Abstract
Aims
ILUMIEN I is the largest prospective, non-randomized, observational study of percutaneous coronary intervention (PCI) procedural practice in patients undergoing intra-procedural pre- and post-PCI fractional flow reserve (FFR) and optical coherence tomography (OCT). We report on the impact of OCT on physician decision-making and the association with post-PCI FFR values and early clinical events.
Methods and results
Optical coherence tomography and documentary FFR were performed pre- and post-PCI in 418 patients (with 467 stenoses) with stable or unstable angina or NSTEMI. Based on pre-PCI OCT, the procedure was altered in 55% of patients (57% of all stenoses) by selecting different stent lengths (shorter in 25%, longer in 43%). After clinically satisfactory stent implantation using angiographic guidance, post-PCI FFR and OCT were repeated. Optical coherence tomography abnormalities deemed unsatisfactory by the implanting physician were identified: 14.5% malapposition, 7.6% under-expansion, 2.7% edge dissection and prompted further stent optimization based on OCT in 25% of patients (27% of all stenoses) using additional in-stent post-dilatation (81%, 101/124) or placement of 20 new stents (12%). Optimization subgroups were identified post hoc: stent placement without reaction to OCT findings (n = 137), change in PCI planning by pre-PCI OCT (n = 165), post-PCI optimization based on post-PCI OCT (n = 41), change in PCI planning, and post-PCI optimization based on OCT (n = 65). Post-PCI FFR values were significantly different (P = 0.003) between optimization groups (lower in cases with pre- and post-PCI reaction to OCT) but no longer different after post-PCI stent optimization. MACE events at 30 days were low: death 0.25%, MI 7.7%, repeat PCI 1.7%, and stent thrombosis 0.25%.
Conclusion
Physician decision-making was affected by OCT imaging prior to PCI in 57% and post-PCI in 27% of all cases.
ClinicalTrials.gov Identifier
NCT01663896, Observational Study of Optical Coherence Tomography (OCT) in Patients Undergoing Fractional Flow Reserve (FFR) and Percutaneous Coronary Intervention (ILUMIEN I).
Keywords: Optical coherence tomography, Percutaneous coronary intervention, Stent, Fractional flow reserve, Periprocedural myocardial infarction
See page 3356 for the editorial comment on this article (doi:10.1093/eurheartj/ehv433)
Introduction
Since its introduction in 1977, safety and efficacy outcomes after percutaneous coronary interventions (PCI) have improved significantly through advances in device technology, adjunctive periprocedural pharmacology, and operator experience. Although the majority of PCIs are performed under angiographic guidance, use of fractional flow reserve (FFR) for confirmation of PCI appropriateness1,2 and intracoronary imaging for optimization of procedural technique3,4 may lead towards further improvement of PCI outcomes. Several components of procedural optimization cannot be accurately assessed by angiography, e.g. sizing of stent length and diameter, the presence of residual thrombus, wall coverage, and stent strut apposition. These features can be precisely evaluated with the use of intravascular Fourier domain optical coherence tomography (OCT). Optical coherence tomography acquires longitudinal sequences of cross-sectional images (100 frames/s) in a blood-free environment, resulting in sharp border definition between lumen and vessel wall. Together with high axial resolution (10–15 microns), volumetric segmentation of vessel and wall contours facilitates more rational selection of stent size and length, as well as ascertainment of full stent deployment and expansion.
ILUMIEN I is to date the largest prospective, non-randomized, observational study of PCI practice in patients undergoing pre- and post-PCI FFR and OCT. The objective was to define guidance parameters for stent optimization. This manuscript reports on the impact of OCT on physician decision-making, procedural findings, and early clinical events.
Methods
Study protocol
Inclusion and exclusion criteria
Patients providing written informed consent for inclusion in the study protocol presented with stable angina, unstable angina, or NSTEMI, and underwent elective or ‘ad hoc’ PCI of de novo, single or multivessel coronary artery stenosis. Up to two major vessels and three lesions could be treated, with no more than two lesions per major coronary artery. Subjects with acute STEMI, emergent PCI, cardiogenic shock, restenosis or stent thrombosis, target left main stenosis, aorto-ostial or diffuse disease, extreme angulation, or calcification were excluded, as well as planned use of bare metal stent (see detailed list of inclusion and exclusion criteria in Supplementary material online, Appendix 1). The study was conducted in 35 sites with balanced patient inclusion between continents: USA 36%, Europe 31%, Japan 23%, Asia 6%, Canada 41%, and Australia 3% (list of investigators and clinical sites in Supplementary material online, Appendix 2).
Study flowchart
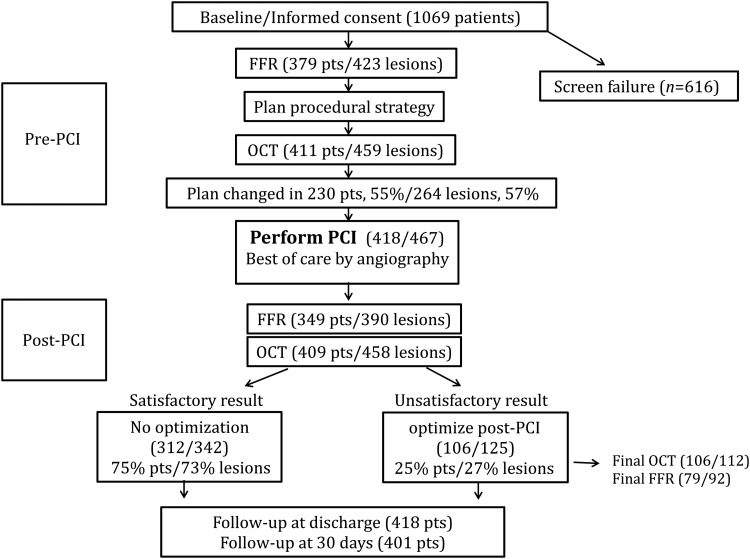
Acquisition of pre- and post-PCI FFR and OCT was required for collection of paired functional and anatomical data, according to standardized technique and reporting. Further to indications for PCI based on clinical grounds, intervention was recommended in target vessels with abnormal FFR (≤0.80). After angiography, investigators were requested to describe the planned PCI strategy based on available angiographic data. Pre-PCI OCT was intended to be documentary, but any changes from the initial plan were carefully recorded. Next, PCI and stent placement were performed using angiographic guidance, per individual investigator standard of care. When a ‘best of care’ angiographic result was obtained, post-PCI standardized documentary FFR was recorded and OCT data were acquired. When operators felt that the OCT result was clinically unsatisfactory, further optimization was performed followed by repeat imaging (Figure 1). Recommendations to intervene or not to intervene based on post-PCI angiographic and OCT findings were not prescriptive and limited to severe abnormalities: flow-limiting edge dissection, significant malapposition, thrombus/tissue protrusion with flow reduction, stent under-expansion ≥30% compared with reference distal lumen. All investigator treatment decisions were recorded on procedural worksheets at the time of intervention.
Figure 1.
Consort diagram: study flowchart, procedural steps, and patient disposal. FFR, fractional flow reserve; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Analysis of angiography, fractional flow reserve, and optical coherence tomography
Devices used in the present study are described in Supplementary material online, Appendix 3. Co-registered images from coronary angiography, FFR, and OCT were analysed by the core laboratory for pre-specified variables including lesion morphology and extent of disease, vessel injury (dissection, thrombus, tissue prolapse) and stent apposition, volume, area, diameter, expansion, positioning, length, and geographical miss4–6 To report on physician decision-making, on-site classified occurrence of malapposition, under-expansion, thrombus/tissue protrusion, and edge dissection are based on the recommended definitions below.
Edge dissection >180° in more than five frames on OCT.
Significant malapposition defined as >200 micron in axial diameter and present in at least five consecutive frames on OCT.
The presence of thrombus and/or tissue protrusion on OCT causing flow reduction (i.e. TIMI < 3 and/or obstruction visible by angiography).
Under-expansion ≥30% by OCT compared with reference distal lumen area and when quantitative coronary angiogram (QCA) shows >20% in-stent residual diameter stenosis.
Event definition and adjudication
MACE events by ARC definitions7,8 included both device-oriented composite endpoint [cardiac death, myocardial infarction (MI) not clearly attributable to a non-target vessel, and target lesion revascularization] and patient-oriented composite endpoint (all-cause mortality, any MI, and any repeat revascularization). Stent thrombosis was classified as definite, probable or possible, and as early or late.7 Cardiac enzymes were collected within 48 h prior to PCI and between 6 and 48 h post-procedure or in case of chest pain or ECG changes. Classification and criteria for biomarker diagnosis of periprocedural MI required troponin or CK-MB >3 times URL, with baseline value <URL. Myocardial infarction was reported according to two definitions: per protocol (ARC definition) as well as type 4a from the Universal MI definition consensus document.9 In the absence of clinical symptoms, the elevation of cardiac enzymes was required to be at least 3 times the UNL and 5 times the baseline value to conservatively classify patients with elevated baseline values. All relevant data were collected at screening, baseline, pre-PCI, during and post-PCI, at hospital discharge, at 1-, 6-, and 12-month follow-up.
The Clinical Event Committee (CEC) adjudicated on an ongoing basis the adverse events reported during the study. The CEC consisted of interventional cardiologists who were blinded to individual subject and site identities (see Supplementary material online, Appendix 4). They classified reported adverse events according to adverse event type/code, severity, relatedness, and unanticipated category. Listings were reviewed on a quarterly basis to check whether events met adjudication criteria. Furthermore, all MACE events (MI, death, revascularization, and stent thrombosis) were adjudicated.
Study objectives, statistical analysis, and reporting
No formal sample size calculation was applied since this was an observational study. The study objective was to define guidance parameters for stent optimization. This manuscript reports on the impact of OCT on physician decision-making and the association with post-PCI FFR values and early clinical events. Data analysis was performed on a per subject basis, unless specified otherwise (lesion or stent-related variables are shown on a per stenosis basis). Demographic variables, procedure characteristics, adverse event rates, and additional characteristics were analysed. Continuous and categorical variables were assessed using an ANOVA F-test and Fishers Exact MCMC P-values, respectively.
Informed consent process
Prior to enrollment, patients were fully informed of the details of the study protocol that was approved by local Medical Ethics Committee and relevant authorities. Written informed consent was obtained from all participating patients.
Results
Demographics and baseline characteristics
Of 1069 patients screened, 418 patients (467 stenoses) qualified for inclusion (Consort diagram on Figure 1). Mean age was 64.6 ± 10.2, 24.5% female, with typical risk factors (family history 34%, BMI 29 ± 15, diabetes 37%, treated arterial hypertension 72%, peripheral vascular disease 10%, present or past smoking 47%, prior or current hyperlipidaemia 76%).
Clinical presentation was stable angina (63%), unstable angina (22%), NSTEMI (11%), or silent ischaemia (4%). Previous MI was present in 24% and prior PCI in 20%. Target vessel was left anterior descending coronary artery in 59%, right coronary artery in 22%, and circumflex in 19%, with mostly PCI of a single lesion (90%) or two lesions in the same vessel (10%). Stenosis severity at baseline was 73 ± 15% diameter stenosis by angiography and 0.72 ± 0.14 by FFR.
Detailed use of medication at baseline and at 30 days is available in Supplementary material online, Appendix 5. At 30 days, aspirin was used in 96% of patients, clopidogrel in 82%, and other antiplatelet agents in 20%. Anticoagulation (mostly for atrial fibrillation) was prescribed in 8% of patients.
Impact of optical coherence tomography on physician decision-making
Following pre-PCI angiography, FFR, and OCT, PCI was performed in 418 patients/467 lesions. In this study population, pre-PCI FFR was obtained in 379 patients/423 lesions, and pre-PCI OCT was obtained in 411 patients/459 lesions (Figure 2). Pre-PCI FFR was not performed in 9.4% and pre-PCI OCT was not performed in 1.7% of the treated lesions. Following pre-PCI OCT, treatment planning was modified in 55% of patients (57% of all stenosis), in 7–80% of cases per site.
Figure 2.
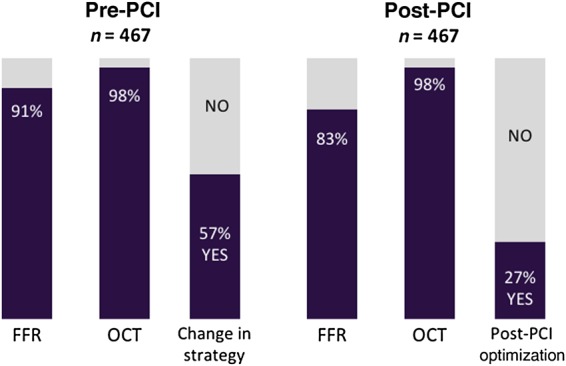
Impact of optical coherence tomography (OCT) on percutaneous coronary intervention (PCI) planning and procedural technique. Per-patient rates of fractional flow reserve (FFR) and optical coherence tomography pre- and post-percutaneous coronary intervention.
Stent implantation was performed based on angiography by best practice. Additional unplanned stent implantation and post-dilatations were performed in 40 cases until the PCI result was seen as satisfactory by angiographic standards, as determined by the investigator. Post-PCI FFR was documented in 83% of patients (84% of all lesions) and post-PCI OCT in 98% of all patients/stenoses (Figure 2). Edge dissection, malapposition, and under-expansion were commonly observed on post-PCI OCT (Table 1). Post-PCI result was deemed as unsatisfactory by OCT imaging which led to further optimization in 106 patients (25%) or 125 stenoses (27% of all lesions), in 8–55% of cases per site.
Table 1.
Rates and types of abnormal findings by post-PCI OCT imaging
| OCT variables | All abnormalities by core Laboratory, n/N | Rate (%) | Abnormalities deemed unsatisfactory by operator, n/N | Rate (%) |
|---|---|---|---|---|
| Edge dissection | 107/388 | 27.6 | 11/408 | 2.7 |
| Malapposition | 126/392 | 32.1 | 59/408 | 14.5 |
| Under-expansion | 159/385 | 41.3 | 31/408 | 7.6 |
| Edge dissection and malapposition | 34/388 | 8.8 | 2/408 | 0.5 |
| Edge dissection and under-expansion | 35/385 | 9.1 | 2/408 | 0.5 |
| Malapposition and tissue protrusion | 44/392 | 11.2 | 2/408 | 0.5 |
| Edge dissection, malapposition, and under-expansion | 14/385 | 3.6 | 0/408 | 0 |
| Thrombus or tissue protrusiona | 100/392 | 25.5 | 4/408 | 1.0 |
FFR, fractional flow reserve; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
aTissue protrusion was qualitatively analysed, and it was defined as intimal tissue protruding and disturbing lumen contour.
Post hoc subgroup analysis per optimization strategy (Table 2) accounts for changes in treatment planning and performance based on OCT findings: pre-PCI only, post-PCI only, pre-PCI and post-PCI, or no change based on OCT (neither pre- or post-PCI). Few baseline variables were significantly different between optimization subgroups (Table 2). Pre- and post-PCI treatment changes based on OCT occurred less often when the lesions were single, in which post-PCI % diameter stenosis was low (13.6 ± 16.0) and post-PCI FFR was high (0.89 ± 0.07). Otherwise, there were no significant differences in clinical background, demographic variables, or PCI indications between subgroups. There was no difference in use of medication at baseline and at 30 days between optimization subgroups (see Supplementary material online, Table S1, Appendix 5).
Table 2.
Demographics and PCI indications (per optimization subgroup)
| Variables | Statistic Mean ± SD (N) (Min, Max) or n/N (%) |
P-value* | |||
|---|---|---|---|---|---|
| PCI optimization without change based on OCT (n = 137) | PCI optimization based on Pre-PCI OCT only (n = 165) | PCI optimization based on post-PCI OCT only (n = 41) | PCI optimization based on pre-PCI and post-PCI OCT (n = 65) | ||
| Age (years) | 64 ± 9.9 (137) (36, 86) | 65.4 ± 10.2 (165) (37, 88) | 65.8 ± 10.7 (41) (43, 87) | 63 ± 10.4 (65) (39, 88) | 0.2825 |
| Gender | |||||
| Female | 27/137 (19.7%) | 45/165 (27.3%) | 14/41 (34.1%) | 14/65 (21.5%) | 0.1900 |
| Male | 110/137 (80.3%) | 120/165 (72.7%) | 27/41 (65.9%) | 51/65 (78.5%) | |
| BMI (kg/m2) | 28.6 ± 6.2 (135) (17.3, 52.4) | 28.5 ± 5.6 (164) (18.6, 64.7) | 33.5 ± 35.2 (41) (17.6, 246.9) | 30 ± 20.9 (65) (18, 191) | 0.2302 |
| CVA | 10/137 (7.3%) | 12/164 (7.3%) | 3/41 (7.3%) | 4/65 (6.2%) | 1.0000 |
| TIA | 3/137 (2.2%) | 0/165 (0%) | 0/41 (0%) | 1/65 (1.5%) | 0.1820 |
| Family history of CAD | 41/137 (29.9%) | 60/164 (36.6%) | 17/41 (41.5%) | 20/64 (31.3%) | 0.4385 |
| Renal insufficiency/failure | 6/137 (4.4%) | 9/164 (5.5%) | 4/41 (9.8%) | 3/65 (4.6%) | 0.5751 |
| Diabetes mellitus | 56/137 (40.9%) | 52/164 (31.7%) | 12/41 (29.3%) | 32/65 (49.2%) | 0.0462 |
| Peripheral vascular occlusive disease | 12/137 (8.8%) | 20/164 (12.2%) | 5/41 (12.2%) | 5/65 (7.7%) | 0.6521 |
| Tobacco use—smoking | 56/137 (40.9%) | 87/164 (53%) | 17/41 (41.5%) | 33/65 (50.8%) | 0.1590 |
| Hyperlipidaemia | 101/137 (73.7%) | 130/164 (79.3%) | 33/41 (80.5%) | 46/65 (70.8%) | 0.4299 |
| Taking hypertension medications at baseline | 97/137 (70.8%) | 123/165 (74.5%) | 31/41 (75.6%) | 45/65 (69.2%) | 0.7845 |
| Previous PCI in target vessel | 26/134 (19.4%) | 41/163 (25.2%) | 7/39 (17.9%) | 9/65 (13.8%) | 0.2607 |
| Previous MI | 29/134 (21.6%) | 37/163 (22.7%) | 9/39 (23.1%) | 23/65 (35.4%) | 0.1884 |
| Pre-procedure indication | |||||
| NSTEMI | 19/137 (13.9%) | 17/165 (10.3%) | 2/41 (4.9%) | 6/65 (9.2%) | 0.7273 |
| Other | 4/137 (2.9%) | 10/165 (6.1%) | 2/41 (4.9%) | 2/65 (3.1%) | |
| Stable angina | 85/137 (62%) | 97/165 (58.8%) | 29/41 (70.7%) | 44/65 (67.7%) | |
| Unstable angina | 29/137 (21.2%) | 41/165 (24.8%) | 8/41 (19.5%) | 13/65 (20%) | |
| Vessel identification | |||||
| Multi vessel | 2/138 (1.4%) | 2/168 (1.2%) | 2/42 (4.8%) | 2/69 (2.9%) | 0.0031 |
| Single vessel, multi lesion | 7/138 (5.1%) | 17/168 (10.1%) | 2/42 (4.8%) | 15/69 (21.7%) | |
| Single vessel, single lesion | 129/138 (93.5%) | 149/168 (88.7%) | 38/42 (90.5%) | 52/69 (75.4%) | |
| Target vessel (per FFR) | |||||
| Circumflex | 30/140 (21.4%) | 34/170 (20%) | 7/44 (15.9%) | 10/70 (14.3%) | 0.1624 |
| LAD | 76/140 (54.3%) | 108/170 (63.5%) | 22/44 (50%) | 45/70 (64.3%) | |
| Right | 34/140 (24.3%) | 28/170 (16.5%) | 15/44 (34.1%) | 15/70 (21.4%) | |
| Diameter stenosis (%) | |||||
| By angiography | 73.9 ± 14.7 (134) (32, 99) | 72.6 ± 15.1 (145) (0.8, 99) | 75.1 ± 12.1 (33) (44, 99) | 70.2 ± 13.4 (51) (50, 99) | 0.3683 |
*Continuous and categorical P-values were calculated using an ANOVA F-test and Fishers Exact MCMC statistics, respectively.
FFR, fractional flow reserve; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Change in percutaneous coronary intervention procedure and resource utilization
Change in planned treatment strategy based on pre-PCI OCT led to changes in selection of stent length (shorter in 25%, longer in 43%, and was unchanged in 32%). Selection of stent diameter decreased in 31%, increased in 8%, and was unchanged in 61%. The number of stents was unchanged in 87%, and the number of implanted stents per patient did not change (1.12 ± 0.34 planned vs. 1.21 ± 0.45 implanted, P = 0.49).
Post-PCI OCT findings prompting further procedural optimization were malapposition, under-expansion (both P < 0.001), and edge dissection (P = 0.0034) (see Supplementary material online, Table S2, Appendix 6). These OCT findings led mostly to additional in-stent post-dilatation (81%, 101/124), new stent placement (13%), or both (3%). Further to implantation of 20 additional stents (in 4% of all stenoses treated), the number of stents per patient was significantly higher after optimization based on post-PCI OCT (Table 3).
Table 3.
Stenosis and procedural characteristics (per optimization subgroup)
| PCI optimization without change based on OCT | PCI optimization based on Pre-PCI OCT only | PCI optimization based on post-PCI OCT only | PCI optimization based on pre-PCI and post-PCI OCT | P-value* | ||
|---|---|---|---|---|---|---|
| Stenoses | n | 146 | 185 | 46 | 79 | |
| Planned number of stents prior to OCT | Mean ± SD | 1.1 ± 0.37 | 1.14 ± 0.41 | 1.17 ± 0.44 | 1.22 ± 0.41 | 0.293 |
| Actual number of stents per patient | Mean ± SD | 1.17 ± 0.41 | 1.22 ± 0.47 | 1.32 ± 0.57 | 1.49 ± 0.75 | 0.0004 |
| Pre-PCI % stenosisa | Mean ± SD | 64.2 ± 16.1 | 64.5 ± 17 | 69.1 ± 20 | 59.5 ± 17.5 | 0.159 |
| Post-PCI % stenosisa | Mean ± SD | 13.6 ± 16 | 14.4 ± 12.7 | 22.3 ± 14.7 | 22.3 ± 19.7 | 0.007 |
| Pre-PCI FFR | Mean ± SD | 0.72 ± 0.14 | 0.73 ± 0.14 | 0.72 ± 0.14 | 0.72 ± 0.13 | 0.931 |
| Post-PCI FFR | Mean ± SD | 0.89 ± 0.07 | 0.89 ± 0.07 | 0.89 ± 0.08 | 0.86 ± 0.09 | 0.0035 |
| Final FFR | Mean ± SD | — | — | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.2417 |
| Pre-PCI MLAb | Mean ± SD | 1.9 ± 1.2 | 1.7 ± 0.8 | 1.6 ± 0.9 | 1.8 ± 0.8 | 0.113 |
| Post-PCI MLAb | Mean ± SD | 6.1 ± 2.5 | 5.2 ± 2.1 | 5.3 ± 1.8 | 5.0 ± 2.0 | 0.004 |
| Fluoroscopy duration | min | 21 ± 14.7 | 25.7 ± 35.2 | 23.9 ± 13.1 | 31.9 ± 25.7 | 0.0536 |
| Procedure duration | min | 87.6 ± 37 | 89.7 ± 34.9 | 93.6 ± 26.1 | 106.4 ± 39.5 | 0.0043 |
| Contrast agent used | mL | 275.5 ± 127.6 | 260.7 ± 122 | 251.8 ± 132 | 256.3 ± 124.5 | 0.6012 |
*Continuous and categorical P-values were calculated using an ANOVA F-test and Fishers Exact MCMC statistics, respectively.
FFR, fractional flow reserve; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
apercent diameter stenosis by OCT (%).
bMLA, minimal luminal area by OCT (mm2).
Fluoroscopy time and procedure duration increased when operators decided to react to OCT findings. Total amount of contrast used was not different and no case of contrast-induced nephropathy or other serious adverse events related to OCT imaging were observed.
Procedural outcomes, in-hospital and 30-day follow-up
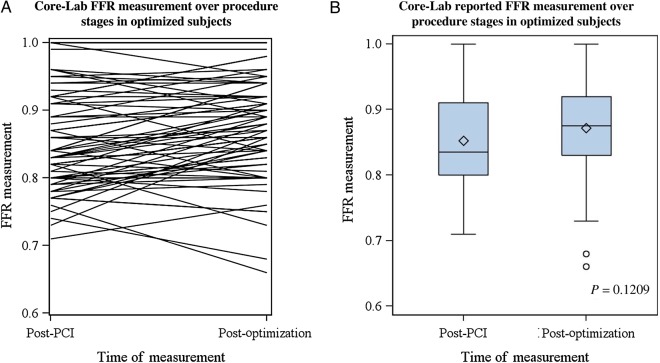
Pre-PCI FFR values were similar between optimization subgroups (Table 3). Functional effect of PCI on FFR measurements was different between optimization subgroups (P = 0.003): 0.89 ± 0.07, 0.89 ± 0.07, 0.89 ± 0.08, and 0.86 ± 0.09, lower in cases with pre-PCI and further post-PCI optimization. Final FFR values were not statistically different between the four optimization subgroups. In the subset of cases with paired final FFR and OCT measurements following optimization, FFR values improved from 0.86 ± 0.07 to 0.90 ± 0.10 (Figure 3) following correction of OCT findings that were deemed unsatisfactory by the operator. Unsatisfactory malapposition decreased from 48 to 9% (P < 0.001), stent under-expansion from 27 to 0% (P < 0.001), and edge dissection from 8 to 0% (P = 0.014).
Figure 3.
Changes in distal fractional flow reserve (FFR) from post-percutaneous coronary intervention (PCI) to final measurement after optical coherence tomography (OCT)-driven percutaneous coronary intervention optimization. Individual (left, panel A) and group (right, panel B) changes are shown in the subset of optimized subjects with paired measurements (n = 70, P = 0.1209).
Low rates of device-oriented and patient-oriented MACE were noted (Table 4) both in hospital and at 30 days. Rates of clinically significant periprocedural MI were found to be different when procedural changes were made based on pre- and post-PCI OCT (P = 0.029). The overall rate of in-hospital MI was 6.9% by ARC and 6.4% by Universal MI definitions. Other event rates were very low, including stent thrombosis (Table 4).
Table 4.
Adverse events in hospital and at 30 days (per optimization subgroup)
| PCI optimization without change based on OCT | PCI optimization based on pre-PCI OCT only | PCI optimization based on post-PCI OCT only | PCI optimization based on pre-PCI and post-PCI OCT | P-value | |
|---|---|---|---|---|---|
| [n events], % patients (n) | [n events], % patients (n) | [n events], % patients (n) | [n events], % patients (n) | ||
| Device-oriented MACE | |||||
| In hospital | [12], 8.8% (137) | [11], 6.7% (165) | [5], 12.2% (41) | [1], 1.5% (65) | 0.118 |
| 30 days | [12], 8.8% (137) | [15], 8% (163) | [5], 12.5% (40) | [1], 1.5% (65) | 0.127 |
| Cardiac death (%) | |||||
| In hospital | 0 | 0 | 0 | 0 | |
| 30 days | 0 | 0 | 0 | 0 | |
| MI—ARC definition | |||||
| In hospital | [12], 8.8% | [11], 6.7% | [5], 12.2% | 0% | 0.023 |
| 30 days | [12], 8.8% | [13], 8% | [5], 12.5% | 0% | 0.024 |
| MI—Third Universal definition | |||||
| In hospital | [11], 8% | [11], 6.7% | [4], 9.8% | 0% | 0.051 |
| 30 days | [11], 8% | [13], 8% | [4], 10% | 0% | 0.047 |
| Target lesion revascularization | |||||
| In hospital | 0% | 0% | 0% | [1], 1.5% | 0.26 |
| 30 days | 0% | [2], 1.2% | 0% | [1], 1.5% | 0.485 |
| Patient-oriented MACE | |||||
| In hospital | [13], 9.5% (137) | [11], 6.7% (165) | [5], 12.2% (41) | [1], 1.5% (65) | 0.091 |
| 30 days | [16], 10.9% (137) | [19], 9.8% (163) | [5], 12.5% (40) | [1], 1.5% (65) | 0.077 |
| All-cause mortality | |||||
| In hospital | [1], 0.7% | 0% | 0% | 0% | 0.596 |
| 30 days | [1], 0.7% | 0% | 0% | 0% | 0.596 |
| MI—ARC definition | |||||
| In hospital | [12], 8.8% | [11], 6.7% | [5], 12.2% | 0% | 0.023 |
| 30 days | [14], 10.2% | [14], 8.6% | [5], 12.5% | 0% | 0.017 |
| MI—Third Universal definition | |||||
| In hospital | [11], 8% | [11], 6.7% | [4], 9.8% | 0% | 0.051 |
| 30 days | [13], 9.5% | [14], 8.6% | [4], 10% | 0% | 0.029 |
| Any revascularization | |||||
| In hospital | 0% | 0% | 0% | [1], 1.5% | 0.26 |
| 30 days | [1], 0.7% | [5], 3.1% | 0% | [1], 1.5% | 0.448 |
| Stent thrombosis | |||||
| Definite | |||||
| In hospital | 0% | 0% | 0% | 0% | |
| 30 days | 0% | [1], 0.6% | 0% | 0% | 1 |
| Probable (%) | |||||
| In hospital | 0 | 0 | 0 | 0 | |
| 30 days | 0 | 0 | 0 | 0 | |
| Possible (%) | |||||
| In hospital | 0 | 0 | 0 | 0 | |
| 30 days | 0 | 0 | 0 | 0 | |
| Early | |||||
| In hospital | 0% | 0% | 0% | 0% | |
| 30 days | 0% | [1], 0.6% | 0% | 0% | 1 |
OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Discussion
ILUMIEN I study aims at defining and evaluating OCT parameters for optimization of PCI procedures and clinical outcomes. The initial procedural findings and 30-day outcomes are important to report based on the high impact of OCT imaging on physician decision-making, as listed below:
– Optical coherence tomography could be applied both pre-PCI and post-PCI with success rates of 98% of patients (91% of all lesions).
– Physician decision-making was influenced by OCT findings either pre-PCI and/or post-PCI in 66% of patients (68% of lesions).
– Physician decision-making was influenced both pre- and post-PCI more often in patients with more complex disease.
– As reported by Prati et al.10 abnormal findings by OCT imaging were common after ‘optimal’ PCI by angiographic standards. Physician decision to react on post-PCI OCT abnormalities identified a subset of coronary lesions with more frequent malapposition, edge dissection, and stent under-expansion.
– Additional in-stent post-dilatations and stent implantations were used to correct unsatisfactory post-PCI results, namely stent under-expansion and malapposition by OCT that were not apparent on angiography.
– MACE events, including stent thrombosis, were very low in all optimization subgroups. Changes in pre- and post-PCI procedure based on OCT imaging were associated with low rates of periprocedural MI.
– Identical final FFR values were obtained through different optimization sequences.
Clinical relevance
Residual risk and opportunities for further outcome improvement of PCI remain, especially in complex patient and lesion subsets currently treated in real-life practice. In patients with multivessel revascularization, MI rates remain high with PCI compared with bypass surgery at all early and later time points.11 In the FAME 2 trial, potential benefit of revascularization over best of medical care was jeopardized by higher event rates in the PCI group than in the medical therapy group (2.2% vs. 0.9%; hazard ratio, 2.49), mostly periprocedural MIs occurring within the first 7 days after PCI.12 In patients undergoing elective PCI, any periprocedural event will jeopardize the potential long-term benefit of revascularization.13 In-hospital findings of ILUMIEN I do raise the intriguing hypothesis that safety of PCI could be further improved by reducing the rates of periprocedural MI. Severe stent under-expansion and malapposition are known to induce turbulences and pressure loss.14 Platelet aggregation and distal emboli may occur, especially with high residual platelet reactivity.15,16 This hypothesis should inform the design of prospective, randomized trials aiming at establishing the clinical superiority of OCT-guided PCI vs. sole angiographic guidance. In addition to anticipated reductions in mortality, death, stent thrombosis, or repeat revascularization in the longer term, prospective guidance trials should be powered to assess a difference in periprocedural MI rates, as an important metric of PCI optimization for improved safety.
Role of optical coherence tomography and fractional flow reserve for percutaneous coronary intervention optimization
Interestingly, pre-PCI OCT imaging had a high impact on the decision-making process, especially pre-PCI when OCT was intended to be documentary in the absence of prescriptive ‘guidance’ recommendations. Indeed the planned strategy was modified in more than half of the cases. Optical coherence tomography imaging post-PCI seemed to offer additional opportunities for optimization of procedural PCI technique. Residual edge dissections have been associated with both DES thrombosis and restenosis.5,10 Stent under-expansion and small minimal luminal area are strong predictors of late stent failure.17 Other ‘abnormalities’ may not be of clinical relevance.18 Analysis of 1-year outcomes in ILUMIEN I, including additional events, will likely contribute to further defining which OCT parameters and degree of abnormality require optimization, along with other studies.10
Using documentary FFR as an intermediate yardstick for PCI optimization was attempted in the present study. Final post-PCI FFR is known as a strong predictor of outcome.19 This parameter is a better indication for reduction of ischaemic flow than angiography and quantitative coronary angiography.20 A detailed analysis of the crosstalk between stent/vessel anatomy by OCT and functional outcome by post-PCI FFR may help qualifying the significance of “abnormal” OCT findings, for potential inclusion in future procedural optimization strategies.21,22
Study limitations
Study population was restricted to elective procedures in patients with either stable or unstable condition. PCI complexity was fair, not excessive; single vessel PCI was dominant; no planned bare metal stents or bioresorbable scaffolds were used. Patients with acute STEMI, left main PCI, severe chronic kidney disease, and a number of other high-risk features were not included. Physicians were provided general recommendations when to and when not to intervene but were not required to follow them in the context of an observational study. In addition, these short-term results, although important, may vary after the 12-month results are tallied in terms of clinical events. Since no prescriptive recommendations were provided in the protocol, a wide variation in physician behavior was expected and observed. This design will allow to evaluate the clinical consequences of a wide range of residual OCT ‘abnormalities’. Given the high rate of procedure planning change after pre-PCI OCT, clear recommendations as to stent length and size selection, based on pre-PCI OCT imaging, will be applied in ILUMIEN III, a prospective, randomized trial comparing PCI optimization strategies using angiography, OCT, or intravascular ultrasound (ClinicalTrials.gov NCT 2471586). There is clear indication that operators reacted to post-PCI OCT findings primarily in the presence of less satisfactory PCI results. Not surprisingly, these were seen more often with advanced or more complex disease. In addition to the cost of the OCT catheter itself, post-PCI optimization resulted in increased resource utilization. Extra cost will be weighed against clinical benefit from the analysis of 1-year outcomes.
The operator's decision to make use of pre- and/or post-PCI OCT findings was associated with varied periprocedural MI rates, an hypothesis-generating finding that remains to be tested prospectively. Overall, periprocedural MI rate was 7.7%, higher than in stent trials. Absolute MI rates tend to be higher even with the use of identical definitions, when more sensitive assays are used.8 Today's clinical practice is based on better assays, with increased ability to detect myocardial damage. Given the study population and definitions applied in the present study, the observed figures are realistic and pertain to clinically relevant MI's.
Conclusion
Short-term results of ILUMIEN I, a prospective, non-randomized, observational study of PCI procedural practice in patients undergoing pre- and post-PCI FFR and OCT show that both physician decision-making and procedural strategy were influenced by OCT findings either pre-PCI and/or post-PCI in the majority of patients.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: Authors from participating sites report institutional study grant from St Jude Medical. W.W. reports research grants from Volcano and Boston Scientific. He is a co-founder, shareholder, and non-executive board member of Argonauts Partners, Cardio3 Biosciences (now Celyad), and Genae. J.S. reports fees from St Jude; Jones is a speaker for St Jude. Price reports fees from St Jude, Boston Scientific, Medtronic, and Terumo; T.A. reports fees from St Jude, Terumo, and Goodman.
Funding
Funding to pay the Open Access publication charges for this article was provided by St Jude Medical.
References
- 1.Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 2001;103:2928–2934. [DOI] [PubMed] [Google Scholar]
- 2.Authors/Task Force members Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 3.Jang IK, Arbustini E, Bezerra HG, Ozaki Y, Bruining N, Dudek D, Radu M, Erglis A, Motreff P, Alfonso F, Toutouzas K, Gonzalo N, Tamburino C, Adriaenssens T, Pinto F, Serruys PW, Di Mario C; Expert's OCT Review Document. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J 2012;33:2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Vito L, Yoon JH, Kato K, Yonetsu T, Vergallo R, Costa M, Bezerra HG, Arbustini E, Narula J, Crea F, Prati F, Jang IK; COICO group (Consortium of Investigators for Coronary OCT). Comprehensive overview of definitions for optical coherence tomography-based plaque and stent analyses. Coron Artery Dis 2014;25:172–185. [DOI] [PubMed] [Google Scholar]
- 5.Chamié D, Bezerra HG, Attizzani GF, Yamamoto H, Kanaya T, Stefano GT, Fujino Y, Mehanna E, Wang W, Abdul-Aziz A, Dias M, Simon DI, Costa MA. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc Interv 2013;6:800–813. [DOI] [PubMed] [Google Scholar]
- 6.Attizzani GF, Bezerra HG, Chamié D, Fujino Y, Spognardi AM, Stanley JR, Yamamoto H, Mehanna E, Wang W, Carlyle WC, McClain JB, Costa MA. Serial evaluation of vascular response after implantation of a new sirolimus-eluting stent with bioabsorbable polymer (MISTENT): an optical coherence tomography and histopathological study. J Invasive Cardiol 2012;24:560–568. [PubMed] [Google Scholar]
- 7.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 8.Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, Serruys PW. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention 2010;5:871–874. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; ESC Committee for Practice Guidelines (CPG); Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG) Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 10.Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, Burzotta F, Trani C, Porto I, Ramazzotti V, Imola F, Manzoli A, Materia L, Cremonesi A, Albertucci M. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 2012;8:823–829. [DOI] [PubMed] [Google Scholar]
- 11.Morice MC, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR, Torracca L, van Es GA, Leadley K, Dawkins KD, Mohr F. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation 2010;121:2645–2653. [DOI] [PubMed] [Google Scholar]
- 12.De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nüesch E, Jüni P; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208–1217. Erratum in: N Engl J Med. 2014;371:1465. [DOI] [PubMed] [Google Scholar]
- 13.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 14.Hanekamp CE, Koolen JJ, Pijls NH, Michels HR, Bonnier HJ. Comparison of quantitative coronary angiography, intravascular ultrasound, and coronary pressure measurement to assess optimum stent deployment. Circulation 1999;99:1015–1021. [DOI] [PubMed] [Google Scholar]
- 15.Folts JD, Crowell EB, Jr, Rowe GG. Platelet aggregation in partially obstructed vessels and its elimination with aspirin. Circulation 1976;54:365–370. [DOI] [PubMed] [Google Scholar]
- 16.Babu GG, Walker JM, Yellon DM, Hausenloy DJ. Peri-procedural myocardial injury during percutaneous coronary intervention: an important target for cardioprotection. Eur Heart J 2011;32:23–31. [DOI] [PubMed] [Google Scholar]
- 17.Maehara A, Ben-Yehuda O, Ali Z, Wijns W, Bezerra HG, Shite J, Généreux P, Nichols M, Jenkins P, Witzenbichler B, Mintz GS, Stone GW. Comparison of Stent Expansion Guided by Optical Coherence Tomography vs. Intravascular Ultrasound: The ILUMIEN II Study. JACC CV Interv 2015;in press. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg DH, Mintz GS, Mandinov L, Yu A, Ellis SG, Grube E, Dawkins KD, Ormiston J, Turco MA, Stone GW, Weissman NJ. Long-term impact of routinely detected early and late incomplete stent apposition: an integrated intravascular ultrasound analysis of the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, long lesion, and direct stent studies. JACC Cardiovasc Interv 2010;3:486–494. [DOI] [PubMed] [Google Scholar]
- 19.Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, Domínguez-Franco AJ, Dupouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jiménez-Navarro MF, Katritsis DG, Kocaman SA, Koo BK, López-Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodés-Cabau J, Sedlis SP, Takeishi Y, Tonino PA, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne B, Gould KL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64:1641–1654. [DOI] [PubMed] [Google Scholar]
- 20.Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, Heyndrickx GR, Bartunek J, Vanderheyden M, Barbato E, Wijns W, De Bruyne B. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J 2014;35:2831–2838. [DOI] [PubMed] [Google Scholar]
- 21.De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, Gould KL, Wijns W. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but ‘Normal’ coronary angiography. Circulation 2001;104:2401–2406. [DOI] [PubMed] [Google Scholar]
- 22.Stefano GT, Bezerra HG, Attizzani G, Chamié D, Mehanna E, Yamamoto H, Costa MA. Utilization of frequency domain optical coherence tomography and fractional flow reserve to assess intermediate coronary artery stenoses: conciliating anatomic and physiologic information. Int J Cardiovasc Imaging 2011;27:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]