Abstract
trans-2-Hexenal, one of the C6 green leaf volatiles, is potentially useful for the control of Bradysia odoriphaga Yang et Zhang. In this study, the biological activity of trans-2-hexenal on B. odoriphaga was assessed in the laboratory. trans-2-Hexenal was observed to kill B. odoriphaga in different developmental stages at a relatively low concentration under fumigation. The respiration rate in the male treatment group decreased from 131.44 to 4.07 nmol/g·min with a prolonged fumigation time, while the respiration rate in females decreased from 128.82 to 24.20 nmol/g·min. Male adults exhibited a more sensitive electroantennogram response at 0.05–500 μl/ml at the dose of 10.0 μl than female adults. Moreover, trans-2-hexenal had a repellent effect on adults based on the results with a Y-tube olfactometer at 10.0 μl, as shown by the deterrent rate of male and female adults with 96.67% and 98.33%, respectively. The results showed that trans-2-hexenal had good biological activity in different developmental stages of B. odoriphaga, which could reduce the need for, and risks associated with, the use of traditional insecticides and enable nonharmful management.
Keywords: Bradysia odoriphaga, trans-2-hexenal, fumigation toxicity, respiratory rate, electroantennogram response
Fungus gnats, Bradysia spp. (Diptera: Sciaridae), are one of the most common insect pests of production nurseries and greenhouse plants (Harris et al. 1995, Jagdale et al. 2004). Severely injured plants generally lose their healthy appearance, become off-color, and dry out eventually. Larvae are capable of spreading fungal pathogens during feeding, and adult flies irritate people and disseminate fungal spores from plant to plant when they migrate in the greenhouse (Gillespie and Menzies 1993, Ludwig and Oetting 2001). The sciarid fly Bradysia odoriphaga is a serious pest of the Chinese chive, Allium tuberosum Rottl. ex Spreng., in China. The larva gather in the roots and stems of the plant, which is hard to be controlled with common strategies and cause a huge yield loss of Chinese chives. Generally, applying of organophosphate insecticides, such as chlorpyrifos and phoxim, is the main method to control pest of Chinese chives. However, public concerning over the residual toxicity of organophosphate insecticides increased calls for new and less poisonous approaches to control this pest in recent years. Alternative control methods have been developed, including botanical secondary metabolites such as thermopsine, cytosine (Yu et al. 2003), the entomopathogenic nematode Heterorhabditis indica LN2 (Sun et al. 2004), and benzothiazole for trapping them (Chen et al. 2014). However, these methods do not provide adequate control; therefore, there is a need for safe insecticides or repellents for use on this fly.
Volatile organic compounds (VOCs), which exist widely in plant essential oils, are secondary metabolites produced in plant metabolism that have low toxicity to humans and wild life and are environmentally safe (Katz et al. 2008). They could interfere with the basic metabolic, biochemical, physiological functions, and behavior of insects (Mohamed and Abdelgaleil 2008). Because of the multiple action mechanisms, the probability of developing a resistant population is low (Abdelgaleil et al. 2008, Rattan 2010). Many VOCs had been investigated for potential use as fumigants against stored products insects (Tunç et al. 2000, Cosimi et al. 2009, Ilboudo et al. 2010, Li et al. 2013) and greenhouse pests (Çalmaşur et al. 2006, Yang et al. 2010). trans-2-Hexenal exists widely in green leaf volatile compounds (Tandon et al. 2000, Farag and Pare 2002, Tapia et al. 2007, Takayama et al. 2012) and can cause electroantennogram (EAG) and olfactory responses in pests (Stelinski et al. 2003, Park and Hardie 2004, Piesik et al. 2011). Fumigation of trans-2-hexenal has been reported to control mold on seedless table grapes Vitis vinifera L. (Archbold et al. 1999), but there are no previous reports on its effect as a poison when used in fumigation. Considering the significant biological activity exhibited by trans-2-hexenal and as part of an ongoing effort to discover environmental and efficient insecticidal VOCs for B. odoriphaga, trans-2-hexenal was found to have potential insecticidal and behavioral effects on B. odoriphaga.
In this article, we studied the biological activity of trans-2-hexenal against different developmental stages of B. odoriphaga. This study aimed to determine the biological activity of trans-2-hexenal on B. odoriphaga and provide a theoretical basis for use of this compound in ecological control of this pest.
Materials and Methods
Chemicals
trans-2-Hexenal was purchased from ACROS (Janssen Pharmaceuticalaan, Geel, Belgium, 99% purity).
Insects
B. odoriphaga eggs and larvae were collected from roots and subterraneous stems in Zhangqiu, Shandong Province, China. The eggs and larvae were reared on the stem of leeks at 25 ± 1°C, 70% humidity in the laboratory, Shandong Agricultural University. Rearing method of B. odoriphaga was referred to Mu et al. (2003).
Bioassay
All toxicity test and behavior experiment were performed at 20 ± 1°C, 60 ± 10% relative humidity in artificial climate chest.
Fumigation Toxicity of trans-2-Hexenal on Adults
Triangular glass flasks (3.75 liter) sealed with Parafilm were used for the bioassays. trans-2-Hexenal added in filter paper directly without any solvent. After the lethal concentration, range was determined by preliminary experiments. trans-2-Hexenal was serially designed into 0.15, 0.20, 0.25, 0.30, and 0.35 μl/liter and dispensed at an appropriate dose on filter paper (Whatman No. 1), and the filter paper was hung in a triangular flask. The control contained a similar filter paper but with nothing. The fumigation test was performed on adults that were divided into a female group and a male group. Approximately 50 new emerged adults were used per concentration and sex. At 0.5, 1, 1.5, and 2 h after treatment, the number of dead adults was recorded. The experiment was repeated for four times.
Fumigation Toxicity of trans-2-Hexenal on Eggs, Larvae, and Pupae
In a glass fumigation box (∼22 by 21.5 by 21 cm), a thin copper wire was fixed along the diagonal. Then, filter paper (cut into 2 cm by 2 cm pieces) with a quantitative trans-2-hexenal (concentration in egg, fourth-instar larvae, and pupae treatment group were 0.16, 0.21, 0.26, 0.31 and 0.36 μl/liter; 0.60, 0.80, 1.00, 1.20, and 1.40 μl/liter; 1.0, 1.5, 2.0, 2.5, and 3.0 μl/liter, respectively) was attached to the copper wire, avoiding contact with the bottom of the box. trans-2-Hexenal added in filter paper directly without any solvent and the control in each experiment contained a similar filter paper but with nothing. The eggs, larvae, and pupae were put in separate 9 cm Petri dishes in the bottom of the box. The eggs and pupae were fumigated with five concentrations separately for 24 h and then moved to normal rearing conditions. Egg hatchability was recorded after 3, 4, 5, and 6 d, and the emergence rate was counted after 2, 3, 4, and 5 d. In the larvae fumigation experiment, the fourth-instar larvae were treated with five concentrations of trans-2-hexenal. Mortality was recorded after 6, 12, 24, 48, and 72 h of exposure. Approximately 50 eggs per larvae per pupae were used for each concentration, and the experiment was repeated five times.
The Effect of trans-2-Hexenal on the Adult Respiratory Rate
Oxygen consumption was determined using a Clark-type electrode (Oxytherm oxygraph, Hansatech Instruments, Norfolk, England). Briefly, ∼100 nonsexed male and female adult insects were put in a triangular flask, respectively. Then, similar to the adult fumigation experiment, the fumigation concentrations (0.243 and 0.207 μl/liter for female and male, respectively) in this experiment were LC50 value after 2.0 h for female and male adults, respectively, while the control group did not receive trans-2-hexenal. Approximately 30 insects were transferred into the Oxytherm every 0.5 h to measure the respiratory rate over 2 min for males and females. Each experiment was repeated for four times.
EAG Recordings
EAG equipment, produced by Syntech (the Netherlands), composed of an INR-5 micro-manipulator, IADAC-4 data acquisition controller, CS-55 odor stimulation control, and EAG record output device. Both flow velocity of the stimulatory odor and continuous flow were 20 ml/min, and the stimulation time was 0.5 s, with a 30 s interval between two stimuli. Because of B. odoriphaga has no EAG response to mineral oil (AMERSCO), so we use mineral oil as control stimulus. trans-2-Hexenal was diluted in mineral oil and 10 μl of each treatment (0.05, 0.5, 5, 50, and 500 μl/ml) was applied to a filter paper strip (4 cm by 0.5 cm), then inserted into a glass Pasteur pipette, constituting an odor cartridge. The control stimulus was a similar pipette containing a filter paper strip impregnated with a 10 μl aliquot of mineral oil. The antennae of unmated B. odoriphaga were cut from the base by sharp blade, and the tip of the antenna was cut off with a sharp razor blade. Two ends of the antennae were fixed on a gel electrode with a conductive adhesive, and the odor-mixing tube was 0.5 cm away from the antenna. For male and female adults, five series of trans-2-henxenal were applied to 10 antennae in the following order: standard stimulus, mineral oil control, series of concentrations of trans-2-hexenal, mineral oil control, and standard stimulus. Benzaldehyde can cause obvious EAG responses on B. odoriphaga, so it was used as the standard stimulus at the beginning and end of a recording series to confirm the activity of each antenna preparation.
The Effect of trans-2-Hexenal on Adult Olfactory Responses
A Y-tube olfactometer was used to test the ability of trans-2-hexenal to repel unmated females and males. An olfactometer (internal diameter: 1.0 cm; length: 10 cm; angle between arms: 50°) was designed for the response experiments. trans-2-Hexenal was injected at an appropriate dose (0.5, 2.5, 5, 7.5, 10 μl) on filter paper (2 cm by 2 cm) using a 10 μl pipetting gun (Eppendorf Research plus, Germany). A piece of filter paper was placed in the container of one arm, and the other container remained empty. Air was pushed through the two arms by a pump at a speed of 0.5 liter/min, and the adults used in the experiment were virgins. Check insect quantity of each tube after 5 min, each treatment was administered to 10 adults and was repeated six times.
Statistical Analyses
Absolute EAG = measured value of EAG – mean value of control stimulus
Standard EAG = mean value of standard stimulus – mean value of control stimulus
Normalized EAG = absolute EAG/Standard EAG
Repellence rate = adult population in control arm/total population
LC50 was calculated by SPSS program. For the analysis of the fumigation experiment, the data were arcsine transformed and respiratory rate data were square root transformed, then subjected to an analysis of variance and Duncan’s multiple range test to determine significant differences between treatments for same treatment time and stages. The EAG and olfactory response were compared by two-sample t tests.
Results
Effect of Fumigation With trans-2-Hexenal on Different Insect Life Stages
Female Adults
trans-2-Hexenal caused 50% mortality after 2.0 h at a concentration of 0.243 μl/liter (Table 1). The mortality increased with increased doses of trans-2-hexenal and exposure time. trans-2-Hexenal was effective in controlling B. odoriphaga. Doses between 0.836 and 0.475 μl/liter achieved 95% lethality in female adults at fumigation times from 0.5 to 2.0 h. In all cases, a significant increase in the susceptibility of adults was observed with extended treatment.
Table 1.
The influence of trans-2-hexenal fumigation on the mortality of female B. odoriphaga adults
| Treatment timea | Slope ± SE | r | LC50 (95% CL)b | LC95 (95% CL)b | df | χ2 |
|---|---|---|---|---|---|---|
| 0.5 | 6.103 ± 0.757 | 0.963 | 0.450 (0.395∼0.554) | 0.836 (0.653∼1.254) | 3 | 1.270 |
| 1 | 5.018 ± 0.454 | 0.981 | 0.369 (0.342∼0.410) | 0.785 (0.653∼1.108) | 3 | 4.951 |
| 1.5 | 5.231 ± 0.390 | 0.983 | 0.301 (0.288∼0.319) | 0.621 (0.547∼0.735) | 3 | 6.719 |
| 2 | 5.302 ± 0.357 | 0.990 | 0.243 (0.234∼0.252) | 0.496 (0.453∼0.558) | 3 | 4.879 |
aTreatment time in hours of fumigation.
bConcentration in μl/liter of trans-2-hexenal. Parentheses contain the 95% lower and upper confidence intervals, CL means confidence limit.
cData are the means of four replications.
Male Adults
Table 2 lists the effect of trans-2-hexenal on male adults at different times. The lethal concentration, LC50, varied between 0.415 and 0.207 μl/liter for different times, while the LC95 was between 0.886 and 0.377 μl/liter. Similar to female adults, the LC50 value decreased with extended fumigation time. It is reasonable to conclude that increased fumigation time can enhance the effect of trans-2-hexenal. In addition, comparing Tables 1 and 2, female adults showed higher tolerance than male adults at the same time point.
Table 2.
The influence of trans-2-hexenal fumigation on the mortality of male B. odoriphaga adults
| Treatment timea | Slope ± SE | r | LC50 (95% CL)b | LC95 (95% CL)b | df | χ2 |
|---|---|---|---|---|---|---|
| 0.5 | 5.040 ± 0.513 | 0.999 | 0.415 (0.377∼0.476) | 0.886 (0.715∼1.213) | 3 | 0.242 |
| 1 | 4.327 ± 0.374 | 0.983 | 0.322 (0.303∼0.348) | 0.772 (0.648∼0.986) | 3 | 5.071 |
| 1.5 | 4.375 ± 0.349 | 0.983 | 0.271 (0.259∼0.285) | 0.644 (0.560∼0.778) | 3 | 5.808 |
| 2 | 6.312 ± 0.378 | 0.993 | 0.207 (0.199∼0.214) | 0.377 (0.356∼0.405) | 3 | 4.610 |
aTreatment time in hours of fumigation.
bConcentration in μl/liter of trans-2-hexenal. Parentheses contain the 95% lower and upper confidence intervals, CL means confidence limit.
cData are the means of four replications.
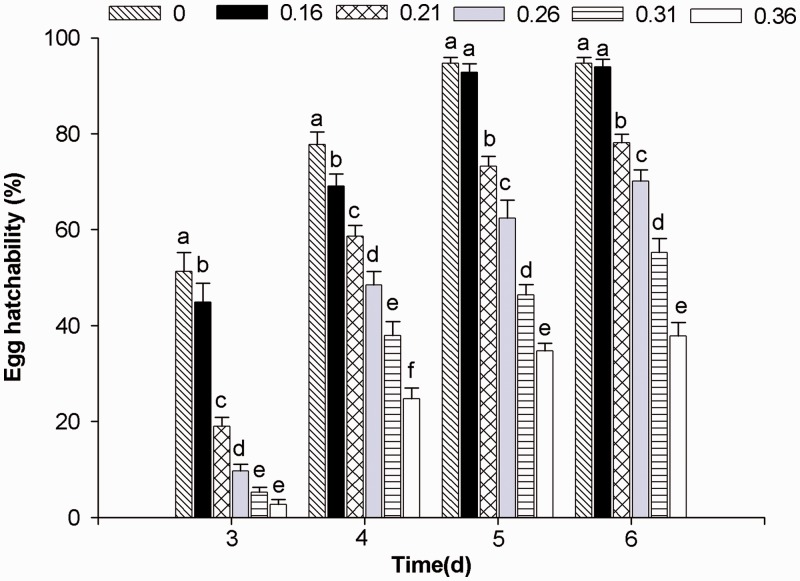
Fig. 1.
Percentage inhibition of egg hatchability (±SE) of B. odoriphaga by different concentrations of trans-2-hexenal and different hatch days. Data in the same treatment time followed by different letters are significantly different at the 0.05 level by Duncan's multiple range test. The same is true for Fig. 2.
Eggs
The results of the analysis of variance, showing the effect of trans-2-hexenal fumigation on egg hatchability, are shown in Fig. 1. There were significant differences (P < 0.05 level) between responses at 3, 4, 5, and 6 d. At a concentration of 0.36 μl/liter, 37.82% hatchability was observed after 6 d compared with 94.62% in the control. This finding shows that trans-2-henxenal had a good fumigation effect on egg hatching.
Larvae
There was a concentration-dependent increase in mortality of fourth-instar larva treated with trans-2-hexenal (Table 3). LC50 varied between 1.908 and 0.633 μl/liter for different treatment times, while the LC95 was between 6.430 and 2.077 μl/liter. Moreover, a significant feeding decrease was found in the treatment group but not the control group.
Table 3.
The influence of trans-2-hexenal fumigation on the mortality of fourth-instar larvae of B. odoriphaga
| Treatment timea | Slope ± SE | r | LC50 (95% CL)b | LC95 (95% CL)b | df | χ2 | |
|---|---|---|---|---|---|---|---|
| 6 | 3.117 ± 0.400 | 0.988 | 1.908 (1.640-2.437) | 6.430 (4.364-12.258) | 3 | 1.494 | |
| 12 | 3.185 ± 0.351 | 0.983 | 1.425 (1.303-1.622) | 4.682 (3.512-7.293) | 3 | 2.905 | |
| 24 | 2.806 ± 0.319 | 0.981 | 1.033 (0.968-1.112) | 3.984 (3.053-6.034) | 3 | 3.067 | |
| 48 | 2.909 ± 0.320 | 0.965 | 0.821 (0.756-0.877) | 3.018 (2.442-4.169) | 3 | 6.119 | |
| 72 | 3.186 ± 0.334 | 0.973 | 0.633 (0.557-0.692) | 2.077 (1.799-2.566) | 3 | 4.717 | |
aTreatment time in hours of fumigation.
bConcentration in μl/liter of trans-2-hexenal. Parentheses contain the 95% lower and upper confidence intervals, CL means confidence limit.
cData are the means of four replications.
Pupae
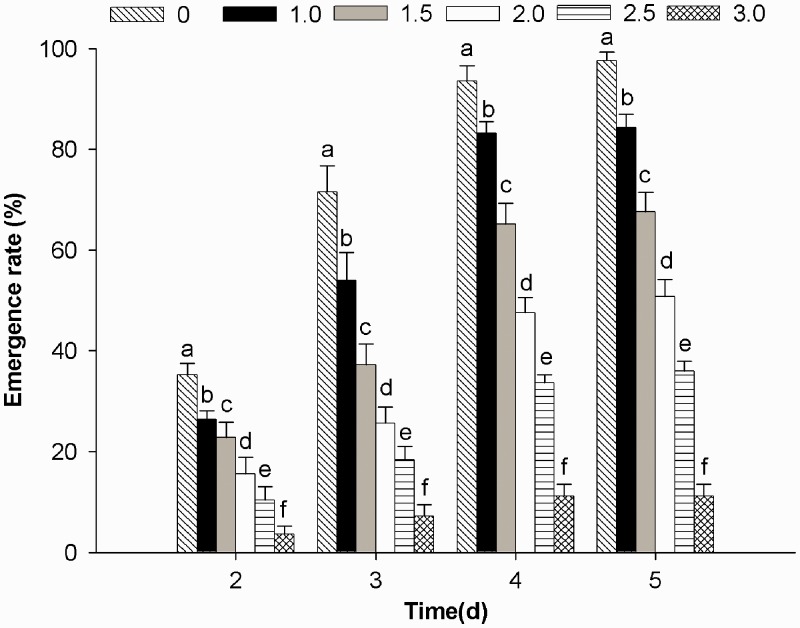
The results showed that trans-2-hexenal had a good insecticidal effect on pupae emergence (Fig. 2). The emergence rate was obviously affected by concentration and treatment time. The effect of concentration at each treatment time was highly significant different (P < 0.05 level). At the highest dose of 3.0 μl/liter of trans-2-hexenal at 5 d, an 11.20% emergence rate was recorded compared with 97.60% in the control.
Fig. 2.
Percentage inhibition of emergence rate (±SE) of B. odoriphaga by different concentrations of trans-2-hexenal and different treatment times.
Respiratory Rate
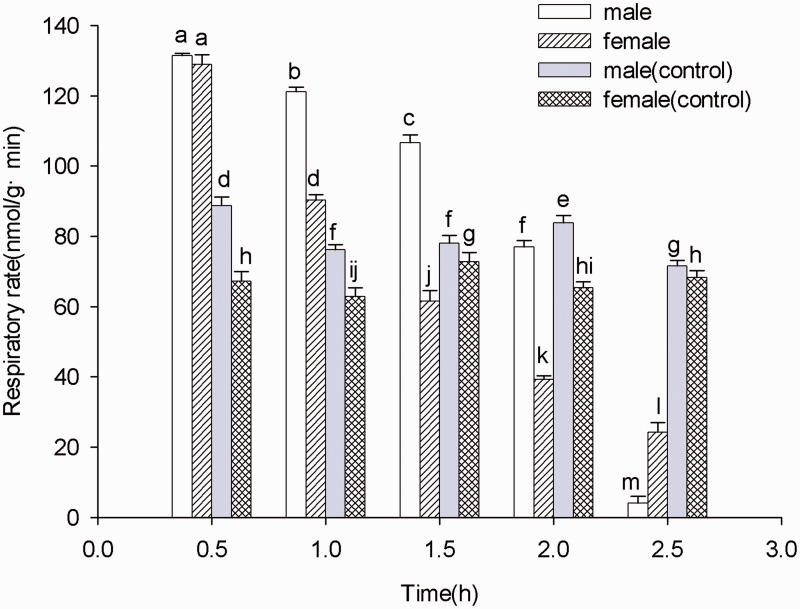
Figure 3 shows the respiratory rate of female and male adults after 2.5 h of treatment with 0.243 and 0.207 μl/liter, respectively. The results showed that the initial respiratory rate of the treatment group was higher than that of control group, then the rates equalized, and the final respiratory rate of females and males were 24.20 and 4.07 nmol/g·min, respectively. The maximum value in the male and female treatment group appeared at 0.5 h. However, the respiratory rate of the control group remained unchanged.
Fig. 3.
Effect of trans-2-hexenal on the respiratory rate of male and female B. odoriphaga adults. Data in the figure are the mean ± SD, and bars with different small letters above them are significantly different at the 0.05 level at the same time point comparing the fumigation treatment and blank control by Duncan’s test.
Electrophysiological Responses to trans-2-Hexenal
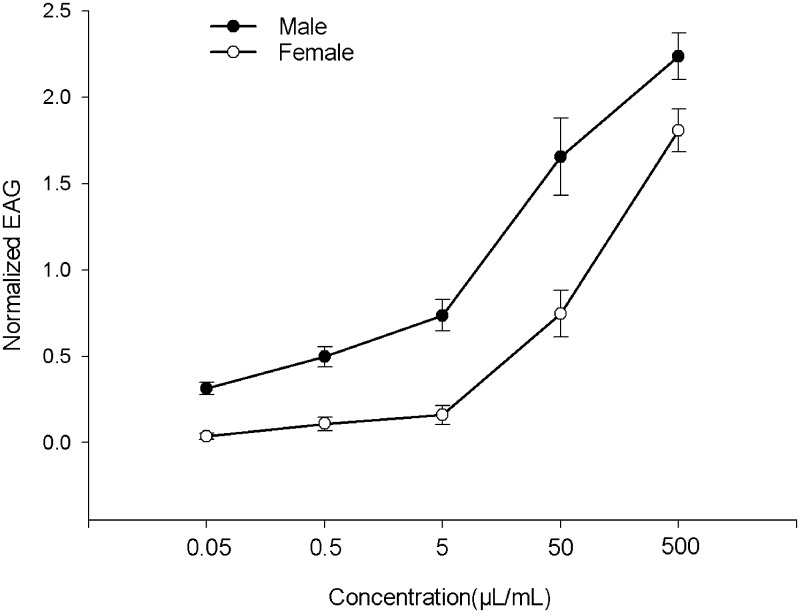
The olfactory responses of insects depend on the interaction of chemicals with antennal sensillae. In this study, we tested the EAG response of each sex of B. odoriphaga at five concentrations. The data were compared with Student’s t-test to determine the significance of EAG responses between female and male adults. All of the concentrations elicited significant EAG responses in unmated male and female B. odoriphaga, with male adults exhibiting a greater EAG response than females (P values of Student’s t-test at 0.05–500 μl/ml were all 0.0001). The EAG responses increased as the trans-2-hexenal concentration increased from 0.05 to 500 μl/ml (Fig. 4). The results showed that male adults were more sensitive to trans-2-hexenal than females in the electrophysiological experiment.
Fig. 4.
EAG dose-response curves to different doses of trans-2-hexenal for male and female B. odoriphaga. The EAG responses were normalized ( ± SE) to mineral oil.
Olfactory Response
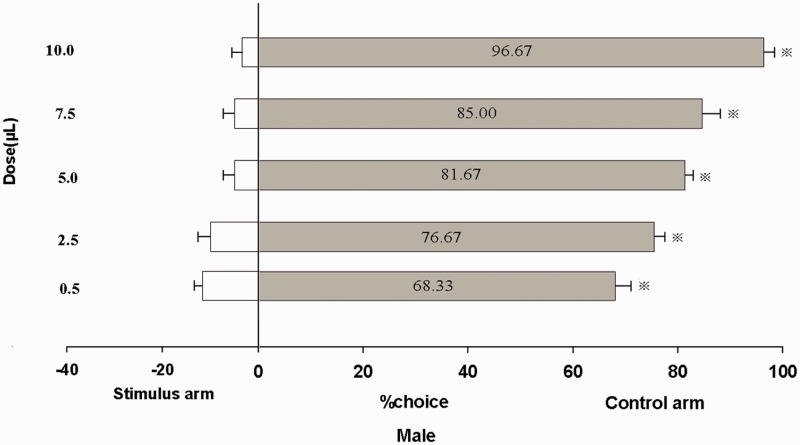
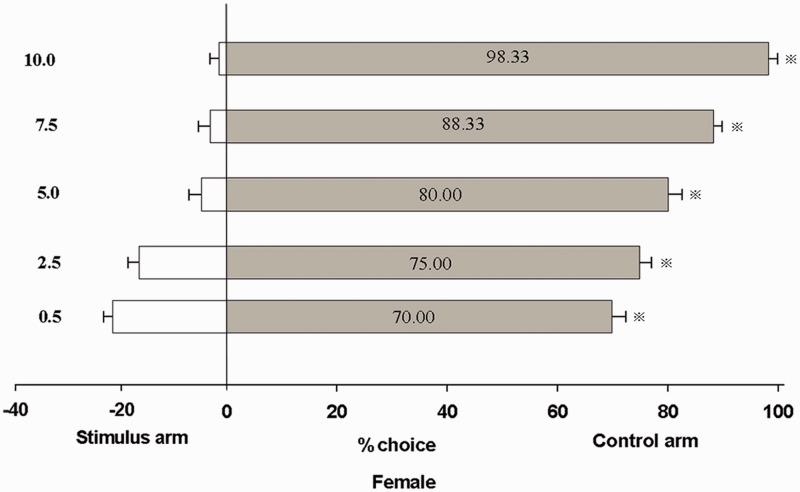
A “Y” shape olfactometer was used to measure the behavioral responses of unmated male and female adults to trans-2-hexenal (Fig. 5). The results demonstrated a significant repellent response of unmated male and female B. odoriphaga adults to trans-2-hexenal, with the repellence rate increasing at higher doses. At least 68.33% and 96.67% of male adults were repelled by 0.5 μl and 10.0 μl trans-2-hexenal (Fig. 4a). Meanwhile, 70.00% and 98.33% of female adults chose the control arm to avoid trans-2-hexenal at 0.5 and 10 μl, respectively (Fig. 6). trans-2-Hexenal shows a good repellent activity at a relatively low dose. In addition, B. odoriphaga exhibited a dose- dependent increase choice behavior in the behavior experiment, in which the repellence rate increased with the dose.
Fig. 5.
Response of unmated male B. odoriphaga to trans-2-hexenal treatments in a Y-tube olfactometer. The responses of unmated male adults when given a choice between air (control) and different doses of trans-2-hexenal stimuli. Gray bars indicate the percentage responses to the control; white bars indicate the percentage responses to the tested stimuli. N = 10 individuals per choice test. Asterisks (*) indicate significant differences within a choice test (P < 0.05, Student’s t-test). The same notation is used in Fig. 6.
Fig. 6.
Response of unmated female B. odoriphaga to trans-2-hexenal stimuli treatments in a Y-tube olfactometer.
Discussion
The Chinese chive grows in sheltered ground mainly in a sunlight greenhouse and is usually no higher than 1 meter. This makes it difficult for conventional spray application of insecticides; therefore, fumigation is a good alternative method of application. In this study, the insecticidal activity of trans-2-hexenal increased with increasing concentration and exposure times. The relationship between mortality and fumigation time was similar to those reported by Collins et al. (2005). Furthermore, female adults showed better tolerance to trans-2-hexenal than males (Tables 1 and 2), which may be caused by the weight difference between these two sexes, given that females are normally three times heavier than males. trans-2-Hexenal caused significant mortality on fourth larvae (2.077 μl/liter killed 95% of the larvae) at 72 h (Table 3) and had an obvious inhibitory effect on eggs and pupae at 0.36 and 3.0 μl/liter after 5 d of exposure (Figs. 1 and 2). So the trans-2-hexenal has a relatively high insecticidal activity against different life stages of B. odoriphaga and is suitable for use in a sunlight greenhouse, and trans-2-hexenal can apply to field by processing into microcapsule suspension which with remarkable sustained-release property to control B. odoriphaga. The mode of action of trans-2-hexenal on egg hatch inhibition and pupae emergence has not been known but it may be due to suffocation and inhibition of various biosynthetic processes of the insect (Chaubey 2008). In addition, compared with other chemical insecticides, trans-2-hexenal might be less harmful to humans given that it has been identified in many fruits and foods (Burdon et al. 2004, Frank et al. 2007, Song et al. 2011) and has been applied as edible flavor in the food industry.
Respiratory metabolism is one of most important physiological and ecological characteristics of insects. Changes in respiratory rate can reflect the influence of toxic drugs or environmental pressure on insects (Dingha et al. 2005, Santos et al. 2011, DeVries and Appel 2013). In our study, the treatment group exhibited a significantly higher respiratory rate than the control group at 0.5 and 1 h (Fig. 3), which is similar to the results of other published studies, such as those on insecticides (Kestler 1991), toxic plant extracts (Harak et al. 1999, Sibul et al. 2004), and handling stress (Harak et al. 1998). With extended fumigation time, the respiratory rate was significantly reduced to 4.07 and 24.20 nmol·g−1·min−1 (male and female, respectively) at 2.5 h, which is lower than that of the control group. Unlike respiratory inhibitors such as rotenone and nitric oxide, where the respiratory rate is significantly reduced after application, the change in the respiratory rate in this experiment indicated that trans-2-hexenal is not a respiratory inhibitor. Its mode of action requires further research.
With the concept of plant protection transforming from kill to management, the influence of volatile compounds on insect behavior has received more and more attention. As a result, there appears to have been successful use of plant-derived repellents against sanitary, stored-product insect pests, and arthropods under laboratory conditions (Liu and Ho 1999, Odalo et al. 2005, Nerio et al. 2010, Yoon et al. 2011).
EAG experiments are a convenient method to assess the overall sensitivity of insects to volatile compounds at physiologically relevant concentrations. trans-2-Hexenal can cause an obvious EAG response in many types of insects (Han and Han 2007, Ngumbi et al. 2010, Chi et al. 2011). In our EAG test, B. odoriphaga adults had a strong EAG response to 500 μl/ml trans-2-hexenal (Fig. 4), and males were more sensitive than females. These results verified again that trans-2-hexenal may play an important role in the behavior regulation on insects. So, it has the potential ability to control pests by changing the way of behavior and our behavior experiment verify this point.
There are many reports on the effect of trans-2-hexenal on insect behaviors (Pope et al. 2004, Han and Han 2007). However, there are no reports concerning the repellent activity of trans-2-hexenal against B. odoriphaga. The results of the Y-tube olfactometer bioassays in this study demonstrated strong repellence of both sexes. We also found that female B. odoriphaga had a stronger olfactory response and more active selective behavior than males when the dose was higher than 5.0 μl. The fundamental reason for this selectivity may be that the antennae receptors in male and female adults have gender-related differences or qualitative differences in olfactory physiology. We can take advantage of the repellent activity to mask the odors of the Chinese chive, so that pests are not able to detect the presence of food and oviposition sites. Furthermore, trans-2-hexenal could also be used to treat sheltered ground to flush out hidden B. odoriphaga before fresh Chinese chive plants are introduced.
Traditional volatile compounds such as methyl bromide, phosphine, chloropicrin mainly used for control storage insect pests and soil fumigation before cultivation due to their low crop safety, so it is not suitable to use in perennial Chinese chives field. This work provides new information on plant volatile compound component trans-2-hexenal, which is toxic to all stages of B. odoriphaga and also has a strong repellent effect on adults of both sexes. These characteristics suggest that trans-2-hexenal could act as a fumigant in sunlit greenhouses or as protective bands to drive pests away from Chinese chives.
For commonly used as flavors and fragrances, the cost for agriculture pest control of many VOCs, including of trans-2-hexenal, are higher than that of traditional chemical insecticides. Large-scale application depends on the exploration of their application effect and scope in future.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (201303027). We also thank Dr. Yongjun Zhang of the State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences for the support in EAG experiments.
References Cited
- Abdelgaleil S.A.M., Abbassy M. A., Belal A.-S.H., Abdel Rasoul M. A. 2008. Bioactivity of two major constituents isolated from the essential oil of Artemisia judaica L. Bioresour. Technol. 99: 5947–5950. [DOI] [PubMed] [Google Scholar]
- Archbold D. D., Hamilton-Kemp T. R., Clements A. M., Collins R. W. 1999. Fumigating ‘Crimson Seedless' table grapes with (E)-2-hexenal reduces mold during long-term postharvest storage. HortScience 34: 705–707. [Google Scholar]
- Burdon J., McLeod D., Lallu N., Gamble J., Petley M., Gunson A. 2004. Consumer evaluation of “Hayward” kiwifruit of different at-harvest dry matter contents. Postharvest. Biol. Technol. 34: 245–255. [Google Scholar]
- Çalmaşur Ö., Aslan İ., Şahin F. 2006. Insecticidal and acaricidal effect of three Lamiaceae plant essential oils against Tetranychus urticae Koch and Bemisia tabaci Genn. Ind. Crops Prod. 23: 140–146. [Google Scholar]
- Chaubey M. K. 2008. Fumigant toxicity of essential oils from some common spices against pulse beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). J. Oleo. Sci., 57: 171–179. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Zhao Y. H., Li H., Zhang P., Mu W., Liu F. 2014. Biological activity of benzothiazole against Bradysia odoriphaga (Diptera: Sciaridae) at different developmental stages. Acta Entomologica Sinica 57: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi D., Li X., Yu J., Xie X., Wang G. 2011. The EAG response and behavior of the Saperda populnea L. to volatiles from poplar branches. Acta Ecol. Sin. 31: 334–340. [Google Scholar]
- Collins P. J., Daglish G. J., Pavic H., Kopittke R. A. 2005. Response of mixed-age cultures of phosphine-resistant and susceptible strains of lesser grain borer, Rhyzopertha dominica, to phosphine at a range of concentrations and exposure periods. J. Stored Prod. Res. 41: 373–385. [Google Scholar]
- Cosimi S., Rossi E., Cioni P. L., Canale A. 2009. Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: Evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J. Stored Prod. Res. 45: 125–132. [Google Scholar]
- DeVries Z. C., Appel A. G. 2013. Standard metabolic rates of Lepisma saccharina and Thermobia domestica: effects of temperature and mass. J. Insect. Physiol. 59: 638–645. [DOI] [PubMed] [Google Scholar]
- Dingha B. N., Appel A. G., Eubanks M. D. 2005. Discontinuous carbon dioxide release in the German cockroach, Blattella germanica (Dictyoptera: Blattellidae), and its effect on respiratory transpiration. J. Insect. Physiol. 51: 825–836. [DOI] [PubMed] [Google Scholar]
- Farag M. A., Pare P. W. 2002. C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61: 545–554. [DOI] [PubMed] [Google Scholar]
- Frank D., O'Riordan P., Varelis P., Zabaras D., Watkins P., Ceccato C., Wijesundera C., Ferguson A., Hewett E., Gunson F. 2007. Deconstruction and recreation of ‘Hayward' volatile flavour using a trained sensory panel, olfactometry and a kiwifruit model matrix, pp. 107-118. In Proceedings of the Sixth International Symposium on Kiwifruit, Rotorua, New Zealand, 20-24 February 2006. International Society for Horticultural Science (ISHS), Belgium. [Google Scholar]
- Gillespie D., Menzies J. 1993. Fungus gnats vector Fusarium oxysporum f. sp. radicislycopersici. Ann. Appl. Biol. 123: 539–544. [Google Scholar]
- Han B. Y., Han B. H. 2007. EAG and behavioral responses of the wingless tea aphid Toxoptera aurantii (Homoptera: Aphididae) to tea plant volatiles. Acta Ecol. Sin. 27: 4485–4490. [Google Scholar]
- Harak M., Kuusik A., Hiiesaar K., Metspalu L., Luik A., Tartes U. 1998. Calorimetric investigations on physiological stress in Tenebrio molitor (Coleoptera, Tenebrionidae) pupae. Thermochim Acta 309: 57–61. [Google Scholar]
- Harak M., Lamprecht I., Kuusik A., Hiiesaar K., Metspalu L., Tartes U. 1999. Calorimetric investigations of insect metabolism and development under the influence of a toxic plant extract. Thermochim Acta 333: 39–48. [Google Scholar]
- Harris M. A., Oetting R. D., Gardner W. A. 1995. Use of entomopathogenic nematodes and a new monitoring technique for control of fungus gnats, Bradysia coprophila (Diptera: Sciaridae), in floriculture. Biol. Control 5: 412–418. [Google Scholar]
- Ilboudo Z., Dabiré L.C.B., Nébié R.C.H., Dicko I. O., Dugravot S., Cortesero A. M., Sanon A. 2010. Biological activity and persistence of four essential oils towards the main pest of stored cowpeas, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 46: 124–128. [Google Scholar]
- Jagdale G. B., Casey M. L., Grewal P. S., Lindquist R. K. 2004. Application rate and timing, potting medium, and host plant effects on the efficacy of Steinernema feltiae against the fungus gnat, Bradysia coprophila, in floriculture. Biol. Control 29: 296–305. [Google Scholar]
- Katz T. M., Miller J. H., Hebert A. A. 2008. Insect repellents: historical perspectives and new developments. J. Am. Acad. Dermatol. 58: 865–871. [DOI] [PubMed] [Google Scholar]
- Kestler P. 1991. Cyclic CO2 release as a physiological stress indicator in insects. Comp. Biochem. Physiol. C Comp. Pharmacol. 100: 207–211. [Google Scholar]
- Li S. G., Li M. Y., Huang Y. Z., Hua R. M., Lin H. F., He Y. J., Wei L. L., Liu Z. Q. 2013. Fumigant activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione S-transferase activities in adult Sitophilus zeamais. J. Pest. Sci. 86: 677–683. [Google Scholar]
- Liu Z. L., Ho S. H. 1999. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst). J. Stored Prod. Res. 35: 317–328. [Google Scholar]
- Ludwig S. W., Oetting R. D. 2001. Evaluation of medium treatments for management of Frankliniella occidentalis (Thripidae: Thysanoptera) and Bradysia coprophila (Diptera: Sciaridae). Pest Manag. Sci. 57: 1114–1118. [DOI] [PubMed] [Google Scholar]
- Mohamed M. I., Abdelgaleil S. A. 2008. Chemical composition and insecticidal potential of essential oils from Egyptian plants against Sitophilus oryzae (L.)(Coleoptera: Curculionidae) and Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). Appl. Entomol. Zool. 43: 599–607. [Google Scholar]
- Mu W., Liu F., Jia Z. M., He M. H., Xiang G. F. 2003. A simple and convenient rearing technique for Bradysia odoriphaga. Entomol. J. East China 12: 87–89. [Google Scholar]
- Nerio L. S., Olivero-Verbel J., Stashenko E. 2010. Repellent activity of essential oils: a review. Bioresour. Technol. 101: 372–378. [DOI] [PubMed] [Google Scholar]
- Ngumbi E., Chen L., Fadamiro H. 2010. Electroantennogram (EAG) responses of Microplitis croceipes and Cotesia marginiventris and their lepidopteran hosts to a wide array of odor stimuli: correlation between EAG response and degree of host specificity? J. Insect Physiol. 56: 1260–1268. [DOI] [PubMed] [Google Scholar]
- Odalo J. O., Omolo M. O., Malebo H., Angira J., Njeru P. M., Ndiege I. O., Hassanali A. 2005. Repellency of essential oils of some plants from the Kenyan coast against Anopheles gambiae. Acta. Trop. 95: 210–218. [DOI] [PubMed] [Google Scholar]
- Park K. C., Hardie J. 2004. Electrophysiological characterisation of olfactory sensilla in the black bean aphid, Aphis fabae. J. Insect Physiol. 50: 647–655. [DOI] [PubMed] [Google Scholar]
- Piesik D., Wenda-Piesik A., Kotwica K., Łyszczarz A., Delaney K. J. 2011. Gastrophysa polygoni herbivory on Rumex confertus: single leaf VOC induction and dose dependent herbivore attraction/repellence to individual compounds. J. Plant Physiol. 168: 2134–2138. [DOI] [PubMed] [Google Scholar]
- Pope T. W., Campbell C.A.M., Hardie J., Wadhams L. J. 2004. Electroantennogram responses of the three migratory forms of the damson-hop aphid, Phorodon humuli, to aphid pheromones and plant volatiles. J. Insect Physiol. 50: 1083–1092. [DOI] [PubMed] [Google Scholar]
- Rattan R. S. 2010. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 29: 913–920. [Google Scholar]
- Santos J. C., Faroni L.R.A., Sousa A. H., Guedes R.N.C. 2011. Fumigant toxicity of allyl isothiocyanate to populations of the red flour beetle Tribolium castaneum. J. Stored Prod. Res. 47: 238–243. [Google Scholar]
- Sibul I., Kuusik A., Voolma K. 2004. Monitoring of gas exchange cycles and ventilatory movements in the pine weevil Hylobius abietis: respiratory failures evoked by a botanical insecticide. Entomol. Exp. Appl. 110: 173–179. [Google Scholar]
- Song S., Zhang X., Hayat K., Liu P., Jia C., Xia S., Xiao Z., Tian H., Niu Y. 2011. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 124: 203–209. [Google Scholar]
- Stelinski L. L., Miller J. R., Ressa N. E., Gut L. J. 2003. Increased EAG responses of tortricid moths after prolonged exposure to plant volatiles: evidence for octopamine-mediated sensitization. J. Insect Physiol. 49: 845–856. [DOI] [PubMed] [Google Scholar]
- Sun R. H., Li A. H., Han R. C., Cao L., Liu X. L. 2004. Factors affecting the control of Bradysia odoriphaga with entomopathogenic nematode Heterorhabditis indicaLN2. Chin. Nat. Enemies Insects 26: 150–155. [Google Scholar]
- Takayama K., Jansen R., van Henten E. J., Verstappen F. W., Bouwmeester H. J., Nishina H. 2012. Emission index for evaluation of volatile organic compounds emitted from tomato plants in greenhouses. Biosyst. Eng. 113: 220–228. [Google Scholar]
- Tandon K., Baldwin E., Shewfelt R. 2000. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum, Mill.) as affected by the medium of evaluation. Postharvest. Biol. Technol. 20: 261–268. [Google Scholar]
- Tapia T., Perich F., Pardo F., Palma G., Quiroz A. 2007. Identification of volatiles from differently aged red clover (Trifolium pratense) root extracts and behavioural responses of clover root borer (Hylastinus obscurus)(Marsham) (Coleoptera: Scolytidae) to them. Biochem. Syst. Ecol. 35: 61–67. [Google Scholar]
- Tunç İ., Berger B. M., Erler F., Dağlı F. 2000. Ovicidal activity of essential oils from five plants against two stored-product insects. J. Stored Prod. Res. 36: 161–168. [Google Scholar]
- Yang N. W., Li A. L., Wan F. H., Liu W. X., Johnson D. 2010. Effects of plant essential oils on immature and adult sweet potato whitefly, Bemisia tabaci biotype B. Crop Prot. 29: 1200–1207. [Google Scholar]
- Yoon C., Moon S.-R., Jeong J.-W., Shin Y.-H., Cho S.-R., Ahn K.-S., Yang J.-O., Kim G.-H. 2011. Repellency of lavender oil and linalool against spot clothing wax cicada, Lycorma delicatula (Hemiptera: Fulgoridae) and their electrophysiological responses. J. Asia Pac. Entomol. 14: 411–416. [Google Scholar]
- Yu X. Y., Rao G., Liao H. F., Li X. J. 2003. Insecticidal activities of several botanical secondary metabolites against larvae of Bradysia odoriphaga Yang et Zhang. Jiangsu J. Agric. Sci. 19: 228–232. [Google Scholar]