Abstract
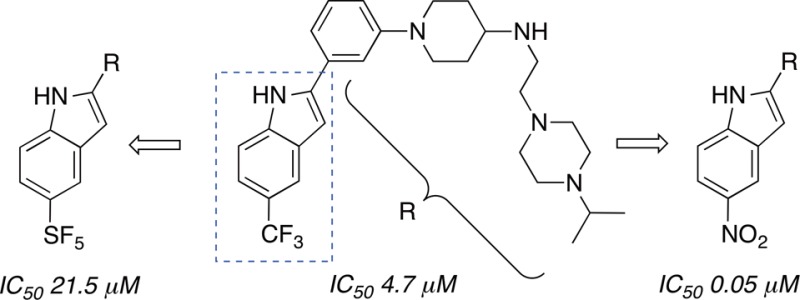
Exploratory SAR studies of a new phenyl indole chemotype for p97 inhibition revealed C-5 indole substituent effects in the ADPGlo assay that did not fully correlate with either electronic or steric factors. A focused series of methoxy-, trifluoromethoxy-, methyl-, trifluoromethyl-, pentafluorosulfanyl-, and nitro-analogues was found to exhibit IC50s from low nanomolar to double-digit micromolar. Surprisingly, we found that the trifluoromethoxy-analogue was biochemically a better match of the trifluoromethyl-substituted lead structure than a pentafluorosulfanyl-analogue. Moreover, in spite of their almost equivalent strongly electron-depleting effect on the indole core, pentafluorosulfanyl- and nitro-derivatives were found to exhibit a 430-fold difference in p97 inhibitory activities. Conversely, the electronically divergent C-5 methyl- and nitro-analogues both showed low nanomolar activities.
Keywords: AAA ATPase, p97 inhibitors, pentafluorosulfanyl-indole, trifluoromethyl-indole, structure−activity relationships, fluorinated substituent effects
Among recent developments in organofluorine chemistry, the pentafluorosulfanyl (SF5) group is notable for its greater electronegativity, lipophilicity, stability, and larger size versus the much more commonly encountered1 trifluoromethyl (CF3) group.2−5 While methods for introducing SF5-groups on aromatic, heteroaromatic, and aliphatic precursors are still in need of further development,6 several pentafluorosulfanyl arenes are commercially available, and the syntheses of an increasing number of other SF5-substituted building blocks, such as pyridines,7 quinolines,8 and indoles9 have been reported.3 SF5-substituted organic molecules have proven to be metabolically very stable.10,11 Therefore, these compounds would appear to be uniquely suited for materials research and the development of agrochemicals and pharmaceuticals, but relatively few case studies have focused on the comparative structure–activity relationship (SAR) of SF5-substituted drug candidates.3,5,8,10,12−16 We now report a systematic investigation of the SAR of SF5-indole inhibitors of the AAA ATPase p97, comparing SF5, a “super-sized” CF3-group sterically equivalent to a t-butyl but electronically most closely related to a nitro functionality, to the corresponding CH3O-, CF3O-, CH3-, CF3-, and NO2-analogues.
The ATPase Associated with various cellular Activities (AAA) p97, also known as Valosin-Containing Protein (VCP), or Cdc48p in yeast, regulates endoplasmic reticulum associated degradation, cell cycle, autophagy, transcription factors, mitochondrial associated degradation, membrane and ubiquitin fusion, and other rather diverse processes that involve protein and membrane processing.17 Binding to ATP followed by hydrolysis to ADP initiates massive conformational changes in the hexameric p97 that can segregate a bound protein from a macromolecular ligand, including other proteins and membrane segments (Figure 1).18
Figure 1.
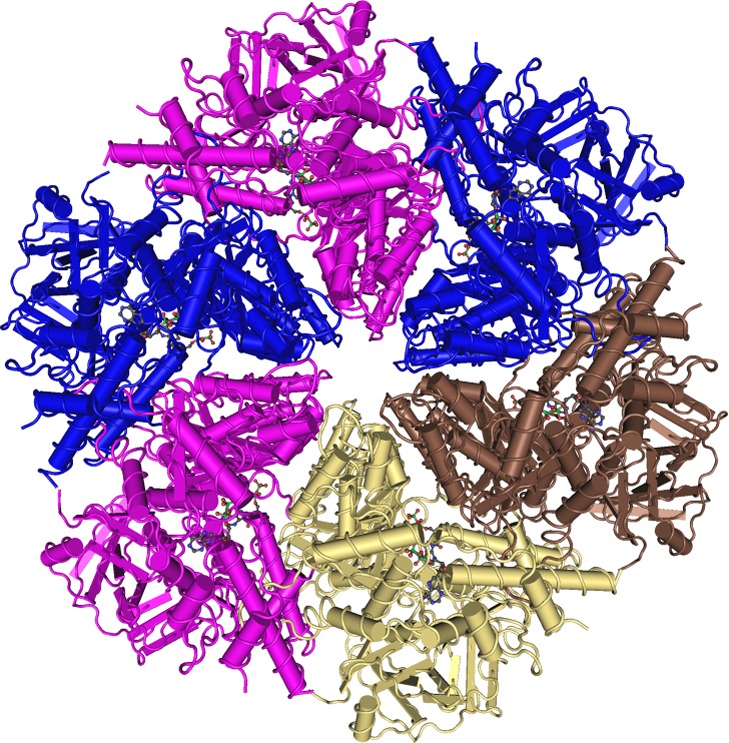
Crystal structure of hexameric, full-length p97 in complex with ADP (PDB ID 3CF3).19
As found for other chaperones,20,21 various forms of cancer, including breast, lung, pancreatic, and colorectal cancer, upregulate p97 as a response to accelerated growth and deteriorating protein quality control.22,23 This property might render cancer cells more sensitive to p97 inhibitors than normal cells. In particular, combinations with proteasome or heat shock protein inhibitors could further widen the therapeutic window, but the test of this hypothesis awaits the development of clinically efficacious p97 antagonists.
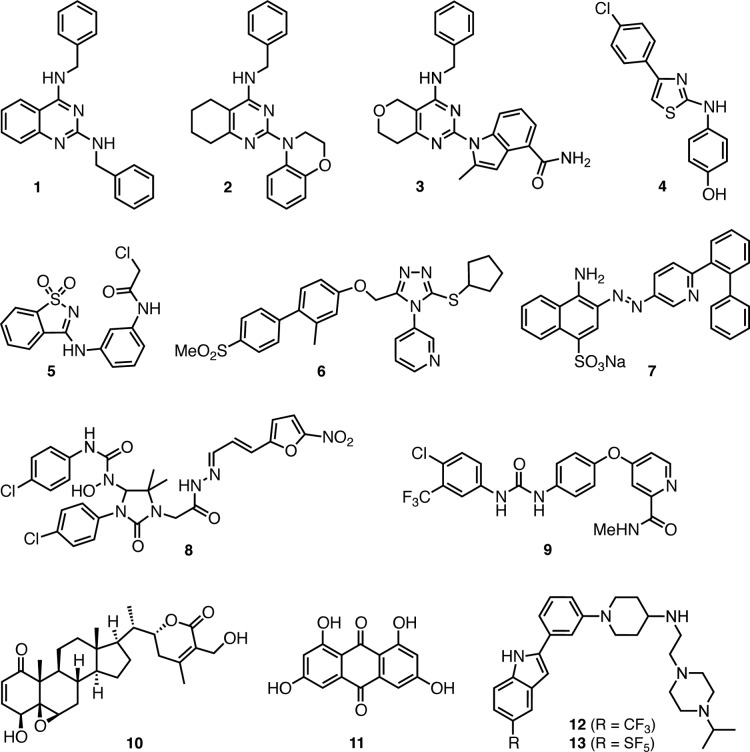
While p97 is not yet a validated clinical cancer target, several small molecule inhibitors have been identified that could enable proof-of-principle of therapeutic efficacy.24 This list includes several amino-heterocycles, such as the diaminoquinazolines 1,252,26 and 3,27 aminothiazole 4,28 and the irreversible inhibitor chloroacetamide 5(29) (Figure 2). 1,2,4-Triazole 6,30 sulfonate 7,31 and imidazolinone 8(32) were identified by high throughput screening campaigns, whereas the discovery of the anticancer agent 9(33) (sorafenib) and the natural products 10(34) (withaferin A) and 11(35) (rheoemodin) as p97 inhibitors was based on specific mechanism of action and targeted lead identification studies.
Figure 2.
Structures of p97 inhibitors.
As part of a medicinal chemistry campaign to optimize p97 inhibitors, we generated the C-5 trifluoromethylated indole 12 as a promising lead structure.36 In the ADPGlo assay,37 we determined a 4.7 ± 2.0 μM IC50 for this compound (Table 1), and we decided to probe the effect of replacing the CF3- with an SF5-group at the C-5 position as shown for compound 13.
Table 1. Biochemical Activities of p97 Inhibitorsa.
| entry | compound/R-group | p97-ADPGlo IC50 [μM] | p97-ADPGlo Std. Dev. [μM] |
|---|---|---|---|
| 1 | 12/CF3 | 4.7 | ±2.0 |
| 2 | 13/SF5 | 21.5 | ±0.4 |
| 3 | 23/NO2 | 0.05 | ±0.04 |
| 4 | 24/CH3 | 0.24 | ±0.11 |
| 5 | 25/OCH3 | 0.71 | ±0.22 |
| 6 | 26/OCF3 | 3.8 | ±0.8 |
Assay conditions: ADPGlo with 20 nM p97 ATPase WT in the presence of 100 μM ATP.37 Assays were run in quadruplicate (12, 13, 25, 26), seven times (24), or nine times (23).
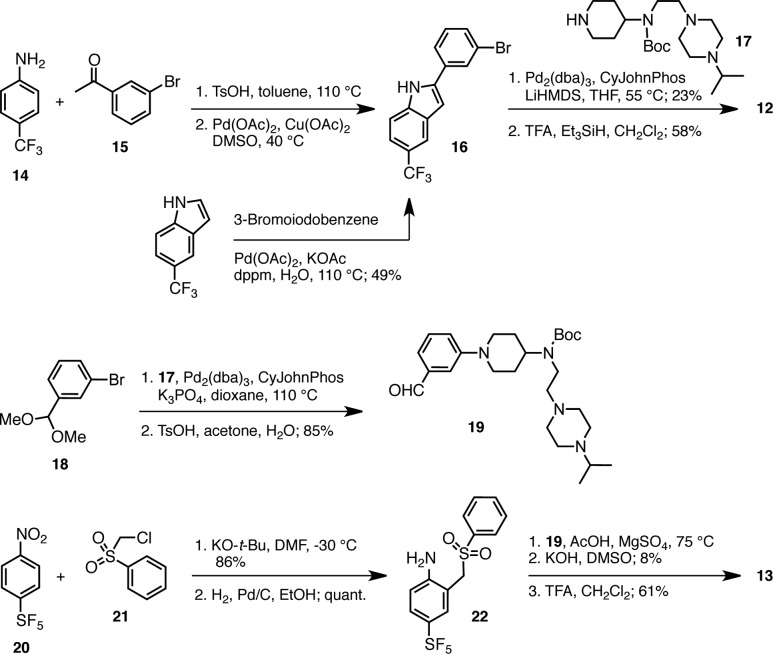
For the initial preparation of 12, we performed a Pd(II)/Cu(I)-catalyzed indole synthesis38 on the imine derived from condensation of amine 14 with ketone 15, followed by a cross coupling39,40 of aryl bromide 16 with secondary amine 17 and Boc-deprotection (Scheme 1).41 Subsequently, a more direct synthesis of 16 was accomplished by a C,H-arylation of commercially available 5-trifluoromethylindole with 3-bromoiodobenzene, which provided indole 16 in 49% yield.42 A different indole synthesis43 was used for the preparation of the pentafluorosulfanyl analogue 13. Pd(0)-catalyzed coupling of bromide acetal 18 with amine 17(41) followed by selective acetal hydrolysis provided aldehyde building block 19. After nucleophilic alkylation of SF5-arene 20 with sulfone 21 and reduction of the nitro group, aniline 22 was condensed with this aldehyde and the indole was formed by cyclization and elimination, albeit in a low 8% yield from 22. Removal of the Boc-group with TFA provided the C-5 pentafluorosulfanyl indole 13 in 61% yield.
Scheme 1. Preparation of CF3- and SF5-Substituted p97 Inhibitors 12 and 13.
Surprisingly, replacement of the trifluoromethyl with a pentafluorosulfanyl group reduced the p97 inhibition almost 5-fold to an IC50 of 21.5 ± 0.4 μM. We hypothesized that this decrease could either be due to the larger size of the SF5 group or its stronger electron-withdrawing effect on the indole ring, and we decided to synthesize the corresponding nitro (23), methyl (24), methoxy (25), and trifluoromethoxy (26) analogues to test these parameters. Electron-density surfaces encoded with electrostatic potential maps for the indole segments of these compounds illustrate both their steric features as well as the range of their inductive effects on the aromatic π-system (Figure 3). Sterically, SF5-analogue 13 and CF3O-analogue 26 are the closest match, but their electronic effects on the indole ring and the indole nitrogen are significantly different. As expected, nitro-analogue 23 is the best electronic match of the pentafluorosulfanyl derivative. Sterically, and, in particular, electronically, CH3O-analogue 25 is most closely related to CH3-analogue 24. Arguably, CF3-analogue 12 is somewhat unique in this series, but sterically its closest match would be CH3-analogue 24, whereas electronically 12 is situated between SF5-analogue 13 and CF3O-analogue 26. Accordingly, we expected that the methylated indole 24 would have similar activity to 12 if steric effects were dominant, whereas electronic effects would likely favor ethers 25 and 26 since the bulkier and more electron-deficient pentafluorosulfanyl had registered a significant drop in activity.
Figure 3.
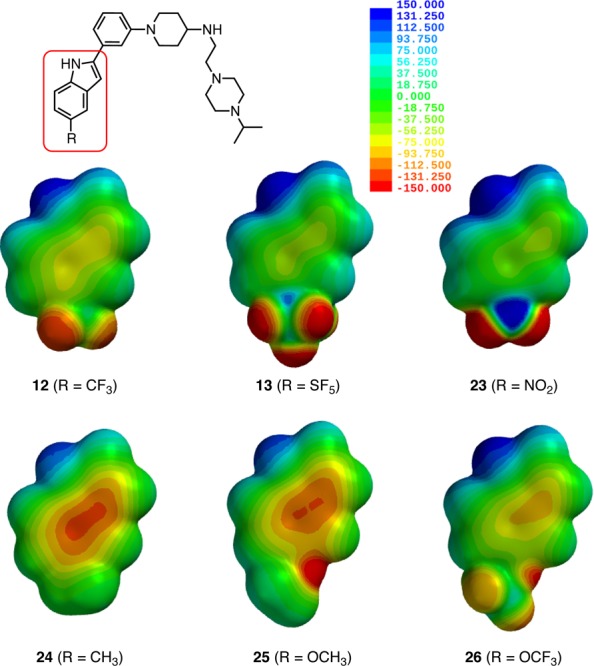
Structures and steric/electronic features46 of new phenyl indole p97 inhibitors. The color coding on the electron-density surface of the indole moiety reflects the electrostatic potential experienced by a positive probe charge (red = attractive, blue = repulsive).
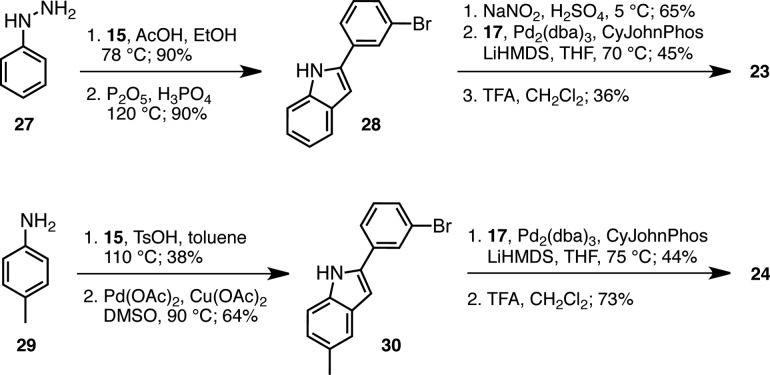
Syntheses of analogues 23–26 are summarized in Schemes 2 and 3. Acid-catalyzed condensation of phenyl hydrazine 27 with bromoacetophenone 15 provided an intermediate hydrazone that was cyclized44 in a Fischer indole synthesis in the presence of P2O5 and H3PO4 to give indole 28. Nitration was selective for the C-5 position of the indole45 and was followed by Pd(0)-catalyzed cross-coupling with Boc-protected tetramine 17. TFA-mediated removal of the Boc-group yielded nitro-indole 23. A related sequence starting with aniline 29 and bromoacetophenone 15 led to an imine intermediate that was subjected to Pd(II)/Cu(I)-catalyzed indole synthesis conditions38 to give 30, and after cross-coupling with 17 and Boc-deprotection, methyl-indole 24 was obtained in 8% yield over the four steps (Scheme 2).
Scheme 2. Preparation of NO2- and CH3-Substituted p97 Inhibitors 23 and 24.
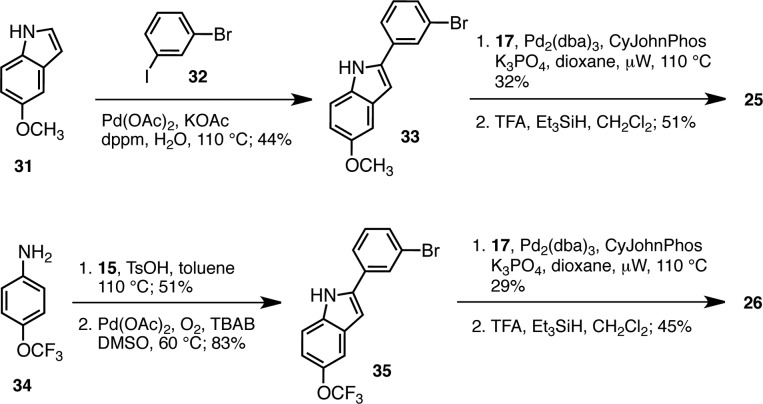
Scheme 3. Preparation of CH3O- and CF3O-Substituted p97 Inhibitors 25 and 26.
The C,H-bond activation42 strategy was selected for the preparation of methoxy-indole intermediate 33 (Scheme 3). C-2 arylation of 31 with 3-bromoiodobenzene 32 led to the coupling product 33 in high selectivity but moderate yield (44%). Cross-coupling with tetramine 17 and deprotection provided the desired CH3O-analogue 25 in 7% yield over the three steps. Starting with the commercially available aniline 34, the corresponding CF3O-analogue 26 was prepared in four steps and 6% overall yield using the alternative imine-cyclization38 strategy.
Evaluation of 23–26 in the p97 ADPGlo assay revealed a remarkable 3 orders of magnitude range of activities between the six indoles (Table 1). Nitro-analogue 23 was found to be a ca. 50 nM inhibitor of the AAA ATPase (entry 3, IC50 0.05 ± 0.04 μM). CF3O-analogue 26 was considerably less active (entry 6, IC50 3.8 ± 0.8 μM), but was slightly more potent than CF3-analogue 12. Methylated 24 and methoxylated 25 had intermediate but still submicromolar IC50s (entries 4 and 5, IC50 0.24 ± 0.11 and 0.71 ± 0.22 μM, respectively).
Most of the literature precedence on the biological activities of SF5-containing compounds versus the corresponding CF3-analogues report relatively minor, but predominantly improved potencies.47 For example, an SF5-containing bosentan analogue was shown to be slightly more active at the human endothelin receptor subtypes A and B, but was less active than the corresponding t-butyl, bicyclo[1.1.1]pentanyl, and cyclopropyl-trifluoromethyl derivatives.10 In the mefloquine series, SF5-substitution of CF3-groups did not change potency.8,16 In a study of cannabinoid receptor ligands, SF5-pyrazoles generally showed slightly higher or equivalent CB1 receptor affinity and selectivity toward the CB2 receptor relative to both CF3- and t-butyl-analogues.14 A recent analysis of matched pairs of CF3/ SF5-analogues from Novartis detected a mean 1.6-fold increase in in vitro potency.13 In contrast, our study of trifluoromethyl and pentafluorosulfanyl indole based inhibitors of the AAA ATPase p97 shows that the bioisosteric replacement strategy with fluorinated building blocks can lead to surprising SAR results, with the possibility for both steric and electronic features contributing to the inhibitory activities. In the series of CH3O-, CF3O-, CH3-, SF5-, and NO2-analogues of the CF3-indole 12, IC50s vary >400-fold, and the SF5-analogue is, in fact, the least active compound. The CF3O-derivative is biochemically the closest match of the CF3 lead structure. Steric factors alone do not account for the differences in potency in this series, as substituents with similar sizes have 20-fold different potencies (e.g., 24 vs 12). If an electron-rich indole is preferred, it would indeed be consistent that 24 and the methyl ether 25 have nanomolar activities, whereas the pentafluorosulfanyl derivative 13 has a double-digit micromolar effect. The outlier in this analysis is, however, clearly nitro-indole 23, which has the strongest electron-depleting effect on the π-system of the indole and yet exhibits the most potent p97 inhibition of this set with a 50 nM IC50, a 5-fold increase in potency compared to the electron rich CH3-analogue 24, and a 430-fold increase compared to the electronically similar SF5-derivative 13. The relative lipophilicity in this series, as expressed by clogP values48 for 12 (2.95), 13 (4.03), 23 (2.01), 24 (2.59), 25 (1.91), and 26 (3.50) can only provide a partial correlation to observed IC50 values. An intriguing alternative explanation is that the indole ring of these p97 inhibitors is involved in π-stacking interactions49 with the protein, which might explain both the affinity for electron-rich π-systems (such as in 24 and 25) as well as the improved activity of the flat, strongly electron-deficient π-system in 23. Structural information on the binding of these inhibitors to p97 will be necessary to further investigate this hypothesis.
In conclusion, we report the first direct comparison of five CF3-isosteres as part of a preliminary SAR analysis of a new series of p97 inhibitors. Four different synthetic approaches to the phenyl indole moiety were developed to provide access to the six indole substitutions. The biological activity in this focused series is highly sensitive to the nature of the C-5 indole substituent, spanning a >400-fold difference in IC50s between the SF5-analogue 13 and the NO2-analogue 23. Contrary to expectation and literature precedence, we found that it was not the SF5- but the CF3O-analogue that was biochemically the closest match to the CF3-substituted lead structure 12 and that, in spite of the similar electron-withdrawing effect on the indole core, SF5- and NO2-derivatives provided the most divergent inhibitors in terms of their in vitro assay activities. Steric or electronic factors alone can not explain this SAR. The preparation of additional analogues that probe more diverse side-chain substituent effects, as well as structural biology studies that might shed light on π-stacking interactions are in progress and will be reported in due course.
Acknowledgments
The authors gratefully acknowledge Ms. Taber S. Lewis (U. Pittsburgh) for QC analysis and operation of LCMS instrumentation. The authors also greatly appreciate the stimulating discussions and valuable suggestions from all members of the CBC p97 team, particularly Drs. Eric Baldwin (Leidos Biomedical Research), Tsui-Fen Chou (UCLA), Raymond Deshaies (Caltech), Andrew Flint (Leidos Biomedical Research), Gunda Georg (U. Minnesota), Neal Green (NIH/NCI), Barbara Mroczkowski (NIH/NCI), Lalith Samankumara (U. Pittsburgh), Shizuko Sei (Leidos Biomedical Research), Gordon Stott (Leidos Biomedical Research), and Michael Walters (U. Minnesota).
Glossary
ABBREVIATIONS
- CyJohnPhos
(1,1′-biphenyl-2-yl)dicyclohexylphosphine
- dppm
1,1-bis(diphenylphosphino)methane
- TBAB
tetra-n-butylammonium bromide
- TFA
trifluoroacetic acid
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00364.
Experimental details and spectral data for all new products (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Chemical Biology Consortium Contract No. HHSN261200800001E Agreement No. 29XS127TO15.
The authors declare no competing financial interest.
Supplementary Material
References
- Gillis E. P.; Eastman K. J.; Hill M. D.; Donnelly D. J.; Meanwell N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- Sheppard W. A. Electrical effect of the sulfur pentafluoride group. J. Am. Chem. Soc. 1962, 84, 3072–3076. 10.1021/ja00875a007. [DOI] [Google Scholar]
- Bowden R. D.; Comina P. J.; Greenhall M. P.; Kariuki B. M.; Loveday A.; Philp D. A new method for the synthesis of aromatic sulfurpentafluorides and studies of the stability of the sulfur pentafluoride group in common synthetic transformations. Tetrahedron 2000, 56, 3399–3408. 10.1016/S0040-4020(00)00184-8. [DOI] [Google Scholar]
- Savoie P. R.; Welch J. T. Preparation and utility of organic pentafluorosulfanyl-containing compounds. Chem. Rev. 2015, 115, 1130–1190. 10.1021/cr500336u. [DOI] [PubMed] [Google Scholar]
- Altomonte S.; Zanda M. Synthetic chemistry and biological activity of pentafluorosulphanyl (SF5) organic molecules. J. Fluorine Chem. 2012, 143, 57–93. 10.1016/j.jfluchem.2012.06.030. [DOI] [Google Scholar]
- Umemoto T.; Garrick L. M.; Saito N. Discovery of practical production processes for arylsulfur pentafluorides and their higher homologs, bis- and tris-(sulfur pentafluorides): beginning of a new era of super-trifluoromethyl arene chemistry and its industry. Beilstein J. Org. Chem. 2012, 8, 461–471. 10.3762/bjoc.8.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanishchev O. S.; Dolbier W. R. Jr. Synthesis and characterization of 2-pyridylsulfur pentafluorides. Angew. Chem., Int. Ed. 2015, 54, 280–284. 10.1002/anie.201409990. [DOI] [PubMed] [Google Scholar]
- Wipf P.; Mo T.; Geib S. J.; Caridha D.; Dow G. S.; Gerena L.; Roncal N.; Milner E. E. Synthesis and biological evaluation of the first pentafluorosulfanyl analogs of mefloquine. Org. Biomol. Chem. 2009, 7, 4163–4165. 10.1039/b911483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakobson G.; Posta M.; Beier P. Synthesis of pentafluorosulfanyl-containing indoles and oxindoles. Synlett 2013, 24, 855–859. 10.1055/s-0032-1318452. [DOI] [Google Scholar]
- Westphal M. V.; Wolfstaedter B. T.; Plancher J.-M.; Gatfield J.; Carreira E. M. Evaluation of tert-butyl isosteres: case studies of physicochemical and pharmacokinetic properties, efficacies, and activities. ChemMedChem 2015, 10, 461–469. 10.1002/cmdc.201402502. [DOI] [PubMed] [Google Scholar]
- Micheli F.; Andreotti D.; Braggio S.; Checchia A. A specific and direct comparison of the trifluoromethyl and pentafluorosulfanyl groups on the selective dopamine D3 antagonist 3-(3-{[4-methyl-5-(4-methyl-1,3-oxazol-5-yl)-4H-1,2,4-triazol-3-yl]thio}propyl)-1-phenyl-3-azabicyclo[3.1.0]hexane template. Bioorg. Med. Chem. Lett. 2010, 20, 4566–4568. 10.1016/j.bmcl.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Karagiannidis L. E.; Haynes C. J. E.; Holder K. J.; Kirby I. L.; Moore S. J.; Wells N. J.; Gale P. A. Highly effective yet simple transmembrane anion transporters based upon ortho-phenylenediamine bis-ureas. Chem. Commun. 2014, 50, 12050–12053. 10.1039/C4CC05519E. [DOI] [PubMed] [Google Scholar]
- Barnes-Seeman D.; Beck J.; Springer C. Fluorinated compounds in medicinal chemistry: recent applications, synthetic advances and matched-pair analyses. Curr. Top. Med. Chem. 2014, 14, 855–864. 10.2174/1568026614666140202204242. [DOI] [PubMed] [Google Scholar]
- Altomonte S.; Baillie G. L.; Ross R. A.; Riley J.; Zanda M. The pentafluorosulfanyl group in cannabinoid receptor ligands: synthesis and comparison with trifluoromethyl and tert-butyl analogues. RSC Adv. 2014, 4, 20164–20176. 10.1039/c4ra01212g. [DOI] [Google Scholar]
- Chia P. W.; Brennan S. C.; Slawin A. M. Z.; Riccardi D.; O’Hagan D. Allosteric agonists of the calcium receptor (CaR): Fluorine and SF5 analogues of cinacalcet. Org. Biomol. Chem. 2012, 10, 7922–7927. 10.1039/c2ob26402a. [DOI] [PubMed] [Google Scholar]
- Mo T.; Mi X.; Milner E. E.; Dow G. S.; Wipf P. Synthesis of an 8-pentafluorosulfanyl analog of the antimalarial agent mefloquine. Tetrahedron Lett. 2010, 51, 5137–5140. 10.1016/j.tetlet.2010.07.113. [DOI] [Google Scholar]
- Woodman P. G. p97, a protein coping with multiple identities. J. Cell Sci. 2003, 116, 4283–4290. 10.1242/jcs.00817. [DOI] [PubMed] [Google Scholar]
- Li G.; Huang C.; Zhao G.; Lennarz W. J. Interprotomer motion-transmission mechanism for the hexameric AAA ATPase p97. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 3737–3741. 10.1073/pnas.1200255109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. M.; Brunger A. T.; Weis W. I. Improved structures of full-length p97, an AAA ATPase: Implications for mechanisms of nucleotide-dependent conformational change. Structure 2008, 16, 715–726. 10.1016/j.str.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Brandvold K. R.; Morimoto R. I. The chemical biology of molecular chaperones-implications for modulation of proteostasis. J. Mol. Biol. 2015, 427, 2931–2947. 10.1016/j.jmb.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos-Turvey A.; Brodsky J.; Wipf P. The effect of structure and mechanism of the Hsp70 chaperone on the ability to identify chemical modulators and therapeutics. Top. Med. Chem. 2015, 16, 1–49. 10.1007/7355_2015_90. [DOI] [Google Scholar]
- Deshaies R. J. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014, 12, 94. 10.1186/s12915-014-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines D. S. p97-containing complexes in proliferation control and cancer: emerging culprits or guilt by association?. Genes Cancer 2010, 1, 753–763. 10.1177/1947601910381381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.; Maksim N.; De La Cruz F.; La Clair J. J. Inhibitors of the AAA+ chaperone p97. Molecules 2015, 20, 3027–3049. 10.3390/molecules20023027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.-F.; Brown S. J.; Minond D.; Nordin B. E.; Li K.; Jones A. C.; Chase P.; Porubsky P. R.; Stoltz B. M.; Schoenen F. J.; Patricelli M. P.; Hodder P.; Rosen H.; Deshaies R. J. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 4834–4839. 10.1073/pnas.1015312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C.-J.; Gui L.; Zhang X.; Moen D. R.; Li K.; Frankowski K. J.; Lin H. J.; Schoenen F. J.; Chou T.-F. Evaluating p97 inhibitor analogues for their domain selectivity and potency against the p97-p47 complex. ChemMedChem 2015, 10, 52–56. 10.1002/cmdc.201402420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.-J.; Parlati F.; Wustrow D.. Preparation of fused pyrimidines as inhibitors of p97 complex. Patent WO2014015291, 2014.
- Bursavich M. G.; Parker D. P.; Willardsen J. A.; Gao Z.-H.; Davis T.; Ostanin K.; Robinson R.; Peterson A.; Cimbora D. M.; Zhu J.-F.; Richards B. 2-Anilino-4-aryl-1,3-thiazole inhibitors of valosin-containing protein (VCP or p97). Bioorg. Med. Chem. Lett. 2010, 20, 1677–1679. 10.1016/j.bmcl.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Magnaghi P.; D’alessio R.; Valsasina B.; Avanzi N.; Rizzi S.; Asa D.; Gasparri F.; Cozzi L.; Cucchi U.; Orrenius C.; Polucci P.; Ballinari D.; Perrera C.; Leone A.; Cervi G.; Casale E.; Xiao Y.; Wong C.; Anderson D. J.; Galvani A.; Donati D.; O’Brien T.; Jackson P. K.; Isacchi A. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat. Chem. Biol. 2013, 9, 548–556. 10.1038/nchembio.1313. [DOI] [PubMed] [Google Scholar]
- Polucci P.; Magnaghi P.; Angiolini M.; Asa D.; Avanzi N.; Badari A.; Bertrand J.; Casale E.; Cauteruccio S.; Cirla A.; Cozzi L.; Galvani A.; Jackson P. K.; Liu Y.; Magnuson S.; Malgesini B.; Nuvoloni S.; Orrenius C.; Sirtori F. R.; Riceputi L.; Rizzi S.; Trucchi B.; O’Brien T.; Isacchi A.; Donati D.; D’alessio R. Alkylsulfanyl-1,2,4-triazoles, a new class of allosteric valosine containing protein inhibitors. Synthesis and structure-activity relationships. J. Med. Chem. 2013, 56, 437–450. 10.1021/jm3013213. [DOI] [PubMed] [Google Scholar]
- Kakizuka A.; Hori S.; Shudo T.; Fuchigami T.. Preparation of 2-(arylazo or heteroarylazo)-4-aminonaphthalene-1-sulfonic acid derivatives as regulators of vasolin-containing protein (VCP). PCT Int. Appl. WO 2012014994, 2012.
- Wang Q.; Li L.; Ye Y. Inhibition of p97-dependent protein degradation by eeyarestatin I. J. Biol. Chem. 2008, 283, 7445–7454. 10.1074/jbc.M708347200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P.; Higa A.; Taouji S.; Bexiga M. G.; Marza E.; Arma D.; Castain C.; Le Bail B.; Simpson J. C.; Rosenbaum J.; Balabaud C.; Bioulac-Sage P.; Blanc J.-F.; Chevet E. Sorafenib-mediated targeting of the AAA+ ATPase p97/VCP leads to disruption of the secretory pathway, endoplasmic reticulum stress, and hepatocellular cancer cell death. Mol. Cancer Ther. 2012, 11, 2610–2620. 10.1158/1535-7163.MCT-12-0516. [DOI] [PubMed] [Google Scholar]
- Tao S.; Tillotson J.; Wijeratne E. M. K.; Xu Y.-M.; Kang M.; Wu T.; Lau E. C.; Mesa C.; Mason D. J.; Brown R. V.; La Clair J. J.; Gunatilaka A. A. L.; Zhang D. D.; Chapman E. Withaferin A analogs that target the AAA+ chaperone p97. ACS Chem. Biol. 2015, 10, 1916–1924. 10.1021/acschembio.5b00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. J.; Wu T.; Wijeratne E. M. K.; Lau E. C.; Mason D. J.; Mesa C.; Tillotson J.; Zhang D. D.; Gunatilaka A. a. L.; La Clair J. J.; Chapman E. Functional chromatography reveals three natural products that target the same protein with distinct mechanisms of action. ChemBioChem 2014, 15, 2125–2131. 10.1002/cbic.201402258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuscript in preparation.
- Chou T.-F.; Bulfer S. L.; Weihl C. C.; Li K.; Lis L. G.; Walters M. A.; Schoenen F. J.; Lin H. J.; Deshaies R. J.; Arkin M. R. Specific inhibition of p97/VCP ATPase and kinetic analysis demonstrate interaction between D1 and D2 ATPase domains. J. Mol. Biol. 2014, 426, 2886–2899. 10.1016/j.jmb.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.; Deb I.; Yoshikai N. Palladium-catalyzed aerobic oxidative cyclization of N-aryl imines: indole synthesis from anilines and ketones. J. Am. Chem. Soc. 2012, 134, 9098–9101. 10.1021/ja3030824. [DOI] [PubMed] [Google Scholar]
- Harris M. C.; Huang X.; Buchwald S. L. Improved functional group compatibility in the palladium-catalyzed synthesis of aryl amines. Org. Lett. 2002, 4, 2885–2888. 10.1021/ol0262688. [DOI] [PubMed] [Google Scholar]
- Kazock J.-Y.; Thery I.; Chezal J.-M.; Chavignon O.; Teulade J.-C.; Gueiffier A.; Enguehard-Gueiffier C. From the particular reactivity of 8-iodoimidazo[1,2-a]pyridine towards copper- and palladium-catalyzed aminations. Bull. Chem. Soc. Jpn. 2006, 79, 775–779. 10.1246/bcsj.79.775. [DOI] [Google Scholar]
- See Supporting Information for experimental details.
- Joucla L.; Batail N.; Djakovitch L. ″On water″ direct and site-selective Pd-catalysed C-H arylation of (NH)-indoles. Adv. Synth. Catal. 2010, 352, 2929–2936. 10.1002/adsc.201000512. [DOI] [Google Scholar]
- Iakobson G.; Pošta M.; Beier P. Synthesis of pentafluorosulfanyl-containing indoles and oxindoles. Synlett 2013, 24, 855–859. 10.1055/s-0032-1318452. [DOI] [Google Scholar]
- Kuehne M. E.; Kitagawa T. Reactions of indoles with benzyne. J. Org. Chem. 1964, 29, 1270–1273. 10.1021/jo01028a514. [DOI] [Google Scholar]
- Noland W. E.; Rush K. R.; Smith L. R. Nitration of indoles. IV. Nitration of 2-phenylindole. J. Org. Chem. 1966, 31, 65–69. 10.1021/jo01339a013. [DOI] [Google Scholar]
- Electron-density surfaces encoded with electrostatic potential maps were calculated in Spartan 10 (Wave Function, Inc., Irvine, CA) with PM3 parametrization.
- Hendriks C. M. M.; Penning T. M.; Zang T.; Wiemuth D.; Gründer S.; Sanhueza I. A.; Schoenebeck F.; Bolm C. Pentafluorosulfanyl-containing flufenamic acid analogs: Syntheses, properties and biological activities. Bioorg. Med. Chem. Lett. 2015, 25, 4437–4440. 10.1016/j.bmcl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- cLogP values were calculated with Spartan 10 (Wave Function, Inc., Irvine, CA) and Instant JChem 15.7.27.0 205. ChemAxon; http://www.chemaxon.com.
- Hunter C. A.; Sanders J. K. M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. 10.1021/ja00170a016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.