Abstract
Background
Smoking increases the risk of numerous cancers; however, an association of smoking with adult gliomas has not been found in a population.
Methods
This case-control study included 4556 glioma cases (ICD-9 code 191.0–191.9) aged ≥30 years and 9112 controls from a national survey of smoking and mortality in China in 1989–1991. Controls from 325 255 surviving spouses of all-cause deaths were randomly assigned to cases in each of 103 areas according to sex and age groups at a ratio of 2:1. Smoking information was ascertained retrospectively by interviewing surviving spouses.
Results
After adjustment for confounders, smoking increased the risk of glioma deaths by 11% (odds ratio [OR] = 1.11; 95% confidence interval [CI]: 1.03–1.21). Compared with non-smokers; the increased risk was 9% (OR = 1.09; 95% CI: 0.99–1.20) in men and 16% (OR = 1.16; 95% CI: 1.00–1.36) in women. The risk increased with age and doses. For individuals aged ≥50 years, smoking was associated with higher risk of glioma death by 25% (OR = 1.25; 95% CI: 1.15–1.38); this increased risk for smokers who smoked ≥20 cigarettes daily for ≥30 years was 53% (OR = 1.53; 95% CI: 1.34–1.74). There were similar findings in both men and women and with either pathology-based or non–pathology-based comparisons.
Conclusions
This study indicates that smoking is associated with glioma deaths in the Chinese population. Long-term heavy smoking could be a factor for risk stratification in individuals attending brain tumor clinics.
Keywords: case-control, death, epidemiological design, glioma, smoking
A brain tumor has become the seventh leading cause of death from malignant tumors, accounting for 2.3% of all malignant tumors, with an increase in the mortality rate of 194% in China in 2008 compared with the 1970s and 101% compared with the 1990s.1 A glioma, the main pathological subtype of brain tumor, is often fatal because of its invasive nature and resistance to current treatments. Currently, the only established modifiable risk factor for gliomas except age, male sex, Caucasian race, and inherited factors, is ionizing radiation exposure.2–4
An association of smoking with gliomas has not been systemically observed in population-based case-control studies4–8 or cohort studies,9–11 although cigarette smoking is a risk factor for numerous cancers. Furthermore, a meta-analysis pooling 6 cohort and 11 case-control studies has also not produced a significant association.12 This is likely related to a protective effect from the blood-brain barrier (BBB), which selectively excludes most endogenous and xenobiotic blood-borne substances from entering the brain.13 However, a recent experimental study showed that nicotine, a major active component of cigarette smoke, stimulates the malignant behavior of glioma cells.14 Experience from developed countries showed that tobacco control accounted for almost all of the improvement in mortality rates for smoking-related cancers during the past 4 decades. However, an association of smoking with relatively less common tumors, such as gliomas, is unclear, and a strategy for the prevention and treatment of these tumors is absent, particularly in China (which has the largest consumption of tobacco in the world, with an estimated 301 million current smokers and poor tobacco control).15,16
The aim of the current study was to explore whether there was evidence of an association between cigarette smoking and adult glioma deaths.
Methods
National Mortality Survey Data
We used a dataset from the China Nationwide Retrospective Mortality Survey conducted from 1989 through 1991. There were 802 032 (70.6%) deaths in 24 urban areas, and 334 304 (29.4%) deaths in 79 rural counties, which were randomly chosen from more than 2000 counties in China. This survey covered 67 million people and included 1 136 336 all-cause deaths of subjects aged 30 years or older during the years 1986–1988. These 103 areas covered all geographic and economic zones across 28 provincial administrative regions of China. The underlying cause of each death was coded according to the World Health Organization's International Classification of Diseases, 9th Edition (ICD-9). Additional details on this survey were described elsewhere.17
Recruitment of Cases and Controls
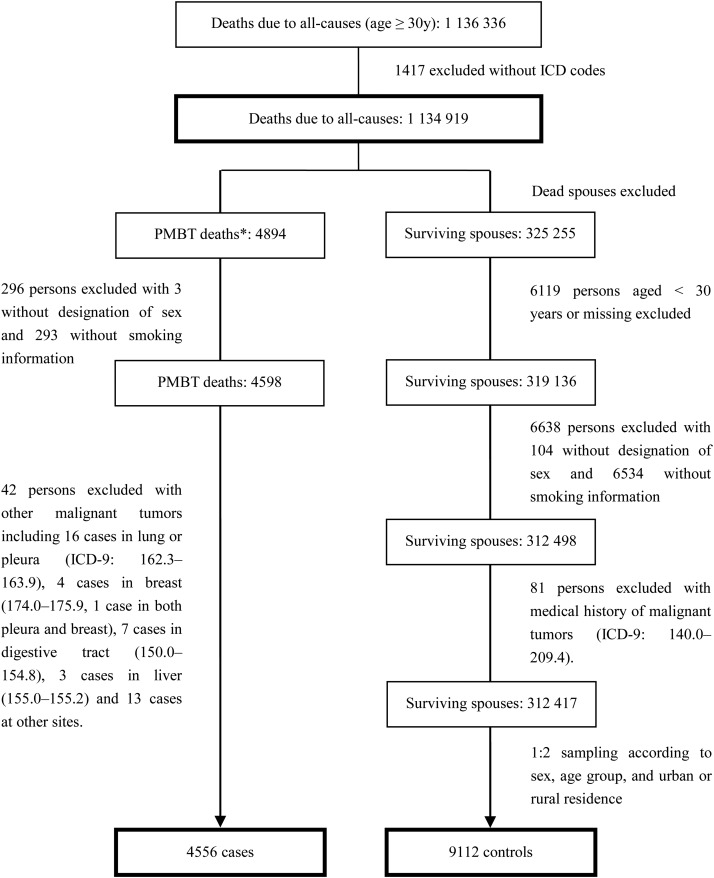
The specific methods used to recruit all cases and controls are shown in Fig. 1. Although a histological code is not available in ICD-9 codes, we collected related information in the survey and labeled individual histologies in this study. A total of 4894 men and women aged 30 years or older and who died from gliomas (ICD-9 codes 191.0–191.9) were initially selected for the study. After excluding subjects with missing information on sex or smoking and other malignant tumors (ICD-9 codes 140.0–189.9 and 192.0–209.4), 4556 (93.0%) were included as cases, with 56.6%, 24.7%, and 11.8% diagnosed in province-, prefecture-, and county-level hospitals, respectively. The diagnosis was confirmed by autopsy, histological test, surgical operation, clinical assessment including imaging or laboratory tests, clinical assessment, and deduction after death in 0.4%, 24.3%, 9.7%, 57.0%, 6.9%, or 0.8% of cases (0.9% unknown), respectively. Pathology-based diagnoses, including autopsies and histological examinations, and surgical procedures that are authorized as the highest category of diagnosis in a national death surveillance system of China, were performed in 34.4% (35.0% for men, 33.6% for women, 36.2% for urban residents, and 26.4% for rural residents, respectively).
Fig. 1.
Flow chart for recruitment of the case and control. *Gliomas (ICD-9: 191.0–191.9) as an underlying cause of deaths.
We selected 325 255 sex and age-span-assigned surviving spouses of all 1 134 919 all-cause deaths as controls in both urban and rural areas. Among these, 12 838 were excluded because of missing data or previous diagnosis of malignant tumors (ICD-9 code 140.0–209.4) at the time of interview. The remaining 312 417 subjects (96.1% of surviving spouses who provided recalled information) were randomly assigned to glioma deaths using a computer-generated allocation algorithm designed for this purpose. Controls were assigned to cases based on a ratio of 2 controls for each case in each of the 103 study areas for statistical power according to sex and 10-year age spans from aged 30 years to more than 80 years. When this assignment could not be performed because of a small sample size in either case or control groups of some rural areas, the corresponding areas were combined with neighboring areas. This control selection procedure was based on the assumption that individuals in the control group were representative of the source population.18
Smoking Survey Data
Spouses or other relatives of all deceased persons were interviewed to obtain information on smoking history. The interviewees described the smoking habit of their deceased family member and of themselves. These data were used to determine whether people had ever smoked by 1980 (a period of time before onset of their disease) to minimize effects of behavior changes after diagnosis of disease. Smoking was defined as at least one cigarette smoked per day for up to one year. For smokers, we defined years of smoking as the age at death for the case or spouse's age at the time of partner's death minus age at onset of daily smoking (given the low rate of quitting smoking).18 The number of cigarettes smoked per day was also recorded.
Statistical Analysis
Odds ratios (ORs) with 95% confidence intervals (CIs) were used to estimate the effects of smoking history on the risk of glioma deaths. Our cases and controls were from the same source population with a complete frequency distribution on sex, age group, and area. Therefore, in a multiple-factor analysis, a non-conditional logistic regression model was used to estimate the risk of smoking and conduct a trend test with adjustment for confounders. Attributable fractions, calculated as (OR–1)/OR, were used to express risk attributing to glioma deaths for the smoking population. All analyses were performed using SAS 9.2 statistical software package (SAS Institute). All P values were 2-sided except for P trend tests, in which 1-sided P values were used; a P value <.05 was considered statistically significant.
Results
The study population comprised 57.1% men and 42.9% women, 82.1% from urban areas and 17.9% from rural areas. The age of cases and controls was comparable (56.7 ± 13.0 years and 56.7 ± 12.8 years, respectively). In general, there was a slightly higher prevalence of smoking of 41.3% (59.7% for men and 16.7% for women) in the case group compared with 39.2% (57.7% for men and 14.6% for women) in the control group. This was associated with a slightly increased risk of glioma deaths by 11% (OR = 1.11; 95% CI: 1.03–1.21) after adjustment for sex, age, and area of residence. The increased risk was 9% (OR = 1.09; 95% CI: 0.99–1.20) in men, 16% (OR = 1.16; 95% CI: 1.00–1.36) in women, 12% (OR = 1.12; 95% CI: 1.02–1.22) in urban populations, and 9% (OR = 1.09; 95% CI: 0.90–1.34) in rural populations.
The numbers of cases and controls in all subgroups, defined by sex and age group, are shown in Table 1. ORs for an association between smoking and glioma deaths were influenced by age, and both crude and adjusted ORs of over 1.00 were observed in both men and women aged 50 years or older. For individuals aged ≥50 years, smoking was associated with higher risk of glioma deaths by 25% (OR = 1.25; 95% CI: 1.15–1.38) adjusted for sex, age, and area of residence. The increased risk was 27% (OR = 1.27; 95% CI: 1.13–1.42) in men, 23% (OR = 1.23; 95% CI: 1.04–1.44) in women, 13% (OR = 1.13; 95% CI: 0.98–1.31) in pathology-based comparison, and 32% (OR = 1.32; 95% CI: 1.19–1.47) in non-pathology-based comparison. We also calculated ORs and attributable fractions according to area of residence and sex as shown in Table 2. In particular, rural residents showed a much higher OR than urban residents, and rural men had a much larger attributable fraction of 32.4% compared with 18.7% and 18.0% in urban men and urban women, respectively.
Table 1.
Current smoking prevalence and odds ratios
| Age Group (years) | N (% of Smokers) |
Crude OR (95% CI) | Adjusted ORa (95% CI) | |
|---|---|---|---|---|
| Case | Control | |||
| Men | ||||
| 30–39 | 310 (49.4) | 620 (59.8) | 0.65 (0.50–0.86) | 0.69 (0.51–0.90) |
| 40–49 | 372 (55.4) | 744 (61.4) | 0.78 (0.61–1.00) | 0.78 (0.61–1.00) |
| 50–59 | 744 (62.5) | 1488 (59.5) | 1.13 (0.95–1.36) | 1.13 (0.95–1.36) |
| 60–69 | 726 (64.6) | 1452 (58.7) | 1.29 (1.07–1.55) | 1.29 (1.07–1.55) |
| ≥70 | 448 (58.0) | 896 (48.2) | 1.49 (1.18–1.87) | 1.47 (1.16–1.85) |
| Total | 2600 (59.7) | 5200 (57.7) | 1.09 (0.99–1.20) | 1.09 (0.99–1.20) |
| Women | ||||
| 30–39 | 293 (2.1) | 586 (2.2) | 0.92 (0.35–2.45) | 0.91 (0.34–2.43) |
| 40–49 | 287 (6.6) | 574 (8.0) | 0.81 (0.47–1.42) | 0.81 (0.47–1.42) |
| 50–59 | 570 (19.7) | 1140 (16.7) | 1.22 (0.94–1.58) | 1.23 (0.95–1.59) |
| 60–69 | 500 (24.4) | 1000 (20.8) | 1.23 (0.95–1.59) | 1.23 (0.95–1.59) |
| ≥70 | 306 (22.2) | 612 (18.8) | 1.24 (0.88–1.73) | 1.40 (0.99–1.98) |
| Total | 1956 (16.7) | 3912 (14.6) | 1.17 (1.01–1.36) | 1.16 (1.00–1.35) |
Abbreviations: CI, confidence interval; OR, odds ratio.
aAdjusted for age and urban or rural residence.
Table 2.
Effects of smoking on glioma deaths in individuals aged 50 years or older
| Crude OR (95% CI) | Age-adjusted OR* (95% CI) | Prevalence of Smoking in the Control Group (%) | AFs (%) | |
|---|---|---|---|---|
| Urban men | 1.23 (1.09–1.39) | 1.23 (1.09–1.39) | 57.0 | 18.7 |
| Urban women | 1.22 (1.03–1.44) | 1.21 (1.02–1.44) | 16.2 | 18.0 |
| Rural men | 1.48 (1.10–1.99) | 1.50 (1.11–2.02) | 60.9 | 32.4 |
| Rural women | 1.36 (0.81–2.28) | 1.37 (0.82–2.30) | 7.4 | – |
Abbreviations: AFs, attributable factors; CI, confidence interval; OR, odds ratio.
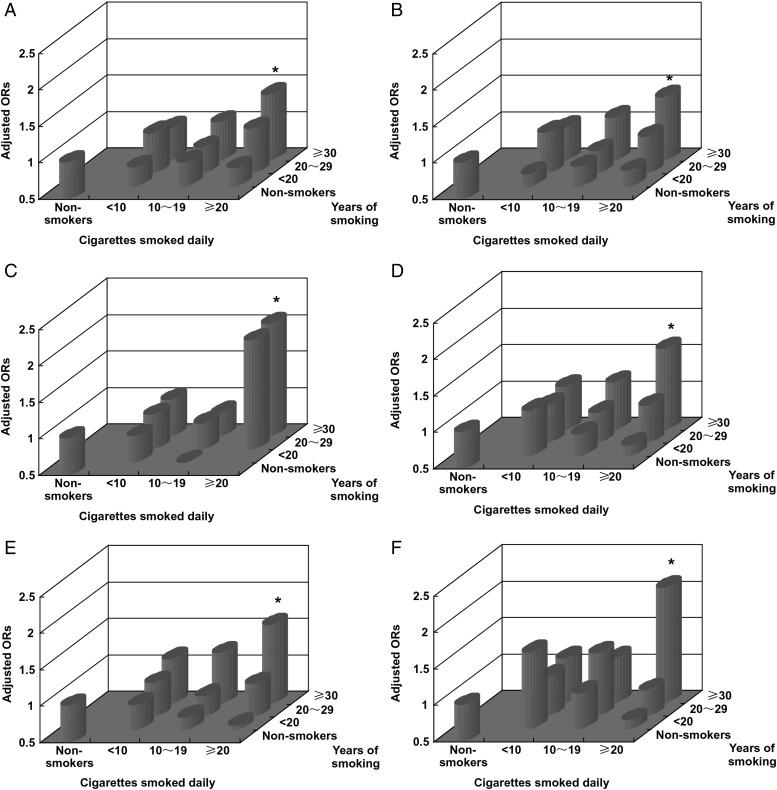
A dose-response relationship between smoking variables, such as years of smoking and number of cigarettes smoked daily and glioma deaths, was found (Table 3). In men, the ORs were 0.70 (95% CI: 0.57–0.85), 0.92 (95% CI: 0.78–1.08), and 1.24 (95% CI: 1.11–1.38) for smokers with <20, 20–29, and ≥30 years of smoking (P for trend <0.001) and 1.00 (95% CI: 0.87–1.16), 1.00 (95% CI: 0.86–1.13), and 1.24 (95% CI: 1.10–1.41) for smokers with <10, 10–19, and ≥20 cigarettes smoked daily (P for trend = .001) after adjustment for age and urban or rural residence compared with non-smokers; this trend was similar in women. For either pathology-based or non-pathology-based comparison with all controls, the ORs adjusted for age and urban or rural residence showed an obvious dose-response relationship between smoking and glioma deaths. We used a common reference group, (ie, non-smokers) based on multivariable logistic models to express the synergistic effects of years of smoking and number of cigarettes smoked daily, and found that the risk dramatically increased with years of smoking and the number of cigarettes smoked daily. In particular, in long-term heavy smokers (defined as individuals with ≥30 smoking years) and ≥20 cigarettes (equivalent to one pack) smoked daily, the risk increased by 53% (OR = 1.53; 95% CI: 1.34–1.74) after adjustment for age, sex, and area of residence; this increased risk was 40% (OR = 1.40; 95% CI: 1.15–1.71) in pathology-based comparisons and 61% (OR = 1.61; 95% CI: 1.39–1.87) in non-pathology-based comparisons. These findings were consistent in both men and women (Fig. 2).
Table 3.
Dose-response relationships between smoking and glioma deaths
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Case | Control | Crude OR (95% CI) | Adjusted OR | Case | Control | Crude OR (95% CI) | Adjusted OR (95% CI)a | |
| N (% of Proportion) | (95% CI)a | N (% of smokers) | ||||||
| Total | ||||||||
| Non-smoker | 1047 (40.3) | 2202 (42.4) | 1.00 | 1.00 | 1629 (83.3) | 3340 (85.4) | 1.00 | 1.00 |
| Years of smoking | ||||||||
| <20 | 211 (8.1) | 510 (9.8) | 0.87 (0.73–1.04) | 0.70 (0.57–0.85) | 40 (2.0) | 80 (2.0) | 1.03 (0.70–1.51) | 1.03 (0.70–1.51) |
| 20∼29 | 287 (11.0) | 607 (11.7) | 0.99 (0.85–1.17) | 0.92 (0.78–1.08) | 58 (3.0) | 109 (2.8) | 1.09 (0.79–1.51) | 1.09 (0.79–1.51) |
| ≥30 | 1055 (40.6) | 1881 (36.2) | 1.18 (1.06–1.31) | 1.24 (1.11–1.38) | 229 (11.7) | 383 (9.8) | 1.23 (1.03–1.46) | 1.22 (1.02–1.46) |
| P for trend | <0.001 | 0.016 | ||||||
| Cigarettes smoked daily | ||||||||
| <10 | 393 (15.1) | 838 (16.1) | 0.99 (0.86–1.14) | 1.00 (0.87–1.16) | 159 (8.1) | 297 (7.6) | 1.10 (0.90–1.34) | 1.09 (0.89–1.34) |
| 10∼19 | 513 (19.7) | 1071 (20.6) | 1.01 (0.89–1.15) | 1.00 (0.86–1.13) | 91 (4.7) | 183 (4.7) | 1.02 (0.79–1.32) | 1.02 (0.79–1.32) |
| ≥20 | 647 (24.9) | 1089 (20.9) | 1.25 (1.11–1.41) | 1.24 (1.10–1.41) | 77 (3.9) | 92 (2.4) | 1.72 (1.26–2.34) | 1.71 (1.25–2.33) |
| P for trend | 0.001 | 0.004 | ||||||
| Pathology-basedb | ||||||||
| Non-smoker | 379 (41.7) | 2202 (42.4) | 1.00 | 1.00 | 565 (85.9) | 3340 (85.4) | 1.00 | 1.00 |
| Years of smoking | ||||||||
| <20 | 97 (10.7) | 510 (9.8) | 1.11 (0.87–1.41) | 0.72 (0.55–0.95) | 9 (1.4) | 80 (2.0) | 0.67 (0.33–1.33) | 0.63 (0.32–1.27) |
| 20–29 | 116 (12.8) | 607 (11.7) | 1.11 (0.89–1.39) | 0.96 (0.76–1.21) | 20 (3.0) | 109 (2.8) | 1.09 (0.67–1.76) | 1.07 (0.66–1.75) |
| ≥30 | 318 (35.0) | 1881 (36.2) | 0.98 (0.84–1.15) | 1.15 (0.97–1.36) | 64 (9.7) | 383 (9.8) | 0.99 (0.75–1.31) | 1.12 (0.83–1.49) |
| P for trend | 0.097 | 0.284 | ||||||
| Cigarettes smoked daily | ||||||||
| <10 | 123 (13.5) | 838 (16.1) | 0.85 (0.69–1.06) | 0.92 (0.74–1.15) | 45 (6.8) | 297 (7.6) | 0.90 (0.65–1.24) | 0.97 (0.69–1.34) |
| 10–19 | 185 (20.3) | 1071 (20.6) | 1.00 (0.83–1.21) | 0.95 (0.79–1.16) | 23 (3.5) | 183 (4.7) | 0.74 (0.48–1.16) | 0.78 (0.50–1.22) |
| ≥20 | 223 (24.5) | 1089 (20.9) | 1.19 (0.99–1.43) | 1.14 (0.95–1.37) | 25 (3.8) | 92 (2.4) | 1.61 (1.02–2.52) | 1.74 (1.11–2.75) |
| P for trend | 0.114 | 0.195 | ||||||
| Non-pathology-basedb | ||||||||
| Non-smoker | 668 (39.5) | 2202 (42.4) | 1.00 | 1.00 | 1064 (82.0) | 3340 (85.4) | 1.00 | 1.00 |
| Years of smoking | ||||||||
| <20 | 114 (6.8) | 510 (9.8) | 0.74 (0.59–0.92) | 0.67 (0.53–0.86) | 31 (2.4) | 80 (2.0) | 1.22 (0.80–1.85) | 1.26 (0.83–1.92) |
| 20–29 | 171 (10.1) | 607 (11.7) | 0.93 (0.77–1.12) | 0.90 (0.73–1.09) | 38 (2.9) | 109 (2.8) | 1.09 (0.75–1.59) | 1.11 (0.76–1.61) |
| ≥30 | 737 (43.6) | 1881 (36.2) | 1.29 (1.14–1.46) | 1.29 (1.13–1.46) | 165 (12.7) | 383 (9.8) | 1.35 (1.11–1.64) | 1.28 (1.04–1.56) |
| P for trend | <0.001 | 0.007 | ||||||
| Cigarettes smoked daily | ||||||||
| <10 | 270 (16.0) | 838 (16.1) | 1.05 (0.89–1.23) | 1.06 (0.90–1.25) | 114 (8.8) | 297 (7.6) | 1.21 (0.96–1.51) | 1.16 (0.92–1.46) |
| 10–19 | 328 (19.4) | 1071 (20.6) | 1.02 (0.87–1.18) | 1.01 (0.87–1.17) | 68 (5.2) | 183 (4.7) | 1.17 (0.88–1.55) | 1.14 (0.86–1.53) |
| ≥20 | 424 (25.1) | 1089 (21.0) | 1.30 (1.13–1.50) | 1.28 (1.11–1.48) | 52 (4.0) | 92 (2.4) | 1.78 (1.26–2.51) | 1.72 (1.21–2.43) |
| P for trend | 0.006 | 0.001 | ||||||
Abbreviations: CI, confidence interval; OR, odds ratio.
aAdjusted for age and urban or rural residence.
bAll controls included in this study were used.
Fig. 2.
Effects combining years of smoking with cigarettes smoked per day on glioma deaths (adjusted for age and urban or rural residence; *P < .01).
Discussion
To the best of our knowledge, this is the first study to indicate an overall association of cigarette smoking with adult gliomas among both men and women. Our main finding was that cigarette smoking increased the risk of glioma deaths, especially in the population aged 50 years or older.
Whether or not suspect agents can cross the BBB or whether environmental exposures can reach the brain by other routes is crucial in the study of risk factors for gliomas.19 Evidence showed that cigarette smoking could severely impair endothelial function by directly affecting endothelial tight junctions and the ionic homeostasis across the endothelium at the cerebrovascular level and specifically at the BBB.13,20,21 More recently, an effect of nicotine on cellular proliferation, migration, signaling, and radiation sensitivity by activation of the epidermal growth factor receptor and protein kinase B, has been shown in gliomas.14 Additionally, intravenously administered N-nitrous compounds in cigarette smoke have been shown to induce gliomas in rats.22
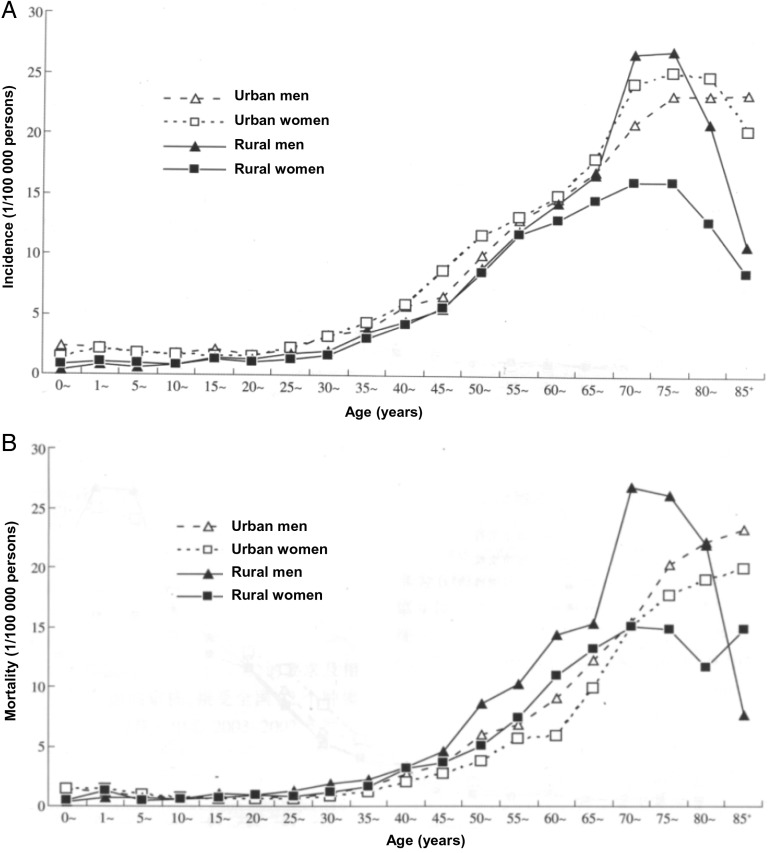
In our study, the duration and dose of exposure to tobacco were highly associated with glioma deaths independent of sex, age, and urban or rural residence. We first hypothesized that carcinogens in cigarette smoke may act to initiate tumors in the brain, with an induction period of at least 30 years, which is similar to other malignant tumors.23 This induction period of 30 smoking years seems to be particularly associated with a higher risk of glioma deaths when combined with a higher number of cigarettes smoked per day (Fig. 2). Our study showed that the proportion of smokers with more than 30 years of smoking was much higher than subjects with fewer years of smoking (Table 3). Furthermore, smokers aged 50 years or older in our study had a significantly increased risk of glioma death. A young age for starting smoking (52.4% of male smokers and 37.4% of female smokers in this study started smoking before the age of 20 years), and a low rate of quitting smoking (eg, 3.5%18) substantially contribute to a large number of long-term smokers. The incidence of brain tumors is also influenced by age, and the majority of cases were aged 50 years or older as shown in Fig. 3. In China, rural men have the highest brain tumor mortality of 3.29 per 100 000 compared with urban men (2.47 per 100 000), urban women (2.00 per 100 000), and rural women (2.40 per 100 000) (1). Our results correspondingly showed not only the highest risk of glioma deaths from smoking by 51%, but also indicated that the greatest preventive effect of smoking cessation would be in rural male smokers aged 50 years or older (ie, 32.4% of all glioma deaths would be prevented if tobacco use ceased in this group). In considering a strategy for smoking cessation, taking into account the induction period for the effect of cigarette smoking, rural men of all ages would be an important population to target. Additionally, it was reported that there was a much higher incidence and an increase in incidence of brain tumors during 2003–2007 in urban areas than that in rural areas (7.07 per 100 000 vs 4.99 per 100 000; 18.26% vs 12.67%).1 Therefore, early detection and preventive strategies are particularly important in urban areas. Long-term heavy smokers in our study had an excess risk of glioma death. Thus, establishing a smoking history for individuals attending brain tumor clinics is likely to be of value.
Fig. 3.
Incidence and mortality of brain tumors in China (2003–2007).1
Our study indicated that the association between smoking and glioma death was likely stronger among women than among men, as supported by previous studies.7,8 For instance, in a cohort of 133 811 subscribers to the Kaiser Permanente Medical Care Program of Northern California aged at least 25 years old with follow-up of up to 21 years, there were 130 glioma cases and an increased risk of gliomas among women of 70% (hazard ratio [HR] = 1.7; 95% CI: 0.9–3.1), 80% (HR = 1.8; 95% CI: 0.8–4.1), and 200% (HR = 3.0; 95% CI: 0.9–10.6) for less than 1 pack, 1–2 packs, and more than 2 packs smoked per day, respectively (P for trend =0.04), compared with non-smokers; but this finding was not observed in men.7 This is particularly meaningful in China, where women have a much higher incidence (5.2 per 100 000) of brain tumors than in the world overall (3.2 per 100 000), less developed regions (2.8 per 100 000), and more developed regions (4.4 per 100 000) and further indicates a need for preventive measures against smoking.1,24
A key strength is that this is the largest population-based case-control study with the most pathology-based cases, to our knowledge, on smoking in relation to gliomas. Because an underlying weak effect of tobacco on gliomas was indicated by previous studies, statistical power could not be achieved by fewer cases. For example, although the National Institutes of Health-AARP Diet and Health Study recruited 477 095 participants, current tobacco exposure rate much lower than ours (12.5% vs 39.2%) was probably difficult to result in more incident cases of gliomas and significant difference between exposure group and controls thus, especially in men, current heavy smoking was at odds shown to be associated with a reduced risk of gliomas.4,25 Next, getting probability sample of controls may be too time consuming or otherwise infeasible in such a huge source population from which cases were identified. Selecting living spouses as controls made it possible to produce an approximate random sample, considering that the distribution of all deaths was at random in the source population, as were their spouses’ deaths.26 This design is novel and has been verified by our serial studies.17,18,26,27 Additionally, living spouses recalled exposure information for both cases and controls; thus, accuracy of the information was guaranteed.
In this study, regardless of pathology-based comparison or not, the association of smoking with gliomas was consistently observed. We excluded 42 cases with ICD-9 codes of both gliomas and other malignant tumors including lung cancer, which is prone to brain metastases, to reduce misclassification and guarantee a case group with a glioma as the underlying cause of death (Fig. 1). Obvious differences in exposure levels by years of smoking and numbers of cigarettes smoked daily were not observed between pathology-based cases and non-pathology-based cases (Table 2); this allowed legitimate comparison of these 2 datasets with controls, although the younger age of pathology-based cases, when compared with non-pathology-based cases (54.1 vs. 58.0 years) likely weakened the association between smoking and gliomas. We also observed robust significance of overall ORs from 1.10 to 1.13 on the base of pathology-based comparisons when 40%, 50%, … ,100% of non-pathology-based cases were assumed as pathology-based cases (see Supplementary material, appendix Table S1 showing 0%, 10%, 20%, … ,100% of non-pathology-based cases were, step by step, involved into pathology-based comparisons). Considering that 81.3% of gliomas, a life-threatening disease, were diagnosed and treated in a tertiary hospital and other malignant tumors were excluded in the case group, a misdiagnosis rate could not be more than 40% where a change from non-significance to significance was observed, as shown in supplementary material, appendix Table S1. Moreover, a 5-year survival rate of gliomas was close to zero at the time of the survey; thus, retrospective collection of smoking information 5 years before death could decrease bias from changes in behavior after diagnosis. As there was little ionizing radiation exposure and cell phone use at the time of the study, there was likely no contribution to bias. Based on these facts, our results are reliable. Finally, China's smoking pattern is stable: in the past 3 decades China had a slight decreasing trend of current smoking prevalence from 33.88% in 1984 to 28.1% in 2010 for adults aged 15 years or older.18 Therefore, we could justifiably derive some implications for current tobacco control policies in China using the etiological linkage (ie, ORs from national survey data collected in 1989–1991).
This study has some limitations. We cannot exclude bias from genetic susceptibility and specific genetic polymorphisms, which are likely risk factors for gliomas.28,29 Also, some confounding factors, such as alcohol intake, were not acquired in such a large-scale field survey, so we could not adjust for these confounders. Next, recall bias, a measurement error from surviving spouses that might attenuate the association between smoking and gliomas, could not be completely excluded. Additionally, we could not exclude diagnostic bias from two-thirds of non-pathology-based cases completely.
In conclusion, this study across 28 provincial administrative regions of China provides the first evidence to support an association between tobacco use and gliomas among both male and female adults. Given the relationships between smoking and various forms of malignant tumor and cardiovascular diseases, it is highly advisable that the government develop strong tobacco prevention and cessation programs in both urban and rural areas of China. In particular, smoking cessation should be targeted at rural men. For individuals attending brain tumor clinics, long-term heavy smoking could be recognized as a factor for risk stratification and behavior intervention; this is important for urban residents, especially urban women.
Supplementary Material
Funding
The Medical Research Council and Imperial Cancer Research Fund in Britain, US National Institutes of Health, the Chinese Ministry of Health, and Chinese Academy of Medical Sciences funded the original survey. Professor Richard Peto, the local government, and the thousands of doctors, nurses, and other workers in the medical field gave great support to this project. This work, including analysis, decision to publish, and preparation of the manuscript, has been supported by a UICC International Cancer Technology Transfer Fellowship under Contract NO ICR/13/073/2013 UICC.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Duan JJ, Chen WQ, Yang NN et al. An analysis on incidence and mortality of brain tumors from 2003 to 2007 in China [in Chinese]. China Cancer. 2012;21(9):644–649. [Google Scholar]
- 2.Sadetzki S, Chetrit A, Freedman L et al. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–432. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Barnholtz-Sloan JS. Current state of our knowledge on brain tumor epidemiology. Curr Neurol Neurosci Rep. 2011;11(3):329–335. [DOI] [PubMed] [Google Scholar]
- 4.Braganza MZ, Rajaraman P, Park Y et al. Cigarette smoking, alcohol intake, and risk of glioma in the NIH-AARP Diet and Health Study. Br J Cancer. 2014;110(1):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson VS, Pirie K, Green J et al. Million Women Study Collaborators. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99(1):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick CN, Giovannucci EL, Rosner B et al. Prospective study of cigarette smoking and adult glioma: dosage, duration, and latency. Neuro Oncol. 2007;9(3):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efird JT, Friedman GD, Sidney S et al. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol. 2004;68(1):57–69. [DOI] [PubMed] [Google Scholar]
- 8.Silvera SA, Miller AB, Rohan TE. Cigarette smoking and risk of glioma: a prospective cohort study. Int J Cancer. 2006;118(7):1848–1851. [DOI] [PubMed] [Google Scholar]
- 9.Zheng T, Cantor KP, Zhang Y et al. Risk of brain glioma not associated with cigarette smoking or use of other tobacco products in Iowa. Cancer Epidemiol Biomarkers Prev. 2001;10(4):413–414. [PubMed] [Google Scholar]
- 10.Hu J, Johnson KC, Mao Y et al. Risk factors for glioma in adults: a case-control study in northeast China. Cancer Detect Prev. 1998;22(2):100–108. [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA). Cancer Causes Control. 1997;8(1):13–24. [DOI] [PubMed] [Google Scholar]
- 12.Mandelzweig L, Novikov I, Sadetzki S. Smoking and risk of glioma: a meta-analysis. Cancer Causes Control. 2009;20(10):1927–1938. [DOI] [PubMed] [Google Scholar]
- 13.Mazzone P, Tierney W, Hossain M et al. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int J Environ Res Public Health. 2010;7(12):4111–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalil AA, Jameson MJ, Broaddus WC et al. Nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation. Brain Tumor Pathol. 2013;30(2):73–83. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364(25):2469–2470. [DOI] [PubMed] [Google Scholar]
- 16.Yang G. Marketing ‘less harmful, low-tar’ cigarettes is a key strategy of the industry to counter tobacco control in China. Tob Control. 2014;23(2):167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu BQ, Peto R, Chen ZM et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317(7170):1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou L, Jiang J, Liu B et al. Association between smoking and deaths due to colorectal malignant carcinoma: a national population-based case-control study in China. Br J Cancer. 2014;110(5):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bondy ML, Scheurer ME, Malmer B et al. Brain Tumor Epidemiology Consortium. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113(7 suppl):1953–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbruscato TJ, Lopez SP, Mark KS et al. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91(12):2525–2538. [DOI] [PubMed] [Google Scholar]
- 21.Abbruscato TJ, Lopez SP, Roder K et al. Regulation of blood-brain barrier Na,K,2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J Pharmacol Exp Ther. 2004;310(2):459–468. [DOI] [PubMed] [Google Scholar]
- 22.Maekawa A, Mitsumori K. Spontaneous occurrence and chemical induction of neurogenic tumors in rats--influence of host factors and specificity of chemical structure. Crit Rev Toxicol. 1990;20(4):287–310. [DOI] [PubMed] [Google Scholar]
- 23.Giovannucci E, Martínez ME. Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst. 1996;88(23):1717–1730. [DOI] [PubMed] [Google Scholar]
- 24.Ferlay J, Shin HR, Bray F et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO report on the global tobacco epidemic, 2008-The MPOWER package. Available at http://www.who.int/tobacco/mpower/2008/en/. Accessed July 28, 2015.
- 26.Jiang JM, Zeng XJ, Chen JS et al. Smoking and mortality from esophageal cancer in China: a large case-control study of 19,734 male esophageal cancer deaths and 104,846 living spouse controls. Int J Cancer. 2006;119(6):1427–1432. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Liu B, Nasca PC et al. Comparative study of control selection in a national population-based case-control study: Estimating risk of smoking on cancer deaths in Chinese men. Int J Med Sci. 2009;6(6):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melean G, Sestini R, Ammannati F et al. Genetic insights into familial tumors of the nervous system. Am J Med Genet C Semin Med Genet. 2004;129C(1):74–84. [DOI] [PubMed] [Google Scholar]
- 29.Bethke L, Webb E, Murray A et al. Comprehensive analysis of the role of DNA repair gene polymorphisms on risk of glioma. Hum Mol Genet. 2008;17(6):800–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.