Abstract
Background:
Nebivolol provides a protective effect on contrast-induced acute kidney injury (CIAKI) in animal models. However, the reports on the efficacy of nebivolol for the prevention of CIAKI in human remain unclear.
Aims:
The objective of this meta-analysis was to assess the effect of nebivolol for the prevention of CIAKI.
Materials and Methods:
Comprehensive literature searches were performed using MEDLINE, EMBASE, and Cochrane Database from inception through February 2015. Studies that reported relative risks, odd ratios, or hazard ratios comparing the risk of CIAKI in patients who received nebivolol versus those who did not were included. Pooled risk ratios (RR) and 95% confidence interval (CI) were calculated using a random-effect, generic inverse variance method.
Results:
Four studies (2 randomized controlled trials and 2 cohort studies) with 543 patients were included in our analysis to assess the risk of CIAKI and the use of nebivolol. Patients in the nebivolol group had an overall lower incidence of CIAKI (14.4%) compared to the control group (18.4%). The pooled RR of CIAKI in patients receiving nebivolol was 0.66 (95% CI: 0.38-1.15, I2 = 0). When meta-analysis was limited only to randomized control trials (RCTs), the pooled RR of CIAKI in patients receiving nebivolol was 0.79 (95% CI: 0.35-1.79, I2 = 0%).
Conclusions:
Despite no statistical significance, there was a trend toward reduced CIAKI risk in patients receiving nebivolol. The findings of our meta-analysis suggest the need of a large RCT with very careful attention to the balance of benefits and harms.
Keywords: B-Blocker, contrast-induced nephropathy, contrast-induced acute kidney injury, meta-analysis, nebivolol
Introduction
Acute kidney injury (AKI) is a frequent clinical syndrome in hospitalized patients. Among causes of AKI,[1,2,3,4,5,6] contrast-induced nephropathy or contrast-induced acute kidney injury (CIAKI) is a common encounter, with an incidence of 2% in the general population without risk factors to more than 40% in high-risk patients.[7,8,9,10,11,12,13] In addition, the incidence of CIAKI has been rising in recent years due to the increasing use of cardiac angiography and percutaneous coronary intervention.[14,15] A number of studies have attempted to identify effective interventions to prevent CIAKI including hydration with intravenous isotonic saline, oral hydration, sodium bicarbonate infusion, N-acetylcysteine, nonionic low-osmolar agents, and statin administration.[10,16,17] However, CIAKI is still it is a growing problem worldwide accounting for approximately 150,000 patients each year.[18] Patients with CIAKI have also been shown to have longer hospitalizations and higher mortality rates.[19] Thus, further studies to investigate interventions to prevent CIAKI are needed.
Nebivolol, a third-generation beta-blocker, has recently been proposed to be a potentially effective agent to help prevent CIAKI, since it provides nitric oxide (NO)-induced vasodilation and antioxidant properties.[20] Previous studies were conducted to assess the efficacy of nebivolol in humans for CIAKI prevention. The results of several studies trended toward the protective effects of nebivolol for CIAKI prevention.[21,22,23] Conversely, a study showed no beneficial effect of nebivolol for the prevention of CIAKI.[24] However, these studies included small sample sizes and the findings did not show a significant role of nebivolol in CIAKI. Thus, we performed this meta-analysis to assess the effect of nebivolol for the pharmacologic prevention of CIAKI.
Materials and Methods
Data sources and searches
We performed a MEDLINE search (through February 28, 2015), Scopus, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov to identify eligible studies using the Medical Subject Headings database search terms “nebivolol AND contrast- induced nephropathy,” “beta-blocker AND contrast-induced nephropathy,” “nebivolol,” “contrast-induced acute kidney injury,” “CIAKI,” and “contrast-induced nephropathy.” The search is limited to studies in humans aged 18 and older. We also include unpublished studies in the form of posters and abstracts in our search strategy.
Study selection
We included randomized and prospective clinical trials examining the incidence of CIAKI and pre- and post-contrast exposure serum creatinine in a patient with any kind of contrast exposure. Nebivolol was required as the intervention for CIAKI prevention. There were no restrictions on sample size, or study duration. Two authors (NT and WC) independently screened the titles and abstracts of all electronic citations, and full text articles were retrieved for comprehensive review and independently re-screened.
Data extraction and quality assessment
The following data were extracted from the studies examined in the study: Year of publication, study design, sample size, percentage of male subjects, mean age of subjects, incidence of CIAKI, precontrast serum creatinine, 48 h postcontrast creatinine, percentage of diabetes, and interventions of treatment and control groups. Differing decisions were resolved by mutual consensus. Study quality was assessed with a modified version of the Jadad et al. scale[25] for randomized control trials (RCTs) and Newcastle-Ottawa quality assessment scale[26] for observational studies.
Data synthesis and analysis
We used random-effects model meta-analyses to assess standardized net changes in continuous outcomes. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird.[27] All pooled estimates are displayed with a 95% confidence interval (CI). Statistical heterogeneity was assessed using the Cochran's Q-test. This statistic is complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. An I2 value of 0-25% represents insignificant heterogeneity, 26-50% low heterogeneity, 51-75% moderate heterogeneity, and >75% high heterogeneity.[28] The presence of publication bias was assessed by funnel plots of the logarithm of odds ratios versus their standard errors.[29] The meta-analyses were performed with Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014).
Results
Characteristics and quality of the studies
A total of 190, potentially relevant studies were identified and screened; 12 full texts were evaluated. Four studies fulfilled our inclusion criteria for the meta-analysis. Finally, 543 patients from 4 studies were included in our analysis to assess the risk of CIAKI and the use of nebivolol.[21,22,23,24] Two studies were RCT[23,24] and other two were cohort studies.[21,22] One study was reported as an abstract for poster presentation.[21] The subjects were patients undergoing scheduled coronary angiography. In the above trials, intravenous isotonic saline was administered before contrast exposure in all patients and nebivolol was given to the intervention group. The trials followed patient creatinine before coronary angiography contrast exposure and at 48 h after exposure, and included sample sizes ranging from 90 to 247 patients.
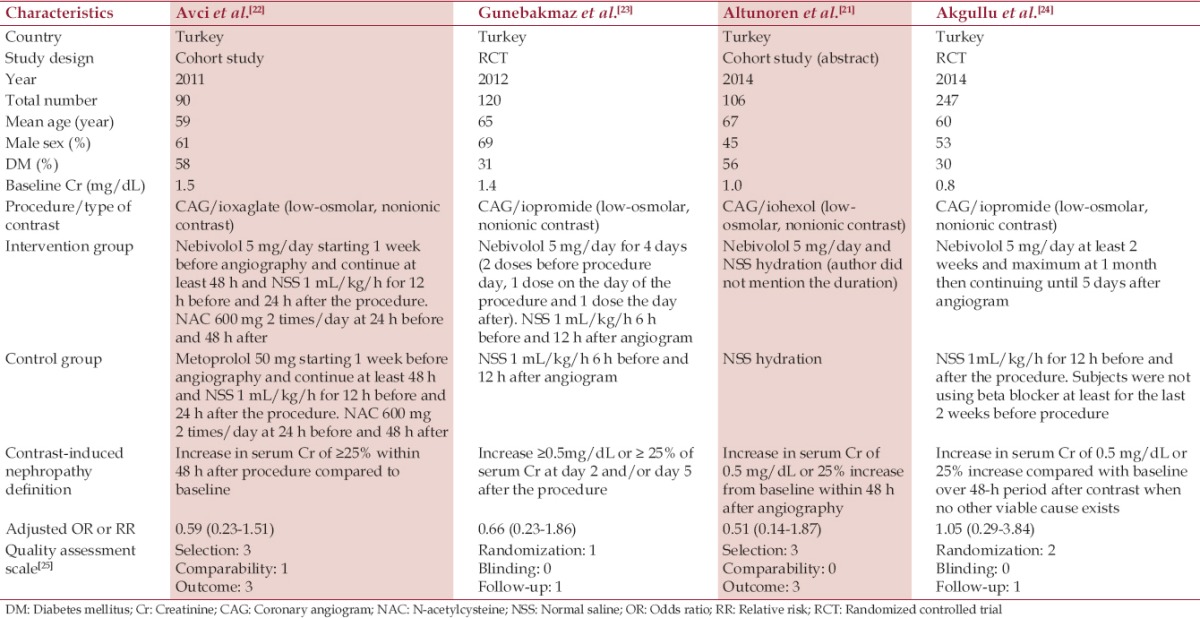
All studies included both diabetic and nondiabetic patients. The mean serum creatinine (SCr) ranged from 0.8 to 1.54 mg/dL and one study was conducted in patients with mild renal insufficiency (SCr >1.2 mg/dL).[22] The mean age of the subjects ranged from 59 to 67 years. Characteristics of the individual trials are displayed in Table 1.
Table 1.
Main characteristics of the studies included in this meta-analysis
The risk of contrast-induced acute kidney injury in nebivolol and control groups
Patients in the nebivolol group had an overall lower incidence of CIAKI (14.4%) compared to the control group (18.4%). The pooled risk ratios (RR) of CIAKI in patients receiving nebivolol was 0.66 (95% CI: 0.38-1.1.15, I2 = 0). Figure 1 shows the forest plot of the included studies. Sensitivity analysis was limited to only RCTs and demonstrated the pooled RR of CIAKI in patients receiving nebivolol of 0.79 (95% CI: 0.35-1.79) as shown in Figure 2. The statistical heterogeneity was insignificant with an I2 of 0%.
Figure 1.
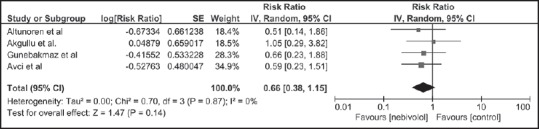
Forest plot of the meta-analysis of all included studies comparing the risk of contrast-induced acute kidney injury in patients who received nebivolol and those who did not; square data markers represent risk ratios; horizontal lines, the 95% confidence intervals with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall risk ratio and 95% confidence interval for the outcome of interest. IV: Inverse variance; SE: Standard error
Figure 2.
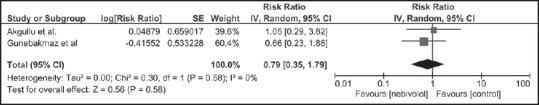
Forest plot of the meta-analysis of randomized controlled trials comparing the risk of contrast-induced acute kidney injury in patients who received nebivolol and those who did not; square data markers represent risk ratios; horizontal lines, the 95% confidence intervals with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall risk ratio and 95% confidence interval for the outcome of interest. IV: Inverse variance; SE: Standard error
Evaluation for publication bias
A funnel plot to evaluate publication bias for the risk of CIAKI in patients receiving nebivolol versus a control group is summarized in Figure 3. The graph is fairly symmetric demonstrating insignificant publication bias.
Figure 3.
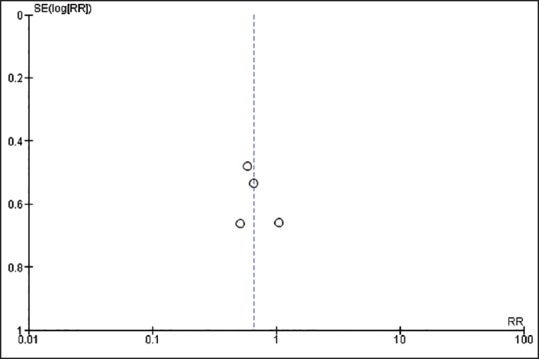
Funnel plot of 4 studies included in the meta-analysis for the risk of CIAKI in patients who received nebivolol. The graph is slight asymmetric and suggests the presence of publication in favor of negative studies. RR: Risk ratio; SE: Standard error.
Discussion
Our meta-analysis showed an overall incidence of CIAKI of 5.3% in patients with contrast exposures. Patients in the nebivolol group had a lower incidence of CIAKI (14.4%) when compared to the control group (18.4%). Based on the pooled incidence of CIAKI in the nebivolol and control groups, the number needed to treat (NNT) (the number of patients) to prevent a CIAKI event using nebivolol is 25. Our study also demonstrated a protective effect of periprocedural statins on the incidence of CIAKI.
The kidney disease: Improving global outcomes AKI guideline suggests that intravascular volume expansion, either with normal saline or mixing with sodium bicarbonate, is the most effective way to prevent CIAKI.[30] Despite these preventative measures, CIAKI is still a common clinical problem among hospitalized patients which results in increased length of hospital stay and mortality. Thus, studies have investigated potential pharmacologic agents to reduce the CIAKI risk.
Although the underlying pathogenesis of CIAKI is not completely understood, proposed mechanisms of CIAKI include direct tubular injury mediated by an increase in free radicals and hemodynamic changes of renal blood flow by modulating the actions of dopamine, adenosine, angiotensin II, endothelin, and NO.[31,32] According to the animal studies,[20,33] nebivolol, a selective beta-1 adrenergic receptor blocker can increase the renal NO excretion, enhance NO bioavailability, and prevent deactivation of NO leading to vasodilatation and improved renal blood flow.[34] In addition, nebivolol was found to have a protective role against CIAKI in albino rats.[20] Rats pretreated with nebivolol were found to have significantly less oxidative stress markers and histological abnormalities compared to the control rats. Our study compared the risk of CIAKI between nebivolol group and standard treatment, which is normal saline infusion. Based on the meta-analysis, nebivolol reduces the incidence of CIAKI (18.4% vs. 14.4%). Despite the statistical insignificance of the complete analysis, there was a trend toward the reduction of CIAKI risk in nebivolol treated patients without significant heterogeneity. The insignificant result is likely due to underpowered studies with relatively small sample sizes, especially since the NNT to prevent a CIAKI event using nebivolol in our meta-analysis was 25. Therefore, the findings of our meta-analysis suggest the need of a large RCT.
Despite no significant heterogeneity or publication bias in our complete analysis, there are several limitations in our study. First, a causal relationship needs to be cautiously interpreted because observational studies were included in our meta-analysis. However, a trend toward the reduction of CIAKI risk in nebivolol existed in the sensitivity analysis limited to the only RCTs. In addition, all included studies were conducted in Turkey. Therefore, the generalizability of potential benefit of nebivolol to different populations is limited and future studies are required.
In summary, our meta-analysis demonstrated a trend toward reduced CIAKI risk in patients receiving nebivolol. A large RCT-with very careful attention to the balance of benefits and harms is needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Thongprayoon C, Cheungpasitporn W, Srivali N, Ungprasert P, Kittanamongkolchai W, Greason KL, et al. Acute kidney injury after transcatheter aortic valve replacement: A systematic review and meta-analysis. Am J Nephrol. 2015;41:372–82. doi: 10.1159/000431337. [DOI] [PubMed] [Google Scholar]
- 2.Thongprayoon C, Cheungpasitporn W, Srivali N, Ungprasert P, Kittanamongkolchai W, Kashani K. The impact of fluid balance on diagnosis, staging and prediction of mortality in critically ill patients with acute kidney injury. J Nephrol. 2015 doi: 10.1007/s40620-015-0211-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, O Corragain OA, Edmonds PJ, et al. Comparison of renal outcomes in off-pump versus on-pump coronary artery bypass grafting: A systematic review and meta-analysis of randomized controlled trials. Nephrology (Carlton) 2015 doi: 10.1111/nep.12506. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Cheungpasitporn W, Thongprayoon C, Srivali N, O’Corragain OA, Edmonds PJ, Ungprasert P, et al. Preoperative renin-angiotensin system inhibitors use linked to reduced acute kidney injury: A systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30:978–88. doi: 10.1093/ndt/gfv023. [DOI] [PubMed] [Google Scholar]
- 5.Thongprayoon C, Cheungpasitporn W, Akhoundi A, Ahmed AH, Kashani KB. Actual versus ideal body weight for acute kidney injury diagnosis and classification in critically ill patients. BMC Nephrol. 2014;15:176. doi: 10.1186/1471-2369-15-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thongprayoon C, Cheungpasitporn W, Kittanamongkolchai W, Srivali N, Ungprasert P, Kashani K, et al. Optimum methodology for estimating baseline serum creatinine for the acute kidney injury classification. Nephrology (Carlton) 2015 doi: 10.1111/nep.12525. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–5. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Briguori C, Airoldi F, D’Andrea D, Bonizzoni E, Morici N, Focaccio A, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): A randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–7. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 9.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–82. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 10.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010;5:4–9. doi: 10.2215/CJN.05200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber W, Eckel F, Hennig M, Rosenbrock H, Wacker A, Saur D, et al. Prophylaxis of contrast material-induced nephropathy in patients in intensive care: Acetylcysteine, theophylline, or both? A randomized study. Radiology. 2006;239:793–804. doi: 10.1148/radiol.2393041456. [DOI] [PubMed] [Google Scholar]
- 13.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 14.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv. 2005;64:442–8. doi: 10.1002/ccd.20316. [DOI] [PubMed] [Google Scholar]
- 16.Cheungpasitporn W, Thongprayoon C, Brabec BA, Edmonds PJ, O’Corragain OA, Erickson SB. Oral hydration for prevention of contrast-induced acute kidney injury in elective radiological procedures: A systematic review and meta-analysis of randomized controlled trials. N Am J Med Sci. 2014;6:618–24. doi: 10.4103/1947-2714.147977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Edmonds PJ, O’Corragain OA, Srivali N, et al. Periprocedural effects of statins on the incidence of contrast-induced acute kidney injury: A systematic review and meta-analysis of randomized controlled trials. Ren Fail. 2015;37:664–71. doi: 10.3109/0886022X.2015.1010939. [DOI] [PubMed] [Google Scholar]
- 18.Feldkamp T, Kribben A. Contrast media induced nephropathy: Definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99:177–96. [PubMed] [Google Scholar]
- 19.Solomon RJ, Mehran R, Natarajan MK, Doucet S, Katholi RE, Staniloae CS, et al. Contrast-induced nephropathy and long-term adverse events: Cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–9. doi: 10.2215/CJN.00550109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toprak O, Cirit M, Tanrisev M, Yazici C, Canoz O, Sipahioglu M, et al. Preventive effect of nebivolol on contrast-induced nephropathy in rats. Nephrol Dial Transplant. 2008;23:853–9. doi: 10.1093/ndt/gfm691. [DOI] [PubMed] [Google Scholar]
- 21.Altunoren O, Balli M, Tasolar H, Eren N, Arpaci A, Caglayan CE, et al. Effect of nebivolol in the prevention of contast induced nephropathy in high risk individuals. Nephrol Dial Transplant. 2014;29:108–9. [Google Scholar]
- 22.Avci E, Yesil M, Bayata S, Postaci N, Arikan E, Cirit M. The role of nebivolol in the prevention of contrast-induced nephropathy in patients with renal dysfunction. Anadolu Kardiyol Derg. 2011;11:613–7. doi: 10.5152/akd.2011.164. [DOI] [PubMed] [Google Scholar]
- 23.Günebakmaz O, Kaya MG, Koc F, Akpek M, Kasapkara A, Inanc MT, et al. Does nebivolol prevent contrast-induced nephropathy in humans? Clin Cardiol. 2012;35:250–4. doi: 10.1002/clc.21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akgüllü Ç, Eryilmaz U, Güngör H, Huyut A, Zencir C, Hekim T. A clinical study about contrast nephropathy: Risk factors and the role of beta blockers. Anatol J Cardiol. 2015;15:232–40. doi: 10.5152/akd.2014.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 30.Lameire N, Kellum JA KDIGO AKI Guideline Work Group. Contrast-induced acute kidney injury and renal support for acute kidney injury: A KDIGO summary (Part 2) Crit Care. 2013;17:205. doi: 10.1186/cc11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gami AS, Garovic VD. Contrast nephropathy after coronary angiography. Mayo Clin Proc. 2004;79:211–9. doi: 10.4065/79.2.211. [DOI] [PubMed] [Google Scholar]
- 32.Katholi RE, Woods WT, Jr, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64–71. doi: 10.1053/ajkd.1998.v32.pm9669426. [DOI] [PubMed] [Google Scholar]
- 33.Kakoki M, Hirata Y, Hayakawa H, Nishimatsu H, Suzuki Y, Nagata D, et al. Effects of vasodilatory beta-adrenoceptor antagonists on endothelium-derived nitric oxide release in rat kidney. Hypertension. 1999;331(Pt 2):467–71. doi: 10.1161/01.hyp.33.1.467. [DOI] [PubMed] [Google Scholar]
- 34.Greven J, Gabriëls G. Effect of nebivolol, a novel beta 1-selective adrenoceptor antagonist with vasodilating properties, on kidney function. Arzneimittelforschung. 2000;50:973–9. doi: 10.1055/s-0031-1300320. [DOI] [PubMed] [Google Scholar]


