Abstract
Glioblastoma (GBM) is a lethal and aggressive brain tumor that is resistant to conventional radiation and cytotoxic chemotherapies. Molecularly targeted agents hold great promise in treating these genetically heterogeneous tumors, yet have produced disappointing results. One reason for the clinical failure of these novel therapies can be the inability of the drugs to achieve effective concentrations in the invasive regions beyond the bulk tumor. In this review, we describe the influence of the blood–brain barrier on the distribution of anticancer drugs to both the tumor core and infiltrative regions of GBM. We further describe potential strategies to overcome these drug delivery limitations. Understanding the key factors that limit drug delivery into brain tumors will guide future development of approaches for enhanced delivery of effective drugs to GBM.
Keywords: blood-brain barrier, drug delivery, efflux transporters, glioma
Glioblastoma (GBM) is the most common and aggressive primary brain tumor that remains essentially incurable despite decades of research on conventional and novel therapeutic strategies. Due to the invasive nature of GBM, aggressive surgical resection is insufficient to control tumors, and following maximal surgical resection, the addition of partial brain irradiation and temozolomide chemotherapy can significantly extend time to recurrence and survival.1 Unfortunately, despite this aggressive treatment regimen, the median survival for GBM patients remains ∼12–15 months, and only 5% of patients survive longer than 5 years.2 As our understanding of molecular mechanisms that mediate gliomagenesis and progression increases, integration of molecularly targeted agents into conventional chemoradiation regimens may provide a significant therapeutic benefit for patients with GBM. However, several factors may limit the efficacy of these promising therapeutic strategies, including molecular heterogeneity, invasion of tumor cells beyond the bulk tumor core delineated by imaging, and the blood–brain barrier (BBB), which prevents accumulation of xenobiotics within the central nervous system. These first 2 issues have been reviewed recently in this journal,3 and here we provide an overview of the influence of the BBB on treatment efficacy in GBM patients and potential strategies to overcome this limitation.
Influence of the Blood–Brain Barrier on Drug Delivery
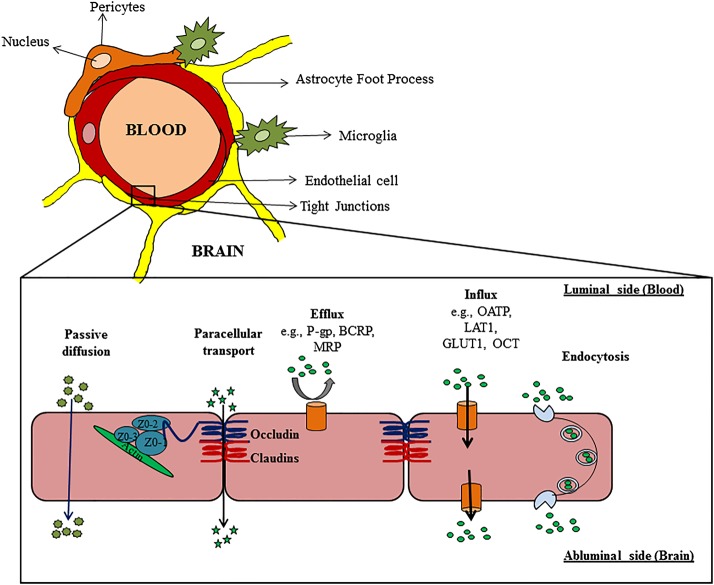
Despite progress in discovery of drug targets for treatment of primary brain tumors, overcoming the BBB and achieving adequate drug delivery into the tumor that remains after resection is a major challenge. The BBB is an anatomical and biochemical barrier that protects the brain from potentially harmful substances in the systemic circulation. Unlike the microvasculature in the periphery, brain capillary endothelial cells are interconnected by tight junctions, with limited fenestrations and pinocytic vesicles that form a physical barrier to prevent unimpeded diffusion into the brain from the bloodstream. This physical barrier significantly limits accumulation of small hydrophilic drugs, as well as large molecules, such as antibodies and antibody-drug conjugates, that cannot diffuse readily across lipid bilayers. Lipophilic molecules that are able to traverse the luminal endothelial cell membrane are often efficiently transported back into the capillary lumen by efflux transporters that reside in the luminal capillary endothelial membrane. Small-molecule drugs are often substrates for these efflux pumps located in the BBB. Therefore, both physical and biochemical barriers within the BBB significantly limit the brain delivery of therapeutic agents, which can limit treatment efficacy (Fig. 1).
Fig. 1.
Schematic representation of the neurovascular unit in relation to the blood–brain barrier and the different pathways for drug transport. Abbreviations: P-gp, P-glycoprotein; BCRP, breast cancer resistance protein; MRP, multidrug resistance protein; OATP, organic anion transporting polypeptide; LAT1, large-neutral amino acid transporter 1; GLUT1, glucose transporter 1; OCT, organic cation transporter.
CNS delivery of hydrophilic molecules and macromolecules is severely restricted by the physical presence of “tight junctions” called zonulae occludentes. The junctional complex holding the endothelial cells together comprises multiple proteins, including claudins (3, 5, and 12), occludins (1, 2, and 3), and junctional-adhesion molecules (JAM-A, -B, and -C).4 Furthermore, the microvessels are surrounded by, and in communication with, a variety of cells, including astrocytic glia cells, pericytes, and neurons. In addition to their structural roles, these supporting cells form a neurovascular unit that modulates the integrity of the BBB and influences drug diffusion into the brain. This physical barrier to paracellular transport is especially important for limiting the accumulation of large macromolecules that otherwise cannot readily traverse the plasma membrane of capillary endothelial cells.
Polarized expression of efflux transporters on the luminal and abluminal side of the BBB significantly limit delivery of numerous therapeutics that can otherwise readily diffuse across plasma membranes. P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) are 2 BBB efflux transporters that have been extensively studied and reported to limit brain distribution of numerous cytotoxic and molecularly targeted agents.5 In contrast, carrier-mediated influx transporters, such as glucose transporter 1, L-type amino acid transporter 1, and organic anion transporter polypeptides, translocate polar nutrients such as glucose, amino acids, vitamins, and hormones across the BBB.6 Receptor-mediated endocytosis can transport large molecules such as insulin, transferrin, and some vitamins (eg, folic acid) into the brain. Various transport pathways that are active within the BBB are depicted in Fig. 1.
Blood–Brain Barrier within Tumors
The majority of GBM patients have variable regions of disruption of the BBB. Clinically, this is visualized by accumulation of gadolinium-based MRI contrast agents within regions of the tumor that have a disrupted BBB. As discussed below, cytokines secreted by GBM tumor cells can lead to disruption of the BBB, and accumulation of contrast in these regions provides a visual demonstration that essentially all GBM patients have significant disruption of the BBB. In the past, this fact had been used by many to argue that the BBB is not a major factor that influences the delivery and, hence, efficacy of therapies in GBM.7
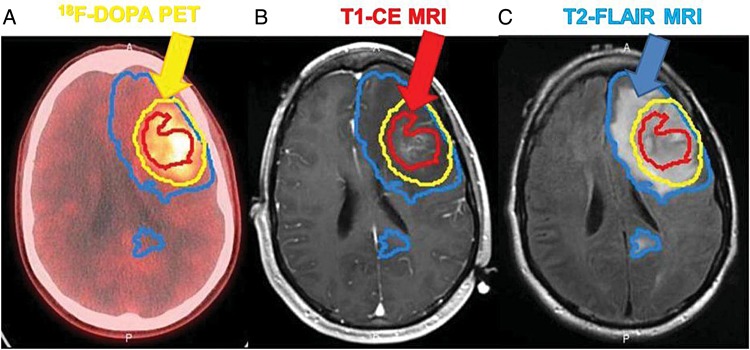
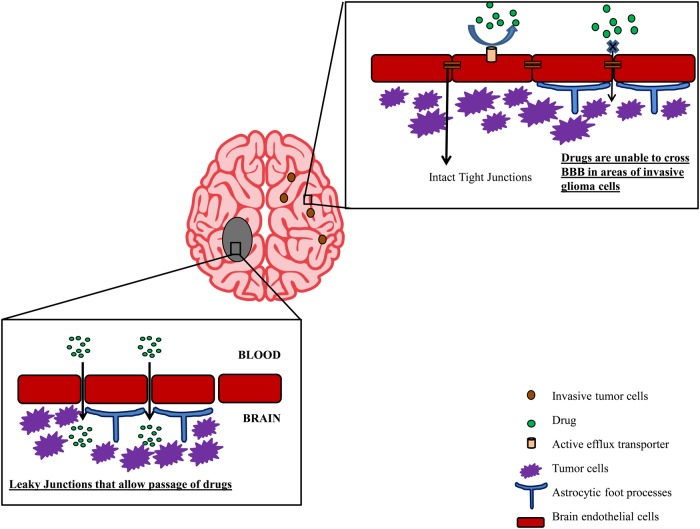
All GBM patients also have regions of tumor with an intact BBB. Even when neurosurgeons achieve a gross total resection of all contrast-enhancing regions of tumor, the tumor will recur within months in all of these patients.8 Several biopsy studies have demonstrated that significant tumor burden exists in the T2/fluid attenuated inversion recovery (FLAIR) regions outside of the contrast-enhancing regions seen on MRI. Moreover, using a 3,4-dihydroxy-6-[18F]fluoro-l-phenylalanine [18F-DOPA]) radiotracer that readily crosses the BBB via L-type amino acid transporter 1–mediated uptake, an anatomical comparison of 18F-DOPA uptake versus MRI contrast enhancement demonstrates significant regions of bulk tumor in which there is no significant accumulation of contrast media (Fig. 2).9 Beyond grossly positive regions of tumor, the highly invasive nature of GBM is well known, and at autopsy, infiltrating single cells have been found throughout the brain.10 In fact, based on surgical biopsy studies demonstrating infiltration of single cells extending beyond the T2/FLAIR tumor volume, radiation target volumes for GBM typically extend 1–2 cm beyond any visible radiographic abnormality.9 Thus, based on well-established surgical and radiographic information on GBM, all GBM patients have regions of a disrupted BBB and tumor regions with an intact BBB sufficient to prevent drug distribution to these invasive tumor cells. Taken together, these data suggest that delivery of therapeutic agents across the BBB to all tumor regions is essential to make significant progress in GBM treatment.5 Therefore it is critical to understand the mechanisms that limit drug distribution to invasive tumor sites (summarized in Fig. 3) and develop strategies to overcome these limitations.
Fig. 2.
Representative image from the same patient of (A) 18F-DOPA uptake imaged by PET-CT, (B) regions of contrast accumulation in the same location defined by a T1 postcontrast enhanced MRI, (C) regions of tumor-associated edema as defined by a T2-FLAIR MRI. Contours of these 3 regions were drawn by a neuroradiologist. The figure illustrates that significant tumor burden exists beyond the disrupted BBB defined by the contrast enhancement (CE). (Copyright9)
Fig. 3.
Schematic representation of the issues for effective delivery of drugs to bulk tumor cells and invasive glioma cells. The presence of intact blood–brain barrier and expression of efflux transporters limit distribution of chemotherapeutics to invasive glioma cells.
Strategies to Improve Drug Delivery to GBM
Penetration of drugs through the BBB, like other membranes in the body, occurs by 3 major mechanisms: passive diffusion, endocytosis, and carrier-mediated transport. These mechanisms can be exploited in various ways to enhance penetration of drugs into the brain. In the sections below, we review various methods that have been explored to increase delivery of chemotherapeutics into the brain.
Disruption of Tight Junctions in the Blood–Brain Barrier
BBB disruption via osmotic mechanisms or ultrasound
Osmotic BBB disruption (BBBD) is a mechanism-based technique that has been investigated for transiently disrupting the BBB to increase permeability to drug molecules. This method relies on administration of hypertonic solutions that lead to shrinkage of the endothelial cells, and this physical disruption of the tight junction complexes creates gaps that allow paracellular diffusion of molecules.11 In 1972, Rapoport and colleagues12 reported that osmotic opening of the BBB by hypertonic arabinose led to staining of the brain tissue by Evans blue in mouse models. Kroll and Neuwelt13 pioneered the clinical application of this concept by using hypertonic mannitol solutions to osmotically open the BBB to enhance drug delivery of chemotherapeutics. In a retrospective analysis of 38 patients with GBM, Neuwelt and colleagues14 reported that patients receiving chemotherapy with BBB modification by intracarotid or intravertebral artery infusion of mannitol had significantly longer survival (17.5 mo) than the control groups (surgery and radiation alone or surgery, radiation, and systemic chemotherapy). In a second retrospective study of 41 patients with high-grade astrocytomas, the same group reported favorable survival for patients treated with mannitol BBBD combined with intra-arterial chemotherapy (∼90 wk) compared with intra-arterial chemotherapy alone (∼50 wk), although direct comparisons between the treatment groups is difficult due to significant potential for selection bias.15 This study did not report the histology of the treatment arms; only half of the 41 patients had GBM, and both treatment with BBBD and anaplastic astrocytoma histology were positive predictors of survival. Nonetheless, toxic side effects and complexity of the procedure limit the usefulness of this technique in general practice.16,17
Due to the toxicity profile of mannitol-based BBBD methods, focused ultrasound (FUS) methods for BBBD have been developed. Focused ultrasound relies on transcranial delivery of low-frequency ultrasound waves that result in opening of the tight junctions between the endothelial cells.18 This technique was first successfully applied to enhance delivery of BBB-impermeable liposome-encapsulated doxorubicin in rats with glioma.19 Recently, the microbubble (MB)-enhanced FUS approach has been shown to temporarily disrupt tight junctions between brain endothelial cells and increase permeability of drugs across the BBB. The oscillation of the MBs in the presence of FUS leads to mechanical disruption of the BBB. Although ultrasound alone can disrupt the BBB, the addition of MBs allows for lower frequencies to be used to achieve the same BBB opening effect. In combination with ultrasound, MBs can collapse the tight junctions and vessel stability at high acoustic pressures.20 In addition, by using MBs to focus the effects of ultrasound on the microvasculature, one can apply FUS transcranially without producing significant skull heating.21 Using this approach, Ting and colleagues22 reported enhanced brain penetration of carmustine (BCNU) into the rat brain using multifunctional MBs and FUS.22 In a similar study, Liu et al23 reported increased brain delivery of BCNU into both tumor and surrounding normal brain using a focused external ultrasound generator. In another study, the effect of FUS BBBD was tested in a preclinical model of glioma and demonstrated that FUS provided both improved delivery and efficacy compared with temozolomide alone.24 Although FUS offers a potential way to disrupt the tight junctions, so far this technique has been evaluated only preclinically. While the ultrasound bursts can produce transient disruption in a focused part of the BBB, the extent of BBBD in human tumors is unknown, and further optimization and proof-of-principle studies will be required to understand the clinical potential of this approach. In addition to these difficulties, FUS-induced BBB disruption has been shown to have undesirable side effects, such as erythrocyte extravasation, edema, and intracerebral hemorrhage,25 that will need to be evaluated if this technique is translated into the clinic. Recent reports by Fan et al26 have demonstrated a decrease in the occurrence of these undesirable effects by removing inertial cavitation through the use of resonance frequency–matched FUS.26 In translating this technique to the clinic, optimization of the FUS-MB method to enhance delivery and limit brain injury will be essential.
Blood–brain barrier disruption via pharmacological agents
Tight junctions are actively regulated complexes that potentially can be pharmacologically manipulated. Bradykinin-like compounds (eg, histamine, leukotrienes, bradykinin) disrupt tight junctions by stimulating B2 receptors expressed on endothelial cells and transiently increasing cytosolic Ca2+, resulting in opening of the tight junctions.17 Further evidence also suggests a role for nitric oxide in selectively increasing the blood–tumor barrier permeability due to higher concentrations of nitric oxide synthase in tumor vasculature compared with normal brain microvessels.27 In an animal model, low-dose bradykinin selectively increased blood–tumor barrier permeability in intracerebral tumors when administered as an infusion through the ipsilateral internal carotid artery.28 However, in a direct comparison of osmotic versus bradykinin BBBD methods, hypertonic mannitol resulted in significant improvement in brain delivery for a variety of agents, whereas bradykinin treatment was not effective in increasing the delivery of drugs in this brain tumor model in nude rats.29 A more potent and B2-selective bradykinin analog, Cereport (RMP-7), also has been tested. RMP-7 is resistant to degradation by bradykinin-metabolizing enzymes30 and increased delivery by 30%–80% of carboplatin to tumor cells in an RG2 rat-glioma model.31,32 However, the same combination showed disappointing results in a randomized, double-blind, placebo-controlled, phase II trial in recurrent malignant glioma patients with no difference in time to tumor progression for patients treated with chemotherapy alone versus RMP-7 plus chemotherapy.33
Another strategy for disruption of the BBB is to intervene in the signaling pathways that maintain its integrity. Accumulating evidence has demonstrated the role of vascular endothelial growth factor, a major permeability and angiogenic factor, in downregulating or relocalizing tight junction proteins (zonula occludens 1, occludins), resulting in destabilization of tight junctions and increase in permeability.34,35 Others have suggested that the Smoothened pathway may be important for signaling to tight junctions within the BBB, and in a recent report, Alvarez et al36 demonstrated focal disruption of the tight functions in the brain of normal mice following treatment with the Smoothened inhibitor cyclopamine. While early in experimental development, these data highlight the idea that pharmacological manipulation of the physical BBB potentially could be used to improve drug delivery into brain tumors.
Overcoming Active Efflux at the Blood–Brain Barrier
Active efflux transport of molecules from the capillary endothelial cells is a major mechanism by which the BBB limits drug delivery to the brain. This biochemical barrier is mediated by numerous active efflux transporters, including P-gp, multidrug resistance proteins (eg, MRP4), and BCRP, which function on the luminal membrane of brain capillary endothelial cells to translocate molecules back into the bloodstream.37 In order to effectively deliver drugs across the BBB and evade active efflux, several approaches have been investigated. These include modification of drug structure to diminish efflux transporter affinity and coadministration of transport inhibitors to enhance delivery of anticancer drugs. Table 1 lists molecularly targeted and nontargeted agents that have demonstrated substrate liability for P-gp and BCRP in preclinical studies. Listed are the brain-to-plasma ratios determined in wild-type mice and transporter-knockout mice. Given the number of agents that are substrates for active efflux at the BBB, the treatment options to effectively administer these compounds for GBM will require some means to overcome active efflux, as discussed below.
Table 1.
List of molecularly targeted and nontargeted agents examined for treatment of glioblastoma and their P-gp/BCRP substrate status reported as brain-to-plasma ratio (B/P) determined in wild-type mice compared with knockout (KO) mice lacking expression of P-gp or BCRP or both
| Drug | Molecular Target | Substrate Status |
B/P in Mouse Models |
References | ||||
|---|---|---|---|---|---|---|---|---|
| P-gp | BCRP | Wild-type Mouse B/P | P-gp KO Mouse B/P | BCRP KO Mouse B/P | Combined P-gp/BCRP KO B/P | |||
| Targeted agents | ||||||||
| Erlotiniba | EGFR | Yes | Yes | 0.14 | 0.41 | 0.14 | 0.58 | 38 |
| Tandutiniba | PDGFR, FLT3 | Yes | Yes | ∼3.2 fold | ∼0.9 fold | ∼13.5 fold | 39 | |
| Gefitinibb | EGFR | Yes | Yes | 0.07 ± 0.02 | 7.3 ± 0.5 | 40 | ||
| Cediranibb | VEGFR | Yes | Yes | 0.25 ± 0.10 | 5.2 ± 1.1 | 0.27 ± 0.01 | 6.3 ± 1.2 | 41 |
| Sorafenibb,c | Raf kinase, VEGFR, PDGFR | Yes | Yes | 0.094 ± 0.007b, 5.3 ± 2.7c | 0.11 ± 0.02b, 5.8 ± 2.2c | 0.36 ± 0.06b, 22.6 ± 5.0c | 0.91 ± 0.29b, 49.4 ± 5.2c | 65,66 |
| Vandetanibd | VEGFR, EGFR | Yes | Yes | 0.21 | 0.64 | 42 | ||
| Pazopanibd | VEGFR, PDGFR, c-kit | Yes | Yes | 0.015 | 0.041 | 67 | ||
| Dasatiniba,c | BCR-Abl, EGFR | Yes | Yes | 6.39 ± 1.39c, 0.12a | 22.7 ± 5.41c | 5.11 ± 0.7c | 84.3 ± 13.3c, 0.93a | 41,68 |
| Sunitinibb,c | VEGFR, PDGFR, c-kit | Yes | Yes | 1.6 ± 1.0c, 0.51 ± 0.26b | 2.8 ± 0.8c, 2.33 ± 0.56b | 2.4 ± 0.9c, 0.73 ± 0.44b | 42.4 ± 10.7c, 17.44 ± 5.08b | 69,70 |
| Imatinibe,f,g | BCR-Abl, PDGFR, c-kit | Yes | Yes | ∼2.73 folde, ∼3.6 foldg, ∼5.5 foldf | ∼2.5 folde | 71–73 | ||
| Lapatinibb | EGFR | Yes | Yes | 0.03 ± 0.01 | 0.09 ± 0.02 | 0.04 ± 0.01 | 1.2 ± 0.42 | 74 |
| Everolimusa | mTOR | Yes | NA | ∼1.3 fold | 75 | |||
| GNE-317h | Dual PI3 K/mTOR | No | No | 1.01 ± 0.05 | 35 | |||
| GDC-0980i | Dual PI3 K/mTOR | Yes | Yes | 0.082 ± 0.008 | 1.0 ± 0.20 | 76 | ||
| GDC-0941g | PI3K | Yes | No | ∼2.24 fold | ∼1.05 fold | ∼29.42 fold | 77 | |
| Axitinibc | VEGFR-1, -2, -3 | Yes | Yes | 94.8 ± 27 | 643.6 ± 183.2 | 47.7 ± 12.7 | 1315 ± 375 | 78 |
| Nontargeted agents | ||||||||
| Vincristineb | Vinca alkaloid | Yes | No | ∼2.1 fold | ∼3.5 fold | 79 | ||
| Cisplatin | Platinum-based drug | Yes | Yes | 80 | ||||
| Doxorubicinj | Anthracycline topoisomerase inhibitor | Yes | Yes | ∼3.3 fold | 81 | |||
| Paclitaxelk | Microtubule inhibitor | Yes | No | ∼7.9 fold | 82 | |||
| Irinotecana | Topoisomerase inhibitor | Yes | Yes | ∼2.1 fold | 83 | |||
| Topotecana | Topoisomerase inhibitor | Yes | Yes | 0.32 | 0.64 | 0.21 | 1.02 | 84 |
Abbreviations: EGFR, epidermal growth factor receptor; PDGFR, platelet derived growth factor receptor; FLT3, Fms-like tyrosine kinase 3; VEGFR, vascular endothelial growth factor receptor; BCR-Abl, breakpoint cluster region–Abelson murine leukemia; NA, not available (not yet established in mouse studies).
aArea under the concentration (AUC) time curve ratios obtained as AUC∞ brain-to-AUC∞ plasma.
bSteady state brain-to-plasma concentration ratios.
cBrain penetration, ie, Pbrain = relative brain accumulation at 6 h after oral administration, calculated by determining the brain concentration relative to plasma AUC0-6.
dBrain-to-plasma concentration ratio obtained after administration with a dual inhibitor (GF120918) of P-gp and BCRP.
eB/P ratios not available.
fUsing in situ brain perfusion to determine fold increase.
gDetermined at a single time point post oral dose.
hDetermined at 2 time points; 1 h after oral dose; B/P ratio in absence of P-gp and BCRP was not reported.
iDetermined at 2 time points, 1 h and 6 h post-dose; here we report 1 h after oral dose.
jDetermined at a single time point post i.v. dose.
kAUC ratio obtained as AUC0-24, brain-to-AUC0-8, plasma.
Structural refinement to allow passive diffusion of drugs
The use of computational models to predict physicochemical properties has allowed the design of compounds that may penetrate the BBB by passive diffusion. Traditional medicinal chemistry approaches have focused on increasing lipophilicity to enhance drug penetration across the BBB. However, with a more complete understanding of the biochemical barriers to drug accumulation in the brain, researchers can develop drug candidates that have favorable BBB penetration based both on their physicochemical properties and on their lack of affinity for efflux transporters. For instance, molecularly targeted dual phosphatidylinositol-3 kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitors GNE-317 and GDC-0084 have been structurally optimized using structural-activity relationships to minimize liability for active efflux by P-gp and BCRP at the BBB.40 Similarly, the pan-PI3K inhibitor BKM120 has excellent brain penetration associated with limited efflux liability and has shown promising results in several in vivo GBM models.42 These results demonstrate that molecular modeling approaches can be used to minimize efflux liability and enhance brain delivery.
Pharmacological inhibition of efflux
Numerous molecularly targeted agents and anticancer drugs have demonstrated substrate affinity to both P-gp and BCRP that limits their capacity to cross the BBB (Table 1). The expression of efflux transporters also has been reported in glioma cells,43 and this presents an additional barrier to drug delivery into tumor cells. Earlier, Agarwal et al5 reviewed the role of the BBB and the brain–tumor cell barrier in restricting brain penetration of molecularly targeted agents. These 2 sequential barriers can critically restrict the delivery of many targeted antitumor agents and other chemotherapeutics into the intracellular space of the glioma cell.
One approach to modulate active efflux of potentially useful targeted agents is by coadministration of pharmacological inhibitors of P-gp and BCRP. Several researchers have reported higher brain concentrations of molecularly targeted agents (eg, gefitinib,44 vandetanib45) with coadministration of elacridar, a dual inhibitor of P-gp and BCRP, in non–tumor bearing wild-type mice (see Table 1). In addition, Agarwal et al46 reported higher concentrations of erlotinib in the brain when it was coadministered with elacridar in a U87 orthotopic rat xenograft model of glioma. Similarly, clinical reports with efflux transport inhibitors have shown interesting results. In 2005, Sasongko et al47 observed enhanced brain penetration of 11C-verapamil with coadministration of a P-gp inhibitor, cyclosporine A, in healthy volunteers. Wagner and colleagues48 reported similar results using another inhibitor of P-gp, tariquidar, in healthy volunteers. These results demonstrate a proof-of-concept for potential clinical use of pharmacological inhibitors to enhance brain delivery of molecularly targeted therapies. However, pharmacological inhibition of efflux transporters at the BBB in combination with targeted agents has not been used in the clinic, in part due to increases in observed toxicities in initial clinical trials examining the combination of elacridar with cytotoxic chemotherapeutics, including doxorubicin and topotecan.49,50 These toxicities were attributable to the increase in plasma concentrations of the cytotoxic agents through inhibition of the transporter-mediated systemic clearance process. It is important to note that most molecularly targeted agents are not susceptible to transporter-driven changes in their overall disposition, since their systemic clearances depend primarily on metabolic processes and not on the action of efflux transporters. Thus, a strategy to combine efflux inhibitors with molecularly targeted agents is interesting and worth pursuing.
Local Delivery Strategies
Convection-enhanced delivery
Convection-enhanced delivery (CED) is an invasive local delivery method that distributes large- and small-molecular-weight compounds into brain tissue. This is driven by a hydrostatic pressure gradient from a prolonged slow infusion in the brain parenchyma through fenestrated catheters placed at the time of surgery.51 CED directly delivers the drug into the intracerebral tumor tissue,52 which results in drug distribution into the peritumoral tissue by bulk flow to provide a relatively constant drug concentration over a distance from the site of infusion.53 This method has been extensively evaluated for delivery of therapeutic proteins, oligonucleotides, liposomes, and viral-mediated therapies. Notably, delivery of large-molecular-weight therapeutic proteins such as Pseudomonas aeruginosa exotoxin (PE), interleukin (IL)4-PE (PRX321), and IL13-PE38QQR (cintredekin besudotox) has been extensively evaluated in phase I and II trials. These clinical trials demonstrated acceptable safety profiles but failed to reach efficacy standards to obtain FDA approval.54 Similarly, trabedersen (AP-12009), an antisense oligonucleotide that targets transforming growth factor–β2, significantly improved the 14-month tumor control rate in an anaplastic astrocytoma subgroup compared with chemotherapy alone (temozolomide or procarbazine/lomustine/vincristine) in phase II studies, although no significant benefit was observed for patients with recurrent or refractory GBM in the same subgroup analysis.55 Thus, the value of CED remains to be validated by a successful clinical trial.
The feasibility of CED is highly dependent on technical expertise and drug characteristics. Catheter placement significantly influences the geographic distribution of the drug and can also influence induction of adverse effects such as chemical meningitis.54 Other factors that can influence drug distribution by CED include pH, osmolarity, ionic composition, and the solubility of the drug.56 Furthermore, the insufficient spatial distribution of the drug in the brain may restrict targeted delivery to invasive glioma cells52 and leave the infiltrative growing edge of the tumor and the invasive glioma cells at distant sites untreated. Acknowledging these technical limitations, CED may prove to be an important tool for delivering otherwise brain-impenetrant payloads, such as virus or antibody-toxin conjugates. The key challenge will be to identify payloads with sufficient activity against GBM to justify the more intensive CED approach.
Other local delivery strategies
Invasive local delivery methods that can be used for GBM therapy include bolus injection of therapeutic agents directly into the tumor or intracerebro-ventricular delivery. Similar to CED, drug administration by these methods bypasses the BBB by locally introducing the drug into the tumor, brain parenchyma, ventricles, or subarachnoid space of the spinal cord. Direct injection into the tumor is commonly used to deliver viral therapies into GBM tumors, even though this technique can result in a highly heterogeneous distribution throughout the tumor. CSF delivery also has limited relevance for GBM, since drug transport by diffusion or convection from the CSF into distant bulk tumor is highly unlikely. Other local delivery methods make use of biodegradable wafers such as Gliadel (carmustine), which are placed in the tumor cavity after surgical resection57 and intrathecal placement of implantable pumps.58 Intranasal drug delivery is another approach that is being explored as a noninvasive alternative to bypass the BBB. Although these methods are not limited by the size of the drug molecule (both small and large molecules can be delivered), the main pharmacological limitation lies in that the drug concentration decreases exponentially as the diffusion distance increases. This phenomenon is known as the “sink effect,” where drugs with high passive permeability have limited distribution through brain tissue due to the drug diffusing into the vasculature and being washed out into the systemic circulation. Thus, in the context of highly infiltrative tumors such as GBM, all of these local delivery techniques are limited by the extent of diffusion possible through the bulk tumor and regions of brain with infiltrating tumor cells.
Nanoparticle Drug Carriers
Recent advances in nanotechnology allow packaging of drugs, which otherwise are poorly distributed to the brain, into nanoparticles with brain targeting properties. These targeted carrier systems enhance delivery of drugs by entrapping or encapsulating the drug in the particle with a targeting peptide/ligand on its surface that results in BBB targeting. These carrier systems cross the BBB by utilizing transcellular pathways such as receptor-mediated endocytosis.59 A major advantage of solid lipid nanoparticles is that their high lipid solubility physically stabilizes the nanoparticle, thereby increasing drug loading capacity and resulting in a controlled rate of drug release.60 Two cytotoxic drugs, doxorubicin and camptothecin, when encapsulated in pegylated solid lipid nanoparticles, exhibited efficient transport across the brain capillary endothelial cells in preclinical studies.61,62 Although nanoparticles and lipid-based formulations have increased systemic circulation times and increased tumor retention by enhanced permeation and retention effect, their major limiting factor is the rapid clearance from the blood circulation by the reticuloendothelial system. Several groups are working to improve brain targeting by decorating nanoparticles with ligands that are substrates for BBB uptake transporters, thereby facilitating transit into the brain. As this technology evolves, this system may prove effective for delivering a wide variety of therapeutic agents with a favorable toxicity profile.
Peptide-Based Drug Delivery
Peptide-like macromolecules can be transported into the endothelial cells of the BBB via adsorption- or receptor-based transcytosis processes.63 In this method, the drug is chemically conjugated by a linker to a targeting moiety that is taken up by a specific receptor and undergoes endocytosis. These vector-based methods can improve delivery of drugs that otherwise have little to no brain penetration. Rousselle et al64 showed enhanced brain uptake in rats when doxorubicin was conjugated to a small peptide (SynB1) compared with doxorubicin alone. Small peptides such as AngioPep-2 have been shown to enhance delivery of small molecules across the BBB via low-density lipoprotein receptor–related protein (LRP1).38 ANG1005/GRN1005 is a conjugate of 3 molecules of paclitaxel and 1 molecule of AngioPep-2 peptide that can significantly increase delivery of paclitaxel in a rat brain perfusion model.39 Clinically, ANG1005 has been tested in phase I trials for recurrent glioma and metastatic brain tumors.41 The efficacy of this approach remains to be demonstrated in phase II or III clinical trials, which potentially could be limited by toxicity or immunogenicity associated with peptide-based therapies.
Conclusions and Future Perspectives
Treatment of GBM is a formidable challenge. The intense investigation of numerous strategies to improve drug delivery in the context of GBM reflects the difficulty posed by the BBB, which provides both physical and biochemical barriers that limit penetration of most drugs into regions of invasive GBM. Overcoming these challenges will be key in refining therapies for GBM, where the reason for failure may be inadequate delivery of an effective drug. Progress in treating this disease will require not only delivering the right drugs to the right targets, but also delivering an adequate amount of drug throughout the entire tumor to effectively modulate the pharmacological targets in all tumor cells.
Conflict of interest statement. J.N.S. has research grants from Merck, Sanofi-Aventis, Basilea, Lilly, and Genentech; W.F.E. has paid consultancy or honoraria from Jazz Pharmaceuticals, Medtronic Inc., Abbvie, Genentech, Novartis, Pfizer, and Teva.
References
- 1.Furnari FB, Fenton T, Bachoo RM et al. . Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neurooncol. 2014;16(Suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vartanian A, Singh SK, Agnihotri S et al. . GBM's multifaceted landscape: highlighting regional and microenvironmental heterogeneity. Neuro Oncol. 2014;16(9):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S, Sane R, Oberoi R et al. . Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood–brain barrier. J Pharm Sci. 2000;89(11):1371–1388. [DOI] [PubMed] [Google Scholar]
- 7.Vick NA, Khandekar JD, Bigner DD. Chemotherapy of brain tumors. Arch Neurol. 1977;34(9):523–526. [DOI] [PubMed] [Google Scholar]
- 8.Hou LC, Veeravagu A, Hsu AR, Tse VC. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20(4):E5. [DOI] [PubMed] [Google Scholar]
- 9.Pafundi DH, Laack NN, Youland RS et al. . Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15(8):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onda K, Tanaka R, Takahashi H et al. . Cerebral glioblastoma with cerebrospinal fluid dissemination: a clinicopathological study of 14 cases examined by complete autopsy. Neurosurgery. 1989;25(4):533–540. [PubMed] [Google Scholar]
- 11.Rapoport SI. Effect of concentrated solutions on blood–brain barrier. Am J Physiol. 1970;219(1):270–274. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport SI, Hori M, Klatzo I. Testing of a hypothesis for osmotic opening of the blood–brain barrier. Am J Physiol. 1972;223(2):323–331. [DOI] [PubMed] [Google Scholar]
- 13.Kroll RA, Neuwelt EA. Outwitting the blood–brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083–1099; discussion 1099–1100. [DOI] [PubMed] [Google Scholar]
- 14.Neuwelt EA, Howieson J, Frenkel EP et al. . Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood–brain barrier modification in glioblastoma. Neurosurgery. 1986;19(4):573–582. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer DF, Fortin D, Neuwelt EA. Chemotherapeutic dose intensification for treatment of malignant brain tumors: recent developments and future directions. Curr Neurol Neurosci Rep. 2002;2(3):216–224. [DOI] [PubMed] [Google Scholar]
- 16.Seto A, Murakami M, Fukuyama H et al. . Ventricular tachycardia caused by hyperkalemia after administration of hypertonic mannitol. Anesthesiology. 2000;93(5):1359–1361. [DOI] [PubMed] [Google Scholar]
- 17.Kemper EM, Boogerd W, Thuis I et al. . Modulation of the blood–brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30(5):415–423. [DOI] [PubMed] [Google Scholar]
- 18.Hynynen K, McDannold N, Vykhodtseva N et al. . Focal disruption of the blood–brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105(3):445–454. [DOI] [PubMed] [Google Scholar]
- 19.Etame AB, Diaz RJ, Smith CA et al. . Focused ultrasound disruption of the blood–brain barrier: a new frontier for therapeutic delivery in molecular neurooncology. Neurosurg Focus. 2012;32(1):E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev. 2014;72C:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDannold N, King RL, Hynynen K. MRI monitoring of heating produced by ultrasound absorption in the skull: in vivo study in pigs. Magn Reson Med. 2004;51(5):1061–1065. [DOI] [PubMed] [Google Scholar]
- 22.Ting CY, Fan CH, Liu HL et al. . Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33(2):704–712. [DOI] [PubMed] [Google Scholar]
- 23.Liu HL, Hua MY, Chen PY et al. . Blood–brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255(2):415–425. [DOI] [PubMed] [Google Scholar]
- 24.Liu H-L, Huang C-Y, Chen J-Y et al. . Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood–brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS One. 2014;9(12):e114311–e114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan CH, Liu HL, Huang CY et al. . Detection of intracerebral hemorrhage and transient blood-supply shortage in focused-ultrasound-induced blood–brain barrier disruption by ultrasound imaging. Ultrasound Med Biol. 2012;38(8):1372–1382. [DOI] [PubMed] [Google Scholar]
- 26.Fan C-H, Ting C-Y, Chang Y-C et al. . Acta Biomaterialia Drug-loaded bubbles with matched focused ultrasound excitation for concurrent blood–brain barrier opening and brain-tumor drug delivery. Acta Biomater. 2015;15:89–101. [DOI] [PubMed] [Google Scholar]
- 27.Nakano S, Matsukado K, Black KL. Increased brain tumor microvessel permeability after intracarotid bradykinin infusion is mediated by nitric oxide. Cancer Res. 1996;56(17):4027–4031. [PubMed] [Google Scholar]
- 28.Inamura T, Black KL. Bradykinin selectively opens blood–tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab. 1994;14(5):862–870. [DOI] [PubMed] [Google Scholar]
- 29.Kroll RA, Pagel MA, Muldoon LL et al. . Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: a comparison of osmotic versus bradykinin modification of the blood–brain and/or blood–tumor barriers. Neurosurgery. 1998;43(4):879–886; discussion 886–889. [DOI] [PubMed] [Google Scholar]
- 30.Doctrow SR, Abelleira SM, Curry LA et al. . The bradykinin analog RMP-7 increases intracellular free calcium levels in rat brain microvascular endothelial cells. J Pharmacol Exp Ther. 1994;271(1):229–237. [PubMed] [Google Scholar]
- 31.Borlongan CV, Emerich DF. Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, Cereport. Brain Res Bull. 2003;60(3):297–306. [DOI] [PubMed] [Google Scholar]
- 32.Elliott PJ, Hayward NJ, Dean RL et al. . Intravenous RMP-7 selectively increases uptake of carboplatin into rat brain tumors. Cancer Res. 1996;56(17):3998–4005. [PubMed] [Google Scholar]
- 33.Prados MD, Schold SJS, Fine HA et al. . A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003;5(2):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argaw AT, Gurfein BT, Zhang Y et al. . VEGF-mediated disruption of endothelial CLN-5 promotes blood–brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106(6):1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280(1):H434–H440. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez JI, Dodelet-Devillers A, Kebir H et al. . The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–1731. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal S, Hartz AM, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17(26):2793–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlieghe P, Khrestchatisky M. Medicinal chemistry based approaches and nanotechnology-based systems to improve CNS drug targeting and delivery. Med Res Rev. 2013;33(3):457–516. [DOI] [PubMed] [Google Scholar]
- 39.Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol. 1984;247(3 Pt 2):H484–H493. [DOI] [PubMed] [Google Scholar]
- 40.Heffron TP, Salphati L, Alicke B et al. . The design and identification of brain penetrant inhibitors of phosphoinositide 3-kinase alpha. J Med Chem. 2012;55(18):8007–8020. [DOI] [PubMed] [Google Scholar]
- 41.Drappatz J, Brenner A, Wong ET et al. . Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19(6):1567–1576. [DOI] [PubMed] [Google Scholar]
- 42.Wen PY, Lee EQ, Reardon DA et al. . Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro Oncol. 2012;14(7):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demeule M, Shedid D, Beaulieu E et al. . Expression of multidrug-resistance P-glycoprotein (MDR1) in human brain tumors. Int J Cancer. 2001;93(1):62–66. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal S, Sane R, Gallardo JL et al. . Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)–mediated active efflux. J Pharmacol Exp Ther. 2010;334(1):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minocha M, Khurana V, Qin B et al. . Co-administration strategy to enhance brain accumulation of vandetanib by modulating P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp1/Abcg2) mediated efflux with m-TOR inhibitors. Int J Pharm. 2012;434(1–2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal S, Manchanda P, Vogelbaum MA et al. . Function of the blood–brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug Metab Dispos. 2013;41(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasongko L, Link JM, Muzi M et al. . Imaging P-glycoprotein transport activity at the human blood–brain barrier with positron emission tomography. Clin Pharmacol Ther. 2005;77(6):503–514. [DOI] [PubMed] [Google Scholar]
- 48.Wagner CC, Bauer M, Karch R et al. . A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood–brain barrier with (R)-11C-verapamil and PET. J Nucl Med. 2009;50(12):1954–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Planting AS, Sonneveld P, van der Gaast A et al. . A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2005;55:91–99. [DOI] [PubMed] [Google Scholar]
- 50.Kuppens IELM, Witteveen EO, Jewell RC et al. . A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13(11):3276–3285. [DOI] [PubMed] [Google Scholar]
- 51.Groothuis DR, Ward S, Itskovich AC et al. . Comparison of 14C-sucrose delivery to the brain by intravenous, intraventricular, and convection-enhanced intracerebral infusion. J Neurosurg. 1999;90(2):321–331. [DOI] [PubMed] [Google Scholar]
- 52.Bobo RH, Laske DW, Akbasak A et al. . Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding D, Kanaly CW, Bigner DD et al. . Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1-1. J Neurooncol. 2010;98(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serwer LP, James CD. Challenges in drug delivery to tumors of the central nervous system: an overview of pharmacological and surgical considerations. Adv Drug Deliv Rev. 2012;64(7):590–597. [DOI] [PubMed] [Google Scholar]
- 55.Bogdahn U, Hau P, Stockhammer G et al. . Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groothuis DR. The blood–brain and blood–tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duntze J, Litre CF, Eap C et al. . Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann Surg Oncol. 2013;20(6):2065–2072. [DOI] [PubMed] [Google Scholar]
- 58.Bhatia G, Lau ME, Gulur P, Koury KM. Intrathecal drug delivery (ITDD) systems for cancer pain. F1000 Res. 2013;2:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herve F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10(3):455–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blasi P, Giovagnoli S, Schoubben A et al. . Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–477. [DOI] [PubMed] [Google Scholar]
- 61.Cirpanli Y, Allard E, Passirani C et al. . Antitumoral activity of camptothecin-loaded nanoparticles in 9L rat glioma model. Int J Pharm. 2011;403(1–2):201–206. [DOI] [PubMed] [Google Scholar]
- 62.Battaglia L, Gallarate M, Peira E et al. . Solid lipid nanoparticles for potential doxorubicin delivery in glioblastoma treatment: preliminary in vitro studies. J Pharm Sci. 2014;103(7):2157–2165. [DOI] [PubMed] [Google Scholar]
- 63.Scherrmann JM. Drug delivery to brain via the blood–brain barrier. Vascul Pharmacol. 2002;38(6):349–354. [DOI] [PubMed] [Google Scholar]
- 64.Rousselle C, Clair P, Lefauconnier JM et al. . New advances in the transport of doxorubicin through the blood–brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57(4):679–686. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336(1):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010;9(2):319–326. [DOI] [PubMed] [Google Scholar]
- 67.Minocha M, Khurana V, Qin B, Pal D, Mitra AK. Enhanced brain accumulation of pazopanib by modulating P-gp and Bcrp1 mediated efflux with canertinib or erlotinib. Int J Pharm. 2012;436(1–2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagas JS, van Waterschoot RA, van Tilburg VA et al. . Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res. 2009;15(7):2344–2351. [DOI] [PubMed] [Google Scholar]
- 69.Tang SC, Lagas JS, Lankheet NA et al. . Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer J Int Cancer. 2012;130(1):223–233. [DOI] [PubMed] [Google Scholar]
- 70.Oberoi RK, Mittapalli RK, Elmquist WF. Pharmacokinetic assessment of efflux transport in sunitinib distribution to the brain. J Pharmacol Exp Ther. 2013;347(3):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther. 2003;304(3):1085–1092. [DOI] [PubMed] [Google Scholar]
- 72.Breedveld P, Pluim D, Cipriani G et al. . The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65(7):2577–2582. [DOI] [PubMed] [Google Scholar]
- 73.Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcbla) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier. J Neurochem. 2007;102(6):1749–1757. [DOI] [PubMed] [Google Scholar]
- 74.Polli JW, Olson KL, Chism JP et al. . An unexpected synergist role of P- glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3- fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino }methyl)-2- furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos. 2009;37(2):439–442. [DOI] [PubMed] [Google Scholar]
- 75.Chu C, Abbara C, Noel-Hudson MS et al. . Disposition of everolimus in mdrla-/1b- mice and after a pre-treatment of lapatinib in Swiss mice. Biochem Pharmacol. 2009;77(10):1629–1634. [DOI] [PubMed] [Google Scholar]
- 76.Salphati L, Pang J, Plise EG et al. . Preclinical assessment of the absorption and disposition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor GDC-0980 and prediction of its pharmacokinetics and efficacy in human. Drug Metab Dispos. 2012;40(9):1785–1796. [DOI] [PubMed] [Google Scholar]
- 77.Salphati L, Lee LB, Pang J, Plise EG, Zhang X. Role of P-glycoprotein and breast cancer resistance protein-1 in the brain penetration and brain pharmacodynamic activity of the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab Dispos. 2010;389:1422–1426. [DOI] [PubMed] [Google Scholar]
- 78.Poller B, Iusuf D, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Differential impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on axitinib brain accumulation and oral plasma pharmacokinetics. Drug Metab Dispos. 2011;39(5):729–735. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Zhou F, Kruh GD, Gallo JM. Influence of blood-brain barrier efflux pumps on the distribution of vincristine in brain and brain tumors. Neuro Oncol. 2010;12(10):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pawłowski KM, Mucha J, Majchrzak K, Motyl T, Król M. Expression and role of PGP, BCRP, MRP1 and MRP3 in multidrug resistance of canine mammary cancer cells. BMC Vet Res. 2013;9:119–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Asperen J, van Tellingen O, Tijssen F, Schinkel aH, Beijnen JH. Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdrla P-glycoprotein. Br J Cancer. 1999;79:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kemper EM, Van Zandbergen aE, Cleypool C et al. . Increased penetration of paclitaxel into the brain by inhibition of P-glycoprotein. Clin Cancer Res. 2003;9(July):2849–2855. [PubMed] [Google Scholar]
- 83.Goldwirt L, Beccaria K, Carpentier A, Farinotti R, Fernandez C. Irinotecan and temozolomide brain distribution: a focus on ABCB1. Cancer Chemother Pharmacol. 2014;74:185–193. [DOI] [PubMed] [Google Scholar]
- 84.DeVries Na, Zhao J, Kroon E, Buckle T, Beijnen JH, Van Tellingen O. P-glycoprotein and breast cancer resistance protein: Two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13(11):6440–6449. [DOI] [PubMed] [Google Scholar]