Abstract
Borrelia burgdorferi, the causative agent of Lyme disease, and Babesia microti, a causative agent of babesiosis, are increasingly implicated in the growing tick-borne disease burden in the northeastern United States. These pathogens are transmitted via the bite of an infected tick vector, Ixodes scapularis, which is capable of harboring and inoculating a host with multiple pathogens simultaneously. Clinical presentation of the diseases is heterogeneous and ranges from mild flu-like symptoms to near-fatal cardiac arrhythmias. While the reason for the variability is not known, the possibility exists that concomitant infection with both B. burgdorferi and B. microti may synergistically increase disease severity. In an effort to clarify the current state of understanding regarding coinfection with B. burgdorferi and B. microti, in this review, we discuss the geographical distribution and pathogenesis of Lyme disease and babesiosis in the United States, the immunological response of humans to B. burgdorferi or B. microti infection, the existing knowledge regarding coinfection disease pathology, and critical factors that have led to ambiguity in the literature regarding coinfection, in order to eliminate confusion in future experimental design and investigation.
1. Introduction
Tick-borne diseases, which affect both humans and other animals, are on the rise in the United States as once uninhabited wilderness continues to be urbanized promoting increased exposure and transmission to humans. Tick-borne diseases can result from several types of pathogens including bacteria, viruses, and protozoa, and most infections are the consequence of an infected tick bite. Further complicating the diagnosis and treatment of this family of emerging diseases is the fact that ticks can harbor multiple pathogens and coinfect individuals with multiple parasites. Two of the most common tick-borne illnesses in the United States are Lyme disease, a condition caused by the bacterium Borrelia burgdorferi, and babesiosis, a disease resulting from infection with Babesia microti. These two pathogens occur in overlapping geographic areas in the United States and use the same vector host Ixodes scapularis. There is considerable confusion in the literature regarding the effect that coinfection with B. burgdorferi and B. microti has on disease severity and prognosis. In an effort to clarify what is understood regarding concomitant infection, in this paper, we review the geographical distribution and pathogenesis of Lyme disease and babesiosis in the United States, the immunological response of humans to B. burgdorferi or B. microti infection, the existing knowledge regarding coinfection disease pathology, and the critical factors that have led to ambiguity in the literature regarding coinfection. A clearer understanding of what is currently known in the literature will serve to help guide future research efforts as well as improve clinical diagnosis and treatment.
2. Lyme Disease and Babesiosis
Lyme disease, a condition caused by the bacterium Borrelia burgdorferi, is transmitted to humans via the bite of Ixodes scapularis, also known by the common names deer tick or blacklegged tick. In 1993, Ixodes dammini was proven to be the same species as I. scapularis. While the use of the name I. scapularis is preferential, I. dammini is still occasionally used [1]. For the purpose of this review, the name I. dammini will be replaced with I. scapularis in order to eliminate confusion. In addition, in the western United States, the Western Blacklegged Tick Ixodes pacificus is responsible for the transmission of B. burgdorferi to humans [2]. The current geographic distribution of I. scapularis in the United States is primarily in the northeastern, southeastern, and midwestern states (Figure 1).
Figure 1.
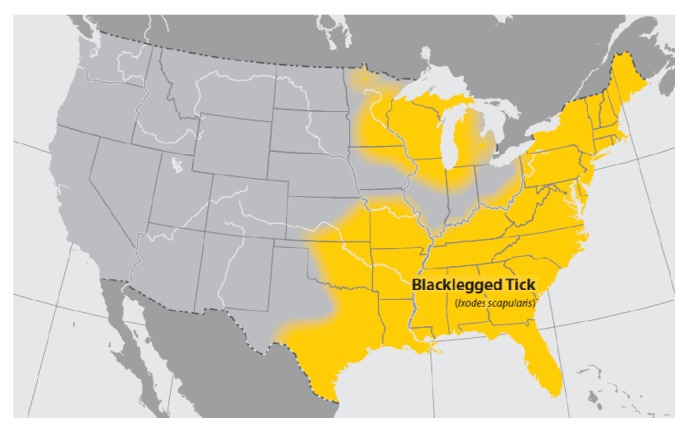
Geographic distribution of I. scapularis within the continental United States. Yellow shaded areas represent the distribution of I. scapularis in the northeastern and upper midwestern United States [2].
Currently considered the most commonly diagnosed tick-transmitted disease in the United States, Lyme disease affected over 25,000 individuals in 2013 alone according to the Center for Disease Control and Prevention [3]. The overall number of confirmed cases has ranged from a low of 11,700 in 1995 to a high of 29,959 in 2009. Lyme disease is caused by infection with the spirochete bacterium B. burgdorferi and initially results in a rash at the site of the infective tick bite followed by flu-like symptoms including fever, chills, fatigue, headache, and general malaise for up to a month. During and after this period, muscular pain, as well as neurological and cardiac pathologies, can occur. In a small percentage of cases, severe complications result such as facial paralysis and dangerous cardiac arrhythmias. Finally, after several months of infection, symptoms similar to those of rheumatoid arthritis begin to occur in joints. A review of the clinical data reveals that infection with B. burgdorferi follows often unpredictable disease progression, producing only mild symptoms in some over a long period of time or causing rapid onset of potentially fatal effects in others. Naturally, this observation leads to the question of what factors might influence the variable and unpredictable progression of Lyme disease in the nearly quarter of a million patients infected in the United States every year [4].
In the United States, I. scapularis is the most well-known vector of Babesia microti, a natural parasite of rodents [5]. Transmitted by the bite of an infected tick and in some rare cases via blood transfusion or congenitally, babesiosis is acquired when sporozoites are introduced into the host's bloodstream by the bite of an infected tick. Following introduction, sporozoites infect erythrocytes, reproduce, and form merozoites that are then capable of erupting from infected erythrocytes and infecting others. The continuation of this cycle yields a large intraerythrocytic population very quickly [5]. B. microti infection mimics mild malaria with the main symptoms stemming from hemolytic anemia. Indeed, B. microti infections are often even diagnosed as malaria due to the similar appearance of infected erythrocytes that can be viewed via a blood smear. While babesiosis can be fatal in immunologically compromised or splenectomized individuals, healthy individuals recover from infection with B. microti spontaneously, requiring only temporary treatment of symptoms [5].
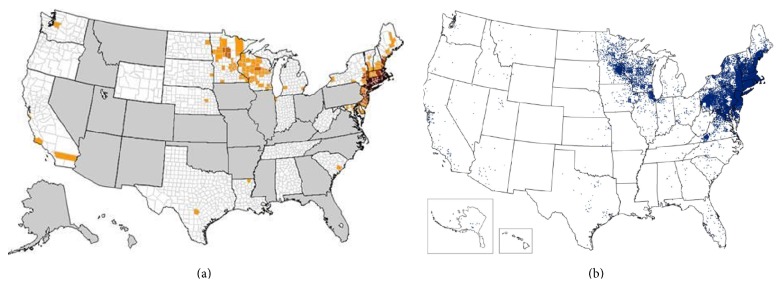
Lyme disease and human babesiosis appear most commonly diagnosed in overlapping geographic areas (Figures 2(a) and 2(b)). Babesiosis has been found to be most prevalent in the northeastern United States, as well as the upper midwest, and is diagnosed in particularly high density areas of the northeast, New Jersey, New York, Minnesota, and Wisconsin [6]. Notably, all of these states are also among those that report the largest number of Lyme disease cases each year. In 2011, 96% of Lyme cases were reported from 13 states: Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Vermont, Virginia, and Wisconsin [7]. The geographic overlap between the occurrence of Lyme disease and babesiosis suggests that the two diseases, both transmitted by the vector I. scapularis, may simultaneously infect a population in a geographic area.
Figure 2.
Geographic distribution of babesiosis and Lyme disease in the United States. (a) Reported cases of at least one case of babesiosis in 22 of the 27 different states that conducted surveillance: white = 0 cases; yellow = 1–5 cases; orange = 6–10 cases; brown = 11–20 cases; and dark brown > 20 cases. Babesiosis was not a reportable disease in the gray states, and health departments in those states did not notify CDC of cases [6]. (b) Reported cases of Lyme disease across the United States. Each blue dot represents a confirmed case [7].
Clinical evidence supporting the idea that coinfection may be possible and more common than originally imagined includes, one, the observation that both pathogens are commonly found in rodents captured in endemic areas; two, the notion that patients diagnosed with Lyme disease are often found to also be seropositive for antibabesial antibodies; and three, the fact that I. scapularis has been shown to be capable of transmitting both pathogens at once [8–10]. In a study of rodent populations in Prudence and Patience, Rhode Island, the fact that greater than 50% of captured Peromyscus leucopus and Microtus pennsylvanicus harbored both B. microti and B. burgdorferi suggests that individual larval I. scapularis ingest and transmit both pathogens [11]. Other research has confirmed that rodents and tick vectors are both frequently coinfected. In a study from tick samples captured in New Jersey, of those positive for B. burgdorferi, B. microti, or human granulocytic ehrlichiosis agent, 20% of ticks were coinfected with at least two pathogens [12]. It is also known that nymphal I. scapularis infected with both B. burgdorferi and B. microti are able to simultaneously transmit both organisms to hamsters [13]. The observation that both pathogens frequently reside together in rodent populations and the tick vector I. scapularis certainly raises suspicion that coinfection in human hosts may be possible [14–16]. Multiple studies measuring human antibody titers do provide some evidence that coinfection does occur. These include a recent study that found that up to 66% of residents of Long Island, New York, who were diagnosed with Lyme disease were also seropositive to antibodies against B. microti [17, 18]. Conversely, in a study in which patients had first been diagnosed with babesiosis, 54% also possessed IgG and IgM antibodies to B. burgdorferi [19]. While the simple presence of antibodies to B. microti or B. burgdorferi in no way guarantees that both infections were acquired from the same tick, it does raise interesting speculation regarding how temporal variations in the acquisition of babesiosis and Lyme disease, be it from the same tick or within short time frame from different ticks, may affect the clinical progression of coinfection.
Although it is well established that both B. microti and B. burgdorferi certainly coinfect rodent hosts and tick vectors, that tick vectors are capable of transmitting both pathogens simultaneously, and that a large percentage of patients suffering from Lyme disease or babesiosis have also been exposed to B. microti or B. burgdorferi, respectively, little research has been amassed regarding the pathophysiological effects of concomitant infection. Citing observations from mice in which babesial infection appears to enhance Lyme disease myocarditis, it has been suggested that coinfection increases the severity of disease and may impair host defense mechanisms [20]. There is some data to support this hypothesis in that patients with coinfections report a longer duration of illness and exacerbated symptoms including myalgia, fatigue, sweats, anorexia, erythema migrans, and conjunctivitis [17, 18]. In one case of coinfection, death as a result of pancarditis even occurred [21]. Other studies, however, report that coinfection does not worsen the long term outcome of patients suffering from infection with both pathogens specifically with regard to the prevalence of constitutional, musculoskeletal, or neurological symptoms [22].
Clearly, while it has been established that both B. microti and B. burgdorferi can coexist in the same organism, infect the same vector, simultaneously infect a mammal host, and cause debilitating symptoms, disagreement is still substantial and research is lacking regarding the synergistic or perhaps only additive effect of concomitant infection. While the debate continues, the incidence of tick-borne infections is quickly on the rise due to a variety of factors such as larger deer populations, increasing tick populations, and increased development of wooded and rural areas bringing humans, deer, and ticks in even closer proximity. As tick-borne infections become more common in the United States and across the globe, the need for research on the clinical manifestation, immunological response, pathophysiological mechanism, and proper treatment of coinfection with tick-borne pathogens is vital.
3. Immunological Response to B. burgdorferi
The cells of the innate immune system constitute the first line of defense against B. burgdorferi. The widely accepted mechanism is that lipid-modified membrane proteins and diacylglycerol-containing glycolipids of the spirochete signal via CD14 and/or Toll-like receptor 2 (TLR2)/TLR1 heterodimers to promote a proinflammatory response during infection [23, 24]. The chemokine receptor CXCR2 also plays a role in the generation of B. burgdorferi induced inflammation [25]. Specifically, the lipoproteins and glycolipids of B. burgdorferi activate the immune system by binding to TLRs, in particular TLR2, leading to cytokines IL-6, IL-10, IL-12, TNF-α, and IL-1β being released from innate immune system cells [26]. These cytokines serve as a link between the innate and adaptive immune systems, influencing the response and polarization of the host's cell mediated and humoral immune response against B. burgdorferi. Subsequently, as T-helper cells are activated, they differentiate into a combination of Th1, Th2, Th17, or T regulatory cells, resulting in a polarized immune response. Different individuals can mount immune responses with varying polarization, and researchers have speculated that the polarization of the cell mediated immune response may influence the overall outcome of B. burgdorferi infection. While not exclusive, the adaptive immune system combats intracellular pathogens via a strong Th1 response, characterized by increased production of IFN-γ, while a strong Th2 response, vital for host defense against extracellular pathogens, is characterized by an increase in IL-4 production [26].
In the late 1990s, two studies found that IFN-γ predominated, compared to IL-4, during B. burgdorferi infection. In one study, researchers noted decreased IL-4 synthesis and increased IFN-γ synthesis in patients infected with B. burgdorferi compared to a control group [27]. The increase in IFN-γ observed in these patients resulted from induced Th1 polarization, contributing to increased pathogenesis of the bacteria, and potential autoimmune reactions. A second report by Ekerfelt and colleagues found similar results in an adult population afflicted with neural B. burgdorferi infection. In this population of individuals, suffering from neuroborreliosis, IFN-γ production was significantly increased while IL-4 production was unusually low [28]. These two seminal studies suggest that, in adults (particularly those with severe courses of infection), strong Th1 polarization of the cell mediated adaptive immune response is characteristic of B. burgdorferi infection.
The observed cytokine response to B. burgdorferi also appears to have temporal variability. Individuals with nonchronic neuroborreliosis have an initial increase in INF-γ followed by an increase in IL-4, corresponding to pathogen clearance, while in individuals who experience chronic neuroborreliosis the initial IFN-γ response is not followed by IL-4 elevation suggesting a persistent Th1 response [29]. Interestingly, both the genetics and age of the host may influence this temporal immune polarization; children are notably predisposed to generating a highly effective balanced Th1/Th2 response, while adults are more likely to generate primarily a Th1 response [30]. In addition, a strong genetic component involved in the differential immune polarization response to B. burgdorferi has been noted in various strains of laboratory mice that exhibit different susceptibilities to B. burgdorferi [31].
One of the most significant characteristics of the B. burgdorferi spirochete is its ability to avoid immune detection, often for many years, by avoiding the host complement system. The complement system is one of the most versatile parts of the immune system, and its activation leads to phagocytosis of target pathogens or the formation of membrane attack complexes (MACs) [32]. In some cases, host complement regulatory factors are recruited by pathogens in order to protect them from MACs. For example, B. burgdorferi recruits host complement proteins factor H (FH) and factor H-like protein-1 (FHL-1) to its own surface, effectively thwarting the host complement attack against the spirochete. Two different borrelial proteins, of the complement regulator-acquiring surface protein (CRASP) family, have been identified as ligands for FH and FHL-1 [33, 34]. Expression of CRASPs directly correlates with serum resistance, in that all serum-resistant isolates express these proteins, whereas all serum-sensitive isolates analyzed to date do not possess proteins with such binding activity [35, 36]. Recently published studies with recombinant outer surface protein OspE suggest that it also functions as a ligand for factor H [37]. Experiments have shown that interference with these surface proteins, particularly OspE, can decrease spirochete survivability making OspE a good therapeutic target [38].
Recently, it was discovered that if B. burgdorferi spirochetes were introduced into a host using a syringe versus an infected tick bite, the inflammatory response in the host's skin was altered. When injected via syringe, without associated vector saliva and salivary molecules, the spirochetes elicited an inflammatory reaction characterized by heightened production of TNF-α and induction of CRAMP, a mouse cathelicidin (antimicrobial peptide). Alternatively, when mice were inoculated with B. burgdorferi via an infected tick bite, the inflammatory response was significantly reduced [39]. Such findings illustrate the importance of natural vector infection and the vital role that vector saliva plays in the establishment of Lyme disease. Initial spirochete multiplication in skin tissue appears to occur prior to dissemination of spirochetes throughout the body, suggesting that if immunity in the skin could be improved or restored via blockade of immunomodulatory and immunosuppressive salivary peptides, disease progression could be delayed or prevented. This idea is further discussed in Natural versus Artificial Inoculation Strategies.
Lastly, the humoral immune response is also vital in protecting the host against deleterious effects of persistent infection. Experiments in a variety of mouse models have shown that both T-cell dependent and T-cell independent mechanisms contribute to activation of B cells and humoral immunity against B. burgdorferi. When severe combined immune deficient (SCID) mice were injected with sera from immune competent mice infected with B. burgdorferi, the SCID mice were protected from disease even when high doses of spirochetes were used. Conversely, in SCID mice in which infection had already been established, injection with immunocompetent mouse sera resulted in resolution of Lyme arthritis but not carditis, indicating that while a humoral response protects against certain aspects of B. burgdorferi infection, cellular and T-cell dependent responses are vital for the complete resolution of infection in all organs [40].
4. Immunological Response to B. microti
The intraerythrocytic parasite B. microti possesses a detailed lifecycle that utilizes several hosts. In nature, both a rodent and tick host are required for survival of the parasite. In the rodent host, most commonly the white-footed mouse Peromyscus leucopus, sporozoites are injected from the bite of an infected tick, commonly I. scapularis, and infect mouse erythrocytes where they either reproduce asexually or undergo gametogony to produce viable gametes ([5] and the references therein). These gametes are then reintroduced into the definitive host, a tick from the genus Ixodes, during a subsequent blood meal. In the definitive host, gametes join to form an ookinete which migrates to the salivary glands and undergoes sporogony producing new sporozoites. In the natural lifecycle of B. microti, these sporozoites would once again be injected into a rodent during the process of a tick blood meal. However, if the infected tick instead seeks its blood meal from a human, the sporozoites are introduced into the human host. Humans can also artificially infect others through the process of blood donation and transfusion, as this parasite resides inside erythrocytes for much of its lifecycle. Though not the primary mechanism of infection, babesiosis is the most common blood transfusion-transmitted infection in the United States with 162 cases reported since 1980 [41–43].
The immunological response of the human host against B. microti reflects its elaborate lifecycle. Within the human host, B. microti infection occurs in three phases: establishment, progression, and resolution [44]. During the establishment phase of infection, sporozoites injected by the bite of an infected tick are free in the plasma. It is during this time that IgG antibodies of a previously exposed host bind to and facilitate the destruction of sporozoites [44]. Once sporozoites penetrate erythrocytes and establish the erythrocytic phase of infection, the innate immune system controls parasite populations during what is referred to as the progression stage. Macrophages producing TNF-α, reactive oxygen species, and nitric oxide, as well as natural killer cells producing IFN-γ, contribute to the innate immune response, although their mechanism of action is still unknown [44]. Production of IL-12 by macrophages and natural killer cells is also vital for host defense during the progression stage, as mice that lack both macrophages and natural killer cells are unusually susceptible to high levels of parasitemia following B. microti infection [45]. The cytokine most vital to the control of parasitemia and resolution of infection may in fact be IFN-γ. Not only is IFN-γ produced by innate immune cells and effector T cells in both progression and resolution stages of B. microti infection, but experimental studies have also found that IFN-γ is vital for the generation of protective immunity. In 1999, Igarashi et al. discovered that IFN-γ deficient mice were completely incapable of mounting any significant protective immune response against B. microti, while blockade of IL-2, IL-4, and TNF-α with monoclonal antibodies did not alter the immune response [46]. Finally, the spleen also aids in the process of parasite control as it helps clear damaged and infected erythrocytes through macrophage phagocytosis.
Approximately ten days after B. microti infection, parasite numbers generally decrease, and the resolution phase, characterized by activation of CD4+, IFN-γ producing T cells, begins [44]. The importance of T-helper cells in defense against B. microti is well established [47]. The humoral response toward B. microti occurs during the resolution stage and results in the production of antibodies specific for surface antigens of merozoites in the plasma. E/S antigens of infected red blood cells help to reduce parasitemia in the blood and protect against future infection. The generation of parasite specific IgG is also essential for the prevention of parasite replication during the resolution stage of B. microti infection [48]. Specifically, the humoral response to acute infection is characterized by IgM production, followed by IgG production, and the immunological memory elicited from this response can prevent or reduce the duration and severity of future infections [44].
Based upon the presence of IL-2 and IFN-γ throughout B. microti infection in mouse models, it is likely that, during the initial stages of infection, establishment, and progression, a Th1 response predominates. IL-2 and IFN-γ are present approximately a week after infection and peak around day 12 during the progression stage [49]. Th2 cytokines, IL-4 and IL-10, are elevated starting approximately 2 weeks after infection and peak at three weeks following infection during the resolution stage [49]. Thus, in the early stages of infection, a Th1 response is likely required for the initial control of parasite population growth, while a Th2 response predominates during the resolution stage of infection to clear aging and damaged parasites from the body. Supporting this hypothesis is the observation that the failure to generate and maintain a strong Th1 response during the initials stages of B. microti infection results in a drastically increased rate of parasite replication [48]. These results can be extrapolated to human populations as researchers have extensively characterized the human course of babesial infection. Even as early as 1977, it was shown that human subjects experienced a slightly delayed response to B. microti with symptoms taking up to two weeks to develop [50].
5. Immunological Response to Coinfection with B. burgdorferi and B. microti
While there is limited research on the host response to concomitant infection with the tick-borne bacterium B. burgdorferi and the parasite B. microti, it has been suggested that coinfection may result in an altered or suppressed immune response when both pathogens are present. Supporting this, Vinasco et al. observed that, in coinfected BALB/c and C3H mice, the quantity of spleen macrophages is drastically reduced impairing the destruction and clearance of parasitized red blood cells [51]. Thus, such a compromised host immune response could lead to intensified pathogenesis and even the development of chronic infection as suggested in the literature [18, 52].
To examine this further, four key studies, published in an effort to establish definitive conclusions regarding the immunological, pathological, and physiological effects resulting from coinfection with B. burgdorferi and B. microti, will be reviewed (Table 1). Those factors that have led to uncertainty in the literature regarding coinfection will be discussed, including variations in experimental model (humans versus mice), inoculation strategy, pathogen strain, and quantitative measures.
Table 1.
Summary of results from four key studies investigating the impact of B. burgdorferi and B. microti coinfection.
| Researchers | Arthritis results | Carditis/other results | Cytokine results | Blood analysis results |
|---|---|---|---|---|
| Krause et al. (1996) [18] | Increased severity and duration of arthralgia and joint swelling in coinfected individuals∗ | Increased severity and duration of splenomegaly, conjunctivitis, neck stiffness, and erythema migrans in coinfected individuals∗ | N/A | Higher detection of spirochete DNA in peripheral blood in coinfected individuals |
|
| ||||
| Wang et al. (2000) [22] | Equal severity of arthralgia and joint swelling in individuals with evidence of previous babesial infection∧ | Equal severity of splenomegaly, conjunctivitis, neck stiffness, and erythema migrans in individuals with evidence of babesial infection∧ | N/A | Higher Babesia seropositivity in individuals with evidence of previous babesial infection, 22% versus 7% (control) |
|
| ||||
| Moro et al. (2002) [54] | Increased severity of arthritis in coinfected BALB/c mice 30 days after infection versus single infection; no change in C3H/HeJ mice | Equal severity of carditis in BALB/c and C3H/HeJ coinfected and singly infected mice | Significant decrease in IL-10 and IL-13 in coinfected BALB/c mice 30 days after infection; no change in C3H/HeJ mice | Significant decrease in IgG in coinfected BALB/c mice 15 days after infection which returned to baseline 30 days after infection; no change in C3H/HeJ mice |
|
| ||||
| Coleman et al. (2005) [55] | Equal severity of arthritis in coinfected versus singly infected C3H/HeN mice or BALB/c mice | Equal spleen weights in C3H/HeN or BALB/c coinfected versus singly infected mice | N/A | Equal parasitemia in C3H/HeN or BALB/c coinfected versus singly infected mice; elevated LDH |
∗Symptom severity assessed by patient self-report.
∧Symptom severity assessed by clinical exam using criteria set forth by the American College of Rheumatology Glossary Joint Exam.
6. Experimental Design Discrepancies
6.1. Human Subjects versus Mouse Models
Much of the confusion in the literature regarding coinfection is a direct result of very large differences in experimental design, in particular epidemiological studies from naturally infected humans versus studies in mouse models synthetically inoculated. In general, human studies pose a variety of challenges, one of which is the identification of a large group of patients with acute concomitant infections. While Krause et al. were able to find 26 individuals with evidence of acute coinfection, in the study by Wang et al., only 4 individuals with acute coinfection were identified [18, 22]. These small sample sizes limit the statistical power of the data produced and ultimately result in questionable accuracy for the studies. Furthermore, the wide variety of uncontrollable, confounding variables in human clinical studies, for example, subject variability with regard to medical history, further reduces the accuracy and reliability of results generated from such studies. Human epidemiological analyses constitute two of the four major studies that provide information on concomitant infection with B. burgdorferi and B. microti. However, the conclusions of these studies are contradictory. Krause et al. found that coinfection with B. burgdorferi and B. microti results in an increase in pathological severity while Wang et al. determined that coinfection did not have an impact on disease or symptom outcomes.
While the aforementioned studies disagree regarding the pathological outcome of coinfection in humans, infections in splenectomized individuals suggest that disease outcomes are indeed synergistic. B. microti infection alone is generally a self-limiting disease in healthy, immunocompetent individuals, but splenectomized individuals exhibit characteristically severe pathology with potentially fatal results. A 2008 case study reported that a middle-aged, splenectomized male diagnosed with both B. microti and B. burgdorferi experienced symptoms of neuroborreliosis only two weeks after an initial diagnosis of babesiosis and failed to respond to multiple antibiotics. The dual infection and associated symptoms were only resolved after complete replacement transfusion with intravenous IgG (IVIG). However, even after five years, the patient still exhibited mild sensory neuropathy in his legs [53].
Although studies using mice models have been able to generate larger sample sizes, control the sample population for previous pathogen exposure and immunological history, regulate timing and mechanism of pathogen exposure, and standardize outcome measurements, these studies, unfortunately, have also produced conflicting results regarding the immunological and pathophysiological effect of coinfection. Some explanations for the continued discrepancy in the literature are that, one, different mouse strains can demonstrate highly varied responses to B. burgdorferi; two, the artificial inoculation strategies used eliminated the important variable of the action of tick salivary molecules; and lastly, although mouse models are extremely important in biomedical research, the relevance of nonhuman models in complex immune responses to multiple pathogens is not clear. Two studies using mouse models to investigate whether pathology was exacerbated in the presence of acute coinfection with B. microti and B. burgdorferi produced conflicting results [54, 55].
Some of the conflict between these studies can be attributed to physiological differences in mouse strains (Table 2). For example, the C3H mouse strain is known to be extremely susceptible to Lyme induced arthritis. In this strain, arthritis severity does not change in coinfected mice versus those singly infected with B. burgdorferi, and moreover, splenic weights are also unchanged [55]. However, coinfection in BALB/c mice, a strain much less susceptible to Lyme related arthritis, does present with increased arthritis and decreased IL-10 and IL-13 levels one month after infection, suggesting that additional infection with B. microti produces a Th1 inflammatory response responsible for the exacerbated arthritis [54]. The lack of a statistically significant increase in arthritis in C3H mice is likely attributable to the fact that singular infection with B. burgdorferi alone already produces an exaggerated Th1 response, possibly due to genetic and immunoinflammatory factors unique to C3H mice; thus, any exacerbation due to coinfection is masked. Interestingly, both mouse strains displayed equal carditis when either singly infected or coinfected. It should be noted that two different substrains of C3H mice were used in these studies, C3H/HeN and C3H/HeJ, which also could lead to variability in experimental results. There is a genetically mediated difference in the two substrains in their response to bacterial endotoxin which is linked to the Toll-like receptor 4 protein; as a result, C3H/HeN mice are endotoxin sensitive, whereas C3H/HeJ mice are endotoxin resistant [56].
Table 2.
Mouse strains.
| Strain | Characteristics |
|---|---|
| BALB/c | Reduced susceptibility to Lyme disease related arthritis Produce IL-4 and develop a Th2 polarized response to B. burgdorferi Reduction in IL-4 experimentally demonstrated to increase severity of arthritis Reduced levels of parasitemia in general when infected with B. microti |
|
| |
| C3H/x∗ | Increased susceptibility to Lyme disease related arthritis Produce IFN-γ and develop a Th1 polarized response to B. burgdorferi Reduction in IFN-γ correlated with reduced arthritis Supplementation with recombinant IL-4 correlated with reduced arthritis |
∗Mouse substrains HeJ and HeN were used in different studies. The difference between the two substrains is that the HeN strain is endotoxin sensitive (normal LPS response) and the HeJ strain is endotoxin resistant.
6.2. Natural versus Artificial Inoculation Strategies
In addition to differences in experimental model, different inoculation strategies have led to disparate results in the literature regarding coinfection with B. burgdorferi and B. microti. Recently, it was discovered that arthropod saliva contains a variety of proteinaceous factors that enhance a pathogen's ability to establish an initial infection in the host [57]. In many cases, such as that of Plasmodium, it appears that arthropod saliva is completely necessary for natural infection to occur. This effect may explain why extremely large doses of B. burgdorferi and B. microti are necessary to establish infection in the laboratory.
Specifically, salivary proteins of I. scapularis were shown to exert unique immunoregulatory effects which aid survival of the pathogens Rickettsia and B. burgdorferi, not only by creating a more hospitable microenvironment at the injection site, but also by directly assisting in pathogen immune evasion and survival. The salivary factors of I. scapularis aid the establishment of Rickettsia by inflammatory cytokine suppression and assist B. burgdorferi in evading host antibodies by producing Salp15, a salivary protein [58–60]. Another salivary protein, TSLPI (Tick Salivary Lectin Pathway Inhibitor), improves B. burgdorferi's transmission through blocking the lectin complement cascade resulting in impaired neutrophil phagocytosis, chemotaxis, and decreased pathogen lysis [61]. The salivary protein Salp25, an antioxidant, reduces ROS concentrations present at the vector-pathogen-host interface via its detoxifying action, thus improving B. burgdorferi's chances of survival and successful infection. Salp25, Salp15, TSLPI, and other arthropod salivary proteins, yet to be elucidated, likely play a vital role in the establishment of initial infection within mammalian hosts. As a result of their actions during the host immune system's first exposure to pathogen, these proteins may also cause long term immunomodulatory effects through modifying polarization patterns. Therefore, the absence of arthropod saliva in studies using mouse models likely accounts for some of the incongruous results when compared with clinical case studies.
In addition to the absence of salivary proteins, another problem with artificial inoculation of mouse models is that the injection routes are not anatomically synonymous with that of natural exposure. In most cases, mice are given intraperitoneal, intravenous, or intramuscular injections with the pathogen while natural infection would occur within the dermis or subcutaneous tissue. Altering the initial site of host-pathogen exposure could yield unexpected changes in immune polarization and response since differences in dermal and mucosal immune responses are well known. In both mouse studies discussed in this review, mice were injected intradermally with B. burgdorferi spirochetes, thus approximating the natural vector-borne infection pattern; however, in both experiments, B. microti parasites were injected intraperitoneally.
6.3. Pathogen Strain
Another confounding element in B. burgdorferi and B. microti coinfection studies is the use of different strains of pathogen. In general, two different strains of B. microti have been used in experiments, one isolated from a strain of P. leucopus, adapted to growth in laboratory mice and maintained by blood passage in C3H/HeN mice [55], and a second strain, MN1, which was isolated from a human patient, inoculated into golden Syrian hamsters for adaptation, and then cryopreserved rather than maintained by repeated blood passage [62]. The preserved blood was subsequently reconstituted in hamsters for amplification; blood was ultimately collected for studies when 80% parasitemia was reached. It has been hypothesized that the strain adapted from P. leucopus may have been attenuated through repeated blood passage, thus producing different results following infection. B. burgdorferi strain N40 was used in all studies examined in this review.
6.4. Quantitative Measurements
To assess the immunological progression and pathology of coinfection with B. burgdorferi and B. microti from multiple studies, it is essential that similar measures and outcomes are compared. Several of the most commonly measured immunopathological outcomes of infection are variations in cytokine level, arthritis severity, and peripheral blood pathogen levels. The lack of consistency and standardization in assessment however has generated contradictions in the literature. In Table 3, the methods used in the four major coinfection research studies are compared. Only one study evaluated any change in cytokine level, while all studies performed some variable level of histopathology and serology.
Table 3.
Summary of experimental methodology from four key studies investigating the impact of B. burgdorferi and B. microti coinfection.
| Krause et al. (1996) [18] | Human subjects, n = 250 B. burgdorferi seropositive: n = 214 B. microti seropositive: n = 10 Coinfected: n = 26 |
Epidemiological analyses Clinical evaluation: physical exam and medical history Serological assessment: blood smears, ELISA, western blot, IFA, and PCR Statistical analyses: χ 2 analysis and Student's t-test |
|
| ||
| Wang et al. (2000) [22] | Human subjects, n = 336 Clinical Lyme disease: n = 171 Acute concurrent Lyme disease and babesiosis: n = 4 B. burgdorferi seropositive: n = 112 B. microti seropositive: n = 48 B. burgdorferi and B. microti seropositive: n = 27 |
Epidemiological analyses Clinical evaluation: physical exam and medical history Serological assessment: western blot and antibody-capture EIA for B. burgdorferi; IFA for B. microti Statistical analyses: χ 2 analysis and Student's t-test |
|
| ||
| Moro et al. (2002) [54] | Mouse model Species: C3H/HeJ and BALB/c |
Histopathological analysis for arthritis and carditis Spirochete quantification: competitive PCR Tissue cytokine analysis: ELISA |
|
| ||
| Coleman et al. (2005) [55] | Mouse model Species: C3H/HeN and BALB/c |
Histopathological analysis for arthritis, carditis, and splenomegaly Spirochete quantification: quantitative PCR Serological assessment: ELISA, indirect immunofluorescence assays (IFA), complete blood count, and blood smears |
In human epidemiological studies, pathology was determined either by patient self-report or by patient reported symptoms combined with a clinical exam [18, 22]. While the clinical examination used by Wang et al. was standardized based upon the American College of Rheumatology Glossary Joint Exam criteria, this study did not assess all disease parameters, that is, arthritis, neurological changes, and infection status. Overall, Krause et al. reported that in coinfected individuals there was slightly less arthralgia, 27%, compared to either singly infected Lyme disease or babesiosis, 36% and 40%, respectively, although splenomegaly, conjunctivitis, and multiple erythema migrans were all significantly higher in coinfected individuals [18]. This study also confirmed coinfection with B. burgdorferi and B. microti by the presence of spirochete DNA in blood samples. Spirochete DNA was more frequently detected in coinfected individuals (27%) versus those infected with B. burgdorferi alone (6%). Moreover, spirochete DNA was also found for a longer period of time in coinfected individuals versus singly infected ones, mean = 91 days versus 12 days, respectively. Wang et al. only used the presence of antibodies to either B. burgdorferi or B. microti combined with an acute clinical diagnosis of Lyme disease or babesiosis to confirm coinfection. This approach likely underestimates the number of current or previously coinfected patients, since past coinfection is impossible to confirm with certainty. Furthermore, based upon the serological analyses performed, it is impossible to determine whether actual concurrent coinfection ever existed or whether exposure occurred separately at different time points possibly even years apart. Therefore, much of the data reported by Wang et al. cannot be interpreted regarding the question of increased severity of disease. However, in four patients undoubtedly identified to be acutely coinfected with both B. burgdorferi and B. microti at the time of the study, disease symptoms indeed lasted longer and were more severe, even resulting in the need for IV medication and prolonged hospitalization in most cases [22].
Unfortunately, mouse studies have not produced any more consistent quantitative outcomes of coinfection [54, 55]. While arthritis in mice is scaled based upon severity by quantifying leukocyte infiltration, there are variations in the scale which are in the range 0–3 versus 0–4. Such variation not only makes objective comparison of results difficult, but also makes replication and verification of results challenging. In addition to different scales of severity being used, the temporal assessment is not consistent across studies. Moro et al. evaluated arthritis severity at 15 and 30 days, while Coleman et al. used a 21-day time point exclusively. Additionally, Moro et al. also quantified IL-4, IL-10, IL-13, and IFN-γ cytokine levels throughout the infection process, while Coleman et al. only measured variance in splenic weight, thus making any comparisons with their results more challenging. Clearly, additional investigations using a wide variety of mouse strains are necessary to determine the degree to which coinfection exaggerates disease pathology before a consensus can be reached.
7. Conclusions
Overall, it is apparent from this review that there is still much confusion in the literature regarding the pathogenesis and immunological response to coinfection with B. burgdorferi and B. microti, mostly resulting from experimental design disparities and subject variability. There is undoubtedly great value in the use of naturally infected human subjects in epidemiological studies. While mouse models offer greater control over potentially confounding variables, the mouse immune response, especially in the case of complex coinfection, is likely to be quite different than that of humans making comparisons difficult. However, the conflicting results produced from human epidemiological studies cannot be resolved until outcome measures for arthritis, carditis, neurological manifestations, and immunological determinants are standardized across studies. The human epidemiological studies reviewed in this work were carried out over a decade ago, and significant technological advances have been made since then that can now improve both the identification and recruitment of study participants as well as diagnostic testing and reliability. If future studies standardize assessment parameters, utilize impartial and objective measurements, that is, cytokine quantification, and incorporate larger sample sizes, it is likely that a definitive conclusion can be determined regarding concomitant infection.
Understanding the outcomes of coinfection is increasingly important as Lyme disease and other tick-borne diseases are on the rise. At the time of Krause's seminal study of coinfection with B. burgdorferi and B. microti, babesiosis appeared to be the primary suspect for coinfection. However, in the years since, I. scapularis has been found to transmit not only B. burgdorferi and B. microti, but also Anaplasma phagocytophilum, the bacterium responsible for anaplasmosis, a disease which became nationally reportable in 1999 [58]. It should be noted that, prior to a taxonomic change in 2001 that identified A. phagocytophilum as belonging to the genus Anaplasma, this bacterium was for many years referred to as Ehrlichia equi or Ehrlichia phagocytophilum, and the disease caused by this bacterium was frequently termed human granulocytic ehrlichiosis (HGE) [63]. Cases of anaplasmosis have been steadily increasing since 1994 and occur in the same geographic area as Lyme disease and babesiosis.
Coinfection with B. burgdorferi and A. phagocytophilum has been found to cause increased dispersal of spirochetes in experimentally infected C3H mice, resulting in greater number of spirochetes in ear, heart, and skin tissue [64]. Coinfection results in increased generation of matrix metalloproteinases (MMPs) and increased permeability of microvasculature in the brain increasing the ability of spirochetes to cross the compromised barrier more easily [65]. However, in 2013, Horowitz et al. suggested that coinfection did not increase the severity of disease symptoms [66]. Furthermore, in recent years, concern regarding coinfection with the bacterium B. miyamotoi, the causative agent of tick-borne relapsing fever, has further encouraged investigation into the realm of coinfection [67, 68]. Finally, not only has concern regarding concomitant anaplasmosis, Lyme disease, tick-borne relapsing fever, and babesiosis increased in the last decade, but so has the fear of tick-borne viruses such as the Powassan virus or POWV. While the Powassan virus has affected only a small number of individuals in the eastern United States, the effects of this virus are devastating, with over 10% of infected individuals dying due to the characteristic encephalitis and meningitis caused by this pathogen [69].
Overall, the rapid expansion of tick-borne disease in the United States, and the potential for human coinfection with multiple parasites, necessitates that more research be conducted to clarify how coinfection affects disease transmission and progression in order to aid in the accurate diagnosis and treatment of these illnesses.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Oliver J. H., Jr., Owsley M. R., Hutcheson H. J., et al. Conspecificity of the ticks Ixodes scapularis and I. dammini (Acari: Ixodidae) Journal of Medical Entomology. 1993;30(1):54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Ticks—Geographic Distribution of Ticks That Bite Humans. 2015. http://www.cdc.gov/ticks/geographic_distribution.html. [Google Scholar]
- 3.Center for Disease Control and Prevention. Lyme Disease, 2015, http://www.cdc.gov/lyme/index.html.
- 4.Adrion E. R., Aucott J., Lemke K. W., Weiner J. P. Health care costs, utilization and patterns of care following Lyme disease. PLoS ONE. 2015;10(2) doi: 10.1371/journal.pone.0116767.0116767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogitsh J., Carter E., Oeltmann T. Human Parasitology. 4th. Oxford, UK: Academic Press; 2005. [Google Scholar]
- 6.Center for Disease Control and Prevention. Parasites-Babesiosis, 2015, http://www.cdc.gov/parasites/babesiosis/data-statistics/index.html#Babesiosis.
- 7.Center for Disease Control and Prevention. Map—Reported Cases of Lyme Disease. 2015, http://www.cdc.gov/lyme/stats/maps/map2013.html.
- 8.Mitchell P. D., Reed K. D., Hofkes J. M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. Journal of Clinical Microbiology. 1996;34(3):724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adelson M. E., Rao R.-V. S., Tilton R. C., et al. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in Northern New Jersey. Journal of Clinical Microbiology. 2004;42(6):2799–2801. doi: 10.1128/jcm.42.6.2799-2801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson K. I., Norris D. E. Co-circulating microorganisms in questing Ixodes scapularis nymphs in Maryland. Journal of Vector Ecology. 2007;32(2):243–251. doi: 10.3376/1081-1710(2007)32[243:cmiqis]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson J. F., Johnson R. C., Magnarelli L. A., Hyde F. W., Myers J. E. Peromyscus leucopus and Microtus pennsylvanicus simultaneously infected with Borrelia burgdorferi and Babesia microti . Journal of Clinical Microbiology. 1986;23(1):135–137. doi: 10.1128/jcm.23.1.135-137.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varde S., Beckley J., Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerging Infectious Diseases. 1998;4(1):97–99. doi: 10.3201/eid0401.980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piesman J., Hicks T. C., Sinsky R. J., Obiri G. Simultaneous transmission of Borrelia burgdorferi and Babesia microti by individual nymphal Ixodes dammini ticks. Journal of Clinical Microbiology. 1987;25(10):2012–2013. doi: 10.1128/jcm.25.10.2012-2013.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnarelli L. A., Stafford K. C., III, Ijdo J. W., Fikrig E. Antibodies to whole-cell or recombinant antigens of Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti in white-footed mice. Journal of Wildlife Diseases. 2006;42(4):732–738. doi: 10.7589/0090-3558-42.4.732. [DOI] [PubMed] [Google Scholar]
- 15.Magnarelli L. A., Williams S. C., Norris S. J., Fikrig E. Serum antibodies to Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti in recaptured white-footed mice. Journal of Wildlife Diseases. 2013;49(2):294–302. doi: 10.7589/2012-06-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnarelli L. A., Anderson J. F., Stafford K. C., III, Dumler J. S. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in white-footed mice. Journal of Wildlife Diseases. 1997;33(3):466–473. doi: 10.7589/0090-3558-33.3.466. [DOI] [PubMed] [Google Scholar]
- 17.Dos Santos C. C., Kain K. C. Two tick-borne diseases in one: a case report of concurrent babesiosis and Lyme disease in Ontario. Canadian Medical Association Journal. 1999;160(13):1851–1853. [PMC free article] [PubMed] [Google Scholar]
- 18.Krause P. J., Telford S. R., Spielman A., et al. Concurrent Lyme disease and babesiosis: evidence for increased severity and duration of illness. Journal of the American Medical Association. 1996;275(21):1657–1660. doi: 10.1001/jama.275.21.1657. [DOI] [PubMed] [Google Scholar]
- 19.Benach J. L., Coleman J. L., Habicht G. S., MacDonald A., Grunwaldt E., Giron J. A. Serological evidence for simultaneous occurrences of Lyme disease and babesiosis. Journal of Infectious Diseases. 1985;152(3):473–477. doi: 10.1093/infdis/152.3.473. [DOI] [PubMed] [Google Scholar]
- 20.Thompson C., Spielman A., Krause P. J. Coinfecting deer-associated zoonoses: lyme disease, babesiosis, and ehrlichiosis. Clinical Infectious Diseases. 2001;33(5):676–685. doi: 10.1086/322681. [DOI] [PubMed] [Google Scholar]
- 21.Marcus L. C., Steere A. C., Duray P. H., Anderson A. E., Mahoney E. B. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis. Demonstration of spirochetes in the myocardium. Annals of Internal Medicine. 1985;103(3):374–376. doi: 10.7326/0003-4819-103-3-374. [DOI] [PubMed] [Google Scholar]
- 22.Wang T. J., Liang M. H., Sangha O., et al. Coexposure to Borrelia burgdorferi and Babesia microti does not worsen the long-term outcome of Lyme disease. Clinical Infectious Diseases. 2000;31(5):1149–1154. doi: 10.1086/317465. [DOI] [PubMed] [Google Scholar]
- 23.Schröder N. W. J., Eckert J., Stübs G., Schumann R. R. Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology. 2008;213(3-4):329–340. doi: 10.1016/j.imbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Hirschfeld M., Kirschning G. J., Schwandner R., et al. Cutting edge: Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. Journal of Immunology. 1999;163(5):2382–2386. [PubMed] [Google Scholar]
- 25.Guerau-de-Arellano M., Huber B. T. Chemokines and Toll-like receptors in Lyme disease pathogenesis. Trends in Molecular Medicine. 2005;11(3):114–120. doi: 10.1016/j.molmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Skogman B. H., Hellberg S., Ekerfelt C., et al. Adaptive and innate immune responsiveness to Borrelia burgdorferi sensu lato in exposed asymptomatic children and children with previous clinical Lyme borreliosis. Clinical and Developmental Immunology. 2012;2012:10. doi: 10.1155/2012/294587.294587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oksi J., Savolainen J., Pène J., Bòusquet J., Laippala P., Viljanen M. K. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infection and Immunity. 1996;64(9):3620–3623. doi: 10.1128/iai.64.9.3620-3623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekerfelt C., Ernerudh J., Bunikis J., et al. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis: secretion of IFN-gamma predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. Journal of Neuroimmunology. 1997;79(2):155–162. doi: 10.1016/s0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 29.Widhe M., Jarefors S., Ekerfelt C., et al. Borrelia-specific interferon-γ and interleukin-4 secretion in cerebrospinal fluid and blood during lyme borreliosis in humans: association with clinical outcome. Journal of Infectious Diseases. 2004;189(10):1881–1891. doi: 10.1086/382893. [DOI] [PubMed] [Google Scholar]
- 30.Widhe M., Skogman B. H., Jarefors S., et al. Up-regulation of Borrelia-specific IL-4- and IFN-γ-secreting cells in cerebrospinal fluid from children with Lyme neuroborreliosis. International Immunology. 2005;17(10):1283–1291. doi: 10.1093/intimm/dxh304. [DOI] [PubMed] [Google Scholar]
- 31.Matyniak J. E., Reiner S. L. T helper phenotype and genetic susceptibility in experimental Lyme disease. Journal of Experimental Medicine. 1995;181(3):1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbas A. K., Lichtman A. H., Pillai S. Cellular and Molecular Immunology. 8th. Philadelphia, Pa, USA: Elsevier; 2015. [Google Scholar]
- 33.Kraiczy P., Hellwage J., Skerka C., et al. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. The Journal of Biological Chemistry. 2004;279(4):2421–2429. doi: 10.1074/jbc.m308343200. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann K., Corvey C., Skerka C., et al. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Molecular Microbiology. 2006;61(5):1220–1236. doi: 10.1111/j.1365-2958.2006.05318.x. [DOI] [PubMed] [Google Scholar]
- 35.Kraiczy P., Skerka C., Kirschfink M., Brade V., Zipfel P. F. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. European Journal of Immunology. 2001;31(6):1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Kraiczy P., Stevenson B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: structure, function and regulation of gene expression. Ticks and Tick Borne Diseases. 2013;4(1-2):26–34. doi: 10.1016/j.ttbdis.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellwage J., Meri T., Heikkilä T., et al. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi . The Journal of Biological Chemistry. 2001;276(11):8427–8435. doi: 10.1074/jbc.m007994200. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharjee A., Oeemig J. S., Kolodziejczyk R., et al. Structural basis for complement evasion by Lyme disease pathogen Borrelia burgdorferi . The Journal of Biological Chemistry. 2013;288(26):18685–18695. doi: 10.1074/jbc.m113.459040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kern A., Collin E., Barthel C., Michel C., Jaulhac B., Boulanger N. Tick saliva represses innate immunity and cutaneous inflammation in a murine model of Lyme disease. Vector-Borne and Zoonotic Diseases. 2011;11(10):1343–1350. doi: 10.1089/vbz.2010.0197. [DOI] [PubMed] [Google Scholar]
- 40.McKisic M. D., Barthold S. W. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infection and Immunity. 2000;68(9):5190–5197. doi: 10.1128/iai.68.9.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lobo C. A., Cursino-Santos J. R., Alhassan A., Rodrigues M. Babesia: an emerging infectious threat in transfusion medicine. PLoS Pathogens. 2013;9(7) doi: 10.1371/journal.ppat.1003387.e1003387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar S., Gubernot D. M., Nakhasi H. L., Mied P. A., Asher D. M., Epstein J. S. Transfusion-transmitted babesiosis in the United States: summary of a workshop. Transfusion. 2009;49(12):2759–2771. doi: 10.1111/j.1537-2995.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 43.Leiby D. A. Transfusion-associated babesiosis: shouldn't we be ticked off? Annals of Internal Medicine. 2011;155(8):556–557. doi: 10.7326/0003-4819-155-8-201110180-00363. [DOI] [PubMed] [Google Scholar]
- 44.Homer M. J., Aguilar-Delfin I., Telford S. R., III, Krause P. J., Persing D. H. Babesiosis. Clinical Microbiology Reviews. 2000;13(3):451–469. doi: 10.1128/CMR.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aguilar-Delfin I., Wettstein P. J., Persing D. H. Resistance to acute babesiosis is associated with interleukin-12- and gamma interferon-mediated responses and requires macrophages and natural killer cells. Infection and Immunity. 2003;71(4):2002–2008. doi: 10.1128/iai.71.4.2002-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Igarashi I., Suzuki R., Waki S., et al. Roles of CD4+ T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infection and Immunity. 1999;67(8):4143–4148. doi: 10.1128/iai.67.8.4143-4148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi M., Omata Y., Oikawa H., et al. Protective immune response of Isospora felis-infected mice against Babesia microti infection. The Journal of Veterinary Medical Science. 1993;55(4):587–590. doi: 10.1292/jvms.55.587. [DOI] [PubMed] [Google Scholar]
- 48.Li Y., Terkawi M. A., Nishikawa Y., et al. Macrophages are critical for cross-protective immunity conferred by Babesia microti against Babesia rodhaini infection in mice. Infection and Immunity. 2012;80(1):311–320. doi: 10.1128/iai.05900-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen D., Copeman D. B., Burnell J., Hutchinson G. W. Helper T cell and antibody responses to infection of CBA mice with Babesia microti . Parasite Immunology. 2000;22(2):81–88. doi: 10.1046/j.1365-3024.2000.00279.x. [DOI] [PubMed] [Google Scholar]
- 50.Perez M., Carson C. A., Ristic M. Cell-mediated immune response in hamsters infected with Babesia microti . Veterinary Parasitology. 1977;3(2):161–167. doi: 10.1016/0304-4017(77)90032-2. [DOI] [Google Scholar]
- 51.Vinasco J., Braga W., Zegarra-Moro O., Moro M. H. Cellular immune responses in a Murine model of Borrelia burgdorferi and Babesia microti coinfection. The Journal of Immunology. 2007;178, article S47 [Google Scholar]
- 52.Martinez-Balzano C., Hess M., Malhotra A., Lenox R. Severe babesiosis and Borrelia burgdorferi coinfection. QJM. 2015;108(2):141–143. doi: 10.1093/qjmed/hcs100. [DOI] [PubMed] [Google Scholar]
- 53.Abrams Y. M. Complications of coinfection with Babesia and Lyme disease after splenectomy. Journal of the American Board of Family Medicine. 2008;21(1):75–77. doi: 10.3122/jabfm.2008.01.060182. [DOI] [PubMed] [Google Scholar]
- 54.Moro M. H., Zegarra-Moro O. L., Bjornsson J., et al. Increased arthritis severity in mice coinfected with Borrelia burgdorferi and Babesia microti . Journal of Infectious Diseases. 2002;186(3):428–431. doi: 10.1086/341452. [DOI] [PubMed] [Google Scholar]
- 55.Coleman J. L., LeVine D., Thill C., Kuhlow C., Benach J. L. Babesia microti and Borrelia burgdorferi follow independent courses of infection in mice. Journal of Infectious Diseases. 2005;192(9):1634–1641. doi: 10.1086/496891. [DOI] [PubMed] [Google Scholar]
- 56.Qureshi S. T., Larivière L., Leveque G., et al. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (Tlr4) Journal of Experimental Medicine. 1999;189(4):615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leitner W., Wali T., Denis A. C.-S. Is arthropod saliva the Achilles' heel of vector-borne diseases? Frontiers in Immunology. 2013;4, article 255 doi: 10.3389/fimmu.2013.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G., Severo M. S., Sohail M., et al. Ixodes scapularis saliva mitigates inflammatory cytokine secretion during Anaplasma phagocytophilum stimulation of immune cells. Parasites & Vectors. 2012;5, article 229 doi: 10.1186/1756-3305-5-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dai J., Wang P., Adusumilli S., et al. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host and Microbe. 2009;6(5):482–492. doi: 10.1016/j.chom.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramamoorthi N., Narasimhan S., Pal U., et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436(7050):573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuijt T. J., Coumou J., Narasimhan S., et al. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the Lyme disease agent. Cell Host and Microbe. 2011;10(2):136–146. doi: 10.1016/j.chom.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stricker R. B., Burrascano J. J., Harris N. S., et al. Coinfection with Borrelia burgdorferi and Babesia microti: bad or worse? Journal of Infectious Diseases. 2006;193(6):901–902. doi: 10.1086/500473. [DOI] [PubMed] [Google Scholar]
- 63.Center for Disease Control and Prevention. Statistics—Anaplasmosis. Center for Disease Control and Prevention; 2013. http://www.cdc.gov/anaplasmosis/stats/#casesbyyear. [Google Scholar]
- 64.Holden K., Hodzic E., Feng S., Freet K. J., Lefebvre R. B., Barthold S. W. Coinfection with Anaplasma phagocytophilum alters Borrelia burgdorferi population distribution in C3H/HeN mice. Infection and Immunity. 2005;73(6):3440–3444. doi: 10.1128/iai.73.6.3440-3444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grab D. J., Nyarko E., Barat N. C., Nikolskaia O. V., Dumler J. S. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clinical and Vaccine Immunology. 2007;14(11):1420–1424. doi: 10.1128/cvi.00308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horowitz H. W., Aguero-Rosenfeld M. E., Holmgren D., et al. Lyme disease and human granulocytic anaplasmosis coinfection: impact of case definition on coinfection rates and illness severity. Clinical Infectious Diseases. 2013;56(1):93–99. doi: 10.1093/cid/cis852. [DOI] [PubMed] [Google Scholar]
- 67.Gugliotta J. L., Goethert H. K., Berardi V. P., Telford S. R., III Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. The New England Journal of Medicine. 2013;368(3):240–245. doi: 10.1056/nejmoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krause P. J., Narasimhan S., Wormser G. P., et al. Human Borrelia miyamotoi infection in the United States. The New England Journal of Medicine. 2013;368(3):291–293. doi: 10.1056/nejmc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powassan Virus (POW) Basics. Minnesota Department of Health, http://www.health.state.mn.us/divs/idepc/diseases/powassan/basics.html.