Abstract
Ethanol can be self-infused directly into the posterior ventral tegmental area (pVTA) and these effects involve activation of local dopamine neurons. However, the neuro-circuitry beyond the pVTA involved in these reinforcing effects has not been explored. Intra-pVTA micro-injection of ethanol increases dopamine release in the nucleus accumbens (NAC), medial prefrontal cortex (mPFC) and ventral pallidum (VP). The current study tested the hypothesis that the reinforcing effects of ethanol within the pVTA involve activation of dopamine projections from the pVTA to the NAC, VP, and mPFC. Following the acquisition of self-infusions of 200 mg% ethanol into the pVTA, either the dopamine D2 receptor antagonist sulpiride (0, 10 or 100 µM), or the D1 receptor antagonist SCH-23390 (0, 10 or 100 µM) was micro-injected into the ipsilateral NAC shell (NACsh), NAC core (NACcr), VP or mPFC immediately prior to the self-infusion sessions to assess the involvement of the different dopamine projections in the reinforcing effects of ethanol. Microinjection of each compound at the higher concentration into the NACsh, VP or mPFC, but not the NACcr, significantly reduced the responses on the active lever (from 40–50 to approximately 20 responses). These results indicate that activation of dopamine receptors in the NACsh, VP, or mPFC, but not the NACcr, is involved in mediating the reinforcing effects of ethanol in the pVTA, suggesting that the ‘alcohol reward’ neuro-circuitry consist of, at least in part, activation of the dopamine projections from the pVTA to the NACsh, VP and mPFC.
Keywords: Dopamine D1 receptor, Dopamine D2 receptor, Ethanol, Intra-cranial self-administration, Medial prefrontal cortex, Nucleus accumbens, Ventral pallidum, Ventral tegmental area
Introduction
Identifying brain regions and circuits involved in the reinforcing effect of ethanol is critical toward understanding the neurobiology of alcohol abuse and addiction. A prior study reported ethanol can be self-infused directly into the ventral tegemental area (VTA), suggesting this region as an anatomical site underlying the reinforcing effects of ethanol (Gatto et al, 1994). Subsequent studies demonstrated that ethanol is self-infused into the posterior, but not anterior, VTA (Rodd-Henricks et al, 2000; Rodd et al, 2005). Pharmacological studies indicate that such effects depended on activation of local dopamine neurons (Rodd et al, 2004), involving local 5-HT3 and 5-HT2A receptors (Ding et al, 2009b; Rodd-Henricks et al, 2003), but not local 5-HT1B or GABAA receptors (Ding et al, 2009b, 2011). So far, the neuro-circuits beyond the pVTA underlying this effect have not been explored.
Dopamine neurons in the VTA project to the forebrain coritco-limbic regions, including the nucleus accumbens (NAC), medial prefrontal cortex (mPFC), and ventral pallidum (VP) (Oades and Halliday, 1987; Swanson, 1982). Dopamine neurotransmission in these regions has been suggested to mediate the rewarding and reinforcing effects of ethanol (Koob and Volkow, 2010). Ethanol can increase dopamine release in the NAC following oral self-administration or systemic injection (Doyon et al, 2003; Imperato and Di Chiara, 1986; Weiss et al, 1993). Inhibition of dopamine receptors in the NAC attenuated oral self-administration of ethanol (Czachowski et al, 2001; Hodge et al, 1997; Rassnick et al, 1992; Samson et al, 1993). Similarly, both passive- or self-administration of ethanol increased extracellular dopamine levels in the VP (Melendez et al, 2003, 2004). In addition, pharmacological manipulation of dopamine receptors in the VP regulated alcohol drinking in the alcohol-preferring P rat (Melendez et al, 2005). In contrast to the NAC and VP, the neurochemical effect of ethanol on dopamine neurotransmission in the mPFC has been controversial. Early studies indicated that systemic injection of ethanol failed to increase extracellular dopamine levels in the mPFC (Engleman et al, 2006; Hegarty and Vogel, 1993). However, one recent study demonstrated that passive intravenous injection of ethanol increased extracellular dopamine levels in the mPFC (Schier et al, 2013). Microinjection of dopamine receptor agents in the mPFC altered oral alcohol operant self-administration (Hodge et al, 1996; Samson and Chappell, 2003). Therefore, the current evidence implicates these three regions in the systemic effects of ethanol. However, the role of dopamine neurotransmission in these regions in the local reinforcing effects of ethanol in the pVTA has not been examined.
Self-infusions of ethanol into the pVTA depend on activation of local dopamine neurons (Rodd et al, 2004). Microinjection of ethanol into the pVTA increased dopamine release in the NAC shell (NACsh), VP and mPFC (Ding et al, 2009a, 2011). These results suggest that stimulation of dopamine projections from the pVTA to the NACsh, VP and mPFC and subsequent activation of dopamine receptors within these regions may constitute a feed-forward circuitry that is critical to mediating the reinforcing effects of ethanol in the pVTA. Therefore, the current study was designed to examine the effects of local administration of dopamine D1 or D2 receptor antagonists within the NAC, VP and mPFC on ethanol self-infusions into the pVTA. The hypothesis is that activation of dopamine neurotransmission to these three regions is involved in mediating the local rewarding effects of ethanol in the pVTA.
Materials and methods
Animals
Experimentally-naïve adult female Wistar rats (body weight 270 to 320 g, Harlan Sprague Dawley, Inc., Indianapolis, IN) were used in the present study. Animals were habituated in pairs in a reverse 12-hr light-dark cycle room (light off at 10:00 am) controlled for temperature and humidity. Food and water were available ad libitum except during the ICSA test. Female rats were used because these rats maintain their head size better than male rats for more accurate stereotaxic placement (Ding et al, 2009b, 2012; Rodd-Henricks et al, 2000). The estrous cycle was not monitored. Previous studies suggested that the estrous cycle did not appear to have a significant effect on ICSA behavior (Ding et al, 2009b, 2012; Rodd-Henricks et al, 2000). Experiments were performed during the dark phase. Protocols used were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Chemical agents
The artificial cerebrospinal fluid (aCSF) consisted of 120 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 2.5 mM CaCl, 10 mM d-glucose, pH 7.2–7.4. Ethyl alcohol (190 proof) was obtained from McCormick Distilling, Weston, MO. The dopamine D2 receptor antagonist (−)-sulpiride and the D1 receptor antagonist R(+)-SCH23390 were purchased from Sigma-Aldrich (St. Louis, MO). All chemicals were dissolved in the aCSF solution to the desired concentrations.
Stereotaxic surgery procedures
Rats were implanted unilaterally with two 22-gauge cannulae (Plastics One Inc., Roanoke, VA) aimed at the right pVTA (AP −5.6 mm, ML +2.1 mm, DV −8.1 mm) and one of the forebrain regions: NAC shell (AP +1.7 mm, ML +2.3 mm, DV −6.9 mm), VP (AP + 0.1 mm, ML + 2.3 mm, DV −7.0 mm), or mPFC (AP + 3.0 mm, ML + 0.7 mm, DV −3.0 mm), according to Paxinos and Watson (Paxinos and Watson, 1998). Cannulae for the pVTA and NACsh were implanted with a 10° angle to the midline, whereas cannulae for the mPFC and VP were implanted with no angle. Stylets were inserted into cannulae when no experiments were being conducted. Since accumulating evidence suggests that the NAC consists of two distinct sub-regions, shell and core, which differ from each other in various aspects (Zahm, 1999), the current study also examined the involvement of the NAC core (NACcr, AP + 1.6 mm, ML + 2.7 mm, DV – 6.0 mm). Rats were individually housed after surgery and were allowed to recover from surgery for approximately 5–7 days prior to tests during which rats were habituated and handled on a daily basis. Each rat was used for only one ICSA experiment.
General test procedure
The ICSA tests followed procedures previously described (Ding et al, 2009b; Rodd-Henricks et al, 2000). Briefly, rats were placed into operant conditioning chambers equipped with two levers, one active and one inactive. The active lever was connected to an isolated pulse stimulator (Model 2100) from A-M systems, Inc (Varlsborg, WA) controlled by an operant conditioning control system. The A-M pulse stimulator was connected to two electrodes that were immersed in a solution-filled cylinder container equipped with a 28-gauge injection cannula. Each response on the active lever (FR1 schedule of reinforcement) activated the pulse stimulator that produced a 5-sec infusion current between the electrodes, resulting in an infusion of 100-nl solution into the p-VTA. Each infusion was followed by a 5-sec timeout period. During both the infusion and timeout periods, responses on the active lever were recorded but did not produce further infusions. The responses on the inactive lever were recorded but did not result in any infusions. The assignment of active and inactive lever was counterbalanced among rats. There were a total of seven sessions conducted with each session being 4-hr long and 48–72 hr between sessions.
Experiment 1 examined the involvement of NACsh dopamine receptors in ethanol self-infusions into the pVTA
Rats started with self-infusions of 200 mg% ethanol into the pVTA during the first 4 sessions (acquisition). For sessions 5 and 6, rats received microinjection of aCSF, the dopamine D2 receptor antagonist sulpiride (10 or 100 µM), or the dopamine D1 receptor antagonist SCH-23390 (10 or 100 µM) in the NACsh immediately prior to the commencement of ethanol self-infusion in the pVTA. During session 7 (reinstatement), rats self-infused 200 mg% ethanol in the pVTA without treatment in the NACsh. All antagonists were injected in a volume of 0.5 µl with a flow rate at 0.25 µl/min. The injector remained in place for another 2 min before being removed. The 200 mg% ethanol is an optimal dose to produce both behavioral and neurochemical effects in the pVTA (Ding et al, 2009a; Rodd-Henricks et al, 2000). Since both antagonists showed efficacy at the 100- µM concentration, this concentration was tested in subsequent experiments.
Experiment 2 examined the involvement of NACcr dopamine receptors in ethanol self-infusions into the pVTA
The ICSA procedure was the same as in experiment 1 except sulpiride (100 µM) or SCH-23390 (100 µM) was microinjected into the NACcr immediately before sessions 5 and 6.
Experiment 3 examined the involvement of VP dopamine receptors in ethanol self-infusions into the pVTA
The ICSA procedure was the same as in experiment 1 except that the microinjection of aCSF, sulpiride (100 µM) or SCH-23390 (100 µM) into the VP was given immediately before sessions 5 and 6.
Experiment 4 examined the involvement of mPFC dopamine receptors in ethanol self-infusions into the pVTA
The ICSA procedure was the same as in experiment 1 except that the microinjection of aCSF, sulpiride (100 µM) or SCH-23390 (100 µM) into the mPFC was given immediately before sessions 5 and 6.
Histology
At the end of experiments, rats were euthanized and bromophenol blue was injected into the target regions, and placement of injection sites were verified following procedures previously described (Ding et al, 2009b).
Statistical analysis
For each individual group, lever discrimination was determined by ‘lever (active vs inactive) x session’ mixed ANOVA with repeated measures on session; t-tests were used to compare responses between active and inactive lever following a significant main effect of lever (p < 0.05). For determination of drug effect, paired t-tests were used to compare the responses on the active lever during sessions 5 and 6 to average responses on the active lever during acquisition sessions 3 and 4 following significant effects of session and interaction (p < 0.05).
Results
Figure 1 depicts the representative non-overlapping placements of injection sites within the pVTA, mPFC, VP, NACsh and NACcr. The pVTA is defined as the VTA region from −5.3 mm to −6.0 mm relative to bregma (Ding et al, 2009a; Rodd-Henricks et al, 2000). The injection sites in the mPFC were mostly within the prelimbic sub-region of the mPFC. The injection sites in the VP were mostly within the ventral portion of the VP.
Figure 1.
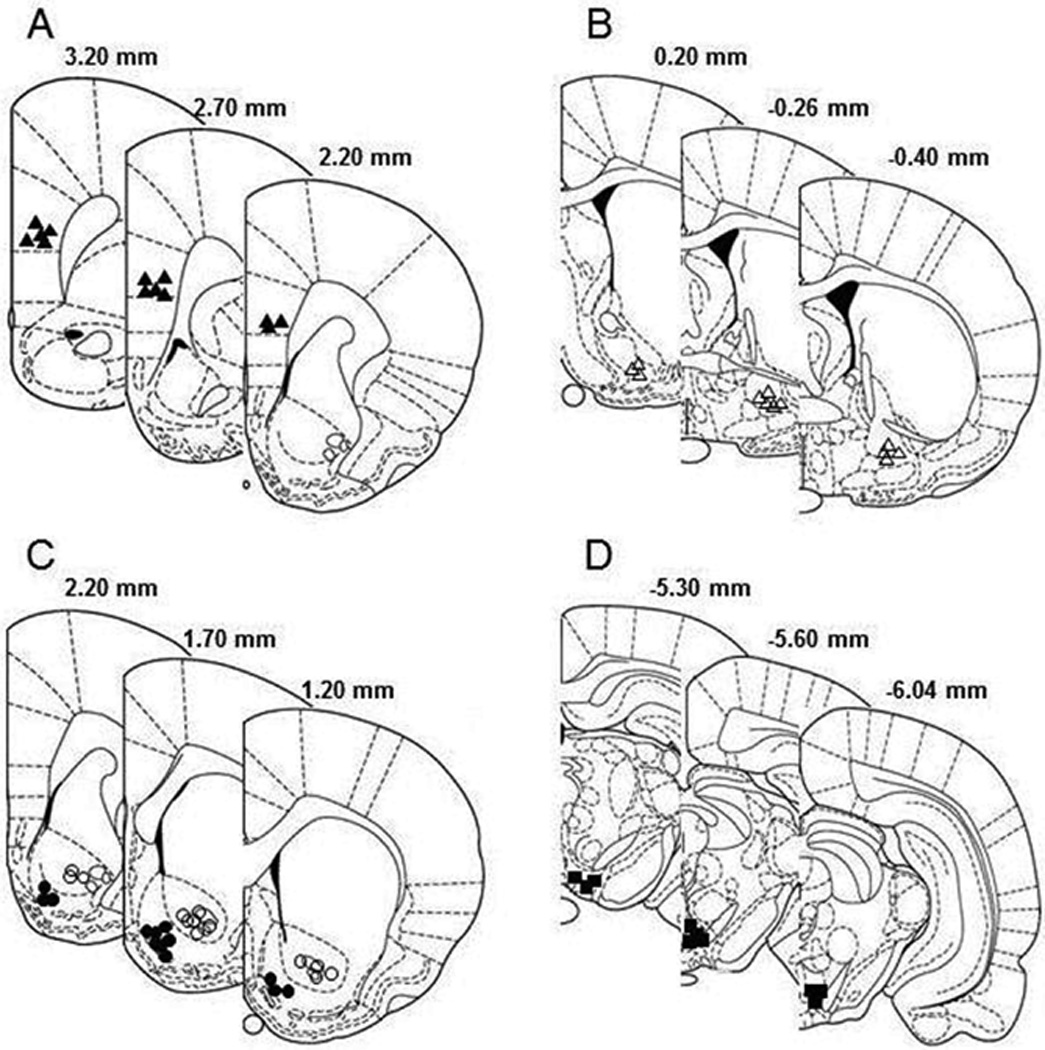
Representative non-overlapping placements of the injection sites within the medial prefrontal cortex (A, filled triangle), ventral pallidum (B, open triangle), nucleus accumbens (C, filled circle for shell sub-region and open circle for core sub-region), and posterior ventral tegmental area (D, filled square), according to the brain atlas of Paxinos and Watson (1998). Placements within the medial prefrontal cortex were mainly within the prelimbic subregion. Placements within the ventral pallidum were mainly in the ventral portion.
The involvement of NACsh dopamine receptors in ethanol ICSA in the pVTA
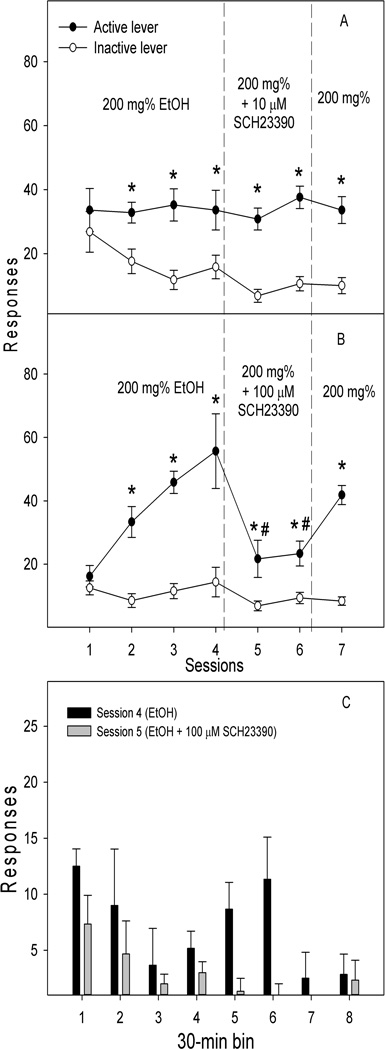
Fig. 2 depicts the lever responses in groups that received ethanol self-infusions into the pVTA and microinjection of aCSF or sulpiride in the NACsh. The “lever × session” repeated ANOVAs were conducted in each group. The aCSF-treated group (Fig. 2A) demonstrated a significant effect of lever (F1, 14 = 21.5, p< 0.001), but no effect of session (F6, 9 = 1.1, p = 0.42) or interaction (F6,9 = 0.9, p = 0.55). Lever discrimination was observed during sessions 3 to 7. The 10 µM sulpiride-treated group demonstrated significant effects of session (F6, 7 = 37.7, p < 0.001), lever (F1, 12 = 17.8, p = 0.001), and interaction (F6, 7 = 10.1, p = 0.004). Lever discrimination was observed during the last two acquisition sessions and the reinstatement session. Microinjection of 10 µM sulpiride significantly reduced responses on the active lever only during the second treatment (Fig. 2B, p < 0.05). The 100 µM sulpiride-treated group (Fig. 2C) demonstrated significant effects of session (F6, 13 = 18.4, p < 0.001), lever (F1, 18 = 16.5, p = 0.001), and interaction (F6, 13 = 6.8, p = 0.002). Lever discrimination was developed during acquisition and reinstatement sessions. Microinjection of 100 µM sulpiride into the NACsh significantly depressed responses on the active lever during both treatment sessions (ps < 0.05). Responses on the active lever during the reinstatement session returned toward the acquisition levels in both sulpiride-treated groups (Fig. 2B&C). In addition, response patterns in 30-min bins (Fig. 2D) indicated that the highest responding on active lever occurred during the first 30 min of session 4, with continued but lower responding occurring throughout the 4-hr session. In contrast, responses on the active lever following microinjection of sulpiride into the NACsh during session 5 were observed essentially only during the first 30-min period.
Figure 2.
Effects of microinjection of vehicle (A, n = 8) or the D2 receptor antagonist sulpiride (B & C, 10 and 100 µM, n = 7 and 10 respectively) into the nucleus accumbens shell on responses (Mean ± SEM) on the active and inactive lever for self-infusions of 200 mg% ethanol into the posterior ventral tegmental area. All rats received self-infusion of 200 mg% ethanol into the posterior ventral tegmental area in all sessions; the microinjection of sulpiride was given immediately prior to sessions 5 and 6. * Responses on the active lever were significantly higher than responses on the inactive lever (p < 0.05). # Responses on the active lever were significantly lower than average basal responses during sessions 3 and 4 (p < 0.05). Response on the active lever in 30-min bins during acquisition session 4 and following treatment with 100 µM sulpiride in session 5 (panel D).
Fig. 3 shows the effects of microinjection of SCH-23390 into the NACsh on ethanol self-infusion into the pVTA. The repeated measures ANOVA revealed significant effect of lever (F1, 8 = 49.1, p < 0.001), but no effect of session (F6, 3 = 2.5, p = 0.25) or interaction (F6, 3 = 1.0, p = 0.54) in the 10 µM SCH-23390 treated group (Fig. 3A). Lever discrimination was observed during sessions 2 to 7. Microinjection of 10 µM SCH-23390 did not alter responses on the active lever. However, the 100 µM SCH-23390-treated group (Fig. 3B) demonstrated significant effects of session (F6, 5 = 11.6, p = 0.008), lever (F1, 10 = 37.8, p < 0.001), and interaction (F6, 5 = 9.2, p = 0.014). Lever discrimination was seen during acquisition sessions 2–4. Microinjection of 100 µM SCH-23390 significantly reduced responses on the active lever during both treatment sessions, but lever discrimination remained. Responses on the active lever returned toward acquisition levels during the reinstatement session. In addition, response patterns (Fig. 3D) indicate that the highest responding on the active lever in session 4 occurred during the 1st and 3rd hr; whereas responses on the active lever were reduced throughout with almost no responding observed after the 1st hr during session 5 following microinjection of SCH23390 into the NACsh.
Figure 3.
Effects of microinjection of the D1 receptor antagonist SCH-23390 (10 and 100 µM, n = 5 and 6, respectively) into the nucleus accumbens shell on the responses (Mean ± SEM) on the active and inactive lever for self-infusions of 200 mg% ethanol into the posterior ventral tegmental area. All rats received self-infusions of 200 mg% ethanol into the posterior ventral tegmental area in all sessions; the microinjection of SCH23390 was given immediately prior to sessions 5 and 6 (panels A and B). * Responses on the active lever were significantly higher than responses on the inactive lever (p < 0.05). # Responses on the active lever were significantly lower than average basal responses during sessions 3 and 4 (p < 0.05). Responses on the active lever in 30-min bins during acquisition session 4 and following treatment with 100 µM SCH 23390 in session 5 (panel C).
The involvement of NACcr dopamine receptors in ethanol ICSA in the pVTA
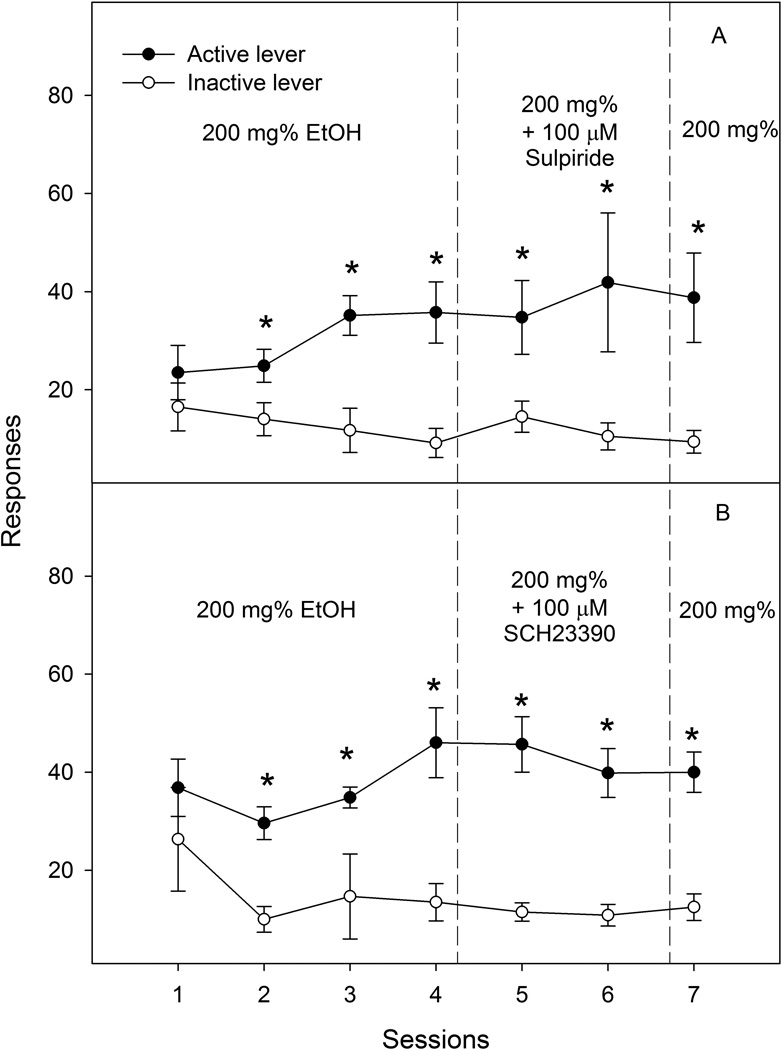
The effects of microinjection of sulpiride or SCH-23390 into the NACcr on lever responses are shown in Fig. 4. The repeated measures ANOVAs revealed a significant effect of lever, but no effect of session or interaction for both the sulpired-treated (Fig. 4A: session F6, 9 = 0.9, p = 0.52; lever F1, 14 = 13.4, p = 0.003; interaction F6, 9 = 2.6, p = 0.1) and SCH-23390-treated (Fig. 4B: session F6, 5 = 0.6; p = 0.70; lever F1, 10 = 25.1, p = 0.001 ; interaction F6, 5 = 0.7, p = 0.65) groups. Lever discrimination was observed in sessions 2 to 7 in both groups. Microinjection of either sulpiride (100 µM) or SCH-23390 (100 µM) did not alter responses on the active lever.
Figure 4.
Effects of microinjection of the D2 receptor antagonist sulpiride (100 µM, n = 8) or the D1 receptor antagonist SCH23390 (100 µM, n = 6) into the nucleus accumbens core on responses (Mean ± SEM) on the active and inactive lever for self-infusions of 200 mg% ethanol into the posterior ventral tegmental area. All rats received self-infusions of 200 mg% ethanol in the posterior ventral tegmental area in all sessions; the microinjection of sulpiride (panel A) or SCH-23390 (panel B) was given immediately prior to sessions 5 and 6. * Responses on the active lever were significantly higher than responses on the inactive lever (p < 0.05).
The involvement of VP dopamine receptors in ethanol ICSA in the pVTA
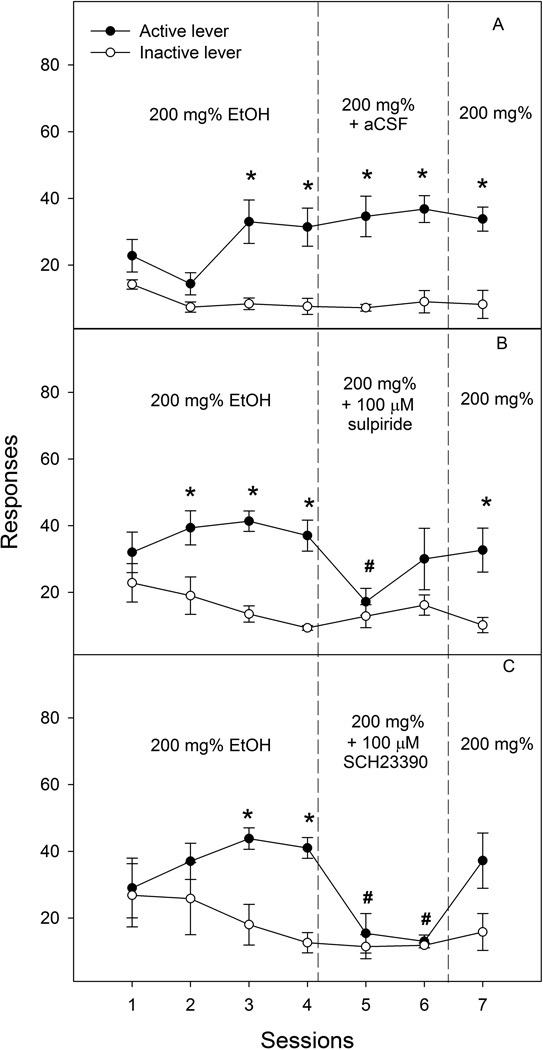
In the aCSF-treated group (Fig. 5A), repeated ANOVA revealed significant effects of session (F6, 3 = 89.2, p = 0.002), lever (F1, 8 = 33.2, p < 0.001), and interaction (F6, 3 = 34.1, p = 0.007). Lever discrimination was observed during sessions 3 to 7. The significant effect of session appeared to be due to lower responses on the active lever during sessions 1 and 2. However, microinjection of aCSF did not alter responding for ethanol. The 100 µM sulpiride-treated group (Fig. 5B) demonstrated significant effects of session (F6, 5 = 8.9, p = 0.015), lever (F1, 10 = 15.9, p = 0.003), and interaction (F6, 5 = 10.1, p = 0.011). Lever discrimination developed during acquisition sessions 2–4. Sulpiride significantly reduced responses on the active lever during the first treatment session compared with those during sessions 3 and 4, but not during the second treatment session. In the 100 µM SCH-23390-treated group, repeated measures ANOVA showed significant effects of session (F6, 3 = 45.3, p = 0.005), lever (F1, 8 = 5.37, p = 0.049), and interaction (F6, 3 = 61.8, p = 0.003). Lever discrimination developed during the acquisition sessions 3 and 4. Microinjection of SCH-233920 significantly reduced responses on the active lever during both treatment sessions to levels seen on the inactive lever. Responses returned toward acquisition levels during the reinstatement session.
Figure 5.
Effects of microinjection of aCSF (n = 5), the D2 receptor antagonist sulpiride (100 µM, n = 6) or the D1 receptor antagonist SCH23390 (100 µM, n = 5) into the ventral portion of the ventral pallidum on the responses (Mean ± SEM) on the active and inactive lever for self-infusions of 200 mg% ethanol into the posterior ventral tegmental area. All rats received self-infusions of 200 mg% ethanol into the posterior ventral tegmental area in all sessions; the microinjection of aCSF (panel A), sulpiride (panel B) or SCH23390 (panel C) was given immediately prior to sessions 5 and 6. * Responses on the active lever were significantly higher than responses on the inactive lever (p < 0.05). # Responses on the active lever were significantly lower than average basal responses during sessions 3 and 4 (p < 0.05).
The involvement of mPFC dopamine receptors in ethanol ICSA in the pVTA
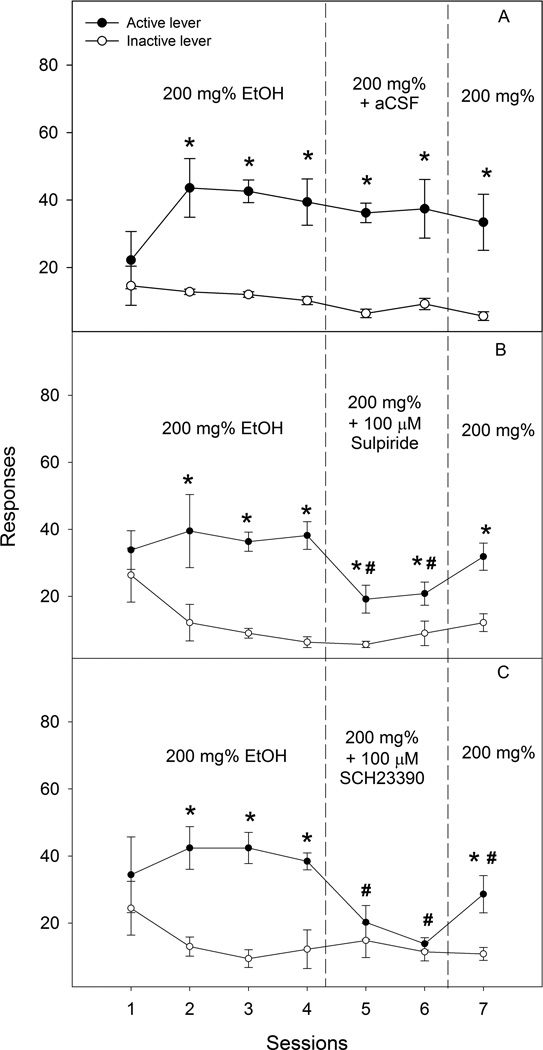
Repeated measures ANOVA in the aCSF-treated group (Fig. 6A) revealed a significant effect of lever (F1, 8 = 27.2, p = 0.001), but not session (F6, 3 = 1.5, p = 0.39) or interaction (F6, 3 = 1.9, p = 0.32). Lever discrimination was observed in sessions 2 to 7. Microinjection of aCSF did not alter responses on the active lever. The 100 µM sulpiride-treated group (Fig. 6B) demonstrated significant effects of session (F6, 5 = 14.9, p = 0.005), lever (F1, 10 = 27.6, p < 0.001), and interaction (F6, 5 = 5.4, p = 0.042). Lever discrimination developed during acquisition sessions 2–4. Sulpiride significantly reduced responses on the active lever during both treatment sessions. However, lever discrimination remained during both treatment sessions. Responses on the active lever returned toward the acquisition levels during the reinstatement session. In the 100 µM SCH-23390-treated group (Fig. 6C), repeated ANOVA shows significant effects of session (F6, 3 = 22.8, p = 0.013), lever (F1, 8 = 16.4, p = 0.004), and interaction (F6, 3 = 36.6, p = 0.007). Lever discrimination developed during acquisition sessions 2–4. Microinjection of SCH-233920 significantly reduced responses on the active lever during both treatment sessions to levels seen on the inactive lever. Active lever responses returned toward the acquisition levels during the reinstatement session.
Figure 6.
Effects of microinjection of aCSF (n = 5), the D2 receptor antagonist sulpiride (100 µM, n = 6) or the D1 receptor antagonist SCH23390 (100 µM, n = 5) into the prelimbic sub-region of the medial prefrontal cortex on responses (Mean ± SEM) on the active and inactive lever for self-infusions of 200 mg% ethanol into the posterior ventral tegmental area. All rats received self-infusions of 200 mg% ethanol into the posterior ventral tegmental area in all sessions; the microinjection of aCSF (panel A), sulpiride (panel B) or SCH23390 (panel C) was given immediately prior to sessions 5 and 6. * Responses on the active lever were significantly higher than responses on the inactive lever (p < 0.05). # Responses on the active lever were significantly lower than average basal responses during sessions 3 and 4 (p < 0.05).
Discussion
The major findings of the current study are that microinjection of either a dopamine D1 or D2 receptor antagonist into the NACsh, the prelimbic sub-region of the mPFC, or ventral portion of the VP, but not the NACcr, reduced ethanol self-infusions into the pVTA. These results suggest that concurrent activation of dopamine receptors in the NACsh, mPFC and VP are required to maintain the rewarding actions of ethanol within the pVTA. Although the activation of dopamine receptors in these regions may involve multiple mechanisms, previous studies found that local application of ethanol into the pVTA increased dopamine release in the NACsh, mPFC and VP (Ding et al, 2009a, 2011), suggesting that activation of dopamine projections from the pVTA to these forebrain regions is one important mechanism mediating the reinforcing effects of ethanol in the pVTA. In addition, the feed forward process of the reinforcing effects of ethanol appears to require concurrent activation of both D1 and D2 receptors, since microinjection of either antagonist was effective in reducing ethanol self-infusions into the pVTA. These findings suggest that the neuro-circuitry underlying the rewarding effects of ethanol include, at least in part, the dopamine projections from the pVTA to the NACsh, ventral VP and prelimbic mPFC, but not the dopamine projections to the NACcr.
Numerous studies have indicated that the NAC is critical for mediating the reinforcing and rewarding effects of ethanol (Gonzales et al, 2004; Koob et al, 1998). Systemic and intra-NAC administration of the non-specific dopamine receptor antagonist fluphenazine attenuated oral operant self-administration of ethanol in rats (Rassnick et al, 1992). The intra-NAC administration of the D1 antagonist SCH 23390 or the D2 receptor antagonist raclopride reduced responding for oral ethanol self-administration (Hodge et al, 1997; Samson et al, 1993). In addition, microinjection of SCH23390 or raclopride into the NAC reduced ethanol seeking behavior (Chaudhri et al, 2009; Czachowski et al, 2001). The current study agrees with these studies and further indicates the involvement of NAC in the reinforcing effects of ethanol in the pVTA.
In the NACsh, both sulpirde and SCH23390 reduced ethanol self-infusions into the pVTA (Figs. 2 & 3), suggesting that a concerted activation of both D1 and D2 receptors within the NACsh is required for processing the rewarding information of ethanol in the pVTA. Both doses of sulpiride appeared to be more effective than doses of SCH23390 (Figs. 2 & 3). The pattern of responding on the active lever following sulpiride indicated it was effective within the first 30 min (Fig. 2D). Since SCH23390 and sulpiride have nM affinity to their respective receptors, their differences on the ICSA of ethanol into the pVTA may be due to differences in the cellular distribution of D1 and D2 receptors on median spiny projection neurons within the NACsh (Lu et al., 1998; Meredith 1999).
On the other hand, inhibition of either D1 or D2 receptors in the NACcr did not alter ethanol self-infusions in the pVTA (Fig. 4). Although it remains possible that concurrent inhibition of both receptors may alter ethanol self-infusions into the pVTA, the present results suggest that activation of each individual receptor may not be involved in the reinforcing effects of ethanol in the pVTA. A previous study suggests that D2 receptors in the NACcr may be involved in systemic reinforcing effects of ethanol, because local injection of a D2 receptor antagonist raclopride into the NACcr reduced operant responding for oral self-administration of ethanol in rats (Samson et al, 2003). It is possible that different behavioral paradigms used (oral operant self-administration vs ICSA) may contribute to such difference.
The differential involvement of the NACsh and NACcr in the reinforcing effects of ethanol in the pVTA agrees with the findings that activation of dopamine receptors in the NACsh, but not NACcr, produced reward (Ikemoto et al, 1997). The mechanisms for this difference remain unknown, but may be associated with substantial difference between these two sub-regions in their afferent and efferent projections (Brog et al, 1993; Zahm, 2000). For example, the NACsh projects mainly to the ventro-medial VP, which feeds to the mediodorsal thalamus, whereas the NACcr projects mainly to the dorsal lateral VP, which projects to the subthalamic nucleus (Zahm, 2000). In addition, different dopamine projections to the NACsh and NACcr may also contribute to this difference. The pVTA projects mainly to the NACsh, while the NACcr receives projections mainly from the anterior VTA (Ikemoto 2007).
Locomotor activity was not monitored in the current study. Several studies have examined the effects of intra-NAC administration of SCH23390 or sulpiride on locomotor activity. The general consensus among these studies is that intra-NAC treatment did not alter locomotor activity at concentrations up to 1.5 mM for SCH23390 (Coccurello et al, 2000; Gerrits et al, 1994; Taslimi et al, 2012; Zarrindast et al, 2012), or up to 700 µM for sulpiride (Coccurello et al, 2000; Taslimi et al, 2012; Zarrindast et al, 2012). These studies suggest that the reduction of ethanol self-infusions into the pVTA by SCH23390 or sulpiride (100 µM each) infusion in the NACsh were not likely due to impairment of general locomotor activity.
Administration of dopamine receptor antagonists into the ventral portion of the VP also reduced self-infusions of ethanol into the pVTA, suggesting that inhibition of dopamine D1 or D2 receptor-mediated transmission in the VP prevented the feed forward information needed for maintaining the reinforcing effects of ethanol within the pVTA (Fig. 5). The reduction of self-infusions of ethanol is unlikely due to impairment to general locomotor activity because the concentrations used in the current study were within the range that was shown not to alter motor activity. Microinjection of SCH23390 (0.5 mM) or sulpiride (58 mM) into the VP did not produce motor responses (Napier, 1992). Microinjection of SCH23390 (1.5–25 mM) or sulpiride (1.2 mM–12 mM) into the VP did not reduce water or ethanol consumption in rats, suggesting no alteration in general locomotor activity (Melendez et al, 2005; Shimura et al, 2006). In addition, the treatments with SCH23390 or sulpiride did not alter responses on the inactive lever (Fig. 5).
These results are consistent with the dopamine-stimulating effects of ethanol on the meso-pallidal pathway, following either systemic or intra-pVTA administration of ethanol (Ding et al, 2011; Melendez et al, 2003). These results also agree with studies suggesting a general role of the VP in mediating the reinforcing and rewarding effects of ethanol (Kemppainen et al, 2012; Melendez et al, 2005). Findings from the current study are in contrast to a previous study that examined the involvement of VP D1 and D2 receptors in ethanol drinking (Melendez et al, 2005). Microinjection of sulpiride into the VP significantly increased ethanol drinking, whereas microinjection of SCH23390 into the VP produced a small, but non-significant decrease of ethanol intake (Melendez et al, 2005). The different animal models may explain the different results in these two studies.
A concerted activation of D1 and D2 receptors in the prelimbic mPFC seems to be critical to the reinforcing effects of ethanol within the pVTA, as evidenced by the results that either sulpiride or SCH23390 was able to attenuate self-infusions of ethanol into the pVTA (Fig. 6). These effects were not likely due to locomotor impairment. A number of studies have shown that microinjection of SCH23390 into the mPFC did not alter locomotor activity at concentrations up to 6 mM (Hall et al, 2009; St Onge et al, 2011), and that microinjection of sulpiride into the mPFC did not alter locomotor activity at concentrations up to 3 mM (Beyer and Steketee, 2001; Steketee and Walsh, 2005). These results are consistent with previous findings that intra-pVTA administration of ethanol increased dopamine release in the mPFC (Ding et al, 2011). In addition, passive intra-venous infusion of ethanol was shown to increase dopamine overflow in the mPFC (Schier et al, 2013). These neurochemical data suggest a role of mPFC dopamine neuro-transmission in the effects of ethanol. Chemical lesion of the mPFC with 6-OHDA altered alcohol drinking behavior (Nielsen et al, 1999). Infusion of the D2 receptor antagonist raclopride into the mPFC reduced oral operant responding for ethanol in rats (Hodge et al, 1996; Samson et al, 2003). So far, the role of D1 receptors in the mPFC in oral operant self-administration or voluntary drinking of ethanol has received little attention.
During reinstatement, there appeared to be a difference in the recovery of responding in the VP compared to the other two regions. It is not clear why differences were observed between the reinstatement following sulpiride in the NACsh (Fig. 2) and VP (Fig. 5), and between the reinstatement in the mPFC (Fig. 6) and VP (Fig. 5) following SCH23390 administration. The differences may be due to a combination of factors, such as the unique neuronal circuitry within the VP, the interactions of the VP with the NACSh and pVTA, and differences in the recovery of the VP neuronal circuits to the dopamine antagonists.
In conclusion, the results of the current study provide evidence for an ‘alcohol reward circuit’ involving the pVTA, NACsh, prelimbic mPFC and ventral VP. The anterior VTA (Rodd-Henricks et al., 2000) and the NACcr (Fig. 4) do not appear to be involved in this circuit. The first step in processing the rewarding effects of ethanol within the pVTA is the concurrent activation of local dopamine neurons projecting to the NACsh, prelimbic mPFC and ventral VP to forward the rewarding signal to circuits that initiate and maintain goal-directed behavior. This activation of pVTA dopamine neurons is mediated, at least in part, by excitatory 5-HT3 receptors (Ding et al., 2011; Rodd-Henricks et al., 2003). The activation of local dopamine neurons results in increased dopamine release in the NACsh, mPFC and VP (Ding et al., 2009a, 2011). Following the release of dopamine, a concerted activation of both D1 and D2 receptors in these three regions appear to be needed to process the rewarding information forward. The major feed forward effects of the dopamine inputs into the (a) NACsh are to regulate GABA outputs to the ventral-medial VP; (b) ventral VP are to regulate GABA outputs to the medio-dorsal thalamus; and (c) the prelimbic mPFC to regulate glutamate outputs to the premotor cortex. The net results of these actions are to initiate and maintain the goal-directed behavior to obtain self-infusions of ethanol into the pVTA. This hypothetical simple scheme does not take into account the multiple interactions these limbic regions have with other structures, nor does it take into account the complex neuronal interactions that occur within each region.
Acknowledgements
This study was supported by research grants AA012262, AA020908, and AA019366. We thank Erin Larrabe for her technical help. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
The authors declare no conflict of interest.
Author contribution
ZMD, WJM and ZAR were responsible for concept development and research design. ZMD and CMI participated in performing experiments and acquiring data. ZMD and WJM performed data analysis and contributed to the writing of the manuscript.
References
- Beyer CE, Steketee JD. Characterization of the role of medial prefrontal cortex dopamine receptors in cocaine-induced locomotor activity. Behav Neurosci. 2001;115:1093–1100. doi: 10.1037//0735-7044.115.5.1093. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the 'accumbens' part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology. 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R, Adriani W, Oliverio A, Mele A. Effect of intra-accumbens dopamine receptor agents on reactivity to spatial and non-spatial changes in mice. Psychopharmacology. 2000;152:189–199. doi: 10.1007/s002130000515. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25:1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hall SR, Engleman EA, Hauser SR, McBride WJ, et al. The stimulating effects of ethanol on ventral tegmental area dopamine neurons projecting to the ventral pallidum and medial prefrontal cortex in female Wistar rats: regional difference and involvement of serotonin-3 receptors. Psychopharmacology. 2011;216:245–255. doi: 10.1007/s00213-011-2208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, McBride WJ, et al. Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: involvement of serotonin-3 receptors. J Pharmacol Exp Ther. 2012;340:202–209. doi: 10.1124/jpet.111.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009a;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Toalston JE, Oster SM, McBride WJ, Rodd ZA. Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology. 2009b;204:381–390. doi: 10.1007/s00213-009-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gerrits M, Ramsey NF, Wolterink G, Van Ree JM. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology. 1994;114:486–494. doi: 10.1007/BF02249340. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hall DA, Powers JP, Gulley JM. Blockade of D1 dopamine receptors in the medial prefrontal cortex attenuates amphetamine- and methamphetamine-induced locomotor activity in the rat. Brain Res. 2009;1300:51–57. doi: 10.1016/j.brainres.2009.08.084. [DOI] [PubMed] [Google Scholar]
- Hegarty AA, Vogel WH. Modulation of the stress response by ethanol in the rat frontal cortex. Pharmacol Biochem Behav. 1993;45:327–334. doi: 10.1016/0091-3057(93)90247-q. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappell AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nuclues accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–239. [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Kiianmaa K. Role for ventral pallidal GABAergic mechanisms in the regulation of ethanol self-administration. Psychopharmacology. 2012;223:211–221. doi: 10.1007/s00213-012-2709-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, McBride WJ, Murphy JM. Alcohol stimulates the release of dopamine in the ventral pallidum but not in the globus pallidus: a dual-probe microdialysis study. Neuropsychopharmacology. 2003;28:939–946. doi: 10.1038/sj.npp.1300081. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Involvement of the mesopallidal dopamine in ethanol reinforcement. Alcohol. 2004;32:137–144. doi: 10.1016/j.alcohol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Dopamine receptor regulation of ethanol intake and extracellular dopamine levels in the ventral pallidum of alcohol preferring (P) rats. Drug Alcohol Depend. 2005;77:293–301. doi: 10.1016/j.drugalcdep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Napier TC. Dopamine receptors in the ventral pallidum regulate circling induced by opioids injected into the ventral pallidum. Neuropharmacology. 1992;31:1127–1136. doi: 10.1016/0028-3908(92)90009-e. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Nielsen DM, Crosley KJ, Keller RW, Jr, Glick SD, Carlson JN. Left and right 6-hydroxydopamine lesions of the medial prefrontal cortex differentially affect voluntary ethanol consumption. Brain Res. 1999;823:59–66. doi: 10.1016/s0006-8993(99)01099-9. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res Rev. 1987;12:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. New York: Academic Press; 1998. [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav. 2003;79:581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull. 1993;30:133–141. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Schier CJ, Dilly GA, Gonzales RA. Intravenous ethanol increases extracellular dopamine in the medial prefrontal cortex of the long-evans rat. Alcohol Clin Exp Res. 2013;37:740–747. doi: 10.1111/acer.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Walsh TJ. Repeated injections of sulpiride into the medial prefrontal cortex induces sensitization to cocaine in rats. Psychopharmacology. 2005;179:753–760. doi: 10.1007/s00213-004-2102-5. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Taslimi Z, Arezoomandan R, Omranifard A, Ghalandari-Shamami M, Riahi E, Vafaei AA, et al. Orexin A in the ventral tegmental area induces conditioned place preference in a dose-dependent manner: involvement of D1/D2 receptors in the nucleus accumbens. Peptides. 2012;37:225–232. doi: 10.1016/j.peptides.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–267. [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Khalifeh S, Rezayof A, Rostami P, Aghamohammadi Sereshki A, Zahmatkesh M. Involvement of rat dopaminergic system of nucleus accumbens in nicotine-induced anxiogenic-like behaviors. Brain Res. 2012;1460:25–32. doi: 10.1016/j.brainres.2012.04.036. [DOI] [PubMed] [Google Scholar]