Summary
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults with a median survival of 16.2 to 21.2 months post diagnosis [1]. Because of its location, complete surgical resection is impossible; additionally because GBM is also resistant to chemotherapeutic and radiotherapy approaches, development of novel therapies is urgently needed. In this chapter we describe the development of preclinical animal models and a conditionally cytotoxic and immune-stimulatory gene therapy strategy that successfully causes tumor regression in several rodent GBM models.
Keywords: GBM models, immunotherapy, adenoviral gene therapy, T cell activation assays
1. Introduction
The advantages of implantation GBM models (syngeneic or xenograft) are their efficient tumorigenesis, their reproducible and relatively fast growth rates and the accurate knowledge of tumor location [2-4]. They exhibit many of the histopathological features of human GBM, i.e. infiltration of tumor cells throughout the surrounding brain parenchyma (Figure 1 and Figure 2), areas of pseudopalisading necrosis, and neovascularization [2, 5, 6]. Hence they have proven to be a valuable tool for the preclinical assessment of novel therapies. Syngeneic GBM models in rodents encompass the implantation of GBM cells that originated in the same mouse or rat breed so that they are immunologically compatible. Since the tumor and the host match immunologically, this tumor model does not require immunodeficient animals, allowing testing of immunotherapeutic strategies. Xenograft tumor formation involves the implantation of human GBM-derived short term primary cultures or cell lines in immunosuppressed or immunodeficient mice. For this reason, they are not suitable for the evaluation of immunotherapeutic approaches and will not be part of our focus here.
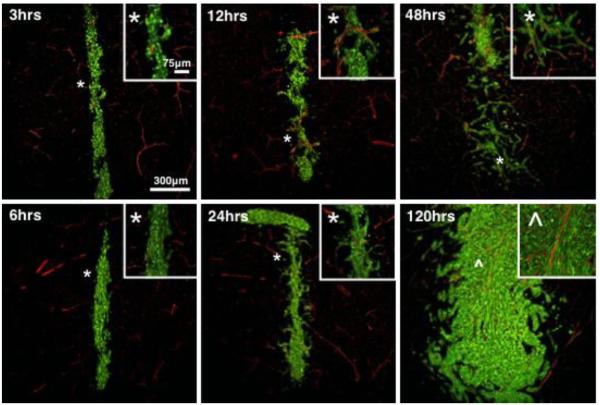
Figure 1. Time-course analysis of early GL26-Cit glioma growth in the striatum in mice.
To visualize perivascular glioma growth GL26-Cit cells were implanted in RA/EGxdelCre mice that express GFP in the brain endothelium. Invasive glioma cells maintain close vascular contact at all time-points analyzed over 120 hrs as they disseminate throughout the brain. GFP+ brain microvasculature has been pseudocolored red. Corresponding high-magnification micrographs (insets) detail perivascular invasion at the tumor border. White asterisks (*) relate the image area shown in the high-magnification micrographs with the corresponding area in the low-magnification micrographs. Perivascular tumor invasion begins 24 hrs post-implantation. Inset denoted by the carrot (^) in the 120 hr micrograph is included to demonstrate the trapping of normal brain microvessels within the growing tumor mass as perivascular invasion is followed by tumor cell proliferation with in the perivascular space. Time-points progress from top to bottom and from left to right.
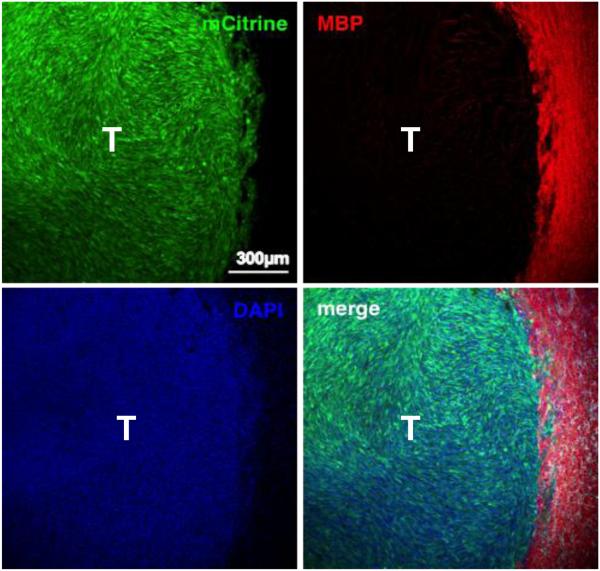
Figure 2. Late-stage GL26-Cit glioma tumor.
Scanning fluorescence confocal micrograph of syngeneic GL26-Cit mouse glioma (shown in green) 21-days post-tumor implantation into the striatum. Glioma cells were genetically modified to express mCitrine fluorescent protein prior to tumor implantation to facilitate direct tumor visualization by fluorescence microscopy. Individual and merged channels are shown. Tumor-bearing brain tissue was sectioned 50 μm thick and immunolabeled with anti-myelin basic protein (MBP) antibodies (shown in red) then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (shown in blue). Note that this late-stage tumor has well-defined borders compared to GL26-Cit tumors at earlier stages, which lack definition in their tumor borders and which exhibit extensive perivascular invasion. Also note that large myelinated axonal bundles that become compressed towards the outside of the growing tumor mass. Scale bar corresponds to 300μm. T denotes tumor area.
A number of key physiological processes such as the relative paucity of dendritic cells (DCs) in the brain, lack of lymphatic drainage, production of anti-inflammatory mediators such as TGF-β and nitric oxide (NO) by cells in the central nervous system (CNS), as well as low MHC-II expression on infiltrating microglia, protect the brain from an immune-mediated attack but at the same time,also contribute to its immune suppressive environment [7-10]. We have succeeded in developing an adenoviral mediated immunotherapy for brain tumors that is dependent on the expression of two genes: Thymidine kinase (TK), that phosphorylates the prodrug Ganciclovir which induces DNA crosslinking followed by cell death and fms-like tyrosine kinase-3 ligand (Flt3L), a potent DC growth factor that recruits DCs within the tumor microenvironment [11-13]. Tumor cell death induced by TK causes the release of intracellular proinflammatory molecules and tumor antigens, which are taken up by surveying DCs that subsequently prime the T cells to elicit an antigen specific cytotoxic anti-tumor immune response [11-13]. Combination therapy using these two adenoviruses induces tumor regression, long term survival and immunological memory in several mouse and rat GBM models [11, 13, 14].
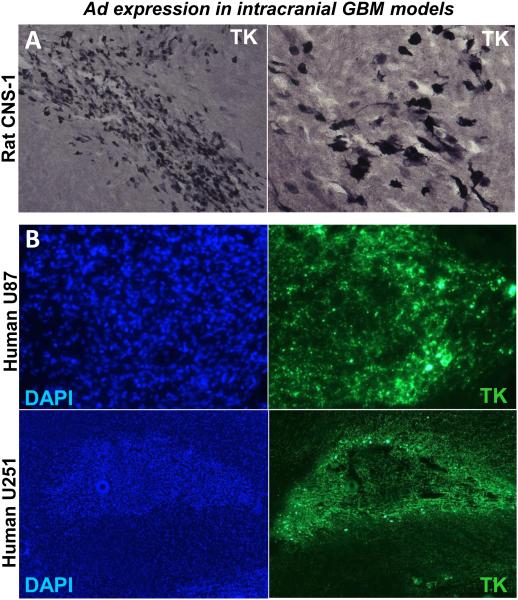
Gene therapy vectors can also be used for the treatment of neurodegenerative diseases. In order to be useful for gene therapy of chronic neurological disorders, vector systems should allow long-term transduction of brain cells in the absence of undesirable long-term side effects. Recombinant adenovirus vectors are powerful tools for gene delivery to the CNS [15, 16]. Adenoviruses can be easily purified to high titers and efficiently transduce differentiated cells such as neurons and glial cells. Additionally, transgene expression is restricted to the area of vector administration or areas that project to the injection site [17, 18]. The majority of current adenoviral-mediated gene therapy protocols utilize first-generation vectors, which are recombinant vectors that are non-replicative because of the deletion of the E1 region from the viral genome [19, 20]. While in the absence of prior immune priming to adenovirus, first-generation adenoviral vectors injected into the brain parenchyma can sustain prolonged transgene expression for months, activation of anti-viral T cells by peripheral immunization leads to loss of vector-mediated transgene expression [18, 21]. To overcome this instability, “gutless,” high-capacity adenoviral (HC-Ad) vectors have been engineered in which all viral encoding genes have been eliminated and replaced with noncoding stuffer DNA sequences. Even in the presence of an anti-adenoviral immune response, expression from HC-Ad vectors remains stable for at least 1 year [22, 23]. Critical parameters that determine the efficiency of transgene expression are (1) dose and volume of adenoviruses administered, (2) vector backbone and the choice of promoter, (3) immune status of the animal and (4) purity of the adenoviral vector stock [24, 25]. In this unit we provide detailed instructions for the implantation of various syngeneic GBM models in rodents and adenoviral-mediated gene therapy using Ad-TK and Ad-Flt3L. Adenoviral-mediated transgene expression can be readily detected in treated brain tumors by immunocytochemistry. Figure 3 shows representative images from synegeneic rat CNS-1 model and, U251 and U87 xenografts models injected intratumorally with Ad adenoviral vectors encoding TK, which . TK expression was assesses by immunohistochemistry and immunofluorescence. ELISPOT and T cell proliferation are extremely useful assays for testing the quality and quantity of T cell antitumor responses induced by immunotherapeutic approaches; hence the protocols for setting up these assays are also described [11, 13, 14, 26-30].
Figure 3. Adenoviral-mediated transgene delivery into intracranial GBM in rodent models.
Microphotographs show TK expression within intracranial GBMs implanted in the rodent brain. Lewis rats bearing intracranial syngeneic CNS-1 GBMs (A) and nude mice bearing intracranial human U251 or U87 GBM (B) were intratumorally injected with Ad vectors encoding thymidin kinase (TK). TK expression was assessed by immunocytochemistry (A) and immunofluorescence (B). Nuclei were stained with DAPI (B, left panels).
2. Materials
2.1 For intracranial tumor implantation
1) Anesthetics/analgesics and other drugs: Ketamine (75 mg/Kg body weight, Orion Pharma), Dexdomitor (0.25 mg/Kg body weight, Orion Pharma), Rimadyl/Carprofen (5 mg/Kg body weight, Orion Pharma), Atipamezole (1 mg/Kg body weight, Orion Pharma), Buprenorphine (20 ul/gm body weight, MWI Vetrinary Supply), Povidone-Iodine solution (Aplicare), Paralube eye ointment (Dechra), Saline solution (0.9 % Sodium Chloride; Hospira).
2) Surgical equipment: Stereotactic frame with adaptor for rats and mice and blunt ear bars (Stoelting), stereomicroscope (Zeiss Stemi 1000 zoom) equipped with 16x eyepieces and 0.4x auxiliary objective lens and mounted on hinged coupling arm and a heavy foot stand, surgical lamp (Philips Burton, CS316W), electric drill (Dremel Stylus) with 0.6 mm drill bit for mice and 1.75 mm drill bit for rats (Stoelting), surgical shavers (3M Surgical clipper), scalpel and blades, skin retractors, ethanol swabs (Kendall-webcol), curved and straight forceps, holding scissors, sharp scissors, 3-0 nylon sutures (Ethicon), 5 μl Hamilton syringe with 26G needle for rats and 33G needle for mice.
2.2 For adenoviral gene therapy
1) Anesthetics/analgesics and other drugs: as above
2) Surgical equipment: as above
3) Adenoviral vectors: Detailed methodologies to clone, purify and quantitate adenoviral vectors for gene therapy are detailed elsewhere [17, 24]. Also included in these references are protocols for the analysis of transgene expression using immunohistochemistry (Figure 3), enzymatic assays and flow cytometry. Protocols for the detection of anti-adenoviral immune responses are also described in this chapter. The adenoviral vectors used in this protocol are first generation Ad.hCMV.hsFLT3L + Ad.hCMV.TK.
4) Phosphate buffered saline: Dulbecco’s PBS, pH 7.4, without Calcium or Magnesium (Life technologies)
5) Ganciclovir: To prepare 7 mg/ml stock solution, weigh 250 mg of Ganciclovir (TSZ Chem) and add 20 ml of Miili-Q H2O to it. Adjust pH to 12 with 1 M NaOH. The solution will clear once the pH reaches 12. Next lower the pH to 11 using 1N HCl and add Milli-Q H2O to a final volume of 35.71 ml. Dilute further with saline for injections.
2.3 For functional assays post gene therapy administration
1) Tyrodes solution: 132 mM NaCl, 1.8 mM CaCl2.H2O, 0.32 mM NaH2PO4, 5.56 mM glucose, 11.6 mM NaHCO3, and 2.68 mM KCl. To prepare 1L add 8 gms NaCl, 0.264 gms CaCl2.H2O, 0.05 gms NaH2PO4, 1 gm glucose, 1 gm NaHCO3 and 0.2 gms of KCl. Add 100 μl of heparin (Sagent Pharmaceuticals) to prevent blood from clotting while perfusion.
2) ACK buffer: Add 8.29 gms NH4Cl, 1 gm KHCO3, 37.2 mgs Na2EDTA to 800 ml of pure H2O. Adjust pH to 7.4 with 1N HCl and top up the volume to 1L with H2O.
3) Complete media: RPMI/DMEM medium supplemented with 10% FBS, 50 units/mL penicillin and 50 μg/mL streptomycin.
4) ELISPOT reagents: Mouse IFN-γ ELISPOT Ready-SET-Go kit (eBioscience), Immobilon-P 96 well plates (Millipore, MSIPS4510), Ethanol (USP grade, mixed 35 % vol/vol in sterile Miili-Q H2O), ELISPOT Wash Buffer (0.01% Tween-20 in PBS), Cell stimulation cocktail (eBioscience), TMB substrate (Mabtech), ELISPOT plate reader (AID Autoimmun Diagnostika GmbH).
5) For T cell proliferation: 5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE) cell proliferation kit (Life technologies), Bovine serum albumin (BSA; Sigma), anti-mouse and anti-rat CD3, CD4 and CD8 fluorochrome conjugated antibodies for flow staining (eBioscience/Biolegend/BD Biosciences), flow staining buffer (2% FBS in PBS).
3. Procedure
3.1 Intracranial GBM implantation
Resuspend glioma cell lines in serum free media at the required concentration (Injectable volume 1-5 μl).
The surgical area needs to be clean and organized with all the required instruments, drugs and sterile tools (see Note 1).
Place the animal under anesthesia by intraperitoneal (i.p) injection of Ketamine and Dexdomitor. Once the animal has lost the footpad reflex it is sedated enough to start the surgical procedure. Administer a subcutaneous injection of Carprofen to ensure analgesia during and after surgery (see Note 2).
Shave the fur from the head of the animal.
Mount the animal onto the stereotactic frame, immobilize its head in the incisor bar and tighten it gently with the ear bars and the nose clamp (see Note 3).
Clean the incision area thoroughly with alcohol wipes and povidone-iodine solution.
Make a ~1 cm (for mice) or ~1.5 cm (for rats) midline incision along the top of the head between the eyes and the ears. Using a mouse or rat skin retractor hold back the skin on both sides of the incision.
Direct the light beams onto the exposed skull and focus the microscope on the bregma, the junction of the sagittal and transverse sutures (see Note 4).
Position the Hamilton syringe using the manipulator arms so that the tip of the needle is exactly over bregma. To inject into the striatum of a mouse or rat, move the manipulator arm forward x mm for the antrio-posterior coordinate and then y mm lateral away from the bregma. (Refer to the table for the x and y coordinates for the various cell lines). Watching through the microscope eyepiece, use a bent 26G1/2 needle to itch a small mark in the skull where the needle will penetrate. Lift the needle so that it does not obstruct the area.
Drill a small burr hole with a 0.6 mm bit for mice and 1.75 mm bit for rats using wide circular drill motions. The burr hole should be wide to provide a large open area for the insertion of the needle. Drilling into the skull generates considerable heat, so it is advisable to drill in short bursts, while intermittently bathing the skull with ice cold saline solution, which can be removed using a cotton swab. Avoid severing any blood vessels during drilling. In the event of bleeding, clean the burr hole to prevent the blood clot from blocking the needle entry and retraction (see Note 5 and 6).
Load the Hamilton syringe (33G needle for mice, 26G for rats) with the proper dose of cells (see Note 7). Lower the needle such that it is leveled with the dura. Read the dorsoventral coordinates and lower the needle to 0.5 mm plus the appropriate coordinates, depending on the GBM model. Pull the needle up toward the dura 0.5 mm and wait for 2 minutes. The extra 0.5 mm provides a pocket for the cells at the time of injection.
Administer the injection slowly over the course of 0.5 μl/min. Keep the needle in place for 5 minutes post injection to allow tumor cells to settle before slowly withdrawing the needle from the brain. Clean the syringe thoroughly by flushing 3 times with saline (see Note 8).
Flush the skull with sterile saline 3 times to remove any residual cells from the brain surface and dry the area with a cotton swab.
Remove the skin retractor and close the incision using 3-0 nylon suture.
Resuscitate the animal by i.p injection of atipamazole. Administer buprenorphine subcutaneously. Monitor the animal until it fully recovers from anesthesia and return them to their cage.
Provide the animals with water soaked chow in a petri dish and monitor for any surgical complications. Remove any remaining sutures at 10-14 days post surgery.
3.2 Adenoviral gene therapy
1) Dilute the adenovirus preparation in sterile PBS such that the required number of infectious units can be administered in the appropriate volume (see Note 9).
2) Anesthetize tumor bearing animals with an i.p injection of ketamine and dexdomitor. Ensure that the animal is fully anesthetized by checking for the lack of responses to footpad and tail pinching. Place the anesthetized mice in a stereotactic apparatus.
3) By now the sutures from the surgery for tumor implantation would have fallen off and the old skin incision would have healed. Using a scalpel, make a 1.5 cm midline incision into the skin at the same location as before. Use the scalpel blade to gently separate the healed skin from the underlying tissue.
4) Use skin retractors to hold back the skin on either side of the incision.
5) Remove the fibrous tissue covering the site of the tumor injection by gently scraping with the scalpel blade. Wash the area with cold saline to stop any bleeding and clean with ethanol swab.
6) The old burr hole should now be clearly visible. At this point, it is not required to drill again through the bone to provide access to the needle into the site of tumor implantation. Use a bent 26G needle to remove the scar tissue that forms at the burr hole. Use ethanol swabs to clean the burr hole to remove any clotted blood.
7) Lower the needle into the brain to the dorsoventral coordinate of tumor injection plus 0.5 mm ventrally and wait for 2 minutes before slowly injecting 1/3rd of the vector suspension. Wait for 1 minute for the vector solution to infuse into the tissue. Move 0.5 mm up dorsally and inject another 1/3rd of the vector and wait a further minute. Repeat for 1 last time to inject the remaining vector suspension. Wait for 5 minutes after the final administration and then slowly draw the needle out.
8) Starting 24 hours post gene therapy, administer 25 mg/kg of Ganciclovir i.p twice daily for 7 days for mice or 10 days for rats (see Note 10). Monitor the animals for signs of moribund behavior (hunched posture, lack of grooming, porphyrin staining around the eyes) and euthanize when their health status reaches the criteria established by the institutional animal care guidelines. Animals will be humanely killed by terminal perfusion with oxygenated, heparinized Tyrode's solution under deep anesthesia.
3.3 Analysis of antigen-specific T cells responses
3.3.1 IFNγ-ELISPOT
1) ELISPOT assays will be carried out using the mouse IFN-γ ELISPOT Ready Set Go! kit (eBiosciences).
2) One day prior to the start of the assay, dilute the IFN-γ capture antibody in sterile ELISPOT Coating Buffer, as noted on Certificate of Analysis included with the reagent set. Coat ELISPOT plate (Immobilon-P plates from Millipore) with 100 μl/well of capture antibody solution diluted in the coating buffer included in the kit. Cover the plate and seal with parafilm. Incubate at 4°C overnight (see Note 11).
3) On the day of the assay, decant or aspirate coating antibody from plate and wash plates 2 times with 200 μl/well sterile ELISPOT coating buffer. Decant.
4) Block plate with 200 μl/well of complete RPMI-1640 at 37° C for 2 hours. Decant or aspirate plate (see Note 12).
5) Harvest the spleens from the treated animals between 7-10 days post gene therapy by making an incision in the abdominal cavity on the left side of the mouse, inferior to the stomach (see Note 13).
6) Place the excised spleen on a 70 micron cell strainer that is put on a 50 ml conical tube.
7) Mash the spleen gently using the plunger end of a 1 ml syringe. Wash the cells through the strainer using 15-20 ml of complete media. Pellet the cells at 1500 rpm for 5 minutes at 4° C. Discard the supernatant.
8) Remove RBCs by ACK lysis as follows: Resuspend the cell pellet in 3 ml of ice cold ACK buffer per spleen. Incubate on ice for 3 minutes. Dilute out the ACK buffer by adding 10 ml of 1X PBS. Pellet and discard the buffer as in step 7. Wash once more with complete media (see Note 14).
9) Resuspend the splenocytes in complete media to a concentration of 0.5 × 106 cells/100 μl. Add 100 μl of this cell suspension to the ELISPOT plate.
10) Aliquot mitogen (cell stimulation cocktail from eBioscience, 1:500 dilution), antigen (100-200 μg/ml) or controls diluted in complete medium to appropriate wells at 100 μl/well.
11) Incubate the plate for 48 hours at 37° C, in a 5% CO2 humidified incubator before discarding the cells (see Note 15).
12) Wash plate 3 times with ELISPOT Wash Buffer. Dilute biotinylated detection antibody in Assay Diluent according to instructions on the Certificate of Analysis provided with the kit. Add 100 μl/well to the plate and incubate at 4°C overnight.
13) Decant antibody solution. Wash 4 times with ELISPOT Wash Buffer.
14) Dilute Avidin-HRP reagent in Assay Diluent according to instructions on the Certificate of Analysis provided with the reagent set. Add 100 μl/well of Avidin-HRP and incubate at room temperature for 2 hours.
15) Decant Avidin-HRP solution. Wash plate 3 times with ELISPOT Wash Buffer, and then 2 times with 1X PBS.
16) Add 100 μl/well of TMB substrate and develop at room temperature for 5-10 minutes; monitor development of spots (see Note 16).
17) Stop the substrate reaction by washing wells 3 times with 200 μl/well distilled water.
18) Air-dry the plate. Count spots using a dissecting microscope or automated ELISPOT plate reader.
3.3.2 T cell Proliferation Assay
1) Purify splenocytes from tumor-bearing mice treated with intracranial injections of Ad-TK + Ad-Flt3L as above in section 3.3.1.
2) Wash splenocytes twice with 1X PBS. Resuspend splenocytes in PBS containing 0.1% BSA at a concentration of 107/ml (see Note 17).
3) Add CFSE dye to a final concentration of 4 μM. Incubate at 37° C for 10 minutes with occasional shaking. At the end of the incubation, add the same volume of complete media and incubate on ice for 5 minutes to quench the staining reaction. Pellet the splenocytes at 1500 rpm for 5 minutes at 4° C followed by one wash with complete media.
4) Plate the cells at a density of 1 × 105 - 5 × 105 splenocytes in 100 μl in a 96 well flat bottom plate. Add appropriate antigens or mitogens in a 100 μl volume to the wells and incubate at 37° C, 5% CO2 humidified incubator for 5 days.
5) At the end of the culture period, pellet the cells as above, wash twice with flow staining buffer.
6) Resuspend the cells in 200 μl of flow staining buffer and add CD3, CD4 and CD8 antibodies at concentrations specified in the respective product sheets. Stain for 30 minutes at 4° C in the dark.
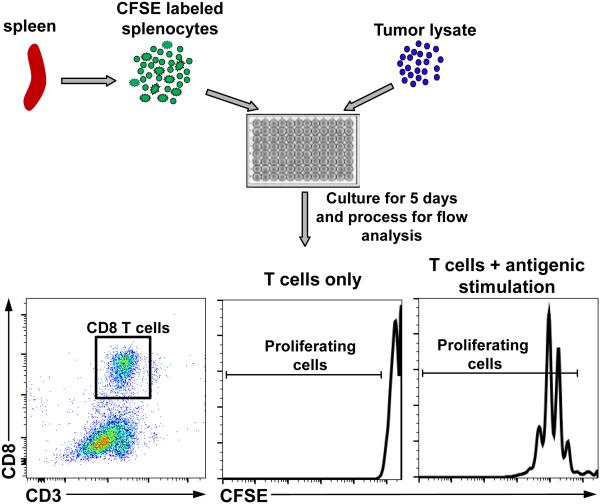
7) Pellet as above and wash 2 times with flow staining buffer and resuspend in 250 μl of flow staining buffer. Acquire data using an appropriate flow cytometer. Figure 4 depicts the workflow for T cell proliferation assay and shows the gating strategy for CD8+ T cells. Histograms show examples of CFSE dye dilution in unstimulated and stimulated CD8+ T cells.
Figure 4. Workflow for T cell proliferation assay.
Purify splenocytes, remove RBCs, label with CFSE dye and culture with tumor lysate followed by processing for flow cytometric analysis. Dot plots show gating strategy for CD8 T cells. Histograms show representative images of CFSE stains in unstimulated and stimulated CD8 T cells.
Table 1.
Cell numbers, implantation coordinates and survival duration for various GBM models are shown.
| Cell Line | Host | Cell number for implantation |
Injection coordinates (AP: anterio-posterior, ML: media-lateral, DV: dorso-ventral) |
Median survival |
|---|---|---|---|---|
| GL261 | C57BL/6 | 2 × 104 | +0.5 mm AP, +2.2 mm ML from bregma and −3.2 mm DV from dura | 28-35 days |
| GL26 | C57BL/6 | 2 × 104 | +0.5 mm AP, +2.1 mm ML from bregma and −3.2 mm DV from dura | 26-32 days |
| SMA560 | VM/Dk | 5 × 103 | +0.5 mm AP, +2.1 mm ML from bregma and −3.2 mm DV from dura | 30 days |
| CNS1 | Lewis rat | 5 × 104 | +1.0 mm AP, +3.0 mm ML from bregma and −5.5, −5.0, and −4.5 mm DV from dura (1μl per site) |
13-19 days |
| 9L | Fisher rat | 5 × 105 | +1.0 mm AP, +3.2 mm ML from bregma and −5.5, −5.0 and −4.5 mm DV from dura (1 μl per site). |
28.1 ± 1.3 days |
| F98 | Fisher rat | 5 × 104 | +1.0 mm AP, +3.2 mm ML from bregma and −6.5, −5.5 and −4.5 mm DV from the dura (1 μl per site). |
29 days |
| RG2 | Fisher rat | 2 × 104 | +1.0 mm AP, +3.0 mm ML from bregma and +6.0 mm DV from dura. | 20 days |
Acknowledgement
This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants U01-NS052465, U01-NS052465-S1, R01-NS074387, and R01-NS057711 to M.G.C.; NIH/NINDS Grants R01-NS054193, R01-NS061107, R01-NS082311, and R21-NS084275 to P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH 2UL1-TR000433; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863. M.C. and M.A.M.A were supported by the Consejo Nacional de Ciencia y Tecnologia (CONICET PIP 114-201101-00353) and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT-2012-0830 and PICT-2013-0310).
Footnotes
Ensure that the Hamilton syringe and the needle are in good working condition prior to commencing the surgery. Only minimal adjustments should be done after positioning the animal.
It is imperative to apply paralube ointment to the eyes prior to surgery to prevent the eyes from drying out.
Care should be taken that the positioning of the animal into the stereotactic frame does not cause any respiratory distress. The breathing rate of the animal should be monitored throughout the surgical procedure.
To prevent the heat from the lamps causing burn injuries to the animals, maintain the lamps at a sufficient distance from the animals and only use them when using the microscope. At other times, the beam can be directed away from the animal.
Stop drilling when the base of the skull becomes translucent. Perforate the remaining thin layer of skull with a sterile needle and use a pair of curved forceps, carefully remove this layer of bone to expose the dura mater. The duramater (which is whitish, opalescent and elastic) and the brain surface (darker and yellowish in color) should be distinguished carefully to prevent lesioning of the brain surface with the needle.
Clear cerebrospinal fluid often leaks into the burr hole when the skull is perforated. Soak up this CSF with an ethanol swab to maintain visibility of the injection site.
It is recommended to load a small amount of extra volume of cells or adenoviral vectors into the Hamilton syringe to expel it onto an ethanol swab to ensure that the syringe is not clogged.
It is not practical to sterilize surgical instruments between every animal when multiple animals are to be injected in succession. In such a situation, thoroughly clean the apparatus including the drill bits and the Hamilton syringe with enzymatic cleaning solutions such as Endozyme followed by rinsing with 70% ethanol and finally sterile saline.
While cell suspension should not be kept on ice for more than 3-4 hours, vector solution can be kept on ice for several hours. It is not advisable to freeze thaw the vectors which leads to a loss in titer.
Ganciclovir when reconstituted in H2O can be stored at 4° C for up to 14 days and -20° C for up to 2 months.
To coat the Immobilon-P plates with the IFN-γ capture antibody, first wet the wells with 35 μl of 35 % ethanol. Immediately rinse 2 times with 100 μl of PBS before the ethanol evaporates. Pre-wetting the membrane with ethanol helps to improve the binding of the capture antibody to the membrane.
Once the plate has been wet, do not allow it to dry out. Always keep the wells wetted with PBS or media.
T cell activity peaks at approximately 7 days after the administration of gene therapy. Therefore 7-10 days post gene therapy is an appropriate time point to examine T cell activation induced by gene therapy.
Splenocytes can be frozen at this stage to continue the assay at a later time point. To freeze the cells, resuspend 50-70 × 106 cells in 1 ml of freezing media and store at -80° C overnight. Then transfer to liquid nitrogen for long-term storage. Splenocytes when frozen carefully can be stored for at least 4-6 weeks before the assay is run.
Fill the empty wells with 200 μl of PBS or media during the 48 hours incubation at 37° C. This will prevent unequal evaporation from different areas of the plate and reduce edge effect.
Filter the TMB substrate through a 0.45 micron syringe filter prior to adding it to the plate for color development. The spot development using TMB substrate is rapid and should be carefully monitored to prevent merging of individual spots.
Proteins in FBS can inhibit CFSE dye uptake by the cells. Therefore wash the cells 2 times with 1X PBS to remove proteins prior to staining with CFSE.
References
- 1.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Candolfi M, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85(2):133–48. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro MG, et al. Gene therapy and targeted toxins for glioma. Curr Gene Ther. 2011;11(3):155–80. doi: 10.2174/156652311795684722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King GD, et al. Gene therapy and targeted toxins for glioma. Curr Gene Ther. 2005;5(6):535–57. doi: 10.2174/156652305774964631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radaelli E, et al. Immunohistopathological and neuroimaging characterization of murine orthotopic xenograft models of glioblastoma multiforme recapitulating the most salient features of human disease. Histol Histopathol. 2009;24(7):879–91. doi: 10.14670/HH-24.879. [DOI] [PubMed] [Google Scholar]
- 6.Baker GJ, et al. Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia. 2014;16(7):543–61. doi: 10.1016/j.neo.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assi H, et al. Gene therapy for brain tumors: basic developments and clinical implementation. Neurosci Lett. 2012;527(2):71–7. doi: 10.1016/j.neulet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finsen B, Owens T. Innate immune responses in central nervous system inflammation. FEBS Lett. 2011;585(23):3806–12. doi: 10.1016/j.febslet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Fabry Z, et al. Sensing the microenvironment of the central nervous system: immune cells in the central nervous system and their pharmacological manipulation. Curr Opin Pharmacol. 2008;8(4):496–507. doi: 10.1016/j.coph.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122(4):1164–71. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali S, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtin JF, et al. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr Top Med Chem. 2005;5(12):1151–70. doi: 10.2174/156802605774370856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghulam Muhammad AK, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15(19):6113–27. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtin JF, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akli S, et al. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3(3):224–8. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- 16.Le Gal La Salle G, et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259(5097):988–90. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 17.Southgate T, et al. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci. 2008:Unit 4 23. doi: 10.1002/0471142301.ns0423s13. Chapter 4. [DOI] [PubMed] [Google Scholar]
- 18.Barcia C, et al. Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2006;2(4):309–22. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng P, Graham FL. Construction of first-generation adenoviral vectors. Methods Mol Med. 2002;69:389–414. doi: 10.1385/1-59259-141-8:389. [DOI] [PubMed] [Google Scholar]
- 20.Bett AJ, et al. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994;91(19):8802–6. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas CE, et al. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3(1):36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 22.Kreppel F, et al. Long-term transgene expression in the RPE after gene transfer with a high-capacity adenoviral vector. Invest Ophthalmol Vis Sci. 2002;43(6):1965–70. [PubMed] [Google Scholar]
- 23.King GD, et al. High-capacity adenovirus vector-mediated anti-glioma gene therapy in the presence of systemic antiadenovirus immunity. J Virol. 2008;82(9):4680–4. doi: 10.1128/JVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puntel M, et al. Gene transfer into rat brain using adenoviral vectors. Curr Protoc Neurosci. 2010:Unit 4 24. doi: 10.1002/0471142301.ns0424s50. Chapter 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glover CP, et al. Long-term transgene expression can be mediated in the brain by adenoviral vectors when powerful neuron-specific promoters are used. J Gene Med. 2003;5(7):554–9. doi: 10.1002/jgm.381. [DOI] [PubMed] [Google Scholar]
- 26.Candolfi M, et al. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011;13(10):947–60. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Candolfi M, et al. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin Cancer Res. 2014;20(6):1555–65. doi: 10.1158/1078-0432.CCR-13-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King GD, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther. 2011;19(10):1793–801. doi: 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mineharu Y, et al. Blockade of mTOR Signaling via Rapamycin Combined with Immunotherapy Augments Antiglioma Cytotoxic and Memory T-Cell Functions. Mol Cancer Ther. 2014;13(12):3024–36. doi: 10.1158/1535-7163.MCT-14-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mineharu Y, et al. Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res. 2011;17(14):4705–18. doi: 10.1158/1078-0432.CCR-11-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]