Abstract
Emerging evidences suggest that GSTM1 and GSTT1 are involved in the detoxification of carcinogens, and polymorphisms in this gene that result in a loss of enzyme activity may increase the risk of renal cell carcinoma (RCC). Thus, to evaluate the association of GSTM1 and GSTT1 polymorphisms and RCC, we performed an updated meta-analysis of 10 case-control studies by RevMan 5.2, and the publication bias was tested using STATA 11.0. The meta-analysis showed that the single locus GSTM1 and GSTT1 polymorphisms were not significantly associated with a risk of RCC in a recessive model. However, that wild-type genotype versus the dual null genotype of GSTM1-GSTT1 showed a positive association with RCC risk (OR = 0.70; 95% CI = 0.51–0.98; P = 0.04). In another analysis of subjects exposed to pesticides, we found that the GSTM1 wild-type genotype was associated with increased RCC risk in Europeans (OR = 2.72; 95% CI = 1.54–4.82; P = 0.0006). We also identified an association between the GSTT1 wild-type and lower RCC TNM staging (I + II versus III + IV: OR = 1.88; 95% CI = 1.09–3.26; P = 0.02). This meta-analysis suggests that there may be a relationship between the GSTM1 and GSTT1 wild-type genotype and RCC.
Renal cell carcinoma (RCC) is the predominant form of kidney malignancy (approximately 90% of all cases) and one of the leading causes of global cancer death. Approximately 300,000 people were diagnosed with kidney cancer in 2008, and 100,000 people died from the disease1. An estimated 63,920 Americans will be diagnosed with renal cancer and 13,860 will die of the disease in the United States in 20142. The morbidity and mortality rates are approximately twice as high for males as for females. The incidence of RCC is increasing worldwide, with the highest incidence occurring in developed countries, especially in Europe, North America, and Australia3,4.
It is well-known that exposure to potential carcinogens is an etiologic factor for RCC5. Glutathione S-transferases (GSTs) are a superfamily of enzymes that are subdivided into 7 classes (α, μ, ω, π, σ, θ, ξ)6, and are known to protect cells by catalyzing the detoxification of electrophilic compounds, including exogenous products (carcinogens, therapeutic drugs, environmental toxins) and endogenous oxidative products, through conjugation with glutathione7. This conjugation reaction is an important step of detoxification and facilitates their excretion from the body, thereby decreasing the associated toxicity. Considering the damage to DNA induced by electrophilic compounds, GSTs are important for maintaining genomic integrity. Therefore, GST enzymes may potentially play a key role in the development of RCC.
GSTM1 and GSTT1 are the most frequently studied enzymes in the GST family. The GSTM1 gene encodes the GST-μ1 enzyme and has been mapped to chromosome 1p13.3. There are two variants of GSTM1 that can occur through a substitution and a deletion, respectively. The substitution polymorphism changes C-to-G at base position 534, resulting in a lysine-to-asparagine switch, which does not appear to affect enzyme function. The deletion variant is a null genotype of GSTM1, which is more commonly studied than the substitution variant because it leads to the absence of GST-μ1 synthesis6. In this article, we only studied the deletion variant of GSTM1. The GSTT gene encodes the GST-θ subfamily, which is located at chromosome 22q11.2 and consists of two genes, GSTT1 and GSTT2. The duplicated GSTT2 is a pseudogene (named GSTT2P). The GSTT1 gene is embedded in a region with extensive homologies and flanked by two 18 kb regions, which were identified as deletion/junction regions of the GSTT1 null allele8. Moreover, the deletion variant of GSTT1 is a null genotype, which occurs less frequently and results in complete absence of the enzyme.
To date, a limited number of molecular epidemiological studies have investigated the association between the GSTM1 and GSTT1 polymorphisms and RCC, and the conclusions have not been consistent9,10,11,12,13,14,15,16,17,18. A single case-control study may fail to demonstrate this complicated genetic relationship due to the small sample size, and thus a meta-analysis could increase the statistical power for detecting overall effects. Recently, a few meta-analyses19,20,21 have attempted to uncover the relationship between the GSTM1 and GSTT1 polymorphisms and RCC. However, Liu et al.20 did not investigate the relationship between GSTT1 polymorphisms and RCC and the relationship between the combination of GSTM1 and GSTT1 polymorphisms and RCC. Cheng et al.19 also studied the association between GSTM1 and GSTT1 polymorphisms and RCC, but the data extracted from the studies by Longuemaux et al. and De Martino et al. were controversial and the data from the studies by Salinas et al. and Buzio et al. were not included. In addition, Yang et al.21 incorrectly extracted duplicate genetic information from the studies by Wiesenhütter and Brüning, as the RCC case and control groups in Wiesenhütter et al. were comprised of case and control groups from Brüning et al. In this study, we conducted an updated meta-analysis on all currently available to validate the further relationship between the GSTM1 and GSTT1 polymorphisms and RCC.
Results
Description of studies
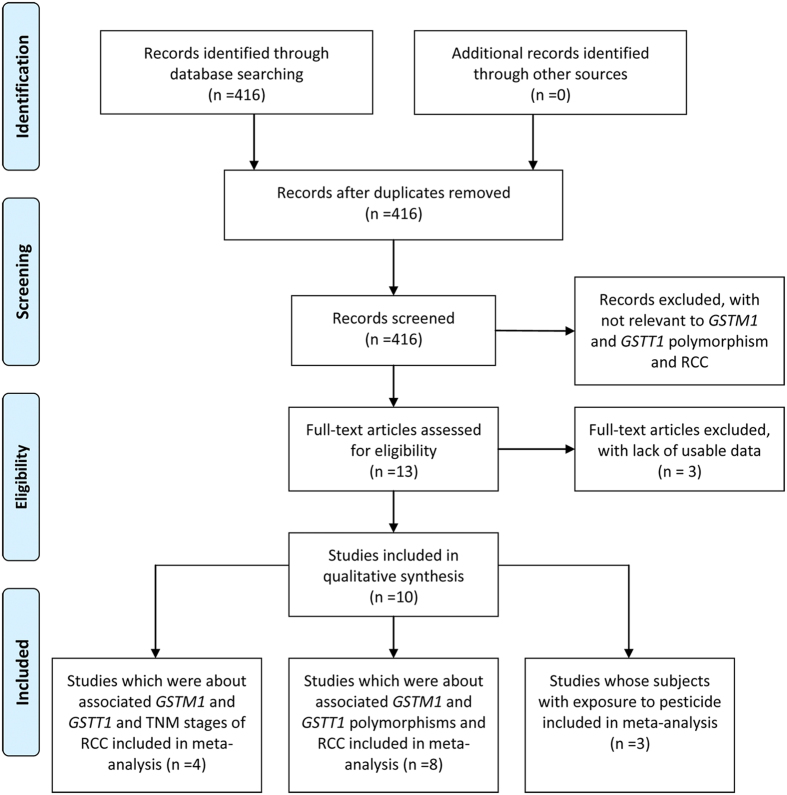
A total of 416 articles were identified through a literature search in PubMed, ISI, Wangfang, and CNKI databases. Ten eligible studies were retrieved for detailed evaluation (Fig. 1). We included eight studies that described an association between GSTM1 and RCC by comparing RCC with healthy controls, which included 1826 cases and 3377 controls. In addition, there were 1831 cases and 3407 controls in the same eight studies regarding GSTT1 and RCC (Table 1). Five of the studies were conducted in Europe, and three studies were conducted in America, Australia, and India, respectively. Four of the eight studies described an association between the dual null genotype of GSTM1 and GSTT1 and risk of RCC, which included 1307 cases and 2057 controls. Moreover, three studies, all conducted in Europe, assessed the association between GSTM1 or GSTT1 and RCC in patients exposed to pesticide or trichloroethene, which both included 107 cases and 101 controls (Table 2). Three studies (2 in Europe, 1 in India) explored the association between GSTM1 and RCC staging, including 224 GSTM1 wild-type patients and 247 GSTM1 null patients. In addition, four studies (3 in Europe, 1 in India) explored the association between GSTT1 and RCC staging, including 335 GSTT1 wild-type patients and 251 GSTT1 null patients (Table 3). The GSTM1 and GSTT1 polymorphisms were detected by PCR in all of the studies.
Figure 1. Flow diagram of study identification.
Table 1. Main characteristics of studies on GSTM1 and GSTT1 polymorphisms and RCC included in the meta-analysis.
| Study | Year | Country | Sample size | GSTM1 |
GSTT1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
Sample size | Case |
Control |
||||||||
| (case/control) | Present | Null | Present | Null | (case/control) | Present | Null | Present | Null | |||
| Ahmad | 2012 | India | 196/250 | 94 | 102 | 134 | 116 | 196/250 | 71 | 125 | 144 | 106 |
| Salinas | 2011 | Spain | 133/193 | 76 | 57 | 115 | 78 | 132/163 | 110 | 22 | 138 | 25 |
| De Martino | 2010 | Austria | 147/112 | 67 | 80 | 53 | 59 | 147/112 | 120 | 27 | 89 | 23 |
| Wiesenhütter | 2007 | German | 98/770 | 47 | 51 | 383 | 358 | 98/768 | 79 | 19 | 624 | 144 |
| Moore | 2007 | Central and Eastern European | 925/1247 | 431 | 424 | 611 | 555 | 861/1199 | 694 | 167 | 990 | 209 |
| Buzio | 2003 | Italy | 100/200 | 50 | 50 | 92 | 108 | 100/200 | 89 | 11 | 165 | 35 |
| Sweeney | 2000 | American | 126/505 | 63 | 63 | 250 | 255 | 126/505 | 90 | 36 | 411 | 94 |
| Longuemaux | 1999 | French | 173/211 | 83 | 88 | 94 | 116 | 171/210 | 146 | 25 | 170 | 40 |
Table 2. Main characteristics of studies under the conditions of all subjects with exposure to pesticides included in the meta-analysis.
| Study | Year | Country | Sample size(case/control) | GSTM1 |
GSTT1 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
Case |
Control |
||||||||
| Present | Null | Present | Null | Present | Null | Present | Null | ||||
| Karami | 2008 | Central and Eastern European | 36/23 | 19 | 17 | 6 | 17 | 28 | 8 | 15 | 8 |
| Buzio | 2003 | Italy | 26/30 | 15 | 11 | 11 | 19 | 20 | 6 | 24 | 6 |
| Brüning | 1997 | German | 45/48 | 27 | 18 | 17 | 31 | 42 | 3 | 37 | 11 |
Table 3. Main characteristics of studies on GSTM1 and GSTT1 polymorphisms and clinical TNM stages of RCC included in the meta-analysis.
| Study | GSTM1 |
GSTT1 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I |
II |
III + IV |
I |
II |
III + IV |
|||||||
| Present | Null | Present | Null | Present | Null | Present | Null | Present | Null | Present | Null | |
| Ahmad | 48 | 22 | 29 | 31 | 17 | 49 | 37 | 33 | 21 | 39 | 13 | 53 |
| De Martino | 27 | 44 | 2 | 1 | 38 | 35 | 60 | 11 | 2 | 1 | 58 | 15 |
| Sweeney | 37 | 35 | 18 | 15 | 8 | 15 | 51 | 21 | 25 | 8 | 17 | 6 |
| Salinas | 29 | 30 | 11 | 9 | 11 | 25 | ||||||
Meta-analysis results
Association between GSTM1 and GSTT1 polymorphisms and RCC risk9,10,11,12,13,14,15,16
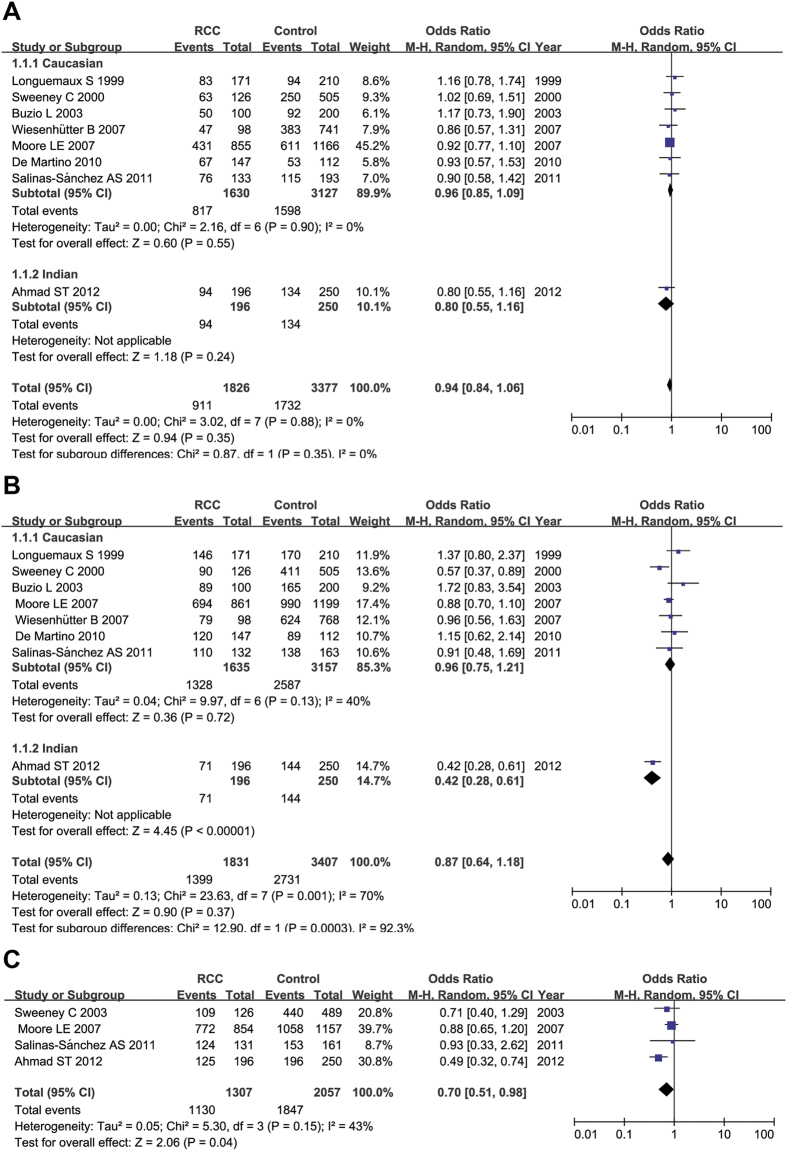
The results of the meta-analysis did not find any association between GSTM1 polymorphisms and RCC susceptibility for the recessive model (for present/present + present/null versus null/null: OR = 0.94, 95% CI = 0.84–1.06; P = 0.35; I2 = 0%, 95% CI = 0%–45%, τ2 = 0.00). After excluding the study conducted by Ahmad et al. because it did not focus on Caucasians, the results of the meta-analysis also did not indicate any association between the GSTM1 polymorphism and RCC susceptibility for the recessive model in Caucasians (for present/present + present/null versus null/null: OR = 0.96, 95%CI = 0.85–1.09; P = 0.55; I2 = 0%, 95% CI = 0%–45%, τ2 = 0.00) (Fig. 2A). We also did not find any association between the GSTT1 polymorphism and RCC susceptibility for the recessive model (for present/present+ present/null versus null/null: OR = 0.87, 95% CI = 0.64–1.18; P = 0.37; I2 = 70%, 95% CI = 38%–86%, τ2 = 0.13). After excluding Ahmad et al., the results of the meta-analysis also did not show any association between GSTT1 polymorphisms and RCC susceptibility for the recessive model in Caucasians (for present/present + present/null versus null/null: OR = 0.96, 95% CI = 0.75–1.21; P = 0.72; I2 = 40%, 95% CI = 0%–75%, τ2 = 0.04) (Fig. 2B). However, the association between the combination of GSTM1 and GSTT1 polymorphisms and risk of RCC for the recessive model (for GSTM1(present)/GSTT1(present) + GSTM1(present)/GSTT1(null) GSTM1(null)/GSTT1(present) versus GSTM1(null)/GSTT1(null): OR = 0.70; 95 %CI = 0.51–0.98; P = 0.04; I2 = 43%, 95% CI = 0–81%, τ2 = 0.05) was statistically significant (Fig. 2C).
Figure 2. Forest plots describing the association of GSTM1 and GSTT1 wild-type genotype with RCC risk.
(A) The association of GSTM1 wild-type genotype with RCC risk. (B) The association of GSTT1 wild-type genotype with RCC risk. (C) The association of combination of GSTM1 and GSTT1 polymorphism with RCC. OR: odds ratio; CI: confidence interval; I2, measure to quantify the degree of heterogeneity in meta-analysis.
Association between GSTM1 and GSTT1 polymorphisms and RCC risk of subjects exposed to occupational pesticides16,17,18
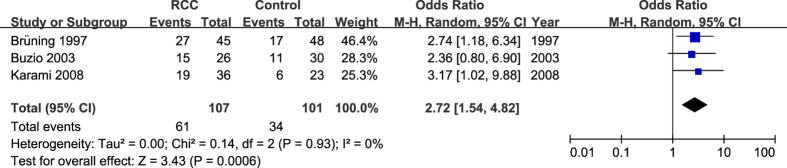
In studies that assessed subjects exposed to pesticides, we found that GSTM1 wild-type was significantly associated with increased RCC risk (present/present+ present/null versus null/null: OR = 2.72, 95% CI = 1.54–4.82; P = 0.0006; I2 = 0%, 95%CI = 0–89%, τ2 = 0.00) (Fig. 3). However, we did not find an association between GSTT1 wild-type and RCC (present/present + present/null versus null/null: OR = 1.83, 95% CI = 0.76–4.38; P = 0.18; I2 = 31%, 95% CI = 0–76%,τ2 = 0.18).
Figure 3. Forest plots describing the association of GSTM1 wild-type genotype with RCC under the condition of all subjects with exposure to pesticides.
OR: odds ratio; CI: confidence interval; I2, measure to quantify the degree of heterogeneity in meta-analysis.
Association between GSTM1 and GSTT1 polymorphisms and clinical TNM stages of RCC11,12,13,14
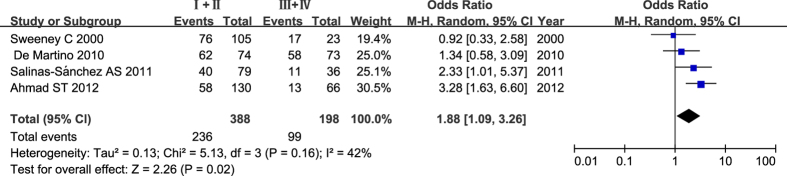
We performed a quantitative assessment of OR of wild-type and null genotype frequency in each TNM stage from the included studies, which assessed the association between GSTM1 and GSTT1 polymorphisms and TNM stages of RCC. The results did not show any significant difference for GSTM1 wild-type (recessive model in stage I versus stage II + III + IV: OR = 1.24, 95% CI = 0.63–2.43; P = 0.54; I2 = 76%, 95% CI = 19–92%, τ2 = 0.27; recessive model in stage I + II versus stage III + IV: OR = 1.71, 95% CI = 0.49–6.01; P = 0.40; I2 = 89%, 95% CI = 68–96%, τ2 = 1.08). However, subjects with GSTT1 wild-type showed an association with lower clinical TNM stages of RCC (recessive model in stage I versus stage II + III + IV: OR = 1.58, 95% CI = 0.89–2.79; P = 0.12; I2 = 58%, 95% CI = 0%-86%, τ2 = 0.19; recessive model in stage I + II versus stage III + + IV: OR = 1.88, 95% CI = 1.09–3.26; P = 0.02; I2 = 42%, 95% CI = 0–80%, τ2 = 0.13) (Fig. 4).
Figure 4. Forest plots describing the association of GSTT1 wild-type genotype with RCC TNM stages (recessive model in stage I + II versus stage III + IV).
OR: odds ratio; CI: confidence interval; I2, measure to quantify the degree of heterogeneity in meta-analysis.
Publication bias
The Begg’s rank correlation method and Egger’s weighted regression method were used to assess publication bias. Because of the number of studies included in the analysis, we only assessed publication bias for the association between GSTM1 and RCC risk (Begg’s test: P = 0.322; Egger’s test: P = 0.536), and GSTT1 and RCC (Begg’s test: P = 0.138; Egger’s test: P = 0.490). No evidence of publication bias was observed in our meta-analysis of the eight studies (Fig. 5).
Figure 5. Publication bias of GSTM1 and GSTT1 polymorphism and RCC risk.
(A) Begg’s publication bias of GSTM1 polymorphism and RCC risk (P = 0.322). (B) Egger’s publication bias of GSTM1 polymorphism and RCC risk (P = 0.536). (C) Begg’s publication bias of GSTT1 polymorphism and RCC risk (P = 0.138). (D) Egger’s publication bias of GSTT1 polymorphism and RCC risk (P = 0.490).
Discussion
The identification of SNPs that affect gene function or expression and contribute to RCC susceptibility play a critical role in helping predict individual and population risk and understanding the pathology of RCC. Deletion variants have been identified in the GSTM1 and GSTT1, which are enzymes that play important roles in phase II detoxification and for maintaining genomic integrity. The GSTM1 and GSTT1 null genotype have been hypothesized to be associated with increased risk for RCC due to reduced protection against endogenous reactive oxidants. The GSTM1 and GSTT1 null polymorphism are associated with lung cancer22, prostate cancer23, hepatocellular carcinoma24, and head and neck squamous cell carcinoma25. However, there are controversies in the relationship between the GSTM1 and GSTT1 polymorphisms and RCC susceptibility. In 1999, Longuemaux et al. first investigated the correlation between the GSTM1 null polymorphisms and RCC, but his and followed studies did not identify a significant correlation9,10,11. With respect to the GSTT1 genotype, Sweeney et al. and Ahmad et al. found it to be associated with an increased risk of RCC, but other studies did not reach the same conclusion. In this meta-analysis, we failed to identify an association between the single locus GSTM1 and GSTT1 polymorphisms and increased risk of RCC. However, with regard to the dual null genotype of GSTM1 and GSTT1, we found this genotype to be significantly associated with an increased risk of RCC. This finding indicates that the protective effects provided by only one of the GSTM1 and GSTT1 wild-type genes are minor, but cumulative. However, when both GSTM1 and GSTT1 genes are deleted, there would be significant loss of detoxification and increased risk of RCC.
In our meta-analysis of the association between the GSTM1 and GSTT1 wild-type genotype and RCC risk of subjects exposed to pesticides, we included a history of trichloroethene exposure, because it is widely used in industrial and agriculture processes, especially in the menstruum of pesticides. In contrast to previous results, we found that subjects exposed to occupational pesticides with the GSTM1 wild-type genotype are significantly associated with an increased risk of RCC. However, the GSTT1 wild-type genotype did not have a significant relationship with RCC risk. These results support the hypothesis that GSTM1 might play a role in the interaction with environmental factors, especially occupational exposure to toxic compounds. However, because these results conflict with previous findings, the role of GSTM1 in RCC remains unclear at this time.
Fortunately, some current studies have provided valuable clues that could help us decipher this puzzle. The biological mechanism of our findings may involve unique biochemical processes in the tubular renal epithelium. Renal tissue has a high metabolic activity and oxygen demand, leading to enhanced exogenous toxic or mutagenic metabolites and endogenous formations of reactive oxidants metabolites. Generally speaking, the GSTM1 enzyme catalyzes the binding of these metabolites to glutathione and the subsequent conversion into mercapturic acids, making their excretion in urine or bile easier in GSTM1 wild-type genotype subjects. However, halogenated compounds, such as trichloroethene or some pesticides, enter the mercapturic acid metabolism pathway, which are subsequently cleaved to S-(1, 2-dichlorovinyl)-L-cysteine. In the renal tubular epithelium, this compound is enriched and creates chlorothioketenes catalyzed by the renal cysteine conjugate β-lyase26. Even worse, chlorothioketenes are highly reactive and can form adducts with protein and DNA27. Therefore, an active GSTM1 enzyme forms more reactive intermediate chlorothioketenes, which can directly damage renal cells. Moreover, recent data suggests that the GSTM1 wild-type genotype is associated with an increased risk of RCC due to involvement with the ASK1 signal transduction pathway28,29. ASK1 is a mitogen activated protein kinase (MAP3K5) that is auto-phosphorylated and activates downstream kinases, such as p38. When cells are exposed to stress, such as heat shock or reactive oxidants, ASK1 activates p38, which leads to the induction of apoptosis28,29. GSTM1 may block ASK1 by binding to ASK1 and forming a complex with the enzyme to inhibit its activity. Patients with the GSTM1 wild-type genotype therefore may possess reduced activity of ASK1, resulting in increased risk of RCC when exposed to pollutants due to a decreased apoptotic activity response to cellular damage. Therefore, taken together, these studies provide a possible explanation for the selective nephrotoxicity and nephrocarcinogenicity of trichloroethene and pesticides in GSTM1 wild-type subjects.
Whether the GSTM1 and GSTT1 polymorphisms have modified the risk of invasiveness and malignancy of RCC was also unclear until recently. A study conducted by Ahmad et al. found that the GSTM1 null polymorphism might be a vital determinant of the advancement of RCC to higher clinical TNM stages11. In addition, Ahmad et al. and Salinas et al. found that patients with the GSTT1 null genotype had more advanced TNM stages11,14. Therefore, we performed a meta-analysis of these studies to investigate the association between GSTM1 and GSTT1 polymorphisms and clinical TNM stages and Fuhrman grades of RCC. As a result, we did not find a significant association between the GSTM1 null genotype and clinical TNM stages and Fuhrman grades of RCC (TNM stage I versus stage II + III + IV, stage I + II versus stage III + IV; Fuhrman grade 1 versus grade 2+3+4 and grade 1+2 versus grade 3+4). However, the GSTT1 null genotype showed an association with more advanced clinical TNM stages and Fuhrman grades in RCC patients (TNM stage I + II versus stage III + IV and Fuhrman grade 1 versus grade 2+3+4). These results indicate that GSTT1 wild-type genotype may be associated with tumors that exhibit a less invasive biological behavior and malignancy. In addition, De Martino et al. reported that the GSTM1 null genotype was associated with a lower Fuhrman grade and a higher survival rate in RCC patients, while the study by Ahmad et al. found GSTT1 null genotype was associated with a higher Fuhrman grade11,12. Due to the small sample size of both studies, the association between the GSTM1 or GSTT1 deletion variant and histological grades and survival rate of RCC remains unclear.
Several limitations should be considered in interpreting the current findings. First, reporting bias is a key threat to meta-analysis and literature-based reviews. Although publication bias was not found by funnel plots, due to small size meta-analysis, we cannot rule out the possibility that publication bias was undetected30. Second, even though I2 and τ2 value are very low which commonly consider as statistically acceptable, these results should be viewed with caution, since there may be still some undetected heterogeneity31. And, random-effects Mantel-Haenszel method for binary variable we used in the present study is hybrid and the weighting is an inverse variance but not strictly Mantel-Haenszel. Third, we didn’t conduct stratified analysis according to the publish year of included studies. It might be a bias in this meta-analysis. Fourth, the high level of environmental chemicals may accentuate or minimize the effect of the GSTM1 and GSTT1 wild-type genotype in oncogenesis. The interactions between genes and between genes and the environment may influence the susceptibility of RCC by more than one gene. Therefore, large-scale case control studies using microarrays, such as current genome-wide association studies (GWAS), will help to unveil potential interactions and susceptible mutations. Fifth, the sample size of studies included in our meta-analysis was not sufficiently large for a comprehensive analysis. And, nearly all of the studies were performed in Caucasian population, and additional studies are needed of other ethnic groups. Sixth, if the detail information of the included studies at an individual level were available, individual patient data meta-analysis may provide more convincing evidence.
In conclusion, our meta-analysis suggests there is an association between GSTM1 and GSTT1 polymorphisms and RCC. However, due to the restriction of sample size, large and well-designed studies are needed to re-evaluate these associations, especially in non-Europeans.
Methods
Publication search
We searched the PubMed, Institute for Scientific Information (ISI), Wangfang, Google Scholar, and Chinese National Knowledge Infrastructure (CNKI) databases for all articles describing an association between the GSTM1 and GSTT1 polymorphisms and RCC risk to December 2014. The key words were “GSTM1”, “GSTT1”, “renal cancer/renal cell carcinoma”, and “polymorphism/variant”. The electronic search was supplemented by checking reference lists. All of the original studies had to meet the following criteria: (1) case-control design; (2) provide sufficient data for estimating an odds ratio (OR) with a 95% confidence interval (95% CI); (3) if multiple studies were encountered that had data that overlapped by the same researchers, the most reliable studies with recently published and largest number of participants were chosen.
Data extraction
Two of the authors (Huang and Shi) extracted all data independently following the selection criteria. The following details were collected from each study: first author’s name, publication year, country of study, ethnicity, number of cases and controls, genotyping method, and OR.
Statistical analysis
The OR with 95% CI was used to assess the association between the GSTM1 and GSTT1 polymorphisms and RCC based on the genotype frequencies in cases and controls. We collected data and performed a meta-analysis of three assessments: (1) the association between the GSTM1 and GSTT1 polymorphisms and RCC; (2) the association between the GSTM1 and GSTT1 polymorphisms and RCC in studies where all cases and controls were exposed to a pesticide; and (3) the association between the GSTM1 and GSTT1 polymorphisms and RCC pathological staging. For the GSTM1 wild-type polymorphism, the meta-analysis examined the genetic susceptibility in the three conditions described above and compared the GSTM1 wild-type to the GSTM1 null genotype in a recessive model (present/present+ present/null versus null/null). The same approach was used for assessment of GSTT1. The heterogeneity among the studies was checked with I2 andτ2 value. For the dichotomous outcomes, we used Mantel-Haenszel method and assessed this meta-analysis by using the random-effects model instead of the fixed-effects model to avoiding heterogeneity32,33,34,35,36. The included studies were stratified according to ethnicity for exploration of heterogeneity. Begg’s funnel plot, which is a scatter plot of effect against study size, was generated as a visual aid to detect bias or systematic heterogeneity37. Publication bias was assessed by Egger’s test (P < 0.05 was considered statistically significant)38. Meta-analysis was performed using RevMan 5.2 software (Cochrane IMS). The Begg’s funnel plot and Egger’s test were performed using STATA version 11.0 (StataCorp, College Station, Texas, USA)
Additional Information
How to cite this article: Huang, W. et al. GSTM1 and GSTT1 polymorphisms contribute to renal cell carcinoma risk: evidence from an updated meta-analysis. Sci. Rep. 5, 17971; doi: 10.1038/srep17971 (2015).
Acknowledgments
This work was financially supported by the Science and Technology Fund of Guangdong Province (no. 2009B030801053), the Science and Technology Fund of Guangzhou City (no. 2009Z1-E381-02), the Natural Science Foundation of Guangdong Province (Grant No. 2015A030310384) and the research project of Health and Family Planning Commission of Shenzhen (Grant No. 20150524).
Footnotes
Author Contributions Conceived and designed the study: Q.H. and W.H. Extracted and analyzed the data: Q.H., H.S. and W.H. Wrote the paper: Q.H. and W.H. Revised the manuscript: Q.H., Z.M. and X.X. All authors reviewed the manuscript.
References
- Ferlay J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127, 2893-2917 (2010). [DOI] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- Ljungberg B. et al. The epidemiology of renal cell carcinoma. Eur Urol 60, 615–621 (2011). [DOI] [PubMed] [Google Scholar]
- Ferlay J., Parkin D. M. & Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J. Cancer 46, 765–781 (2010). [DOI] [PubMed] [Google Scholar]
- Moore L. E., Wilson R. T. & Campleman S. L. Life style factors, exposures, genetic susceptibility, and renal cell cancer risk: a review. Cancer Invest 23, 240–255 (2005). [DOI] [PubMed] [Google Scholar]
- McIlwain C. C., Townsend D. M. & Tew K. D. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene 25, 1639–1648 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. & Strange R. C. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 61, 154–166 (2000). [DOI] [PubMed] [Google Scholar]
- Sprenger R. et al. Characterization of the glutathione S-transferase GSTT1 deletion: discrimination of all genotypes by polymerase chain reaction indicates a trimodular genotype-phenotype correlation. Pharmacogenetics 10, 557–565 (2000). [DOI] [PubMed] [Google Scholar]
- Longuemaux S. et al. Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: a study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res 59, 2903–2908 (1999). [PubMed] [Google Scholar]
- Moore L. E. et al. Glutathione S-transferase polymorphisms, cruciferous vegetable i ntake and cancer risk in the Central and Eastern European Kidney Cancer Study. Carcinogenesis 28, 1960–1964 (2007). [DOI] [PubMed] [Google Scholar]
- Ahmad S. T., Arjumand W., Seth A., Kumar S. A. & Sultana S. Impact of glutathione transferase M1, T1, and P1 gene polymorphisms in the genetic susceptibility of North Indian population to renal cell carcinoma. DNA Cell Biol 31, 636–643 (2012). [DOI] [PubMed] [Google Scholar]
- De Martino M. et al. Renal cell carcinoma Fuhrman grade and histological subtype correlate with complete polymorphic deletion of glutathione S-transferase M1 gene. J Urol 183, 878–883 (2010). [DOI] [PubMed] [Google Scholar]
- Sweeney C. et al. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma: a case-control study. Cancer Epidemiol Biomarkers Prev 9, 449–454 (2000). [PubMed] [Google Scholar]
- Salinas-Sanchez A. S. et al. GSTT1, GSTM1, and CYP1B1 gene polymorphisms and susceptibility to sporadic renal cell cancer. Urol Oncol 30, 864–870 (2012). [DOI] [PubMed] [Google Scholar]
- Wiesenhutter B., Selinski S., Golka K., Bruning T. & Bolt H. M. Re-assessment of the influence of polymorphisms of phase-II metabolic enzymes on renal cell cancer risk of trichloroethylene-exposed workers. Int Arch Occup Environ Health 81, 247–251 (2007). [DOI] [PubMed] [Google Scholar]
- Buzio L. et al. Glutathione S-transferases M1-1 and T1-1 as risk modifiers for renal cell cancer associated with occupational exposure to chemicals. Occup Environ Med 60, 789–793 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami S. et al. Renal cell carcinoma, occupational pesticide exposure and modification by glutathione S-transferase polymorphisms. Carcinogenesis 29, 1567–1571 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning T. et al. Influence of polymorphisms of GSTM1 and GSTT1 for risk of renal cell cancer in workers with long-term high occupational exposure to trichloroethene. Arch Toxicol 71, 596–599 (1997). [DOI] [PubMed] [Google Scholar]
- Cheng H. Y., You H. Y. & Zhou T. B. Relationship between GSTM1/GSTT1 null genotypes and renal cell carcinoma risk: a meta-analysis. Ren Fail 34, 1052–1057 (2012). [DOI] [PubMed] [Google Scholar]
- Liu R., Wang X. H., Liu L. & Zhou Q. No association between the GSTM1 null genotype and risk of renal cell carcinoma: a meta-analysis. Asian Pac J Cancer Prev 13, 3109–3112 (2012). [DOI] [PubMed] [Google Scholar]
- Yang X. et al. Glutathione S-Transferase Polymorphisms (GSTM1, GSTT1 and GSTP1) and Their Susceptibility to Renal Cell Carcinoma: An Evidence-Based Meta-Analysis. PLoS One 8, e63827 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten C., Sagoo G. S., Frodsham A. J., Burke W. & Higgins J. P. Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol 167, 759–774 (2008). [DOI] [PubMed] [Google Scholar]
- Gong M. et al. Genetic polymorphisms of GSTM1, GSTT1, and GSTP1 with prostate cancer risk: a meta-analysis of 57 studies. PLoS One 7, e50587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K., Yi J., Shen X. & Cai Y. Genetic polymorphisms of glutathione S-transferase genes GS TM1, GSTT1 and risk of hepatocellular carcinoma. PLoS One 7, e48924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Association between GSTM1 and GSTT1 allelic variants and head and neck squamous cell cancinoma. PLoS One 7, e47579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harth V., Bruning T. & Bolt H. M. Renal carcinogenicity of trichloroethylene: update, mode of action, and fundamentals for occupational standard setting. Rev Environ Health 20, 103–118 (2005). [PubMed] [Google Scholar]
- Dekant W. et al. Bacterial beta-lyase mediated cleavage and mutagenicity of cysteine conjugates derived from the nephrocarcinogenic alkenes trichloroethylene, tetrachloroethylene and hexachlorobutadiene. Chem Biol Interact 60, 31–45 (1986). [DOI] [PubMed] [Google Scholar]
- Cho S. G. et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem 276, 12749–12755 (2001). [DOI] [PubMed] [Google Scholar]
- Dorion S., Lambert H. & Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J Biol Chem 277, 30792–30797 (2002). [DOI] [PubMed] [Google Scholar]
- Sterne J. A., Gavaghan D. & Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53, 1119–29 (2000). [DOI] [PubMed] [Google Scholar]
- Kontopantells E., Springate D. A. & Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One 8, e69930 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockwell S. E. & Gordon I. R. A comparison of statistical methods for meta-analysis. Stat Med 20, 825–40 (2001). [DOI] [PubMed] [Google Scholar]
- Kontopantells E. & Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: A simulation study. Stat Methods Med Res 21, 409–26 (2012). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–748 (1959). [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey S. G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]