Abstract
Background
Primary malignant brain tumors (PMBTs) are devastating malignancies with poor prognosis. Optimizing psychosocial and supportive care is critical, especially in the later stages of disease.
Methods
This retrospective cohort study compared early versus late hospice enrollment of PMBT patients admitted to the home hospice program of a large urban, not-for-profit home health care agency between 2009 and 2013.
Results
Of 160 patients with PMBT followed to death in hospice care, 32 (22.5%) were enrolled within 7 days of death. When compared with patients referred to hospice more than 7 days before death, a greater proportion of those with late referral were bedbound at admission (97.2% vs 61.3%; OR=21.85; 95% CI, 3.42–919.20; P < .001), aphasic (61.1% vs 20.2%; OR = 6.13; 95% CI, 2.59–15.02; P < .001), unresponsive (38.9% vs 4%; OR = 14.76,;95% CI, 4.47–57.98; P < .001), or dyspneic (27.8% vs 9.7%; OR = 21.85; 95% CI, 3.42–10.12; P = .011). In multivariable analysis, male patients who were receiving Medicaid or charitable care and were without a health care proxy were more likely to enroll in hospice within 1 week of death.
Conclusions
Late hospice referral in PMBT is common. PMBT patients enrolled late in hospice are severely neurologically debilitated at the time hospice is initiated and therefore may not derive optimal benefit from multidisciplinary hospice care. Men, patients with lower socioeconomic status, and those without a health care proxy may be at risk for late hospice care and may benefit from proactive discussion about end-of-life care in PMBT, but prospective studies are needed.
Keywords: glioblastoma, malignant brain tumor, palliative care, quality of life
Hospice provides essential psychosocial and supportive care for individuals at the end of life (EOL). For patients with cancer, including those with primary malignant brain tumors (PMBTs), initiation of early multidisciplinary hospice care is associated with better pain relief, quality of life, reduced cost, and less aggressive care at the EOL as well as less psychiatric morbidity for caregivers.1–6 Despite the benefits of hospice care in advanced cancer, late referral to hospice remains common, and hospice services are underutilized.7–9 Transition to hospice within 1 month of death was associated with undignified death in one study of PMBT patients.10
PMBTs are devastating and often incurable cancers. Patients with PMBT endure a heavy burden of symptoms at the EOL including cognitive changes, aphasia, immobility, headache, seizures, and incontinence.11–15 Caregivers experience significant psychological burden in caring for those with PMBT as a result of patients' neurologic dysfunction and behavioral changes.16,17 Home hospice care in PMBT is associated with improved patient function and quality of life as well as caregiver well-being.18 Despite the salient hospice needs of PMBT patients and caregivers at EOL, little is known about the frequency of and the characteristics associated with late hospice referral in PMBT.
Materials and Methods
Study Sample
This study included all patients with PMBT who were admitted to a large urban, not-for-profit home hospice agency in New York City between 2009 and 2013. Eligibility criteria included a diagnosis of malignant neoplasm of the brain (by ICD-9 code), age of at least 18 years, and death in the agency's home hospice care by the end of 2013. Patients without histopathologic diagnosis, but a PMBT documented by neuroimaging, were included in the study. All charts were reviewed manually to exclude patients with systemic cancers and brain metastases erroneously coded as PMBT. For patients with multiple episodes of home hospice care during the study period, only the first episode was included. Participating institutional review boards approved all study procedures. A waiver of informed consent was authorized because patients were deceased at the time their records were reviewed.
Study Data and Measures
Demographic, clinical, and service data were obtained from standardized assessment forms captured through the agency's hospice database. Unless otherwise noted, all clinical characteristics were extracted from the database and were obtained within 3 days of hospice admission. Twelve patients were missing data elements from the clinical assessment, and this information was obtained by reviewing their clinical progress notes.
Demographic characteristics included sex, age, race/ethnicity, marital status, primary language spoken at home, insurance provider, and the presence or absence of a health care proxy (HCP) at the time of hospice admission. Specific PMBT diagnosis was extracted from clinical notes and classified into one of 4 categories: glioblastoma (GBM), non-GBM glioma of any grade (astrocytoma, oligodendroglioma, or mixed glioma), other brain tumor, or those not specified. A score for each patient on the Palliative Performance Scale (PPS),19 a reliable and valid tool for evaluating a patient's clinical and functional status specifically in the palliative care setting, was extracted from the hospice admission assessment. The PPS score, scaled from 0 (deceased) to 100 (no evidence of disease and no limitations on any aspect of daily life) was assigned to these patients by a hospice nurse. The score represents an evaluation of the domains of ambulation, activity level, evidence of disease, and self-care abilities.20 A dichotomous variable was also created to distinguish patients with a PPS score of 30 or less, which characterizes patients who are entirely bedbound, have reduced consciousness, and are unable to do any activities of daily living. Binary indicators of the following patient characteristics were captured: whether the patient was bedbound, unable to communicate (aphasic), unresponsive, incontinent (bowel and/or bladder), experiencing pain of any severity, nausea of any severity, or dyspnea of any severity.
Length of service (LOS) was calculated as the number of days from admission date to the date of death, with late hospice care defined as LOS <7 days, as in previous studies.21,22 Visit records were used to characterize 7 types of home services received including skilled nursing, social work, home health aide, spiritual counseling, and others. Dichotomous variables were created to indicate receipt of each service. Service intensity was calculated for all patients within each service discipline as the total number of visits (or hours for service provided by home health aides) divided by the number of weeks enrolled in hospice care.
Statistical Analyses
Descriptive statistics, including means and frequencies, were employed to summarize patients' demographic, clinical, functional, and service characteristics. Our primary outcome of interest was late referral to hospice as defined above. A multivariable logistic regression model was used to examine sociodemographic predictors of late referral. Fisher's exact tests were employed to estimate bivariate associations between clinical characteristics and late referral status. All analyses were performed using R (version 3.1.2, “Pumpkin Helmet”).23
Results
Sample Characteristics
The characteristics of 160 patients meeting all study criteria are described in Table 1. The mean age of the sample was 63.4 years (SD, 15.6), and a majority of patients (92; 58%) were male. One hundred forty-seven (73%) patients had GBM or non-GBM glioma. Six (4%) had another primary brain tumor including primary central nervous system lymphoma, malignant meningioma, and others. Thirty-seven (23%) had no histopathologic diagnosis recorded or had not undergone biopsy. Fifty-eight (36%) patients did not have an appointed HCP at the time of home hospice admission. Most patients (131; 82%) died at home, and death occurred at an affiliated hospice facility for 29 (18%) patients.
Table 1.
Demographic characteristics of study population (N = 160)
| All Patients (N = 160) |
Non-late (N = 124) |
Late (N = 36) |
P | ||||
|---|---|---|---|---|---|---|---|
| N | % or Mean (SD) | N | % or Mean (SD) | N | % or Mean (SD) | ||
| Sex | |||||||
| Men | 92 | 58 | 65 | 52.4 | 27 | 75 | .02 |
| Women | 68 | 42 | 59 | 47.6 | 9 | 25 | |
| Age (y) | 63.4 (15.6) | 64.23 (14.65) | 60.73 (18.43) | .30 | |||
| ≤65 | 84 | 52.5 | 64 | 51.6 | 20 | 55.6 | .71 |
| >65+ | 76 | 47.5 | 60 | 48.4 | 16 | 44.4 | |
| Race/ethnicity | |||||||
| White non-Hispanic | 99 | 62 | 80 | 64.5 | 19 | 52.8 | .60 |
| Hispanic | 23 | 14 | 17 | 13.7 | 6 | 16.7 | |
| Asian/Pacific Islander | 16 | 10 | 11 | 8.9 | 5 | 13.9 | |
| African American | 12 | 8 | 8 | 6.5 | 4 | 11.1 | |
| Other/unknown | 10 | 6 | 8 | 6.5 | 2 | 5.6 | |
| Marital status | |||||||
| Married | 88 | 55 | 69 | 55.6 | 19 | 52.8 | .85 |
| Not married | 72 | 45 | 55 | 44.4 | 17 | 47.2 | |
| Primary language spoken | |||||||
| English | 141 | 88 | 111 | 89.5 | 30 | 83.3 | .38 |
| Language other than English | 19 | 12 | 13 | 10.5 | 6 | 16.7 | |
| Payer | |||||||
| Medicare or private insurance | 126 | 79 | 103 | 83.1 | 23 | 63.9 | .02 |
| Medicaid or charitable care | 34 | 21 | 21 | 16.9 | 13 | 36.1 | |
| Health care proxy status | |||||||
| Patient has health care proxy | 102 | 64 | 86 | 69.4 | 16 | 44.4 | .01 |
| Does not have health care proxy | 58 | 36 | 38 | 30.6 | 20 | 55.6 | |
Clinical Characteristics and Utilization of Homecare Services
Clinical characteristics at the time of hospice admission are summarized in Table 2. Patients' PPS scores ranged from 10–60, with a median score of 30 (interquartile range = 13.4). Ninety-one (56.9%) had a PPS score ≤ 30 at admission. A large number of patients were bedbound at the time of admission (111; 69.4%), and a substantial proportion were aphasic (47; 29.4%) or unresponsive (19; 11.9%). Pain of any severity was reported by 34 (21.2%) patients, nausea by 15 (9.4%), and shortness of breath by 22 (13.8%). Eighty-two (38.7%) patients were incontinent of bladder and/or bowel.
Table 2.
Clinical characteristics of study population at hospice admission (N = 160)
| All Patients (N = 160) |
Non-late (N = 124) |
Late (N = 36) |
P | ||||
|---|---|---|---|---|---|---|---|
| N | % or Mean (SD) | N | % or Mean (SD) | N | % or Mean (SD) | ||
| Tumor Type | |||||||
| Glioblastoma | 104 | 65 | 81 | 65.3 | 23 | 63.9 | .08 |
| Glioma | 13 | 8.1 | 100 | 8.1 | 3 | 8.3 | |
| Other | 6 | 3.8 | 2 | 1.6 | 4 | 11.1 | |
| Not specified/biopsied | 37 | 23.1 | 31 | 25.0 | 6 | 16.7 | |
| Palliative performance scale | 32.1 (13.4) | ||||||
| Score of 30 or higher | 69 | 43.1 | 65 | 52.4 | 4 | 11.1 | <.001 |
| Score of 29 or below | 91 | 56.9 | 59 | 47.6 | 32 | 88.9 | |
| Activity limitation | |||||||
| Bedbound | 111 | 69.4 | 76 | 61.3 | 35 | 97.2 | <.001 |
| Not bedbound | 49 | 30.6 | 48 | 38.7 | 1 | 2.8 | |
| Ability to communicate | |||||||
| Uncommunicative | 47 | 29.3 | 25 | 20.2 | 22 | 61.1 | <.001 |
| Not uncommunicative | 113 | 70.7 | 99 | 79.8 | 14 | 38.9 | |
| Responsiveness | |||||||
| Unresponsive | 19 | 11.8 | 5 | 4.0 | 14 | 38.9 | <.001 |
| Not unresponsive | 141 | 88.2 | 119 | 96.0 | 22 | 61.1 | |
| Pain | |||||||
| Yes | 34 | 21.2 | 27 | 21.8 | 7 | 19.4 | >.99 |
| No | 126 | 78.8 | 97 | 78.2 | 29 | 8.6 | |
| Nausea | |||||||
| Yes | 15 | 9.4 | 13 | 10.5 | 2 | 5.6 | .52 |
| No | 145 | 90.6 | 111 | 89.5 | 34 | 94.4 | |
| Shortness of breath | |||||||
| Yes | 22 | 13.8 | 12 | 9.7 | 10 | 27.8 | .01 |
| No | 138 | 86.2 | 112 | 90.3 | 26 | 72.2 | |
| Incontinence | |||||||
| Yes | 67 | 42 | 43.5 | 54 | 36.1 | 13 | .45 |
| No | 93 | 58 | 56.5 | 70 | 63.9 | 23 | |
Abbreviations: SD, standard deviation.
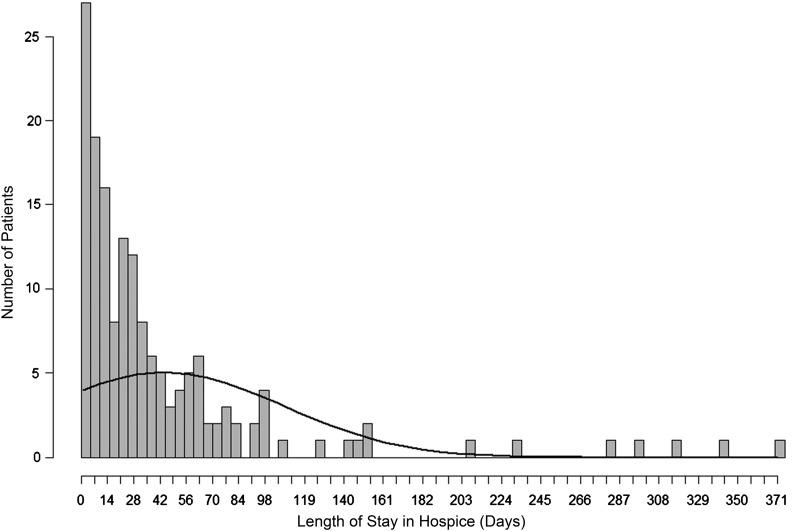
The mean LOS was 44.3 days (SD, 63.5; Fig. 1, Table 3). Fifty-six patients (35%) had LOS <14 days, and 32 (20%) were enrolled in hospice < 7 days. All patients had home nursing care with a mean of 3.2 visits per week, 198 (93.4%) had visits by a social worker (mean, 1.2 visits per week), and 100 (47.2%) had a physician visit the home (mean, 0.5 visits per week). One hundred thirty-three (62.7%) patients had a home health aide (mean, 20.9 hours per week of service), and 126 (59.4%) had spiritual counseling at home (mean, 1.0 visits per week).
Fig. 1.
Histogram of length of stay in hospice for patients with primary malignant brain tumors.
Table 3.
Hospice service characteristics of study population
| N | % or Mean (SD) | |
|---|---|---|
| Average length of service (days) | 44.3 (63.5) | |
| < 7 days | 32 | 20.0 |
| 7–14 days | 24 | 15.0 |
| 14–30 days | 39 | 24.3 |
| 31–60 days | 31 | 19.4 |
| > 60 days | 34 | 21.3 |
| Services received | ||
| Nursing | 160 | 100.0 |
| Social work | 150 | 93.8 |
| Home health aide | 101 | 63.1 |
| Counselor | 101 | 63.1 |
| Physician | 80 | 50.0 |
| Physical/occupational therapy | 27 | 16.9 |
| Other services | 17 | 10.1 |
| Service intensity per week | N | Mean (SD) |
| Nursing visits | 160 | 3.7 (3.2) |
| Social work visits | 150 | 1.2 (1.0) |
| Home health aide hours | 101 | 19.6 (21.1) |
| Spiritual counselor visits | 101 | 0.8 (1.0) |
| Physician visits | 80 | 0.5 (0.5) |
| Therapy visits | 27 | 0.5 (0.4) |
| Other visits | 17 | 0.4 (0.4) |
Abbreviation: SD, standard deviation.
Late Versus Earlier Referral to Home Hospice
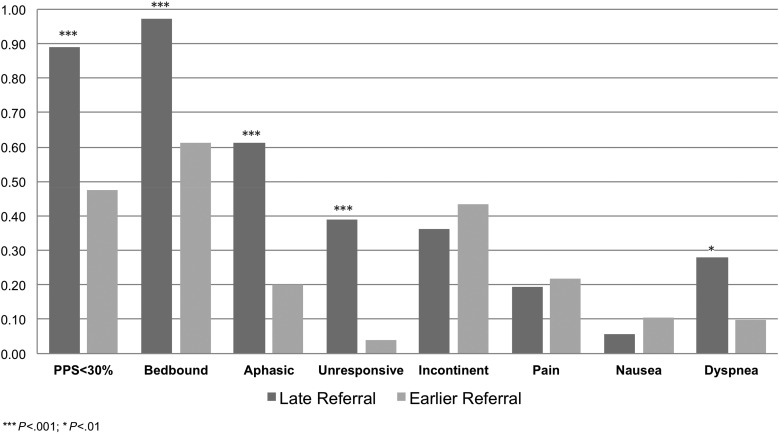
Comparison of clinical characteristics between patients with late versus non-late hospice referral prior to death is presented in Table 3. As compared with patients referred to hospice longer than 7 days before death, a greater proportion of those with late hospice referral had poorer PPS at admission (88.9% with PPS < 30 vs 47.6%; OR = 8.71; 95% CI, 2.85–35.89; P < .001, see Fig. 2); were bedbound at admission (97.2% vs 61.3%; OR = 21.85; 95% CI, 3.42–919.20; P < .001); aphasic (61.1% vs 20.2%; OR = 6.13; 95%, CI, 2.59–15.02; P < .001); unresponsive (38.9% vs 4%, OR = 14.76, 95% CI, 4.47–57.98, P < .001), or dyspneic (27.8% vs 9.7%, OR = 21.85, 95% CI, 3.42–10.12; P = .011, Table 3). There was no significant difference between patients with late versus non-late hospice referral with respect to incontinence, pain, or nausea. Sociodemographic correlates of late hospice referral in a multivariate logistic regression model (Table 4) included male sex (75% of late referrals vs 52.4%; OR = 7.09; 95% CI, 2.45–24.26; P < .001), having Medicaid or charitable care (36.1% of late referral vs 17%; OR = 6.35; 95%, CI, 1.85–23.89; P < .001), and not having an appointed HCP at the time of hospice admission (56% of late referral vs 31%; OR = 6.35; 95%, CI 1.53–9.25; P < .001). Age, marital status, English as primary language, and race/ethnicity were not associated with late hospice enrollment. As compared with non-late referrals, late-referred patients required more nursing visits per week (6.8 vs 2.9; P < .001), more social work visits per week (1.8 vs 0.9; P < .001), more spiritual counseling visits (0.9 vs 0.4; P = .05), and received fewer physical therapy visits (0 vs 0.1; P < .001).
Fig. 2.
Clinical characteristics of home hospice patients with primary malignant brain tumors PMBT by late referral status. Patients initiating home hospice care within 7 days of death were more frequently debilitated by Palliative Performance Scale score, bedbound, aphasic, and dyspneic at the time of admission as compared with those admitted prior to one week before death.
Table 4.
Correlates of late referral status among home hospice deaths (N = 160)
| % late | % Non-late | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Multivariable model of sociodemographic correlates | |||||
| Sex | |||||
| Female | 25.0 | 47.6 | 1.00 | (ref) | (ref) |
| Male | 75.0 | 52.4 | 7.09 | 2.45–24.26 | <.001 |
| Age (y) | |||||
| Age <65 | 55.6 | 51.6 | 1.00 | (ref) | (ref) |
| Age >65 | 44.4 | 48.4 | 2.07 | 0.80–5.71 | .143 |
| Marital status | |||||
| Not married | 47.2 | 44.4 | 1.00 | (ref) | (ref) |
| Married | 52.8 | 55.6 | 0.71 | 0.28–1.79 | .465 |
| English | |||||
| English is primary language | 83.3 | 89.5 | 1.00 | (ref) | (ref) |
| English is not primary language | 16.7 | 10.5 | 0.70 | 0.16–2.74 | .623 |
| Race/ethnicity | |||||
| Non-Hispanic | 52.8 | 64.5 | 1.00 | (ref) | (ref) |
| Hispanic | 16.7 | 13.7 | 1.37 | 0.35–4.91 | .633 |
| Asian/Pacific Islander | 13.9 | 8.9 | 0.53 | 0.10–2.35 | .428 |
| African American | 11.1 | 6.5 | 1.88 | 0.38–8.47 | .417 |
| Other/unknown | 5.6 | 6.5 | 1.23 | 0.17–6.12 | .812 |
| Payer | |||||
| Medicare or private insurance | 63.9 | 83.1 | 1.00 | (ref) | (ref) |
| Medicaid or charitable care insurance | 36.1 | 16.9 | 6.35 | 1.85–23.89 | .004 |
| Health care proxy status | |||||
| Yes | 44.4 | 69.4 | 1.00 | (ref) | (ref) |
| No | 55.6 | 30.6 | 3.68 | 1.53–9.25 | .004 |
| Bivariate model of clinical correlates at admission | |||||
| Palliative performance scale score <30% | 88.9 | 47.6 | 8.71 | 2.85–35.89 | <.001 |
| Bedbound | 97.2 | 61.3 | 21.85 | 3.42–912.20 | <.001 |
| Aphasic | 61.1 | 20.2 | 6.13 | 2.59–15.02 | <.001 |
| Unresponsive | 38.9 | 4.0 | 14.76 | 4.47–57.96 | <.001 |
| Incontinent | 36.1 | 43.5 | 0.73 | 0.31–1.67 | .450 |
| Pain | 19.4 | 21.8 | 0.87 | 0.29–2.32 | .482 |
| Nausea | 5.6 | 10.5 | 0.50 | 0.05–2.40 | .523 |
| Shortness of breath | 27.8 | 9.7 | 3.55 | 1.23–10.12 | .011 |
Abbreviations: CI, confidence interval; OR, odds ratio
Discussion
This study of home hospice care prior to death in patients with PMBT examined clinical correlates and sociodemographic features associated with referral to hospice within 7 days of death. Our observation was that home hospice enrollment was generally late: 22.5% of our study population entered hospice within 7 days of death, 35% of the study population entered within 14 days, and 59.4% entered within 30 days of death. Those entering hospice within 7 days of death were more functionally impaired (immobile, aphasic, or unresponsive) and symptomatic (dyspneic) at the time of initiation to hospice care. Multivariable analysis demonstrated that male sex, Medicaid insurance coverage, and absence of a HCP were significantly associated with late referral. These are important findings given the particular clinical and prognostic context of glioma, glioblastoma, and other PMBTs. First, malignant glioma remains an incurable disease, and therefore it is the timing (“when”) of EOL care, not its necessity (“if”), that is at issue for each and every patient and caregiver. PMBT patients endure a heavy burden of symptoms13–15 and diminished quality of life at the end of life.24 Therefore, the finding that hospice referral is predominantly late, together with the demonstration that patients referred late are densely neurologically impaired and more symptomatic, suggest that a substantial proportion of PMBT patients in the later stages of disease are underserved with respect to symptom management. If patients are aphasic or comatose at the initiation of hospice care, the opportunity to palliate symptoms and provide meaningful psychosocial care may have been missed. While it is to be expected intuitively that patients closer to death will be more symptomatic and impaired than those who are not, our data demonstrate the high degree of clinical debilitation at which late-referred patients first receive hospice care. Our data indicate a potential population at risk for late hospice referral, namely men and those of lower socioeconomic status as reflected by insurance payer, and it is possible that educational interventions about hospice care should be directed to these more vulnerable groups. Finally, these data suggest that the assignation of a HCP may be particular important for the timing of hospice referral for patients with brain tumors.
The reason for late hospice referral in PMBT is not clear. Insofar as referral to hospice takes place in the larger context of discussion about prognosis, prognostic communication has been found to be a particularly challenging process in PMBT.25,26 A systematic review about prognostic communication in malignant glioma27 suggests (from limited literature) that a substantial proportion of patients with malignant glioma are unaware of the incurability of the disease. Frequency of inaccurate awareness of prognosis has been described in the context of nonneurologic cancers28; the prevalence of such beliefs and the underlying reasons for them in neurologic as compared with nonneurologic cancers has not been studied.
Patterns of sociodemographic predictors similar to those found here have been demonstrated in studies of other cancers. Male sex predicted later hospice referral in a study of lung and colorectal cancer7 and in a sample of mixed malignant diseases.29 The reasons for this are not known, although the association between male sex and aggressive care in cancer has been demonstrated previously.30,31 Lower socioeconomic status was found to be associated with decreased likelihood of hospice use in lung cancer32,33 and ovarian cancer patients.34 Similar to our findings, a study of lung cancer in New York State demonstrated that those with Medicaid were less likely to enroll in hospice care.35 Why there is an association between lower socioeconomic status and decreased hospice use has not been established. It has been postulated that Medicaid patients are less likely to have an able-bodied informal caregiver at home and are more likely to receive care in the context of training or rotating physicians, rendering the care environment less amenable to advanced planning of EOL care.35
To our knowledge, late hospice referral or other components of aggressive EOL care have not been previously found to be associated with the absence of an assigned HCP. In the health care agency studied here, designation of a HCP is encouraged but not required for home hospice care. Frequent absence of a HCP has been shown in other cancers; one study of patients with hematologic malignancies reported that 32% had no HCP;36 in a retrospective review of admissions into an oncologic ICU, 37% had no living will or HCP.37 Our data suggest that there may be a uniquely important role for a HCP in the context of neurologic malignancy, perhaps because of patients' impaired cognition. It has been found in nonneurologic cancers that caregiver preferences for EOL care are more predictive of late medical decisions for patients with mild cognitive impairment as opposed to those with no impairments.38 This is an area of needed investigation in neuro-oncologic diseases.
PMBTs are not only cancers but also progressive neurologic diseases, and it is illuminating to consider similar findings regarding EOL care in these entities as well. Men with ALS are more likely to die in an acute care facility than at home.39 Male sex and Medicaid enrollment are associated with decreased use of hospice in patients who have suffered stroke,40 and male sex is associated with decreased hospice use in patients with Alzheimer disease.41 Kiely et al. found that for patients with advanced dementia, HCP's awareness of prognosis was associated with greater hospice enrollment. This finding points to the likely importance of the role of the HCP and caregiver in initiating hospice care in neurologic diseases. Furthermore, these similarities suggest that the study of EOL care in brain tumors may be enriched by considering the context of neurologic diseases as well as that of other cancers.
There are predictors of late hospice care in other cancers, which we did not observe, that were demonstrated in other studies. There are many studies of various cancers demonstrating later hospice referral among individuals of non-white race7,32–34,42,43 and among those living in minority non-white areas.31 It is possible that our sample was not large enough to detect an association between race or ethnicity and hospice referral, or alternatively it is possible that they are less predictive of EOL outcomes in neuro-oncologic diseases. The underlying reasons for racial difference in EOL care are not known, although one observation is that prognostic conversations and EOL discussions, even when taking place, affect EOL outcomes less for non-white patients than for white patients.42,44 One could speculate that cognitive changes in brain cancer could alter either treatment preferences or dynamics of patient-physician communication. It has been demonstrated that patients with brain tumors are frequently unable to participate in medical decision-making by virtue of cognitive impairment45,46 and that minor cognitive deficits may present obstacles to full understanding of their options for medical care.47 Further dedicated study may illuminate the interaction between cognitive impairment and the frequency of prognostic discussions. Other studies have demonstrated associations between late hospice referral and discussion about prognosis and EOL treatments34,35 as well as aspects of religiosity.48–50 These issues were not evaluated in this retrospective study. Further, information pertaining to the circumstances and discussion regarding hospice referral for patients included in our study was not available for review.
There may be practical implications for the clinician treating PMBT patients to draw from these findings, although with the caveat that there is insufficient evidence to substantiate firm recommendations. First, assignation of a responsible HCP appears from these data to be of particular importance in PMBT, and this study would suggest that this be accomplished earlier, rather than later, in the disease trajectory. Furthermore, PMBT patients may benefit from efforts made to inform the HCP about prognosis and survival in PMBT to increase the likelihood of earlier hospice referral and therefore maximal benefit from multidisciplinary palliative care. Patients who are male or of lower socioeconomic status may benefit from earlier and proactive discussion about prognosis, assignation of a HCP, advanced directives, and EOL care.
There are several limitations to this study. First, our sample is limited to a single home hospice agency in New York City, limiting the generalizability of our findings. At the same time, our population was heterogeneous with respect to referral sources and included patients referred from both academic and nonacademic settings. Second, our data were collected retrospectively from existing administrative databases, and therefore several important dimensions of medical care known to be associated with hospice referral were not captured. These include patients' and caregivers' treatment preferences, physician beliefs and experiences, communication events, and others. Also, by virtue of the retrospective nature of the study, we can describe associations between sociodemographic characteristics and timing of hospice referral, but we cannot assert causal relationships between study variables. Additionally, by focusing chiefly on the specific outcome of time before death in hospice, we studied only patients who died in hospice care, and we did not account for PMBT patients who did not pursue hospice care or who were discharged from hospice prior to death. These are other dimensions of EOL care in PMBT that merit investigation. Furthermore, a strength of this study was the uniformity of available data elements for all patients, and this would have been compromised by including patients lost to follow up.
This study is, to our knowledge, the first effort to examine timing of hospice referral in PMBT, its predictors, and associated clinical features. Our study suggests that late referral to hospice is common for patients with PMBT and that such referral may be at the expense of comprehensive EOL care. As has been found in other cancers, men and those with Medicaid payers may be at particular risk for late referral. The presence and assignation of a HCP may be of unique importance in neuro-oncologic diseases. Further research is necessary to corroborate these findings in prospective studies.
Funding
This work was supported by the Translation and Integrative Medicine Award from the Department of Medicine at Memorial Sloan Kettering Cancer Center and the Brain Tumor Center at Memorial Sloan Kettering Cancer Center (awarded to E.L.D. and A.J.A).
Acknowledgments
Dr. Diamond and Dr. Russell had full access to all data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior presentations: None.
Conflicts of interest statement. Dr. Diamond has no conflict of interest. Dr. Russell has no conflict of interest. Dr. Kryza-Lacombe has no conflict of interest. Dr. Bowles has no conflict of interest. Dr. Applebaum has no conflict of interest. Ms. Jeanne Dennis has no conflict of interest. Dr. DeAngelis has no conflict of interest. Dr. Prigerson has no conflict of interest.
References
- 1.Amano K, Morita T, Tatara R, Katayama H, Uno T, Takagi I. Association between early palliative care referrals, inpatient hospice utilization, and aggressiveness of care at the end of life. J Palliat Med. 2015;18(3):270–273. [DOI] [PubMed] [Google Scholar]
- 2.Bergman J, Saigal CS, Lorenz KA et al. . Hospice use and high-intensity care in men dying of prostate cancer. Arch Intern Med. 2011;171(3):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright AA, Zhang B, Ray A et al. . Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng SY, Dy S, Hu WY, Chen CY, Chiu TY. Factors affecting the improvement of quality of dying of terminally ill patients with cancer through palliative care: a ten-year experience. J Palliat Med. 2012;15(8):854–862. [DOI] [PubMed] [Google Scholar]
- 5.Temel JS, Greer JA, Muzikansky A et al. . Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 6.Greer JA, Pirl WF, Jackson VA et al. . Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30(4):394–400. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21(4):728–735. [DOI] [PubMed] [Google Scholar]
- 8.Tang ST, Huang EW, Liu TW, Wang HM, Rau KM, Chen JS. Aggressive end-of-life care significantly influenced propensity for hospice enrollment within the last three days of life for Taiwanese cancer decedents. J Pain Symptom Manage. 2011;41(1):68–78. [DOI] [PubMed] [Google Scholar]
- 9.Aldridge MD, Canavan M, Cherlin E, Bradley EH. Has Hospice Use Changed? 2000–2010 Utilization Patterns. Med Care. 2015;53(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sizoo EM, Taphoorn MJ, Uitdehaag B et al. . The end-of-life phase of high-grade glioma patients: dying with dignity? Oncologist. 2013;18(2):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koekkoek JA, Dirven L, Sizoo EM et al. . Symptoms and medication management in the end of life phase of high-grade glioma patients. J Neurooncol. 2014;120(3):589–595. [DOI] [PubMed] [Google Scholar]
- 12.Sizoo EM, Braam L, Postma TJ et al. . Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12(11):1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gofton TE, Graber J, Carver A. Identifying the palliative care needs of patients living with cerebral tumors and metastases: a retrospective analysis. J Neurooncol. 2012;108(3):527–534. [DOI] [PubMed] [Google Scholar]
- 14.Pace A, Di Lorenzo C, Guariglia L, Jandolo B, Carapella CM, Pompili A. End of life issues in brain tumor patients. J Neurooncol. 2009;91(1):39–43. [DOI] [PubMed] [Google Scholar]
- 15.Walbert T, Khan M. End-of-life symptoms and care in patients with primary malignant brain tumors: a systematic literature review. J Neurooncol. 2014;117(2):217–224. [DOI] [PubMed] [Google Scholar]
- 16.Finocchiaro CY, Petruzzi A, Lamperti E et al. . The burden of brain tumor: a single-institution study on psychological patterns in caregivers. J Neurooncol. 2012;107(1):175–181. [DOI] [PubMed] [Google Scholar]
- 17.McConigley R, Halkett G, Lobb E, Nowak A. Caring for someone with high-grade glioma: a time of rapid change for caregivers. Palliat Med. 2010;24(5):473–479. [DOI] [PubMed] [Google Scholar]
- 18.Pompili A, Telera S, Villani V, Pace A. Home palliative care and end of life issues in glioblastoma multiforme: results and comments from a homogeneous cohort of patients. Neurosurg Focus. 2014;37(6):E5. [DOI] [PubMed] [Google Scholar]
- 19.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. Spring 1996;12(1):5–11. [PubMed] [Google Scholar]
- 20.Myers J, Kim A, Flanagan J, Selby D. Palliative performance scale and survival among outpatients with advanced cancer. Support Care Cancer. 2015;23(4):913–918. [DOI] [PubMed] [Google Scholar]
- 21.Enzinger AC, Zhang B, Weeks JC, Prigerson HG. Clinical trial participation as part of end-of-life cancer care: associations with medical care and quality of life near death. J Pain Symptom Manage. 2014;47(6):1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright AA, Mack JW, Kritek PA et al. . Influence of patients’ preferences and treatment site on cancer patients’ end-of-life care. Cancer. 2010;116(19):4656–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The R Project for Statistical Computing (http://www.r-project.org).
- 24.Sizoo EM, Pasman HR, Dirven L et al. . The end-of-life phase of high-grade glioma patients: a systematic review. Support Care Cancer. 2014;22(3):847–857. [DOI] [PubMed] [Google Scholar]
- 25.Diaz JL, Barreto P, Gallego JM, Barbero J, Bayes R, Barcia JA. Proper information during the surgical decision-making process lowers the anxiety of patients with high-grade gliomas. Acta Neurochir (Wien). 2009;151(4):357–362. [DOI] [PubMed] [Google Scholar]
- 26.Ford E, Catt S, Chalmers A, Fallowfield L. Systematic review of supportive care needs in patients with primary malignant brain tumors. Neuro Oncol. 2012;14(4):392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond EL, Corner GW, De Rosa A, Breitbart W, Applebaum AJ. Prognostic awareness and communication of prognostic information in malignant glioma: a systematic review. J Neurooncol. 2014;119(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weeks JC, Catalano PJ, Cronin A et al. . Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor NR, Hu R, Harris PS, Ache K, Casarett DJ. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas JS, Earle CC, Orav JE et al. . Lower use of hospice by cancer patients who live in minority versus white areas. J Gen Intern Med. 2007;22(3):396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardy D, Chan W, Liu CC et al. . Racial disparities in the use of hospice services according to geographic residence and socioeconomic status in an elderly cohort with nonsmall cell lung cancer. Cancer. 2011;117(7):1506–1515. [DOI] [PubMed] [Google Scholar]
- 33.Nayar P, Qiu F, Watanabe-Galloway S et al. . Disparities in end of life care for elderly lung cancer patients. J Community Health. 2014;39(5):1012–1019. [DOI] [PubMed] [Google Scholar]
- 34.Fairfield KM, Murray KM, Wierman HR et al. . Disparities in hospice care among older women dying with ovarian cancer. Gynecol Oncol. 2012;125(1):14–18. [DOI] [PubMed] [Google Scholar]
- 35.Mack JW, Chen K, Boscoe FP et al. . Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol. 2013;31(20):2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loberiza FR Jr., Swore-Fletcher BA, Block SD et al. . Coping styles, health status and advance care planning in patients with hematologic malignancies. Leuk Lymphoma. 2011;52(12):2342–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halpern NA, Pastores SM, Chou JF, Chawla S, Thaler HT. Advance directives in an oncologic intensive care unit: a contemporary analysis of their frequency, type, and impact. J Palliat Med. 2011;14(4):483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Prigerson HG, Diamond EL et al. . Minor cognitive impairments in cancer patients magnify the effect of caregiver preferences on end-of-life care. J Pain Symptom Manage. 2013;45(4):650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goutman SA, Nowacek DG, Burke JF, Kerber KA, Skolarus LE, Callaghan BC. Minorities, men, and unmarried amyotrophic lateral sclerosis patients are more likely to die in an acute care facility. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5–6):440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.du Preez AE, Smith MA, Liou JI et al. . Predictors of hospice utilization among acute stroke patients who died within thirty days. J Palliat Med. 2008;11(9):1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karikari-Martin P, McCann JJ, Hebert LE, Haffer SC, Phillips M. Do Community and Caregiver Factors Influence Hospice Use at the End of Life Among Older Adults With Alzheimer Disease? J Hosp Palliat Nurs. 2012;14(3):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loggers ET, Maciejewski PK, Paulk E et al. . Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J Clin Oncol. 2009;27(33):5559–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith AK, McCarthy EP, Paulk E et al. . Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson KS, Kuchibhatla M, Tulsky JA. What explains racial differences in the use of advance directives and attitudes toward hospice care? J Am Geriatr Soc. 2008;56(10):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sizoo EM, Pasman HR, Buttolo J et al. . Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer. 2012;48(2):226–232. [DOI] [PubMed] [Google Scholar]
- 46.Triebel KL, Martin RC, Nabors LB, Marson DC. Medical decision-making capacity in patients with malignant glioma. Neurology. 2009;73(24):2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerstenecker A, Meneses K, Duff K, Fiveash JB, Marson DC, Triebel KL. Cognitive predictors of understanding treatment decisions in patients with newly diagnosed brain metastasis. Cancer. 2015;121(12):2013–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phelps AC, Maciejewski PK, Nilsson M et al. . Religious coping and use of intensive life-prolonging care near death in patients with advanced cancer. JAMA. 2009;301(11):1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balboni TA, Vanderwerker LC, Block SD et al. . Religiousness and spiritual support among advanced cancer patients and associations with end-of-life treatment preferences and quality of life. J Clin Oncol. 2007;25(5):555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.True G, Phipps EJ, Braitman LE, Harralson T, Harris D, Tester W. Treatment preferences and advance care planning at end of life: the role of ethnicity and spiritual coping in cancer patients. Ann Behav Med. 2005;30(2):174–179. [DOI] [PubMed] [Google Scholar]