| Title: |
Sulfonamide Compounds as Voltage Gated Sodium Channel Modulators |
| Patent Application Number: |
WO2015151001Al |
Publication date: |
October 8th, 2015 |
| Priority Application: |
1170/MUM/2014 |
Priority date: |
March 29th, 2014 |
| 566/MUM/2015 |
February 20th, 2015 |
| Inventors: |
Ramdas, V.; Loriya, R. M.; Das, A. K.; Khan, T. H. ; Banerjee, M.; Palle, V. P.; Kamboj, R. K. |
| Assignee Company: |
Lupin Limited |
| Disease Area: |
Pain |
Biological Target: |
Nav1.7 |
| Summary: |
Maintaining a specific membrane potential is critical to many cellular functions of mammalian cells. The heart, central and peripheral nervous system, and muscle function all depend on the proper regulation and timing of changes in membrane potential. Voltage gated sodium channels play a key role in these processes. To date, nine voltage gated sodium channels, Nav1.1 to Nav1.9, have been identified, and improper NaV activity can lead to a number of negative health consequences. Nav1.7, for example, has been linked to pain sensation. Loss of function mutations of this ion channel have been documented in humans and linked to congenital insensitivity to painful stimuli. Increased Nav1.7, however, has been associated with increased sensitivity to pain. These observations indicate that Nav1.7 blockade may be a viable method of treating chronic pain. Compounds such as Raxatrigine (CNV1014802), a Nav1.7 blocker that recently completed phase II clinical trials for the treatment of lumbar radiculopathy (sciatica), appear to have validated this hypothesis. The present disclosure describes a series of sulfonamides capable of modulating Nav1.7 activity and their use as treatment for pain and pain related disorders. |
| Important Compound Classes: |
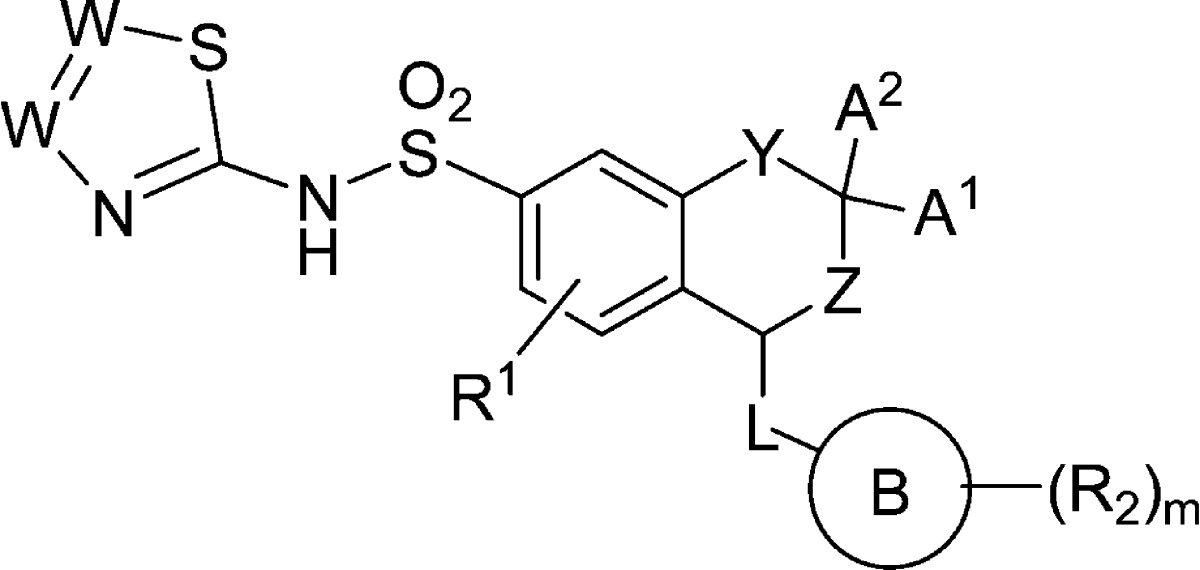 |
| Definitions: |
Y is selected from CH2, O and NR; |
| L is a bond or O; |
| R is hydrogen or substituted or unsubstituted alkyl; |
| Al and A2 are independently hydrogen or substituted or unsubstituted alkyl; or |
| Al and A2, together with the carbon atom to which they are attached, form a substituted or unsubstituted 3- to 6-membered cycloalkyl ring or 4–6-membered heterocyclyl ring; |
| Z is selected from CH2 or – CH2–CH2; |
| Rl is selected from hydrogen, halogen, cyano, substituted or unsubstituted alkyl, and substituted or unsubstituted alkoxy; |
| Ring B is substituted or unsubstituted aryl or substituted or unsubstituted heteroaryl; |
| R2, which may be same or different at each occurrence, is independently selected from halogen, cyano, substituted or unsubstituted alkyl, substituted or unsubstituted haloalkyl, substituted or unsubstituted alkoxy, substituted or unsubstituted hydroxyalkyl, substituted or unsubstituted alkoxyalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and substituted or unsubstituted heterocyclyl; |
| W at each occurrence is independently selected from N or CR3; |
| R3 is selected from hydrogen, halogen, cyano, substituted or unsubstituted alkyl, and substituted or unsubstituted alkoxy; |
| ‘m’ is an integer ranging from 0 to 3, both inclusive; |
| Definitions: (cont.) |
wherein the substituents for substituted or unsubstituted alkyl, substituted or unsubstituted alkoxy, substituted or unsubstituted cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted hydroxyalkyl, substituted or unsubstituted alkoxyalkyl, and substituted or unsubstituted halo alkyl are one or more same or different and independently selected from the group consisting of hydroxy, halogen, carboxy, cyano, nitro, oxo (=O), thio, (=S), alkyl, haloalkyl, hydroxyalkyl, alkoxyalkyl, alkenyl, alkynyl, aryl, aryl alkyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, heteroaryl, heterocyclic ring, heterocyclylalkyl, heteroarylalkyl, −C(O)ORx, −C(O)Ry, −C(S)Ry, −C(O)NRxRz, −NRxC(O)NRxRz, N(Rx)S(O)2Ry, −NRxRz, −NRxC(O)Ry, −NRxC(S)Ry, −NRxC(S)NRxRz, −S(O)2NRxRz, −ORx, −OC(O)Ry, −C(RaRb)1–3C(O)ORx, −C(RaRb)1–3C(O)NRxRz, −OC(RaRb)2–3-ORx, −OC(RaRb)2–3-NRxRz, −OC(RaRb)2–3-S(O)0–2Ry, −C(RaRb)1–3-NRxRz, −C(RaRb)1–3-S(O)0–2Ry, −OC(RaRb)1–3–C(O)NRxRz, −OC(RaRb)1–3–C(O)ORx, and −S(O)0–2Ry; |
| Rx, which may be same or different at each occurrence, is independently selected from hydrogen, alkyl, haloalkyl, alkenyl, alkynyl, aryl, arylalkyl, cycloalkyl, cycloalkenyl, heteroaryl, heterocyclic ring, heterocyclylalkyl, and heteroarylalkyl; |
| Ry, which may be same or different at each occurrence, is independently selected from alkyl, haloalkyl, alkenyl, alkynyl, aryl, aryl alkyl, cycloalkyl, cycloalkenyl, heteroaryl, heterocyclic ring, heterocyclylalkyl, and heteroarylalkyl; and |
| |
Rz, which may be same or different at each occurrence, is independently selected from hydrogen, alkyl, haloalkyl, alkenyl, alkynyl, aryl, arylalkyl, cycloalkyl, cycloalkenyl, heteroaryl, heterocyclic ring, heterocyclylalkyl, and heteroarylalkyl; or Rx and Rz together with the nitrogen atom to which they are attached form a substituted or unsubstituted, saturated or unsaturated 4- to 8-membered cyclic ring, wherein the unsaturated cyclic ring may have one or two double bonds. |
| Key Structures: |
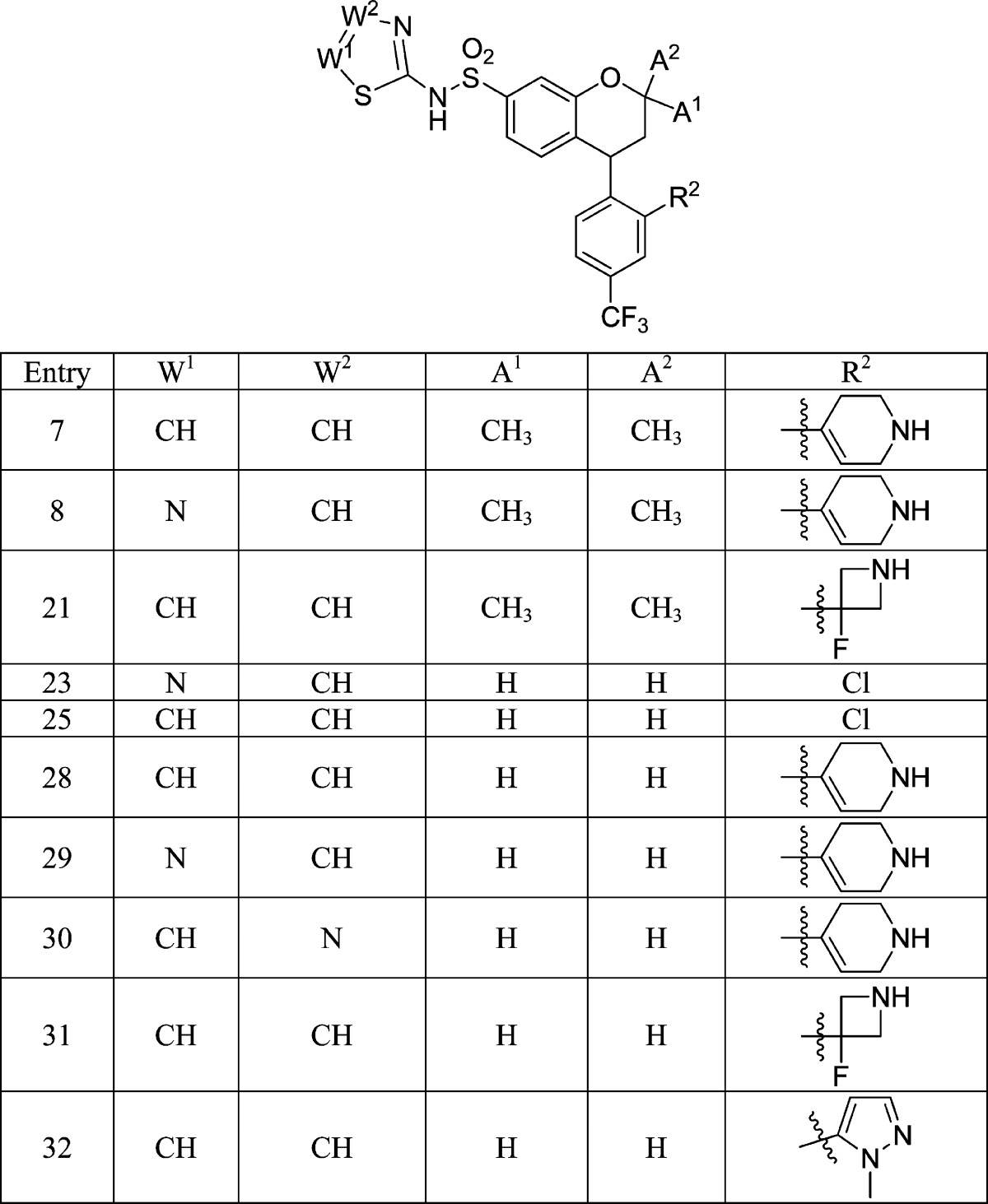 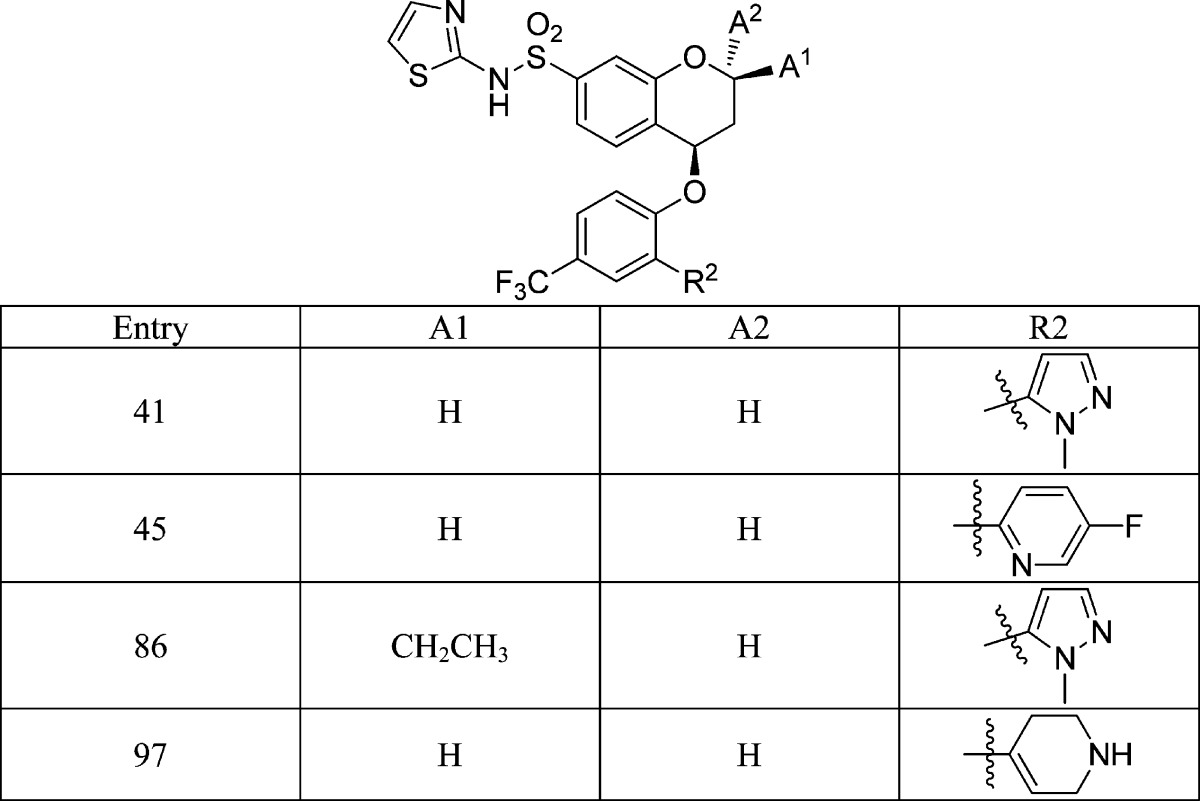
|
| Recent Review Articles: |
Sun S.; Cohen C. J.; Dehnhardt C. M. Inhibitors of voltage-gated sodium channel Nav1.7: patent applications since 2010. Pharmaceutical Patent Analyst 2014, 3 ( (5), ), 509–521. |
| King G. F.; Vetter I. No gain, no pain: NaV1.7 as an analgesic target. ACS Chem. Neurosci. 2014, 5 ( (9), ), 749–751. |
| Dib-Hajj S. D.; Yang Y.; Black J. A.; Waxman S. G. The NaV1.7 sodium channel: from molecule to man. Nat. Rev. Neurosci. 2013, 14 ( (1), ), 49–62. |
| Biological Assay: |
HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30,000 cells/well and incubated at 37 °C/5% CO2 for 48 h. The assay was carried out using the Red Membrane Potential Dye (Molecular Devices) following the manufacturer’s instructions. Briefly, the cells were incubated with IX Red Membrane Potential Dye for 1.5 h. The cells were then treated with various concentrations of the test compounds for 15–20 min followed by depolarization with 10–30 μM Veratridine. The fluorescence was read following excitation at 510–545 nm and emission at 565–625 nm in FLIPR. The “max–min” fluorescence values were used to calculate the % inhibition. IC50 values were calculated by plotting % inhibition against concentration and curve fitting into a sigmoidal dose response. |
| Biological Data: |
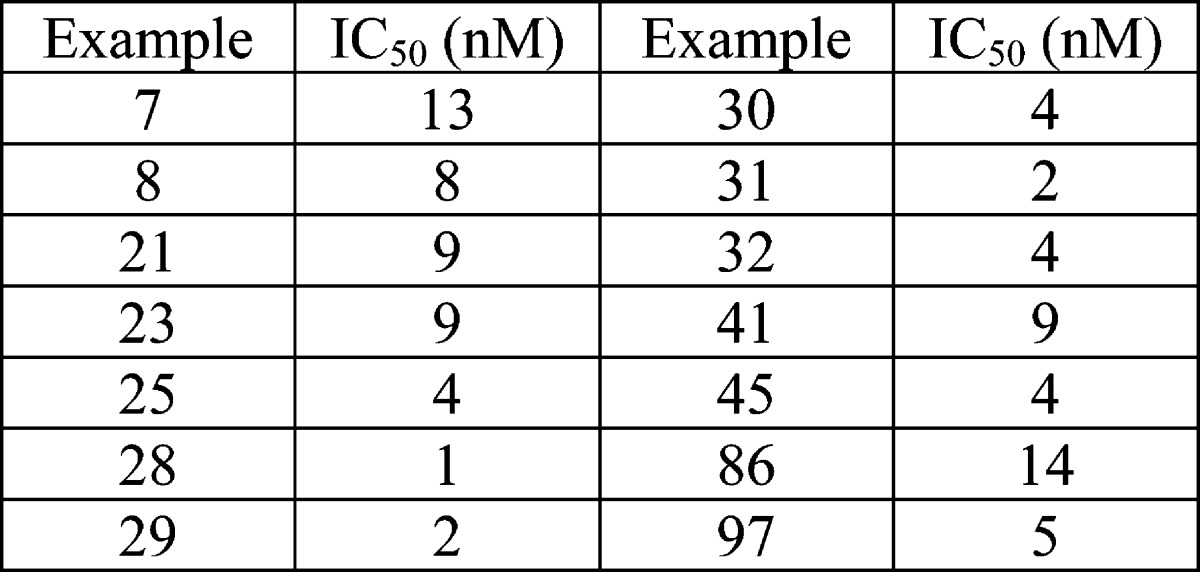 |
| Claims: |
30 total claims |
| 22 composition of matter claims |
| 6 method of use claims |
| 2 process claims |






