Abstract
Ethanol is reinforcing within the nucleus accumbens shell (NACsh), but the underlying mechanisms remain unclear. Ethanol can potentiate the function of the GABAA, GABAB, and 5-HT3 receptors. Therefore, the current study tested the hypothesis that activation of these receptors would be involved in the reinforcing effects of ethanol in the NACsh. An intracranial self-administration (ICSA) procedure was used to assess the reinforcing effects of ethanol in the NACsh of alcohol preferring (P) rats. The ICSA consisted of 7 sessions: 4 sessions to establish 150 mg% ethanol self-infusion into the NACsh; sessions 5 and 6 with co-infusion of ethanol plus one concentration of the GABAA antagonist bicuculline (10 or 100 µM), the GABAB antagonist SCH 50911 (50, 75 or 100 µM), or the 5-HT3 receptor antagonist zacopride (10 or 100 µM); and session 7 with 150 mg% ethanol alone. All groups self-infused ethanol into the NACsh and readily discriminated the active from inactive lever during the acquisition sessions. Co-infusion of 100 µM, but not 10 µM, bicuculline or zacopride significantly decreased active responses during sessions 5 and 6. Co-infusion of 75 µM, but not 50 or 100 µM, SCH 50911 significantly attenuated responses for ethanol. Overall, the results suggest that the reinforcing effects of ethanol in the NACsh may be modulated by activation of local GABAA, GABAB and 5-HT3 receptors.
Keywords: Intracranial self-administration, nucleus accumbens, ethanol, GABAA receptor, GABAB receptor, 5-HT3 receptor
Introduction
Identification of brain reward mechanisms underlying the reinforcing effects of ethanol is critical to the development of effective therapies for combating alcohol abuse and alcoholism. A recent intracranial self-administration (ICSA) study demonstrated that ethanol can be readily self-infused into the nucleus accumbens (NAC) of the rat; the self-infusion was sub-region dependent with self-infusion into the NAC shell (NACsh) but not NAC core (NACcr), and genetically influenced with alcohol preferring (P) rats being more sensitive than stock Wistar rats to the rewarding effects of ethanol (Engleman et al. 2009). These findings suggest that the NACsh is one neuro-anatomical substrate supporting the reinforcing and rewarding effects of ethanol. However, neurochemical mechanisms underlying these effects remain unknown and elucidation of such mechanisms are important.
The NAC is a heterogeneous nucleus and approximately 90% of its neurons are GABAergic medium spiny neurons (MSNs); it receives synaptic inputs from various brain regions (Meredith 1999; Sesack and Grace 2010). GABA synapses provide a major inhibitory regulation of MSN activity. These GABAergic inputs originate mainly from GABA neurons in the VTA (Van Bockstaele and Pickel 1995) and ventral pallidum (Groenewegen et al. 1999), as well as intrinsic axonal collaterals from local MSNs (Meredith 1999). Moderate expression of both GABAA and GABAB receptors were found in the NAC (Bowery et al. 1987; Schwarzer et al. 2001). In addition, the NAC receives modulatory serotonin (5-HT) afferents from the dorsal raphe nucleus (Van Bockstaele and Pickel 1993). The presence of 5-HT receptors has been demonstrated in the NAC (Meredith 1999), including the serotonin-3 (5-HT3) receptor (Ge et al. 1997). The interactions between GABA/5-HT and their respective receptors play an important role in regulating the MSN activity and other neurotransmitter systems converging on the NAC (Meredith 1999). It has been proposed that the rewarding information is encoded in the activity of MSNs in the NAC and forwarded to downstream structures including the ventral pallidum (Carlezon and Thomas 2009).
Substantial evidence suggests that GABA neurotransmission is involved in the effects of ethanol (Koob 2004). Electrophysiological studies indicate that acute ethanol can potentiate GABAA- and GABAB- receptor mediated inhibitory currents (Allan et al. 1987; Federici et al. 2009; Grobin et al. 1998). Systemic administration of GABAA receptor antagonists or an inverse benzodiazepine agonist reduced voluntary alcohol drinking and the motivation in rats to respond for ethanol self-administration in an oral operant setting (McBride et al. 1988; Petry 1997; Rassnick et al. 1993; Samson et al. 1987). GABAA receptors within the mesolimbic system appeared to mediate such effects (Hodge et al. 1995; Hyytia and Koob 1995). Similarly, pharmacological manipulations of GABAB receptor activity can regulate the reinforcing and rewarding effects of ethanol (Maccioni and Colombo 2009; Vlachou and Markou 2010). In addition, acute ethanol can facilitate 5-HT3 receptor-mediated ion currents (Lovinger and White 1991). The 5-HT3 receptor has been implicated in the effects of drugs of abuse (Engleman et al. 2008). Systemic administration of 5-HT3 receptor antagonists suppressed voluntary ethanol consumption in rats under 24-hr free-choice conditions (Knapp and Pohorecky 1992; McKinzie et al. 1998). Nonetheless, the involvement of these receptors in the local reinforcing effects of ethanol within the NACsh has not been examined. Given the above-mentioned findings, it is possible that activation of these receptors may be involved in mediating the self-infusion of ethanol into the NACsh. The current study was designed to test this hypothesis by examining local self-infusion of ethanol in the presence of antagonists for these receptors.
Materials and methods
Animals
Experimentally-naïve adult female alcohol preferring (P) rats (body weight 250 to 300 g, Indiana University) were housed in-pairs in a reverse 12-hr light-dark cycle room (light off at 10:00 am) with temperature and humidity maintained constant for at least 2 weeks before the commencement of experiment. Food and water were available ad libitum except during the ICSA test sessions. Female rats were used in the present study due to their ability to maintain their head size better than male rats for more accurate stereotaxic placements (Ding et al. 2009; Rodd-Henricks et al. 2000a). The estrous cycle was not monitored in the present study. Experiments were performed during the dark phase. Protocols used were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Chemical agents
The artificial cerebrospinal fluid (aCSF) consisted of 120 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 1.2 mM CaCl2, 10 mM d-glucose, pH 7.2–7.4. Ethyl alcohol (190 proof) was obtained from Decon Laboratories, Inc, (King of Prussia, PA). The GABAA receptor antagonist bicuculline, the GABAB receptor antagonist SCH 50911 (Bolser et al. 1995), and the 5-HT3 receptor antagonist zacopride were obtained from Tocris (Ellisville, MO). All chemicals were dissolved in the aCSF solution to the desired concentrations prior to use.
Stereotaxic surgery procedures
Rats were implanted unilaterally with a 22-gauge cannula (Plastics One Inc., Roanoke, VA) aimed at the right NACsh (AP + 1.7 mm, ML + 2.4 mm, DV – 7.0 mm), as described previously (Engleman et al. 2009). Stylets were inserted into cannulae when no experiments were being conducted. Rats were individually housed after surgery and were allowed to recover from surgery for at least 7 days prior to tests. Rats were habituated and handled on a daily basis during the 7-day period.
Intracranial self-administration (ICSA) procedure
The ICSA tests followed procedures previously described (Ding et al. 2009; Engleman et al. 2009). Briefly, rats were placed into standard two-lever operant chambers on test days. One active lever was connected to an isolated pulse stimulator (A–M Systems, Inc, Carlsborg, WA) controlled by an operant control system Graphic State 3.02 (Coulbourn Instruments, Allentown, PA). The pulse stimulator was connected to two electrodes that were immersed in a drug-filled tank equipped with a 28-gauge injection cannula inserted into the NACsh. Each response on the active lever (FR1 reinforcement schedule) produced a pulse infusion of 100 nl of solution into the NACsh during a 5-sec period. Each infusion was followed by a 5-sec timeout period. During both the infusion and timeout periods, responses on the active lever were recorded but did not produce further infusions. The responses on the inactive lever were recorded but did not result in any infusions. The assignment of active and inactive lever was counterbalanced among rats.
There were a total of 7 sessions conducted every other day with the duration of each session being 4 hr. The first four sessions were acquisition sessions with 150 mg% ethanol available for self-infusion for each rat. This concentration of ethanol is physiologically relevant and can be readily achieved with free-choice voluntary drinking by P rats (Murphy et al., 1986). In addition, this concentration of ethanol was demonstrated to be the optimal concentration for self-infusion into the NACsh by P rats (Engleman et al. 2009). Sessions 5 and 6 were co-infusion sessions, during which 150 mg% ethanol plus one concentration of the GABAA antagonist bicuculline (10 or 100 µM), the GABAB receptor antagonist SCH 50911 (50, 75 or 100 µM), or the 5-HT3 receptor antagonist zacopride (10 or 100 µM) was self-infused. Session 7 was a session with the return of self-infusion of only 150 mg% ethanol. This procedure has been carried out in the past to examine receptor mechanisms involved in ethanol ICSA within the posterior VTA (Ding et al. 2012; Rodd-Henricks et al. 2003). In addition, the Engleman et al. study (2009) showed that replacement of ethanol with aCSF during sessions 5 & 6 resulted in extinction of lever responding. Therefore, such a group was not included in the current study.
Histology
At the end of the experiments, rats were euthanized and bromophenol blue was injected into the NACsh, as described previously (Engleman et al. 2009). Brains were quickly removed and frozen at −20 oC. Brain sections (40 µm thick) were sliced in a cryostat microtome and stained with cresyl violet for the verification of the placement of injection sites according to the rat brain atlas of Paxinos & Watson (Paxinos and Watson 1998).
Statistical analysis
For each group, lever discrimination was determined by ‘lever (active vs inactive)×session’ mixed ANOVA with repeated measures on session; t-tests were used to compare responses between active and inactive lever following a significant main effect (p < 0.05). For determination of drug effect, paired t-tests were used to compare the responses on the active lever and number of infusions during sessions 5 and 6 to average responses on the active lever and number of infusions during acquisition sessions 3 and 4.
Results
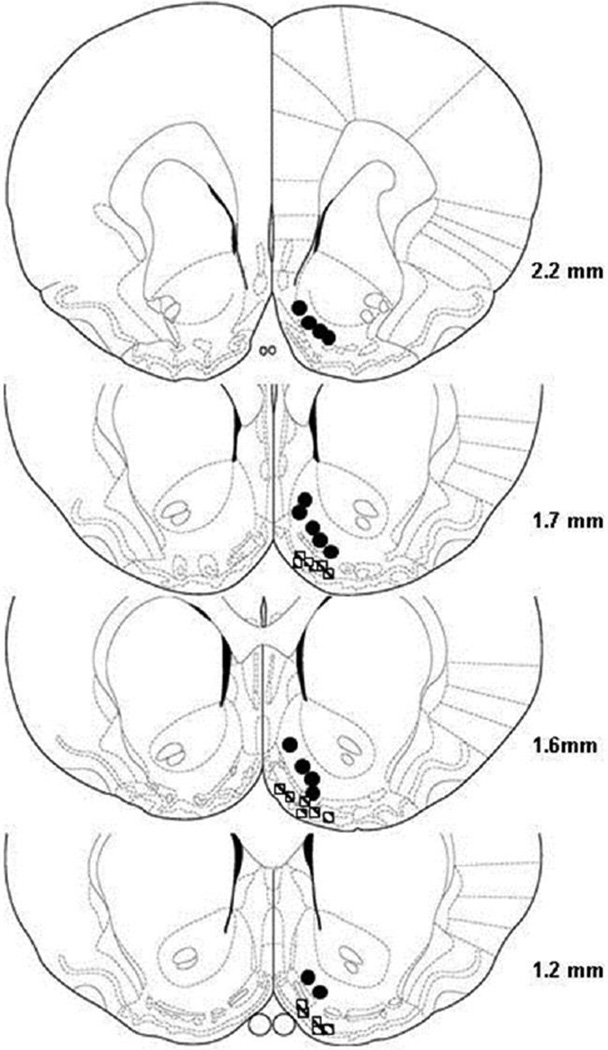
Figure 1 depicts the representative non-overlapping placements of injection sites within the NACsh. A majority of injection sites were located in the medial and ventral portions of the NACsh. Only rats with correct placements in the NACsh were included in analysis. There were six rats with injection sites ventral to the NACsh (Fig. 1). These rats displayed low responses on active and inactive levers (8 ± 2 vs 5 ± 1 responses/session, respectively), as well as low infusions per session (5 ±1). These results, along with the previous study (Engleman et al., 2009), suggest that adjacent areas outside the NACsh do not support the self-infusion of ethanol.
Figure 1.
Representative placements of microinjection sites in the nucleus accumbens shell for the self-infusion of ethanol. For clarity purpose, the overlapping sites are not included. Circles represent sites within the nucleus accumbens shell and squares represent sites ventral to the nucleus accumbens shell.
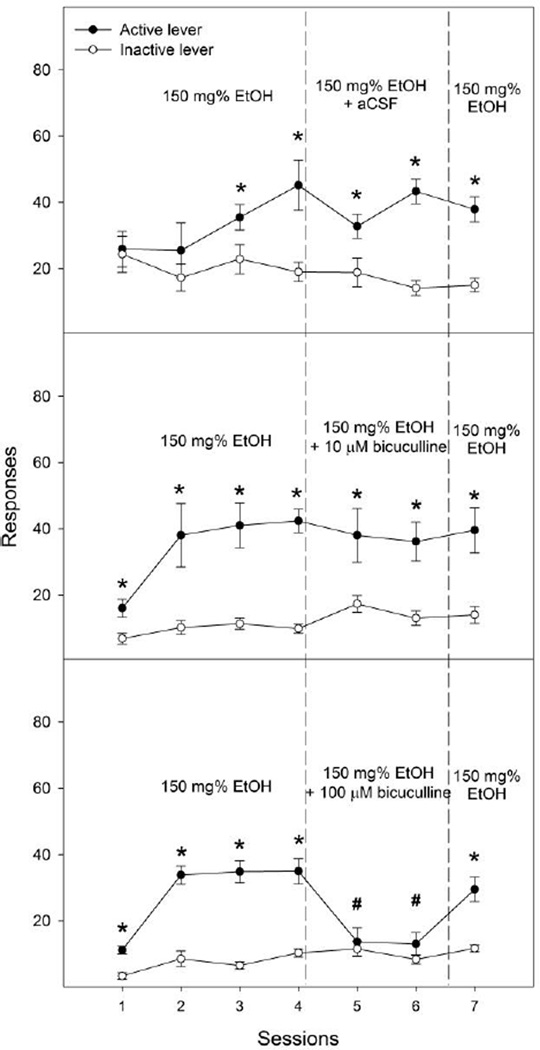
Lever responses for the bicuculline co-infusion groups are shown in Fig. 2. The ‘lever × session’ mixed ANOVAs indicated that the control group (Fig. 2, upper panel) demonstrated a significant effect of lever (F1, 12 = 20.3, p = 0.001) and interaction (F6,72 = 2.8, p = 0.02), but no effect of session (F6, 72 = 1.3, p = 0.28). Lever discrimination was observed during sessions 3 through 7. The 10 µM bicuculline group demonstrated significant effects of session (F6, 60 = 5.3, p < 0.001), lever (F1, 10 = 21.2, p = 0.001) and interaction (F6, 60 = 2.5, p = 0.03). Lever discrimination was observed during each session. Microinjection of 10 µM bicuculline did not significantly reduce responses on the active lever (Fig. 2, middle panel, e.g., responses in session 6 vs average during sessions 3 & 4: 36 ± 6 vs 42 ± 5, t5 = 0.9, p = 0.39) or number of infusions (Table 1) during either treatment session. The significant effects of session and interaction resulted mainly from the significant difference in responses on the active lever between session one and the other sessions. The 100 µM bicuculline group (Fig. 2, bottom panel) demonstrated significant effects of session (F6, 60 = 11.7, p < 0.001), lever (F1, 10 = 21.2, p = 0.001), and interaction (F6, 60 = 9.0, p < 0.001). Lever discrimination was evident during sessions 1 to 4, and session 7. Co-infusion of 100 µM bicuculline with ethanol significantly depressed responses on the active lever (e.g., responses in session 6 vs average during sessions 3 & 4: 13 ± 3 vs 35 ± 2, t5 = 7.4, p = 0.001) and number of infusions (Table 1) during both treatment sessions. Responses on the active lever during session 7 returned to levels observed during the acquisition sessions.
Figure 2.
Effects of co-infusion of aCSF, 10 or 100 µM bicuculline (a GABAA receptor antagonist) with 150 mg% ethanol on lever responses for the intracranial self-infusion of ethanol into the nucleus accumbens shell (n = 6–7 / group). * p < 0.05, significantly higher responses than those on the inactive lever. # p < 0.05, significantly lower responses than the average responses on the active lever during acquisition sessions 3 and 4.
Table 1.
Effects of co-infusion of an antagonist for the GABAA, GABAB or 5HT3 receptor with 150 mg% ethanol on number of infusions into the NACsh
| Group | Sessions 3 & 4 | Session 6 | t value | p value |
|---|---|---|---|---|
| 150 mg% EtOH + aCSF | 24 ± 3 | 25 ± 3 | 0.3 | 0.76 |
| 150 mg% ethanol + 10 µM bicuculline | 23 ± 4 | 22 ± 4 | 0.4 | 0.72 |
| 150 mg% ethanol + 100 µM bicuculline | 17 ± 1 | 8 ± 2 | 5.6 | 0.002 |
| 150 mg% ethanol + 50 µM SCH 50911 | 21 ± 4 | 19 ± 3 | 1.2 | 0.29 |
| 150 mg% ethanol + 75 µM SCH 50911 | 18 ± 1 | 8 ± 1 | 8.0 | < 0.001 |
| 150 mg% ethanol + 100 µM SCH 50911 | 22 ± 2 | 32 ± 5 | 1.6 | 0.16 |
| 150 mg% ethanol + 10 µM zacopride | 19 ± 2 | 14 ± 3 | 1.4 | 0.22 |
| 150 mg% ethanol + 100 µM zacopride | 17 ± 1 | 10 ± 1 | 17.0 | < 0.001 |
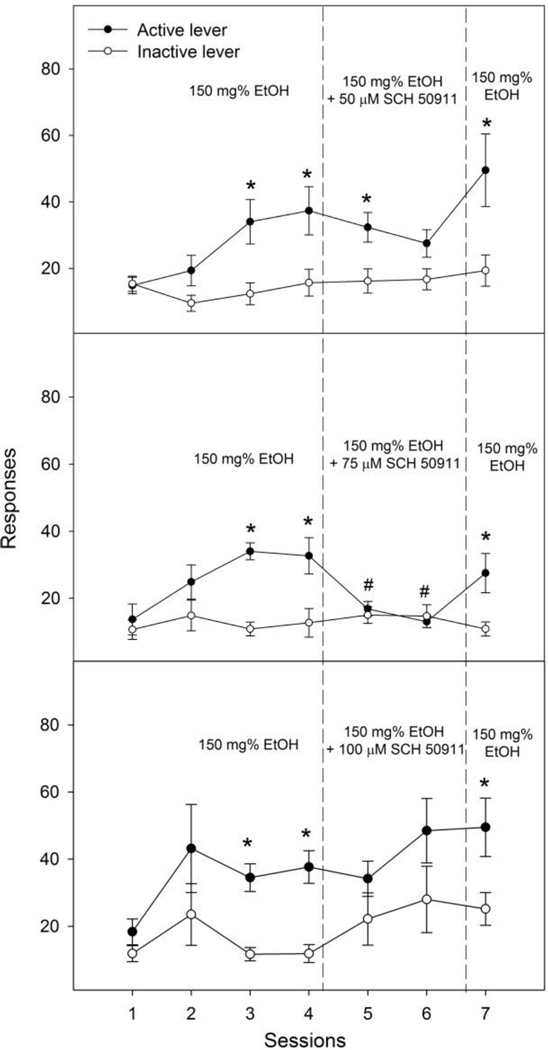
Lever responses in the SCH 50911 co-infusion groups are shown in Fig. 3. The 50 µM SCH 50911 group (Fig. 3, upper panel) demonstrated significant effects of session (F6, 60 = 4.7, p = 0.001), lever (F1, 10 = 13.8, p = 0.004) and interaction (F6, 60 = 2.5, p = 0.03). Lever discrimination was observed during sessions 3 to 5, and 7. SCH 50911 at 50 µM slightly reduced active responses during session 6. The 75 µM SCH 50911-treated group demonstrated significant effects of session (F6, 60 = 3.8, p = 0.003), lever (F1, 10 = 8.3, p = 0.02) and interaction (F6, 60 = 5.3, p < 0.001). Lever discrimination was observed during sessions 3, 4, and 7. Co-infusion of 75 µM SCH 50911 with ethanol significantly depressed responses on the active lever during both co-infusion sessions (Fig. 3 middle panel, e.g., responses during session 6 vs average during sessions 3 & 4: 13 ± 2 vs 33 ± 4, t5 = 5.3, p = 0.003). The number of infusions was also reduced (Table 1). The 100 µM SCH 50911 group (Fig. 3, bottom panel) demonstrated significant effects of session (F6, 60 = 2.9, p = 0.02), lever (F1, 10 = 18.9, p = 0.001), but no significant effect of interaction (F6, 60 = 0.8, p = 0.79). Lever discrimination was observed during sessions 3, 4 and 7. Co-infusion of 100 µM SCH 50911 did not significantly alter responses on the active lever during either co-infusion session (e.g., responses during session 6 vs average during sessions 3 & 4: 36 ±3 vs 49 ± 10, t5 = 1.2, p = 0.29). However, co-infusion of 100 µM SCH 50911 disrupted lever discrimination during both treatment sessions, as a result of a trend to increase responses on the inactive lever (Fig. 3, bottom panel).
Figure 3.
Effects of co-infusion of 50, 75 or 100 µM SCH 50911 (a GABAB receptor antagonist) with 150 mg% ethanol on lever responses for the intracranial self-infusion of ethanol into the nucleus accumbens shell (n = 6 / group). * p < 0.05, significantly higher responses than those on the inactive lever. # p < 0.05, significantly lower responses than the average responses on the active lever during acquisition sessions 3 and 4.
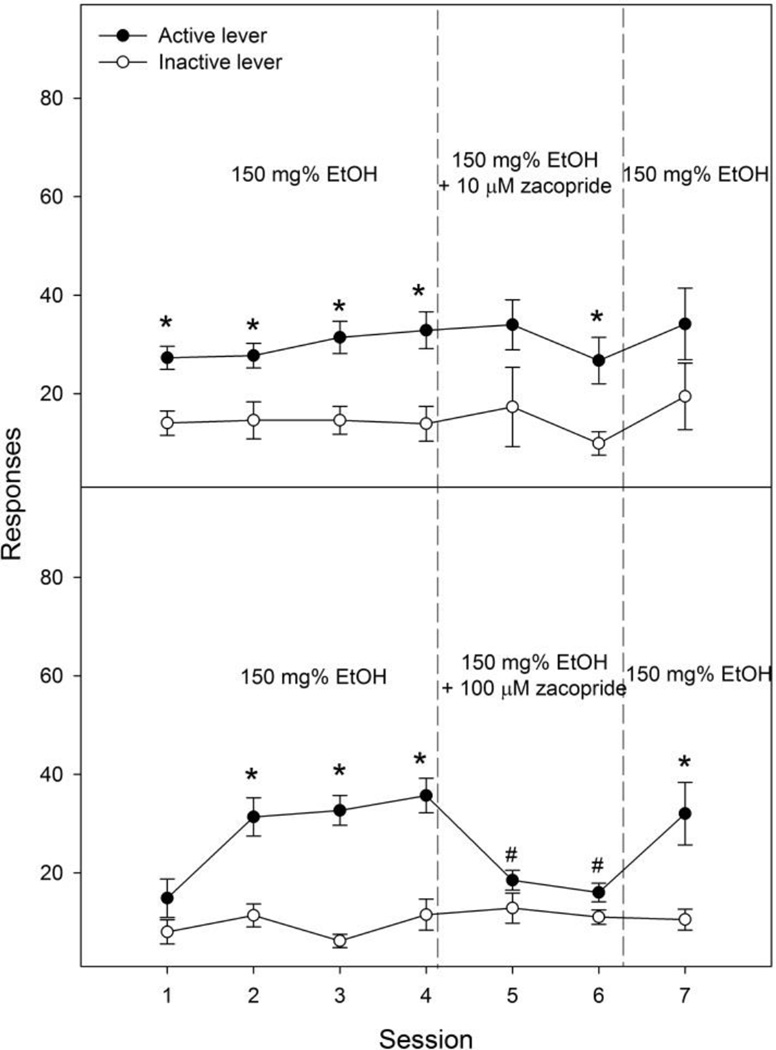
Lever responses in the zacopride co-infusion groups are shown in Fig. 4. The 10 µM zacopride group (Fig. 4, upper panel) demonstrated significant effects of lever (F1, 12 = 15.58, p = 0.002), but no significant effect of session (F6, 72 = 1.1, p = 0.35) or interaction (F6, 72 = 0.15, p = 0.99). Lever discrimination was observed during sessions 1 to 4, and session 6. Co-infusion of 10 µM zacopride did not significantly alter responses on the active lever (e.g., responses during session 6 vs average during sessions 3 & 4: 27 ± 5 vs 32 ± 2, t6 = 1.0, p = 0.34) or number of infusions (Table 1) in either co-infusion session. The 100 µM zacopride group demonstrated significant effects of session (F6, 60 = 5.8, p < 0.001), lever (F1, 10 = 32.3, p < 0.001) and interaction (F6, 60 = 6.3, p < 0.001). Lever discrimination was observed during sessions 2, 3, 4 and 7. Co-infusion of 100 µM zacopride with ethanol significantly decreased responses on the active lever (Fig. 4, bottom panel, e.g., responses during session 6 vs average during sessions 3 & 4: 16 ± 2 vs 34 ± 3, t5 = 7.1, p = 0.001) and number of infusions (Table 1) during both co-infusion sessions. Responses on the active lever returned to baseline levels during session 7.
Figure 4.
Effects of co-infusion of 10 or 100 µM zacopride (a 5-HT3 receptor antagonist) with 150 mg% ethanol on lever responses for the intracranial self-infusion of ethanol into the nucleus accumbens shell (n = 6–7 / group). * p < 0.05, significantly higher responses than those on the inactive lever. # p < 0.05, significantly lower responses than the average responses on the active lever during acquisition sessions 3 and 4.
Discussion
The major findings of the current study are that co-infusion of antagonists for the GABAA, GABAB or 5-HT3 receptor with ethanol attenuated the responses on the active lever and the self-infusion of ethanol into the NACsh, suggesting that the reinforcing effects of ethanol in the NACsh may be mediated, at least in part, by activating local GABAA, GABAB and 5-HT3 receptors. None of the antagonists, at any concentration, appeared to reduce responses on the inactive lever. These results suggest that a general effect of motor impairment cannot account for the reduced responses on the active lever. In addition, the highest concentration of the GABAB receptor antagonist disrupted lever discrimination mainly by increasing the responses on the inactive lever, which suggests a non-specific effect of the antagonist at this concentration.
The finding with bicuculline is in agreement with previous studies demonstrating that both systemic administration (McBride et al. 1988; Petry 1997; Samson et al. 1987) and local microinjection of GABAA receptor antagonists into the NACsh (Hyytia and Koob 1995) impaired oral ethanol self-administration in the rat. Another study (Hodge et al.1995) also indicated that microinjection of bicuculline into the NAC reduced oral ethanol self-administration, although a detailed histological map of injection sites was not presented. In addition, bicuculline did not significantly alter responses on the inactive lever (Fig. 2), suggesting the effects of bicuculline may not involve alteration of locomotor activity. Moreover, a previous study (Znamensky et al. 2001) demonstrated that bilateral microinjection of bicuculline (up to 1.6 mM) into the NACsh did not significantly alter food intake in the rat, providing support that bicuculline is not impairing general motor activity. Taken together, these findings indicate the important role of NACsh GABAA receptors in modulating the local positive motivational properties of ethanol (Koob 2004). A recent study using a similar ICSA procedure indicated that co-infusion of bicuculline with ethanol did not appear to alter ethanol self-infusion directly into the posterior VTA (Ding et al. 2011). These results suggest that local GABAA receptors may be differentially involved in ethanol self-infusion between the posterior VTA and NACsh.
In addition, these findings are in line with in vitro electrophysiological studies demonstrating that ethanol can potentiate GABAA receptor-mediated currents (Allan et al. 1987). In the NAC, ethanol may potentiate GABAA receptor function through both pre- and post-synaptic mechanisms (Weiner and Valenzuela 2006). In a NAC slice preparation, application of ethanol produced a dose-dependent enhancement of GABAA receptor-mediated currents in a subpopulation of NAC neurons; the optimal concentration of ethanol (44 mM or ~ 200 mg%) was comparable to that used in the current study (150 mg%) and increased GABAA current amplitude by approximately 40% in about 45% of neurons examined (Nie et al. 2000). Furthermore, ethanol was shown to increase presynaptic GABA release in the NAC slice (Weiner and Valenzuela 2006). Therefore, the net effect of ethanol through the GABAA receptor on these MSNs may be an enhanced inhibition of their activity and reduce GABA release in the ventral pallidum. In line with this idea, previous findings indicated that ethanol administration decreased spike activity in approximately 30% of NAC neurons, and reduced extracellular GABA levels in the ventral pallidum (Janak et al. 1999; Kemppainen et al. 2010). It should be noted that the exact location of ethanol-sensitive GABAA receptors within the NAC sub-regions remains unknown in these electrophysiological studies due to the lack of detailed histological presentation of electrode placements. Furthermore, GABAA receptor subunits were also found in non-GABAergic interneurons and axonal processes converging on the NAC (Schwarzer et al. 2001). Activation of these receptors can modulate release of acetylcholine, GABA and/or dopamine (Aono et al. 2008; Rada et al. 1993; Yoshida et al. 1997), and differentially alter the activity of MSNs. For example, activation of these receptors on GABAergic terminals may inhibit GABA release, resulting in attenuated inhibition of post-synaptic MSNs. Indeed, ethanol was shown to decrease GABAA-mediated currents and increase the excitability of a subpopulation of NAC neurons (Janak et al. 1999; Nie et al. 2000). Moreover, the sensitivity of GABAA receptors to ethanol enhancement depends on the subunit compositions of the GABAA receptor (Kumar et al. 2009), which are heterogeneously expressed within the NAC (Schwarzer et al. 2001). Therefore, the action of ethanol on MSNs depends on micro-circuits within the NAC that may differ in the relative location of GABAA receptors and their subunit composition. Taken together, it is possible that multiplex mechanisms may contribute to the self-infusion of ethanol and the effects of bicuculline on ethanol self-infusion into the NACsh.
Similar to GABAA receptors, activation of local GABAB receptors appeared to mediate the reinforcing effects of ethanol within the NACsh, as indicated by the results that 75 µM SCH 50911 attenuated ethanol self-infusion. This finding is consistent with in vitro evidence that the GABAB receptor-mediated inhibitory postsynaptic potential is sensitive to ethanol potentiation (Federici et al. 2009). The highest concentration of SCH 50911 disrupted lever discrimination between the active and inactive levers due in part to increased responding on the inactive lever (Fig. 3, bottom panel). These results suggest possible non-specific actions of SCH 50911. The mechanism underlying these effects are unknown, but may be due to different subtypes of GABAB receptors, GABAB receptors being located on both pre- and post-synaptic membrane, and multiple actions of SCH 50911 occurring at the highest concentration. For example, a high concentration of SCH 50911 was shown to act as an inverse agonist for the GABAB receptor to increase cAMP production in a cell culture (Grunewald et al. 2002).
In addition to the GABA receptors, activation of local 5-HT3 receptors also appeared to be involved in mediating the reinforcing effects of ethanol, as suggested by the findings that co-infusion of zacopride with ethanol dampened ethanol self-infusion into the NACsh. A previous study (Herges & Taylor 2000) showed that antagonism of 5-HT3 receptors within the NAC with ondansetron did not significantly alter basal locomotor activity. In addition, zacopride did not appear to alter responses on the inactive lever in the current study (Fig. 4). All these findings suggest that the effects of zacopride may not involve impairment of locomotor activity. This finding is in line with electrophysiological findings that ethanol can potentiate 5-HT3 receptor mediated currents in vitro (Lovinger and White 1991; Machu and Harris 1994). Our results provide mechanistic evidence supporting the involvement of activation of NACsh 5-HT3 receptors in mediating systemic ethanol self-administration in rodents (Engleman et al. 2008; Knapp and Pohorecky 1992; McKinzie et al. 2000; Rodd-Henricks et al. 2000b). Interestingly, local 5-HT3 receptors within the VTA also seem to be involved in the reinforcing and rewarding effects of ethanol (Rodd-Henricks et al. 2003; Rodd et al. 2010). These studies suggest a close relationship between ethanol self-administration and 5-HT3 receptors within the mesolimbic system.
Autoradiographic studies indicated that low to moderate densities of 5-HT3 receptor binding sites were present in the NAC (Barnes et al. 1990; Ge et al. 1997; Kilpatrick et al. 1987). However, the relative cellular location and distribution in each NAC sub-region of these receptors remains unclear. In situ hybridization studies (Morales et al. 2004; Tecott et al. 1993) did not detect 5-HT3 receptor mRNA in the NAC, which agrees with an immunocytochemistry study showing that very low levels of 5-HT3 receptor-expressing cells were detected in the NAC (Morales et al. 1998). These studies suggest that these receptors may be preferentially localized on synaptic terminals as opposed to NAC MSNs. One likely possibility is on dopaminergic terminals. Microdialysis studies demonstrated that local perfusion of a 5-HT3 receptor agonist could increase basal and ethanol-evoked dopamine release in the NAC, whereas antagonizing local 5-HT3 receptors could attenuate ethanol-induced dopamine release in the NAC (Campbell and McBride 1995). In addition, systemic administration of 5-HT3 receptor antagonists was shown to attenuate ethanol-induced dopamine release in the NAC (Carboni et al. 1989; Wozniak et al. 1990). These findings suggest that 5-HT3 receptors on dopaminergic terminals may contribute to ethanol self-infusion and the effects of zacopride observed in the current study.
In summary, the current study demonstrated that antagonism of local GABAA, GABAB or 5-HT3 receptors attenuated ethanol self-infusion into the NACsh, suggesting that the local reinforcing effects of ethanol in the NACsh may be mediated by activation of these receptors. These results are consistent with previous findings and suggest these receptors within the NACsh may contribute to the global reinforcing and rewarding effects of ethanol. On the other hand, it remains unknown whether there are possible interactions among these different receptors and how they interact in mediating ethanol reinforcement, which warrants future studies.
Acknowledgements
This study was supported in part by NIH grants AA012262 and AA07611. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
References
- Allan AM, Huidobro-Toro JP, Bleck V, Harris RA. Alcohol and the GABA receptor-chloride channel complex of brain. Alcohol Alcohol. 1987;(Suppl 1):643–646. [PubMed] [Google Scholar]
- Aono Y, Saigusa T, Mizoguchi N, Iwakami T, Takada K, Gionhaku N, Oi Y, Ueda K, Koshikawa N, Cools AR. Role of GABAA receptors in the endomorphine-1, but not endomorphin-2, induced dopamine efflux in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 2008;580:87–94. doi: 10.1016/j.ejphar.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Champaneria S, Costall B, Naylor RJ. Characterisation and autoradiographic localisation of 5-HT3 recognition sites identified with [3H]-(S)-zacopride in the forebrain of the rat. Neuropharmacology. 1990;29:1037–1045. doi: 10.1016/0028-3908(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Blythin DJ, Chapman RW, Eqan RW, Hey JA, Rizzo C, Kuo SC, Kreutner W. The pharmacology of SCH 50911: a novel, orally-active GABA-B receptor antagonist. J Pharmacol Exp Ther. 1995;274:1393–1398. [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav. 1995;51:835–842. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Frau R, Di Chhiara G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur J Pharmacol. 1989;164:515–519. doi: 10.1016/0014-2999(89)90259-8. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(S1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hall SR, Engleman EA, Hauser SR, McBride WJ, Rodd ZA. The stimulating effects of ethanol on ventral tegmental area dopamine neurons projecting to the ventral pallidum and medial prefrontal cortex in female Wistar rats: regional difference and involvement of serotonin-3 receptors. Psychopharmacology. 2011;216:245–255. doi: 10.1007/s00213-011-2208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, McBride WJ, Rodd ZA. Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: involvement of serotonin-3 receptors. J Pharmacol Exp Ther. 2012;340:202–209. doi: 10.1124/jpet.111.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Toalston JE, Oster SM, McBride WJ, Rodd ZA. Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology. 2009;204:381–390. doi: 10.1007/s00213-009-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Rodd ZA, Bell RL, Murphy JM. The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol Disord Drug Targets. 2008;7:454–467. doi: 10.2174/187152708786927886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici M, Nistico R, Giustizieri M, Bernardi G, Mercuri NB. Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. Eur J Neurosci. 2009;29:1369–1377. doi: 10.1111/j.1460-9568.2009.06700.x. [DOI] [PubMed] [Google Scholar]
- Ge J, Barnes JM, Towers P, Barnes NM. Distribution of S(−)-zacopride-insensitive [125I]R(+)-zacopride binding sites in the rat brain and peripheral tissues. Eur J Pharmacol. 1997;332:307–312. doi: 10.1016/s0014-2999(97)01091-1. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Grunewald S, Schupp BJ, Ikeda SR, Kuner R, Steigerwald F, Kornau HC, Kohr G. Importance of the γ-aminobutyric acidB receptor C-termini for G-protein coupling. Mol Pharmacol. 2002;61:1070–1080. doi: 10.1124/mol.61.5.1070. [DOI] [PubMed] [Google Scholar]
- Herges S, Taylor DA. Involvement of 5-HT3 receptors in the nucleus accumbens in the potentiation of cocaine-induced behaviors in the rat. Br J Pharmacol. 2000;131:1294–1302. doi: 10.1038/sj.bjp.0703687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 1999;817:172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Nurmi H, Kiianmaa K. GABA and glutamate overflow in the VTA and ventral pallidum of alcohol-preferring AA and alcohol-avoiding ANA rats after ethanol. Alcohol Alcohol. 2010;45:111–118. doi: 10.1093/alcalc/agp086. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Pohorecky LA. Zacopride, a 5-HT3 receptor antagonist, reduces voluntary ethanol consumption in rats. Pharmacol Biochem Behav. 1992;41:847–850. doi: 10.1016/0091-3057(92)90237-a. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Mattews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Maccioni P, Colombo G. Role of the GABAB receptor in alcohol-seeking and drinking behavior. Alcohol. 2009;43:555–558. doi: 10.1016/j.alcohol.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li T-K. Effects of Ro 15-4513, fluoxetine and desipramine on the intake of ethanol, water and food by the alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 1988;30:1045–1050. doi: 10.1016/0091-3057(88)90137-2. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Eha RD, Cox R, Stewart RB, Dyr W, Murphy JM, McBride WJ, Lumeng L, Li T-K. Serotonin3 receptor antagonism of alcohol intake: effects of drinking conditions. Alcohol. 1998;15:291–298. doi: 10.1016/s0741-8329(97)00132-8. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, McBride WJ, Murphy JM, Lumeng L, Li T-K. Effects of MDL 72222, a serotonin3 antagonist, on operant responding for ethanol by alcohol-preferring P rats. Alcohol Clin Exp Res. 2000;24:1500–1504. [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;385:385–401. [PubMed] [Google Scholar]
- Morales M, Wang SD, Diaz-Ruiz O, Jho DH. Cannabinoid CB1 receptor and serotinin 3 receptor subunit A (5-HT3A) are co-expressed in GABA neurons in the rat telencephalon. J Comp Neurol. 2004;468:205–216. doi: 10.1002/cne.10968. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances γ-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. The Journal of Pharmacology and Experimental Therapeutics. 2000;293:654–661. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. New York: Academic Press; 1998. [Google Scholar]
- Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol. 1997;5:183–194. [PubMed] [Google Scholar]
- Rada PV, Mark GP, Hoebel BG. In vivo modulation of acetylcholine in the nucleus accumbens of freely moving rats: II. inhibition by γ-aminobutyric acid. Brain Res. 1993;619:105–110. doi: 10.1016/0006-8993(93)91601-n. [DOI] [PubMed] [Google Scholar]
- Rassnick S, D'Amico E, Riley E, Koob GF. GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol Clin Exp Res. 1993;17:124–130. doi: 10.1111/j.1530-0277.1993.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, Murphy JM. Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol. 2010;44:245–55. doi: 10.1016/j.alcohol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000a;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Edmundson VE, Dagon CL, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of 5-HT3 receptor antagonists on daily alcohol intake under acquisition, maintenance, and relapse conditions in alcohol-preferring (P) rats. Alcohol. 2000b;21:73–85. doi: 10.1016/s0741-8329(00)00083-5. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi KG, Mills FG. Oral ethanol reinforcement in the rat: effects of the partial inverse benzodiazepine agonist RO15-4513. Pharmacol Biochem Behav. 1987;27:517–519. doi: 10.1016/0091-3057(87)90357-1. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major γ-aminobutyric acidA receptor subunits in the basal ganglia and associated brain areas of the adult rat. J Comp Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. Ultrastructure of serotonin-immuno-reactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol. 1993;334:603–617. doi: 10.1002/cne.903340408. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. GABAB receptors in reward process. Adv Pharmacol. 2010;58:315–71. doi: 10.1016/S1054-3589(10)58013-X. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Pert A, Linnoila M. Antagonism of 5-HT3 receptors attenuates the effects of ethanol on extracellular dopamine. Eur J Pharmacol. 1990;187:287–289. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Nakahara K, Tomita M, Hamada N, Ishikawa M, Hatakeyama J, Tanaka M, Nagatsu I. Local muscimol disinhibits mesolimbic dopaminergic activity as examined by brain microdialysis and Fos immunohistochemistry. Brain Res. 1997;767:356–360. doi: 10.1016/s0006-8993(97)00474-5. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Echo JA, Lamonte N, Christian G, Ragnauth A, Bodnar RJ. γ-Aminobutyric acid receptor subtype antagonists differentially alter opiod-induced feeding in the shell region of the nucleus accumbens in rats. Brain Res. 2001;906:84–91. doi: 10.1016/s0006-8993(01)02558-6. [DOI] [PubMed] [Google Scholar]