contributions of regions like the striatum and dlPFC to specific aspects of cognitive processing of explicit fear-associated learning like interoceptive awareness, probability assessment, working memory, and anticipation. Although only a handful of meta-analytic studies involving fear-associated learning have been reported so far in the literature, the convergence of evidence from different studies provides initial support to the overall picture outlined above. Furthermore, these findings underscore the complexity involved in the emotional learning process in humans and the need to carefully consider future experimental design to allow valid interpretations. As explicit cognitive processing during emotional learning is ubiquitous in humans, we believe that this contribution might be particularly important to the understanding of human emotional learning and human psychopathology. This is especially true considering the (at times) liberal application of insights gained from rodent studies. Moreover, studies of individual differences have also demonstrated that levels of specific neurotransmitters, as well as stress and sex hormones, modulate regional brain activation during fear learning and extinction, both adding levels of complexity and providing a potential link to the abnormalities in fear-associated learning that might be present in various psychiatric conditions. These initial findings are new, exciting, and carry great promise, as they might help in integrating genetic, molecular, cellular, and brain circuitry information in understanding emotional learning, as well as understanding the mechanisms underlying many psychiatric disorders that involve abnormalities in related systems and functions.
Human fear-associated learning studies have also started delineating disorder-specific abnormalities in the brain circuitry of PTSD, GAD, specific phobia, and schizophrenia patients. The majority of published work has naturally involved disorders of fear and anxiety, as abnormal fear-associated learning had been postulated to contribute to their pathophysiology. Interestingly, the converging findings in PTSD patients suggest the presence of abnormalities in brain regions that modulate extinction retention and renewal, potentially implicating altered contextual processing and the hippocampal-prefrontal circuit. Clearly, additional research is needed to replicate existing findings and to extend these findings to additional patient populations. Furthermore, experiments using fear-associated learning tasks also offer a potentially useful model to examine the effect of therapeutic interventions. For example, one recent study of cognitive reappraisal showed that this strategy seems to reduce fear acquisition and enhance fear extinction, based on psychophysiological and regional activation changes in structures like the ACC, insula, hippocampus, and vmPFC (Hermann et al, 2014). This type of work, as well as studies described earlier that have examined changes in fear learning following CBT in various patient populations, suggest that fear-associated learning might prove to be a useful and less subjective tool to examine and compare therapeutic effects. Similarly, investigating the effect of pharmacological interventions like cannabinoids and antidepressants on fear-associated learning may provide insight into how modification of neurotransmitter systems involved in emotional learning processes can be potentially harnessed to achieve better therapeutic effects. Finally, the effect of sleep on fear learning and extinction is another exciting and relatively novel area of research that is currently gaining momentum. One recent study, for example, reported an association between vmPFC activity during conditioning and REM sleep the following night, as well as an association between hippocampal activity during conditioning and stage 2 and stage 4 sleep (Spoormaker et al, 2014). Sleep before fear extinction has been shown to enhance extinction learning in spider phobics (Pace-Schott et al, 2012) and healthy controls, with accompanying changes in hippocampal and amygdalar activation (Hauner et al, 2013). These types of results, together with a growing understanding of sleep physiology and its underlying cellular and molecular processes, might offer an opportunity to understand the key role that sleep plays in healthy and abnormal emotional learning. These studies are particularly relevant given the prevalence of sleep disturbances in psychiatric disorders. Future studies examining sleep architecture, as well as other phases of fear-associated learning (in both healthy subjects and patient populations), should provide valuable insight into these relationships.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank the Joshua Judson Stern Foundation for its generous support of their work. In addition, we acknowledge Arash Javanbakht for his assistance in the preparation of this review.
References
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C (2008). Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci 28: 6211–6219. Evidence showing involvement of amygdala and hippocampus in contextual conditioning, as well as functional connectivity of amygdala with emotional learning regions during fear acquisition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatta M, Fendt M, Muhlberger A, Wieser MJ, Imobersteg S, Yarali A et al (2012). Onset and offset of aversive events establish distinct memories requiring fear and reward networks. Learn Mem 19: 518–526. [DOI] [PubMed] [Google Scholar]
- Andreatta M, Glotzbach-Schoon E, Muhlberger A, Schulz SM, Wiemer J, Pauli P (2015). Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex 63: 352–363. [DOI] [PubMed] [Google Scholar]
- Armony JL, Dolan RJ (2002). Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia 40: 817–826. [DOI] [PubMed] [Google Scholar]
- Bach DR, Weiskopf N, Dolan RJ (2011). A stable sparse fear memory trace in human amygdala. J Neurosci 31: 9383–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann AW, Sedgley TL, Jackson RV, Gibson JN, Young RM, Torpy DJ (2005). Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology 30: 297–306. [DOI] [PubMed] [Google Scholar]
- Balderston NL, Schultz DH, Baillet S, Helmstetter FJ (2014). Rapid amygdala responses during trace fear conditioning without awareness. PLoS One 9: e96803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O (2007). The role of the dorsal striatum in reward and decision-making. J Neurosci 27: 8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S, Kattoor J, Kullmann JS, Hofmann S, Engler H, Forsting M et al (2014). Towards understanding sex differences in visceral pain: enhanced reactivation of classically-conditioned fear in healthy women. Neurobiol Learn Mem 109: 113–121. [DOI] [PubMed] [Google Scholar]
- Boll S, Gamer M, Gluth S, Finsterbusch J, Buchel C (2013). Separate amygdala subregions signal surprise and predictiveness during associative fear learning in humans. Eur J Neurosci 37: 758–767. [DOI] [PubMed] [Google Scholar]
- Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC et al (2008). Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry 69: 1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G et al (2013). Response to learned threat: an FMRI study in adolescent and adult anxiety. Am J Psychiatry 170: 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ (1999). Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci 19: 10869–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ (1998). Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 20: 947–957. Early study of fear-associated learning using fMRI, showing that involvement of amygdala, insula, and ACC during fear conditioning is conserved in humans. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ (2009). Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciaglia R, Nees F, Pohlack ST, Ruttorf M, Winkelmann T, Witt SH et al (2013). A risk variant for alcoholism in the NMDA receptor affects amygdala activity during fear conditioning in humans. Biol Psychol 94: 74–81. [DOI] [PubMed] [Google Scholar]
- Cacciaglia R, Pohlack ST, Flor H, Nees F (2014). Dissociable roles for hippocampal and amygdalar volume in human fear conditioning. Brain Struct Funct. [DOI] [PubMed]
- Cha J, Greenberg T, Carlson JM, Dedora DJ, Hajcak G, Mujica-Parodi LR (2014). Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. J Neurosci 34: 4043–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-J, Yang C-H, Liang Y-C, Yeh C-M, Huang C-C, Hsu K-S (2009). Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus 19: 1142–1150. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Steele JS, Lenow JK, Smitherman S, Everett B, Messias E et al (2014). Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. J Psychiatr Res 48: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653–663. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M (2003). Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15: 353–375. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA (2009). Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Phelps EA (2011). Neural systems underlying aversive conditioning in humans with primary and secondary reinforcers. Front Neurosci 5: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 58: 275–285. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC (2008). Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage 40: 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, LaBar KS (2012). Brain activity associated with omission of an aversive event reveals the effects of fear learning and generalization. Neurobiol Learn Mem 97: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS (2011). Neurobehavioral mechanisms of human fear generalization. Neuroimage 55: 1878–1888. Study using a fear generalization task, showing that generalized stimuli are associated with differential activation in several fear-related regions, including amygdala, insula, striatum, and periaqueductal gray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Gamer M, Buchel C (2012). Neurobiological mechanisms underlying the blocking effect in aversive learning. J Neurosci 32: 13164–13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald H, Glotzbach-Schoon E, Gerdes AB, Andreatta M, Muller M, Muhlberger A et al (2014). Delay and trace fear conditioning in a complex virtual learning environment-neural substrates of extinction. Front Hum Neurosci 8: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ et al (2012). Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med 42: 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM et al (2015). Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex 64: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Feng T, Chen Z, Lei X (2014). Memory consolidation of fear conditioning: bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Soc Cogn Affect Neurosci 9: 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A et al (2015). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. Comprehensive meta-analysis of human neuroimaging studies of fear conditioning, including data from 677 subjects. [DOI] [PubMed]
- Furmark T, Fischer H, Wik G, Larsson M, Fredrikson M (1997). The amygdala and individual differences in human fear conditioning. Neuroreport 8: 3957–3960. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM et al (2014). Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci 34: 13435–13443. Study of extinction retention and fear renewal in humans, with evidence that renewal is impaired in patients with PTSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J, Erdeniz B, Done J (2013). Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 37: 1297–1310. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Williston SK, Paulus LA, Lasko NB, Gurvits TV, Shenton ME et al (2007). Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol Psychiatry 62: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ et al (2012). Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry 72: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B et al (2013). Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci 38: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Sunaert S, Peeters R, Griez EJ, Schruers KR (2007). Amygdala hyperfunction in phobic fear normalizes after exposure. Biol Psychiatry 62: 1119–1125. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, de Wit H, Phan KL (2015). Cannabinoid modulation of amygdala subregion functional connectivity to social signals of threat. Int J Neuropsychopharmacol 18: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ (2004). Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci 7: 1144–1152. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ (2002). Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci 22: 10829–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsch C, Kattoor J, Icenhour A, Forsting M, Schedlowski M, Gizewski ER et al (2014). Learning pain-related fear: neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain. Neurobiol Learn Mem 116: 36–45. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR (2013. a). Neural reactivity tracks fear generalization gradients. Biol Psychol 92: 2–8. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR (2013. b). Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety 30: 242–250. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B et al (2013). Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry 18: 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ (2004). The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 174: 151–162. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA (2011). Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex 21: 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer D, Weis F, Papassotiropoulos A, Schmoeckel M, Beiras-Fernandez A, Lieke J et al (2011). Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit Care Med 39: 643–650. [DOI] [PubMed] [Google Scholar]
- Hauner KK, Howard JD, Zelano C, Gottfried JA (2013). Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat Neurosci 16: 1553–1555. Evidence that sleep enhances extinction learning, with associated changes in hippocampal and amygdalal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner KK, Mineka S, Voss JL, Paller KA (2012). Exposure therapy triggers lasting reorganization of neural fear processing. Proc Natl Acad Sci USA 109: 9203–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Keck T, Stark R (2014). Dispositional cognitive reappraisal modulates the neural correlates of fear acquisition and extinction. Neurobiol Learn Mem 113: 115–124. [DOI] [PubMed] [Google Scholar]
- Hermann A, Kupper Y, Schmitz A, Walter B, Vaitl D, Hennig J et al (2012). Functional gene polymorphisms in the serotonin system and traumatic life events modulate the neural basis of fear acquisition and extinction. PLoS One 7: e44352. Evidence of an association between 5-HTTPLR genotypes with differences in activation in several important fear-associated learning regions, including amygdala, insula, dACC, and vmPFC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Vouimba R-M, Garcia R (1999). Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J Neurophysiol 82: 2827–2832. [DOI] [PubMed] [Google Scholar]
- Hindi Attar C, Finckh B, Buchel C (2012). The influence of serotonin on fear learning. PLoS One 7: e42397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR (2012). Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry 69: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP et al (2009). Extinction memory is impaired in schizophrenia. Biol Psychiatry 65: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF (2005). Neural systems responding to degrees of uncertainty in human decision-making. Science 310: 1680–1683. [DOI] [PubMed] [Google Scholar]
- Icenhour A, Langhorst J, Benson S, Schlamann M, Hampel S, Engler H et al (2015). Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil 27: 114–127. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, Bishop SJ (2011). Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 69: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Singh L, Stein DJ (2013). Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci 67: 311–322. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ (2006). Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci 26: 9503–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattoor J, Gizewski ER, Kotsis V, Benson S, Gramsch C, Theysohn N et al (2013). Fear conditioning in an abdominal pain model: neural responses during associative learning and extinction in healthy subjects. PLoS One 8: e51149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattoor J, Thurling M, Gizewski ER, Forsting M, Timmann D, Elsenbruch S (2014). Cerebellar contributions to different phases of visceral aversive extinction learning. Cerebellum 13: 1–8. [DOI] [PubMed] [Google Scholar]
- Kircher T, Arolt V, Jansen A, Pyka M, Reinhardt I, Kellermann T et al (2013). Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biol Psychiatry 73: 93–101. [DOI] [PubMed] [Google Scholar]
- Klucken T, Alexander N, Schweckendiek J, Merz CJ, Kagerer S, Osinsky R et al (2013). Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Soc Cogn Affect Neurosci 8: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Tabbert K, Schweckendiek J, Merz CJ, Kagerer S, Vaitl D et al (2009). Contingency learning in human fear conditioning involves the ventral striatum. Hum Brain Mapp 30: 3636–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA (2005). The role of the human amygdala in the production of conditioned fear responses. Neuroimage 26: 1193–1200. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, Bandettini PA (2009). Neural substrates of explicit and implicit fear memory. Neuroimage 45: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA (2010). Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage 49: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I (2012). Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem 19: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I (2012). Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience 223: 163–173. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM et al (2008). Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci 11: 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2013). Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry 73: 70–74. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20: 937–945. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA (2005). Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav Neurosci 119: 677–686. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bucking A, Zeidan MA et al (2012). Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol Mood Anxiety Disord 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B (2013). Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA (1997). Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 22: 443–453. [DOI] [PubMed] [Google Scholar]
- Linnman C, Zeffiro TA, Pitman RK, Milad MR (2011). An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol Mood Anxiety Disord 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR (2012). Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry 169: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Pitman RK, Milad MR (2012). Resting cerebral metabolism correlates with skin conductance and functional brain activation during fear conditioning. Biol Psychol 89: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC et al (2014). Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci 9: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Glaubitz B, Uengoer M, Tegenthoff M (2013). Hippocampal activation during extinction learning predicts occurrence of the renewal effect in extinction recall. Neuroimage 81: 131–143. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindstrom KM, Haaker J, Ohman A, Schalling M et al (2014). BDNFval66met affects neural activation pattern during fear conditioning and 24 h delayed fear recall. Soc Cogn Affect Neurosci 10: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Straube B, Konrad C, Wittchen HU, Strohle A, Wittmann A et al (2013). Neural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia. Am J Psychiatry 170: 1345–1355. [DOI] [PubMed] [Google Scholar]
- Lueken U, Straube B, Reinhardt I, Maslowski NI, Wittchen HU, Strohle A et al (2014). Altered top-down and bottom-up processing of fear conditioning in panic disorder with agoraphobia. Psychol Med 44: 381–394. [DOI] [PubMed] [Google Scholar]
- Lueken U, Straube B, Wittchen HU, Konrad C, Strohle A, Wittmann A et al (2015). Therapygenetics: anterior cingulate cortex-amygdala coupling is associated with 5-HTTLPR and treatment response in panic disorder with agoraphobia. J Neural Transm 122: 135–144. [DOI] [PubMed] [Google Scholar]
- Ma Q, Huang Y, Wang L (2013). Left prefrontal activity reflects the ability of vicarious fear learning: a functional near-infrared spectroscopy study. ScientificWorldJournal 2013: 652542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Maier S, Szalkowski A, Kamphausen S, Perlov E, Feige B, Blechert J et al (2012). Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? PLoS One 7: e50120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SJ, Szalkowski A, Kamphausen S, Feige B, Perlov E, Kalisch R et al (2014). Altered cingulate and amygdala response towards threat and safe cues in attention deficit hyperactivity disorder. Psychol Med 44: 85–98. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14: 417–428. A comprehensive review and conceptual formulation of contextual processing in the brain and how this affects emotional learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ (2004). Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R (2013). Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology 38: 2529–2541. [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C (2008). Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci 28: 9030–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R (2010). A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage 49: 1760–1768. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Hermann A, Stark R, Wolf OT (2014). Cortisol modifies extinction learning of recently acquired fear in men. Soc Cogn Affect Neurosci 9: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R et al (2010). Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35: 33–46. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R et al (2012). Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci 7: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R (2013). Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology 38: 2529–2541. Evidence for differential effects of stress on fear conditioning in men and women following the Trier Social Stress Test, corroborating past research using hydrocortisone administration. [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ et al (2013). Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry 70: 608–618. quiz 554. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. Original study of extinction and extinction retention in PTSD, with results that have now been replicated in numerous studies in humans and animal models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL (2005). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA 102: 10706–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL (2007). A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 62: 1191–1194. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL (2007). The role of the orbitofrontal cortex in anxiety disorders. Ann NY Acad Sci 1121: 546–561. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL et al (2010). The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 168: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE (1995). Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109: 681. [DOI] [PubMed] [Google Scholar]
- Moses SN, Houck JM, Martin T, Hanlon FM, Ryan JD, Thoma RJ et al (2007). Dynamic neural activity recorded from human amygdala during fear conditioning using magnetoencephalography. Brain Res Bull 71: 452–460. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry 77: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller EM, Panitz C, Hermann C, Pizzagalli DA (2014). Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J Neurosci 34: 7059–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J et al (2009). 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet 150b: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA (2007). Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci 2: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Verga PW, Bennett TS, Spencer RM (2012). Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res 46: 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette V, Levesque J, Mensour B, Leroux JM, Beaudoin G, Bourgouin P et al (2003). ‘Change the mind and you change the brain': effects of cognitive-behavioral therapy on the neural correlates of spider phobia. Neuroimage 18: 401–409. [DOI] [PubMed] [Google Scholar]
- Pejic T, Hermann A, Vaitl D, Stark R (2013). Social anxiety modulates amygdala activation during social conditioning. Soc Cogn Affect Neurosci 8: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Li B (2014). Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci 34: 2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L et al (2015). The paraventricular thalamus controls a central amygdala fear circuit. Nature 519: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS et al (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43: 897–905. One of the early neuroimaging studies of extinction in humans, establishing the role of amygdala in early extinction and suggesting that vmPFC plays an important role in the retention of the extinction memory. [DOI] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Liebscher C, Cacciaglia R, Diener SJ, Ridder S et al (2012). Hippocampal but not amygdalar volume affects contextual fear conditioning in humans. Hum Brain Mapp 33: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Schad LR, Flor H (2012). Activation of the ventral striatum during aversive contextual conditioning in humans. Biol Psychol 91: 74–80. [DOI] [PubMed] [Google Scholar]
- Pollak DD, Rogan MT, Egner T, Perez DL, Yanagihara TK, Hirsch J (2010). A translational bridge between mouse and human models of learned safety. Ann Med 42: 115–122. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23: R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I et al (2014). Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol Learn Mem 113: 125–134. Evidence that cannibinoid administration improves fear extinction retention, suggesting that these compounds may improve emotional learning deficits seen in PTSD and other psychiatric disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczka KA, Mechias ML, Gartmann N, Reif A, Deckert J, Pessiglione M et al (2011). Empirical support for an involvement of the mesostriatal dopamine system in human fear extinction. Transl Psychiatry 1: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt I, Jansen A, Kellermann T, Schuppen A, Kohn N, Gerlach AL et al (2010). Neural correlates of aversive conditioning: development of a functional imaging paradigm for the investigation of anxiety disorders. Eur Arch Psychiatry Clin Neurosci 260: 443–453. [DOI] [PubMed] [Google Scholar]
- Ridder S, Treutlein J, Nees F, Lang S, Diener S, Wessa M et al (2012). Brain activation during fear conditioning in humans depends on genetic variations related to functioning of the hypothalamic-pituitary-adrenal axis: first evidence from two independent subsamples. Psychol Med 42: 2325–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Charney DR, Vytal K, Grillon C (2013). Stress increases aversive prediction error signal in the ventral striatum. Proc Natl Acad Sci USA 110: 4129–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A et al (2009). Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res 174: 210–216. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA (2003). The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci 23: 11054–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J et al (2011). Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther 17: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA (2008). From fear to safety and back: reversal of fear in the human brain. J Neurosci 28: 11517–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DH, Balderston NL, Helmstetter FJ (2012). Resting-state connectivity of the amygdala is altered following Pavlovian fear conditioning. Front Hum Neurosci 6: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweckendiek J, Klucken T, Merz CJ, Tabbert K, Walter B, Ambach W et al (2011). Weaving the (neuronal) web: fear learning in spider phobia. Neuroimage 54: 681–688. [DOI] [PubMed] [Google Scholar]
- Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD et al (2014). Sex differences in extinction recall in posttraumatic stress disorder: a pilot fMRI study. Neurobiol Learn Mem 113: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS et al (2010). A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327: 863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, Gvozdanovic GA, Samann PG, Czisch M (2014). Ventromedial prefrontal cortex activity and rapid eye movement sleep are associated with subsequent fear expression in human subjects. Exp Brain Res 232: 1547–1554. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Schroter MS, Andrade KC, Dresler M, Kiem SA, Goya-Maldonado R et al (2012). Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp 33: 2362–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, Sturm A, Andrade KC, Schroter MS, Goya-Maldonado R, Holsboer F et al (2010). The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J Psychiatr Res 44: 1121–1128. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Garfinkel SN, Liberzon I (2013). Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front Hum Neurosci 7: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube B, Lueken U, Jansen A, Konrad C, Gloster AT, Gerlach AL et al (2014. a). Neural correlates of procedural variants in cognitive-behavioral therapy: a randomized, controlled multicenter FMRI study. Psychother Psychosom 83: 222–233. [DOI] [PubMed] [Google Scholar]
- Straube B, Reif A, Richter J, Lueken U, Weber H, Arolt V et al (2014. b). The functional −1019C/G HTR1A polymorphism and mechanisms of fear. Transl Psychiatry 4: e490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WH (2006). Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage 29: 125–135. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT et al (2010). Cortisol enhances neural differentiation during fear acquisition and extinction in contingency aware young women. Neurobiol Learn Mem 94: 392–401. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT et al (2011). Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Soc Cogn Affect Neurosci 6: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA (2007). The neural basis of loss aversion in decision-making under risk. Science 315: 515–518. [DOI] [PubMed] [Google Scholar]
- Tuescher O, Protopopescu X, Pan H, Cloitre M, Butler T, Goldstein M et al (2011). Differential activity of subgenual cingulate and brainstem in panic disorder and PTSD. J Anxiety Disord 25: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H et al (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschoppe J, Nees F, Banaschewski T, Barker GJ, Buchel C, Conrod PJ et al (2014). Aversive learning in adolescents: modulation by amygdala-prefrontal and amygdala-hippocampal connectivity and neuroticism. Neuropsychopharmacology 39: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Well S, Visser RM, Scholte HS, Kindt M (2012). Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cogn Affect Behav Neurosci 12: 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51: 32–58. [DOI] [PubMed] [Google Scholar]
- Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH (2014). Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem 116: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Sreenivasan KR, Wood KH, Ver Hoef LW, Deshpande G, Knight DC (2014). Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage 102 Pt 2: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer J, Schulz SM, Reicherts P, Glotzbach-Schoon E, Andreatta M, Pauli P (2014). Brain activity associated with illusory correlations in animal phobia. Soc Cogn Affect Neurosci 10: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann T, Grimm O, Pohlack ST, Nees F, Cacciaglia R, Dinu-Biringer R et al (2015). Brain morphology correlates of interindividual differences in conditioned fear acquisition and extinction learning. Brain Struct Funct. (e-pub ahead of print). [DOI] [PubMed]
- Wood KH, Kuykendall D, Ver Hoef LW, Knight DC (2013). Neural substrates underlying learning-related changes of the unconditioned fear response. Open Neuroimag J 7: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC (2012). Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. Neuroimage 60: 787–799. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A et al (2011). Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70: 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]