Abstract
Modern-day stenting procedures leverage advances in pharmacotherapy and device innovation. Patients treated with contemporary antiplatelet agents, peri-procedural antithrombin therapy and new-generation drug-eluting stents (DES) have excellent outcomes over the short to medium term. Indeed, coupled with the reducing costs of these devices in most countries there remain very few indications where patients should be denied treatment with standard-of-care DES therapy. The two major causes of stent failure are stent thrombosis (ST) and in-stent restenosis (ISR). The incidence of both has reduced considerably in recent years. Current clinical registries and randomized trials with broad inclusion criteria show rates of ST at or <1% after 1 year and ∼0.2–0.4% per year thereafter; rates of clinical ISR are 5% respectively. Angiographic surveillance studies in large cohorts show rates of angiographic ISR of ∼10% with new-generation DES. The advent of high-resolution intracoronary imaging has shown that in many cases of late stent failure neoatherosclerotic change within the stented segment represents a final common pathway for both thrombotic and restenotic events. In future, a better understanding of the pathogenesis of this process may translate into improved late outcomes. Moreover, the predominance of non-stent-related disease as a cause of subsequent myocardial infarction during follow-up highlights the importance of lifestyle and pharmacological interventions targeted at modification of the underlying disease process. Finally, although recent developments focus on strategies which circumvent the need for chronically indwelling stents—such as drug-coated balloons or fully bioresorbable stents—more data are needed before the wider use of these therapies can be advocated.
Keywords: Bioresorbable stents, Coronary artery disease, Drug-eluting stents, In-stent restenosis, Neoatherosclerosis, Stent thrombosis
Historical background
On 16 September 1977 Andreas Grüntzig performed the first percutaneous coronary intervention (PCI) in an awake human at the Universitätsspital in Zürich, Switzerland.1 Using a rudimentary balloon angioplasty catheter fashioned on his kitchen table, he treated a high-grade stenosis in the proximal left anterior descending artery of a 38-year-old man with very satisfactory acute and late results (Figure 1).2 Although his work would not have been possible without the pioneering endeavours of earlier physician investigators, this procedure is rightly remembered as a landmark in the history of cardiovascular medicine.3
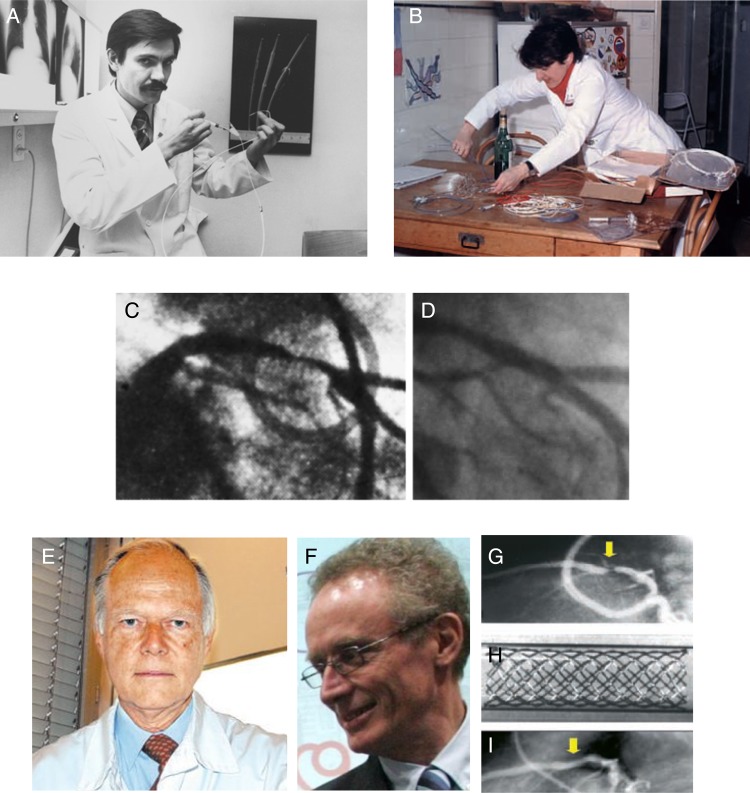
Figure 1.
Historical perspectives on the development of percutaneous coronary intervention and coronary stenting. The first coronary angioplasty in an awake human was performed by Andreas Grünzig (A) on 16 September 1977 using a balloon catheter fashioned on his kitchen table (B). The patient had a high-grade stenosis of the proximal left anterior descending artery and the initial and late follow-up result (D) was very satisfactory. The first coronary stents implanted in man were performed by Ulrich Sigwart (E) in Lausanne and Jaques Puel (F) in Toulouse in March and April 1986. An angiogram from an initial patient shows high grade stenosis of the proximal left anterior descending artery (G), which was treated with a bare metal Wallstent (H) with a good acute result (I).
The limitations of balloon angioplasty however included an unpredictable acute result—due to early abrupt vessel closure—and a relatively high rate of restenosis at the site of the treated lesion—due mainly to plaque prolapse, vessel recoil, and constrictive remodelling. In this respect, it was the modification of this procedure to include the implantation of a metallic stent in the treated vessel that proved the tipping point to enable widespread uptake of PCI therapy. First human coronary stent implantation was performed in Toulouse and Lausanne within weeks of each other in March and April 1986. (Figure 1).4 By splinting angioplasty-induced arterial dissections and sealing disrupted plaques stent implantation resulted in less acute vessel thrombosis. Moreover, the additional advantage in terms of mechanical strength with stent implantation resulted in greater acute gain in luminal calibre and negation of the effects of vessel recoil and constrictive remodelling. This translated into a significantly lower rate of subsequent restenosis.
As the advantages of stent implantation saw its evolution from a ‘bail out’ after complicated balloon angioplasty to a standard treatment strategy5,6 two important limitations were recognized. First, a not insignificant number of cases continued to result in early acute vessel closure due to stent thrombosis (ST). An early study reported complete occlusion occurred ∼25% of cases mostly within the first 14 days after implantation.7 Moreover, these complications occurred despite the fact that early stent procedures were often undertaken with very large doses of heparin (up to 15 000 units)—sometimes with dextran or urokinase infusions—as well as overlapping oral anti-coagulation. This in turn resulted in significant morbidity and mortality due to haemorrhagic complications—with major bleeding occurring in 9% of patients in an early study at our centre.8 Indeed, arguably one of the most important developments in the evolution of stent therapy was the demonstration that dual antiplatelet therapy (DAPT) with aspirin and an ADP-receptor inhibitor could reduce both ST and bleeding complications in comparison with oral antithrombotic therapy.9,10 Together with technical refinements such as the use of routine high-pressure stent deployment,11 these developments facilitated the widespread adoption of coronary stenting for the treatment of a broad range of obstructive coronary artery disease.
The second important limitation was late stent failure due to in-stent restenosis (ISR).12 Although stenting was important in resisting acute and late constrictive mechanical forces, the stent implantation procedure resulted in increased acute vessel injury at the time of PCI and an enhanced healing response leading to varying degrees of neointimal hyperplasia.13 It was this issue that prompted research leading to the development of drug-eluting stents (DES).
Drug-eluting stent devices have proved highly effective in reducing the incidence of stent failure and enabled the expansion of PCI to treat high-risk patient and lesion subsets. Iterative development has focused on thinner stent struts and more biocompatible polymer coatings as well as stents that are fully bioresorbable.14,15 A wide range of DES is currently available. A recent systematic review of DES devices by a European Society of Cardiology-European Association of Percutaneous Coronary Intervention Task Force identified at least 68 DES with CE-mark approval as of June 2014.16 The key characteristics of selected DES devices with published large-scale randomized clinical trial data are shown in Figure 2. Indeed, although it is a subject of considerable debate, the high efficacy and excellent safety of current generation devices may be associated with not just improvement in quality of life but also in survival in treated patients.17
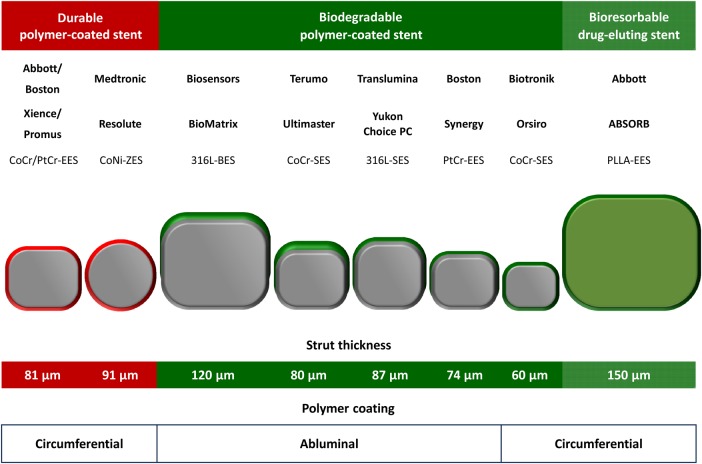
Figure 2.
Overview of principal characteristics of selected current generation durable polymer and biodegradable polymer drug-eluting stents and fully bioresorbable drug-eluting stents with published large-scale randomized controlled trial data. BES, biolimus-eluting stent; CoCr, cobalt chromium; CoNi, cobalt Nickel; EES, everolimus-eluting stent; PtCr, platinum chromium; SES, sirolimus-eluting stent; ZES, zotarolimus-eluting stent.
Stent thrombosis
An overview of histopathology, risk factors, incidence, and intravascular imaging features of ST is provided in Figure 3A–D, respectively.
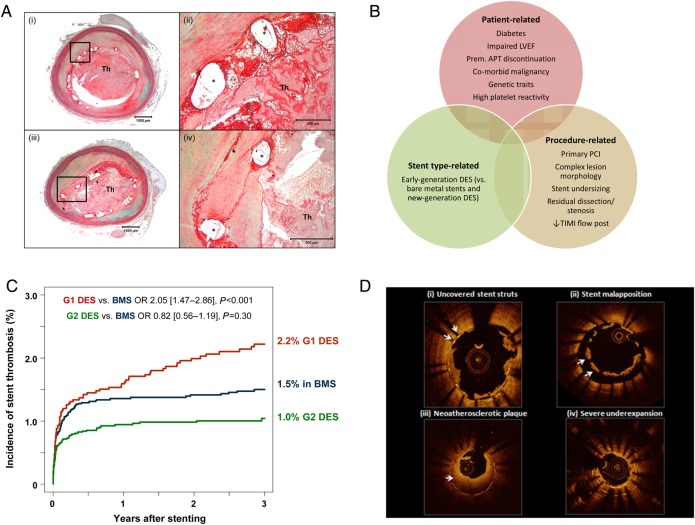
Figure 3.
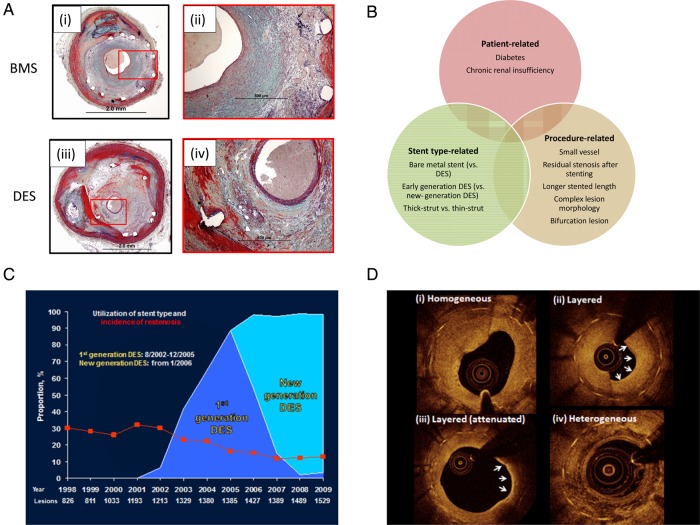
Stent thrombosis: central illustration of histopathology, risk factors, incidence, and intravascular imaging features. (A) Representative case showing late stent thrombosis with uncovered struts following drug-eluting stent implantation. Histologic section from a 47-year-old male who had overlapped drug-eluting stents (paclitaxel-eluting stent in the proximal segment and sirolimus-eluting stent in the distal segment) implanted 10 months prior to death. A low-power image (i) shows a platelet-rich occlusive thrombus in the lumen in paclitaxel-eluting stent. A high-power image (ii) of boxed area in (i) shows uncovered struts with peri-strut fibrin. Image (iii) also shows a platelet-rich occlusive thrombus. A high-power image (iv) of boxed area in (iii) shows partially covered struts with neointima (Asterisk indicates stent strut.). (B) Principal risk factors for stent thrombosis classified according to patient-related, stent type-related, and procedure-related risk factors. (C) Incidence of stent thrombosis after bare metal stents, early-generation drug-eluting stents (G1 DES), and new-generation drug-eluting stents (G2 DES); adapted from Tada et al.22 (D) Representative optical coherence tomography findings from patients presenting with stent thrombosis: (i) persistent uncovered stent struts late after implantation; (ii) marked stent malapposition in the target vessel, this may have been present at the time of implantation or acquired due to late positive remodelling; (iii) neoatherosclerotic plaque formation: diffuse low-signal intensity with higher backscatter in deeper neointimal layers may indicate underlying lipid-rich atherosclerotic tissue; (iv) severe stent underexpansion at site of overlap of multiple stent layers.
Incidence and time course
Stent thrombosis is typically characterized by angiographic or post-mortem evidence of recently formed thrombus in a previously stented segment (Figure 3A). Study of thrombus aspirates from patients presenting with ST have shown a mix of thrombotic and inflammatory components including platelet-rich thrombus, fibrin fragments, and leukocytes of both neutrophil and eosinophil lineage.18 In order to standardize reporting across clinical trials universal definitions were agreed upon in 2006 by a group of experts known as the Academic Research Consortium.19 This definition classified evidence of ST as definite, probable, or possible as well as according to timing after the initial stent implantation (Table 1). In practice, because of differing pathophysiology and risk factors20,21 it can be useful to dichotomize events into two major categories: early ST is defined as thrombosis within the first 30 days and late ST is thrombosis occurring beyond 30 days. In general, early ST is more common than late, accounting for ∼50–70% of all cases depending on the overall time frame of reference.20,21
Table 1.
Definition of diagnostic criteria for and timing of stent thrombosis
| Diagnostic criteria for ST |
|---|
| Definite ST |
| Presence of an acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion |
| Probable ST |
| Unexplained deaths within 30 days after the procedure or acute myocardial infarction involving the target-vessel territory without angiographic confirmation |
| Possible ST |
| All unexplained deaths occurring at least 30 days after the procedure |
| Timing of ST in relation to index stent implantation |
| Acute STa |
| Occurring between 0 and 24 h after the index PCI |
| Subacute STa |
| Occurring between 24 h and 30 days after the index PCI |
| Late ST |
| Occurring between 31 and 360 days after the index PCI |
| Very late ST |
| Occurring later than 360 days after the index PCI |
aThe term early stent thrombosis can be used to refer to all stent thrombosis occurring within the first 30 days.
In recent years, important progress has been made in reducing the incidence of ST. Recent large-scale registries show that with contemporary antithrombotic therapies and modern generation DES the rate of early ST is <1% (Figure 3C).22–24 Moreover, a recent systematic review of randomized trials with DES reporting results at 9–12 months showed a median incidence of definite ST of 0.61%.16 In addition, overall rates of early and late ST out to 3 years have halved in recent years from ∼3.0 to 1.5%. (Figure 3C).22,23
Risk factors for stent thrombosis
In general, it can be useful to classify risk factors as patient-, procedure-, or device specific (Figure 3B).
Early stent thrombosis
In terms of early ST procedural risk factors are the most important. Stent undersizing, presence of residual dissection, impaired TIMI flow and residual disease proximal or distal to the stent lesion are important predictors of ST.21 A French study showed that lesion complexity and index PCI in the setting of acute myocardial infarction were strong predictors of subsequent ST.25 In addition, patient-specific risk factors such as reduced left ventricular function and impaired response to ADP-antagonist therapy confer important increased risk. Indeed, premature discontinuation of antiplatelet therapy in the initial 30 days after stenting is arguably the most important predictor of ST.26 Moreover, considerable interest has focused on predicting risk based on response to ADP-antagonist therapy. First, pharmacogenetic testing seems to be able to identify patients-at-risk based on genetic polymorphism related to enzymes required for clopidogrel metabolism.27–29 Second, many studies have shown an association between high on-treatment platelet reactivity in platelet function testing and subsequent ST.24,30 For example, a registry study from our centre showed a 9-fold risk of early ST in patients with high on-treatment platelet reactivity.30 Importantly, however, while both pharmacogenetic and platelet function testing are attractive for identifying patients at risk no trial has yet been able to show significant improvement in outcomes if treatment is modified (i.e. intensity increased) on the basis of this data.31–33
Finally, although device-specific factors were thought to be of lesser importance in determining the risk of early ST, recent studies suggest that there may be important differences. Analysis of large datasets suggests that rates of early ST seem to be slightly higher with bare metal vs. drug-eluting stents.34 Indeed bench work suggests that polymer-coatings may reduce acute thrombogenicity possibly by improving stent-blood interactions.35 Moreover, randomized trial data in the setting of stent implantation for acute myocardial infarction suggests some evidence of reduced early ST with polymer-coated vs. bare metal stents.36
Late stent thrombosis
Although significant technical shortcomings in the index procedure will more likely manifest as early stent failure, such factors can also play an important role in late ST where significant mechanical issues—e.g. stent undersizing or underexpansion—remain after the time point of DAPT discontinuation.21 Malapposition (or incomplete stent apposition) is often observed on intravascular imaging in patients with ST.37 However, definitive evidence of its clinical importance based on appropriately designed case–control studies is lacking at present. In particular, the threshold at which malapposition distance and extent become clinically relevant is not well defined.38 Patient-specific risk factors also remain important for late ST. In particular, reduced left ventricular function and diabetes mellitus are associated with increased risk.21 In addition, impaired response to ADP-antagonist therapy also confer important increased risk for late ST.29
An important role in late ST is played by stent type-related factors. Controversy generated by presentations at the European Society of Cardiology annual meeting in 2006 focused attention on a possible increased risk of cardiac death with early-generation DES devices, mediated through higher rates of ST.39 Indeed, a number of meta-analyses that appeared shortly afterwards showed evidence of a small but significant increase in the risk of ST with both sirolimus- and paclitaxel-eluting stents.34,40,41 Moreover, registry reports showed evidence of an ongoing risk of ST out to 4–5 years with no clear evidence of an attenuation of this effect with time.42 The underlying substrate for this excess risk was identified in autopsy studies to be delayed arterial healing, a pathophysiological process characterized by impaired endothelial coverage, persistent fibrin deposition, and ongoing vessel wall inflammation.43 Although clearly multifactorial in origin, it seemed to be that inflammatory reaction to the durable polymer coatings used in early-generation devices played an important role.44,45 Indeed, the passage of time has shown us that delayed healing likely underlies a spectrum of adverse clinicopathological entities including not just late ST but also delayed late luminal loss (which may contribute to late re-stenosis),46 persistent vasomotor dysfunction proximal and distal to the stented segment,47 and de novo in-stent atherosclerosis.48 Newer generation DES seem to have addressed this healing problem in a meaningful way by incorporating thinner stent struts (which reduce acute vessel injury), more biocompatible polymer coatings (both nonerodable and biodegradable), and lower dosages of sirolimus-analogue drugs (Figure 2).22,23 Although each of these iterative developments may be clinically relevant in isolation, it should not be forgotten that overall clinical performance of DES is due to aggregate effects of both the backbone and the drug-matrix coating.
Clinical consequences and treatment of stent thrombosis
Stent thrombosis is a very serious clinical event typically resulting in ST-elevation myocardial infarction in the majority of cases20,49,50 and mortality rates that may be as high as 20–40%.50 Although detailed consideration of management of ST is beyond the scope of this review, most registries of ST report that thrombus aspiration and balloon angioplasty are frequently used with repeat stenting in ∼30–50% of cases.20,49
Dual antiplatelet therapy duration and prevention of stent thrombosis
Key to the prevention of ST is the prescription of an appropriate duration of DAPT after PCI. Randomized clinical trials in the 1990s demonstrated conclusively that DAPT was superior to anti-coagulation for the prevention of complications after bare metal stenting.9,10 Early randomized clinical trials with DES implantation included a recommendation for 3–6 months of DAPT after PCI,51,52 though concerns soon emerged about a possible increase in late ST after DES implantation. Two important consequences of these were that (i) guideline authorities recommended—on the basis of expert opinion—a more prolonged duration of DAPT of typically at least 12 months and (ii) a number of large-scale clinical trials were initiated to define more precisely the optimal duration of DAPT after DES implantation (see Table 2). Data from all of these studies have now been reported permitting reappraisal of recommendations relating to DAPT duration.53–62
Table 2.
Main characteristics of patients enrolled in trials comparing different duration of dual antiplatelet therapy after coronary stenting
| Trial or subgroup | Patients, n | Age (years) | Males (%) | Diabetics (%) | ACS at admission | Newer DES (%) | Thienopyridines |
|---|---|---|---|---|---|---|---|
| Prolonged up to 12 months | |||||||
| EXCELLENT53 | 1443 | 62.7 | 64.5 | 38.2 | 51.1 | 74.8 | Clopidogrel |
| ISAR SAFE54 | 4000 | 67.2 | 80.6 | 24.5 | 40.0 | 88.6 | Clopidogrel |
| OPTIMIZE55 | 3119 | 61.6 | 63.3 | 35.3 | 5.4§ | N/A | Clopidogrel |
| RESET56 | 2148 | 62.4 | 63.6 | 29.3 | 58.6 | 44.8 | Clopidogrel |
| SECURITY57 | 1399 | 65.2 | 77.2 | 30.9 | 38.4† | 100 | Clopidogrel |
| Prolonged beyond 12 months | |||||||
| ARCTIC-Interruption58 | 1259 | 64.0 | 80.5 | 33.5 | N/R | 63.0 | Clopidogrel, Prasugrel |
| DAPT (DES)59 | 9961 | 61.7 | 74.6 | 30.6 | 42.6 | 47.2 | Clopidogrel, Prasugrel, Ticagrelor |
| DES LATE (extended)60 | 5045 | 62.4 | 69.3 | 28.1 | 60.7 | 34.2 | Clopidogrel |
| ITALIC/ITALIC plus61 | 1850 | 61.6 | 80 | 38.0 | 23.4 | 100 | Clopidogrel, Prasugrel, Ticagrelor |
| PRODIGY62 | 1970 | 67.8 | 76.7 | 24.2 | 74.4 | 50.2 | Clopidogrel |
Overall mean values are reported; any DES other than Cypher (Cordis, Warren, NJ, USA), Taxus (Boston Scientific, Natick, MA, USA), and Endeavor (Medtronic Inc., Santa Rosa, California, USA) is defined as ‘newer DES’. ACS, acute coronary syndrome; DES, drug-eluting stent; N/R, not reported; N/A, not applicable.
Results from the initial studies to report data showed that prolongation of DAPT did not reduce ischaemic adverse events but did lead to an excess of major bleeding events.63 However, none of the individual studies were powered to show a reduction in ST, all were open-label, and by virtue of randomizing patients at the time point of stent implantation rather than of treatment divergence, many of these trials may have been biased towards a null effect. In this respect, the recently published results of the DAPT trial are of high clinical relevance.59 Overall, 9921 patients treated with DES were randomized to prolonged duration (30 months) or standard duration (12 months) DAPT. The main findings were that prolonged DAPT duration significantly reduced ST [0.4 vs. 1.4%; hazard ratio, 0.29 (95% CI, 0.17–0.48); P < 0.001] and overall composite cardiac events [4.3 vs. 5.9%; hazard ratio, 0.71 (95% CI, 0.59–0.85); P < 0.001] at the expense of a significant increase in major bleeding [2.5 vs. 1.6%; hazard ratio 1.61 (95% CI, 1.21–2.16); P = 0.001]. Results were consistent according to clinical presentation at the time of index stenting.64 However, a number of aspects should be considered when interpreting the data. First, although no interaction with stent type was observed, a clustering of ST events occurred in patients treated with early-generation DES, stents which have now fallen out of use—e.g. 27% of enrolled patients were treated with paclitaxel-eluting stents, while 57% of all ST occurred in patients treated with this stent. Second, there is some cause for concern due to a higher rate of death in patients treated with prolonged duration DAPT. However, an association with bleeding events is not clear and it remains possible that this is a chance observation. Third, despite the best efforts of investigators only selected patients were included: the majority of screened patients were not represented in the randomized controlled trial. Fourth, it must of course be recognized that prolongation of DAPT not only offers the possibility to mitigate the risk of ST but also to prevent ischaemic complications not related to the stented lesion; in this respect, the demonstrated reduction in the overall incidence of myocardial infarction [2.1 vs. 4.1%; hazard ratio, 0.47 (95% CI, 0.37–0.61); P < 0.001] is an important finding. Finally, an update meta-analysis of published trials on DAPT duration supports the individual findings of the DAPT trial (Figure 4).65 The clear reduction in ST at the expense of increased major bleeding highlights the importance of clinical judgement in individualizing treatment duration for our patients in clinical practice. Accordingly, while a general time window for optimal duration of DAPT—between 6 months and 30 months duration—might be recommended, a one size fits all approach for DAPT duration is likely not optimal. Moreover, DAPT duration in clinical practice is a dynamic decision, which might be re-assessed at regular intervals during follow-up.
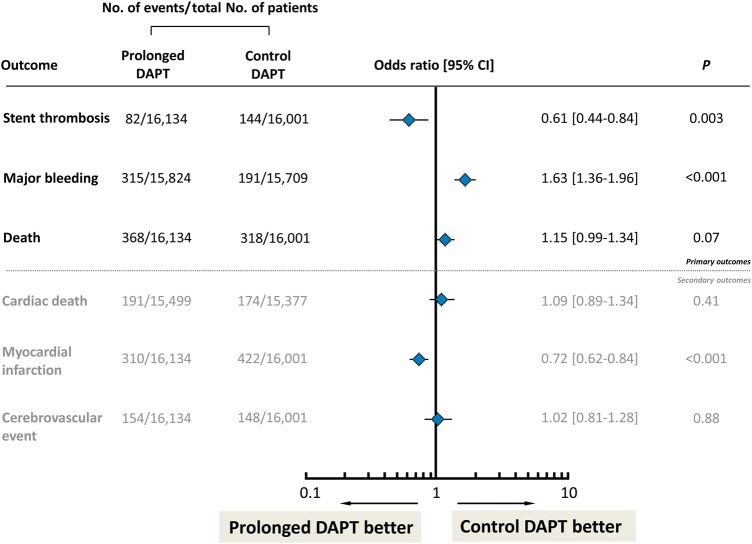
Figure 4.
Summary results of meta-analysis of trials investigating prolonged duration vs. standard duration dual antiplatelet therapy after drug-eluting stent implantation. Odds ratio with (95% confidence interval) associated with prolonged vs. control dual antiplatelet therapy accounting for events occurred at the longest follow-up available in each included studies. The diamonds and the horizontal lines indicate the odds ratio and the (95% confidence interval) derived from meta-analysis. DAPT, dual antiplatelet therapy; figure based on analysis of data from Cassese et al.65
Some other issues in relation to DAPT duration deserve brief mention. First, concern exists about a possible clustering of late ST events after discontinuation of DAPT.66 In a small platelet reactivity trial, however, we could not detect difference in platelet aggregation between patients randomized to clopidogrel tapering vs. abrupt cessation at the end of planned DAPT.67 In addition, a larger trial powered for clinical endpoints also failed to detect a difference between tapering and abrupt cessation, though this study was stopped prematurely and had low event rates.68 Against this, there is some recent evidence supporting a rebound effect after DAPT discontinuation in the DAPT trial.59
Secondly, some observational studies have provided support for the concept that <6 months duration of DAPT may be safe following implantation of newer generation stents.69,70 Importantly however, these registries are limited by confounding: the reasons for therapy discontinuation (e.g. physician directed, short interruption, or patient noncompliance) are a critical factor.71 Moreover, the basis on which DES have obtained CE-mark approval for short duration DAPT in the absence of appropriately designed RCTs remain unclear and highlights some problems with current approval processes for medical devices in Europe.16 In our practice, we do not recommended DAPT durations shorter than 6 months [in patients not treated with oral anticoagulants (OAC)].
Thirdly, optimal management of patients receiving OAC who are treated with stent implantation is unclear. Industry-support for randomized trials has been lacking and only two modest-sized investigator-initiated RCTs have been completed. The WOEST investigators showed overall comparable outcomes between OAC plus clopidogrel vs. OAC plus dual antiplatelet therapy for 12 months.72 In the ISAR-TRIPLE trial, we also showed broadly similar outcomes between a strategy of 6 weeks DAPT vs. 6 months DAPT.73 However, both of these trials were significantly underpowered to detect differences in safety endpoints. Presently, a number of large-scale industry-supported RCTs are examining the best treatment for this important patient subgroup.74 Finally, more potent oral ADP-receptor antagonists significantly reduced the incidence of ST in comparison with clopidogrel; however, these agents are currently recommended only in patients undergoing stenting following presentation with an acute coronary syndrome. Moreover, application of novel intravenous ADP-receptor antagonists may further reduce rates of ST in the acute phase after stenting.75
In-stent restenosis
An overview of histopathology, risk factors, incidence, and intravascular imaging features of ISR is provided in Figure 5A–D, respectively.
Figure 5.
In-stent restenosis: central illustration of histopathology, risk factors, incidence, and intravascular imaging features. (A) Representative histopathological cases showing in-stent restenosis after coronary stenting: (i) low-power magnification of in-stent restenosis in a bare metal stent; (ii) high-power magnification shows predominance of smooth muscle cell-rich neointimal; (iii) low-power magnification of in-stent restenosis in a sirolimus-eluting stent; (iv) higher magnification shows a stent strut with surrounding proteoglycan-rich neointimal tissue and presence of foam cells and neovascularization. (B) Risk factors for stent thrombosis classified according to patient-related, stent type-related, and procedure-related risk factors. (C) Proportion of patients treated with bare metal stents, early-generation drug-eluting stents, and new-generation drug-eluting stents over time and rates of binary angiographic restenosis (red line) in a large registry of patients with angiographic surveillance after stent implantation. Adapted from Cassese et al.63 (D) Optical coherence tomography imaging of patients with in-stent restenotic tissue during surveillance after stenting; tissue with homogeneous-signal intensity (i) is typical after bare metal stenting; heterogeneous, attenuated, or layered signal intensity tissue (ii–iv) is typical after drug-eluting stents.
The higher degree of vessel injury with stent implantation in comparison with balloon angioplasty alone increased the extent of neointimal hyperplasia in the intervened segment and this is the dominant cause of restenosis after bare metal stent implantation.13 Restenosis after PCI has been characterized as a distinct pathophysiological process rather than merely an accelerated form of post-intervention atherosclerosis.76 In general, terms inflammatory response to vessel wall injury during PCI plays a central role in restenosis after stenting with vessel wall inflammation driving fibroblast growth and smooth muscle cell hyperplasia. Mechanistically contributing factors to restenosis after vascular intervention may be divided into five categories: (i) acute or subacute prolapse of the disrupted plaque, (ii) elastic recoil of the vessel wall, (iii) constrictive remodelling, (iv) neointimal hyperplasia (due to extracellular matrix deposition and smooth muscle cell hyperplasia), and (v) de novo in-stent atherosclerosis (neoatherosclerosis).77
Angiographic restenosis is commonly adjudicated as a binary event defined as a re-narrowing of >50% of the vessel diameter as determined by coronary angiography.77 Intravascular imaging modalities acquire data in three dimensions, using these modalities restenosis is defined as a re-narrowing of >75% of the reference vessel area in cross-section. The term clinical restenosis is sometimes used to refer to restenosis of the treated lesion accompanied by requirement for re-treatment, for example, due to symptoms or signs of ischaemia. Rates of clinical restenosis are usually considerably lower than rates of restenosis detected by imaging as not all restenotic lesions cause ischaemia or elicit symptoms.
From a pathological standpoint, it appears that there are considerable differences between restenosis that occurs after bare metal stenting vs. after DES (Table 3; Figure 5).78,79 The main difference is that restenosis after bare metal stenting is typically characterized by neointimal hyperplasia consisting of a proteoglycan matrix and high proportion of vascular smooth muscle cells. In contrast, restenosis after DES is typically characterized by a proteoglycan-rich neointimal hyperplasia with relatively few smooth muscle cells. Moreover, neoatherosclerotic change within the restenotic tissue is seen earlier and more frequently in DES restenosis. Intravascular imaging with OCT also reveal distinct differences between the two processes: imaging of bare stent restenosis tends to show high-volume homogeneous-signal tissue and in DES restenosis layered pattern or homogeneous tissue types tend to predominate (Figure 5).80
Table 3.
Comparison of principle features of bare metal and drug-eluting stent restenosis
| Characteristic | Bare metal stent restenosis | Drug-eluting stent restenosis |
|---|---|---|
| Imaging features | ||
| Angiographic appearance | Diffuse pattern more common | Focal pattern more common |
| Time course of late luminal loss | Late loss maximal by 6–8 months | Ongoing late loss out to 5 years |
| Optical coherence tomography tissue properties | Homogenous, high-signal band typical | Layered structure or heterogeneous typical |
| Histopathological features | ||
| Smooth muscle cellularity | Rich | Hypocellular |
| Proteoglycan content | Moderate | High |
| Peri-strut fibrin and inflammation | Occasional | Frequent |
| Complete endothelialisation | 3–6 months | Up to 48 months |
| Thrombus present | Occasional | Occasional |
| Neoatherosclerosis | Relatively infrequent, late after stenting | Relatively frequent, accelerated course |
Incidence and time course
Systematic angiographic surveillance in >10 000 patients undergoing coronary stenting at our centres showed rates of angiographic restenosis of ∼30% after bare metal stenting.81 By inhibiting vascular smooth muscle migration the introduction of DES led to a significant reduction in rates of angiographic restenosis to ∼15% with early-generation devices and 12% with newer generation devices (Figure 5). On the other hand, contemporary randomized trials without angiographic surveillance typically demonstrate rates of clinically relevant restenosis of <5% at 12 months.82
Angiographic surveillance studies have shown that neointimal formation after bare metal stenting tends to peak at 6 months after stenting and thereafter remain stable or regress somewhat over the medium term.12,83 This is in keeping with completion of vessel healing, contraction of neointima, and positive remodelling of the vessel wall. Interestingly, more prolonged follow-up of series out to 7–11 and 15–20 years indicated some further luminal re-narrowing beyond 4 years.84,85 After DES implantation however, the time course of restenosis seems to be rather different. In a large serial angiographic follow-up registry, we found that ongoing erosion of luminal calibre between 6 and 8 months and 2 years post-stenting is a feature of DES therapy.46 Räber et al. showed incremental late loss in patients treated with first-generation DES who had surveillance angiography at 6–8 months and 5 years.86 The observation of ongoing delayed late loss with DES beyond the 6- to 8-month time window supports the hypothesis of DES-associated delayed arterial healing seen in autopsy and preclinical studies and suggest that the temporal course of restenosis with DES may be significantly right-shifted compared with bare metal stents. Moreover, some of this late erosion of luminal calibre may be attributable to the higher incidence of in-stent neoatherosclerosis formation in DES.48
Risk factors for in-stent restenosis
The occurrence of ISR may have an important impact on long-term prognosis after PCI87 and identification of patients at risk is an important undertaking. Risk factors for ISR can also be classified as patient-, procedure-, or device-specific (see Figure 5).88,89
Early studies in the DES era showed that the major risk factors for restenosis after DES were vessel size, final diameter stenosis, and type of DES (i.e. SES were more effective in preventing ISR than paclitaxel-eluting stent).90 By reducing the extent of injury at the time of implantation thinner stent struts are also associated with a reduced restenotic risk in comparison with thicker struts.91 In the largest analysis to date, we investigated the risk factors for restenosis in a series of 10 004 patients with angiographic follow-up after coronary stenting.81 Binary restenosis was detected in 26% of patients overall. At multivariate analysis, smaller vessel size (odds ratio 1.59 [95% confidence interval, 1.52–1.68] for each 0.5-mm decrease), total stented length (1.27 [1.21–1.33]), complex lesion morphology (1.35 [1.20–1.51]), diabetes mellitus (1.32 [1.19–1.46]), and history of bypass surgery (1.38 [1.20–1.58]) were independently associated with restenosis. Moreover, use of first-generation DES vs. bare metal stents (0.35 [0.31–0.39]) and second-generation DES vs. first-generation DES (0.67 [0.58–0.77]) were independent predictors of lower rates of restenosis. Overall in terms of therapeutic measures to reduce restenosis, the most important issues are likely to be meticulous attention to procedural detail and use of high-performance DES. Other approaches including systemic pharmacotherapy have been associated with mixed results and are discussed in detail elsewhere.92
In terms of risk prediction using biomarkers, a number of studies have investigated an association between inflammatory biomarkers at the time of stenting and subsequent restenosis though the clinical utility of such an approach is unclear. At our centre, we showed that although baseline CRP levels did not seem to correlate with restenosis, the change between baseline and peak post-intervention CRP values strongly correlated with angiographic restenosis.93 A subsequent study from Park et al. also failed to show an association between baseline CRP and restenosis94 as did a larger analysis of four ISAR randomized trials.95
Clinical consequences and treatment of in-stent restenosis
Although ISR may be associated with a recurrence of stable angina symptoms, it is well recognized that up to a third of patients present with myocardial infarction or unstable angina96 and in contemporary clinical trials of patients with ISR ∼20% of patients have biomarker positive acute coronary syndrome. Most patients requiring treatment are amenable to repeat catheter intervention and the most effective strategies seem to be repeat stenting with new-generation DES or angioplasty with drug-coated balloons.97
Neoatherosclerosis as a common pathway in late stent failure
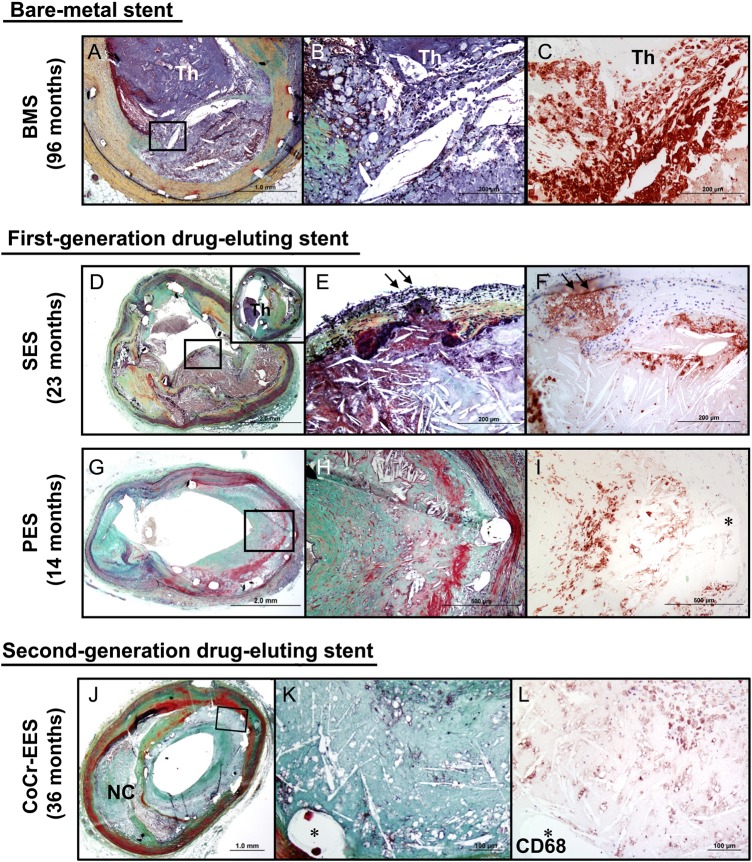
Neoatherosclerosis is a term coined to describe the development of atherosclerotic plaque inside an implanted coronary stent. Histopathologically, the process is characterized by three main stages: (i) early foamy macrophage infiltration, (ii) manifest atherosclerotic plaque development, and (iii) necrotic core plaque formation with or without thin fibrous caps (Figure 6). Although neoatherosclerosis is also observed after bare metal stenting, it occurs earlier and more frequently after stenting with DES. The first systematic report of this process described findings in a series of autopsy segments after drug-eluting and bare metal stenting.98 Neoatherosclerotic change inside the stent was seen in a higher proportion of cases after DES when compared with after bare metal stent implantation (35 vs. 10%; P = 0.0004). Moreover, early neoatherosclerotic changes—such as foamy macrophage infiltration—were as early as 4 months after DES when compared with only beyond 2 years after bare metal stent implantation. These findings were confirmed in a subsequent large series of 384 autopsy specimens.48 Moreover, in spite of iterative development in device technology, the incidence of neoatherosclerosis seems comparable between early- and new-generation DES.48 The observations may be explained by the fact that under normal circumstances, arterial walls are protected from infiltration by circulating lipid particles by a healthy endothelial cell barrier. However, DES are well known to cause anatomical and functional endothelial impairment—features characteristic of delayed arterial healing. Accordingly, it can be hypothesized that the presence of incompetent endothelium after DES is more likely to lead to accelerated and perhaps more frequent neoatherosclerosis.
Figure 6.
Representative cases showing neoatherosclerotic change following bare-metal stent, first-generation drug-eluting stent, and second-generation drug-eluting stent implantation. (A–C) Histologic section from a 47-year-old male who had a bare metal stents implanted 8 years prior to death. Note occlusive thrombus in the lumen and ruptured plaque (boxed area in A), which is shown at higher magnification in (B) with large number of macrophages within the lumen as well as at the ruptured cap. Note large number of CD68-positive macrophages at the site of rupture (C). (D–F) Histological sections from a 59-year-old male with sirolimus-eluting stents implanted for 23 months who died from stent thrombosis (D). Note thin-cap fibroatheroma with fibrous cap disruption in (E) (arrows) from boxed area in (D). The thrombus was more apparent in the distal section (D; inset). (F) CD68-positive macrophages in the fibrous cap and in the underlying necrotic core. (G–I) Histologic sections from a 65-year-old woman with a paclitaxel-eluting stent implanted in the left circumflex artery 14 months antemortem, who died of traumatic brain injury. A low-power image shows a patent lumen with moderate neointimal growth (G), foamy macrophage infiltration and necrotic core formation with cholesterol clefts is seen at high magnification in (H). (i) Same section as (H) showing CD68-positive macrophages in the neointima. (A)–(I) were reproduced with permission from Nakazawa et al.111 (J–L) Histologic sections from a 73-year-old man with cobalt chromium everolimus-eluting stent implanted in the mid left anterior descending for 3 years. A low-power image (J) (Movat) shows moderate luminal narrowing with moderate neointimal growth (69% stenosis) and underlying fibroatheroma. A high-power image (K) of the boxed area in (J) shows necrotic core formation within the neointima where CD68-positive macrophages are identified (L). (J)–(L) were reproduced with permission from Otsuka et al.112
In parallel with these reported autopsy series, the increasing availability of high-resolution intravascular imaging with optical coherence tomography (OCT) permitted the improved characterization of in-stent tissue in patients presenting with late stent failure. Evaluation of tissue extent and homogeneity, signal attenuation, and neointimal tissue contours permits identification of possible areas of neoatherosclerosis with or without necrotic core formation and plaque rupture or erosion in clinical practice.78 Moreover, development of techniques for quantitative analysis of OCT signal intensity offers potential for more accurate identification of these tissue types in future studies.99 A series of 33 patients with OCT imaging during intervention for late ST showed neoatherosclerotic plaque in ∼70% of cases.100 Initial reports in patients with ISR suggested that ca. 50% of cases had tissue type consistent with neoatherosclerosis.101 In another study, predictors of neoatherosclerosis in patients with in-stent neointimal hyperplasia included stent age (>48 months), DES as stent type, current smoking, and chronic renal insufficiency.102 Taken together, both human autopsy and clinical imaging studies suggest that in many cases neoatherosclerosis is the final common pathway for late stent failure.48 Important limitations of existing datasets are a paucity of histopathological correlation studies and the absence of an established preclinical model of neoatherosclerosis.78 Although OCT imaging is intuitively attractive, the routine use of this imaging modality for guiding treatment of stent failure is presently not supported by clinical trial data.
New developments: bioresorbable stents
Bioresorbable stents (BRS) are an important technological development with potential to enhance the outcomes of patients treated by PCI and radically change future stenting practices. The basic concept is based upon the degradation of the stent backbone to inert particles after its useful function is served; once the stent has been fully degraded, this theoretically removes the risks associated with both ST and restenosis. Moreover, these devices offer additional potential benefits, including restoration of normal vasomotor tone of the stented segment and increase in lumen calibre due to positive vessel remodelling associated with stent degradation. This might in turn translate into improvements in coronary physiology and reduction in angina symptom burden.14
Clinical trial reports in selected patients with comparatively straightforward lesion morphology has shown some encouraging results with BRS technology.103 However, the generalizability of these observations is unclear. Recently, a number of registry studies from real-world practice have been published and these data are of interest for a number of reasons.104–106 First, the overall clinical performance of these stents seems satisfactory—at least over short-term follow-up—though more data are needed in larger patient numbers. Secondly, restenosis rates seen with these stents in clinical practice seems low and in line with observations seen in early clinical trials. Thirdly, the rates of ST seen with BRS in the first 6–12 months seems higher than that observed with current generation metallic DES. Moreover, the majority of these events occur within the first 30 days. This means that their occurrence is likely related to the implantation procedure and could be influenced by the expertise of the operator. Specifically, careful selection of patients and lesions is critical and meticulous attention to implantation detail is vital, including a low threshold to use intravascular imaging for optimization of stent deployment. Overall, although BRS is undoubtedly a potential breakthrough technology, concern exists regarding greater complexity in implantation technique and a possible excess of early ST.107 Current devices likely represent relatively immature iterations of the technology requiring careful patient selection and meticulous attention to implantation technique. Further device iteration—with improved backbones and optimized radial strength—will likely be required before widespread adoption can be recommended.
Perspective
The great progress made with coronary stenting and antithrombotic therapies over the course of the last 25–30 years means that the vast majority of our patients who require PCI—including those with unstable presentations, complex disease patterns, and multiple co-morbidities—can be successfully treated in a safe and effective manner. Although nowadays PCI almost always involves implantation of a permanent metallic prosthesis, the excellent clinical outcomes over the short- to medium-term support the effectiveness of this approach. However, a number of areas of unmet need still exist. First, we need to remain focused on the fact that while stent implantation relieves symptoms and may improve prognosis, it remains a downstream therapy which targets the final common pathway of cardiovascular disease rather than the underlying disease process. Indeed, even at a follow-up interval as short as 3-years after stenting, natural history studies show that progression in non-target lesions starts to predominate as a cause of subsequent myocardial infarction.108 In this respect, the cornerstone of therapy remains risk modification, though lifestyle intervention and treatment with disease modifying agents; the impact of application of PCSK9 inhibitors—potent systemic therapies for lipid lowering—on both overall disease progression as well as late stent failure will be potentially considerable.109 Secondly, the availability of high-resolution intravascular imaging has increased our awareness of neotherosclerosis—a final common pathway in many cases of thrombotic or restenotic late stent failure.48 A better understanding of this disease process as well as the development of targeted therapies to prevent its occurrence will likely have a significant impact on the late outcomes of our patients. Thirdly, there remain lesion and patient subtypes in need of improved interventional device options: these include stent implantation in the setting of acute myocardial infarction, bifurcation lesions, chronically occluded vessels, lesions with severe calcification, ISR, and lesions in patients with diabetes. For this reason, we need to ensure that safe and effective methods of evaluation of innovative coronary stent devices remain available to our patients.16 Finally, although recent developments focusing on strategies which circumvent the need for chronically indwelling stents—a so-called leave nothing behind approach—are promising, more data are needed before the wider use of these therapies can be advocated. In particular, though drug-coated balloon therapy seems effective for ISR,110 it remains unestablished for the treatment of de novo coronary disease. In addition, though treatment with fully bioresorbable stents is feasible and intuitively attractive, concerns related to unacceptable rates of early stent failure need to be addressed most likely through further iterative development. These issues and concerns notwithstanding, as we look back at the progress of the last decades, we must acknowledge that it has been a remarkable journey from the kitchen table of Andreas Grüntzig to the contemporary procedures and excellent outcomes available to our patients today. It remains a great pity that his premature death meant that he did not live to bear witness to these important developments.
Authors’ contributions
R.A.B., M.J., A.K.: handled funding and supervision, acquired the data, conceived and designed the research, drafted the manuscript, and made critical revision of the manuscript for key intellectual content.
Funding
This work was supported in part by the European Union Seventh Framework FP7/2007-2013 under grant agreement n° HEALTH-F2-2010-260309 (PRESTIGE) and funding from this source was used to provide open access publication.
Conflict of interest: R.A.B. reports receiving lecture fees from B. Braun Melsungen AG, Biotronik, and Boston Scientific. M.J. reports institutional research support from Abbott Vascular, BioSensors International, Biotronik, Boston Scientific, CeloNova, Medtronic, MicroPort, Stentys, OrbusNeich Medical, SINO Medical Technology, Terumo Corporation, and W.L. Gore. A.K. reports patent applications related to drug-eluting stent coatings.
References
- 1.Grüntzig A. Transluminal dilatation of coronary artery stenosis. The Lancet 1978;311:263-263. [DOI] [PubMed] [Google Scholar]
- 2.Meier B. The first patient to undergo coronary angioplasty – 23-year follow-up. N Engl J Med 2001;344:144–145. [DOI] [PubMed] [Google Scholar]
- 3.Monagan D. Journey into the Heart. USA: Gotham Books and Penguin; 2007. [Google Scholar]
- 4.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701–706. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, Belardi J, Sigwart U, Colombo A, Goy JJ, van den Heuvel P, Delcan J, Marie-angele Morel for the Benestent Study Group. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994;331:489–495. [DOI] [PubMed] [Google Scholar]
- 6.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, Cleman M, Heuser R, Almond D, Teirstein PS, Fish RD, Colombo A, Brinker J, Moses J, Shaknovich A, Hirshfeld J, Bailey S, Ellis S, Rake R, Goldberg S. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994;331:496–501. [DOI] [PubMed] [Google Scholar]
- 7.Serruys PW, Strauss BH, Beatt KJ, Bertrand ME, Puel J, Rickards AF, Meier B, Goy J-J, Vogt P, Kappenberger L, Sigwart U. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med 1991;324:13–17. [DOI] [PubMed] [Google Scholar]
- 8.Schomig A, Kastrati A, Mudra H, Blasini R, Schuhlen H, Klauss V, Richardt G, Neumann FJ. Four-year experience with Palmaz-Schatz stenting in coronary angioplasty complicated by dissection with threatened or present vessel closure. Circulation 1994;90:2716–2724. [DOI] [PubMed] [Google Scholar]
- 9.Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, Pocock SJ, Kuntz RE. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998;339:1665–1671. [DOI] [PubMed] [Google Scholar]
- 10.Schomig A, Neumann FJ, Kastrati A, Schuhlen H, Blasini R, Hadamitzky M, Walter H, Zitzmann-Roth EM, Richardt G, Alt E, Schmitt C, Ulm K. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996;334:1084–1089. [DOI] [PubMed] [Google Scholar]
- 11.Colombo A, Hall P, Nakamura S, Almagor Y, Maiello L, Martini G, Gaglione A, Goldberg SL, Tobis JM. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation 1995;91:1676–1688. [DOI] [PubMed] [Google Scholar]
- 12.Kastrati A, Schomig A, Dietz R, Neumann FJ, Richardt G. Time course of restenosis during the first year after emergency coronary stenting. Circulation 1993;87:1498–1505. [DOI] [PubMed] [Google Scholar]
- 13.Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation 1999;99:44–52. [DOI] [PubMed] [Google Scholar]
- 14.Wiebe J, Nef HM, Hamm CW. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol 2014;64:2541–2551. [DOI] [PubMed] [Google Scholar]
- 15.Stefanini GG, Taniwaki M, Windecker S. Coronary stents: novel developments. Heart 2014;100:1051–1061. [DOI] [PubMed] [Google Scholar]
- 16.Byrne RA, Serruys PW, Baumbach A, Escaned J, Fajadet J, James S, Joner M, Oktay S, Juni P, Kastrati A, Sianos G, Stefanini GG, Wijns W, Windecker S. Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J 2015;36:2608–2620. [DOI] [PubMed] [Google Scholar]
- 17.Windecker S, Stortecky S, Stefanini GG, da Costa BR, Rutjes AW, Di Nisio M, Silletta MG, Maione A, Alfonso F, Clemmensen PM, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head S, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter D, Schauerte P, Sousa Uva M, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Kolh P, Juni P. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ 2014;348:g3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, ten Berg JM, Adriaenssens T, Guagliumi G, Godschalk TC, Neumann F-J, Trenk D, Feldman LJ, Steg PG, Desmet W, Alfonso F, Goodall AH, Wojdyla R, Dudek D, Philippi V, Opinaldo S, Titova A, Malik N, Cotton J, Jhagroe DA, Heestermans AACM, Sinnaeve P, Vermeersch P, Valina C, Schulz C, Kastrati A, Massberg S. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study. Eur Heart J 2015; doi:10.1093/eurheartj/ehv419. Published online ahead of print 30 August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 20.Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, Mitsudo K, Miyazaki S, Yamaguchi T, Hiyoshi E, Nishimura E, Isshiki T, RESTART Investigators. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation 2010;122:52–61. [DOI] [PubMed] [Google Scholar]
- 21.van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009;53:1399–1409. [DOI] [PubMed] [Google Scholar]
- 22.Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 2013;6:1267–1274. [DOI] [PubMed] [Google Scholar]
- 23.Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, Wenaweser P, Daemen J, Meier B, Juni P, Serruys PW, Windecker S. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 2012;125:1110–1121. [DOI] [PubMed] [Google Scholar]
- 24.Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, Gurbel PA, Xu K, Parise H, Kirtane AJ, Brodie BR, Mehran R, Stuckey TD, ADAPT-DES Investigators. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 2013;382:614–623. [DOI] [PubMed] [Google Scholar]
- 25.Cayla G, Hulot JS, O'Connor SA, Pathak A, Scott SA, Gruel Y, Silvain J, Vignalou JB, Huerre Y, de la Briolle A, Allanic F, Beygui F, Barthelemy O, Montalescot G, Collet JP. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA 2011;306:1765–1774. [DOI] [PubMed] [Google Scholar]
- 26.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126–2130. [DOI] [PubMed] [Google Scholar]
- 27.Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, Schulz S, Mayer K, Bernlochner I, Schomig A, Kastrati A. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J 2011;32:1605–1613. [DOI] [PubMed] [Google Scholar]
- 28.Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dorrler K, Morath T, Schomig A, Kastrati A, von Beckerath N. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J 2009;30:916–922. [DOI] [PubMed] [Google Scholar]
- 29.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 2010;304:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schomig A, Kastrati A, von Beckerath N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol 2009;53:849–856. [DOI] [PubMed] [Google Scholar]
- 31.Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Muller U, Richardt G, Jakubowski JA, Neumann FJ. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity in Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy with Prasugrel) study. J Am Coll Cardiol 2012;59:2159–2164. [DOI] [PubMed] [Google Scholar]
- 32.Collet JP, Cuisset T, Range G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrie D, Boueri Z, Belle L, Van Belle E, Rousseau H, Aubry P, Monsegu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Barthelemy O, Beygui F, Silvain J, Vicaut E, Montalescot G, ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100–2109. [DOI] [PubMed] [Google Scholar]
- 33.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ, GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097–1105. [DOI] [PubMed] [Google Scholar]
- 34.Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, Menichelli M, Sabate M, Suttorp MJ, Baumgart D, Seyfarth M, Pfisterer ME, Schomig A. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007;356:1030–1039. [DOI] [PubMed] [Google Scholar]
- 35.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011;123:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Gomez-Hospital JA, Baz JA, Martin-Yuste V, van Geuns RJ, Alfonso F, Bordes P, Tebaldi M, Masotti M, Silvestro A, Backx B, Brugaletta S, van Es GA, Serruys PW. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012;380:1482–1490. [DOI] [PubMed] [Google Scholar]
- 37.Attizzani GF, Capodanno D, Ohno Y, Tamburino C. Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition. J Am Coll Cardiol 2014;63:1355–1367. [DOI] [PubMed] [Google Scholar]
- 38.Foin N, Gutierrez-Chico JL, Nakatani S, Torii R, Bourantas CV, Sen S, Nijjer S, Petraco R, Kousera C, Ghione M, Onuma Y, Garcia-Garcia HM, Francis DP, Wong P, Di Mario C, Davies JE, Serruys PW. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ Cardiovasc Interv 2014;7:180–189. [DOI] [PubMed] [Google Scholar]
- 39.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 2007;115:1440–1455; discussion 1455. [DOI] [PubMed] [Google Scholar]
- 40.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007;356:998–1008. [DOI] [PubMed] [Google Scholar]
- 41.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Juni P. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 2007;370:937–948. [DOI] [PubMed] [Google Scholar]
- 42.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 2007;369:667–678. [DOI] [PubMed] [Google Scholar]
- 43.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol 2007;27:1500–1510. [DOI] [PubMed] [Google Scholar]
- 44.Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol 2009;57:567–584. [PubMed] [Google Scholar]
- 45.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007;115:1051–1058. [DOI] [PubMed] [Google Scholar]
- 46.Byrne RA, Iijima R, Mehilli J, Pinieck S, Bruskina O, Schomig A, Kastrati A. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv 2009;2:291–299. [DOI] [PubMed] [Google Scholar]
- 47.Togni M, Raber L, Cocchia R, Wenaweser P, Cook S, Windecker S, Meier B, Hess OM. Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int J Cardiol 2007;120:212–220. [DOI] [PubMed] [Google Scholar]
- 48.Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 2015;36:2147–2159. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong EJ, Feldman DN, Wang TY, Kaltenbach LA, Yeo KK, Wong SC, Spertus J, Shaw RE, Minutello RM, Moussa I, Ho KK, Rogers JH, Shunk KA. Clinical presentation, management, and outcomes of angiographically documented early, late, and very late stent thrombosis. JACC Cardiovasc Interv 2012;5:131–140. [DOI] [PubMed] [Google Scholar]
- 50.Schulz S, Schuster T, Mehilli J, Byrne RA, Ellert J, Massberg S, Goedel J, Bruskina O, Ulm K, Schomig A, Kastrati A. Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J 2009;30:2714–2721. [DOI] [PubMed] [Google Scholar]
- 51.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315–1323. [DOI] [PubMed] [Google Scholar]
- 52.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773–1780. [DOI] [PubMed] [Google Scholar]
- 53.Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012;125:505–513. [DOI] [PubMed] [Google Scholar]
- 54.Schulz-Schüpke S, Byrne RA, ten Berg JM, Neumann FJ, Han Y, Adriaenssens T, Tölg R, Seyfarth M, Maeng M, Zrenner B, Jacobshagen C, Mudra M, von Hodenberg H, Wöhrle J, Angiolillo DJ, von Merzljak B, Rifatov N, Kufner S, Morath T, Feuchtenberger A, Ibrahim T, Janssen PW, Valina C, Li Y, Desmet W, Abdel-Wahab M, Tiroch K, Hengstenberg C, Bernlochner I, Fischer M, Schunkert H, Laugwitz K-L, Schömig A, Mehilli J, Kastrati A. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 versus 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015;36:1252–1263. [DOI] [PubMed] [Google Scholar]
- 55.Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB, III, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ, Jr, Nicolela EL, Jr, Perin MA, Devito FS, Labrunie A, Salvadori D, Jr, Gusmao M, Staico R, Costa JR, Jr, de Castro JP, Abizaid AS, Bhatt DL, OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013;310:2510–2522. [DOI] [PubMed] [Google Scholar]
- 56.Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, Lee SH, Yoon JH, Hong BK, Kwon HM, Jang Y, RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60:1340–1348. [DOI] [PubMed] [Google Scholar]
- 57.Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, Oteo Dominguez JF, Steffanon L, Tarantini G, Presbitero P, Menozzi A, Pucci E, Mauri J, Cesana BM, Giustino G, Sardella G. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY Randomized Clinical Trial. J Am Coll Cardiol 2014;64:2086–2097. [DOI] [PubMed] [Google Scholar]
- 58.Collet JP, Silvain J, Barthelemy O, Range G, Cayla G, Van Belle E, Cuisset T, Elhadad S, Schiele F, Lhoest N, Ohlmann P, Carrie D, Rousseau H, Aubry P, Monsegu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Beygui F, Vicaut E, Montalescot G, ARCTIC Investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-interruption): a randomised trial. Lancet 2014;384:1577–1585. [DOI] [PubMed] [Google Scholar]
- 59.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR, Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM, DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee CW, Ahn JM, Park DW, Kang SJ, Lee SW, Kim YH, Park SW, Han S, Lee SG, Seong IW, Rha SW, Jeong MH, Lim DS, Yoon JH, Hur SH, Choi YS, Yang JY, Lee NH, Kim HS, Lee BK, Kim KS, Lee SU, Chae JK, Cheong SS, Suh IW, Park HS, Nah DY, Jeon DS, Seung KB, Lee K, Jang JS, Park SJ. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014;129:304–312. [DOI] [PubMed] [Google Scholar]
- 61.Gilard M, Barragan P, Noryani AA, Noor HA, Majwal T, Hovasse T, Castellant P, Schneeberger M, Maillard L, Bressolette EE, Wojcik J, Delarche N, Blanchard D, Jouve B, Ormezzano O, Paganelli F, Levy G, Sainsous J, Carrie D, Furber A, Berland J, Darremont O, Le Breton H, Lyuycx-Bore A, Gommeaux A, Cassat C, Kermarrec A, Cazaux P, Druelles P, Dauphin R, Armengaud J, Dupouy P, Champagnac D, Ohlmann P, Endresen KK, Benamer H, Kiss RG, Ungi I, Boschat JJ, Morice MC. Six-month versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients non-resistant to aspirin: ITALIC, a randomized multicenter trial. J Am Coll Cardiol 2015;65:777–786. [DOI] [PubMed] [Google Scholar]
- 62.Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fuca G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R, Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study I. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125:2015–2026. [DOI] [PubMed] [Google Scholar]
- 63.Cassese S, Byrne RA, Tada T, King LA, Kastrati A. Clinical impact of extended dual antiplatelet therapy after percutaneous coronary interventions in the drug-eluting stent era: a meta-analysis of randomized trials. Eur Heart J 2012;33:3078–3087. [DOI] [PubMed] [Google Scholar]
- 64.Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ, Gershlick AH, Cutlip DE, Cohen DJ, Tanguay JF, Jacobs A, Wiviott SD, Massaro JM, Iancu AC, Mauri L, DAPT Study Investigators. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol 2015;65:2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassese S, Byrne RA, Ndrepepa G, Schunkert H, Fusaro M, Kastrati A. Prolonged dual antiplatelet therapy after drug-eluting stenting: meta-analysis of randomized trials. Clin Res Cardiol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 66.Charlot M, Nielsen LH, Lindhardsen J, Ahlehoff O, Olsen AM, Hansen ML, Hansen PR, Madsen JK, Kober L, Gislason GH, Torp-Pedersen C. Clopidogrel discontinuation after myocardial infarction and risk of thrombosis: a nationwide cohort study. Eur Heart J 2012;33:2527–2534. [DOI] [PubMed] [Google Scholar]
- 67.Sibbing D, Stegherr J, Braun S, Mehilli J, Schulz S, Seyfarth M, Kastrati A, von Beckerath N, Schomig A. A double-blind, randomized study on prevention and existence of a rebound phenomenon of platelets after cessation of clopidogrel treatment. J Am Coll Cardiol 2010;55:558–565. [DOI] [PubMed] [Google Scholar]
- 68.Fiedler KA, Mehilli J, Kufner S, Schlichting A, Ibrahim T, Sibbing D, Ott I, Schunkert H, Laugwitz KL, Kastrati A, Schulz S. Randomised, double-blind trial on the value of tapered discontinuation of clopidogrel maintenance therapy after drug-eluting stent implantation. Intracoronary Stenting and Antithrombotic Regimen: CAUTION in Discontinuing Clopidogrel Therapy – ISAR-CAUTION. Thromb Haemost 2014;111:1041–1049. [DOI] [PubMed] [Google Scholar]
- 69.Silber S, Kirtane AJ, Belardi JA, Liu M, Brar S, Rothman M, Windecker S. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimus-eluting stent implantation. Eur Heart J 2014;35:1949–1956. [DOI] [PubMed] [Google Scholar]
- 70.Genereux P, Rutledge DR, Palmerini T, Caixeta A, Kedhi E, Hermiller JB, Wang J, Krucoff MW, Jones-McMeans J, Sudhir K, Simonton CA, Serruys PW, Stone GW. Stent thrombosis and dual antiplatelet therapy interruption with everolimus-eluting stents: Insights from the Xience V Coronary Stent System Trials. Circ Cardiovasc Interv 2015;8:e001362. [DOI] [PubMed] [Google Scholar]
- 71.Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, Berger PB, Iakovou I, Dangas G, Waksman R, Antoniucci D, Sartori S, Krucoff MW, Hermiller JB, Shawl F, Gibson CM, Chieffo A, Alu M, Moliterno DJ, Colombo A, Pocock S. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013;382:1714–1722. [DOI] [PubMed] [Google Scholar]
- 72.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van 't Hof AW, ten Berg JM, WOEST Study Investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
- 73.Fiedler KA, Maeng M, Mehilli J, Schulz-Schupke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, Kristensen SD, Ibrahim T, Massberg S, Schunkert H, Laugwitz KL, Kastrati A, Sarafoff N. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J Am Coll Cardiol 2015;65:1619–1629. [DOI] [PubMed] [Google Scholar]
- 74.Capodanno D, Lip GY, Windecker S, Huber K, Kirchhof P, Boriani G, Lane D, Gilard M, Collet JP, Valgimigli M, Byrne RA. Triple antithrombotic therapy in atrial fibrillation patients with acute coronary syndromes or undergoing percutaneous coronary intervention or transcatheter aortic valve replacement. EuroIntervention 2015;10:1015–1021. [DOI] [PubMed] [Google Scholar]
- 75.Keating GM. Cangrelor: a review in percutaneous coronary intervention. Drugs 2015;75:1425–1434. [DOI] [PubMed] [Google Scholar]
- 76.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation 2005;111:2257–2273. [DOI] [PubMed] [Google Scholar]
- 77.Byrne RA, Joner M, Alfonso F, Kastrati A. Treatment of in-stent restenosis. In: Bhatt DL, (ed.). Interventional Cardiology: A Companion to Braunwald‘s Heart Disease. Elsevier; 2015. [Google Scholar]
- 78.Byrne RA, Joner M, Tada T, Kastrati A. Restenosis in bare metal and drug-eluting stents: distinct mechanistic insights from histopathology and optical intravascular imaging. Minerva Cardioangiol 2012;60:473–489. [PubMed] [Google Scholar]
- 79.Nakano M, Otsuka F, Yahagi K, Sakakura K, Kutys R, Ladich ER, Finn AV, Kolodgie FD, Virmani R. Human autopsy study of drug-eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J 2013;34:3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonzalo N, Serruys PW, Okamura T, van Beusekom HM, Garcia-Garcia HM, van Soest G, van der Giessen W, Regar E. Optical coherence tomography patterns of stent restenosis. Am Heart J 2009;158:284–293. [DOI] [PubMed] [Google Scholar]
- 81.Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014;100:153–159. [DOI] [PubMed] [Google Scholar]
- 82.Raungaard B, Jensen LO, Tilsted HH, Christiansen EH, Maeng M, Terkelsen CJ, Krusell LR, Kaltoft A, Kristensen SD, Botker HE, Thuesen L, Aaroe J, Jensen SE, Villadsen AB, Thayssen P, Veien KT, Hansen KN, Junker A, Madsen M, Ravkilde J, Lassen JF, Scandinavian Organization for Randomized Trials with Clinical Outcome (SORT OUT). Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non-inferiority trial. Lancet 2015;385:1527–1535. [DOI] [PubMed] [Google Scholar]
- 83.Kimura T, Yokoi H, Nakagawa Y, Tamura T, Kaburagi S, Sawada Y, Sato Y, Yokoi H, Hamasaki N, Nosaka H, Nobuyoshi M. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med 1996;334:561–566. [DOI] [PubMed] [Google Scholar]
- 84.Kimura T, Abe K, Shizuta S, Odashiro K, Yoshida Y, Sakai K, Kaitani K, Inoue K, Nakagawa Y, Yokoi H, Iwabuchi M, Hamasaki N, Nosaka H, Nobuyoshi M. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation 2002;105:2986–2991. [DOI] [PubMed] [Google Scholar]
- 85.Yamaji K, Kimura T, Morimoto T, Nakagawa Y, Inoue K, Soga Y, Arita T, Shirai S, Ando K, Kondo K, Sakai K, Goya M, Iwabuchi M, Yokoi H, Nosaka H, Nobuyoshi M. Very long-term (15 to 20 years) clinical and angiographic outcome after coronary bare metal stent implantation. Circ Cardiovasc Interv 2010;3:468–475. [DOI] [PubMed] [Google Scholar]
- 86.Raber L, Wohlwend L, Wigger M, Togni M, Wandel S, Wenaweser P, Cook S, Moschovitis A, Vogel R, Kalesan B, Seiler C, Eberli F, Luscher TF, Meier B, Juni P, Windecker S. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the sirolimus-eluting versus paclitaxel-eluting stents for coronary revascularization LATE trial. Circulation 2011;123:2819–2828. [DOI] [PubMed] [Google Scholar]
- 87.Cassese S, Byrne RA, Schulz S, Hoppman P, Kreutzer J, Feuchtenberger A, Ibrahim T, Ott I, Fusaro M, Schunkert H, Laugwitz KL, Kastrati A. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J 2015;36:94–99. [DOI] [PubMed] [Google Scholar]
- 88.Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014;100:153–159. [DOI] [PubMed] [Google Scholar]
- 89.Rathore S, Terashima M, Katoh O, Matsuo H, Tanaka N, Kinoshita Y, Kimura M, Tuschikane E, Nasu K, Ehara M, Asakura K, Asakura Y, Suzuki T. Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: contemporary practice in real world patients. EuroIntervention 2009;5:349–354. [DOI] [PubMed] [Google Scholar]
- 90.Kastrati A, Dibra A, Mehilli J, Mayer S, Pinieck S, Pache J, Dirschinger J, Schomig A. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation 2006;113:2293–2300. [DOI] [PubMed] [Google Scholar]
- 91.Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C, Muller M, Dirschinger J, Schomig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol 2003;41:1283–1288. [DOI] [PubMed] [Google Scholar]
- 92.Guerra E, Byrne RA, Kastrati A. Pharmacological inhibition of coronary restenosis: systemic and local approaches. Expert Opin Pharmacother 2014;15:2155–2171. [DOI] [PubMed] [Google Scholar]
- 93.Dibra A, Mehilli J, Braun S, Hadamitzky M, Baum H, Dirschinger J, Schuhlen H, Schomig A, Kastrati A. Inflammatory response after intervention assessed by serial C-reactive protein measurements correlates with restenosis in patients treated with coronary stenting. Am Heart J 2005;150:344–350. [DOI] [PubMed] [Google Scholar]
- 94.Park DW, Yun SC, Lee JY, Kim WJ, Kang SJ, Lee SW, Kim YH, Lee CW, Kim JJ, Park SW, Park SJ. C-reactive protein and the risk of stent thrombosis and cardiovascular events after drug-eluting stent implantation. Circulation 2009;120:1987–1995. [DOI] [PubMed] [Google Scholar]
- 95.Iijima R, Byrne RA, Ndrepepa G, Braun S, Mehilli J, Berger PB, Schomig A, Kastrati A. Pre-procedural C-reactive protein levels and clinical outcomes after percutaneous coronary interventions with and without abciximab: pooled analysis of four ISAR trials. Heart 2009;95:107–112. [DOI] [PubMed] [Google Scholar]
- 96.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J 2006;151:1260–1264. [DOI] [PubMed] [Google Scholar]
- 97.Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol 2014;63:2659–2673. [DOI] [PubMed] [Google Scholar]
- 98.Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovasc Imaging 2009;2:625–628. [DOI] [PubMed] [Google Scholar]
- 99.Malle C, Tada T, Steigerwald K, Ughi GJ, Schuster T, Nakano M, Massberg S, Jehle J, Guagliumi G, Kastrati A, Virmani R, Byrne RA, Joner M. Tissue characterization after drug-eluting stent implantation using optical coherence tomography. Arterioscler Thromb Vasc Biol 2013;33:1376–1383. [DOI] [PubMed] [Google Scholar]
- 100.Kang SJ, Lee CW, Song H, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, Mintz GS, Park SW, Park SJ. OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc Imaging 2013;6:695–703. [DOI] [PubMed] [Google Scholar]
- 101.Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, Lee SW, Kim YH, Whan Lee C, Park SW, Park SJ. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation 2011;123:2954–2963. [DOI] [PubMed] [Google Scholar]
- 102.Yonetsu T, Kato K, Kim SJ, Xing L, Jia H, McNulty I, Lee H, Zhang S, Uemura S, Jang Y, Kang SJ, Park SJ, Lee S, Yu B, Kakuta T, Jang IK. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging 2012;5:660–666. [DOI] [PubMed] [Google Scholar]
- 103.Serruys PW, Chevalier B, Dudek D, Cequier A, Carrie D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L, Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2014;385:43–54. [DOI] [PubMed] [Google Scholar]
- 104.Capodanno D, Gori T, Nef H, Latib A, Mehilli J, Lesiak M, Caramanno G, Naber C, Di Mario C, Colombo A, Capranzano P, Wiebe J, Araszkiewicz A, Geraci S, Pyxaras S, Mattesini A, Naganuma T, Munzel T, Tamburino C. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015;10:1144–1153. [DOI] [PubMed] [Google Scholar]
- 105.Kraak RP, Hassell ME, Grundeken MJ, Koch KT, Henriques JP, Piek JJ, Baan J, Jr, Vis MM, Arkenbout EK, Tijssen JG, de Winter RJ, Wykrzykowska JJ. Initial experience and clinical evaluation of the absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention 2015;10:1160–1168. [DOI] [PubMed] [Google Scholar]
- 106.Jaguszewski M, Ghadri JR, Zipponi M, Bataiosu DR, Diekmann J, Geyer V, Neumann CA, Huber MA, Hagl C, Erne P, Luscher TF, Templin C. Feasibility of second-generation bioresorbable vascular scaffold implantation in complex anatomical and clinical scenarios. Clin Res Cardiol 2015;104:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Byrne RA, Kastrati A. Bioresorbable drug-eluting stents: an immature technology in need of mature application. JACC Cardiovasc Interv 2015;8:198–200. [DOI] [PubMed] [Google Scholar]
- 108.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 109.Petrides F, Shearston K, Chatelais M, Guilbaud F, Meilhac O, Lambert G. The promises of PCSK9 inhibition. Curr Opin Lipidol 2013;24:307–312. [DOI] [PubMed] [Google Scholar]
- 110.Byrne RA, Joner M, Alfonso F, Kastrati A. Drug-coated balloon therapy in coronary and peripheral artery disease. Nat Rev Cardiol 2014;11:13–23. [DOI] [PubMed] [Google Scholar]
- 111.Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 2011;57:1314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014;129:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]