SUMMARY
In rodents, ubiquitous α1-Na+,K+-ATPase is inhibited by ouabain and other cardiotonic steroids (CTS) at ~103-fold higher concentrations than those effective in other mammals. To examine the specific roles of the CTS-sensitive α1S- and CTS-resistant α1R-Na+,K+-ATPase isoforms, we compared the effects of ouabain on intracellular Na+ and K+ content, cell survival, and mitogen-activated protein kinases (MAPK) in human and rat vascular smooth muscle cells (HASMC and RASMC), human and rat endothelial cells (HUVEC and RAEC), and human and rat brain astrocytes. Six-hour exposure of HASMC and HUVEC to 3 μM ouabain dramatically increased the intracellular [Na+]/[K+] ratio to the same extend as in RASMC and RAEC treated with 3,000 μM ouabain. In 24, 3 μM ouabain triggered the death of all types of human cells used in this study. Unlike human cells, we did not detect any effect of 3,000-5,000 μM ouabain on the survival of rat cells, or smooth muscle cells from mouse aorta (MASMC). Unlike in the wild-type α1R/R mouse, ouabain triggered death of MASMC from α1S/S mouse expressing human α1-Na+,K+-ATPase. Furthermore, transfection of HUVEC with rat α1R-Na+,K+-ATPase protected them from the ouabain-induced death. In HUVEC, ouabain led to phosphorylation of p38 MAPK, whereas in RAEC it stimulated phosphorylation of ERK1/2. Overall, our results, demonstrate that the drastic differences in cytotoxic action of ouabain on human and rodent cells are caused by unique features of α1S/α1R-Na+,K+-ATPase, rather than by any downstream CTS-sensitive/resistant components of the cell death machinery.
Keywords: Na+/K+ pump, ouabain, intracellular Na and K, cell death
1. Introduction
Mg2+-dependent (Na++K+)-stimulated adenosine triphosphatase (Na+,K+-ATPase) is an integral plasma membrane protein consisting of α- and β-subunits and detected in all types of animal cells. ATP hydrolysis by the larger α-subunit (~110 kD) leads to phosphorylation of Asp369 residue that triggers E1-E2 conformational transition and provides energy for electrogenic ion transport (3Na+ vs 2K+). In addition to the ubiquitous α1-isoform, 3 other Na+,K+-ATPase α-subunits were identified by screening c-DNA libraries. These isoforms are expressed in a tissue-specific manner with high abundance in neural cells (α3 and α2), heart (α2), skeletal muscle (α3, α2), and testis (α4). Four isoforms of β-subunits encoding ~ 35 kD proteins have also been found in mammals. It has been shown that β-subunits are obligatory for the enzymatic activity of α subunits and control their delivery, conformational stability, and affinity for extracellular potassium (K+o) and intracellular sodium (Na+i). For more details, see [1;2].
The beneficial effects of extracts from the leaves of Digitalis on the patients with heart failure led to isolation of several plant-derived cardiotonic steroids (CTS) known also as cardenolides. Besides cardenolides, other members of the CTS superfamily, bufadienolides, have been isolated from amphibians [3;4]. Numerous studies demonstrated that in rat and mouse, CTS inhibits α1-Na+,K+-ATPase at concentrations ~103-fold higher than in other mammals. Lingrel and co-workers were the first to demonstrate that the presence in rodents of CTS-resistant α1R-Na+,K+-ATPase is caused by substitution of Gln111 and Asn122, detected in CTS-sensitive α1S-Na+,K+-ATPase from other mammalians, with Arg and Asp, respectively (for review, see [5]). Because affinity for the CTS of α2 and α3 subunits in mouse and other mammals is about the same [6], transgenic rodents with α2R- and/or α1S-subunits were successfully used to examine the relative contributions of these isoforms in blood pressure regulation [7;8], cardiac and skeletal muscle function [9;10], and renal salt handling [11].
More recently, several groups found that in contrast to the ubiquitous impact of CTS on Na+i,K+i-dependent cell functions, their actions on cell survival are tissue- and species-specific. For instance, ouabain triggers massive death of renal epithelial cells from the Madin-Darby canine kidney (MDCK) [12;13], endothelial cells from the pig aorta [14], human prostatic smooth muscle cells [15], human prostate adenocarcinoma cells [16], human monocytes [17], human erythroleukemia cell line (HEL) [18], human neuroblastoma cell line SH-SY5Y [19], and human neuronal precursor NT2 cells [20;21]. In striking contrast, we did not detect any impact of 48-h exposure to high concentrations of ouabain on survival of rat vascular smooth muscle cells [22], rat endothelial cells and rat astrocytes [23]. In additional experiments, we found that in contrast to CTS, 24-h inhibition of Na+,K+-ATPase in K+-free medium did not affect survival of MDCK and pig endothelial cells [14;24]. Subsequently, this intriguing observation was confirmed by Contreras and co-workers [25]. Importantly, reduction of [K+]o strongly increased the efficacy of CTS blocking Na+/K+ pump and triggering cell death [24;26]. These data allowed us to propose that the cytotoxic effect of CTS is mediated by their interaction with the Na+,K+-ATPase α-subunit but is not directly related to elevation of the [Na+]i/[K+]i ratio [27].
In the present study, we compared the effects of ouabain on intracellular Na+ and K+ content and appearance of the cell death markers in several types of cells isolated from human and rat tissues. Furthermore, to demonstrate the critical role of the α1R and α1S subunits, we compared the effects of ouabain on VSMC from aorta of wild-type mouse and mouse expressing human α1S-Na+,K+-ATPase, as well as in human endothelial cells transfected with rat α1R-Na+,K+-ATPase. Our results definitively demonstrate the crucial role for α1R- and α1S-Na+,K+-ATPase, rather than downstream intermediates of the CTS-induced intracellular signalling, in survival and death of cell exposed.
2. Materials and methods
2.1 Cell cultures
Human umbilical vein endothelial cells (HUVEC), human aortic smooth muscle cells (HASMC) and rat aortic vascular smooth muscle cells (RASMC) were purchased from Lonza (Walkersville, MD, USA) and passaged 4-12 times in media recommended by the manufacturer. Endothelial cells from rat aorta (RAEC) were kindly provided by Dr. Thorin-Trescases (Institute of Cardiology, University of Montreal, Canada). These cells were isolated and passaged 3-4 times as described elsewhere [23;28]. Primary human astrocytes were acquired from ScienCell Research Laboratories (Carlsbad, CA) and their passages 2–4 were cultivated in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% FBS, 50 U/ml penicillin and 50 μg/ml streptomycin (Pen-Strep). These cells were stained with anti-GFAP antibody to confirm their astrocytic origin [29].
Primary rat astrocytes were prepared from brain cortical tissue of 1- to 2-day-old Sprague-Dawley rats per protocol approved by the Albany Medical Center Institutional Animal Use and Care Committee. Briefly, pups were euthanized by rapid decapitation, the cerebral cortices were dissected from the meninges, hippocampi, and basal ganglia and transferred to ice-cold OptiMEM medium (Life Sciences/Invitrogen). Cortical tissue was minced and transferred into solution of the recombinant protease TrypLE (Life Sciences/Invitrogen) diluted with OptiMEM (1:1 vol:vol). Cells were extracted at 37°C using three 10-min incubations with the TrypLE additionally supplemented with bovine pancreatic DNAse I (Sigma). The first extraction was discarded, while the second and the third extractions were combined with DMEM containing 10% heat-inactivated horse serum (HIHS), and Pen-Strep. After each extraction cells were sedimented by a brief centrifugation (1,000 g for 1.5 min) and then resuspended in DMEM-HIHS. Dissociated cells were seeded on poly-D-lysine-coated T75 culture flasks (Techno Plastic Products, TPP, Trasadingen, Switzerland) at the density of 250,000 cells/flask, and grown to confluency. For more details, see [23;30].
Mice with ouabain-sensitive α1-isoform (α1S/S) were generated by amino acid substitutions at positions 111 and 122 in the first extracellular loop of the α-subunit, as previously described [31;32] and kindly provided by Dr. John N. Lingrel (University of Cincinnati, OH, USA). Mouse aorta smooth muscle cells (MASMC) were isolated from α1S/S and wild-type α1R/R mouse as described in details elsewhere [33] per protocol approved by the University of Chicago Institutional Animal Use and Care Committee.
All cell cultures were maintained in a humidified atmosphere containing 5% CO2/balance air at 37 °C. When applicable, to establish quiescence, cells were incubated for 24 h in the media in which concentration of FBS was reduced to 0.2%. Cell morphology was evaluated by a phase-contrast microscopy without preliminary fixation.
2.2 Stable transfection
HUVEC were transfected with pRC-CMV plasmid containing containing a gene encoding neomycin/G418 resistance and rat α1R cDNA provided by Dr. Lingrel. Cells grown in 10-cm Petri plates up to ~70% of confluency were treated in serum-free DMEM for 6 hr with 25 μg plasmid DNA and 60 μl Lipefectamin 2000, washed with DMEM and incubated for 24 hr in DMEM containing 10% FBS. Then, transfected cells were trypsinized, seeded in 10-cm Petri plates and grown in DMEM with 10% FBS and 0.8 mg/ml geneticin (G418) as a selected reagent. After 2 weeks of selection, transfected cells were used for further experiments.
2.3 Intracellular content of K+ and Na+
Cellular content of exchangeable K+ and Na+ was measured as accumulation of 86Rb and 22Na, respectively. To establish isotope equilibrium, cells growing in 12-well plates were preincubated for 3 h in DMEM containing 0.5 μCi/ml 86RbCl or 3 μCi/ml 22NaCl, and then ouabain was added for the next 3 h. After 3 h, the cells were transferred onto ice, washed 4 times with 2 ml of ice-cold medium W containing 100 mM MgCl2 and 10 mM HEPES-Tris buffer (pH 7.4). The washing medium was aspirated and cells were lysed in a solution of 1% SDS and 4 mM EDTA. Radioactivity of incubation media and cell lysates was quantified, and the intracellular cation content was calculated as A/am, where A was the radioactivity of the samples (cpm), a was the specific radioactivity of 86Rb (K+) and 22Na in the medium (cpm/nmol), and m was the protein content (mg). For more details, see [26].
2.4 Cell viability and apoptosis assays
Cell viability was quantitatively assessed by a lactate dehydrogenase (LDH) release assay, and pro-apoptotic changes were quantified by measuring activity of caspase-3 and chromatin cleavage. LDH release was measured using colorimetric CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (Promega) and following the manufacture’s protocol.
To measure caspase-3 activity, cells growing in 6-well plates were transferred onto ice, scraped off with a rubber cell scraper, sedimented at 5,000g for 10 min at 4 °C, and washed twice with 3 ml of ice-cold PBS. Then, the pellet was mixed with 150 μl of medium containing 0.32 M sucrose, 5 mM EDTA, 10 mM tris-HCl (pH 8.0), 1% triton X100, 2 mM dithiothreitol, 1 mM PMSF, 10 μg/ml pepstatin A, and 10 μg/ml aprotinin. The samples were centrifuged (14,000 rpm, 10 min, 4 °C), and 100 μl of the supernatant were frozen with liquid nitrogen and kept at -80 °C. To measure enzyme activity, 20-μl samples was transferred into 400 μl of buffer containing 5 mM MgCl2, 1 mM EGTA, 50 mM tris-HCl (pH 7.0), 0.1% CHAPS, 1 mM dithiothreitol, 40 μM DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-AMC) with or without 2 μM of the caspase-3 inhibitor Ac-DEVD-CHO. After 2-3 h incubation at 37 °C, the reaction was stopped by the addition of 1 ml of 0.5 M glycine-NaOH buffer (pH 10.0). The samples were diluted with water, and fluorescence was measured with a SPEX FluoroMax spectrofluorimeter at λex = 365 nm and λem = 465 nm (slits 4 and 20 nm, respectively). The fluorescent signal was calibrated with 7-amino-4-methylcoumarin (AMC) in the 0.01 to 0.3 μM range. Caspase-3 activity was calculated as the difference of DEVDase activity in the absence and presence of Ac-DEVD-CHO. For additional details, see [34].
Chromatin cleavage was quantified by previously-described technique [22] with the minor modifications listed below. Cells in 24-well dishes were supplied with DMEM containing serum and 0.1 μCi/ml [3H]-thymidine. After 24 h, they were washed twice with 2 ml of DMEM and incubated for 48 h in DMEM with serum and compounds indicated in the figure and table legends. Then, the medium was collected and centrifuged at 900g for 10 min. Next, the supernatant was transferred for the measurement of radioactivity in a liquid scintillation spectrometer (fraction F1), and the cell pellet and cells remaining in the plates were treated for 15 min with ice-cold lysis buffer (10 mM EDTA, 10 mM tris-HCl, 0.5% triton X100, pH 8.0). Then, the cell lysates were clarified by centrifugation (12,000 rpm, 10 min), and the supernatant was transferred for radioactivity measurement (fraction F2). The remaining radioactivity from the pellets and wells was extracted with a 1% SDS/4 mM EDTA mixture (fraction F3). The relative content of intracellular chromatin fragments was determined as a percentage of total [3H]-labelled DNA: F2/(F1+F2+F3)×1 x 100%.
2.5. Western blot analysis
After treatment with ouabain in 6-well plates, cells were lysed on ice in 0.250 ml of buffer containing 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 2 mM EGTA, 25 mM HEPES (pH 7.5), 10% glycerol, 1 mM NaF, 200 μM Na3VO4, and protease inhibitors (1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM PMSF). The lysates were cleared of the insoluble material by centrifugation at 20,000 g for 10 min, treated for 5 min at 95°C, and subjected to SDS-polyacrylamide gel electrophoresis using 4% and 10% polyacrylamide in stacking and resolving gels, respectively. Proteins were transferred to Hybond-C Extra nitrocellulose membranes (Amersham Biosciences, Mississauga, ON, Canada), blocked for 1 h at room temperature with 5% dry fat-free milk dissolved in TBS, and incubated overnight with primary antibodies at 4°C. Subsequently, the membranes were treated with the horseradish peroxide-conjugated secondary antibodies for 1 h at room temperature. The immunoreactivity was detected using an ECL Western blotting kit (Amersham) in accordance with the manufacturer’s instructions.
2.6. Materials
Lipofectamin 2000 and geneticin (G418) were from Invitrogen (Burlington, ON); methyl-[3H]-thymidine was purchased from ICN Biomedicals, Inc. (Irvine, CA); 22NaCl and 86RbCl were obtained from PerkinElmer (Waltham, MA). DEVD-AMC, DEVD-CHO and z-VAD.fmk were from BIOMOL Research Laboratories (Plymouth Meeting, PA). Anti-phospho-ERK, phospho-JNK and phospho-p38 antibodies were obtained from Cell Signlaing Technology, Inc (Danvers, MA). The remaining chemicals and culture media were from Life Technologies/Gibco (Gaithersburg, MO), EMD Millipore/Calbiochem (La Jolla, CA), Sigma Aldrich (St. Louis, MO) and Anachemia (Montreal, QC).
2.7. Statistical analysis
All experimental data are presented as mean values ± standard errors of mean (S.E.M.), with the number of independent experiments indicated in figure and table legends. The statistical difference in between experimental groups was analyzed by Student’s t-test or one-way ANOVA with Bonferroni correction for multiple comparisons. Probability values less than 0.05 were considered significant.
3. Results
In the initial experiments, we compared the actions of low and high doses of ouabain on intracellular contents of Na+ and K+ in human and rat cells. Table 1 shows that 6-h incubation with 3 μM ouabain led to ~10-fold elevation of Na+i and massive loss of K+i in smooth muscle and endothelial cells from human aorta but did not affect these parameters in rats cells. In rat cells, elevation of ouabain concentration up to 3,000 μM led to about the same increment of [Na+]i and decrement of [K+]i as in human cells treated with 3 μM ouabain. These results are consistent with ~1,000-fold decrease in the apparent affinity of α1-Na+,K+-ATPase in mice and rats as compared to humans and numerous other mammalians species studied so far [4].
Table 1.
Effect of ouabain on intracellular Na+ and K+ content in human (HASMC, HUVEC) and rat (RASMC, RAEC) cells
| Type of cells | Ouabain, μM | Intracellular Na+ (nmol/mg protein) |
Intracellular K+ (nmol/mg protein) |
|---|---|---|---|
| HASMC | 0 | 64±20 | 1211±60 |
| 3 | 701±38* | 108±11* | |
| 3000 | 766±90* | 132±38* | |
|
| |||
| HUVEC | 0 | 114±24 | 1311±89 |
| 3 | 978±53* | 133±20* | |
| 3000 | 1016±97* | 129±8* | |
|
| |||
| RASMC | 0 | 111±23 | 1211±93 |
| 3 | 178±11 | 1097±68 | |
| 3000 | 876±71* | 156±27* | |
|
| |||
| RAEC | 0 | 56±8 | 722±59 |
| 3 | 69±12 | 693±82 | |
| 3000 | 766±54* | 86±21* | |
Cells were incubated in DMEM medium with or without ouabain for 6 h. Means ± S.E. from experiments performed in triplicates are shown.
p<0.001 compared to ouabain-free medium.
With the knowledge that addition of 3 and 3,000 μM ouabain causes approximately the same elevation of the [Na+]i/[K+]i ratio in human and rat cells, respectively, we further compared the action of these concentrations of ouabain on cell survival. Table 2 demonstrates that 24-h exposure to 3 μM ouabain resulted in the apoptotic death of HASMC and HUVEC, manifested in up-to 8-fold elevation of caspase-3 activity and chromatin cleavage. Unlike for human cells, we did not detect any significant elevation of these parameters in RASMC and RAEC treated with 3,000 μM ouabain.
Table 2.
Effect of ouabain on caspase 3 activity and chromatin cleavage in human (HASMC, HUVEC) and rat (RASMC, RAEC) cells
| Type of cells | Ouabain, μM | Caspase-3, nmol/mg prot/min |
Chromatin cleavage1), % |
|---|---|---|---|
|
| |||
| HASMC | 0 | 1.01±0.22 | 4±2 |
| 3 | 7.59±1.10** | 25±6* | |
|
| |||
| HUVEC | 0 | 0.56±0.07 | 3±1 |
| 3 | 3.12±0.31*** | 21±4** | |
|
| |||
| RASMC | 0 | 0.78±0.17 | 6±2 |
| 3000 | 0.67±0.09 | 5±1 | |
|
| |||
| RAEC | 0 | 0.33±0.08 | 3±1 |
| 3000 | 0.51±0.15 | 5±2 | |
Cells were incubated in DMEM medium with or without ouabain for 24 hr.
The total content of [3H]-labelled DNA was taken as 100%. The means ± S.E. from experiments performed in triplicate (caspase-3 activity) or quadruplicate (cell detachment and chromatin cleavage) are given.
, - p<0.05, respectively, as compared to ouabain-untreated cells.
, - p<0.01, respectively, as compared to ouabain-untreated cells. and
- p<0.001, respectively, as compared to ouabain-untreated cells.
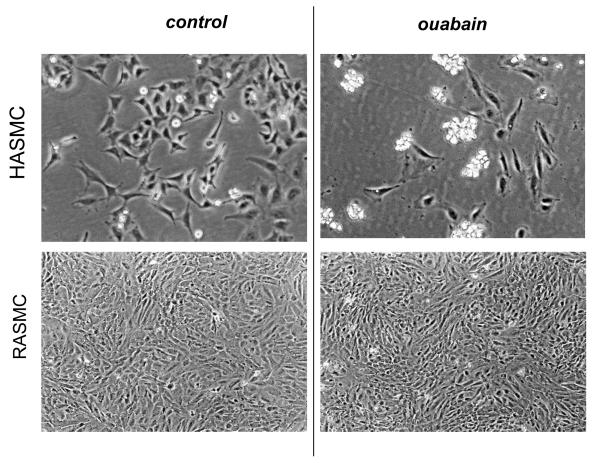
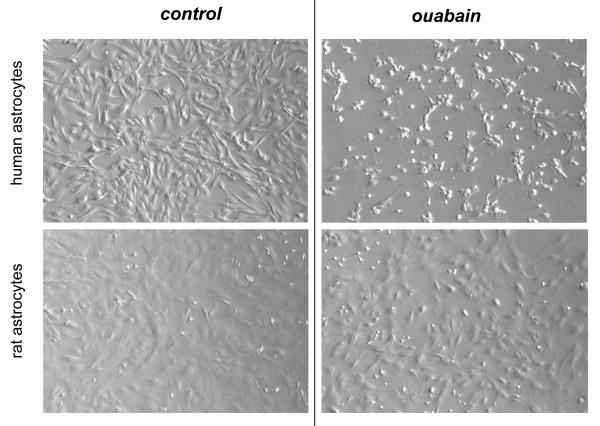
Distinct effects of ouabain on survival of human and rat vascular smooth muscle and endothelial cells were further confirmed by phase-contrast microscopy. Indeed, 24 h exposure to 3,000 μM ouabain did not affect morphology of RASMC (Fig. 1) and RAEC (Fig.2), whereas incubation of HASMC and HUVEC with 3 μM ouabain resulted in massive accumulation of detached and rounded cells (also Fig. 1 and 2, respectively). The latter experiments were also performed in human and rat astrocytes. While human astrocytes showed extensive cell rounding and death upon 24-h exposure to 20 μM ouabain, we did not observe any morphological changes or signs of cell death in rat astrocytes exposed in parallel experiments to 5,000 μM ouabain for 24 h (Fig. 3). In fact, rat astrocytes retained their morphology and viability after 48-h exposure to ouabain (data not shown).
Fig. 1.
Phase-contrast microscopy of smooth muscle cells from human (HASMC) and rat (RASMC) aorta exposed to ouabain. HASMC and RASMC were incubated for 24 h in DMEM containing 3 and 3,000 μM ouabain, respectively. These representative images were captured using a Nikon phase-contrast microscope with 100× magnification.
Fig. 2.
Phase-contrast microscopy demonstrating the effect of ouabain on endothelial cells isolated from human umbilical vein (HUVEC) and rat aorta (RAEC). HUVEC and RAEC were incubated for 24 h in EMB-2 and DMEM containing 3 and 3,000 μM ouabain, respectively. These representative images were captured using a Nikon phase-contrast microscope with 100× magnification
Fig. 3.
Hoffman modulation contrast microscopy images demonstrating the effects of ouabain on human and rat astrocytes. Human and rat astrocytes were incubated for 24 h in DMEM containing 10% fetal bovine serum and 20 and 5,000 μM ouabain for human and rat cells, respectively. These representative images were captured using an Olympus IX-71 setup with 100× magnification.
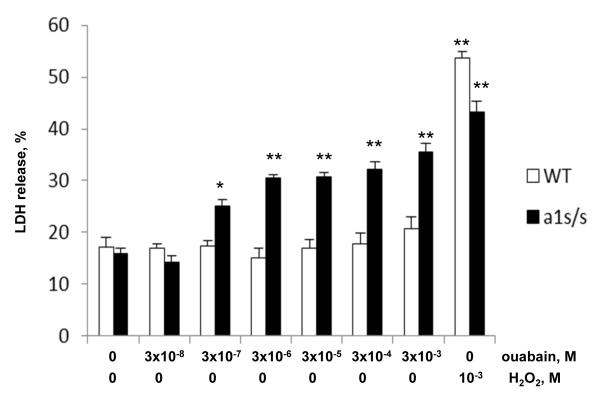
To examine the role of the α1S- and α1R-Na+,K+-ATPase subunits in promoting distinct effects of ouabain on survival and death of human and rat cells, we compared the dose-dependent actions of this compound on smooth muscle cells isolated from aorta of the wild-type (α1R/R) and genetically engineering α1S/S mouse, in which the α1R Na+,K+-ATPase subunit was replaced with the human α1S-Na+,K+-ATPase with high affinity for ouabain. Figure 4 shows that similar to the RASMC, addition of 3,000 μM ouabain did not affect survival of MASMC from the wild-type mice as indicated by no changes in LDH release, as compared to baseline. As a positive control, the cytotoxic compound H2O2 more than tripled LDH release from 17.2±1.9 to 55.7±1.3%. Unlike in the wild-type α1R/R cell cultures, addition of 3 μM ouabain to MASMC from α1S/S mouse increased LDH release from 15.9±1.2 to 30.4±0.7% (p<0.001) with further elevation of extracellular LDH content in the concentration range from 3 to 3,000 μM (Fig. 4).
Fig. 4.
Effects of ouabain and H2O2 on LDH release from smooth muscle cells isolated from aorta of wild-type (WT) mice (open bars) and α1S/S mice, expressing human α1S-Na+,K+-ATPase (solid bars). Cells were serum-starved for 24 h and then treated for additional 24 h with drugs at concentrations indicated at the bottom. The total content of LDH was taken as 100%. Means ± S.E. from 3 experiments performed in triplicates are shown. *, ** p<0.005 and 0.001 as compared to untreated cells.
To further explore the distinct roles of α1S- and α1R-Na+,K+-ATPase, we compared dose-dependent actions of ouabain on intracellular sodium content and LDH release from control and α1R-transfected HUVEC. In mock-transfected cells, ~10-fold elevation of [Na+]i was observed after 6 h incubation with 10 μM ouabain, whereas in cells transfected with α1R subunit the same elevation of [Na+]i was detected in the range from 300 to 3000 μM (Fig. 5A). These results are consistent with previously reported attenuated sensitivity of intracellular Na+/K+ homeostasis handling to ouabain in human HeLa and canine MDCK cells transfected with α1R-Na+,K+-ATPase [35;36]. In mock-transfected cells, LDH release was detected at 24 hr after ouabain addition at the concentrations higher than 0.3 μM ouabain, and was increased by ~ 6-fold in the presence of 3 μM ouabain (Fig. 5B). In contrast, elevation of ouabain levels up to 3000 μM did not affect survival of HUVEC transfected with α1R-Na+,K+-ATPase. As a positive control, H2O2 caused similar ~7-fold elevation of LDH release from both mock-transfected and α1R-cells.
Fig. 5.
Dose-dependent effects of ouabain on the intracellular Na+ content (A) and LDH release (B) from mock- and α1R-transfected HUVEC. Cells were serum-starved for 24 h and then treated for additional 6 (A) or 24 (B) h with ouabain at concentrations indicated at the X-axes. The total content of LDH was taken as 100%. Treatment with 1000 mM H2O2 was used as a positive control for the cell death. Means ± S.E. from 3 experiments performed in triplicates (A) or quadruplicate (B) are shown.
Numerous studies demonstrated the distinct impact of mitogen-activated protein kinases (MAPK) on cell survival and death (for review, see [37-39]). It is generally accepted that phosphorylation of extracellular signal-regulated kinase (ERK) plays a major role in regulation cell proliferation and differentiation. In contrast, phosphorylation of c-Jun N-terminal kinase (JNK) and p38 MAPK is frequently associated with stress and inflammatory responses and in many instances promotes cell death [40;41]. Recently, we demonstrated that phosphorylation of p38 MAPK precedes death of the ouabain-treated MDCK cells [42;43]. Here, we compared the effects of ouabain on phosphorylation of MAPKs in HUVEC and RAEC, which have contrasting sensitivity to ouabain-induced cell death. Figure 6 shows that 6-hr incubation with ouabain resulted in ~2- and 5-fold elevation of ERK1 and ERK2 phosphorylation in RAEC but very modest changes in phosphorylation of these enzymes in HUVEC. Unlike ERK1/2, we detected ~2-fold increase in p38 phosphorylation in HUVEC without any changes in RAEC. In both type of cells, we did not observe any significant impact of ouabain on phosphorylation of JNK1/2 MAPKs (Fig. 6).
Fig. 6.
Effects of ouabain on phosphorylation of the mitogen-activated protein kinases in HUVEC and RAEC. A, Representative Western blots showing the effects of ouabain on the content of phosphorylated ERK1/2, JNK1/2 and p38 MAPK. HUVEC and RAEC were treated during 6 hr with 3 and 3,000 μM ouabain, respectively. B, Normalized changes in the immunoreactivity of phosphorylated ERK1/2, JNK1/2 and MAPK in ouabain-treated HUVEC and RAEC. The immunoreactivity of phosphorylated MAPKs in control cells, which were not treated with ouabain, was taken as 1.00. Means ± S.E. from 3 experiments are shown.
4. Discussion
We report here that such diverse primary rat cell lines as astrocytes, vascular smooth muscle cells, and the endothelial cells from rat aorta are highly resistant to the long-term incubation with ouabain. The lack of cytotoxic action of ouabain in rat cells was in striking contrast to a massive cell death seen in ouabain-treated human primary astrocytes, endothelial cells from human umbilical vein, and smooth muscle cells from human aorta (Table 2, Figs. 1-3).
At least 3 hypotheses can be proposed to explain the distinct impact of ouabain on survival of human and rat cells. First, the lack of cytotoxic effect of ouabain in rat cells is caused by its minor impact on the [Na+]i/[K+]i ratio. This hypothesis, however, has been clearly ruled out by our data demonstrating that 3,000 μM ouabain causes the same elevation of the [Na+]i/[K+]i ratio in rat cells, as its lower concentrations (3 μM) in human cells (Table 1). Second, human cells express an unknown CTS-sensitive component of the cells death machinery, or rat cells contain yet unidentified inhibitor of the CTS-induced cell death signaling. Third, the cell death can be triggered by interaction of ouabain with α1S- but nor with α1R-Na+,K+-ATPase due to different conformation and functional properties of these two αsubunit isoforms, leading to stimulation of alternative signaling pathways in human and rodent cells.
To examine the latter 2 hypotheses, we compared the dose-dependent actions of ouabain on vascular smooth muscle cells isolated from the wild-type mice, expressing α1R-Na+,K+-ATPase, and the mice expressing human ouabain-sensitive α1-isoform (α1S/S). We found that in contrast to the lack of cytotoxic action 3,000 μM ouabain in smooth muscle cells from wild-type α1R/R mouse, the same CTS dose-dependently decreased survival of cells isolated from aorta of α1S/S mice (Fig. 4). Since in these cell types only one protein, the α1-isoform of the Na+,K+-ATPase, has been replaced, these data clearly suggest that distinct outcomes of exposure to CTS on survival of human and rodent cells are determined by properties of α1S- and α1R-Na+,K+-ATPase rather than by distinct set of downstream intermediates of signal transduction. We also observed that stable transfection with α1R-Na+,K+-ATPase rescues HUVEC from cytotoxic action of high doses of ouabain (Fig. 5), suggesting that the rodent α1R-Na+,K+-ATPase activates cytoprotective signaling cascade(s) in a dominant fashion.
Unlike endothelial and smooth muscle cells, which have dominant expression of α1-isoform [44;45], human and rodent neurons additionally express substantial levels of the α3-, while human and rodent astrocytes have significant levels of the α2- Na+,K+-ATPase isoform [46;47]. The functional significance of α2- and α3-isoforms in human brain may be illustrated by clinical findings of α2-subunit mutations (presumably astrocytic) causing familial hemiplegic migraine type 2, and α3 mutations (largely neuronal) leading to a rapid onset dystonia and Parkinsonism [48]. Both α2- and α3-Na+,K+-ATPase possess about the same affinity for CTS in rodents and other mammals [4;49;50]. Taking these latter data into account, our findings that rat astrocytes are highly resistant to the presence of high doses of ouabain (Fig. 3) strongly suggest the negligible impact of CTS-sensitive α2-subunit in the triggering cell death machinery. Unlike rat astrocytes, mouse cortical neurons die after 24-h exposure to 80 μM ouabain [51]. Therefore, additional experiments should be performed to examine the role of α3-subunit in the death of CTS-treated cells.
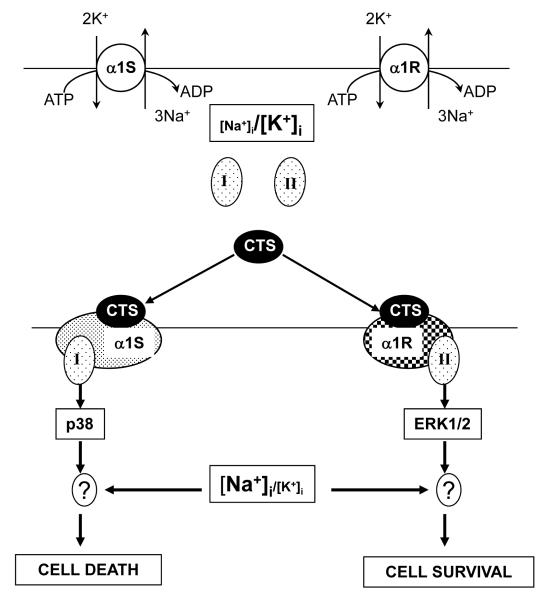
Based on findings of this work we propose a hypothetical mechanism for the distinct action of CTS on the survival of cells expressing α1S- and α1R-Na+,K+-ATPase (Fig. 7). In addition to the well-established effect on the Na+,K+ pump and elevation of the [Na+]i/[K+]i ratio, CTS trigger distinct conformation transitions of α1S- and α1R-Na+,K+-ATPase resulting in their interaction with hypothetical adaptor proteins I and II, respectively. In the case of rodent cells, ouabain is not toxic, possibly because the signaling cascade triggered by its interaction with α1R-Na+,K+-ATPase leads to cytoprotective activation of ERK1/2. This is in contrast to activation of p38 MAPK seen in ouabain-treated human cells (Fig. 6). Indeed, it was previously shown that both p38 phosphorylation and death of ouabain-treated MDCK cells expressing α1S-Na+,K+-ATPase were suppressed by p38 inhibitor SB 202190 [42]. Upstream intermediates of MAPK signaling induced by CTS may include non-receptor tyrosine kinase Src, phosphatidyl inositol 3-kinase (PI3K) [52], as well as recently discovered functional interaction of the Na+,K+-ATPase with Bcl-2 proteins BclXL and Bak [53]. Recently, we demonstrated that sustained elevation of the [Na+]i/[K+]i ratio resulted in differential expression of hundreds of ubiquitous and cell-type specific genes, including potent regulators of cell differentiation, proliferation and death [54;55]. Additional experiments should be performed to examine the role of Na+i/K+i-mediated excitation-transcription coupling in the survival and death of cells expressing α1R- and α1S-Na+,K+-ATPase, respectively.
Fig. 7.
Hypothetical mechanisms underlying the distinct impact of cardiotonic steroids (CTS) on survival of cells, expressing the CTS-sensitive (α1S) and the CTS-resistant (α1R) Na+,K+-ATPase subunits. In both cases, saturating levels of CTS strongly increase the [Na+]i/[K+]i ratio. In addition, CTS trigger distinct conformational changes in α1S and α1R isoforms that, in turn, affect their interactions with unknown protein partner(s) I and II. These subsequent signaling events lead to activation of p38 and ERK1/2 MAPK and result in cell death and survival, respectively. ? – steps showing possible additional effects of the transcriptomic changes, which can be directly induced by elevation of the [Na+]i/[K+]i ratio. For more details, see text.
One critical implication of the present findings is related to development of anticancer therapies based on CTS. Epidemiological observations identified decreased occurrence of leukemia as well as breast, prostate and lung cancer in the patients with heart failure, who were treated with digitalis [56-58]. Therefore, numerous studies screened for the novel anticancer CTS compounds, using rodents injected with human malignant cells (for review, see [59-61]). This study demonstrates that such an approach can be highly problematic since low concetrations of ouabain may trigger undesirable cell death in human but not rodent tissues.
In conclusion, our results, demonstrate, that unlike human cells their rodents counterparts perfectly survive in the presence of ouabain in spite of the complete inhibition of the Na+,K+-ATPase and inversion of the [Na+]i/[K+]i ratio. The dramatic differences in actions of ouabain on rat and mouse cells are determined by unique features of α1-Na+,K+-ATPase, rather than by any other differences in CTS-sensitive/resistant intracellular signaling cascades.
HIGHLIGHTS.
Ouabain triggers the death of human astrocytes, smooth muscle and endothelial cells
In spite of the same elevation of Na+i, ouabain did not affect survival of rat cells
Ouabain triggers the death of cells from mice expressing human α1-Na+,K+-ATPase
Transfection with rat α1-Na+,K+-ATPase protects human cells from ouabain
In rodent but not human cells, ouabain prompted activation of Erk1/2
Acknowledgements
This study was supported in part by grants from the Russian Foundation for Fundamental Research 09-0073/04, 14-04-31705 and 15-04-00101 (to S.N.O. and A.O.A.), grant from the Russian Scientific Foundation 14-15-00006 (to A.O.A. and S.N.O.), grant from the National Institutes of Health (R01 NS061953 to A.A.M.) and Predoctoral Fellowship from American Heart Association 14PRE18360017 to J.L.
References
- 1.Therien AG, Blostien R. Mechanisms of sodium pump regulation. Am.J.Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- 2.Scheiner-Bobis G. The sodium pump. Its molecular properties and mechanisc of ion transport. Eur.J.Biochem. 2002;269:2424–2433. doi: 10.1046/j.1432-1033.2002.02909.x. [DOI] [PubMed] [Google Scholar]
- 3.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol.Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their role in hypertension, salt metabolism, and cell growth. Am.J.Physiol.Cell Physiol. 2007;293:C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 5.Lingrel JB, Argьello JM, Van Huysse JW, Kuntzweiler TA. Cation and cardiac glycoside binding sites of the Na,K-ATPase. Ann.N.Y.Acad.Sci. 1997;843:194–206. doi: 10.1111/j.1749-6632.1997.tb52251.x. [DOI] [PubMed] [Google Scholar]
- 6.Lingrel JB, Williams MT, Vorhees CV, Moseley AE. Na,K-ATPase and the role of a isoforms in behaviour. J.Bioenerg.Bioeng. 2007;39:385–389. doi: 10.1007/s10863-007-9107-9. [DOI] [PubMed] [Google Scholar]
- 7.Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc.Natl.Acad.Sci.USA. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou X, Theriault SF, Dostanic-Larson I, Moseley AE, Lingrel JB, Wu H, Dean S, Van Huysse JW. Enhanced pressor response to increased CSF sodium concentration and to central angiotensin I in heterozygous a2 Na,K-ATPase knockout mice. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2009;295:R1427–R1438. doi: 10.1152/ajpregu.00809.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dostanic I, Lorenz JN, Schultz JEJ, Grupp IL, Neumann JC, Wani MA, Lingrel JB. The α2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J.Biol.Chem. 2003;278:53026–53034. doi: 10.1074/jbc.M308547200. [DOI] [PubMed] [Google Scholar]
- 10.Radzyukevich TL, Lingrel JB, Heiny JA. The cardiac glycoside binding site of the Na,K-ATPase α2 isoform plays a role in the dynamic regulation of active transport in skeletal muscle. Proc.Natl.Acad.Sci.USA. 2009;106:2565–2570. doi: 10.1073/pnas.0804150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-sensitive α1 Na,K-ATPase enhances natriuretic response to saline load. J.Am.Soc.Nephrol. 2008;19:1947–1954. doi: 10.1681/ASN.2008020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledbetter ML, Young GJ, Wright ER. Cooperation between epithelial cells demonstrated by potassium transfer. Am.J.Physiol. 1986;250:C306–C313. doi: 10.1152/ajpcell.1986.250.2.C306. [DOI] [PubMed] [Google Scholar]
- 13.Bolivar JJ, Lazaro A, Fernandez S, Stefani E, Pena-Cruz V, Lechene C, Cereijido M. Rescue of a wild-type MDCK cell by a ouabain-resistant mutant. Am.J.Physiol. 1987;253:C151–C161. doi: 10.1152/ajpcell.1987.253.1.C151. [DOI] [PubMed] [Google Scholar]
- 14.Orlov SN, Thorin-Trescases N, Pchejetski D, Taurin S, Farhat N, Tremblay J, Thorin E, Hamet P. Na+/K+ pump and endothelial cell survival: [Na+]i/[K+]i-independent necrosis triggered by ouabain, and protection against apoptosis mediated by elevation of [Na+]i. Pflugers Archiv. 2004;448:335–345. doi: 10.1007/s00424-004-1262-9. [DOI] [PubMed] [Google Scholar]
- 15.Chueh S-C, Guh J-H, Chen J, Lai M-K, Teng C-M. Dual effect of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J.Urol. 2001;166:347–353. [PubMed] [Google Scholar]
- 16.McConkey DJ, Lin Y, Nutt LK, Ozel HZ, Newman RA. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000;60:3807–3812. [PubMed] [Google Scholar]
- 17.Kurosawa M, Tani Y, Nishimura S, Numazawa S, Yoshida T. Distinct PKC isozymes regulate buffalin-induced diiferentiation and apoptosis in human monocyte cells. Am.J.Physiol.Cell Physiol. 2001;280:C459–C464. doi: 10.1152/ajpcell.2001.280.3.C459. [DOI] [PubMed] [Google Scholar]
- 18.Perne A, Muellner MK, Steinrueck M, Craig-Mueller N, Mayerhofer J, Schwarzinger I, Sloane M, Uras IZ, Hoermann G, Nijman SMB. Cardiotonic glycosides induce cell death in human cells by inhibiting general protein synthesis. PLoS One. 2009;4:e8292. doi: 10.1371/journal.pone.0008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulikov A, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Ouabain activates signalin patthways associated with cell death in human neuroblastoma. Biochim.Biophys.Acta. 2007;1768:1691–1702. doi: 10.1016/j.bbamem.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Hennion JP, el-Masri MA, Huff MO, el-Mailakh RS. Evaluation of neuroprotection by lithium and valproic acid against ouabain-induced cell damage. Bipolar Disord. 2002;4:201–206. doi: 10.1034/j.1399-5618.2002.01162.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosen H, Glukmann V, Feldman T, Fridman E, Lichtstein D. Cardiac steroids induce changes in recyling of the plasma membrane in human NT2 cells. Mol.Biol.Cell. 2004;15:1044–1054. doi: 10.1091/mbc.E03-06-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlov SN, Thorin-Trescases N, Kotelevtsev SV, Tremblay J, Hamet P. Inversion of the intracellular Na+/K+ ratio blocks apoptosis in vascular smooth muscle at a site upstream of caspase-3. J.Biol.Chem. 1999;274:16545–16552. doi: 10.1074/jbc.274.23.16545. [DOI] [PubMed] [Google Scholar]
- 23.Akimova OA, Mongin AA, Hamet P, Orlov SN. The rapid decline of MTT reduction is not a marker of death signaling in ouabain-treated cells. Cell.Mol.Biol. 2006;52(No 8):71–77. [PubMed] [Google Scholar]
- 24.Pchejetski D, Taurin S, der Sarkissian S, Lopina OD, Pshezhetsky AV, Tremblay J, DeBlois D, Hamet P, Orlov SN. Inhibition of Na+,K+-ATPase by ouabain triggers epithelial cell death independently of inversion of the [Na+]i/[K+]i ratio. Biochem.Biophys.Res.Commun. 2003;301:735–744. doi: 10.1016/s0006-291x(02)03002-4. [DOI] [PubMed] [Google Scholar]
- 25.Contreras RG, Flores-Maldonado C, Lazaro A, Shoshani L, Flores-Benitez D, Larre I, Cereijido M. Ouabain binding to Na+,K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J.Membr.Biol. 2004;198:147–158. doi: 10.1007/s00232-004-0670-2. [DOI] [PubMed] [Google Scholar]
- 26.Akimova OA, Bagrov AY, Lopina OD, Kamernitsky AV, Tremblay J, Hamet P, Orlov SN. Cardiotonic steroids differentially affect intracellular Na+ and [Na+]i/[K+]i-independent signaling in C7-MDCK cells. J.Biol.Chem. 2005;280:832–839. doi: 10.1074/jbc.M411011200. [DOI] [PubMed] [Google Scholar]
- 27.Orlov SN, Hamet P. The death of cardiotinic steroid-treated cells: evidence of Na+i,K+i-independent H+i-sensitive signaling. Acta Physiologica (Oxford) 2006;187:231–240. doi: 10.1111/j.1748-1716.2006.01546.x. [DOI] [PubMed] [Google Scholar]
- 28.Voghel G, Thorin-Trescases N, Farhat N, Nguyen A, Villeneuve L, Mamarbachi AM, Fortier A, Perrault LP, Carrier M, Thorin E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress asociated with cardiovascular risk factors. Mechanisms Ageing & Development. 2007;128:662–671. doi: 10.1016/j.mad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Kuo Y-H, Abdullaev IF, Hyzinski-Garcia MC, Mongin AA. Effects of alternative splicing on the function of bestrophin-1 calcium-activated chlroide channels. Biochem.J. 2014;458:575–583. doi: 10.1042/BJ20121546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mongin AA, Hyzinski-Garcia MC, Vincent MY, Keller RW. A simple method for measuring intracellular activities of glutamate synthetase and glutaminase in glial cells. Am.J.Physiol.Cell Physiol. 2011;301:C814–C822. doi: 10.1152/ajpcell.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dostanic I, Schultz JEJ, Lorenz JN, Lingrel JB. The α1 isoform of Na,K-ATPase regulates contractility and functionally interacts and co-localizes with the Na/Ca-exchanger in heart. J.Biol.Chem. 2004 doi: 10.1074/jbc.M410737200. in press. [DOI] [PubMed] [Google Scholar]
- 32.Wansapura AN, Lasko VM, Lingrel JB, Lorenz JN. Mice expressing ouabain-sensitive a1-Na,K-ATPase have increased susceptibility to pressure overload-induced cardiac hypertrtophy. Am.J.Physiol.Heart Circ.Physiol. 2011;300:H347–H355. doi: 10.1152/ajpheart.00625.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a sngle murine aorta. Methods Cell Sci. 2002;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 34.Orlov SN, Thorin-Trescases N, Dulin NO, Dam T-V, Fortuno MA, Tremblay J, Hamet P. Activation of cAMP signaling transiently inhibits apoptosis in vascular smooth muscle cells in a site upstream of caspase 3. Cell Death Differ. 1999;6:661–672. doi: 10.1038/sj.cdd.4400539. [DOI] [PubMed] [Google Scholar]
- 35.Jewell EA, Lingrel JB. Comparison of the substrate dependence properties of the rat Na,K,ATPase α1, α2, and α3 isoforms expressed in HeLa cells. J.Biol.Chem. 1991;266:16925–16930. [PubMed] [Google Scholar]
- 36.Akimova OA, Tremblay J, Van Huysse JW, Hamet P, Orlov SN. Cardiotonic steroid-resistant α1-Na+,K+-ATPase rescues renal epithelial cells from the cytotoxic action of ouabain: evidence for a Na+i,K+i-independent mechanism. Apoptosis. 2010;15:55–62. doi: 10.1007/s10495-009-0429-4. [DOI] [PubMed] [Google Scholar]
- 37.Thornton TM, Rincon M. Non-classical p38 MAP kinase functions: cell cycle checkpoints and survival. Int.J.Biol.Sci. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol.Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 39.Kim EK, Choi EJ. Pathological toles of MAPK signaling pathways in human diseases. Biochim.Biophys.Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Munshi A, Ramesh R. Mitogen-activated protein-kinases and their role in radiation response. Genes Cancer. 2013;4:401–408. doi: 10.1177/1947601913485414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Chen M, Liu H, Yang L, Yang T, He G. The dual role of ERK signaling in the apoptotosis of nerones. Front.Biosci.(Landmark Ed) 2014;19:1411–1417. doi: 10.2741/4291. [DOI] [PubMed] [Google Scholar]
- 42.Akimova OA, Lopina OD, Rubtsov AM, Gekle M, Tremblay J, Hamet P, Orlov SN. Death of ouabain-treated renal epithelial cells: evidence for p38 MAPK-mediated Na+i/K+i-independent signaling. Apoptosis. 2009;14:1266–1273. doi: 10.1007/s10495-009-0404-0. [DOI] [PubMed] [Google Scholar]
- 43.Akimova OA, Lopina OD, Rubtsov AM, Hamet P, Orlov SN. Investigation of mechanism of activation of p38 MAPK by cardiotonic steroids in epithelial cells from distal tubules. Biochemistry (Moscow) 2010;75:1070–1078. doi: 10.1134/s0006297910080043. [DOI] [PubMed] [Google Scholar]
- 44.Pierre S, Compe E, Grillasca JP, Plannells R, Sampol J, Pressley TA, Maixent JM. RP-PCR detection of Na,K-ATPase subunit isoforms in human unbilical vein endothelial cells (HUVEC): evidence for the presence of α1 and β3. Cell.Mol.Biol. 2001;47:319–324. [PubMed] [Google Scholar]
- 45.Hansen O. Quantification of α-subunit isoforms of Na-K-ATPase in rat resistant vessels. Acta Physiol.Scand. 2004;180:49–56. doi: 10.1046/j.0001-6772.2003.01224.x. [DOI] [PubMed] [Google Scholar]
- 46.Golovina VA, Song H, James PF, Lingrel JB, Blaustein MP. Na+ pump α2-subunit expression modulates Ca2+ signaling. Am.J.Physiol. 2003;284:C475–C486. doi: 10.1152/ajpcell.00383.2002. [DOI] [PubMed] [Google Scholar]
- 47.Song H, Thompson SM, Blaustein MP. Nanomolar ouabain augments Ca2+-signalling in rat hippocampal nerones and glia. J.Physiol. 2013;591:1671–1689. doi: 10.1113/jphysiol.2012.248336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benarrroch EE. Na+,K+-ATPase: functions in the nervous system and involvement in the neurologic disease. Neurology. 2011;76:287–293. doi: 10.1212/WNL.0b013e3182074c2f. [DOI] [PubMed] [Google Scholar]
- 49.Mobasheri A, Avila J, Cozar-Castellano I, Brownleader MD, Trevan M, Francis MJO, Lamb JF, Martin-Vassalo P. Na+,K+-ATPase isozyme diversity: comparative biochemistry and physiological implications of novel functional interactions. Biosci.Rep. 2000;20:51–91. doi: 10.1023/a:1005580332144. [DOI] [PubMed] [Google Scholar]
- 50.Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu.Rev.Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurones. J.Neurosci. 2002;22:1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Xie Z. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter traficking. Biochim.Biophys.Acta. 2010;1802:1237–1245. doi: 10.1016/j.bbadis.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauf PK, Alqahtani T, Flues K, Meller J, Adragna NC. Interaction between Na-K-ATPase and Bcl-2 proteins BclXL and Bak. Am.J.Physiol.Cell Physiol. 2015;308:C51–C60. doi: 10.1152/ajpcell.00287.2014. [DOI] [PubMed] [Google Scholar]
- 54.Koltsova SV, Trushina Y, Haloui M, Akimova OA, Tremblay J, Hamet P, Orlov SN. Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: evidence for Ca2+i-independent excitation-transcription coupling. PLoS One. 2012;7:e38032. doi: 10.1371/journal.pone.0038032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orlov SN, Hamet P. Salt and gene expression: evidence for Na+i,K+i-mediated signaling pathways. Pflugers Arch.- Eur.J.Physiol. 2015;467:475–487. doi: 10.1007/s00424-014-1650-8. [DOI] [PubMed] [Google Scholar]
- 56.Goldin AC, Safa AR. Digitalis and cancer. Lancet. 2015;1134 doi: 10.1016/s0140-6736(84)92556-x. [DOI] [PubMed] [Google Scholar]
- 57.Haux J, Kiepp O, Spigset O, Tretli S. Digitoxin medication and cancer: case control and inernal dose response studies. BMC Cancer. 2001;1:11. doi: 10.1186/1471-2407-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Platz EA, Yegnasubramanian S, Liu JO, Chong CR, Shim JS, Kenfield SA, Stampfler MJ, Willett WC, Giovannucci E, Nelson WG. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discov. 2011;1:68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Molecular Interventions. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 60.Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, Kiss R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim.Biophys.Acta. 2007;1776:32–57. doi: 10.1016/j.bbcan.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Weidemann H. Na/K-ATPase, endogenous digitalis-like compounds and cancer development - a hypothesis. Front.Biosci. 2005;10:2165–2176. doi: 10.2741/1688. [DOI] [PubMed] [Google Scholar]