Abstract
A s an important class of non-coding regulatory RNA s, microRNA s (miRNAs) play a key role in a range of biological processes. These molecules serve as post-transcriptional regulators of gene expression and their regulatory activity has been implicated in disease pathophysiology and pharmacological traits. We sought to investigate the impact of miRNAs on cellular proliferation to gain insight into the molecular basis of complex traits that depend on cellular growth, including, most prominently, cancer. We examined the relationship between miRNA expression and intrinsic cellular growth (iGrowth) in the HapMap lymphoblastoid cell lines derived from individuals of different ethnic backgrounds. We found a substantial enrichment for miRNAs (53 miRNAs, FDR < 0.05) correlated with cellular proliferation in pooled CEU (Caucasian of northern and western European descent) and YRI (individuals from Ibadan, Nigeria) samples. Specifically, 119 miRNAs (59 %) were significantly correlated with iGrowth in YRI; of these miRNAs, 18 were correlated with iGrowth in CEU. To gain further insight into the effect of miRNAs on cellular proliferation in cancer, we showed that over-expression of miR-22, one of the top iGrowth-associated miRNAs, leads to growth inhibition in an ovarian cancer cell line (SKOV3). Furthermore, over-expression of miR-22 down-regulates the expression of its target genes (MXI1 and SLC25A37) in this ovarian cancer cell line, highlighting an miRNA-mediated regulatory network potentially important for cellular proliferation. Importantly, our study identified miRNAs that can be used as molecular targets in cancer therapy.
Introduction
Cellular growth is fundamental to cell biology. Thus, a greater understanding of contributing factors to this trait can improve our understanding of biological phenomena, and as a translational application, enable the development of therapeutic strategies against pathological conditions (e.g., cancers and aging).
It has been shown that cellular growth is a heritable trait and genetic variation is likely to contribute to phenotypic variability (Stark et al. 2010). Many genes are known to affect cellular growth, including those controlling cell cycles (Schafer 1998; Whitfield et al. 2006). More recently, micro-RNA s (miRNAs), a class of small non-coding regulatory RNA s, have been implicated in a wide range of biological processes (Bartel 2009; Suzuki and Miyazono 2011). They also play a key role in tumorigenesis and tumor suppression in cancers (Hwang and Mendell 2006). Thus, we hypothesized that miRNAs might be the important regulators of cellular proliferation and set out to perform a genome-wide study of the impact of miRNA expression on cellular growth.
For genome-wide discovery, we utilized a set of the International HapMap lymphoblastoid cell lines (LCLs). These HapMap LCLs have extensive publicly available genomic information (Frazer et al. 2007; The 1000 Genomes Project Consortium 2010), as well as other omics data (Duan et al. 2008; Stranger et al. 2007; Pickrell et al. 2010). More recently, we have generated whole-genome miRNA expression data on a subset of the HapMap samples (Gamazon et al. 2012) (GEO accession number GSE34406). The depth of this publicly available information makes HapMap LCLs an unparalleled resource to conduct “omics” research in human complex traits. In this study, we performed genome-wide analysis using miRNA expression, mRNA expression and cellular growth as a phenotype. We then evaluated whether the miRNA /gene(s) identified in the HapMap cell lines also affect cellular growth in an ovarian cancer cell line.
Results
Intrinsic cellular growth (iGrowth) associated miRNA expression
We have previously generated an intrinsic cellular growth (iGrowth) phenotype for over 500 HapMap LCLs using a mixed effect model averaging (MEM) method to control for various environmental conditions [e.g., passage of cells, time of experiment, different experimental conditions (batch of serum/media)] (Im et al. 2012).
To detect significant correlations between iGrowth and genome-wide miRNA expression, we performed a pooled analysis of the combined CEU (Centre d’Etude du Polymorphisme Humain (CEPH) people from Utah, USA) and YRI (Yoruba people from Ibadan, Nigeria) samples (see “Materials and methods”). As a result, we found 53 miRNAs that were significantly correlated with iGrowth (false discovery rate [FDR] <0.05, listed in Table 1). Among them, 14 (nearly 7 %) showed FDR < 0.001.
Table 1.
miRNA whose expression levels are significantly correlated with iGrowth
| miRNA | iGrowth correlation p value | |||
|---|---|---|---|---|
| Pooled analysis | YRI | CEU | ||
| Raw p value | FDR | |||
| hsa-miR-210 | 3.51E−09 | 7.05E−07 | 1.41E−06 | 4.31E−04 |
| hsa-miR-768-5p | 1.39E−07 | 1.40E−05 | 6.51E−06 | 2.44E−03 |
| hsa-miR-18a | 2.55E−07 | 1.71E−05 | 1.53E−06 | 9.21E−03 |
| hsa-miR-106a | 8.59E−07 | 4.32E−05 | 1.75E−08 | 1.75E−01 |
| hsa-miR-22 | 1.70E−06 | 6.13E−05 | 9.83E−08 | 6.73E−02 |
| hsa-miR-18b | 1.83E−06 | 6.13E−05 | 2.48E−06 | 3.72E−02 |
| hsa-miR-17 | 2.81E−06 | 8.07E−05 | 5.58E−08 | 2.37E−01 |
| hsa-miR-765 | 4.18E−06 | 1.05E−04 | 4.72E−05 | 3.22E−02 |
| hsa-miR-20a | 1.04E−05 | 2.30E−04 | 3.93E−07 | 1.53E−01 |
| hsa-miR-20b | 1.14E−05 | 2.30E−04 | 6.86E−06 | 1.42E−01 |
| hsa-miR-1 | 1.32E−05 | 2.41E−04 | 1.91E−05 | 1.16E−01 |
| hsa-miR-148b | 3.81E−05 | 6.39E−04 | 4.66E−03 | 2.93E−03 |
| hsa-miR-130b | 5.71E−05 | 8.82E−04 | 1.17E−04 | 9.05E−02 |
| hsa-miR-600 | 8.09E−05 | 1.16E−03 | 1.05E−02 | 1.83E−03 |
| hsa-miR-768-3p | 1.10E−04 | 1.41E−03 | 5.80E−04 | 3.62E−02 |
| hsa-miR-518a-5p/hsa-miR-527 | 1.14E−04 | 1.41E−03 | 4.16E−04 | 4.66E−02 |
| hsa-miR-17* | 1.19E−04 | 1.41E−03 | 1.92E−07 | 9.62E−01 |
| hsa-miR-198 | 1.38E−04 | 1.54E−03 | 1.77E−04 | 1.21E−01 |
| hsa-miR-92a | 2.29E−04 | 2.32E−03 | 3.29E−07 | 7.28E−01 |
| hsa-miR-30b* | 2.31E−04 | 2.32E−03 | 9.91E−05 | 2.13E−01 |
| hsa-miR-33a | 2.62E−04 | 2.50E−03 | 4.63E−06 | 7.53E−01 |
| hsa-miR-103 | 3.20E−04 | 2.92E−03 | 5.85E−06 | 8.42E−01 |
| hsa-miR-361-3p | 3.49E−04 | 3.05E−03 | 2.95E−04 | 1.46E−01 |
| hsa-miR-196a* | 3.83E−04 | 3.20E−03 | 3.53E−02 | 4.78E−03 |
| hsa-miR-19a | 6.46E−04 | 5.19E−03 | 1.51E−06 | 3.43E−01 |
| hsa-miR-516a-5p | 6.90E−04 | 5.26E−03 | 7.53E−05 | 3.21E−01 |
| hsa-miR-365 | 7.06E−04 | 5.26E−03 | 2.64E−02 | 1.01E−02 |
| hsa-miR-19b | 1.07E−03 | 7.71E−03 | 1.35E−05 | 2.73E−01 |
| hsa-miR-519e* | 1.12E−03 | 7.80E−03 | 4.90E−04 | 2.69E−01 |
| hsa-miR-921 | 1.22E−03 | 8.03E−03 | 5.80E−04 | 2.99E−01 |
| hsa-miR-146a | 1.24E−03 | 8.03E−03 | 4.53E−04 | 3.38E−01 |
| hsa-miR-193b | 1.33E−03 | 8.36E−03 | 2.18E−02 | 2.72E−02 |
| hsa-miR-185* | 1.55E−03 | 9.21E−03 | 3.98E−02 | 6.89E−03 |
| hsa-miR-301a | 1.59E−03 | 9.21E−03 | 2.21E−03 | 2.30E−01 |
| hsa-miR-193b* | 1.60E−03 | 9.21E−03 | 2.08E−03 | 1.70E−01 |
| hsa-miR-518c* | 1.79E−03 | 9.86E−03 | 1.56E−04 | 5.03E−01 |
| hsa-miR-34a | 1.81E−03 | 9.86E−03 | 8.21E−02 | 8.00E−03 |
| hsa-miR-106b | 2.54E−03 | 1.34E−02 | 7.70E−04 | 3.76E−01 |
| hsa-miR-223 | 2.73E−03 | 1.41E−02 | 1.89E−02 | 6.38E−02 |
| hsa-miR-146b-5p | 2.94E−03 | 1.46E−02 | 1.98E−03 | 3.24E−01 |
| hsa-miR-93 | 2.99E−03 | 1.46E−02 | 2.94E−04 | 6.92E−01 |
| hsa-miR-483-5p | 3.41E−03 | 1.63E−02 | 6.70E−05 | 4.87E−01 |
| hsa-miR-132 | 4.99E−03 | 2.33E−02 | 1.24E−02 | 2.15E−01 |
| hsa-miR-423-3p | 5.38E−03 | 2.46E−02 | 5.03E−05 | 9.82E−01 |
| hsa-miR-342-3p | 6.00E−03 | 2.68E−02 | 3.93E−04 | 6.91E−01 |
| hsa-miR-30c-2* | 7.80E−03 | 3.41E−02 | 1.47E−03 | 6.84E−01 |
| hsa-miR-675 | 8.37E−03 | 3.58E−02 | 4.12E−03 | 4.20E−01 |
| hsa-miR-637 | 8.89E−03 | 3.65E−02 | 5.49E−01 | 5.75E−03 |
| hsa-let-7d | 8.90E−03 | 3.65E−02 | 1.20E−01 | 2.83E−02 |
| hsa-miR-129* | 9.51E−03 | 3.82E−02 | 6.01E−02 | 7.92E−02 |
| hsa-miR-583 | 1.11E−02 | 4.36E−02 | 8.03E−04 | 9.82E−01 |
| miRPlus_17952 | 1.18E−02 | 4.58E−02 | 7.13E−04 | 8.52E−01 |
| hsa-miR-148a | 1.30E−02 | 4.94E−02 | 4.31E−04 | 7.58E−01 |
Only those miRNAs whose expression are correlated with iGrowth in pooled CEU and YRI samples are shown here (FDR < 0.05). miRNAs in bold have iGrowth correlation in both CEU and YRI samples with p value <0.05
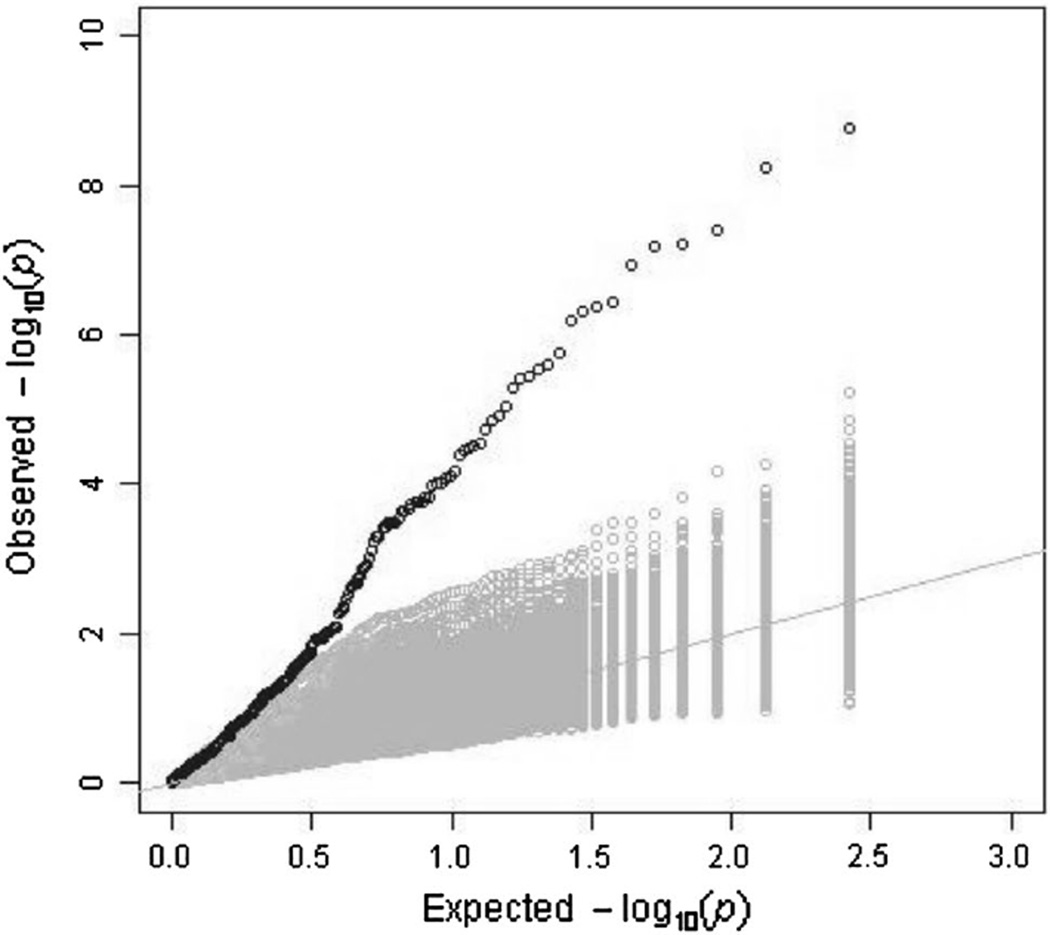
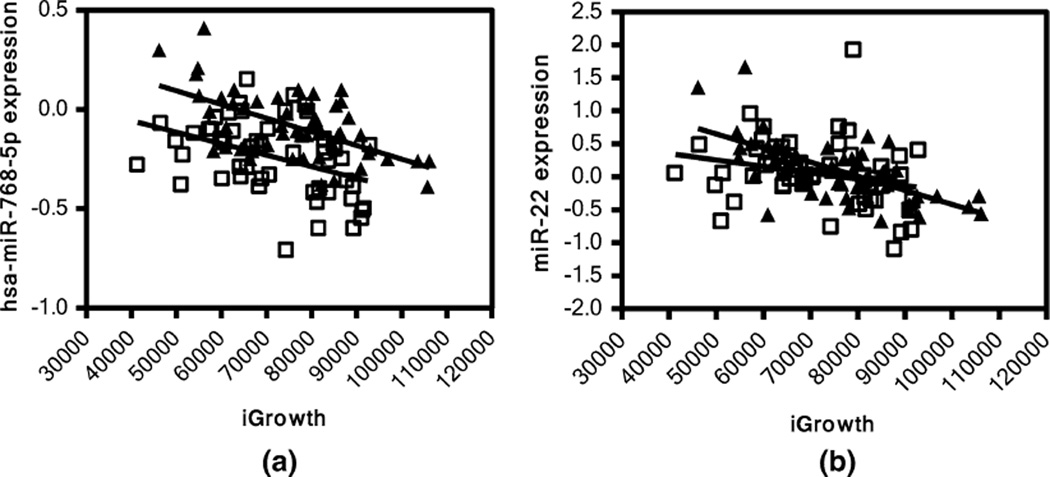
In addition, we performed linear regression analysis between iGrowth and genome-wide miRNA expression in the YRI and CEU samples separately. A Q–Q plot shows a substantial enrichment for significant associations with iGrowth among the miRNAs in the YRI samples (Fig. 1). Indeed, 119 miRNAs were found to be significantly correlated with iGrowth in these samples (FDR < 0.05). Eighteen of these 119 miRNAs were also correlated with iGrowth in CEU samples (Supplementary Table 1). Among 18 miRNAs, 15 miRNAs were also significantly correlated with iGrowth in the pooled analysis. Interestingly, eight (miR-185*, miR-18b, miR-365, miR-600, miR-768-3p, miR-768-5p, miR-939 and miRPlus_42521) of the 18 miRNAs with significant correlation with iGrowth in both populations are differentially expressed between the two panels (p < 0.05). An example is shown in Fig. 2a. Here, miR-768-5p is correlated with iGrowth in both CEU and YRI samples (p = 0.0024 and p < 0.0001 in CEU and YRI, respectively); however, higher miR-768-5p expression was observed in YRI relative to CEU (p < 0.05). Furthermore, we identified other patterns of miRNA-iGrowth relationship. For example, miR-22 expression was found to be significantly and negatively correlated with iGrowth in YRI samples (p < 0.0001) but showed only modest correlation with iGrowth in CEU (p = 0.067) (Fig. 2b).
Fig. 1.
Q–Q plot of iGrowth-associated miRNAs in YRI samples. We observed an excess of significant correlations of miRNAs with iGrowth in YRI. The Q–Q plot, in black, shows the observed distribution of p values from the correlation between miRNA and iGrowth. We also generated permuted datasets (n = 1000) by shuffling the iGrowth phenotype while preserving the miRNA correlation structure. The Q–Q plots, in gray, show the distributions of p values from the correlation between miRNA and iGrowth in the permuted datasets
Fig. 2.
Pattern of correlation between miRNA and iGrowth in CEU and YRI. a miR-768-5p is significantly correlated with iGrowth in both CEU (p = 0.0024) and YRI (p < 0.0001) samples; b miR-22 is significantly correlated with iGrowth in YRI (p < 0.0001) but only modestly in CEU (p = 0.0673). Triangle represents YRI samples and square represents CEU samples
Biological targets of miRNA involved in cellular growth
To explore the underlying mechanism for the observed miRNA and cellular growth associations, we further examined the relationships between iGrowth-associated mRNA s (reported previously in Im et al. 2012) and iGrowth-associated miRNAs identified in the pooled analysis. Of the 53 miRNAs associated with iGrowth in the pooled CEU and YRI samples (FDR < 0.05), 22 miRNAs are negatively correlated with one of 105 growth-associated mRNA s (p < 0.0001, FDR < 0.05) in CEU and 42 miRNAs are negatively correlated with one of 211 growth-associated mRNA s (p < 0.0001, FDR < 0.05) in YRI. These iGrowth-associated mRNA s were evaluated using Database for Annotation, Visualization and Integrated Discovery (DAV ID) for gene ontology (GO) annotations (Huang et al. 2009). GO analysis yielded various biological processes, molecular functioning and cellular component pathways listed in Supplementary Table 2 (p < 0.05). Not surprisingly, among them were the regulation of cellular proliferation and regulation of cellular growth pathways (p = 0.0029 and p = 0.0035, respectively).
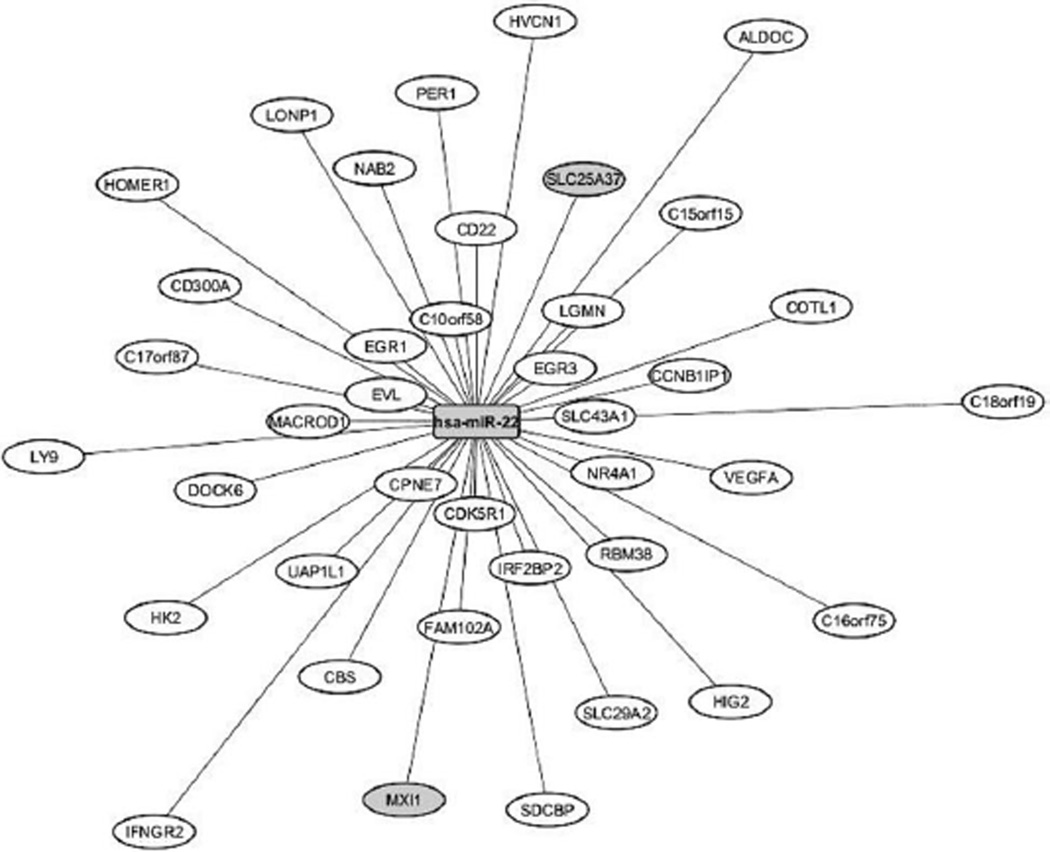
Among the top iGrowth-correlated miRNAs, miR-22 was negatively correlated with the expression levels of 39 genes in the YRI samples (p < 0.0001, FDR < 0.05, Fig. 3). Notably, the expression of miR-22 and all 39 genes was correlated with iGrowth in these samples. Two gene/miR-22 relationships [Max-interacting protein (MXI1) and Solute carrier family 25, member 37 (SLC25A37)] are also supported by the miRbase miRanda prediction algorithm.
Fig. 3.
Negative expression correlation of miR-22 and 39 genes. The expression levels of all genes are correlated with iGrowth in YRI samples. miR-22 ranked 5th in association with iGrowth in pooled analysis of CEU and YRI (p = 1.7 × 10−6). The distance between the central node, miR-22, and the other nodes (comprising of the 39 genes) reflects the p value of the expression correlation between miRNA and gene: the shorter the distance, the more significant is the miR-gene expression correlation. The graph was generated using the cytoscape software
Evaluation of the function of miR-22 in an ovarian cancer cell line
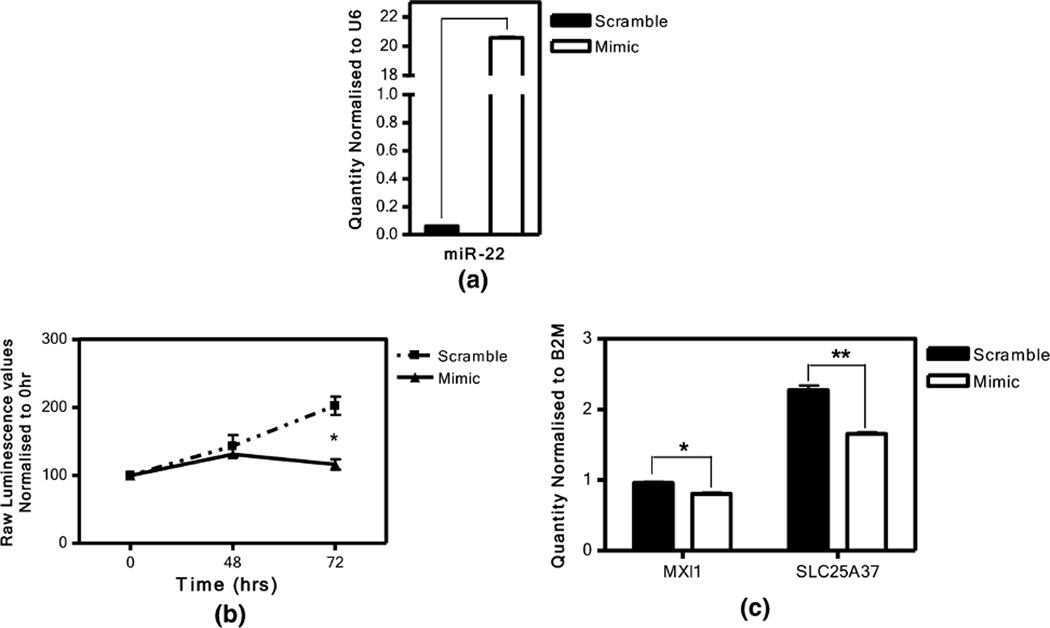
Given that miR-22 was among the very top iGrowth-correlated miRNAs (i.e., 5th) and was negatively correlated with 39 gene expression phenotypes, we performed additional studies on the function of this miRNA in cancer. We conducted miR-22 over-expression experiment in an ovarian cancer cell line (SKOV3). As expected, the transfection of miR-22 mimic (over-expression) resulted in significantly increased expression of miR-22 (compared to control, t test p = 2.4 × 10−05, Fig. 4a) at 24 h post-transfection. Subsequently, we observed a significant inhibition in cellular growth, measured by cellular AT P levels (see “Materials and methods”), 72 h after over-expressing miR-22 when compared to that of scramble control (p = 0.0014, Fig. 4b). Over-expression of miR-22 also resulted in significant decrease in expression levels of MXI1 and SLC25A37 (negatively correlated and predicted by miRbase miRanda prediction algorithm) in this cancer cell line (p = 0.003 and p = 0.001, respectively, Fig. 4c).
Fig. 4.
The effect of miR-22 over-expression in SKOV3 cell line. a Relative miR-22 expression level 24 h post-transfection. RNU6 was used as housekeeping control. The transfection of miR-22 mimic (over-expression) resulted in increased expression of miR-22 at 24 h post-transfection in comparison to that of transfected scramble control (t test p = 2.4 × 10−05). b Significant inhibition of cell growth observed in SKOV3 at 72 h post-transfection of miR-22 mimic (t test p = 0.0014). Cellular growth was determined using CellTiter Glo assay. c Relative MXI1 and SLC25A37 expression levels 24 h post-addition of miR-22 mimic (p = 0.003 and p = 0.001, respectively). B2M was used as housekeeping control for PCR. “mimic” represents over-expression while “scramble” represents the control experiment
Discussion
Through a genome-wide analysis, we found a set of miRNAs whose expression correlated with intrinsic cellular growth in LCLs. Furthermore, as a proof of concept, we demonstrated that over-expression of one of the top cell growth-associated miRNAs can significantly inhibit the proliferation of ovarian cancer cells.
From the pooled analysis and the single-population analyses, we identified 15 miRNAs whose expression levels are correlated with iGrowth in both CEU and YRI samples. Eight of these miRNAs have been previously implicated in cellular proliferation and cancer-related pathways. For example, miR-210 was found to be over-expressed in late stages of lung cancer (Puisségur et al. 2011), and disturbed mitotic progression (He et al. 2012). miR-148b was previously reported to be a tumor suppressor in gastric cancer (Song et al. 2011a), colorectal cancer (Song et al. 2011b), oral squamous cell carcinoma (Yu et al. 2009), as was miR-185 (Imam et al. 2010). miR-365 regulates lung cancer through translational repression of TTF1 (Thyroid transcription factor 1) (Qi et al. 2012). When down-regulated, miR-193b was shown to contribute to the development of T-cell lymphoblastic lymphoma (González-Gugel et al. 2013) and tumor progression in human breast cancer (Li et al. 2009). Let-7d was reported to inhibit neural stem cell proliferation in mammalian brains (Zhao et al. 2013) and involved in regulation of PBX3 (pre-leukemia transcription factor 3) expression in prostate cancer cell lines (Ramberg et al. 2011). miR-34a was involved in inhibition of prostate cancer cell growth (Kashat et al. 2012). miR-34a was also regarded as tumor suppressor in hepatocellular carcinoma (Dang et al. 2013) and regulator of growth factor signaling (Lal et al. 2011). Finally, miR-18b was found to be up-regulated in four out of five breast cancer cell lines, and ectopic inhibition of miR-18b suppressed the migration of two breast cancer cell models in vitro (Fonseca-Sanchéz et al. 2013). Our observation on the effect of the remaining 7 miRNAs on cellular proliferation is, to our knowledge, a novel finding. We note that, for each of the miRNAs, expression is correlated with cellular proliferation in two independent sample sets.
Among the miRNAs with significant correlation with cellular proliferation in the pooled analysis, miR-22 has been found to play a key role in a number of biological pathways. For example, miR-22 was reported to directly target estrogen alpha (Pandey and Picard 2009; Xiong et al. 2010a), MYCBP (Xiong et al. 2010b), PTEN (Bar and Dikstein 2010; Liu et al. 2010) and HDAC4 (Huang et al. 2013). It also affects MyC (Xiong et al. 2010b), p53-dependent apoptosis (Tsuchiya et al. 2011), NFkB (Takata et al. 2011), Wnt signaling (Kaur et al. 2011), AKT (Bar and Dikstein 2010), and PKC/ERK (Ting et al. 2010) pathways. Because of its extensive influence on these pathways, miR-22 has been reported to play a role in cellular senescence induction (Xu et al. 2011), migration (Liu et al. 2010; Xu et al. 2011; Li et al. 2010), angiogenic (Yamakuchi et al. 2011) and cell cycle arrest (Ting et al. 2010) in various cancer cell lines. Moreover, miR-22 expression has been shown to be associated with radioresistance (Zheng et al. 2011). In an ovarian cancer cell line, miR-22 was previously identified as a potential metastasis inhibitor (Li et al. 2010). Previous studies have also showed that ectopic expression of miR-22 significantly inhibits cell proliferation and tumorigenicity in hepatocellular carcinoma (Zhang et al. 2010). Our study confirmed a similar effect of miR-22 on ovarian cancer cell proliferation, with over-expression of miR-22 resulting in growth inhibition.
miRNAs are known to regulate gene expression at the post-transcriptional and translational stage (Morris et al. 2004). Based on DAV ID gene ontology, functional enrichment analysis of the genes (negatively correlated to miRNA from pooled analysis) revealed their involvement in pathways regulating cellular proliferation and cellular growth. The analysis also revealed that they were mostly present in cytosol involved in protein binding. Among the relationships of iGrowth-associated miRNA and iGrowth- associated mRNA, we found that 39 genes were negatively correlated with miR-22, suggesting a potential miR-22 regulated gene network that may mediate the cellular growth phenotype. In an ovarian cancer cell line, we showed that overexpression of miR-22 resulted in decreased gene expression level for putative targets MXI1 and SLC25A37, which may suggest a biological mechanism underlying the miR-22 and iGrowth correlation. MXI1 forms a heterodimer with Max, similar to that of Myc-Max (Zervos et al. 1993), which is involved in controlling cell proliferation and tumorigenesis in different biological contexts (Hurlin and Dezfouli 2004). SLC25A37 is a solute carrier that transports iron into the mitochondria of erythroid cells for the synthesis of heme and iron sulfur clusters (Chen et al. 2009). It has been reported that over-expression of SLC25A37 causes cancer-related fatigue in patients with non-metastatic prostate cancer during external beam radiation therapy (Hsiao et al. 2013). It is important to note that although we showed that over-expression of miR-22 leads to decreased expression of MXI1 and SLC25A37, we did not perform the same functional study on the expression of the remaining 37 (potential target) genes, some of which are supported by previous studies such as the reduction of tumor cell proliferation when Vascular Endothelial Growth Factor A (VEGFA) was targeted in mouse model with ovarian granulosa cell tumor (Tsoi et al. 2013). Additional studies of miR-22 and its target genes in cancers and other pathological contexts are warranted.
In summary, our genome-wide study identified and replicated several miRNAs with significant effect on cellular proliferation. We performed additional functional studies to confirm the effect of miR-22 on cellular growth in LCLs and in an ovarian cancer cell line. These miRNAs are great candidates for future investigations of growth-mediated phenotypes and may be potential targets for cancer therapeutics.
Materials and methods
Cell lines
EBV-transformed B-LCLs from the International HapMap consortium were purchased from the Coriell Institute for Medical Research (Camden, NJ, USA). Fifty-three unrelated CEU and 54 unrelated YRI samples were used for this study. These LCLs were maintained as suspension cultures in RPMI 1640 with supplements described previously (Huang et al. 2007). For functional studies, SKOV3, an ovarian cancer cell line, was procured from AT CC (Manassas, VA, USA) and grown as an adherent culture in McCoy’s 5A medium with 10 % fetal bovine serum (Atlanta Biologicals, GA).
Intrinsic cellular growth rate and genomic, transcriptomic, miRNA expression information
Utilizing a mixed effects model averaging (MEM) method, our group has previously generated an intrinsic cellular growth (iGrowth) phenotype in over 500 HapMap LCLs (Im et al. 2012). This method allowed us to pool data from multiple replicated measurements over a 5-year time period and obtain an intrinsic cell growth phenotype after controlling for various environmental conditions [e.g., passage of cells, time of experiment, different experimental conditions (batch of serum/media)]. The iGrowth for the CEU and YRI samples was used in this study.
We performed genome-wide miRNA expression profiling, as previously reported (Gamazon et al. 2012), using the Exiqon miRCURY LNA arrays v.10.0 (Exiqon array) on 107 LCLs (53 CEU I and 54 YRI I). These data have been deposited into GEO (GSE34406). The baseline genome-wide gene expression data in CEU and YRI were quantified using the Affymetrix GeneChip Human Exon 1.0 ST array (Affymetrix exon array, GEO access #: GSE7761) (Zhang et al. 2008). In the present study, we evaluated the set of genes previously shown to be correlated with iGrowth (Im et al. 2012).
iGrowth-associated miRNA expression
Linear regression analysis was performed between 201 miRNAs that are reliably expressed in LCLs and iGrowth in the HapMap CEU and YRI samples jointly and separately using an R package (lm). In a pooled analysis using the combined CEU and YRI samples, we performed linear regression of miRNA expression against iGrowth with the ancestral group as covariate. For multiple testing adjustment, we used an FDR approach (Storey and Tibshirani 2003). FDR < 0.05 was used as the threshold for significance.
We performed permutation analysis to assess the enrichment of miRNAs significantly correlated with iGrowth. In this analysis, we permuted the iGrowth trait values (n = 1000 permuted datasets) while preserving the correlation structure of the miRNAs. Correlation analysis was performed on each permuted dataset and the distribution of p values for each permuted dataset was plotted in a Q–Q plot along with the observed distribution of p values.
We have reported 2983 mRNA s that are correlated with iGrowth in these cell lines previously. In this study, to explore the potential mechanism underlying the observed miRNA and iGrowth relationships, we further examined the negative correlation between iGrowth-associated miRNAs and mRNA s; p < 0.0001 was used as a cutoff, which corresponds to FDR < 0.05. Gene ontology analysis was performed using the functional annotation tool in DAV ID Bioinformatics resources v6.7 (Huang et al. 2009).
Functional validation
For the functional validation of iGrowth-associated miRNA expression, miR-22 over-expression was performed in SKOV3, an ovarian cancer cell line. miR-22 mimic and scrambled control (AllStars negative control siRNA), purchased from Qiagen, were independently transfected into SKOV3 using DharmaFECT 1 (Thermo scientific) according to Thermoscientific DharmaFECT siRNA transfection protocol for 6 h. The cellular growth rate was measured using CellTiter-GLo luminescent cell viability assay (QIAGEN) at 0, 48 and 72 h post-transfection. Student t test was performed to compare cellular growth rate obtained at 72 h post-transfection between miR-22 over-expressed samples and control with scramble treatment. p < 0.05 was considered statistically significant. The over-expression was confirmed by performing quantitative polymerase chain reaction (qPCR) on 24 h post-miR-22 transfection samples. Further, the gene expression levels of MXI1 and SLC25A37 were quantified through qPCR 24 h post-transfection using TaqMan® assays (Hs00365651_m1 and Hs00249769_m1, respectively, Life Technologies).
Supplementary Material
Acknowledgments
We are grateful for the excellent technical support provided by Pharmacogenomics of Anti-cancer Agent Research (PAAR) group cell line core. This study was supported by National Institute of Health/National Cancer Institute grant (R21 CA139278) and by National Institute of Health/National Institute of General Medical Sciences grant (UO1GM61393). RSH also received support from National Institute of Health/National Institute of General Medical Sciences grant (K08GM089941), Circle of Service Foundation Early Career Investigator award, University of Chicago Cancer Center Support Grant (#P30 CA14599), Breast Cancer SPORE Career Development Award (CA125183) a Conquer Cancer Foundation of ASCO Translational Research Professorship award In Memory of Merrill J. Egorin, MD, and pilot grant from National Institute of Health/National Center Advancing Translational Sciences grant (UL1RR024999).
Abbreviations
- miRNA
microRNA
- iGrowth
Intrinsic cellular growth
- LCLs
Lymphoblastoid cell lines
- CEU
Centre d’Etude du Polymorphisme Humain (CEPH) people from Utah, USA
- YRI
Yoruba people from Ibadan, Nigeria
- MEM
Mixed effects model averaging
- FDR
False discovery rate
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- GO
Gene ontology
- DOHH
Deoxyhypusine hydroxylase
Footnotes
Electronic supplementary material T he online version of this article (doi:10.1007/s00439-014-1434-4) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Divya Lenkala, Section of Hematology/Oncology, Department of Medicine, The University of Chicago, 900 E 57th Street, KCBD, Chicago, IL 60637, USA.
Bonnie LaCroix, Section of Hematology/Oncology, Department of Medicine, The University of Chicago, 900 E 57th Street, KCBD, Chicago, IL 60637, USA.
Eric R. Gamazon, Section of Genetic Medicine, Department of Medicine, The University of Chicago, Chicago, IL 60637, USA
Paul Geeleher, Section of Hematology/Oncology, Department of Medicine, The University of Chicago, 900 E 57th Street, KCBD, Chicago, IL 60637, USA.
Hae Kyung Im, Department of Health Studies, The University of Chicago, Chicago, IL 60637, USA.
R. Stephanie Huang, Email: rhuang@medicine.bsd.uchicago.edu, Section of Hematology/Oncology, Department of Medicine, The University of Chicago, 900 E 57th Street, KCBD, Chicago, IL 60637, USA.
References
- Bar N, Dikstein R. miR-22 forms a regulatory loop in PTEN/AKT pathway and modulates signaling kinetics. PLoS One. 2010;5:e10859. doi: 10.1371/journal.pone.0010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J, et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci USA. 2009;106:16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Luo D, Rong M, Chen G. Underexpression of miR-34a in hepatocellular carcinoma and its contribution towards enhancement of proliferating inhibitory effects of agents targeting c-MET. PLoS One. 2013;8:e61054. doi: 10.1371/journal.pone.0061054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Huang RS, Zhang W, Bleibel WK, Roe CA, Clark TA, Chen TX, Schweitzer AC, Blume JE, Cox NJ, et al. Genetic architecture of transcript-level variation in humans. Am J Hum Genet. 2008;82:1101–1113. doi: 10.1016/j.ajhg.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, et al. A second generation **human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Sanchéz MA, Pérez-Plasencia C, Fernández-Retana J, Arechaga-Ocampo E, Marchat LA, Rodríguez-Cuevas S, Bautista-Piña V, Arellano-Anaya ZE, Flores-Pérez A, Diaz-Chávez J, et al. microRNA-18b is upregulated in breast cancer and modulates genes involved in cell migration. Oncol Rep. 2013;30:2399–2410. doi: 10.3892/or.2013.2691. [DOI] [PubMed] [Google Scholar]
- Gamazon ER, Ziliak D, Im HK, LaCroix B, Park DS, Cox NJ, Huang RS. Genetic architecture of microRNA expression: implications for the transcriptome and complex traits. Am J Hum Genet. 2012;90:1046–1063. doi: 10.1016/j.ajhg.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gugel E, Villa-Morales M, Santos J, Bueno MJ, Malumbres M, Rodríguez-Pinilla SM, Piris MÁ, Fernández-Piqueras J. Down-regulation of specific miRNAs enhances the expression of the gene smoothened and contributes to T-cell lymphoblastic lymphoma development. Carcinogenesis. 2013;34(4):902–908. doi: 10.1093/carcin/bgs404. [DOI] [PubMed] [Google Scholar]
- He J, Wu J, Xu N, Xie W, Li M, Li J, Jiang Y, Yang BB, Zhang Y. MiR-210 disturbs mitotic progression through regulating a group of mitosis-related genes. Nucleic Acids Res. 2012;41(1):498– 508. doi: 10.1093/nar/gks995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C-P, Wang D, Kaushal A, Saligan L. Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nurs. 2013;36(3):189–197. doi: 10.1097/NCC.0b013e318263f514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RS, Kistner EO, Bleibel WK, Shukla SJ, Dolan ME. Effect of population and gender on chemotherapeutic agent-induced cytotoxicity. Mol Cancer Ther. 2007;6:31–36. doi: 10.1158/1535-7163.MCT-06-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ, Dezfouli S. Functions of myc:max in the control of cell proliferation and tumorigenesis. Int Rev Cytol. 2004;238:183–226. doi: 10.1016/S0074-7696(04)38004-6. [DOI] [PubMed] [Google Scholar]
- Huang Z-P, Chen J, Seok HY, Zhang Z, Kataoka M, Hu X, Wang D-Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ Res. 2013;112:1234–1243. doi: 10.1161/CIRCRESAHA.112.300682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAV ID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hwang H-W, Mendell JT. MicroRNA s in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HK, Gamazon ER, Stark AL, Huang RS, Cox NJ, Dolan ME. Mixed effects modeling of proliferation rates in cell-based models: consequence for pharmacogenomics and cancer. PLoS Genet. 2012;8:e1002525. doi: 10.1371/journal.pgen.1002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam JS, Buddavarapu K, Lee-Chang JS, Ganapathy S, Camosy C, Chen Y, Rao MK. MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene. 2010;29:4971–4979. doi: 10.1038/onc.2010.233. [DOI] [PubMed] [Google Scholar]
- Kashat M, Azzouz L, Sarkar SH, Kong D, Li Y, Sarkar FH. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res. 2012;4:432–442. [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Pandey AK, Srivastava S, Srivastava AK, Datta M. Comprehensive miRNome and in silico analyses identify the Wnt signaling pathway to be altered in the diabetic liver. Mol Biosyst. 2011;7:3234–3244. doi: 10.1039/c1mb05041a. [DOI] [PubMed] [Google Scholar]
- Lal A, Thomas MP, Altschuler G, Navarro F, O’Day E, Li XL, Concepcion C, Han Y-C, Thiery J, Rajani DK, et al. Capture of microRNA-bound mRNA s identifies the tumor suppressor miR-34a as a regulator of growth factor signaling. PLoS Genet. 2011;7:e1002363. doi: 10.1371/journal.pgen.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-F, Yan P-J, Shao Z-M. Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene. 2009;28:3937–3948. doi: 10.1038/onc.2009.245. [DOI] [PubMed] [Google Scholar]
- Li J, Liang S, Yu H, Zhang J, Ma D, Lu X. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol. 2010;119:543–548. doi: 10.1016/j.ygyno.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Liu L, Jiang Y, Zhang H, Greenlee AR, Yu R, Yang Q. miR-22 functions as a micro-oncogene in transformed human bronchial epithelial cells induced by anti-benzo[a]pyrene-7,8-diol-9,10-epoxide. Toxicol In Vitr. 2010;24:1168–1175. doi: 10.1016/j.tiv.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Morris KV, Chan SW-L, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- Pandey DP, Picard D. miR-22 inhibits estrogen signaling by directly targeting the estrogen receptor α mRNA. Mol Cell Biol. 2009;29:3783–3790. doi: 10.1128/MCB.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell JK, Marioni JC, Pai AA, Degner JF, Engelhardt BE, Nkadori E, Veyrieras J-B, Stephens M, Gilad Y, Pritchard JK. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature. 2010;464:768–772. doi: 10.1038/nature08872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisségur M-P, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–478. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Rice SJ, Salzberg AC, Runkle EA, Liao J, Zander DS, Mu D. MiR-365 regulates lung cancer and developmental gene thyroid transcription factor 1. Cell Cycle. 2012;11:177–186. doi: 10.4161/cc.11.1.18576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramberg H, Alshbib A, Berge V, Svindland A, Taskén KA. Regulation of PBX3 expression by androgen and Let-7d in prostate cancer. Mol Cancer. 2011;10:50. doi: 10.1186/1476-4598-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35:461–478. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- Song Y-X, Yue Z-Y, Wang Z-N, Xu Y-Y, Luo Y, Xu H-M, Zhang X, Jiang L, Xing C-Z, Zhang Y. MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol Cancer. 2011a;10:1. doi: 10.1186/1476-4598-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Xu Y, Wang Z, Chen Y, Yue Z, Gao P, Xing C, Xu H. MicroRNA-148b suppresses cell growth by targeting cholecystokinin-2 receptor in colorectal cancer. Int J Cancer. 2011b;1051:1042–1051. doi: 10.1002/ijc.26485. [DOI] [PubMed] [Google Scholar]
- Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, Ingle CE, Dunning M, Flicek P, Koller D, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AL, Zhang W, Mi S, Duan S, O’Donnell PH, Huang RS, Dolan ME. Heritable and non-genetic factors as variables of pharmacologic phenotypes in lymphoblastoid cell lines. Pharmacogenomics J. 2010;10:505–512. doi: 10.1038/tpj.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genome-wide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki HI, Miyazono K. Emerging complexity of microRNA generation cascades. J Biochem. 2011;149:15–25. doi: 10.1093/jb/mvq113. [DOI] [PubMed] [Google Scholar]
- Takata A, Otsuka M, Kojima K, Yoshikawa T, Kishikawa T, Yoshida H, Koike K. MicroRNA-22 and microRNA-140 suppress NF-κB activity by regulating the expression of NF-κB coactivators. Biochem Biophys Res Commun. 2011;411:826–831. doi: 10.1016/j.bbrc.2011.07.048. [DOI] [PubMed] [Google Scholar]
- The 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting Y, Medina DJ, Strair RK, Schaar DG. Differentiation-associated miR-22 represses Max expression and inhibits cell cycle progression. Biochem Biophys Res Commun. 2010;394:606–611. doi: 10.1016/j.bbrc.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Tsoi M, Laguë M-N, Boyer A, Paquet M, Nadeau M-È, Boerboom D. Anti-VEGFA therapy reduces tumor growth and extends survival in a murine model of ovarian granulosa cell tumor. Transl Oncol. 2013;6:226–233. doi: 10.1593/tlo.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Izumiya M, Ogata-Kawata H, Okamoto K, Fujiwara Y, Nakai M, Okabe A, Schetter AJ, Bowman ED, Midorikawa Y, et al. Tumor suppressor miR-22 determines p53-dependent cellular fate through post-transcriptional regulation of p21. Cancer Res. 2011;71:4628–4639. doi: 10.1158/0008-5472.CAN-10-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield ML, George LK, Grant GD, Perou CM. Common markers of proliferation. Nat Rev Cancer. 2006;6:99–106. doi: 10.1038/nrc1802. [DOI] [PubMed] [Google Scholar]
- Xiong J, Yu D, Wei N, Fu H, Cai T, Huang Y, Wu C, Zheng X, Du Q, Lin D, et al. An estrogen receptor alpha suppressor, microRNA-22, is downregulated in estrogen receptor alpha-positive human breast cancer cell lines and clinical samples. FEBS J. 2010a;277:1684–1694. doi: 10.1111/j.1742-4658.2010.07594.x. [DOI] [PubMed] [Google Scholar]
- Xiong J, Du Q, Liang Z. Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene. 2010b;29:4980–4988. doi: 10.1038/onc.2010.241. [DOI] [PubMed] [Google Scholar]
- Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi R-U, Takata T, Shimamoto A, et al. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Yagi S, Ito T, Lowenstein CJ. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS One. 2011;6:e20291. doi: 10.1371/journal.pone.0020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Wang X, Gong R, Li A, Yang S, Cao Y, Wen Y, Wang C, Yi X. The expression profile of microRNA s in a model of 7,12-dimethyl-benz[a]anthrance-induced oral carcinogenesis in Syrian hamster. J Exp Clin Cancer Res. 2009;28:64. doi: 10.1186/1756-9966-28-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos AS, Gyuris J, Brent R. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell. 1993;72:223–232. doi: 10.1016/0092-8674(93)90662-a. [DOI] [PubMed] [Google Scholar]
- Zhao C, Sun G, Ye P, Li S, Shi Y. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu S, Wu M, Pan Z, Zhou W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer. 2010;103:1215–1220. doi: 10.1038/sj.bjc.6605895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Duan S, Kistner EO, Bleibel WK, Huang RS, Clark TA, Chen TX, Schweitzer AC, Blume JE, Cox NJ, et al. Evaluation of genetic variation contributing to differences in gene expression between populations. Am J Hum Genet. 2008;82:631–640. doi: 10.1016/j.ajhg.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z-F, Su H-F, Zou Y, Peng Z, Wu S-X. Expression profiles of microRNA s in radioresistant esophageal cell line. Zhonghua Yi Xue Za Zhi. 2011;91:639–642. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.