Abstract
Background
Years of potential life lost (YPLL) complement incidence and survival rates by measuring how much a patient's life is likely to be shortened by his or her cancer. In this study, we examine the impact of death due to brain and other central nervous system (CNS) tumors compared to other common cancers in adults by investigating the YPLL of adults in the United States.
Methods
Mortality and life table data were obtained from the Centers for Disease Control and Prevention's National Center for Health Statistics Vital Statistics Data for 2010. The study population included individuals aged 20 years or older at death who died from one of the selected cancers. YPLL was calculated by taking an individual's age at death and finding the corresponding expected remaining years of life using life table data.
Results
The cancers with the greatest mean YPLL were other malignant CNS tumors (20.65), malignant brain tumors (19.93), and pancreatic cancer (15.13) for males and malignant brain tumors (20.31), breast cancer (18.78), and other malignant CNS tumors (18.36) for females. For both sexes, non-Hispanic whites had the lowest YPLL, followed by non-Hispanic blacks, and Hispanics.
Conclusion
Malignant brain and other CNS tumors have the greatest mean YPLL, thereby reflecting their short survival time post diagnosis. These findings will hopefully motivate more research into mitigating the impact of these debilitating tumors.
Keywords: CBTRUS, central nervous system tumors, NCHS, years of potential life lost
Introduction
Cancer is the second leading cause of death in the United States (US), with an expected 589 430 cancer deaths in 2015.1 The relative rank of cancer as a cause of death varies over the lifespan. It is the third most common cause of death for those aged 25–44 years, the most common cause of death for those aged 45–64 years, and the third most common cause of death for those aged 65 years and older.2
Although not as common as other cancers, brain and other CNS tumors have a significant impact on the population, with a median age of diagnosis of 59 years.1 In 2011, brain and other CNS tumors were the second leading cause of cancer mortality in men aged 20 to 39 years and the fifth leading cause of cancer mortality in women aged 20 to 39 years.1 Malignant brain and CNS tumors over the last 5 years (2007–2011) had an incidence rate of 27.85 per 100 000, and a much lower 5-year relative survival rate compared to that of female breast, prostate, and colorectal cancer.3 Although the incidence of malignant brain and other CNS tumors has significantly decreased in males from 2008 to 2010, the incidence has remained stable in women. The incidence of nonmalignant brain and other CNS tumors has significantly increased in both sexes from 2004 to 2010.4
The most common cancers in adults (aged 20+ y) diagnosed between 2000 and 2010 were prostate cancer (incidence rate [IR]: 215.96 per 100 000), female breast cancer (IR: 173.65 per 100 000), lung and bronchial cancer (IR: 95.40 per 100 000), and colorectal cancer (IR: 68.52 per 100 000).4 From 2000 to 2010, the incidence of lung and bronchus cancer in both men (annual percent change [APC] = −4.0) and women (APC = −2.2), as well as the incidence of colorectal cancer in both men (APC = −4.0) and women (APC = −4.2), decreased significantly. The incidence of prostate cancer decreased (APC = −2.1), while female breast cancer incidence increased (APC = 0.3).1
The most recent data show that overall mortality due to cancer has declined in the last 5 years (2007–2011) in both adult men and women (1.8% and 1.4% declines, respectively), likely driven by the decrease in incidence of the most common cancers.1 Lung and bronchus cancer mortality has significantly decreased in both men (APC = −2.9) and women (APC = −1.9), prostate cancer mortality significantly decreased (APC = −3.2), female breast cancer mortality significantly decreased (APC = −1.9), and colorectal cancer significantly decreased in both men (APC = −2.6) and women (APC = −3.0).1
The aim of this study is to quantify the impact of death due to brain and other CNS tumors occurring in adults by estimating the years of potential life lost (YPLL). YPLL measures the average time an individual would have lived had he or she not died prematurely, in an attempt to highlight the economic and social impacts of premature mortality.5 Accurate estimations of YPLL are imperative for evaluating cancer management and understanding disease burden on society. Comparing brain and other CNS tumors with more common cancers provides context for the importance of looking at other measures, such as YPLL, to describe disease burden, rather than more typical measures such as incidence. We compare YPLL calculations for brain and other CNS tumors in adults to those of other common cancers in adults in the US to emphasize the increased health burden for this diagnosis.
Materials and Methods
Data Collection
The Centers for Disease Control and Prevention's (CDC) National Center for Health Statistics (NCHS) reports complete period life tables for the US by race, Hispanic ethnicity, and sex based on age-specific death rates, as part of the National Vital Statistics System (NVSS). The most current available life tables were for 2010, and thus 2010 was the year chosen for all of the analyses in this study. The years of potential life lost (YPLL) were calculated stratified by sex, race, and ethnicity using the corresponding period life tables. Cause of death on death certificates is coded using the International Classification of Diseases, 10th edition (ICD-10). Mortality data, abstracted from death certificate data, were obtained from the CDC's NCHS Vital Statistics Data via the downloadable data files (http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm). In this study, malignant brain tumors were defined by the following ICD-10 codes: C71.0, C71.1, C71.2, C71.3, C71.4, C71.5, C71.6, C71.7, C71.8, and C71.9. Other malignant CNS tumors (tumors that occurred in non-brain locations in the CNS) were defined by the following ICD-10 codes: C70.0, C70.1, C70.9, C72.0, C72.1, C72.2, C72.3, C72.4, C72.5, C72.8, C72.9, C75.1, C75.2, and C75.3. Nonmalignant brain and CNS tumors were defined by the following ICD-10 codes: D32.0, D32.1, D32.9, D33.0, D33.1, D33.2, D33.3, D33.4, D33.7, D33.9, D35.2, D35.3, and D35.4. Cancer incidence and mortality rates per 100 000 population were obtained from US Cancer Statistics (USCS) via the CDC Wide-ranging Online Data for Epidemiologic Research (WONDER) (http://wonder.cdc.gov/cancer.HTML), which represent 100% of the US population for the period examined. Incidence rates per 100 000 population for brain and CNS tumors were estimated using the Central Brain Tumor Registry of the United States (CBTRUS) analytic file, which is comprised of the largest aggregation of population-based incidence data limited to primary brain and CNS tumors in the United States. The CBTRUS data represent ∼99.9% of the US population during the time period examined.3 Other information regarding primary brain and CNS tumors, along with all other cancers, is available through USCS.
Study Population
This analysis specifically estimated YPLL for adults (age 20+ y) at the time of their death due to cancer. Individuals whose Hispanic ethnicity was listed as “unknown” were excluded (n = 4237). The cancers selected for this study included the 4 most common cancers in adults (lung and bronchial, colorectal, prostate, and breast) and 3 less common cancers that have high mortality after diagnosis (pancreatic, brain and other CNS, and ovarian). Breast cancer was limited to females only. The causes of death for the comparison cancers were defined using ICD-10 codes: lung and bronchus (C34.0–C34.3, C34.8, and C34.9), colorectal (C18.0–C18.9, C19, C20, and C21.8), pancreatic (C25.0–C25.9), prostate (C61), breast (C50.0–C50.9), and ovarian (C56 and C57.8).6
Statistical Analyses
YPLL were calculated by combining the life table data with the mortality data. For example, if a Hispanic female (Life Table 12) died at age 31 (based on mortality data), her YPLL would be the years of life remaining at age 31 for Hispanic females (based on life table data). Thus, her total YPLL is 53.6 years. Total and mean YPLL were computed for each of the selected cancers and compared between sexes and between race/ethnicities in order to quantify and compare the burden of these cancers by sex and race/ethnicity. The YPLL calculations were performed with SAS version 9.4 (http://www.sas.com/en_us/home.html), and graphs were created using R version 3.1.2 (http://www.r-project.org/). Incidence rates for brain and CNS tumors using CBTRUS data were calculated with SEER*Stat 8.1.5 (http://seer.cancer.gov/seerstat/).
Results
Incidence and Mortality Rates
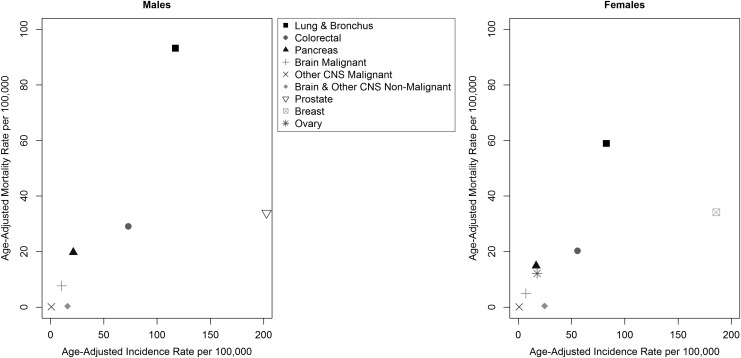
Figure 1 shows the relationship between incidence and mortality for the cancers studied as compared with malignant brain, malignant other CNS, and nonmalignant brain and other CNS tumors. While incidence of malignant brain tumors is rare, their mortality is high compared with other cancers, such as breast and prostate, which have high incidence but lower mortality. For lung and bronchial cancer, the incidence and mortality are both high for males and females.
Fig. 1.
Incidence rate vs mortality rate for selected cancers.
Cohort Demographics
The total number of individuals 20 years and older whose primary cause of death in 2010 was one of the selected cancers was 269 517 (132 923 males and 136 594 females). Of these individuals, 84.69% were non-Hispanic white, 11.44% were non-Hispanic black, and 3.87% were Hispanic. Out of the total number of deaths, 0.96% were among those aged 20–39 years, 17.86% were among those aged 40–59 years, 51.54% were among those aged 60–79 years, and 29.64% were among those aged 80 years and older. For males, lung and bronchial cancer caused the largest proportion of deaths, followed by prostate cancer, colorectal cancer, pancreatic cancer, and malignant brain tumors (Table 1). For females, lung and bronchial cancer caused the largest proportion of deaths, followed by breast, colorectal, pancreatic, and malignant brain tumors (Table 1). For both males and females aged 20–39 years, the leading cause of cancer death in this study was malignant brain tumors. For males aged 40–59 years, the leading cause of cancer death was malignant brain tumors, and for females of the same age group, the leading cause of cancer death was female breast, followed closely by malignant brain tumors.
Table 1.
Demographics for persons 20+ years old who died of selected cancers in 2010 (NVSS)
| Selected Cancer | Lung & Bronchus |
Colorectal |
Pancreas |
Brain (malignant) |
Other CNS (malignant) |
Brain & Other CNS (nonmalignant) |
Prostate | Breast | Ovary | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Female | |
| Total deaths | 69 177 (52.04%) |
55 568 (40.68%) |
20 962 (15.77%) |
19 278 (14.11%) |
14 545 (10.94%) |
13 982 (10.24%) |
5 901 (4.44%) |
4 484 (3.28%) |
99 (0.08%) |
98 (0.07%) |
267 (0.20%) |
429 (0.32%) |
21 972 (16.53%) |
31 564 (23.11%) |
11 191 (8.19%) |
269 517 |
| Race/ethnicity | ||||||||||||||||
| Non-Hispanic white | 59 744 (86.36%) |
49 059 (88.29%) |
17 033 (81.26%) |
15 838 (82.16%) |
12 293 (84.52%) |
11 587 (82.87%) |
5 286 (89.58%) |
4 008 (89.38%) |
84 (84.85%) |
74 (75.51%) |
220 (82.40%) |
357 (83.22%) |
17 420 (79.28%) |
25 555 (80.96%) |
9 692 (86.61%) |
228 250 (84.69%) |
| Non-Hispanic black | 7 354 (10.63%) |
5 243 (9.43%) |
2 743 (13.08%) |
2 513 (13.03%) |
1 579 (10.85%) |
1 687 (12.07%) |
312 (5.29%) |
260 (5.80%) |
11 (11.11%) |
18 (18.37%) |
30 (11.23%) |
52 (12.12%) |
3 567 (16.24%) |
4 524 (14.33%) |
954 (8.52%) |
30 847 (11.44%) |
| Hispanic | 2 079 (3.01%) |
1 266 (2.28%) |
1 186 (5.66%) |
927 (4.81%) |
673 (4.63%) |
708 (5.06%) |
303 (5.13%) |
216 (4.82%) |
4 (4.04%) |
6 (6.12%) |
17 (6.37%) |
20 (4.66%) |
985 (4.48%) |
1 485 (4.71%) |
545 (4.87%) |
10 420 (3.87%) |
| Age at death group, y | ||||||||||||||||
| 20–39 | 191 (0.28%) |
165 (0.30%) |
318 (1.52%) |
243 (1.26%) |
81 (0.56%) |
64 (0.46%) |
357 (6.05%) |
222 (4.95%) |
13 (13.13%) |
7 (7.14%) |
13 (4.87%) |
8 (1.87%) |
2 (0.01%) |
737 (2.34%) |
158 (1.41%) |
2 579 (0.96%) |
| 40–59 | 11 639 (16.82%) |
8 814 (15.86%) |
4 315 (20.58%) |
3 199 (16.59%) |
2 947 (20.26%) |
1 920 (13.73%) |
1 868 (31.66%) |
1 131 (25.22%) |
25 (25.25%) |
19 (19.39%) |
51 (19.10%) |
58 (13.52%) |
1 120 (5.10%) |
8 667 (27.46%) |
2 375 (21.22%) |
48 148 (17.86%) |
| 60–79 | 41 230 (59.60%) |
31 246 (56.23%) |
10 307 (49.17%) |
7 586 (39.35%) |
8 071 (55.49%) |
6 803 (48.66%) |
2 884 (48.87%) |
2 185 (48.73%) |
42 (42.43%) |
36 (36.74%) |
110 (41.20%) |
146 (34.03%) |
9 210 (41.92%) |
13 413 (42.49%) |
5 638 (50.38%) |
138 907 (51.54%) |
| 80+ | 16 117 (23.30%) |
15 343 (27.61%) |
6 022 (28.73%) |
8 250 (42.80%) |
3 446 (23.69%) |
5 195 (37.15%) |
792 (13.42%) |
946 (21.10%) |
19 (19.19%) |
36 (36.73%) |
93 (34.83%) |
217 (50.58%) |
11 640 (52.97%) |
8 747 (27.71%) |
3 020 (26.99%) |
79 883 (29.64%) |
Life Expectancy
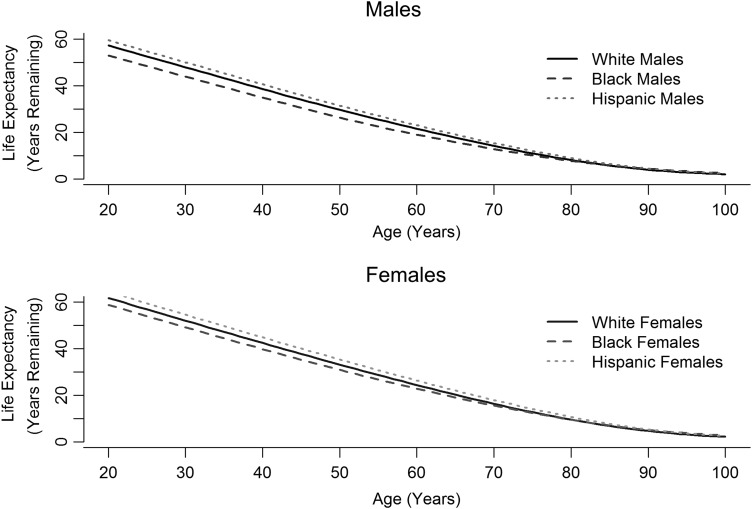
From the period life tables provided by the NVSS, life expectancy was plotted by age, by race/ethnicity, and by sex (Fig. 2) for the year 2010. Females had a higher life expectancy at a given year than males. For both sexes, Hispanics had the greatest life expectancy at a given age, and non-Hispanic blacks had the lowest life expectancy at a given age. Black males had the lowest life expectancy at a given age, followed by white males.
Fig. 2.
Life expectancy by race/ethnicity and sex.
Age at Death
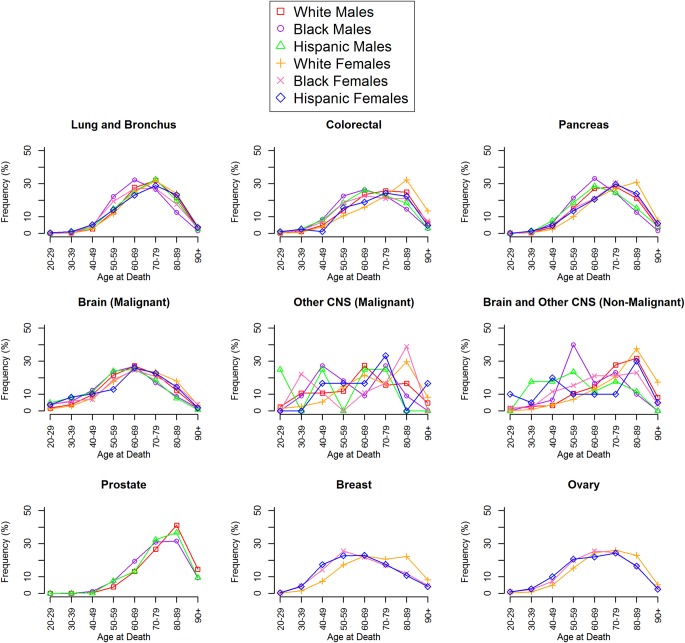
Age at death distributions are provided in Fig. 3 for each selected cancer and plotted by each sex and race/ethnicity combination. Overall, the distributions for most cancer types were consistent between sexes and race/ethnicities. For males, the cancers with the lowest mean age at death were other malignant CNS tumors (62.90 y) and malignant brain (63.33 y), while the cancers with the highest mean age at death were prostate cancer (78.71 y) and nonmalignant brain and other CNS tumors (70.99 y). For females, the cancers with the lowest mean age at death were malignant brain tumors (66.39 y) and breast cancer (68.49 y), while the cancers with the highest mean age at death were nonmalignant brain and other CNS tumors (75.77 y) and colorectal cancer (74.05 y).
Fig. 3.
Age-at-death distributions for selected cancers by race/ethnicity and sex.
Years of Potential Life Lost
The total YPLL and the mean YPLL are presented in Table 2. The total YPLL in 2010 was 1 871 704 for males aged 20 years and older, with 53.49% from lung and bronchial cancer, 16.82% from colorectal cancer, 11.76% from pancreatic cancer, 11.32% from prostate cancer, 6.29% from malignant brain tumors, 0.21% from nonmalignant brain and other CNS tumors, and 0.11% from other malignant CNS tumors. The total YPLL in 2010 was 2 274 071 for females aged 20 years and older, with 39.30% from lung and bronchial cancer, 26.07% from breast cancer, 12.57% from colorectal cancer, 9.08% from pancreatic cancer, 8.63% from ovarian cancer, 4.01% from malignant brain tumors, 0.26% from nonmalignant brain and other CNS tumors, and 0.08% from other malignant CNS tumors. The total YPLL is significantly affected by total number of deaths for each cancer; hence, the mean YPLL provides a better estimate of the impact of each cancer on individual life. The cancers contributing the highest mean YPLL for males were other malignant CNS tumors (20.65), malignant brain tumors (19.93), and pancreatic cancer (15.13). The cancers contributing the highest mean YPLL for females were malignant brain tumors (20.31), breast cancer (18.78), and other malignant CNS tumors (18.36). The mean and median age at death for each selected cancer by sex are also presented in Table 2.
Table 2.
Mean and total YPLL (with mean and median age at death) in 2010 (NVSS, NCHS)
| Cancer Type | Total Deaths | Total YPLL | Mean YPLL | Age at Death |
|
|---|---|---|---|---|---|
| Mean | Median | ||||
| Males | |||||
| Lung & bronchus | 69 177 | 1 001 235 | 14.47 | 70.53 | 71.0 |
| Colorectal | 20 962 | 314 871 | 15.02 | 70.23 | 71.0 |
| Pancreas | 14 545 | 220 133 | 15.13 | 69.76 | 70.0 |
| Brain (malignant) | 5 901 | 117 619 | 19.93 | 63.33 | 64.0 |
| Other CNS (malignant) | 99 | 2 045 | 20.65 | 62.90 | 63.0 |
| Brain & other CNS (nonmalignant) | 267 | 3 948 | 14.78 | 70.99 | 74.0 |
| Prostate | 21 972 | 211 853 | 9.64 | 78.71 | 80.0 |
| Total | 132 923 | 1 871 704 | – | – | – |
| Females | |||||
| Lung & bronchus | 55568 | 893 727 | 16.08 | 71.54 | 72.0 |
| Colorectal | 19278 | 285 946 | 14.83 | 74.05 | 77.0 |
| Pancreas | 13 982 | 206 562 | 14.77 | 73.68 | 71.0 |
| Brain (malignant) | 4484 | 91066 | 20.31 | 66.39 | 67.0 |
| Other CNS (malignant) | 98 | 1800 | 18.36 | 69.33 | 73.5 |
| Brain & other CNS (nonmalignant) | 429 | 5 928 | 13.82 | 75.77 | 80.0 |
| Breast | 31564 | 592779 | 18.78 | 68.49 | 68.0 |
| Ovary | 11 191 | 196 263 | 17.54 | 69.88 | 75.0 |
| Total | 136 594 | 2 274 071 | – | – | – |
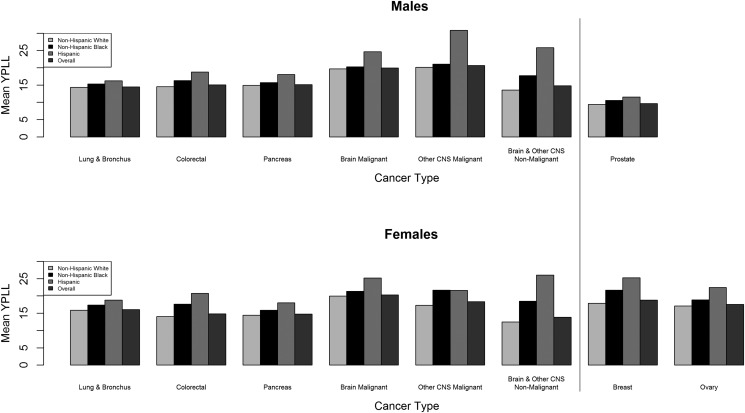
Mean YPLL varies considerably by race/ethnicity, as shown in Fig. 4. For both sexes, non-Hispanic whites had the lowest YPLL, and Hispanics had the highest YPLL for all histologies, with the exception of other malignant CNS tumors in females, where non-Hispanic blacks (21.63) had a higher mean YPLL than Hispanics (21.60). The largest differences in mean YPLL by race/ethnicity in males were malignant brain, other malignant CNS, and nonmalignant brain and other CNS tumors. In females, the largest differences in mean YPLL by race/ethnicity were in colorectal cancer, breast cancer, and nonmalignant brain and other CNS tumors.
Fig. 4.
Mean YPLL for selected cancers by race/ethnicity and sex.
Discussion
YPLL is a measure of cancer impact that is an important complement to routinely reported incidence and survival statistics. Previous studies have shown the importance of estimating YPLL in cancer and its use for quantifying potential social and economic impact.5,7,8 YPLL in more common cancers such as female breast, lung and bronchus, prostate, and colorectal cancer has been well documented.9–11 While brain and other CNS tumors are a relatively rare cancer, they are highly aggressive, as seen in the disproportion between high mortality and morbidity compared to their low incidence. Estimation of YPLL for these tumors more accurately depicts the impact of these tumors on individual lives when examined in the context of other more common cancers in adults.
Life expectancy provides a context for YPLL. In 2010, the overall life expectancy at birth was 78.7 years.2 Hispanics had the greatest life expectancy overall (81.4 y) and non-Hispanic blacks had the lowest life expectancy overall (74.7 y).2 This difference in life expectancy can be attributed to several known factors, such as the Hispanic Epidemiological Paradox (HEP), cultural norms, social support networks, accessibility to health care, and other health and life risks that are disproportionately more present in non-Hispanic black communities. HEP describes a complex in which, despite having lower than average income/education, Hispanic health outcomes, such as life expectancy, are similar to or better than their white counterparts.12
Cancers with the lowest average age at death are considered to be the most deadly cancers. While not the most common cancer, malignant brain tumors had the overall lowest age of death, with a mean age of death of ∼63 years for males and 66 years for females. Other malignant CNS tumors in males had a mean age of death of 63 years. These numbers are lower than the mean age at death for breast (68 y), colorectal (70 y, males; 74 y, females), lung (71 y, males; 72 y, females), and prostate cancers (79 y). This demonstrates the significant impact malignant brain tumors have on the population and cancer death rates. For most cancer types, females had a higher mean age at death than males. Hispanics, contrary to having a higher life expectancy, tended to have worse outcomes than whites for the same cancers. These differences may be attributed to differences in socioeconomic status and access to health care.
In 2010, the cancers with the highest total YPLL were lung and bronchus, colorectal, prostate, breast, and pancreatic. Total YPLL is strongly influenced by total number of incident cases, and mean YPLL provides a sensitive estimation of the impact of cancer death due to more rare cancers, such as brain and other CNS tumors.13 Mean YPLL provides a measure of the burden of cancer to the individual patient, rather than the population as a whole, thus quantifying how much a patient's life is shortened by his or her cancer. Looking at measures such as incidence and mortality rates may not provide a complete picture of disease effect, as shown in Fig. 1. Malignant brain had the highest mean YPLL in both males and females, averaging around 20 years. The mean YPLL for the other common cancers in adults ranged from 14 to 18 years. These differences may be explained by numerous factors, including but not limited to differences in age at diagnosis, in age at death, and/or in total survival time after diagnosis. Also, public health efforts to increase screening and prevention of the most common cancers often result in their diagnosis at earlier—and more treatable—points in individual patient disease trajectory and may contribute to these differences.
This study is subject to certain limitations. Both malignant CNS and nonmalignant brain and other CNS tumors had small sample sizes, leading to a potential overestimation of their effects. However, using population-based data sources representing 99%–100% of the United States for all analyses makes these results the most representative experience in the US for the 2010 time period. Other limitations may include the stipulations by which causes of death are considered primary or antecedent and the overall quality of death certificate data collected by data sources for analysis. This study also had several strengths. The NVSS mortality data are a fundamental source of demographic and cause-of-death information for the entire US and therefore provide a comprehensive mortality profile for all US deaths in 2010. Cancer incidence and mortality from USCS for other cancers representing 100% of the US population and the CBTRUS incidence representing 99.9% of the US population in 2010 also provide comprehensive, reliable data sources for this study.
Conclusion
Although other cancers have higher age-adjusted incidence and mortality rates than brain and CNS tumors, brain and CNS tumors have the highest mean YPLL and demonstrate the devastating effect these cancers have despite their rarity. For all cancer types, there is significant variability in mean age at death and mean YPLL by sex and race/ethnicity. To date, routine screening for brain tumors is not currently available, as it is for some common cancers (e.g, prostate, breast, colorectal). However, an earlier diagnosis may result in a better prognosis and longer survival, thus helping to reduce total YPLL. The results in this study help further our knowledge of the implications of brain and CNS tumors on premature deaths in adults in the US and may, hopefully, motivate more research into mitigating not only the social impact but the less well-known economic impact of these debilitating tumors.
Funding
Funding of the CBTRUS database supporting this study was provided by the Centers for Disease Control and Prevention under agreement 5U58DP00381-04, The Sontag Foundation (www.sontagfoundation.org), Genentech (www.gene.com), Novocure, Inc. (www.novocure.com), along with the Musella Foundation (www.virtualtrials.com), Elekta (www.elekta.com), the National Brain Tumor Society (http://braintumor.org), and the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Acknowledgments
Conflict of interests statement. There are no financial disclosures from any authors.
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2015. Ca Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep. 2013;62(6):1–96. [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman HR, Liao PL et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncology. 2014;16(Suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gittleman HR, Ostrom QT, Rouse CD et al. . Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner JW, Sanborn JS. Years of potential life lost (YPLL)—what does it measure? Epidemiology. 1990;1(4):322–329. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. ICD-10 Classifications of Mental and Behavioral Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 7.Ekwueme DU, Chesson HW, Zhang KB et al. . Years of potential life lost and productivity costs because of cancer mortality and for specific cancer sites where human papillomavirus may be a risk factor for carcinogenesis–United States, 2003. Cancer. 2008;113(S10):2936–2945. [DOI] [PubMed] [Google Scholar]
- 8.Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: the cost of lost productivity due to premature cancer-related mortality in Europe . Int J Cancer. 2015;136(4):E136–E145. [DOI] [PubMed] [Google Scholar]
- 9.Liu PH, Wang JD, Keating NL. Expected years of life lost for six potentially preventable cancers in the United States. Prev Med. 2013;56(5):309–313. [DOI] [PubMed] [Google Scholar]
- 10.Gravena AA, Brischiliari SC, Gil LM et al. . Years of potential life lost due to breast and cervical cancer: a challenge for Brazilian public policy. Asian Pac J Cancer Prev. 2014;15(23):10313–10317. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Ekwueme DU. Years of potential life lost caused by prostate cancer deaths in the United States—projection from 2004 through 2050. Cancer Epidemiol. 2010;34(4):368–372. [DOI] [PubMed] [Google Scholar]
- 12.Lariscy JT, Hummer RA, Hayward MD. Hispanic older adult mortality in the United States: new estimates and an assessment of factors shaping the Hispanic paradox. Demography. 2015;52(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP. Years of life lost (YLL) from cancer is an important measure of population burden—and should be considered when allocating research funds. Br J Cancer. 2005;92(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]