Abstract
The insect central nervous system (CNS) is the target for many insecticides, and changes in transcript levels could be expected after insecticide applications. In this study, differentially expressed genes in the locust (Locusta migratoria manilensis) CNS in response to imidacloprid treatments at low dose (LD, 10% mortality) and high dose (HD, 80% mortality) were identified. Two nicotine acetylcholine receptor (nAChR) subunits genes and 18 interacting protein genes were regulated at LD, and only one nAChR subunit gene and 11 interacting proteins were regulated at HD. Among the 110 annotated P450 unigenes, 43 unigenes were regulated at LD and 34 unigenes were regulated at HD. Most of the differentially expressed P450 unigenes were mapped to CYP4, in which most unigenes were upregulated at LD, but downregulated at HD. Totally, the numbers and regulation levels of the regulated genes were more at LD than that at HD. Seventeen unigenes were selected to test their expression changes following insecticide treatments by qRT-PCR, in which the changes in more than half of the selected genes were verified. The results revealed the variation in the response of locusts to different insecticide pressure, such as different doses.
Keywords: Locusta migratoria manilensis, imidacloprid, nAChR, interacting protein, P450
In insects, nicotinic acetylcholine receptors (nAChRs) are neurotransmitter-gated ion channels that mediate fast cholinergic synaptic transmission (Sattelle 1980, Matsuda et al. 2001). As the main neuro-transmitter receptors, nAChRs are ideal targets for several kinds of insecticides, such as neonicotinoids. Several proteins, such as chaperones, regulators, and modulators, play important roles in the synthesis, degradation, and functional regulation of insect nAChRs, in vivo and in vitro (Lansdell et al. 2005, Choo et al. 2008, Millar 2008). RIC-3 and Lynx are two noted regulators of nAChRs in insects (Choo et al. 2008, Millar 2008, Liu et al. 2009, Yang et al. 2010). RIC-3 is necessary for proper folding/assembly of nAChRs, functioning as a molecular chaperone (Millar 2008). Lynx proteins improved the efficiency of protein folding and assembly in Pyrocoelia rufa and Nilaparvata lugens (Choo et al. 2008, Liu et al. 2009, Yang et al. 2010). Recent studies showed that nAChR subunit expression was regulated by imidacloprid, which changed the sensitivity to neonicotinoid insecticides (Markussen and Kristensen 2010, Yu et al. 2011, Taillebois et al. 2014). Thus, insect nAChRs and their interacting proteins might be regulated by insecticides during transcription.
The important proteins in insect central nervous system (CNS) are targets for many insecticides and insects can use the CNS as the last line of defense against insecticides. In insects, P450 monooxygenases are the most important detoxification enzymes to metabolize endogenous and exogenous compounds such as insecticides. In vertebrate CNS, P450s in the brain could catalyze neurosteroids and drugs (Strobel et al. 2001, Miksys and Tyndale 2009). Some P450 enzymes in insects, such as Drosophila melanogaster CYP6G1, Bemisia tabaci CYP6CM1vQ, and Nilaparvata lugens CYP6AY1 could metabolize imidacloprid (Joussen et al. 2008, Karunker et al. 2009, Ding et al. 2013). The induction/repression mechanisms of detoxification enzymes, in response to xenobiotic exposure, have been elucidated in some insect species (Feyereisen 1999). Expression of CYP4 and CYP9 genes could be induced by compounds present in the midgut of Manduca sexta (Snyder et al. 1995, Stevens et al. 2000). However, there are few reports regarding the induction of P450s in insect CNS by insecticides. Therefore, the variation in P450 expression in insect CNS in response to imidacloprid exposure merits an investigation.
The locust [Locusta migratoria manilensis (Meyen)] is an ideal model hemimetabolous insect and causes tremendous loss of agricultural production (Hassanali et al. 2005). In this study, we used the Illumina sequencing platform to analyze genes differentially expressed in the locust CNS following application of various doses of imidacloprid. The putative interacting proteins of nAChRs and P450s showed marked variation in their expressions. The expression variations of some unigenes were validated by qRT-PCR. This study provides useful information regarding gene regulation in insect CNS in response to insecticide application.
Materials and Methods
Insects and Chemicals
Locusts were purchased from the Hongguang Medicinal Animal Co. Ltd. (Jurong, Jiangsu, China).
Imidacloprid (97%) was purchased from Red Sun Group Corporation (Nanjing, China).
Bioassays and Sample Collection
Bioassays were performed in triplicate using 30 adult locusts with mixed sexes. Each locust was fed with 10 μL of insecticide solution with pipettes. The locusts that could not take in the entire insecticide dose were eliminated from the study. Imidacloprid was first dissolved in acetone and then diluted into different imidacloprid solutions using distilled water with the final proportion for acetone and Triton X-100 of 0.1 and 0.5% by volume. Five imidacloprid concentrations from 10 to 160 mg/liter were used. Water with acetone and Triton X-100 at 0.1 and 0.5% by volume was applied as the control (CK). All tests were conducted at 25 ± 1°C and mortalities were recorded after 24 h. The data were analyzed using DPS software (Tang and Zhang 2013).
The low (LD10), high (LD80) doses of imidacloprid, and the control (CK) were applied to adult locusts. After 24 h, the entire CNS from five surviving locusts as one sample was isolated by dissection for subsequent RNA isolation.
RNA Isolation, Library Construction, and Illumina Sequencing
The entire CNS was homogenized and RNA was extracted utilizing the TRIzol reagent (Invitrogen, USA), following the manufacturer’s instructions. RNase-free DNase I (Takara Biotechnology, China) was applied to eliminate genomic DNA contamination.
cDNA library preparation and sequencing reactions were performed by the BGI (Shenzhen, China). The library was sequenced using Illumina HiSeq 2000 (Illumina Inc., San Diego, CA).
Analysis of Illumina Sequencing Results
Reads from each library were assembled separately after elimination of low-quality reads. Trinity software was used for de novo transcriptome assembly (Grabherr et al. 2011) to generate contigs and unigenes. In the final step, the transcripts were searched for similarity against the protein databases NR, Swiss-Prot, KEGG, and COG, using BLASTX with a cut-off E-value of 10−5. The best-aligned results were used to determine the direction of sequences. These unigenes were initially aligned using protein databases, in the priority order NR, Swiss-Prot, KEGG, and COG. If a unigene could not be aligned to any database, its sequence direction and predicted coding region were determined using the ESTcan software (Iseli et al. 1999).
Differential Expression of Unigenes
Unigene expression was determined using the fragment per kilobase of transcript per million fragments (FPKM) method. The FPKM and reads per kilobase per million read (RPKM) formulae are identical (Mortazavi et al. 2008). However, when both pairs of reads were aligned to a gene, they were treated as one fragment with FPKM, but two reads by RPKM. An algorithm was developed to identify genes differentially expressed between two samples (Audic and Claverie 1997). False discovery rate (FDR) control is a statistical method used in multiple hypothesis testing. Sequences according to ‘FDR ≤ 0.001 and the absolute value of log2Ratio ≥ 1’ were regarded as being significantly differentially expressed.
qRT-PCR Validation of Gene Expression
Seventeen unigenes were selected to test their expression in the locust CNS following imidacloprid treatments at low and high doses. The total RNA was extracted from the locust CNS, and at least three replicate experiments were prepared for each sample. Reverse transcription to cDNA was performed using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Tokyo, Japan) and qRT-PCR was performed with the SYBR Premix Ex Taq (TaKaRa) on a 7500 Real-Time PCR System (Applied Biosystems) with 10 μL reaction buffer (SYBR Premix Ex Taq), 0.4 μL each primer (10 μM), 0.4 × Rox Reference Dye II, H2O up to 20 μL, following the manufacturer instructions. Gene expression difference was calculated by the 2 − ΔΔCt method (Livak and Schmittgen 2001). For normalization, two reference genes (β-actin and GAPDH) were validated experimentally for each generation and treatment, with the geometric mean of the selected genes then used for normalization according to the strategy described previously (Vandesompele et al. 2002). All PCR primer sequences are shown in Table 1.
Table 1.
The specific primers used in qRT-PCR
| Unigene ID | Putative annotation | Primers |
|---|---|---|
| CL2087-1 | CYP6AM1 | F—ATCGACTACACTTACTGGAA |
| R—GAGTAAATACCGACGAAATC | ||
| U5019 | CYP4G43 | F—GCCTTCTTGGACCTTATG |
| R—GTCCTGAATGTCCTGATG | ||
| CL2702-3 | CYP6BK17 | F—CTCGACAAGGTCGTCTCAGAGACG |
| R—GAAGTAGTGAGGGTCGTGATGGAG | ||
| U33624 | Protein kinase C | F—CCTGAATGGTGGAGATCTGATG |
| R—GCGGATATGGCCTTCATAGTC | ||
| CL2319-1 | Mito 12A2 | F—GTTCGCCGAGCTAGAGGTCTAC |
| R—GTCTCTGTCGATGACCTTGAAC | ||
| U13389 | CYP4C1 | F—CAAACGTGAAGATCGATTGCGC |
| R—GGGTGAAGGAAGGCATACATAC | ||
| CL2611-1 | CYP4C1 | F—CAGGATCAGTGAACTCACCATG |
| R—CTTGGATTGGGCTCCGTTCG | ||
| U21296 | Spectrin alpha chain | F—GAACCTGTTGAGCGTGTTGAGGAG |
| R—CATCCTCCCTTCGAACATTCCACC | ||
| U29431 | Spectrin alpha chain | F—ATCTATGATGGAAGGTTCTG |
| R—CTGTGCTCTGTATACCTATT | ||
| CL4026-1 | CYP6AM1 | F—AAAGCTATTTCACTCTGGAG |
| R—TTCAGTACCTCGTCTATCTC | ||
| U6027 | CYP4C1 | F—CACCTACGTCATGCTCTACA |
| R—AGTAATGAGGTCGCTGCTAC | ||
| CL2853-2 | CYP4C62 | F—CCGATGTTCAGGAGAAGGCATAC |
| R—CGTAAGCGTACGGCCTATGAAAG | ||
| U31578 | Mito 2U1 | F—CTGATATACACGGCGCACCACC |
| R—GACAGAGATGGGAGTGAGAGTCC | ||
| U31293 | Mito 302A1 | F—GCAGGGACTGTGGTTGTCACACAG |
| R—GAGCGATACAGCTGCGTGGTCCATG | ||
| CL181-1 | Dynamin 1 | F—ACTAAGGTTCCTGTGGGAGATC |
| R—CATCTGGATCAACACTCTTGGC | ||
| U38505 | CYP4D2 | F—CTGCACTTCTGGCGCAAGATGC |
| R—GTCACGTACTCGTCGCGCTC | ||
| CL3168-1 | Nlα8 | F—CAACTACGAGGTGACGCTGATG |
| R—CCGTTGTAAGTCCACGATCCAA | ||
| β-actin | β-actin | F—CGAAGCACAGTCAAAGAGAGGTA |
| R—GCTTCAGTCAAGAGAACAGGATG | ||
| GAPDH | GAPDH | F—GATGTGAAAGCCGAAGGAAACTG |
| R—GTTGGAGATGACCTTGTAAGAGG |
Results and Discussion
Bioassays
Feeding bioassays were used to assess imidacloprid toxicity in locusts. The toxicological regression line (LD-P) was computed as y = 1.0398x + 5.2032. The LD50 of imidacloprid for the locust was 0.64 mg (95% CI 0.51–0.80), with control mortality of 4.44%. According to the LD-P line, LD10 (0.037 mg) and LD80 (4.11 mg) were selected as the low-dose (LD) and high-dose (HD) to treated locusts, respectively.
Expression of nAChR Subunits
To gain insight into the molecular biology of the response to insecticide stress, transcript levels of the target genes, nAChR subunits, were analyzed. A total of 22 nAChR subunit-encoding sequences, originated from CK, LD, and HD reads, were identified in the locust CNS transcriptome. An algorithm (see ‘Materials and Methods’ section) was used to identify genes differentially expressed at LD and HD. Partial data are shown in Table 2 and detailed data in Supp Table 1 (online only). The unigenes annotated as Locα4 and Locα8 showed a significant decrease in expression at LD, and Locα8 expression was also significantly regulated by HD imidacloprid (Table 2). At HD, there are three unigenes annotated as α8 subunit, in which one unigene was upregulated and another one was downregulated. Therefore, the expression variation of Locα8 required further verification.
Table 2.
The expression of nAChR subunits after exposure to imidacloprid
| Name | Unigene ID | Log2Ratio (CK-LD) | Log2Ratio (CK-HD) |
|---|---|---|---|
| α1 | CL4363-2 | −0.93 | 0.02 |
| α2 | U7233 | −0.39 | 0.06 |
| α4 | CL3101-1 | −1.75 | −0.41 |
| CL4363-1 | −1.10 | −0.33 | |
| α5 | U4231 | 0.52 | −0.30 |
| α6 | U17972 | 0.27 | 0.24 |
| α7 | CL2382-2 | 0.42 | 0.57 |
| α8 | CL3168-1 | 0.50 | 1.20 |
| U21252 | −1.26 | 0.02 | |
| U8705 | −8.44 | −2.57 | |
| α9 | U4117 | 0.33 | 0.19 |
| β1 | U23042 | −0.66 | −0.42 |
Recent studies have shown that changes in nAChR subunit expression caused decreased sensitivity or resistance to neonicotinoids (Markussen and Kristensen 2010, Yu et al. 2011, Taillebois et al. 2014). We suggest that the variation in nAChR subunit expression induced by imidacloprid treatment might contribute to the decreased sensitivity to imidacloprid. In previous studies of neonicotinoid resistance, the selection of a resistant strain or population was indispensable, which always was time-consuming. By contrast, the findings on changes in the subunit expression following imidacloprid treatments may allow rapid identification of insensitivity due to changes in expression levels of nAChR subunits.
nAChRs-Interacting Proteins
The intracellular factors involved in the regulation of nAChR function could modulate neonicotinoid sensitivities (Bodereau-Dubois et al. 2012). The cAMP-dependent protein kinase and protein phosphatase 1/2A are known to interact with nAChRs and regulate the functions of nAChRs through their involvement in the phosphorylation or dephosphorylation process. These protein kinases have been shown to reduce the potency of imidacloprid on insect nAChRs (Courjaret and Lapied 2001). Therefore, it is appropriate to determine whether insecticides regulate the function of nAChRs through the modulation of expression of these regulator and modulator proteins.
For nAChRs interacting proteins, 18 and 11 unigenes were regulated at LD and HD, respectively. Partial data are provided in Table 3 and detailed data in Supp Table 2 (online only). A total of 15 genes (14 upregulated and one downregulated genes), encoding interacting proteins, were expressed significantly differently at LD. All of the 11 significantly differentially expressed genes at HD were upregulated. Some proteins interacted with nAChRs and potentially regulated the expression of insect nAChRs. These genes, encoding RIC-3, SAP102, ubiquilin-1, PICK1, N-ethylmaleimide-sensitive factor, pyruvate kinase 3, spectrin alpha chain and protein kinase C, were upregulated at both LD and HD (Table 2). The log2Ratio values of these genes at LD were higher than those at HD. Several genes, such as VAMP-1/2 and dynamin 1, were specifically regulated at LD. There were also some genes specifically regulated at HD, such as the lysozyme C-1 precursor and 14–3–3 protein. These results suggested that the expression of nAChR-interacting proteins was differently regulated by imidacloprid at different doses. These proteins might regulate the function of nAChRs through changes in their expression levels in response to insecticide-induced stress. Of course, these factors merit further investigation.
Table 3.
The expression of some nAChR-associated proteins after exposure to imidacloprid
| Name | Unigene ID | Log2Ratio (CK-LD) | Log2Ratio (CK-HD) | Function |
|---|---|---|---|---|
| RIC-3 | U31006 | 1.15 | 1.02 | nAChR maturation (Millar 2008) |
| SAP102 | CL2460-1 | 1.65 | 0.77 | Anchor postsynaptic proteins (Conroy et al. 2003) |
| CL2460-4 | 0.94 | 1.02 | ||
| ubiquilin-1 | CL1030-2 | 3.02 | 1.83 | nAChR subunit degradation (Ficklin et al. 2005) |
| PICK1 | CL1823-1 | 1.34 | 1.10 | Reduce nAChR clusters (Baer et al. 2007) |
| VAMP-1/2 | U16086 | 1.46 | 0.48 | Synaptic vesicle protein (Farias et al. 2007) |
| COP9 signalosome complex subunit 6 | U10234 | 1.22 | 0.52 | interacting with human β1 (Stelzl et al. 2005) |
| Clathrin heavy chain | CL490-1 | 1.92 | 0.93 | nAChR trafficki (Kabbani et al. 2007) |
| N-ethylmaleimide-sensitive factor | U545 | 1.86 | 1.20 | nAChR trafficking (Kabbani et al. 2007) |
| eEF2 | U17330 | 1.61 | 0.70 | nAChR maturation (Kabbani et al. 2007) |
| G-protein (Goα) | CL4547-1 | 1.31 | 0.69 | interacting with mouse β2 (Kabbani et al. 2007) |
| Pyruvate kinase 3 | CL4699-3 | 1.53 | 1.26 | interacting with mouse β2 (Kabbani et al. 2007) |
| Spectrin alpha chain | U21296 | 2.37 | 2.24 | Cytoskeleton component (Kabbani et al. 2007) |
| Protein kinase C | U33624 | 3.74 | 2.34 | Serine/threonine phosphorylation (Kabbani et al. 2007) |
| creatine kinase | CL4483-1 | 1.35 | 0.49 | interacting with mouse β2 (Kabbani et al. 2007) |
| Dynamin 1 | CL181-1 | −1.30 | −0.72 | nAChR trafficking (Kabbani et al. 2007) |
| Lysozyme C-1 precursor | CL305-1 | −1.27 | −0.17 | interacting with mouse β2 (Kabbani et al. 2007) |
| CL3823-2 | −0.42 | 1.82 | ||
| CL691-2 | 4.38 | −0.58 | ||
| 14–3–3 protein | CL1627-1 | 2.45 | 1.27 | nAChR stabilization (Jeanclos et al. 2001) |
| CL1627-3 | −2.20 | −0.56 | ||
| Synaptotagmin | CL2343-1 | 1.67 | 1.31 | interacting with mouse β2 (Kabbani et al. 2007) |
| CL298-2 | −2.45 | −0.93 |
Dynamin1 (CL181-1) was downregulated at LD, but was unaffected at HD. The different regulation patterns for this gene by an applied dose could be regarded as a signal to define that the dose was a lethal dose or a sub-lethal dose. Upon application of a fixed insecticide dose, insects might be regarded relatively susceptible if the regulation pattern is consistent with that of HD and relatively resistant if consistent with LD. So, after the confirmation in different populations, these differently regulated genes could be developed into molecular markers to estimate the resistance levels in insects.
Expression of P450-Encoding Genes
The expression levels of 54 P450-enoding genes in the locust CNS transcriptome were found to vary (Supp Table 3 [online only]). A total of 43 P450 unigenes, including 32 upregulated unigenes and 11 downregulated unigenes, was differentially expressed at LD. Thirty-four P450 unigenes, including 21 upregulated and 13 downregulated unigenes, were differentially expressed at HD. For both LD and HD, the CYP4 subfamily had the greatest number of differentially expressed genes of all P450 subfamilies. Fourteen upregulated and three downregulated unigenes at LD belonged to the CYP4 subfamily, and the number was 16 at HD, including seven upregulated and nine downregulated unigenes (Table 4). This may be because the CNS P450s are primarily involved in producing hormones rather than metabolize xenobiotics (Ishizaki and Suzuki 1994, Bogus and Scheller 1996). For example, the CYP4 gene family in insect CNS is involved in regulating the production of molting hormone and juvenile hormone (Sutherland et al. 1998, 2000, Maibeche-Coisne et al. 2000, Aragon et al. 2002, Niwa et al. 2011). It is reasonable to assume that a greater number of genes in the CYP4 subfamily, compared with the CYP6 subfamily, were regulated by the insecticide imidacloprid in the locust CNS. P450 CYP4G15 is expressed specifically in the CNS of D. melanogaster (Maibeche-Coisne et al. 2000). Some unigenes were annotated as CYP4G15 in the locust CNS and regulated by imidacloprid treatment.
Table 4.
Numbers of genes encoding P450 proteins whose expression was regulated by imidacloprid treatments
| LD |
HD |
|||
|---|---|---|---|---|
| Upregulated | Downregulated | Upregulated | Downregulated | |
| CYP4 | 14 | 3 | 7 | 9 |
| CYP6 | 9 | 4 | 7 | 2 |
| CYP9 | 4 | 2 | 3 | 0 |
| Mito | 5 | 2 | 4 | 2 |
| Total | 32 | 11 | 21 | 13 |
In insects, the CYP6 P450 subfamily plays important roles in metabolizing imidacloprid (Ding et al. 2013, Riaz et al. 2013, Yang et al. 2013). In our study, 13 and 9 protein differentially expressed unigenes were identified in CYP6 subfamilies at LD and HD, respectively. The expression of most CYP6 unigenes was upregulated (Table 4) CYP6BQ9 in the CNS of Tribolium castaneum and CYP6D1 in the CNS of Musca domestica are involved in insecticide metabolism and so are associated with insecticide resistance (Korytko and Scott 1998, Zhu et al. 2010). Thus, the upregulation of these CYP6 genes in the locust CNS might be involved in variation in imidacloprid sensitivity.
A greater number of genes were regulated by LD than HD. The levels of regulation were also higher at LD than that at HD. This is consistent with a previous report that the expression of few metabolic enzyme genes was induced by high insecticide concentrations (Willoughby et al. 2006). Among 23 genes regulated at both LD and HD, the regulation levels of 19 P450 unigenes were higher at LD than at HD. Meanwhile, more CYP4 subfamily unigenes were upregulated than that downregulated at LD. In contrast, more unigenes were downregulated than that upregulated at HD. The response might be due to the induction of different regulatory mechanisms in response to sublethal and lethal insecticide doses. In response to sublethal dose, insects mainly try to generate the tolerance to insecticides via gene expression regulation. However, in response to a lethal dose, gene expression regulation is focused on survival.
Validation of Differential Gene Expression
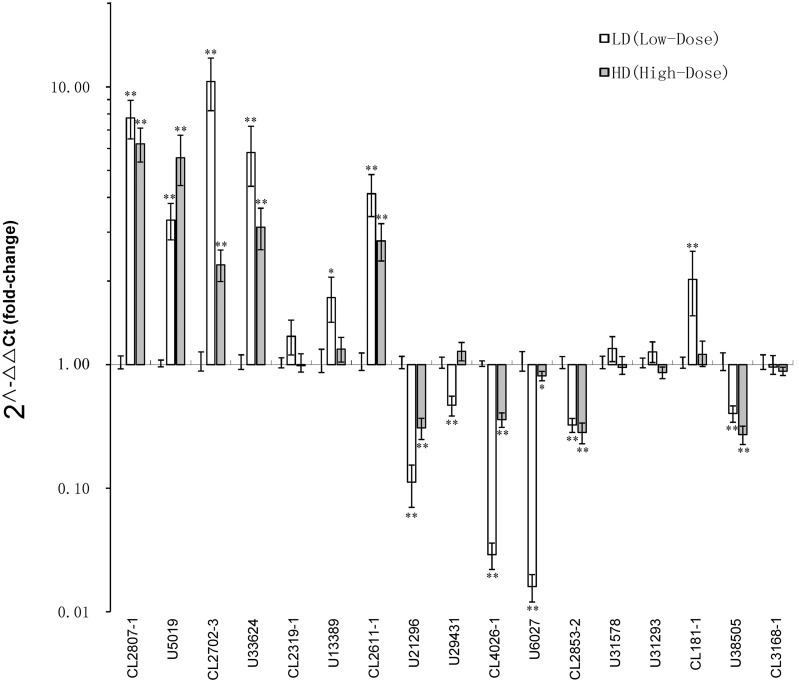
To evaluate the validity of Illumina analysis, a total of 17 differentially expressed unigenes, including one nAChR subunit (Locα8), four nAChRs-interacting proteins unigenes, and 12 P450s unigenes were selected to test expression changes by qRT-PCR following imidacloprid treatments at LD and HD. The expression variation of these unigenes is shown in Table 5 and Figure 1. Among 17 differentially expressed unigenes from the transcriptome analysis, the expression changes in most (more than half) unigenes were confirmed by qRT-PCR in either LD or HD treatments, including upregulation and downregulation. These results indicated that the transcriptome analysis could generally, although not completely, reflect the changes of gene expression with some admissible error. The results of qRT-PCR also supported the conclusion that a greater number of unigenes were regulated by LD than that by HD. However, the changes of some unigenes were not validated by qRT-PCR, such as the downregulation of U21296 and U29431 in LD, which were upregulated in the transcriptome analysis. So, the results from transcriptome analysis always need validation from qRT-PCR or other methods.
Table 5.
The expression variations of 17 unigenes from transcriptome and qRT-PCR analysis
| Unigene ID | LD (transcriptome) |
CK |
qRT-PCR(LD) |
HD (transcriptome) |
qRT-PCR(HD) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log2Ratio (CK-LD) | Fold change | Means | SEM | Means | SEM | Log2Ratio (CK-HD) | Fold change | Means | SEM | |
| CL2087-1 | 4.98 | 31.559 | 1.000 | 0.075 | 7.724 | 1.223** | 2.25 | 4.757 | 6.236 | 0.870** |
| U5019 | 4.45 | 21.857 | 1.000 | 0.039 | 3.311 | 0.498** | 3.90 | 14.929 | 5.557 | 1.144** |
| CL2702-3 | 4.06 | 16.679 | 1.000 | 0.112 | 10.460 | 2.250** | 2.60 | 6.063 | 2.289 | 0.295** |
| U33624 | 3.74 | 13.361 | 1.000 | 0.085 | 5.802 | 1.416** | 2.34 | 5.063 | 3.126 | 0.533** |
| CL2319-1 | 3.32 | 9.987 | 1.000 | 0.058 | 1.265 | 0.181 | 4.10 | 17.148 | 0.983 | 0.112 |
| U13389 | 2.81 | 7.013 | 1.000 | 0.136 | 1.744 | 0.322* | 2.43 | 5.389 | 1.138 | 0.116 |
| CL2611-1 | 2.77 | 6.821 | 1.000 | 0.102 | 4.123 | 0.710** | 2.34 | 5.063 | 2.790 | 0.428** |
| U21296 | 2.37 | 5.169 | 1.000 | 0.071 | 0.112 | 0.042** | 2.24 | 4.724 | 0.308 | 0.060** |
| U29431 | 1.12 | 2.173 | 1.000 | 0.065 | 0.470 | 0.086** | 0.59 | 1.505 | 1.117 | 0.085 |
| CL4026-1 | −7.30 | 0.006 | 1.000 | 0.032 | 0.029 | 0.007** | −1.92 | 0.264 | 0.359 | 0.048** |
| U6027 | −3.69 | 0.077 | 1.000 | 0.114 | 0.016 | 0.004** | −1.42 | 0.374 | 0.812 | 0.071* |
| CL2853-2 | −3.50 | 0.088 | 1.000 | 0.070 | 0.325 | 0.042** | −2.64 | 0.160 | 0.283 | 0.054** |
| U31578 | −1.81 | 0.285 | 1.000 | 0.072 | 1.144 | 0.118 | −1.21 | 0.432 | 0.952 | 0.117 |
| U31293 | −1.71 | 0.306 | 1.000 | 0.056 | 1.113 | 0.095 | −2.52 | 0.174 | 0.864 | 0.093 |
| CL181-1 | −1.30 | 0.406 | 1.000 | 0.064 | 2.029 | 0.530** | −0.72 | 0.607 | 1.090 | 0.125 |
| U38505 | 0.98 | 1.972 | 1.000 | 0.103 | 0.403 | 0.061** | −2.92 | 0.132 | 0.272 | 0.046** |
| CL3168-1 | 0.50 | 1.414 | 1.000 | 0.085 | 0.957 | 0.122 | 1.20 | 2.297 | 0.886 | 0.069 |
* Significantly different at 0.05 level; **significantly different at 0.01 level.
Fig. 1.
The fold change in expression level of 17 unigenes between different dose treatments (LD and HD) and CK determined by qRT-PCR. The expression levels in LD and HD were normalized to that in CK. Values are plotted as means ± SE of at least three repeats. *Significantly different at 0.05 level; **Significantly different at 0.01 level.
In conclusion, the response to different imidacloprid doses differed significantly in locusts at the transcription level of related genes. The numbers and levels of nAChRs, interacting proteins, and P450 genes regulated by imidacloprid application were different between LD and HD treatments. Thus, different regulatory mechanisms would be motivated to mediate the response to stresses induced by different insecticide concentrations. Alternatively, it is possible that the expression variation of related genes could modulate neonicotinoid sensitivities by influencing insecticide detoxifications or by regulating target sensitivities, which might be potential factors for insecticide resistance.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
This work was supported by the National Key Technology Research and Development Program (2012BAD19B01), Jiangsu Science for Distinguished Young Scholars (BK20130028), and the Innovation Project for Graduate Students of Jiangsu Province (KYLX_0579).
References Cited
- Aragon S., Claudinot S., Blais C., Maibeche M., Dauphin-Villemant C. 2002. Molting cycle-dependent expression of CYP4C15, a cytochrome P450 enzyme putatively involved in ecdysteroidogenesis in the crayfish, Orconectes limosus. Insect Biochem. Mol. Biol. 32: 153–159. [DOI] [PubMed] [Google Scholar]
- Audic S., Claverie J. M. 1997. The significance of digital gene expression profiles. Genome Res. 7: 986–995. [DOI] [PubMed] [Google Scholar]
- Baer K., Buerli T., Huh K.-H., Wiesner A., Erb-Voegtli S., Goeckeritz-Dujmovic D., Moransard M., Nishimune A., Rees M. I., Henley J. M., et al. 2007. PICK1 interacts with alpha 7 neuronal nicotinic acetylcholine receptors and controls their clustering. Mol. Cell. Neurosci. 35: 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodereau-Dubois B., List O., Calas-List D., Marques O., Communal P.-Y., Thany S. H., Lapied B. 2012. Transmembrane potential polarization, calcium influx, and receptor conformational state modulate the sensitivity of the imidacloprid-insensitive neuronal insect nicotinic acetylcholine receptor to neonicotinoid insecticides. J. Pharmacol. Exp. 341: 326–339. [DOI] [PubMed] [Google Scholar]
- Bogus M., Scheller K. 1996. Allatotropin released by the brain controls larval molting in Galleria mellonella by affecting juvenile hormone synthesis. Int. J. Dev. Biol. 40: 205–210. [PubMed] [Google Scholar]
- Choo Y. M., Lee B. H., Lee K. S., Kim B. Y., Li J., Kim J. G., Lee J. H., Sohn H. D., Nah S. Y., Jin B. R. 2008. Pr-lynx1, a modulator of nicotinic acetylcholine receptors in the insect. Mol. Cell. Neurosci. 38: 224–235. [DOI] [PubMed] [Google Scholar]
- Conroy W. G., Liu Z. P., Nai Q., Coggan J. S., Berg D. K. 2003. PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron 38: 759–771. [DOI] [PubMed] [Google Scholar]
- Courjaret R., Lapied B. 2001. Complex intracellular messenger pathways regulate one type of neuronal alpha-bungarotoxin-resistant nicotinic acetylcholine receptors expressed in insect neurosecretory cells (dorsal unpaired median neurons). Mol. Pharmacol. 60: 80–91. [PubMed] [Google Scholar]
- Ding Z., Wen Y., Yang B., Zhang Y., Liu S., Liu Z., Han Z. 2013. Biochemical mechanisms of imidacloprid resistance in Nilaparvata lugens: over-expression of cytochrome P450 CYP6AY1. Insect Biochem. Mol. Biol. 43: 1021–1027. [DOI] [PubMed] [Google Scholar]
- Farias G. G., Valles A. S., Colombres M., Godoy J. A., Toledo E. M., Lukas R. J., Barrantes F. J., Inestrosa N. C. 2007. Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J. Neurosci. 27: 5313–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyereisen R. 1999. Insect P450 enzymes. Annu. Rev. Entomol. 44: 507–533. [DOI] [PubMed] [Google Scholar]
- Ficklin M. B., Zhao S. L., Feng G. P. 2005. Ubiquilin-1 regulates nicotine-induced up-regulation of neuronal nicotinic acetylcholine receptors. J. Biol. Chem. 280: 34088–34095. [DOI] [PubMed] [Google Scholar]
- Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanali A., Njagi P. G. N., Bashir M. O. 2005. Chemical ecology of locusts and related acridids. Annu. Rev. Entomol. 50: 223–245. [DOI] [PubMed] [Google Scholar]
- Iseli C., Jongeneel C. V., Bucher P. 1999. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc. Intl Conf. Intell. Syst. Mol. Biol. 138–148. [PubMed] [Google Scholar]
- Ishizaki H., Suzuki A. 1994. The brain secretory peptides that control molting and metamorphosis of the sikmoth, Bombyx-mori. Int. J. Dev. Biol. 38: 301–310. [PubMed] [Google Scholar]
- Jeanclos E. M., Lin L., Treuil M. W., Rao J., DeCoster M. A., Anand R. 2001. The chaperone protein 14-3-3 eta interacts with the nicotinic acetylcholine receptor alpha 4 subunit—evidence for a dynamic role in subunit stabilization. J. Biol. Chem. 276: 28281–28290. [DOI] [PubMed] [Google Scholar]
- Joussen N., Heckel D. G., Haas M., Schuphan I., Schmidt B. 2008. Metabolism of imidacloprid and DDT by P450 GYP6G1 expressed in cell cultures of Nicotiana tabacum suggests detoxification of these insecticides in Cyp6g1-overexpressing strains of Drosophila melanogaster, leading to resistance. Pest Manag. Sci. 64: 65–73. [DOI] [PubMed] [Google Scholar]
- Kabbani N., Woll M. P., Levenson R., Lindstrom J. M., Changeux J.-P. 2007. Intracellular complexes of the beta 2 subunit of the nicotinic acetylcholine receptor in brain identified by proteomics. Proc. Natl. Acad. Sci. U. S. A. 104: 20570–20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunker I., Morou E., Nikou D., Nauen R., Sertchook R., Stevenson B. J., Paine M. J. I., Morin S., Vontas J. 2009. Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high levels of imidacloprid resistance. Insect Biochem. Mol. Biol. 39: 697–706. [DOI] [PubMed] [Google Scholar]
- Korytko P. J., Scott J. G. 1998. CYP6D1 protects thoracic ganglia of houseflies from the neurotoxic insecticide cypermethrin. Arch. Insect Biochem. Physiol. 37: 57–63. [DOI] [PubMed] [Google Scholar]
- Lansdell S. J., Gee V. J., Harkness P. C., Doward A. I., Baker E. R., Gibb A. J., Millar N. S. 2005. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol. Pharmacol. 68: 1431–1438. [DOI] [PubMed] [Google Scholar]
- Liu Z., Cao G., Li J., Bao H., Zhang Y. 2009. Identification of two Lynx proteins in Nilaparvata lugens and the modulation on insect nicotinic acetylcholine receptors. J. Neurochem. 110: 1707–1714. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Maibeche-Coisne M., Monti-Dedieu L., Aragon S., Dauphin-Villemant C. 2000. A new cytochrome P450 from Drosophila melanogaster, CYP4G15, expressed in the nervous system. Biochem. Biophys. Res. Commun. 273: 1132–1137. [DOI] [PubMed] [Google Scholar]
- Markussen M. D. K., Kristensen M. 2010. Low expression of nicotinic acetylcholine receptor subunit Md alpha 2 in neonicotinoid-resistant strains of Musca domestica L. Pest Manag. Sci. 66: 1257–1262. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Buckingham S. D., Kleier D., Rauh J. J., Grauso M., Sattelle D. B. 2001. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 22: 573–580. [DOI] [PubMed] [Google Scholar]
- Miksys S., Tyndale R. F. 2009. Brain drug-metabolizing cytochrome P450 enzymes are active in vivo, demonstrated by mechanism-based enzyme inhibition. Neuropsychopharmacology 34: 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar N. S. 2008. RIC-3: a nicotinic acetylcholine receptor chaperone. Br. J. Pharmacol. 153: S177–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A., Williams B. A., McCue K., Schaeffer L., Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5: 621–628. [DOI] [PubMed] [Google Scholar]
- Niwa R., Sakudoh T., Matsuya T., Namiki T., Kasai S., Tomita T., Kataoka H. 2011. Expressions of the cytochrome P450 monooxygenase gene Cyp4g1 and its homolog in the prothoracic glands of the fruit fly Drosophila melanogaster (Diptera: Drosophilidae) and the silkworm Bombyx mori (Lepidoptera: Bombycidae). Appl. Entomol. Zool. 46: 533–543. [Google Scholar]
- Riaz M. A., Chandor-Proust A., Dauphin-Villemant C., Poupardin R., Jones C. M., Strode C., Regent-Kloeckner M., David J. P., Reynaud S. 2013. Molecular mechanisms associated with increased tolerance to the neonicotinoid insecticide imidacloprid in the dengue vector Aedes aegypti. Aquat. Toxicol. 126: 326–337. [DOI] [PubMed] [Google Scholar]
- Sattelle D. B. 1980. Acetylcholine receptors of insects. Adv. Insect Physiol. 15:215–315. [Google Scholar]
- Snyder M. J., Stevens J. L., Andersen J. F., Feyereisen R. 1995. Expression of cytochrome-P450 genes of the CYP4 family in midgut and fat-body of the tobacco hornworm, Manduca sexta. Arch. Biochem. Biophys. 321: 13–20. [DOI] [PubMed] [Google Scholar]
- Stelzl U., Worm U., Lalowski M., Haenig C., Brembeck F. H., Goehler H., Stroedicke M., Zenkner M., Schoenherr A., Koeppen S., et al. 2005. A human protein–protein interaction network: a resource for annotating the proteome. Cell 122: 957–968. [DOI] [PubMed] [Google Scholar]
- Stevens J. L., Snyder M. J., Koener J. F., Feyereisen R. 2000. Inducible P450s of the CYP9 family from larval Manduca sexta midgut. Insect Biochem. Mol. Biol. 30: 559–568. [DOI] [PubMed] [Google Scholar]
- Strobel H. W., Thompson C. M., Antonovic L. 2001. Cytochromes P450 in brain: function and significance. Curr. Drug Metab. 2: 199–214. [DOI] [PubMed] [Google Scholar]
- Sutherland T. D., Unnithan G. C., Andersen J. F., Evans P. H., Murataliev M. B., Szabo L. Z., Mash E. A., Bowers W. S., Feyereisen R. 1998. A cytochrome P450 terpenoid hydroxylase linked to the suppression of insect juvenile hormone synthesis. Proc. Natl. Acad. Sci. U. S. A. 95: 12884–12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland T. D., Unnithan G. C., Feyereisen R. 2000. Terpenoid omega-hydroxylase (CYP4C7) messenger RNA levels in the corpora allata: a marker for ovarian control of juvenile hormone synthesis in Diploptera punctata. J. Insect Physiol. 46: 1219–1227. [DOI] [PubMed] [Google Scholar]
- Taillebois E., Beloula A., Quinchard S., Jaubert-Possamai S., Daguin A., Servent D., Tagu D., Thany S. H., Tricoire-Leignel H. 2014. Neonicotinoid binding, toxicity and expression of nicotinic acetylcholine receptor subunits in the aphid Acyrthosiphon pisum. PLoS One 9: e96669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.-Y., Zhang C.-X. 2013. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 20: 254–260. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: 0034.1–0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby L., Chung H., Lumb C., Robin C., Batterham P., Daborn P. J. 2006. A comparison of Drosophila melanogaster detoxification gene induction responses for six insecticides, caffeine and phenobarbital. Insect Biochem. Mol. Biol. 36: 934–942. [DOI] [PubMed] [Google Scholar]
- Yang B., Yao X., Gu S., Zhang Y., Liu Z., Zhang Y. 2010. Selectivity of lynx proteins on insect nicotinic acetylcholine receptors in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 19: 283–289. [DOI] [PubMed] [Google Scholar]
- Yang X., Xie W., Wang S.-l., Wu Q.-J., Pan H.-P., Li R.-M., Yang N.-N., Liu B.-M., Xu B.-Y., Zhou X., et al. 2013. Two cytochrome P450 genes are involved in imidacloprid resistance in field populations of the whitefly, Bemisia tabaci, in China. Pestic. Biochem. Physiol. 107: 343–350. [DOI] [PubMed] [Google Scholar]
- Yu X., Wang M., Kang M., Liu L., Guo X., Xu B. 2011. Molecular-cloning and characterization of two nicotine acetylcholine receptor beta subunit genes from Apis cerana cerana. Arch. Insect Biochem. Physiol. 77: 163–178. [DOI] [PubMed] [Google Scholar]
- Zhu F., Parthasarathy R., Bai H., Woithe K., Kaussmann M., Nauen R., Harrison D. A., Palli S. R. 2010. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc. Natl. Acad. Sci. U. S. A. 107: 8557–8562. [DOI] [PMC free article] [PubMed] [Google Scholar]